Detection of Dimethyl Methyl Phosphonate by Silica Molecularly Imprinted Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. SAW Device Manufacturing
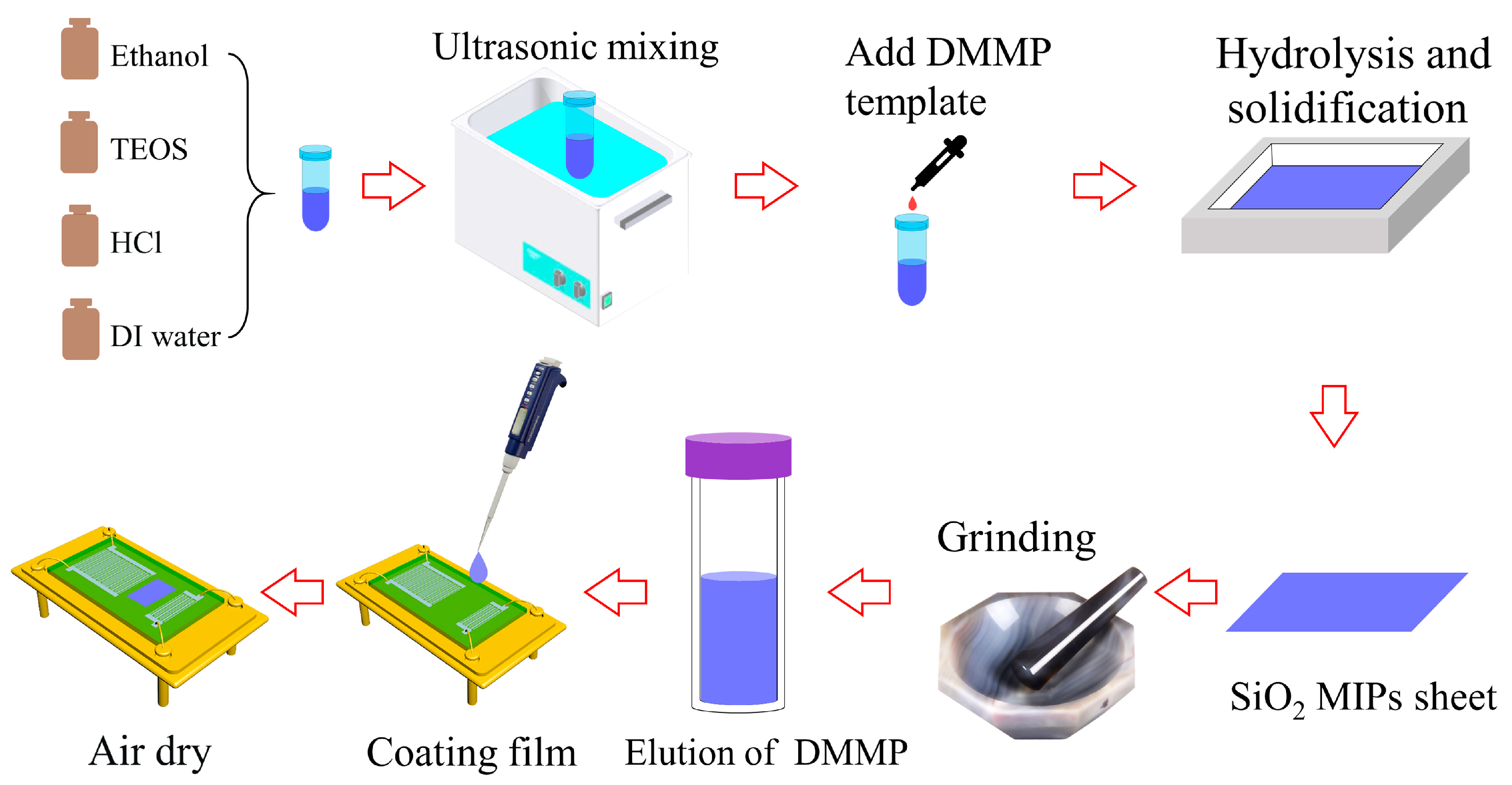
2.2. Synthesis of SiO2 MIPs
2.3. Material Characterization
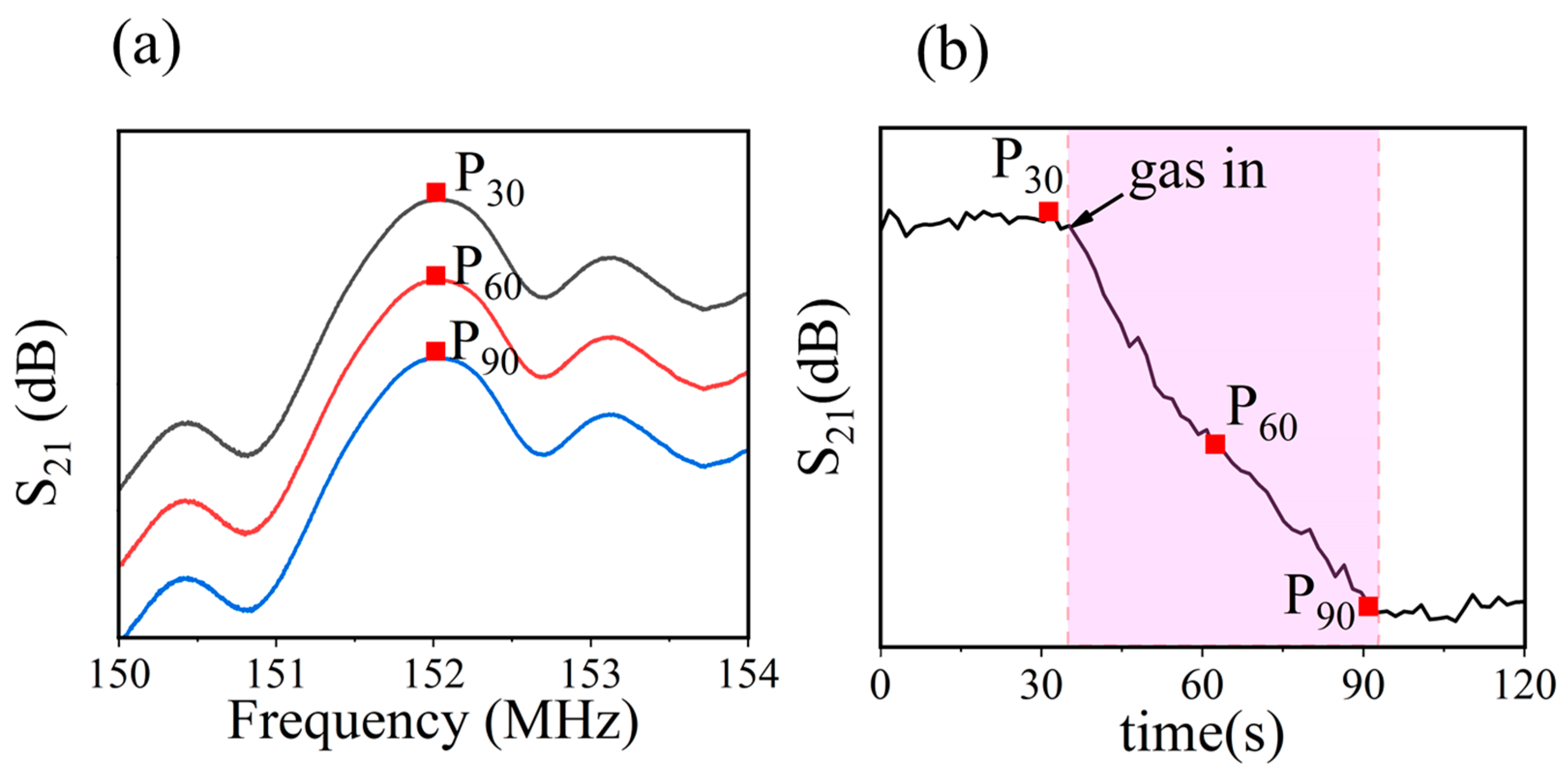
2.4. Sensor Test
2.5. Material Calculation
3. Results
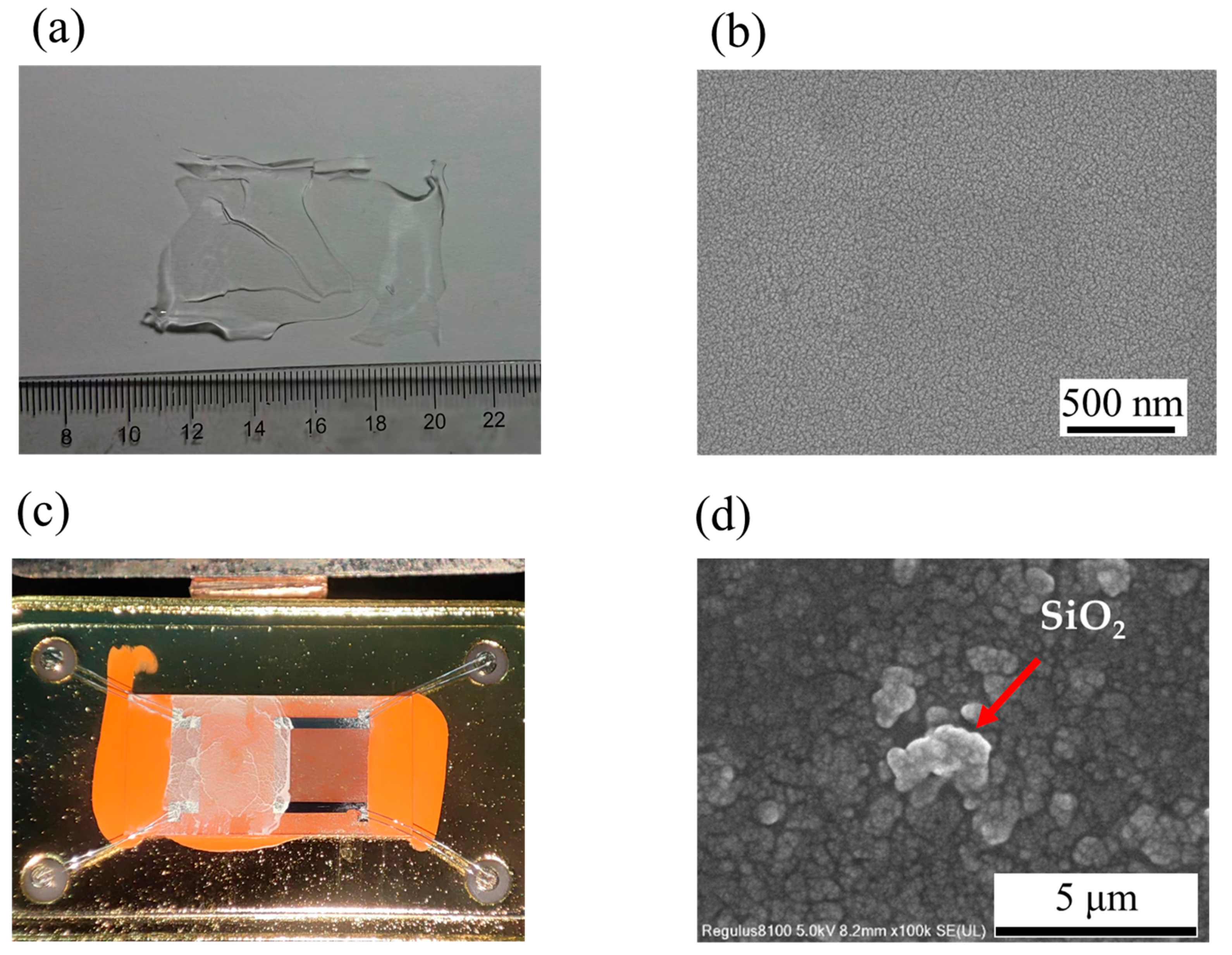
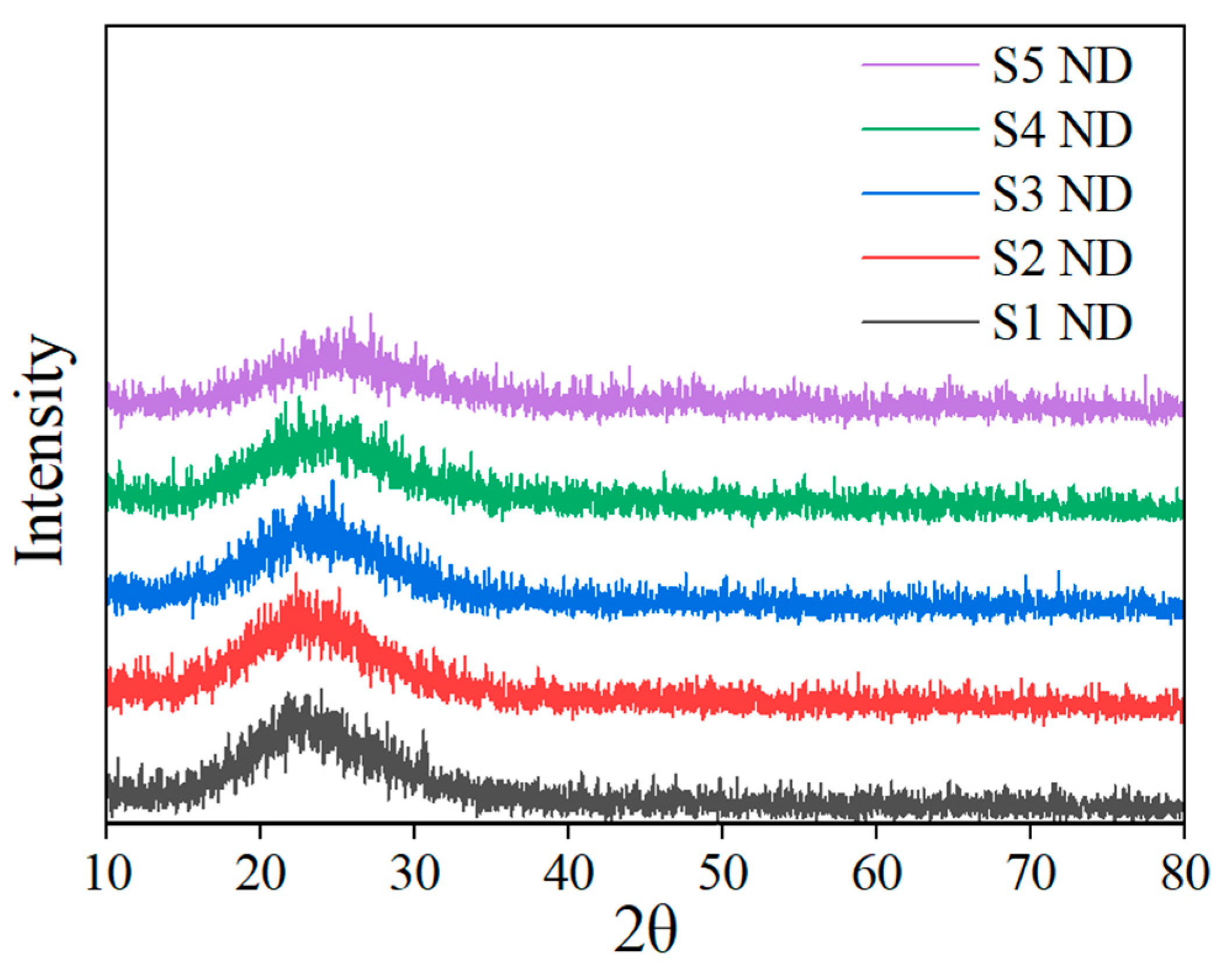
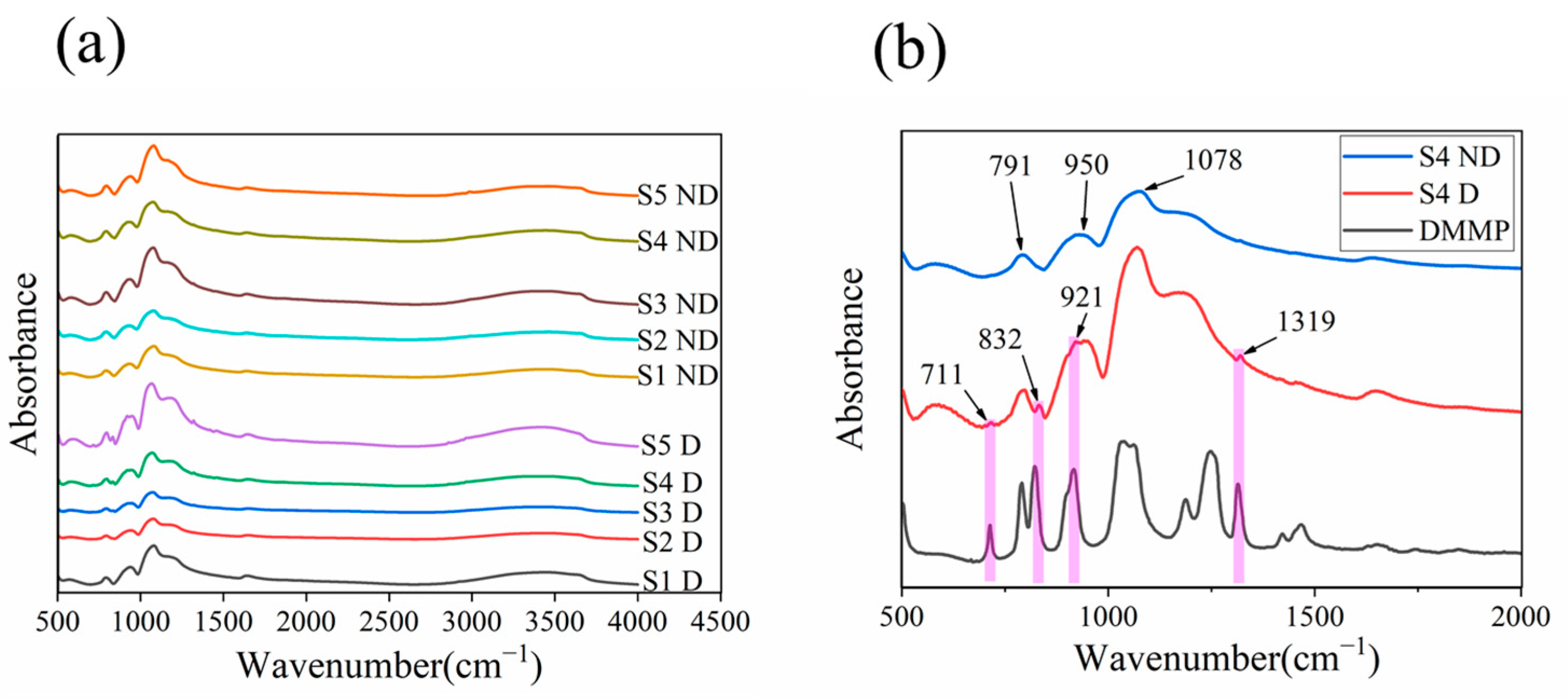
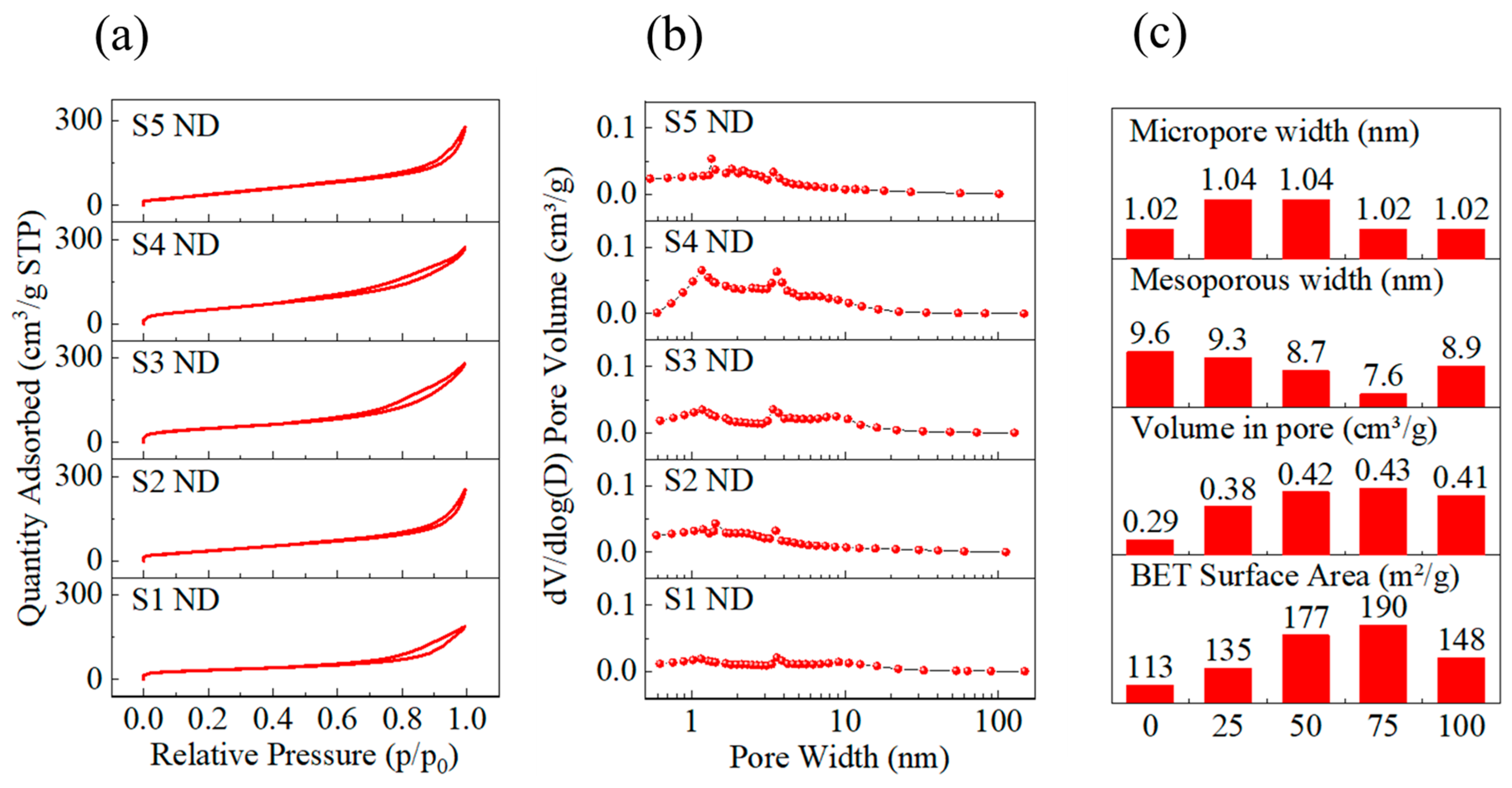
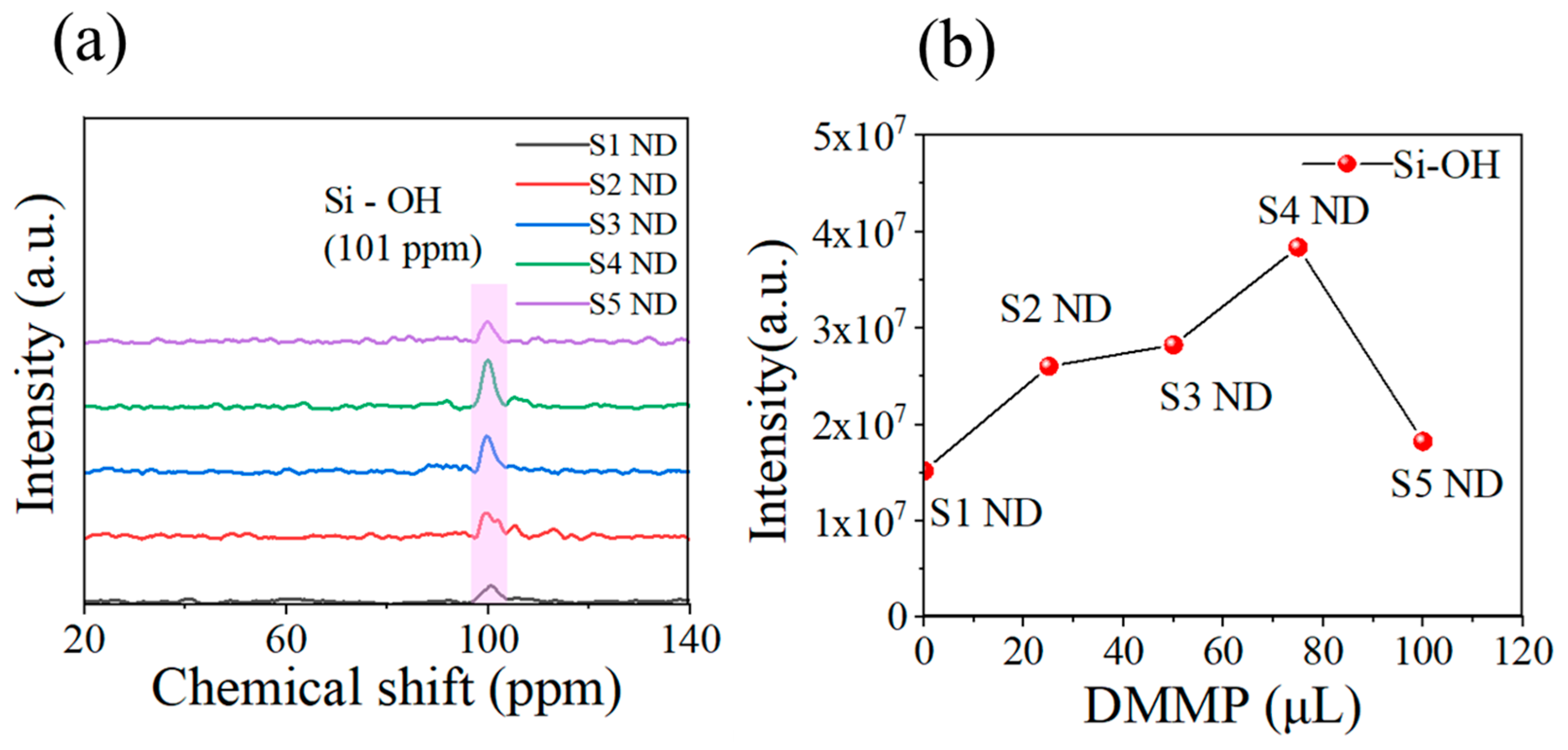
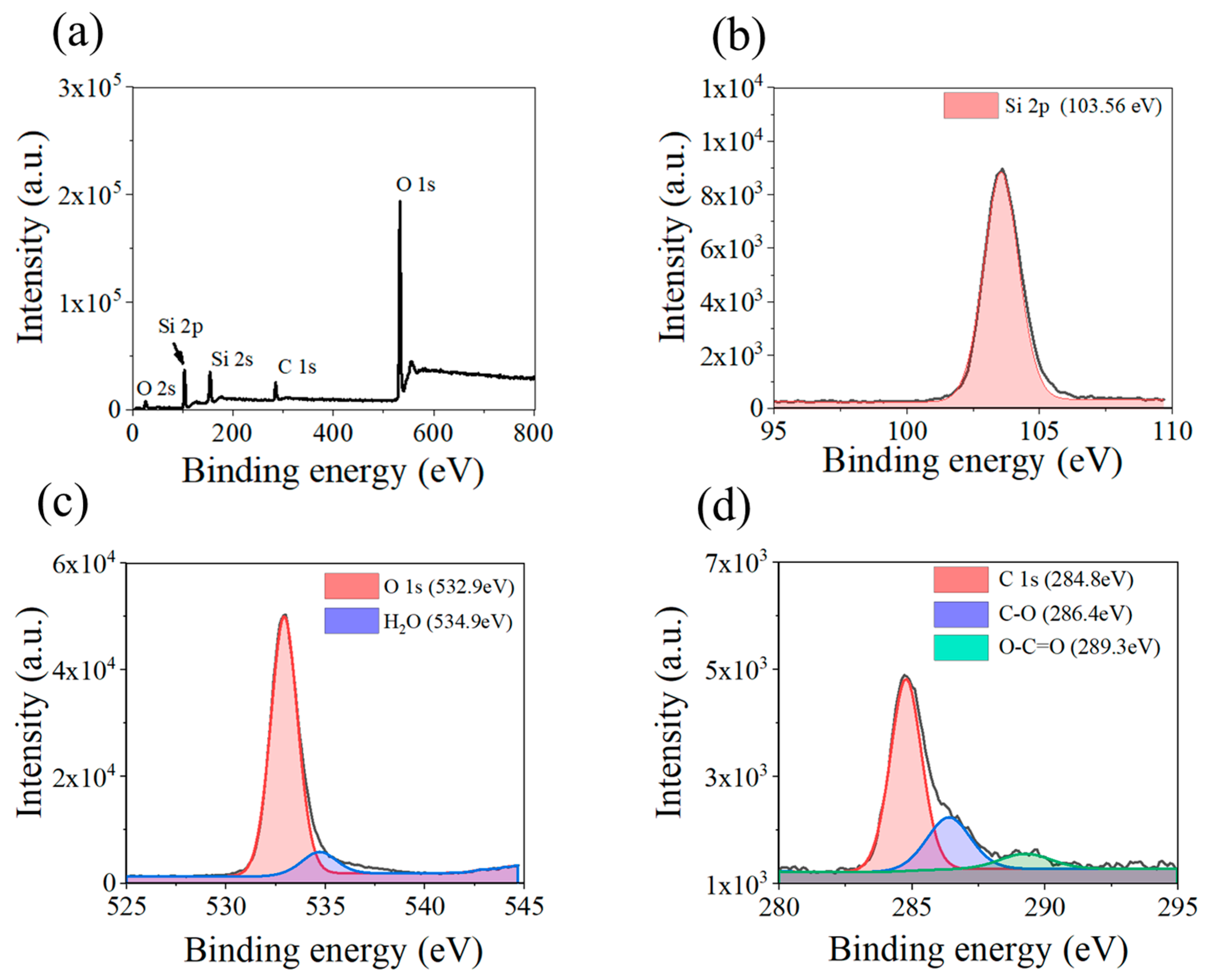
3.1. Characterization Results
3.2. Performance of Gas Sensor
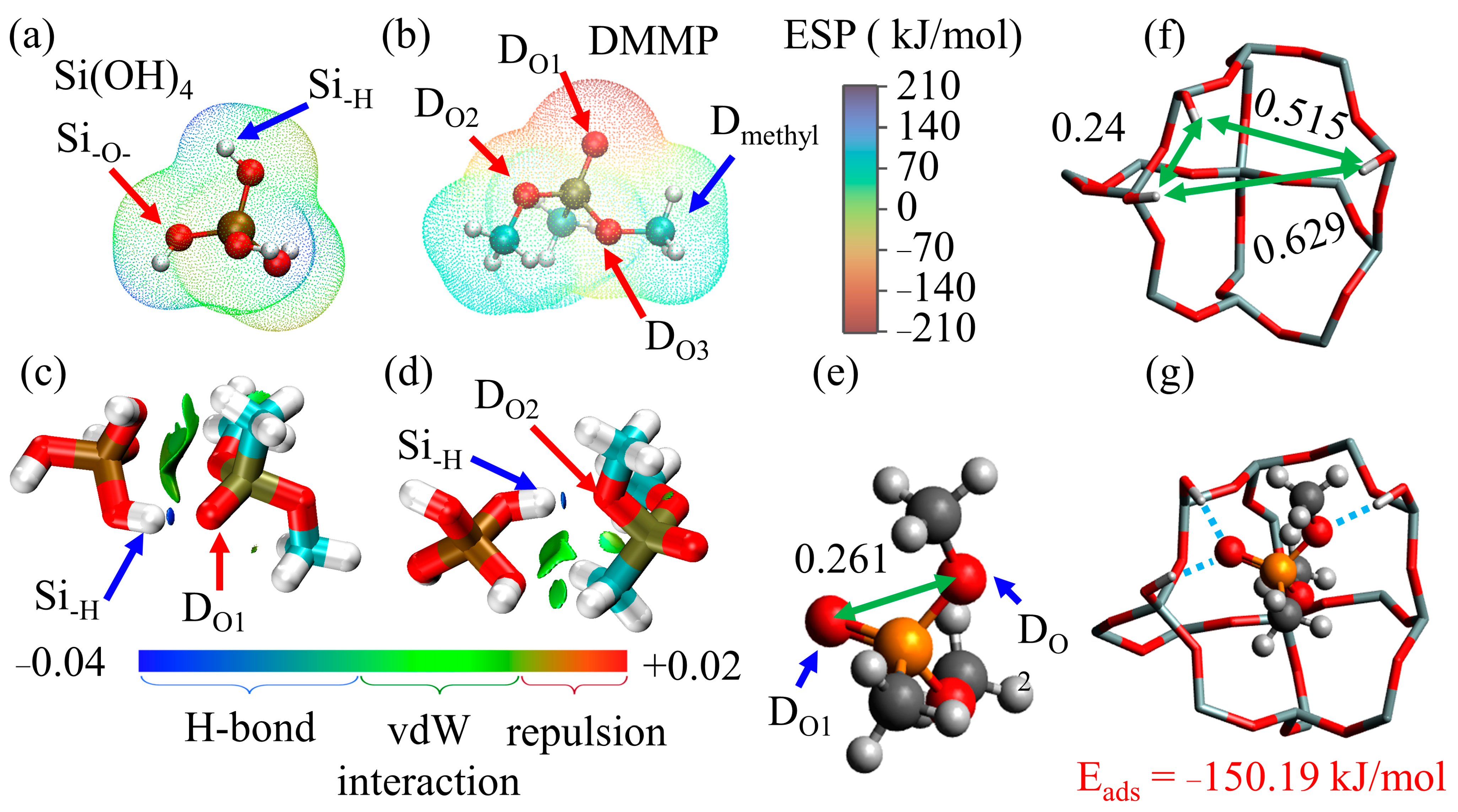
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Phar-macology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Li, Y.; Du, X.S.; Wang, Y.; Tai, H.L.; Qiu, D.; Lin, Q.H.; Jiang, Y.D. MEMS-based gas chromatography column for the analysis of chemical warfare agent(CWA) simulates. Appl. Mech. Mater. 2014, 475–476, 1294–1298. [Google Scholar] [CrossRef]
