Electrostatic Self-Assembled Synthesis of Amorphous/Crystalline g-C3N4 Homo-Junction for Efficient Photocatalytic H2 Production with Simultaneous Antibiotic Degradation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
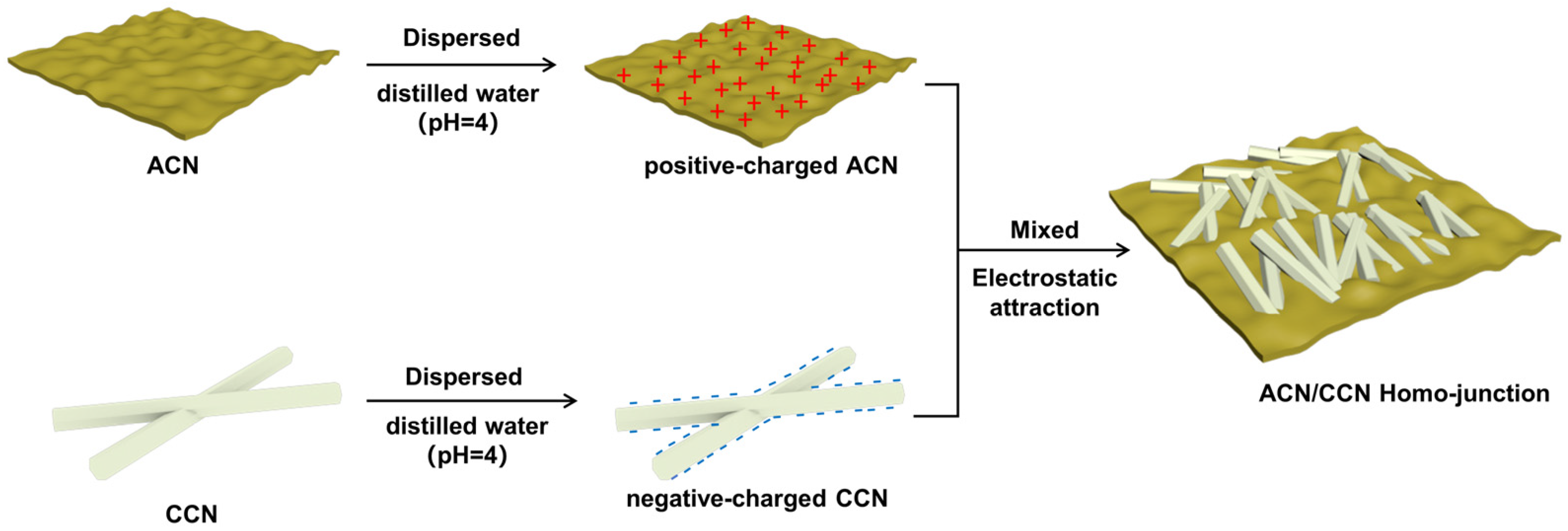
2.2. Synthesis of Amorphous and Crystalline g-C3N4 Homo-Junction
2.3. Material Characterization
2.4. Photoelectrochemical Analysis
2.5. Photocatalytic Performance Analysis
3. Results and Discussion
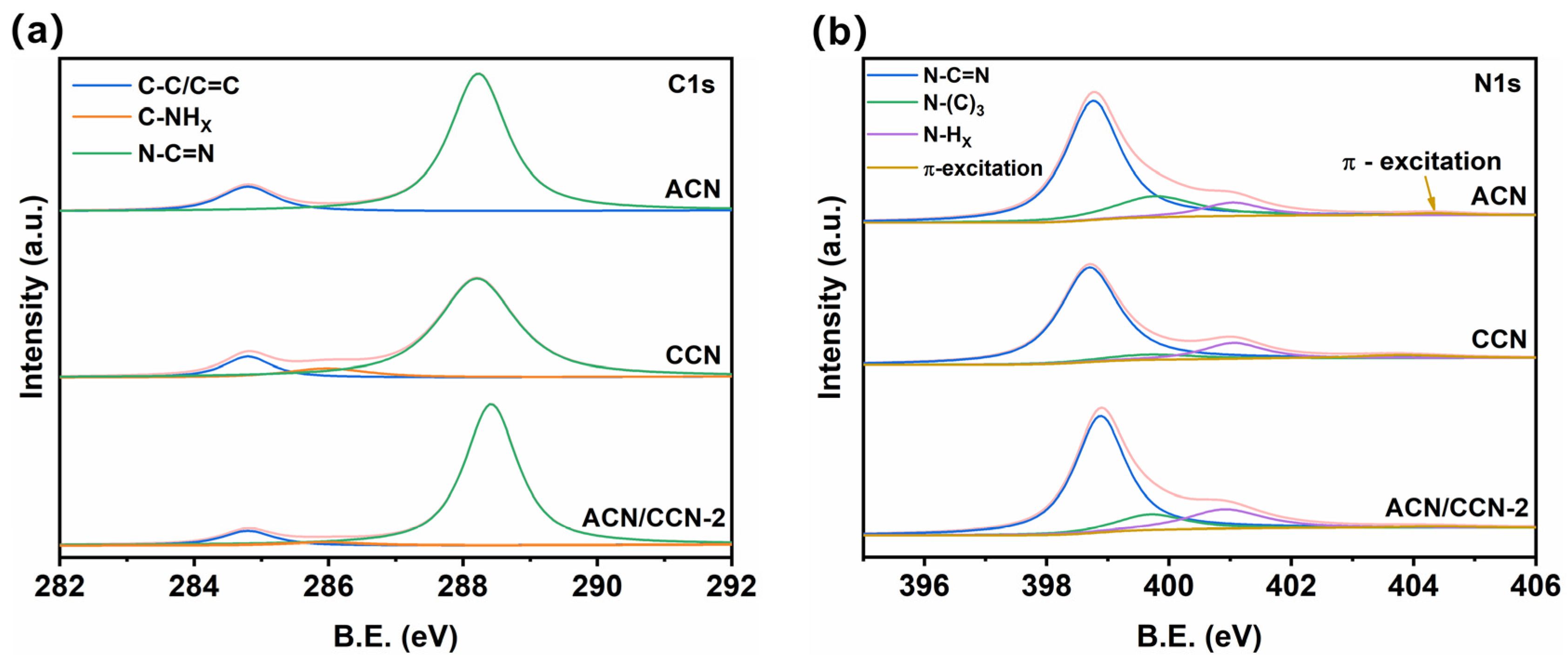
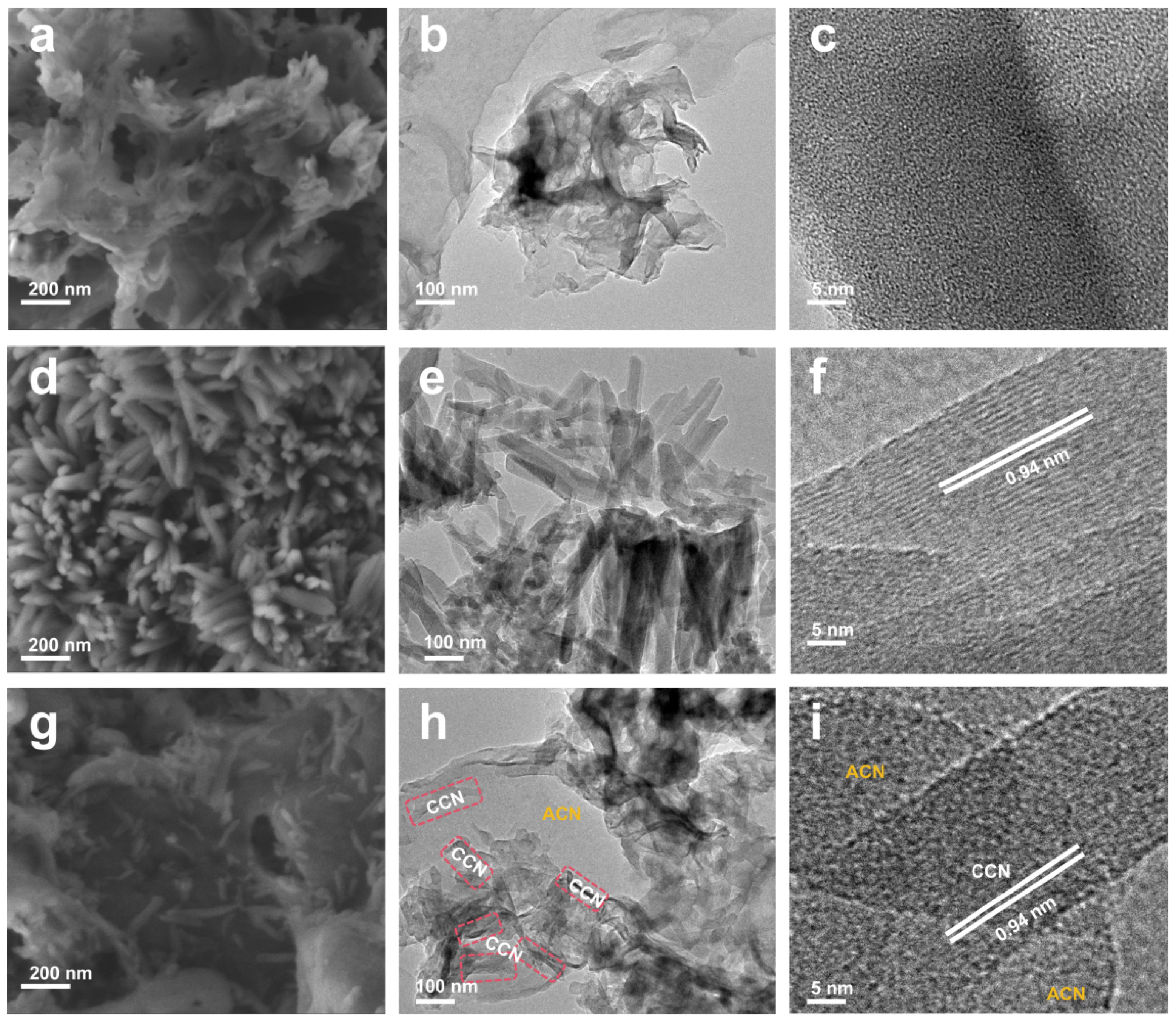
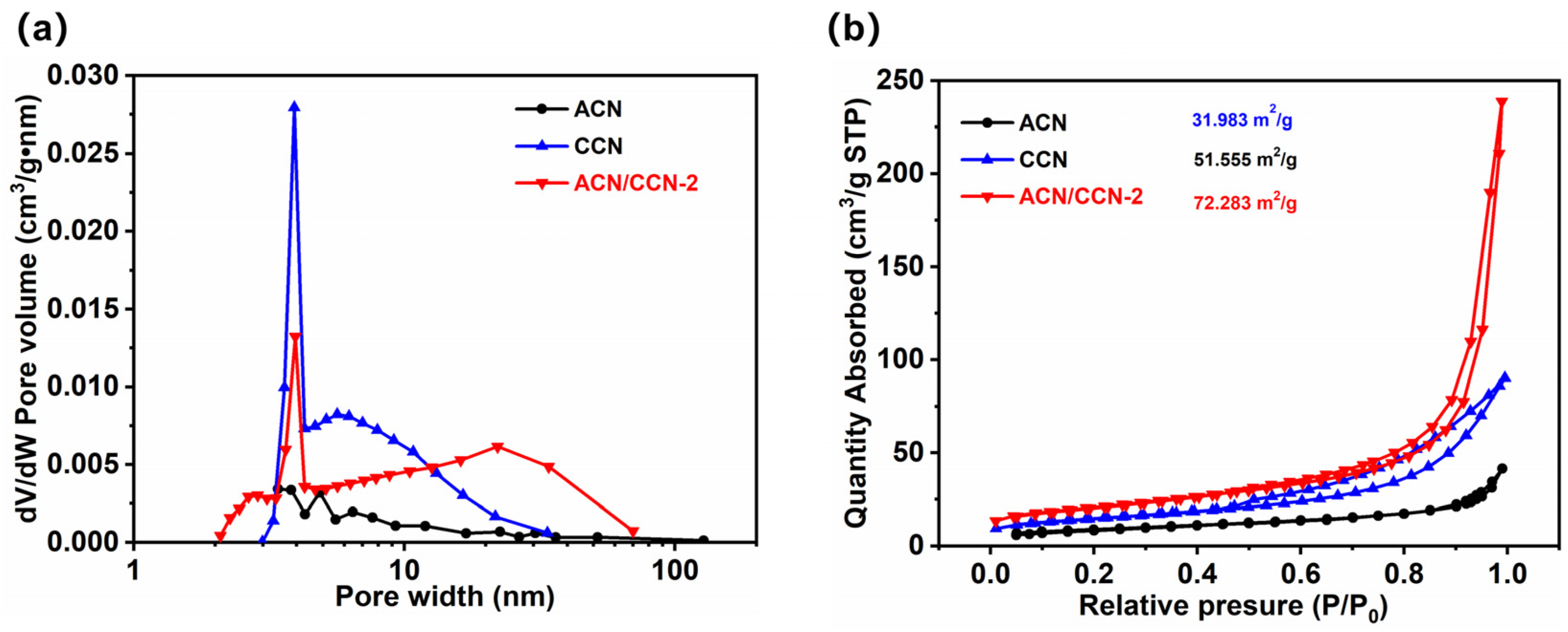
3.1. Morphology and Structure
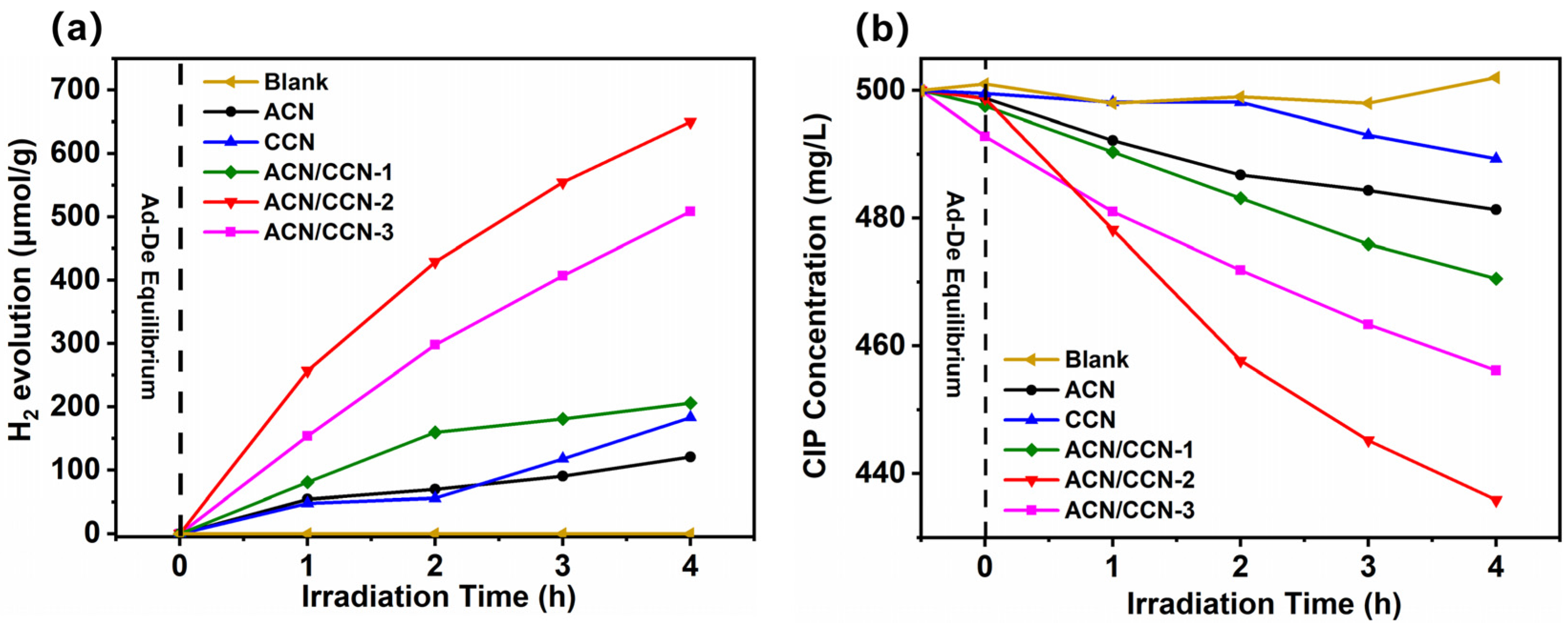
3.2. Photocatalytic H2 Evolution from Wastewater
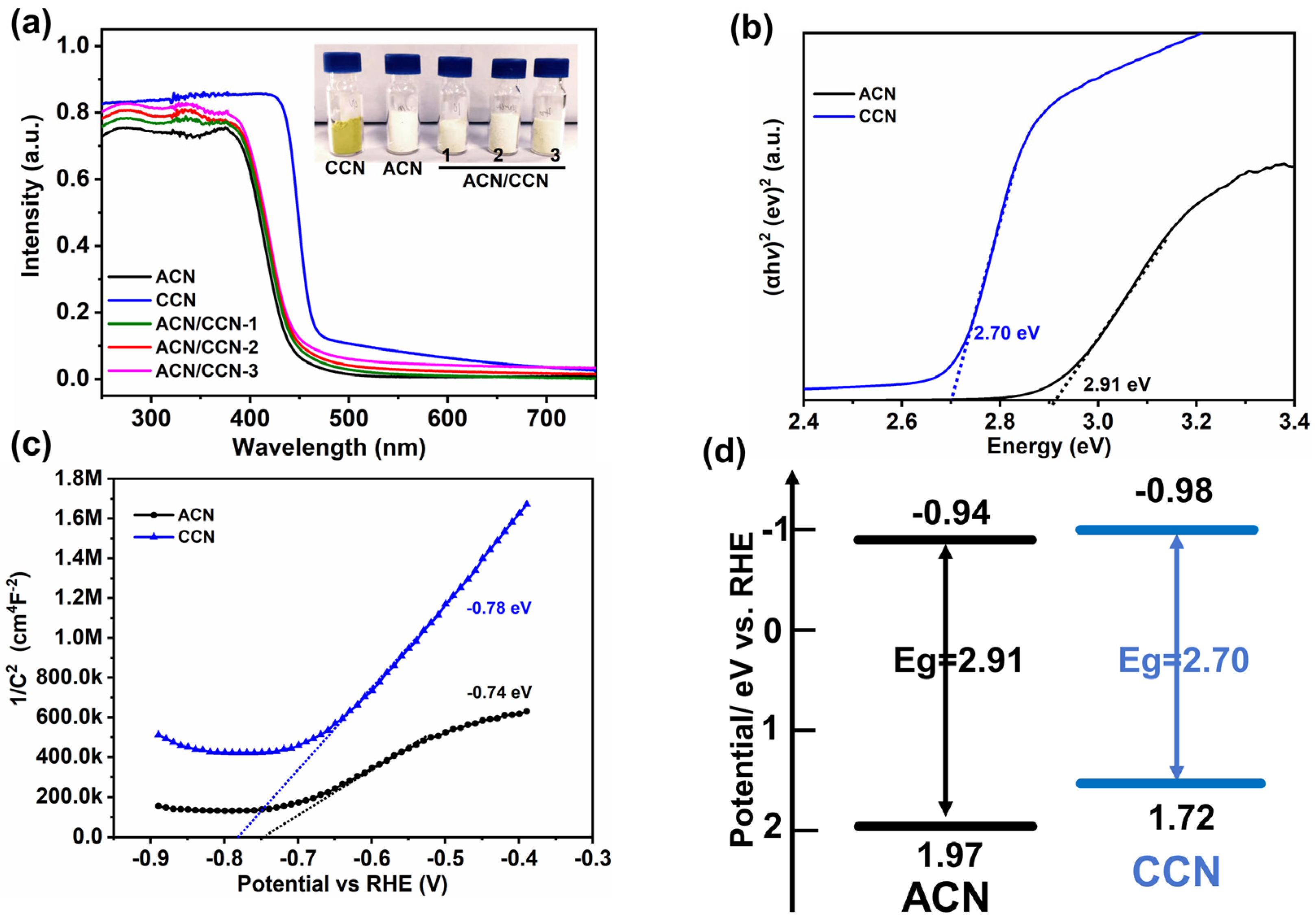
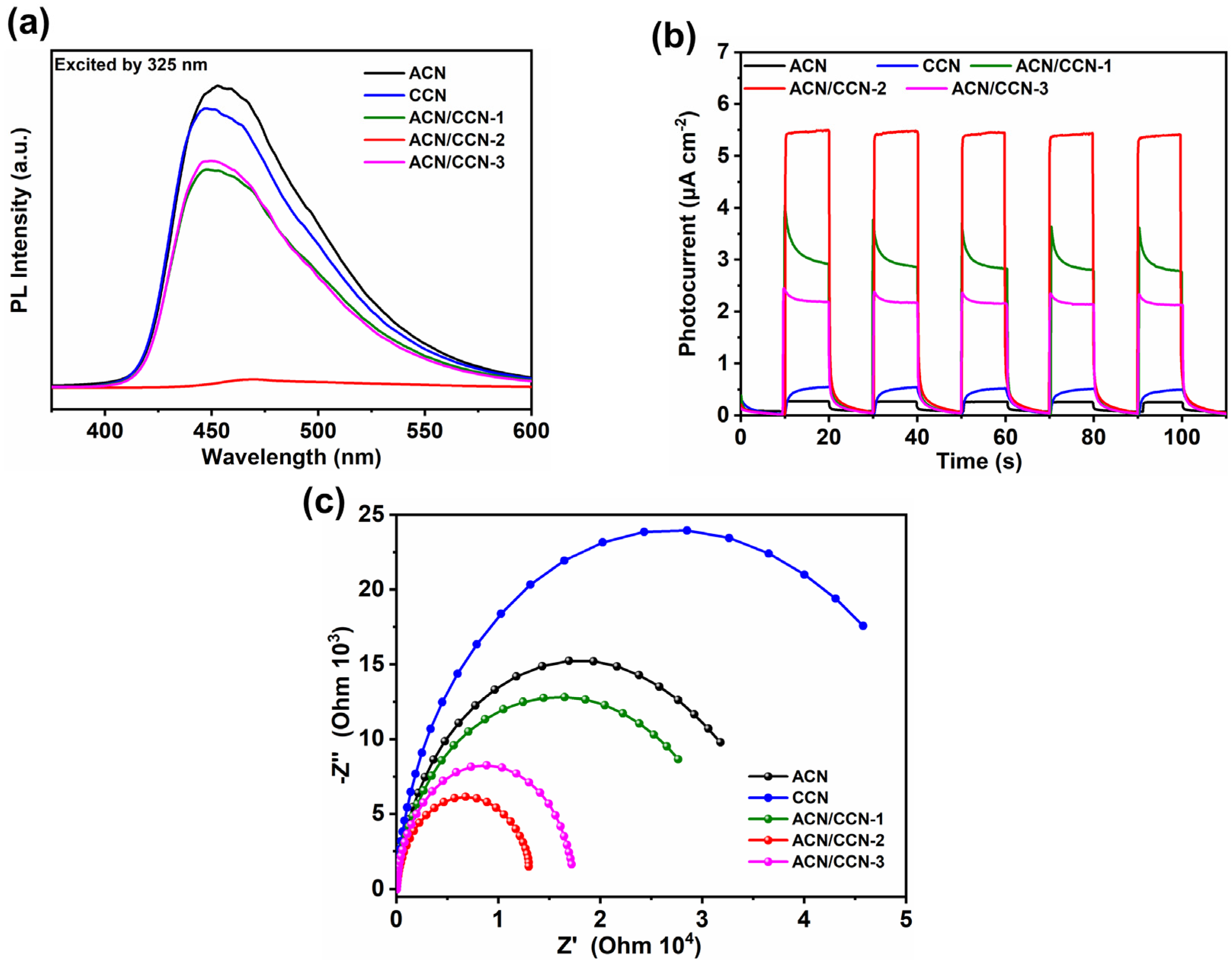
3.3. Photophysical Properties of the ACN/CCN Homo-Junction
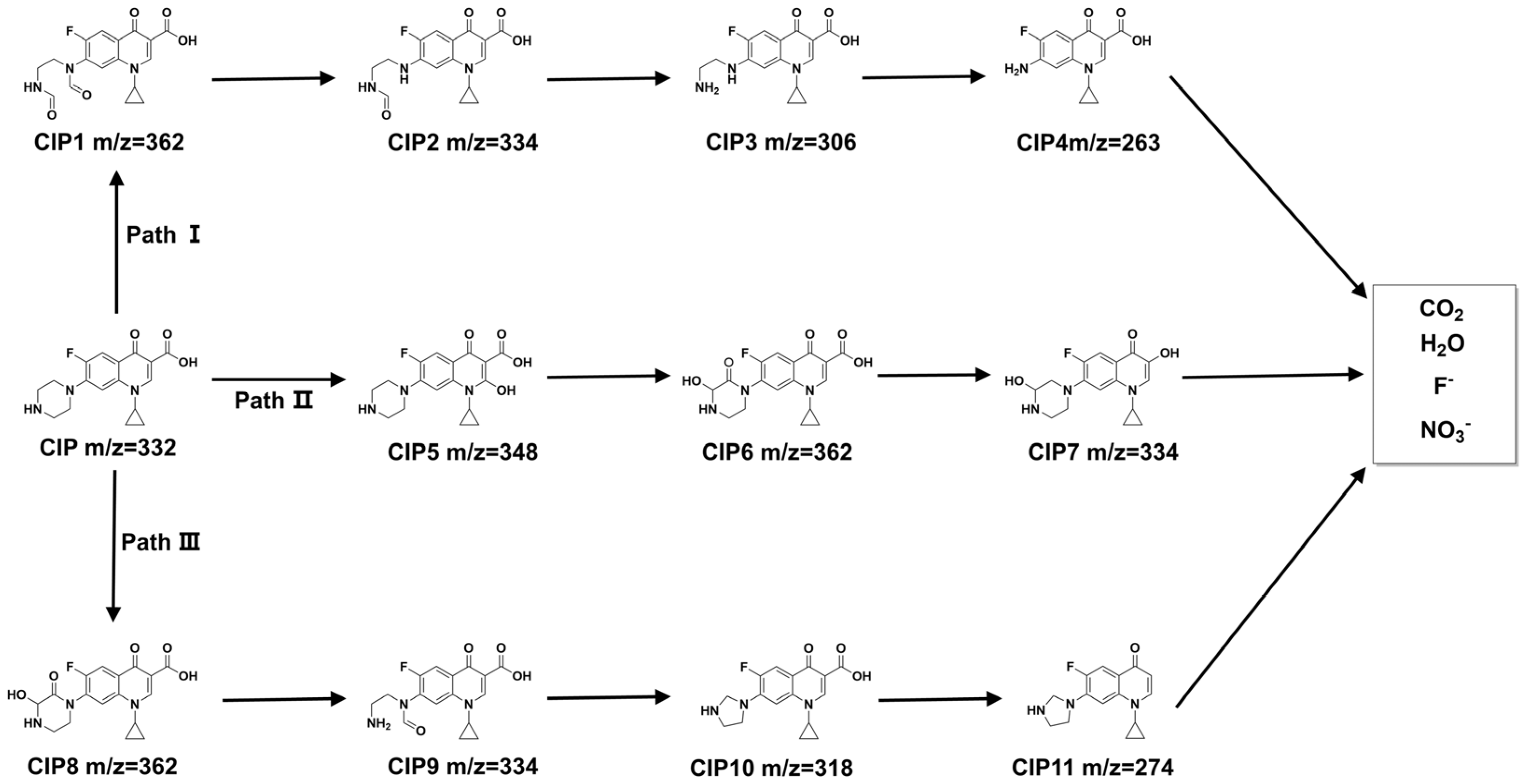
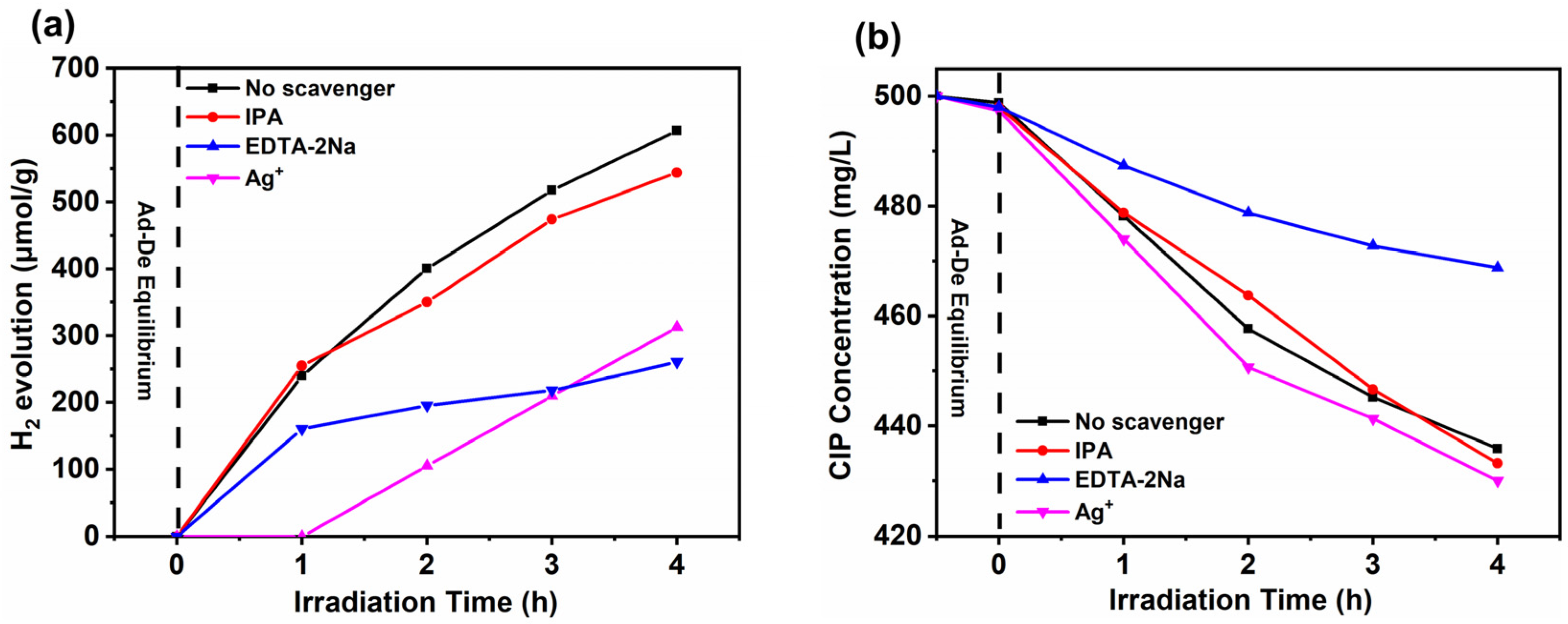
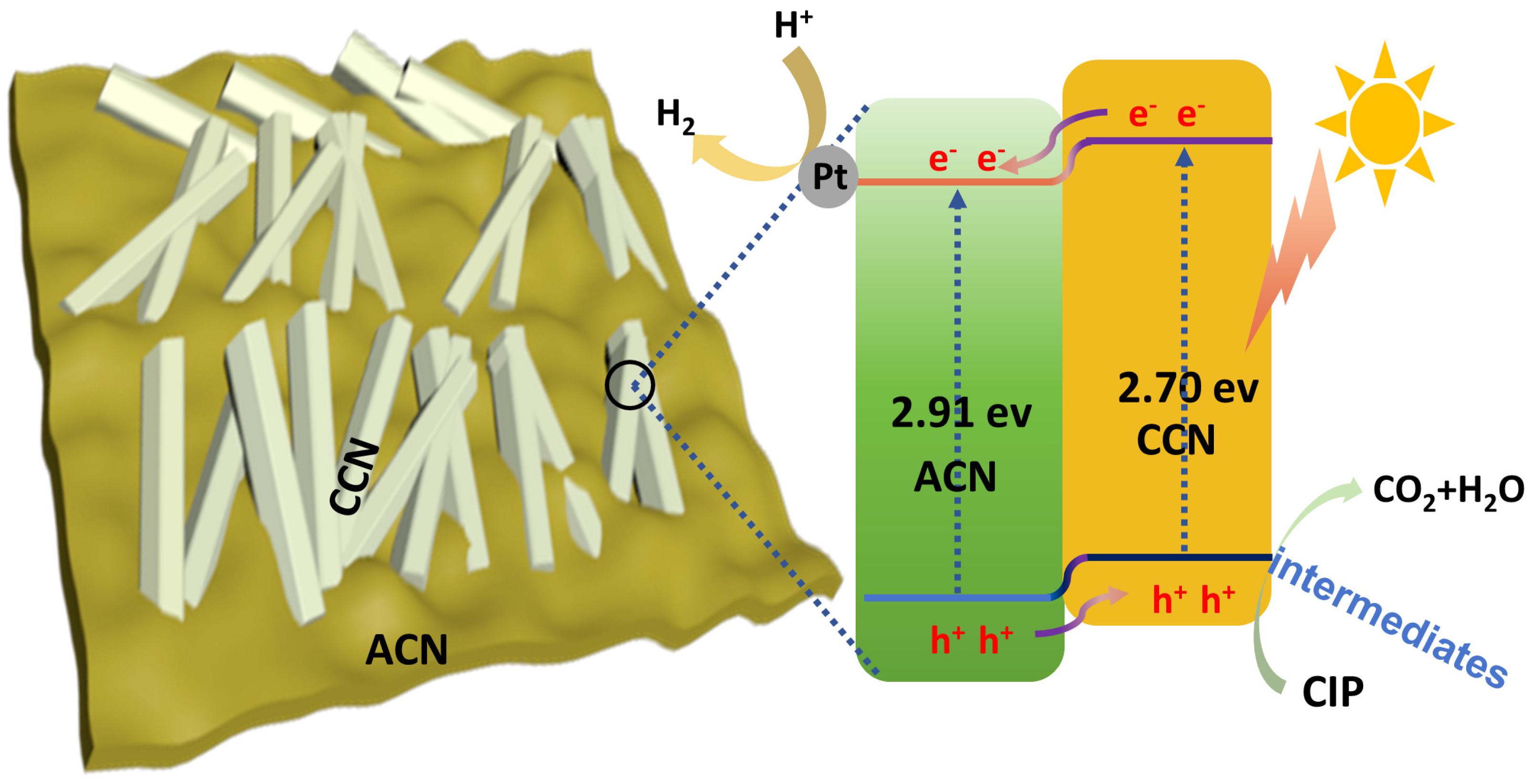
3.4. Photocatalytic Mechanism of H2 Evolution from Wastewater
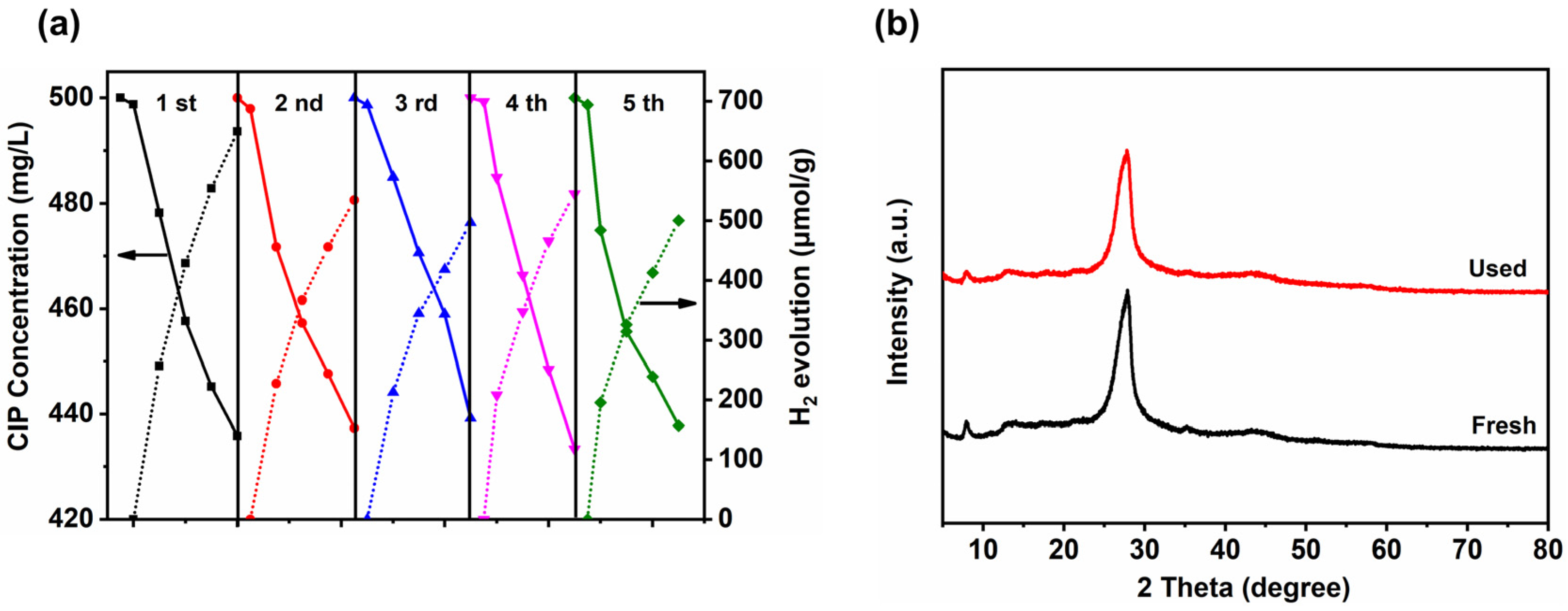
3.5. Stability of ACN/CCN for Photocatalytic H2 Evolution from Wastewater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kawawaki, T.; Kawachi, M.; Yazaki, D.; Akinaga, Y.; Hirayama, D.; Negishi, Y. Development and Functionalization of Visible-Light-Driven Water-Splitting Photocatalysts. Nanomaterials 2022, 12, 344. [Google Scholar] [CrossRef]
- Jiang, D.; Yuan, H.; Liu, Z.; Chen, Y.; Li, Y.; Zhang, X.; Xue, G.; Liu, H.; Liu, X.; Zhao, L.; et al. Defect-anchored single-atom-layer Pt clusters on TiO2−x/Ti for efficient hydrogen evolution via photothermal reforming plastics. Appl. Catal. B 2023, 339, 123081. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Z.; Zhang, G. Combining heterogeneous photocatalysis and enzymatic catalysis via membrane: Conversion of biomass for H2 production from water. Appl. Catal. B 2023, 338, 123069. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Y.; Zhu, J.; Li, Y.; He, C.; Ren, X.; Zhang, P.; Mi, H. Enhanced photocatalytic H2 production independent of exciton dissociation in crystalline carbon nitride. Appl. Catal. B 2023, 338, 123049. [Google Scholar] [CrossRef]
