Temperature- and Size-Dependent Photoluminescence of CuInS2 Quantum Dots
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of CuInS2 QDs
2.3. Methods
3. Results and Discussion
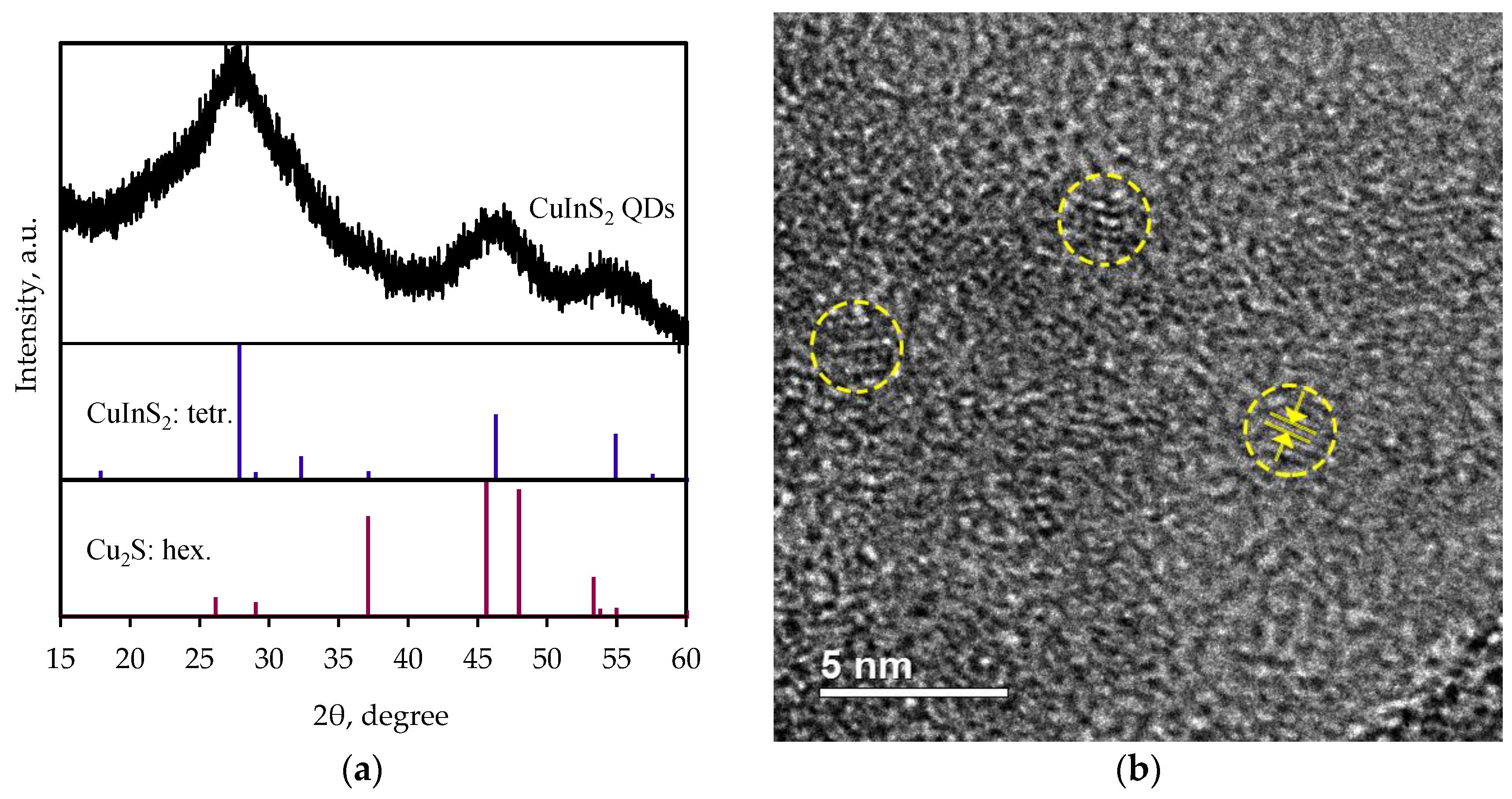
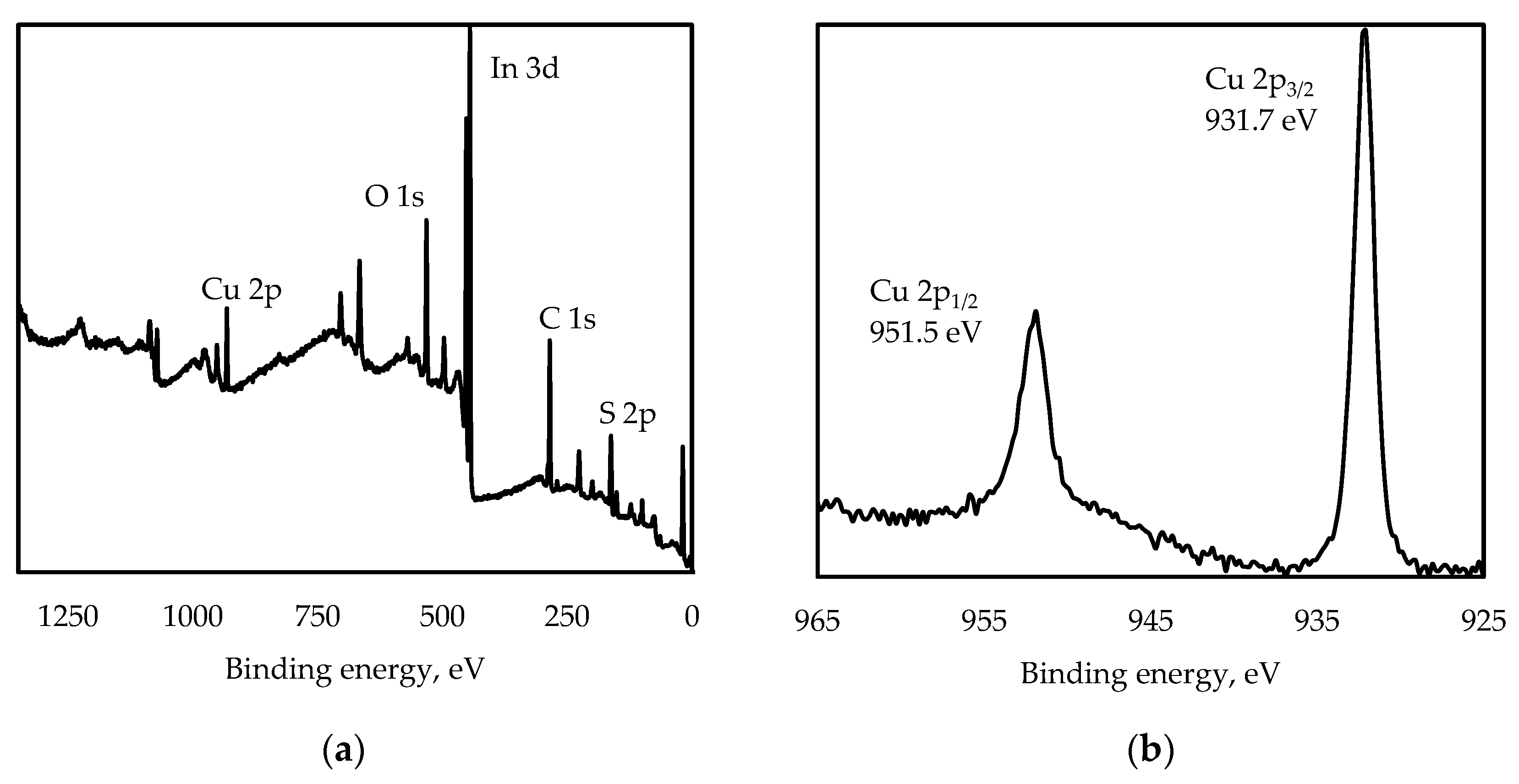
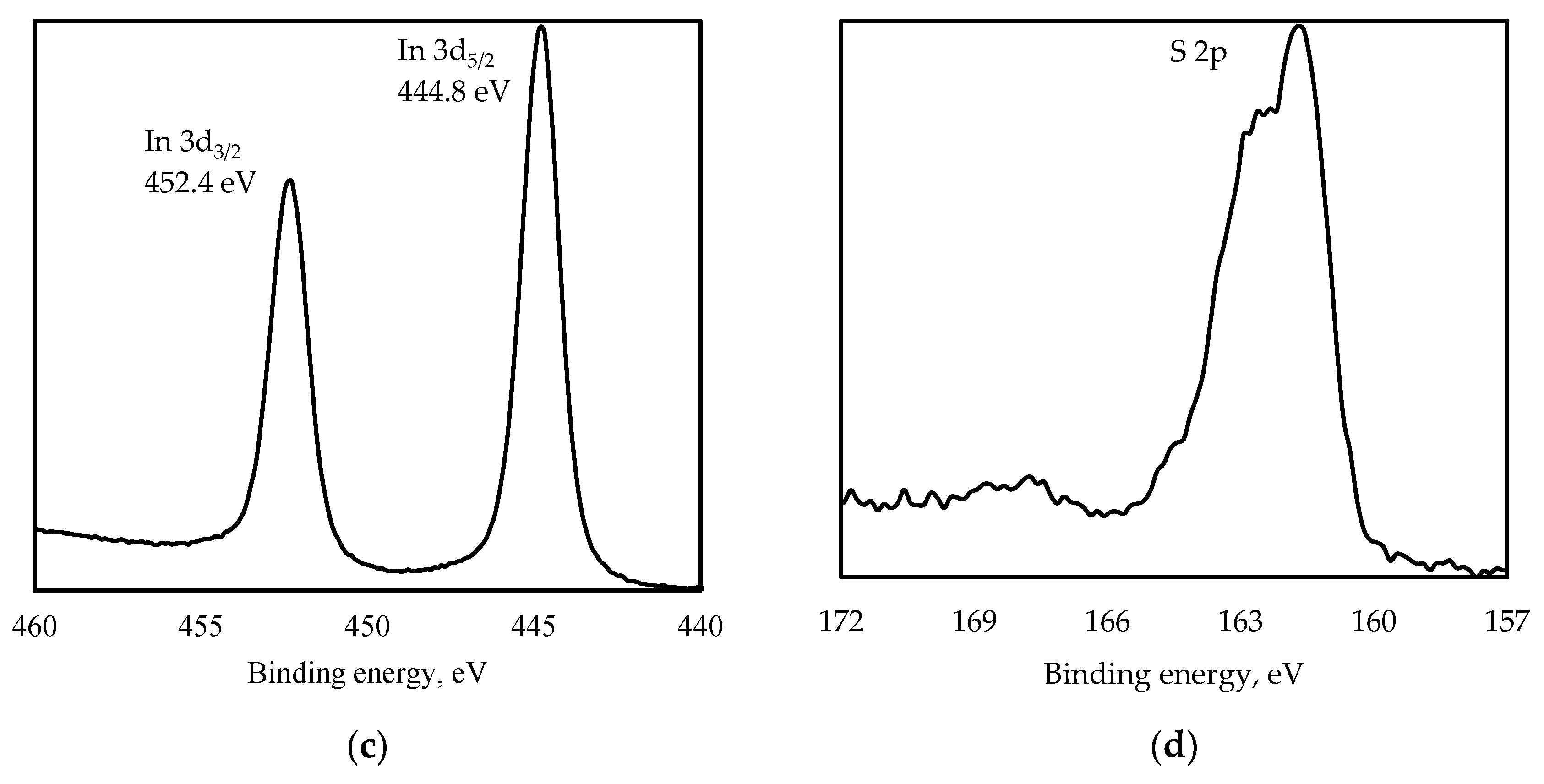
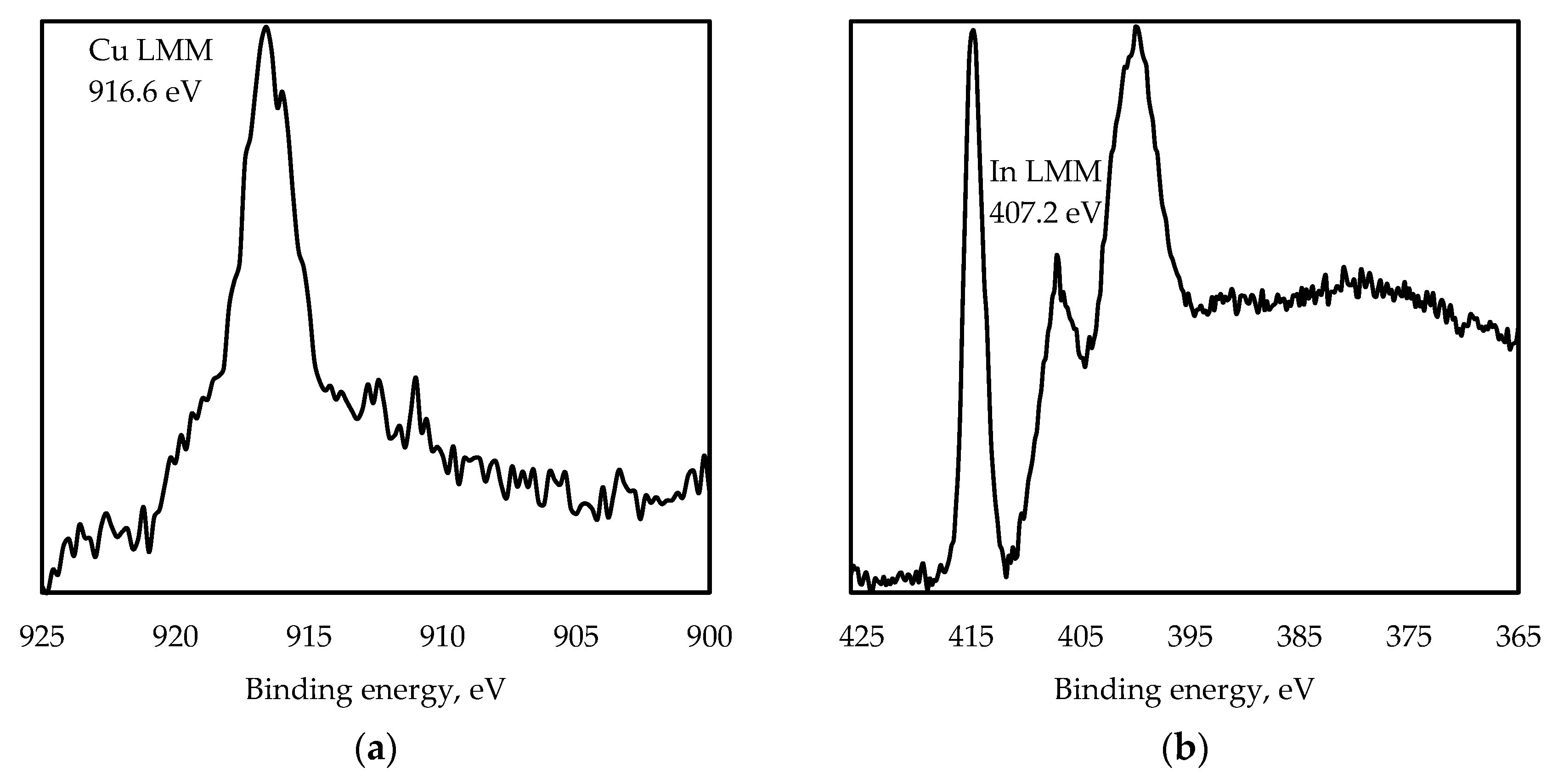
3.1. Structure Characterization
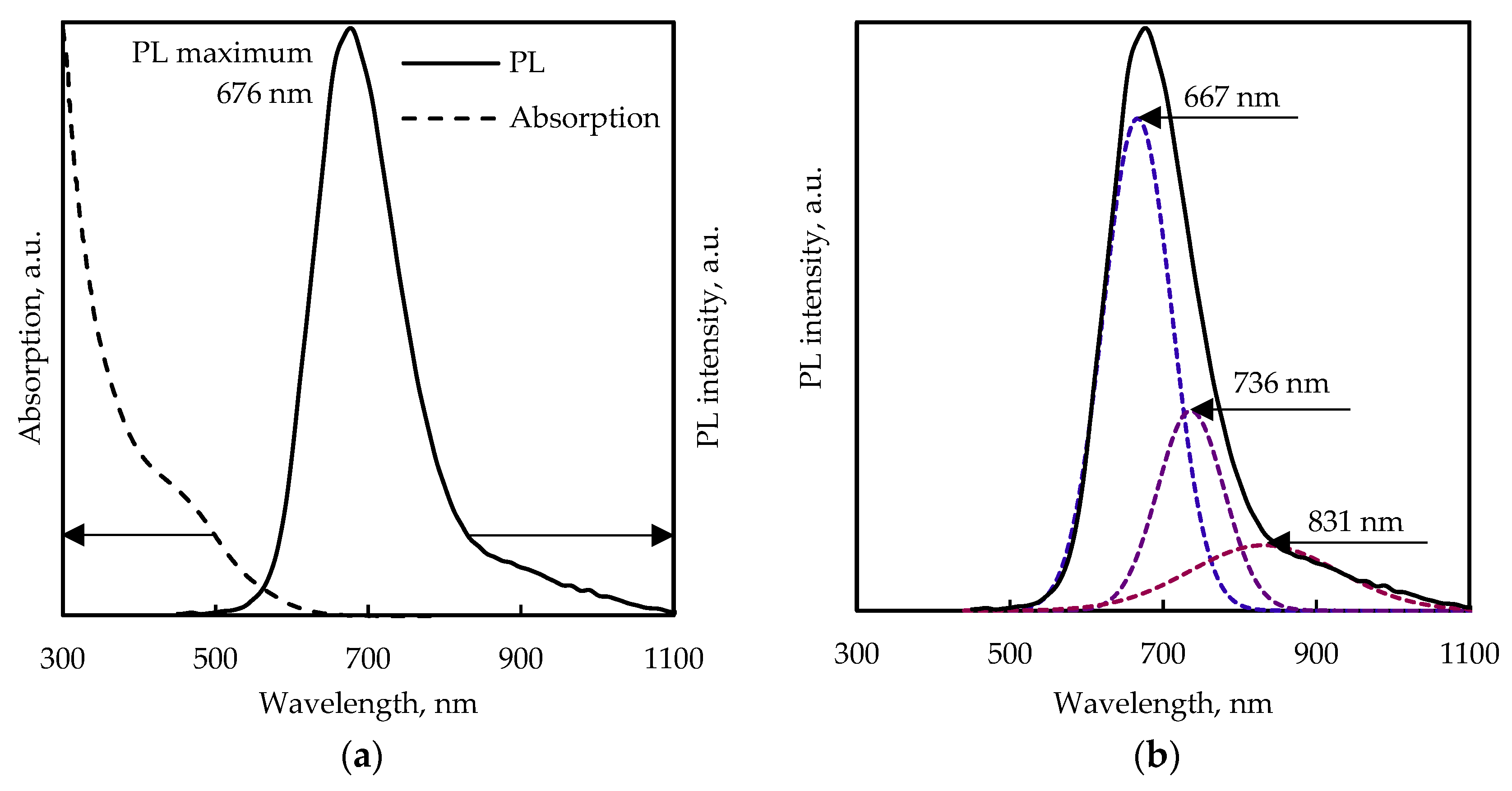
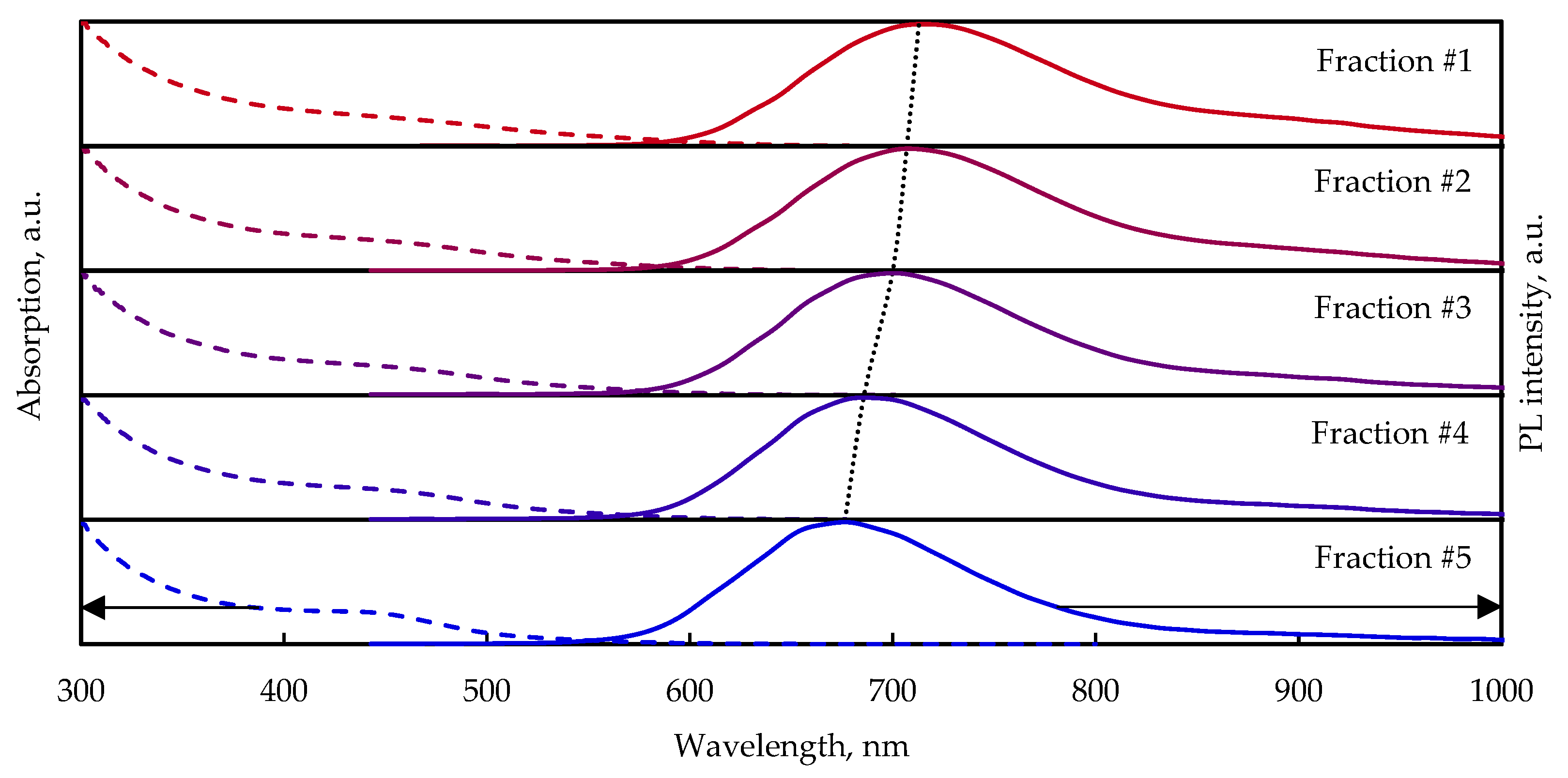
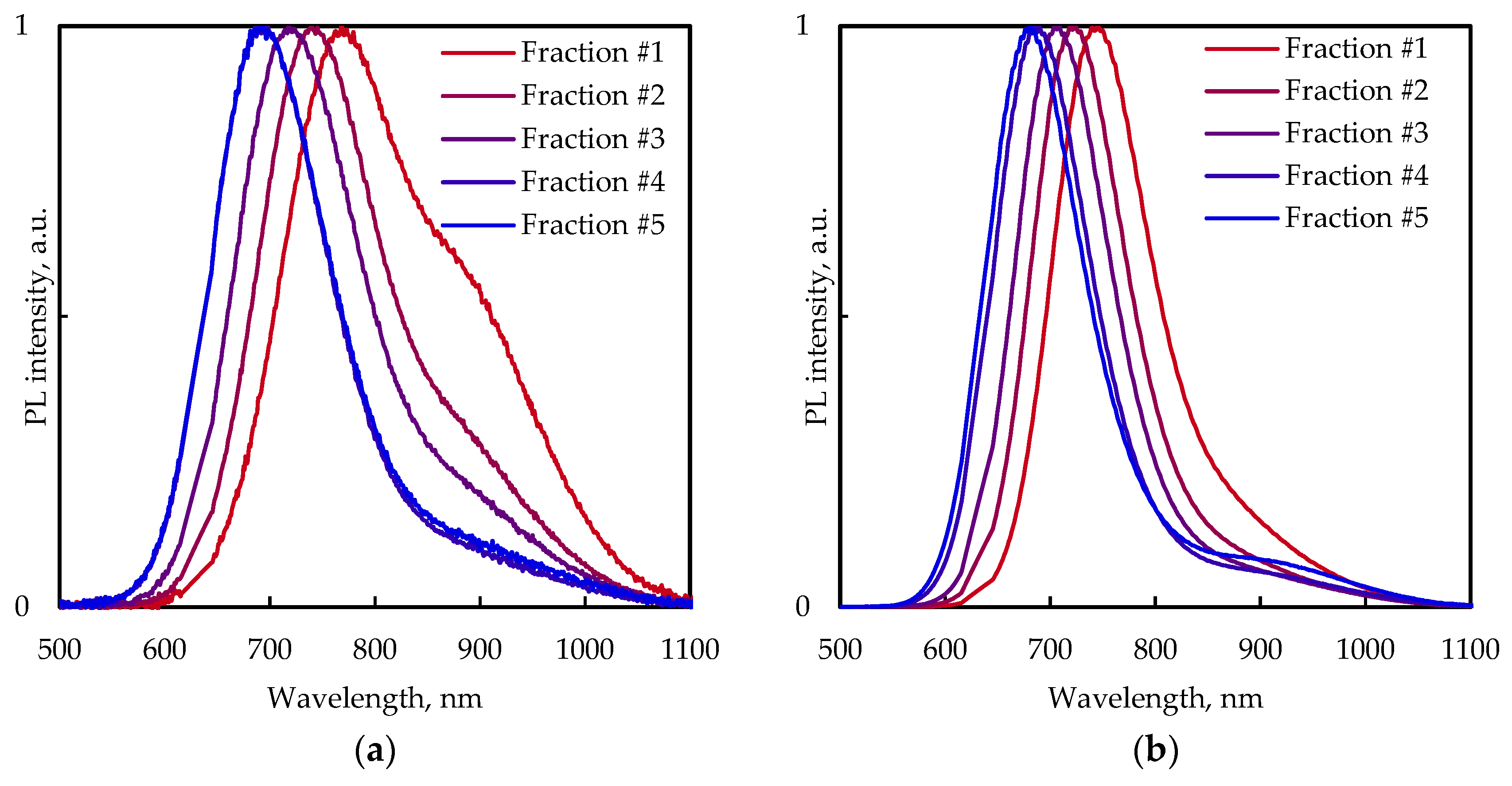
3.2. Absorption and PL Spectra
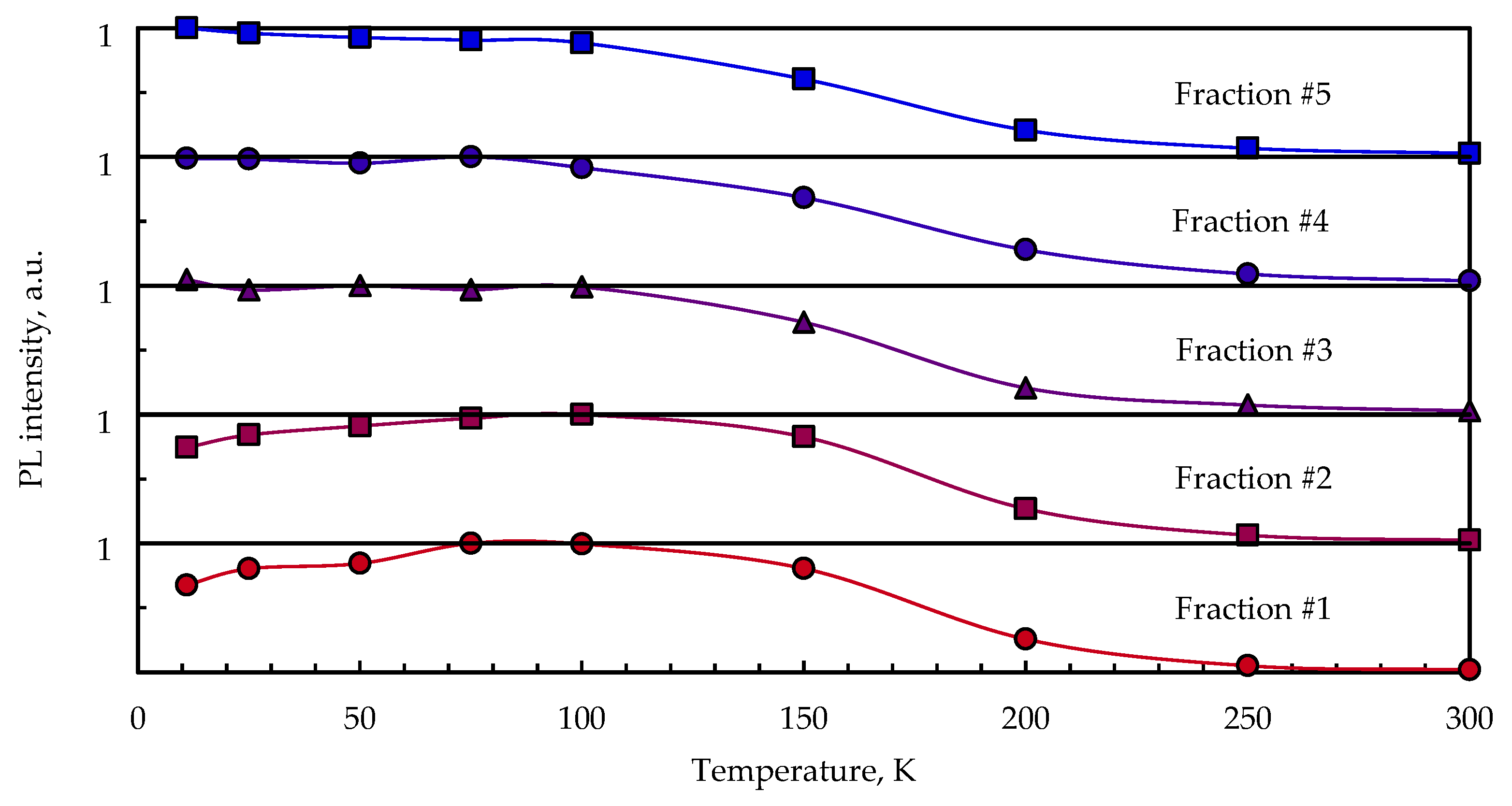
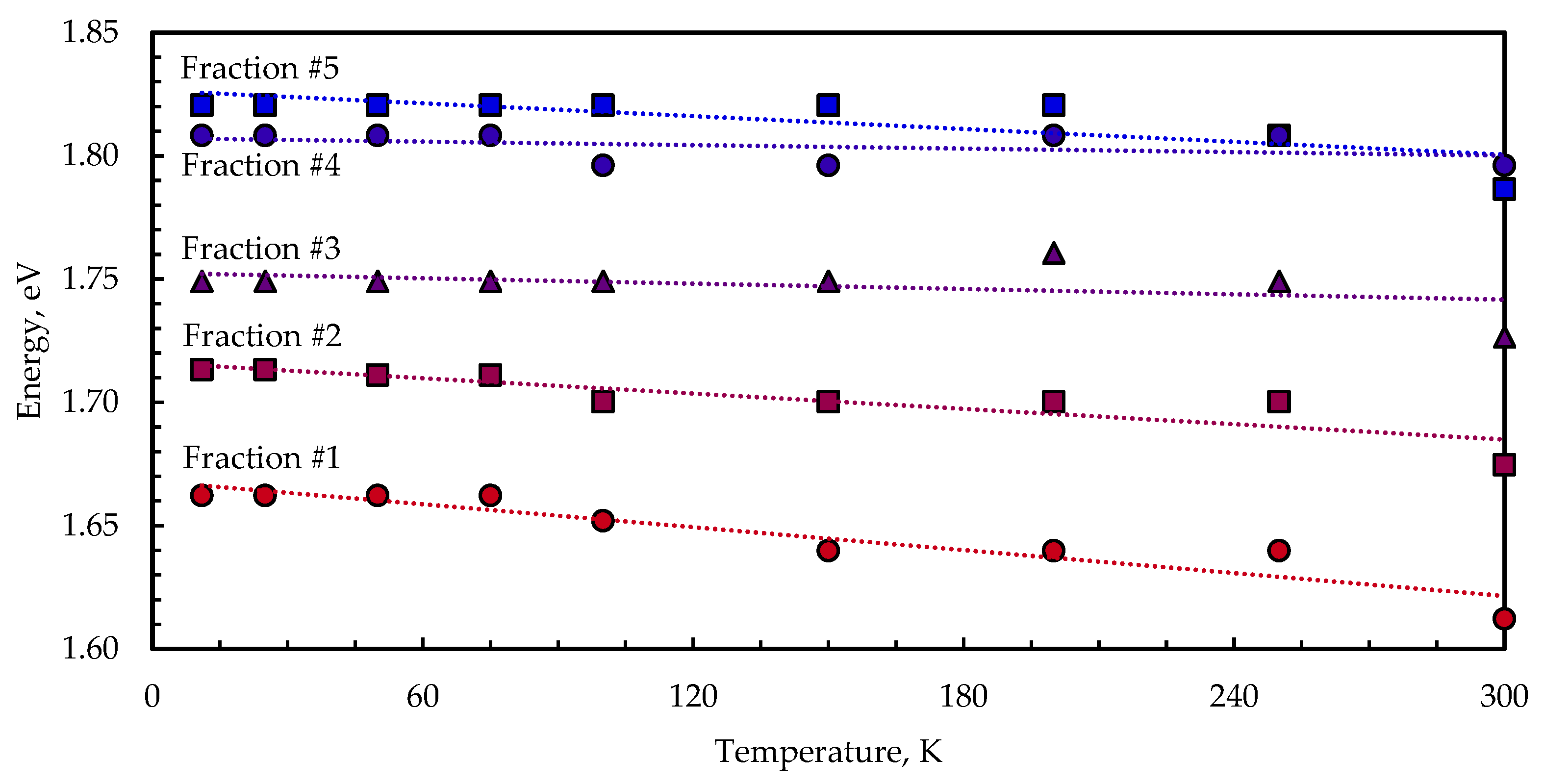
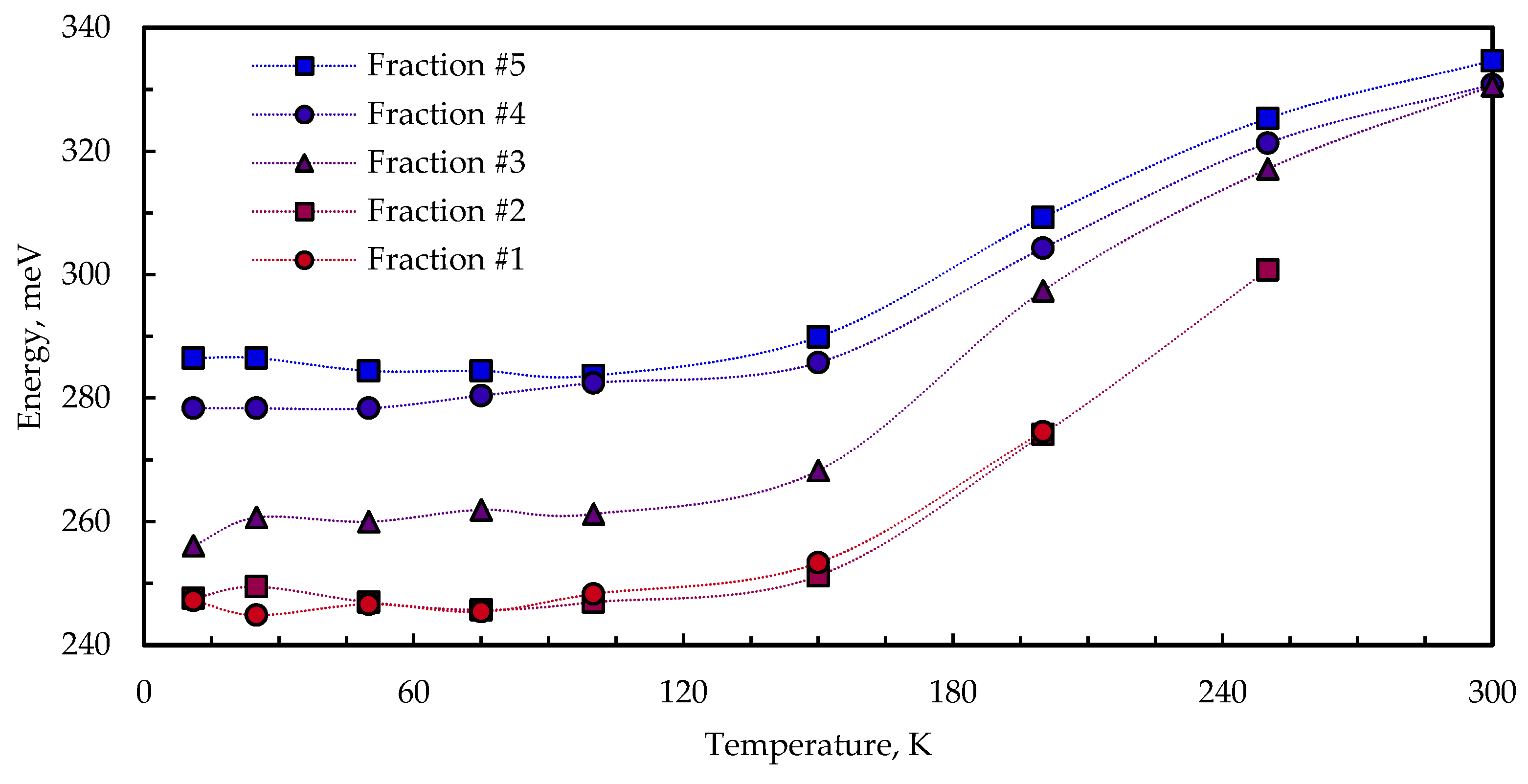
3.3. Temperature-Dependent Photoluminescence
- —the temperature-independent intrinsic inhomogeneous line width;
- —the longitudinal optical (LO) phonon–exciton coupling coefficient;
- —the LO phonon energy.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, X.; Zhang, C.; Guo, Y.; Yang, Y.; Xing, Y.; Que, W. PbS QD-Based Photodetectors: Future-Oriented near-Infrared Detection Technology. J. Mater. Chem. C 2021, 9, 417–438. [Google Scholar] [CrossRef]
- Matea, C.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum Dots in Imaging, Drug Delivery and Sensor Applications. IJN 2017, 12, 5421–5431. [Google Scholar] [CrossRef] [PubMed]
- Reshma, V.G.; Mohanan, P.V. Quantum Dots: Applications and Safety Consequences. J. Lumin. 2019, 205, 287–298. [Google Scholar] [CrossRef]
- Bera, D.; Qian, L.; Tseng, T.-K.; Holloway, P.H. Quantum Dots and Their Multimodal Applications: A Review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, C.-H.; Hyun, B.-R.; Sher, C.-W.; Lv, Z.; Luo, B.; Jiang, F.; Wu, T.; Ho, C.-H.; Kuo, H.-C.; et al. Micro-Light-Emitting Diodes with Quantum Dots in Display Technology. Light Sci. Appl. 2020, 9, 83. [Google Scholar] [CrossRef]