- Terzic, O.; Swahn, I.; Cretu, G.; Palit, M.; Mallard, G. Gas chromatography-full scan mass spectrometry determina-tion of traces of chemical warfare agents and their impurities in air samples by inlet based thermal desorption of sorbent tubes. J. Chromatogr. A 2012, 1225, 182–192. [Google Scholar] [CrossRef]
- Phillips, C.M.; Tan, H.W. FTIR Gas Analysis with Improved Sensitivity and Selectivity for CWA and TIC Detection. In Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Sensing XI; SPIE: Bellingham, WA, USA, 2010; Volume 7665. [Google Scholar] [CrossRef]
- Armenta, S.; Alcala, M.; Blanco, M. A review of recent, unconventional applications of ion mobility spectrometry (IMS). Anal. Chim. Acta 2011, 703, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Puton, J.; Namiesnik, J. Ion mobility spectrometry: Current status and application for chemical warfare agents detection. Trac-Trends Anal. Chem. 2016, 85, 10–20. [Google Scholar] [CrossRef]
- Patil, L.A.; Deo, V.V.; Shinde, M.D.; Bari, A.R.; Patil, D.M.; Kaushik, M.P. Improved 2-CEES sensing performance of spray pyrolized Ru-CdSnO3 nanostructured thin films. Sens. Actuators B Chem. 2014, 191, 130–136. [Google Scholar] [CrossRef]