- Dessì, A.; Monai, M.; Bessi, M.; Montini, T.; Calamante, M.; Mordini, A.; Reginato, G.; Trono, C.; Fornasiero, P.; Zani, L. Towards Sustainable H2 Production: Rational Design of Hydrophobic Triphenylamine-based Dyes for Sensitized Ethanol Photoreforming. ChemSusChem 2018, 11, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Cheng, K.; Zhang, Y.; Ran, J.; Wu, G. Incorporation of Nonmetal Group Dopants into g-C3N4 Framework for Highly Improved Photocatalytic H2 Production. Nanomaterials 2021, 11, 1480. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Guo, W.; Wang, H.; Zhu, B.; Yu, J.; Qiao, S.-Z. Metal-Free 2D/2D Phosphorene/g-C3N4 Van der Waals Heterojunction for Highly Enhanced Visible-Light Photocatalytic H2 Production Metal-Free 2D/2D Phosphorene/g-C3N4 Van der Waals Heterojunction for Highly Enhanced Visible-Light Photocatalytic H2 Production. Adv. Mater. 2018, 30, 1800128–1800133. [Google Scholar] [CrossRef]
- She, P.; Qin, J.s.; Sheng, J.; Qi, Y.; Rui, H.; Zhang, W.; Ge, X.; Lu, G.; Song, X.; Rao, H. Dual-Functional Photocatalysis for Cooperative Hydrogen Evolution and Benzylamine Oxidation Coupling over Sandwiched-Like Pd@TiO2@ZnIn2S4 Nanobox. Small 2022, 18, 2105114–2105122. [Google Scholar] [CrossRef]
- Musa, E.N.; Kaur, S.; Gallagher, T.C.; Anthony, T.M.; Stickle, W.F.; Árnadóttir, L.; Stylianou, K.C. Two Birds, One Stone: Coupling Hydrogen Production with Herbicide Degradation over Metal–Organic Framework-Derived Titanium Dioxide. ACS Catal. 2023, 13, 3710–3722. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Sun, Y.; Luo, Y. Bifunctional Ag-Decorated CeO2 Nanorods Catalysts for Promoted Photodegradation of Methyl Orange and Photocatalytic Hydrogen Evolution. Nanomaterials 2021, 11, 1104. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, J.; Yan, J.; Song, Y.; Mo, Z.; Qian, J.; Wu, X.; Yuan, S.; Li, H.; Xu, H. Cryo-mediated liquid-phase exfoliated 2D BP coupled with 2D C3N4 to photodegradate organic pollutants and simultaneously generate hydrogen. Appl. Surf. Sci. 2019, 490, 117–123. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, X.; Wang, Z. Noble Metal-Free TiO2-Coated Carbon Nitride Layers for Enhanced Visible Light-Driven Photocatalysis. Nanomaterials 2020, 10, 805. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zeng, F.; Zhong, R.; Xie, Y.; Wang, J.; Ye, H.; Ling, Y.; Guo, R.; Zhao, J.; Li, S.; et al. TiO2 Nanowires with Doped g-C3N4 Nanoparticles for Enhanced H2 Production and Photodegradation of Pollutants. Nanomaterials 2021, 11, 254. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, S.; Li, N.; Wu, F.; Chen, S.; Lu, W.; Chen, W. Waste-to-Energy Conversion on Graphitic Carbon Nitride: Utilizing the Transformation of Macrolide Antibiotics to Enhance Photoinduced Hydrogen Production. ACS Sustain. Chem. Eng. 2017, 5, 9667–9672. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, W.; Duan, W.; Wang, S.; Jia, L.; Zhang, G.; Zhu, B.; Huang, W.; Zhang, S. Constructing Co3O4/g-C3N4 Ultra-Thin Nanosheets with Z-Scheme Charge Transfer Pathway for Efficient Photocatalytic Water Splitting. Nanomaterials 2021, 11, 3341. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhang, M.; Qu, Z.H.; Cui, X.; Liu, Z.Y.; Piao, C.C.; Li, S.G.; Wang, J.; Song, Y.T. Fabrication of highly active Z-scheme Ag/g-C3N4-Ag-Ag3PO4 (110) photocatalyst photocatalyst for visible light photocatalytic degradation of levofloxacin with simultaneous hydrogen production. Chem. Eng. J. 2020, 382, 122394. [Google Scholar] [CrossRef]
- Li, G.; Deng, X.X.; Chen, P.; Wang, X.D.; Ma, J.; Liu, F.; Yin, S.F. Sulphur vacancies-VS2@C3N4 drived by in situ supramolecular self-assembly for synergistic photocatalytic degradation of real wastewater and H2 production: Vacancies taming interfacial compact heterojunction and carriers transfer. Chem. Eng. J. 2022, 433, 134505. [Google Scholar] [CrossRef]
- Deng, Q.; Li, H.; Ba, G.; Huo, T.; Hou, W. The pivotal role of defects in fabrication of polymeric carbon nitride homojunctions with enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2021, 586, 748–757. [Google Scholar] [CrossRef]