- Kauffer, F.-A.; Merlin, C.; Balan, L.; Schneider, R. Incidence of the Core Composition on the Stability, the ROS Production and the Toxicity of CdSe Quantum Dots. J. Hazard. Mater. 2014, 268, 246–255. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.; Zhang, J.; Liu, J.; Chen, G.; Pope, C. In Vitro and In Vivo Toxicity of CdTe Nanoparticles. J. Nanosci. Nanotechnol. 2007, 7, 497–503. [Google Scholar] [CrossRef]
- Tarantini, A.; Wegner, K.D.; Dussert, F.; Sarret, G.; Beal, D.; Mattera, L.; Lincheneau, C.; Proux, O.; Truffier-Boutry, D.; Moriscot, C.; et al. Physicochemical Alterations and Toxicity of InP Alloyed Quantum Dots Aged in Environmental Conditions: A Safer by Design Evaluation. NanoImpact 2019, 14, 100168. [Google Scholar] [CrossRef]
- Liu, N.; Tang, M. Toxicity of Different Types of Quantum Dots to Mammalian Cells in Vitro: An Update Review. J. Hazard. Mater. 2020, 399, 122606. [Google Scholar] [CrossRef]
- Girma, W.M.; Fahmi, M.Z.; Permadi, A.; Abate, M.A.; Chang, J.-Y. Synthetic Strategies and Biomedical Applications of I–III–VI Ternary Quantum Dots. J. Mater. Chem. B 2017, 5, 6193–6216. [Google Scholar] [CrossRef]
- Zhong, H.; Bai, Z.; Zou, B. Tuning the Luminescence Properties of Colloidal I–III–VI Semiconductor Nanocrystals for Optoelectronics and Biotechnology Applications. J. Phys. Chem. Lett. 2012, 3, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-T.; Yoon, S.-Y.; Wu, B.-H.; Lu, K.-M.; Lin, C.-M.; Yang, H.; Liu, R.-S. Ultra-Broadband near-Infrared Emission CuInS2/ZnS Quantum Dots with High Power Efficiency and Stability for the Theranostic Applications of Mini Light-Emitting Diodes. Chem. Commun. 2020, 56, 8285–8288. [Google Scholar] [CrossRef] [PubMed]