- Kong, L.T.; Wang, J.; Fu, X.C.; Zhong, Y.; Meng, F.L.; Luo, T.; Liu, J.H. p-Hexafluoroisopropanol phenyl covalently functionalized single-walled carbon nanotubes for detection of nerve agents. Carbon 2010, 48, 1262–1270. [Google Scholar] [CrossRef]
- Long, Y.; Wang, Y.; Du, X.S.; Cheng, L.H.; Wu, P.L.; Jiang, Y.D. The Different Sensitive Behaviors of a Hydrogen-Bond Acidic Polymer-Coated SAW Sensor for Chemical Warfare Agents and Their Simulants. Sensors 2015, 15, 18302–18314. [Google Scholar] [CrossRef]
- Grate, J.W. Hydrogen-Bond Acidic Polymers for Chemical Vapor Sensing. Chem. Rev. 2008, 108, 726–745. [Google Scholar] [CrossRef] [PubMed]
- Grate, J.W. Acoustic wave microsensor arrays for vapor sensing. Chem. Rev. 2000, 100, 2627–2647. [Google Scholar] [CrossRef] [PubMed]
- Ballantine, D.S.; Rose, S.L.; Grate, J.W.; Wohltjen, H. Correlation of surface acoustic wave device coating responses with solubility properties and chemical structure using pattern recognition. Anal. Chem. 1986, 58, 3058–3066. [Google Scholar] [CrossRef]