- Hu, S.; Ma, L.; Li, F.; Fan, Z.; Wang, Q.; Bai, J.; Kang, X.; Wu, G. Construction of g-C3N4/S-g-C3N4 metal-free isotype heterojunctions with an enhanced charge driving force and their photocatalytic performance under anoxic conditions. RSC Adv. 2015, 5, 90750–90756. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, L.; Zhu, Y.; Zhang, Y.; Liu, J.; Wang, J.; Feng, C.; Qiao, L.; Chen, Y. Conjugated polymers S-scheme homojunction with large internal electric field and matching interface for efficient visible light photocatalytic degradation of ciprofloxacin. J. Clean. Prod. 2023, 419, 138199. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, L.; Li, G.; Zhang, Y.; Savateev, A.; Zafeiratos, S.; Wang, X.; Antonietti, M. Ionothermal Synthesis of Triazine–Heptazine-Based Copolymers with Apparent Quantum Yields of 60% at 420 nm for Solar Hydrogen Production from “Sea Water”. Angew. Chem. Int. Ed. 2018, 57, 9372–9376. [Google Scholar] [CrossRef]
- Sun, H.; Shi, Y.; Shi, W.; Guo, F. High-crystalline/amorphous g-C3N4 S-scheme homojunction for boosted photocatalytic H2 production in water/simulated seawater: Interfacial charge transfer and mechanism insight. Appl. Surf. Sci. 2022, 593, 153281. [Google Scholar] [CrossRef]
- Tang, R.; Gong, D.; Zhou, Y.; Deng, Y.; Feng, C.; Xiong, S.; Huang, Y.; Peng, G.; Li, L. Unique g-C3N4/PDI-g-C3N4 homojunction with synergistic piezo-photocatalytic effect for aquatic contaminant control and H2O2 generation under visible light. Appl. Catal. B 2022, 303, 120929. [Google Scholar] [CrossRef]
- He, B.; Feng, M.; Chen, X.; Cui, Y.; Zhao, D.; Sun, J. Fabrication of potassium ion decorated 1D/2D g-C3N4/g-C3N4 homojunction enabled by dual-ions synergistic strategy for enhanced photocatalytic activity towards degradation of organic pollutants. Appl. Surf. Sci. 2022, 575, 151695. [Google Scholar] [CrossRef]
- Cao, J.; Pan, C.; Ding, Y.; Li, W.; Lv, K.; Tang, H. Constructing nitrogen vacancy introduced g-C3N4 p-n homojunction for enhanced photocatalytic activity. J. Environ. Chem. Eng. 2019, 7, 102984. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, Y.; Chen, Z.; Zhang, Z.; Fang, X. Constructing a novel ternary Fe(III)/graphene/g-C3N4 composite photocatalyst with enhanced visible-light driven photocatalytic activity via interfacial charge transfer effect. Appl. Catal. B 2016, 183, 231–241. [Google Scholar] [CrossRef]