- Chetty, S.S.; Praneetha, S.; Vadivel Murugan, A.; Govarthanan, K.; Verma, R.S. Human Umbilical Cord Wharton’s Jelly-Derived Mesenchymal Stem Cells Labeled with Mn2+ and Gd3+ Co-Doped CuInS2–ZnS Nanocrystals for Multimodality Imaging in a Tumor Mice Model. ACS Appl. Mater. Interfaces 2020, 12, 3415–3429. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, Z.; Lin, Z.; Su, X. CuInS2 Quantum Dots/Poly(l-Glutamic Acid)–Drug Conjugates for Drug Delivery and Cell Imaging. Analyst 2014, 139, 831. [Google Scholar] [CrossRef] [PubMed]
- Korepanov, O.A.; Mazing, D.S.; Aleksandrova, O.A.; Moshnikov, V.A. Synthesis and Study of Colloidal Nanocrystals Based on Ternary Chalcogenides for Active Media of Heavy Metal Ions Sensors. In Proceedings of the 2019 IEEE Conference of Russian Young Researchers in Electrical and Electronic Engineering (EIConRus), Saint Petersburg/Moscow, Russia, 28–31 January 2019; IEEE: Saint Petersburg and Moscow, Russia, 2019; pp. 771–773. [Google Scholar]
- Jain, S.; Bharti, S.; Bhullar, G.K.; Tripathi, S.K. Synthesis, Characterization and Stability Study of Aqueous MPA Capped CuInS2/ZnS Core/Shell Nanoparticles. J. Lumin. 2022, 252, 119279. [Google Scholar] [CrossRef]
- Mazing, D.S.; Chernaguzov, I.S.; Shulga, A.I.; Korepanov, O.A.; Aleksandrova, O.A.; Moshnikov, V.A. Synthesis of Ternary Chalcogenide Colloidal Nanocrystals in Aqueous Medium. J. Phys. Conf. Ser. 2018, 1038, 012050. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, Q.; Wang, X.; Lin, Z.; Zhang, H.; Liu, L.; Su, X. A Novel Fluorescent Nanosensor for Detection of Heparin and Heparinase Based on CuInS2 Quantum Dots. Biosens. Bioelectron. 2014, 54, 617–622. [Google Scholar] [CrossRef]
- An, X.; Zhang, Y.; Wang, J.; Kong, D.; He, X.; Chen, L.; Zhang, Y. The Preparation of CuInS2-ZnS-Glutathione Quantum Dots and Their Application on the Sensitive Determination of Cytochrome c and Imaging of HeLa Cells. ACS Omega 2021, 6, 17501–17509. [Google Scholar] [CrossRef]