- Grate, J.W.; Rose-Pehrsson, S.L.; Venezky, D.L.; Klusty, M.; Wohltjen, H. Smart sensor system for trace organophos-phorus and organosulfur vapor detection employing a temperature-controlled array of surface acoustic wave sen-sors, automated sample preconcentration, and pattern recognition. Anal. Chem. 1993, 65, 1868–1881. [Google Scholar] [CrossRef]
- Devkota, J.; Greve, D.W.; Hong, T.; Kim, K.J.; Ohodnicki, P.R. An 860 MHz Wireless Surface Acoustic Wave Sensor with a Metal-Organic Framework Sensing Layer for CO2 and CH4. IEEE Sens. J. 2020, 20, 9740–9747. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Holthoff, E.; Li, L.; Hiller, T.; Turner, K. A molecularly imprinted polymer (MIP)-coated microbeam MEMS sensor for chemical detection. In Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Sensing XVI; SPIE: Bellingham, WA, USA, 2015; Volume 9455. [Google Scholar] [CrossRef]
- Goerigk, L.; Hansen, A.; Bauer, C.; Ehrlich, S.; Najibi, A.; Grimme, S. A look at the density functional theory zoo with the advanced GMTKN55 database for general main group thermochemistry, kinetics and noncovalent interactions. Phys. Chem. Chem. Phys. 2017, 19, 32184–32215. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T.; Manzetti, S. Wavefunction and reactivity study of benzo[a]pyrene diol epoxide and its enantiomeric forms. Struct. Chem. 2014, 25, 1521–1533. [Google Scholar] [CrossRef]
- Manzetti, S.; Lu, T. The geometry and electronic structure of Aristolochic acid: Possible implications for a frozen resonance. J. Phys. Org. Chem. 2013, 26, 473–483. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, T. Efficient evaluation of electrostatic potential with computerized optimized code. Phys. Chem. Chem. Phys. 2021, 23, 20323–20328. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q.X. Independent gradient model based on Hirshfeld partition: A new method for visual study of in-teractions in chemical systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef]
- Sun, J.F.; Xu, Z.Q.; Li, W.F.; Shen, X.D. Effect of Nano-SiO2 on the Early Hydration of Alite-Sulphoaluminate Cement. Nanomaterials 2017, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Dubey, R.S. Synthesis of SiO2 Nanoparticles by Sol-Gel Method and Their Optical and Structural Properties. Rom. J. Inf. Sci. Technol. 2020, 23, 105–112. [Google Scholar]
- Ramalla, I.; Gupta, R.; Bansal, K. Effect on superhydrophobic surfaces on electrical porcelain insulator, improved technique at polluted areas for longer life and reliability. Int. J. Eng. Technol. 2015, 4, 509. [Google Scholar] [CrossRef]
- Lin, C.; Rüssel, C.; van Wüllen, L. Phase Separation and Nanocrystallization in KF–ZnF2–SiO2 Glasses: Lessons from Solid-State NMR. J. Phys. Chem. B 2019, 123, 1688–1695. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C. Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Wen, W.; Shitang, H.; Shunzhou, L.; Minghua, L.; Yong, P. Enhanced sensitivity of SAW gas sensor coated molecu-larly imprinted polymer incorporating high frequency stability oscillator. Sens. Actuators B Chem. 2007, 125, 422–427. [Google Scholar] [CrossRef]
- Du, X.; Ying, Z.; Jiang, Y.; Liu, Z.; Yang, T.; Xie, G. Synthesis and evaluation of a new polysiloxane as SAW sensor coatings for DMMP detection. Sens. Actuators B Chem. 2008, 134, 409–413. [Google Scholar] [CrossRef]