- Jing, H.; You, M.; Yi, S.; Li, T.; Ji, H.; Wang, Y.; Zhang, Z.; Zhang, R.; Chen, D.; Yang, H. Precursor-Engineering Coupled Microwave Molten-Salt Strategy Enhances Photocatalytic Hydrogen Evolution Performance of g-C3N4 Nanostructures. ChemSusChem 2020, 13, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Savateev, A.; Pronkin, S.; Epping, J.D.; Willinger, M.G.; Wolff, C.; Neher, D.; Antonietti, M.; Dontsova, D. Potassium Poly(heptazine imides) from Aminotetrazoles: Shifting Band Gaps of Carbon Nitride-like Materials for More Efficient Solar Hydrogen and Oxygen Evolution. ChemCatChem 2016, 9, 167–174. [Google Scholar] [CrossRef]
- Savateev, A.; Tarakina, N.V.; Strauss, V.; Hussain, T.; Ten Brummelhuis, K.; Sanchez Vadillo, J.M.; Markushyna, Y.; Mazzanti, S.; Tyutyunnik, A.P.; Walczak, R.; et al. Potassium Poly(Heptazine Imide): Transition Metal-Free Solid-State Triplet Sensitizer in Cascade Energy Transfer and [3+2]-cycloadditions. Angew. Chem. Int. Ed. 2020, 59, 15061–15068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Liu, C.; Liu, Q.; Mo, Z.; Zhang, D. Salt-Mediated Structural Transformation in Carbon Nitride: From Regulated Atomic Configurations to Enhanced Photocatalysis. Catalysts 2023, 13, 717. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, D.; Yang, S.; Tian, Z.; Cheng, B.; Fan, J. Novel g-C3N4/g-C3N4 S-scheme isotype heterojunction for improved photocatalytic hydrogen generation. Appl. Surf. Sci. 2019, 495, 143555. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, C.; Lang, J.; Zhou, Y.; Zhou, B.; Hu, Y.H.; Long, M. Modulation of Lewis acidic-basic sites for efficient photocatalytic H2O2 production over potassium intercalated tri-s-triazine materials. Appl. Catal. B 2020, 277, 119225. [Google Scholar] [CrossRef]
- Zou, H.; Yan, X.; Ren, J.; Wu, X.; Dai, Y.; Sha, D.; Pan, J.; Liu, J. Photocatalytic activity enhancement of modified g-C3N4 by ionothermal copolymerization. J. Materiomics 2015, 1, 340–347. [Google Scholar] [CrossRef]