- Amaral-Júnior, J.C.; Mansur, A.A.P.; Carvalho, I.C.; Mansur, H.S. Tunable Luminescence of Cu-In-S/ZnS Quantum Dots-Polysaccharide Nanohybrids by Environmentally Friendly Synthesis for Potential Solar Energy Photoconversion Applications. Appl. Surf. Sci. 2021, 542, 148701. [Google Scholar] [CrossRef]
- Korepanov, O.; Aleksandrova, O.; Firsov, D.; Kalazhokov, Z.; Kirilenko, D.; Kozodaev, D.; Matveev, V.; Mazing, D.; Moshnikov, V. Polyvinylpyrrolidone as a Stabilizer in Synthesis of AgInS2 Quantum Dots. Nanomaterials 2022, 12, 2357. [Google Scholar] [CrossRef]
- Haouari, M.; Maaoui, A.; Saad, N.; Bulou, A. Optical Temperature Sensing Using Green Emissions of Er3+ Doped Fluoro-Tellurite Glass. Sens. Actuators A Phys. 2017, 261, 235–242. [Google Scholar] [CrossRef]
- Meert, K.W.; Morozov, V.A.; Abakumov, A.M.; Hadermann, J.; Poelman, D.; Smet, P.F. Energy Transfer in Eu3+ Doped Scheelites: Use as Thermographic Phosphor. Opt. Express 2014, 22, A961. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Chen, D.; Peng, Y.; Lu, Y.; Chen, X.; Li, X.; Ji, Z. A Review on Nanostructured Glass Ceramics for Promising Application in Optical Thermometry. J. Alloys Compd. 2018, 763, 34–48. [Google Scholar] [CrossRef]
- Iida, K.; Kim, D. Temperature-Dependent Photoluminescence Properties of Water-Soluble CuInS2 and CuInS2/ZnS Quantum Dots. J. Appl. Phys. 2022, 132, 194306. [Google Scholar] [CrossRef]
- Xia, C.; Wu, W.; Yu, T.; Xie, X.; Van Oversteeg, C.; Gerritsen, H.C.; De Mello Donega, C. Size-Dependent Band-Gap and Molar Absorption Coefficients of Colloidal CuInS2 Quantum Dots. ACS Nano 2018, 12, 8350–8361. [Google Scholar] [CrossRef]