- Matatagui, D.; Fernández, M.J.; Fontecha, J.; Santos, J.P.; Gràcia, I.; Cané, C.; Horrillo, M.C. Love-wave sensor array to detect, discriminate and classify chemical warfare agent simulants. Sens. Actuators B Chem. 2012, 175, 173–178. [Google Scholar] [CrossRef]
- Di Pietrantonio, F.; Benetti, M.; Cannatà, D.; Verona, E.; Palla-Papavlu, A.; Dinca, V.; Dinescu, M.; Mattle, T.; Lippert, T. Volatile toxic compound detection by surface acoustic wave sensor array coated with chemoselective polymers deposited by laser induced forward transfer: Application to sarin. Sens. Actuators B Chem. 2012, 174, 158–167. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, G.W.; Guo, T.X.; Liu, X.L.; Zhang, C.H.; Yang, J.C.; Cao, B.Q.; Zhang, C.; Wang, W. Environmental characteristics of surface acoustic wave devices for sensing organophosphorus vapor. Sens. Actuators B Chem. 2020, 315. [Google Scholar] [CrossRef]
- Wu, Q.; Li, X.; Wang, X.M.; Yuan, Y.B.; Bu, X.R.; Wu, H.Y.; Li, X.; Han, C.Y.; Wang, X.L.; Liu, W.H. High-performance p-hexafluoroisopropanol phenyl functionalized multi-walled carbon nanotube film on surface acoustic wave device for organophosphorus vapor detection. Nanotechnology 2022, 33, 375501. [Google Scholar] [CrossRef] [PubMed]