- An, W.; Zhi, X.; Zhai, B.; Niu, P.; Wang, S.; Li, L. Crystallinity improvement of poly(heptazine imide) for high photocatalytic hydrogen evolution. Scr. Mater. 2022, 221, 114992. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Nie, R.; Ning, Y.; Zhao, C.; Xia, Z.; Niu, P.; Li, L.; Wang, S. Effective modification of photocatalytic and piezocatalytic performances for poly(heptazine imide) by carbon dots decoration. Dalton Trans. 2022, 51, 13015–13021. [Google Scholar] [CrossRef]
- Zhong, X.; Zhu, Y.; Jiang, M.; Sun, Q.; Yao, J. Photochemical Synthesis of Porous Triazine-/Heptazine-Based Carbon Nitride Homojunction for Efficient Overall Water Splitting. ChemSusChem 2023, 16, e202202059. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Liu, Y.; Zhao, Y.; Xie, C.; Song, Y.; Yang, P. Crystallinity and phase controlling of g-C3N4/CdS hetrostructures towards high efficient photocatalytic H2 generation. Int. J. Hydrogen Energy 2019, 44, 30151–30159. [Google Scholar] [CrossRef]
- Sayed, M.; Khan, J.A.; Shah, L.A.; Shah, N.S.; Shah, F.; Khan, H.M.; Zhang, P.; Arandiyan, H. Solar Light Responsive Poly(vinyl alcohol)-Assisted Hydrothermal Synthesis of Immobilized TiO2/Ti Film with the Addition of Peroxymonosulfate for Photocatalytic Degradation of Ciprofloxacin in Aqueous Media: A Mechanistic Approach. J. Phys. Chem. C 2017, 122, 406–421. [Google Scholar] [CrossRef]
- Hu, K.; Li, R.; Ye, C.; Wang, A.; Wei, W.; Hu, D.; Qiu, R.; Yan, K. Facile synthesis of Z-scheme composite of TiO2 nanorod/g-C3N4 nanosheet efficient for photocatalytic degradation of ciprofloxacin. J. Clean. Prod. 2020, 253, 120055. [Google Scholar] [CrossRef]
- Wei, X.; Chen, J.; Xie, Q.; Zhang, S.; Ge, L.; Qiao, X. Distinct Photolytic Mechanisms and Products for Different Dissociation Species of Ciprofloxacin. Environ. Sci. Technol. 2013, 47, 4284–4290. [Google Scholar] [CrossRef]
- Zheng, X.; Xu, S.; Wang, Y.; Sun, X.; Gao, Y.; Gao, B. Enhanced degradation of ciprofloxacin by graphitized mesoporous carbon (GMC)-TiO2 nanocomposite: Strong synergy of adsorption-photocatalysis and antibiotics degradation mechanism. J. Colloid Interface Sci. 2018, 527, 202–213. [Google Scholar] [CrossRef]
- Zhao, X.; Xia, Y.; Li, H.; Wang, X.; Wei, J.; Jiao, X.; Chen, D. Oxygen vacancy dependent photocatalytic CO2 reduction activity in liquid-exfoliated atomically thin BiOCl nanosheets. Appl. Catal. B 2021, 297, 120426. [Google Scholar] [CrossRef]