- Shi, A.; Wang, X.; Meng, X.; Liu, X.; Li, H.; Zhao, J. Temperature-Dependent Photoluminescence of CuInS2 Quantum Dots. J. Lumin. 2012, 132, 1819–1823. [Google Scholar] [CrossRef]
- Mir, I.A.; Das, K.; Akhter, T.; Ranjan, R.; Patel, R.; Bohidar, H.B. Eco-Friendly Synthesis of CuInS2 and CuInS2@ZnS Quantum Dots and Their Effect on Enzyme Activity of Lysozyme. RSC Adv. 2018, 8, 30589–30599. [Google Scholar] [CrossRef]
- Speranskaya, E.S.; Sevrin, C.; De Saeger, S.; Hens, Z.; Goryacheva, I.Y.; Grandfils, C. Synthesis of Hydrophilic CuInS2/ZnS Quantum Dots with Different Polymeric Shells and Study of Their Cytotoxicity and Hemocompatibility. ACS Appl. Mater. Interfaces 2016, 8, 7613–7622. [Google Scholar] [CrossRef]
- Firsov, D.D.; Komkov, O.S.; Solov’ev, V.A.; Kop’ev, P.S.; Ivanov, S.V. Temperature-Dependent Photoluminescence of InSb/InAs Nanostructures with InSb Thickness in the above-Monolayer Range. J. Phys. D Appl. Phys. 2016, 49, 285108. [Google Scholar] [CrossRef]
- Landry, C.C.; Barron, A.R. Synthesis of Polycrystalline Chalcopyrite Semiconductors by Microwave Irradiation. Science 1993, 260, 1653–1655. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, H.; Zhang, W.; Tan, Z.; Li, Y.; Yu, C.; Zhai, T.; Bando, Y.; Yang, S.; Zou, B. Highly Emissive and Color-Tunable CuInS2-Based Colloidal Semiconductor Nanocrystals: Off-Stoichiometry Effects and Improved Electroluminescence Performance. Adv. Funct. Mater. 2012, 22, 2081–2088. [Google Scholar] [CrossRef]
- Berends, A.C.; Rabouw, F.T.; Spoor, F.C.M.; Bladt, E.; Grozema, F.C.; Houtepen, A.J.; Siebbeles, L.D.A.; de Mello Donegá, C. Radiative and Nonradiative Recombination in CuInS2 Nanocrystals and CuInS2-Based Core/Shell Nanocrystals. J. Phys. Chem. Lett. 2016, 7, 3503–3509. [Google Scholar] [CrossRef]
- Fuhr, A.S.; Yun, H.J.; Makarov, N.S.; Li, H.; McDaniel, H.; Klimov, V.I. Light Emission Mechanisms in CuInS2 Quantum Dots Evaluated by Spectral Electrochemistry. ACS Photonics 2017, 4, 2425–2435. [Google Scholar] [CrossRef]
- Zang, H.; Li, H.; Makarov, N.S.; Velizhanin, K.A.; Wu, K.; Park, Y.-S.; Klimov, V.I. Thick-Shell CuInS2/ZnS Quantum Dots with Suppressed “Blinking” and Narrow Single-Particle Emission Line Widths. Nano Lett. 2017, 17, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Rinnert, H.; Jambois, O.; Vergnat, M. Photoluminescence Properties of Size-Controlled Silicon Nanocrystals at Low Temperatures. J. Appl. Phys. 2009, 106, 023501. [Google Scholar] [CrossRef]
- Sun, J.; Ikezawa, M.; Wang, X.; Jing, P.; Li, H.; Zhao, J.; Masumoto, Y. Photocarrier Recombination Dynamics in Ternary Chalcogenide CuInS2 Quantum Dots. Phys. Chem. Chem. Phys. 2015, 17, 11981–11989. [Google Scholar] [CrossRef]
- Kalytchuk, S.; Zhovtiuk, O.; Kershaw, S.V.; Zbořil, R.; Rogach, A.L. Temperature-Dependent Exciton and Trap-Related Photoluminescence of CdTe Quantum Dots Embedded in a NaCl Matrix: Implication in Thermometry. Small 2016, 12, 466–476. [Google Scholar] [CrossRef]
- Jing, P.; Zheng, J.; Ikezawa, M.; Liu, X.; Lv, S.; Kong, X.; Zhao, J.; Masumoto, Y. Temperature-Dependent Photoluminescence of CdSe-Core CdS/CdZnS/ZnS-Multishell Quantum Dots. J. Phys. Chem. C 2009, 113, 13545–13550. [Google Scholar] [CrossRef]
- Joshi, A.; Narsingi, K.Y.; Manasreh, M.O.; Davis, E.A.; Weaver, B.D. Temperature Dependence of the Band Gap of Colloidal CdSe/ZnS Core/Shell Nanocrystals Embedded into an Ultraviolet Curable Resin. Appl. Phys. Lett. 2006, 89, 131907. [Google Scholar] [CrossRef]