- Tian, N.; Huang, H.; Liu, C.; Dong, F.; Zhang, T.; Du, X.; Yu, S.; Zhang, Y. In situ co-pyrolysis fabrication of CeO2/g-C3N4 n–n type heterojunction for synchronously promoting photo-induced oxidation and reduction properties. J. Mater. Chem. A 2015, 3, 17120–17129. [Google Scholar] [CrossRef]
- Su, E.-C.; Huang, B.-S.; Liu, C.-C.; Wey, M.-Y. Photocatalytic conversion of simulated EDTA wastewater to hydrogen by pH-resistant Pt/TiO2–activated carbon photocatalysts. Renew. Energy 2015, 75, 266–271. [Google Scholar] [CrossRef]
- Nie, Y.-C.; Yu, F.; Wang, L.-C.; Xing, Q.-J.; Liu, X.; Pei, Y.; Zou, J.-P.; Dai, W.-L.; Li, Y.; Suib, S.L. Photocatalytic degradation of organic pollutants coupled with simultaneous photocatalytic H2 evolution over graphene quantum dots/Mn-N-TiO2/g-C3N4 composite catalysts: Performance and mechanism. Appl. Catal. B 2018, 227, 312–321. [Google Scholar] [CrossRef]
- Li, K.; Zeng, Z.; Yan, L.; Huo, M.; Guo, Y.; Luo, S.; Luo, X. Fabrication of C/X-TiO2 @C3N4 NTs (X = N, F, Cl) composites by using phenolic organic pollutants as raw materials and their visible-light photocatalytic performance in different photocatalytic systems. Appl. Catal. B 2016, 187, 269–280. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, X.; Liu, C.; Wang, L.; Xia, Y.; Zhang, S.; Luo, S.; Pei, Y. Scalable one-step production of porous oxygen-doped g-C3N4 nanorods with effective electron separation for excellent visible-light photocatalytic activity. Appl. Catal. B 2018, 224, 1–9. [Google Scholar] [CrossRef]
- Jiang, X.-H.; Wang, L.-C.; Yu, F.; Nie, Y.-C.; Xing, Q.-J.; Liu, X.; Pei, Y.; Zou, J.-P.; Dai, W.-L. Photodegradation of Organic Pollutants Coupled with Simultaneous Photocatalytic Evolution of Hydrogen Using Quantum-Dot-Modified g-C3N4 Catalysts under Visible-Light Irradiation. ACS Sustain. Chem. Eng. 2018, 6, 12695–12705. [Google Scholar] [CrossRef]
- Xu, F.; Mo, Z.; Yan, J.; Fu, J.; Song, Y.; El-Alami, W.; Wu, X.; Li, H.; Xu, H. Nitrogen-rich graphitic carbon nitride nanotubes for photocatalytic hydrogen evolution with simultaneous contaminant degradation. J. Colloid Interface Sci. 2020, 560, 555–564. [Google Scholar] [CrossRef]