- Alphandéry, E.; Nicholas, R.J.; Mason, N.J.; Lyapin, S.G.; Klipstein, P.C. Photoluminescence of Self-Assembled InSb Quantum Dots Grown on GaSb as a Function of Excitation Power, Temperature, and Magnetic Field. Phys. Rev. B 2002, 65, 115322. [Google Scholar] [CrossRef]
- Eliseev, P.G.; Osinski, M.; Lee, J.; Sugahara, T.; Sakai, S. Band-Tail Model and Temperature-Induced Blue-Shift in Photoluminescence Spectra of InxGa1-xN Grown on Sapphire. J. Elec. Mater. 2000, 29, 332–341. [Google Scholar] [CrossRef]
- Knowles, K.E.; Nelson, H.D.; Kilburn, T.B.; Gamelin, D.R. Singlet–Triplet Splittings in the Luminescent Excited States of Colloidal Cu+:CdSe, Cu+:InP, and CuInS2 Nanocrystals: Charge-Transfer Configurations and Self-Trapped Excitons. J. Am. Chem. Soc. 2015, 137, 13138–13147. [Google Scholar] [CrossRef]
- Bol, A.A.; Ferwerda, J.; Bergwerff, J.A.; Meijerink, A. Luminescence of Nanocrystalline ZnS:Cu2+. J. Lumin. 2002, 99, 325–334. [Google Scholar] [CrossRef]
- Suyver, J.F.; van der Beek, T.; Wuister, S.F.; Kelly, J.J.; Meijerink, A. Luminescence of Nanocrystalline ZnSe:Cu. Appl. Phys. Lett. 2001, 79, 4222–4224. [Google Scholar] [CrossRef]
- Stouwdam, J.W.; Janssen, R.A.J. Electroluminescent Cu-Doped CdS Quantum Dots. Adv. Mater. 2009, 21, 2916–2920. [Google Scholar] [CrossRef]
- Meulenberg, R.W.; van Buuren, T.; Hanif, K.M.; Willey, T.M.; Strouse, G.F.; Terminello, L.J. Structure and Composition of Cu-Doped CdSe Nanocrystals Using Soft X-Ray Absorption Spectroscopy. Nano Lett. 2004, 4, 2277–2285. [Google Scholar] [CrossRef][Green Version]
- Uehara, M.; Watanabe, K.; Tajiri, Y.; Nakamura, H.; Maeda, H. Synthesis of CuInS2 Fluorescent Nanocrystals and Enhancement of Fluorescence by Controlling Crystal Defect. J. Chem. Phys. 2008, 129, 134709. [Google Scholar] [CrossRef] [PubMed]