- Martha, S.; Mansingh, S.; Parida, K.M.; Thirumurugan, A. Exfoliated metal free homojunction photocatalyst prepared by a biomediated route for enhanced hydrogen evolution and Rhodamine B degradation. Mater. Chem. Front. 2017, 1, 1641–1653. [Google Scholar] [CrossRef]
- Zhou, Q.; Guo, Y.; Ye, Z.; Fu, Y.; Guo, Y.; Zhu, Y. Carbon nitride photocatalyst with internal electric field induced photogenerated carriers spatial enrichment for enhanced photocatalytic water splitting. Mater. Today 2022, 58, 100–109. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Che, H.; Liu, C.; Li, C. Facile construction of O-doped crystalline/non-crystalline g-C3N4 embedded nano-homojunction for efficiently photocatalytic H2 evolution. Carbon 2021, 172, 602–612. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Qiao, K.; Ning, C.; Wang, X.; Liu, Z.; Chen, Z. Electrostatic Self-Assembled Synthesis of Amorphous/Crystalline g-C3N4 Homo-Junction for Efficient Photocatalytic H2 Production with Simultaneous Antibiotic Degradation. Nanomaterials 2023, 13, 2964. https://doi.org/10.3390/nano13222964
Pan Y, Qiao K, Ning C, Wang X, Liu Z, Chen Z. Electrostatic Self-Assembled Synthesis of Amorphous/Crystalline g-C3N4 Homo-Junction for Efficient Photocatalytic H2 Production with Simultaneous Antibiotic Degradation. Nanomaterials. 2023; 13(22):2964. https://doi.org/10.3390/nano13222964
Chicago/Turabian StylePan, Yilin, Kai Qiao, Chuangyu Ning, Xin Wang, Zhiquan Liu, and Zhihong Chen. 2023. "Electrostatic Self-Assembled Synthesis of Amorphous/Crystalline g-C3N4 Homo-Junction for Efficient Photocatalytic H2 Production with Simultaneous Antibiotic Degradation" Nanomaterials 13, no. 22: 2964. https://doi.org/10.3390/nano13222964
APA StylePan, Y., Qiao, K., Ning, C., Wang, X., Liu, Z., & Chen, Z. (2023). Electrostatic Self-Assembled Synthesis of Amorphous/Crystalline g-C3N4 Homo-Junction for Efficient Photocatalytic H2 Production with Simultaneous Antibiotic Degradation. Nanomaterials, 13(22), 2964. https://doi.org/10.3390/nano13222964