- Courtel, F.M.; Paynter, R.W.; Marsan, B.; Morin, M. Synthesis, Characterization, and Growth Mechanism of n-Type CuInS2 Colloidal Particles. Chem. Mater. 2009, 21, 3752–3762. [Google Scholar] [CrossRef]
- Miropoltsev, M.; Wegner, K.D.; Häusler, I.; Hodoroaba, V.-D.; Resch-Genger, U. Influence of Hydrophilic Thiol Ligands of Varying Denticity on the Luminescence Properties and Colloidal Stability of Quaternary Semiconductor Nanocrystals. J. Phys. Chem. C 2022, 126, 20101–20113. [Google Scholar] [CrossRef]
- Karczewski, G.; Maćkowski, S.; Kutrowski, M.; Wojtowicz, T.; Kossut, J. Photoluminescence Study of CdTe/ZnTe Self-Assembled Quantum Dots. Appl. Phys. Lett. 1999, 74, 3011–3013. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korepanov, O.; Kozodaev, D.; Aleksandrova, O.; Bugrov, A.; Firsov, D.; Kirilenko, D.; Mazing, D.; Moshnikov, V.; Shomakhov, Z. Temperature- and Size-Dependent Photoluminescence of CuInS2 Quantum Dots. Nanomaterials 2023, 13, 2892. https://doi.org/10.3390/nano13212892
Korepanov O, Kozodaev D, Aleksandrova O, Bugrov A, Firsov D, Kirilenko D, Mazing D, Moshnikov V, Shomakhov Z. Temperature- and Size-Dependent Photoluminescence of CuInS2 Quantum Dots. Nanomaterials. 2023; 13(21):2892. https://doi.org/10.3390/nano13212892
Chicago/Turabian StyleKorepanov, Oleg, Dmitriy Kozodaev, Olga Aleksandrova, Alexander Bugrov, Dmitrii Firsov, Demid Kirilenko, Dmitriy Mazing, Vyacheslav Moshnikov, and Zamir Shomakhov. 2023. "Temperature- and Size-Dependent Photoluminescence of CuInS2 Quantum Dots" Nanomaterials 13, no. 21: 2892. https://doi.org/10.3390/nano13212892
APA StyleKorepanov, O., Kozodaev, D., Aleksandrova, O., Bugrov, A., Firsov, D., Kirilenko, D., Mazing, D., Moshnikov, V., & Shomakhov, Z. (2023). Temperature- and Size-Dependent Photoluminescence of CuInS2 Quantum Dots. Nanomaterials, 13(21), 2892. https://doi.org/10.3390/nano13212892