- Zhuravlev, L.T. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf. A 2000, 173, 1–38. [Google Scholar] [CrossRef]
- Plotnichenko, V.G.; Sokolov, V.O.; Dianov, E.M. Hydroxyl groups in high-purity silica glass. Inorg. Mater. 2000, 36, 404–410. [Google Scholar] [CrossRef]
- Lafuente, M.; Sanz, D.; Urbiztondo, M.; Santamaria, J.; Pina, M.P.; Mallada, R. Gas phase detection of chemical warfare agents CWAs with portable Raman. J. Hazard. Mater. 2020, 384, 121279. [Google Scholar] [CrossRef]
- Wang, J.; Duan, G.; Liu, G.; Li, Y.; Chen, Z.; Xu, L.; Cai, W. Detection of dimethyl methylphosphonate by thin water film confined surface-enhanced Raman scattering method. J. Hazard. Mater. 2016, 303, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wen, B.; Liu, W.; Fu, W.; Kong, J.; Li, J. Rapidly Detection of Chemical Warfare Agent Simulants by Surface Enhanced Raman Spectroscopy. Spectrosc. Spectr. Anal. 2022, 42, 110–114. [Google Scholar] [CrossRef]
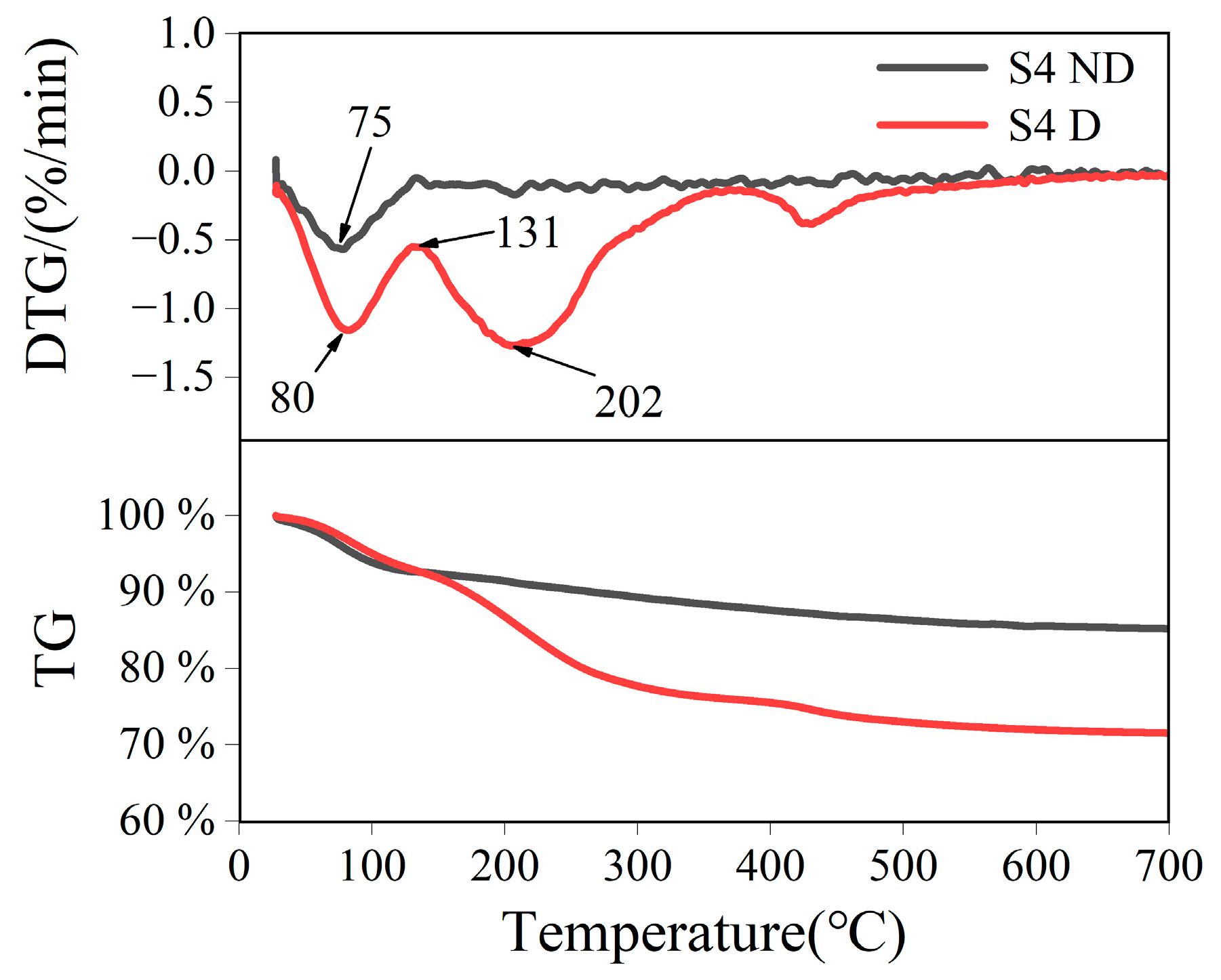
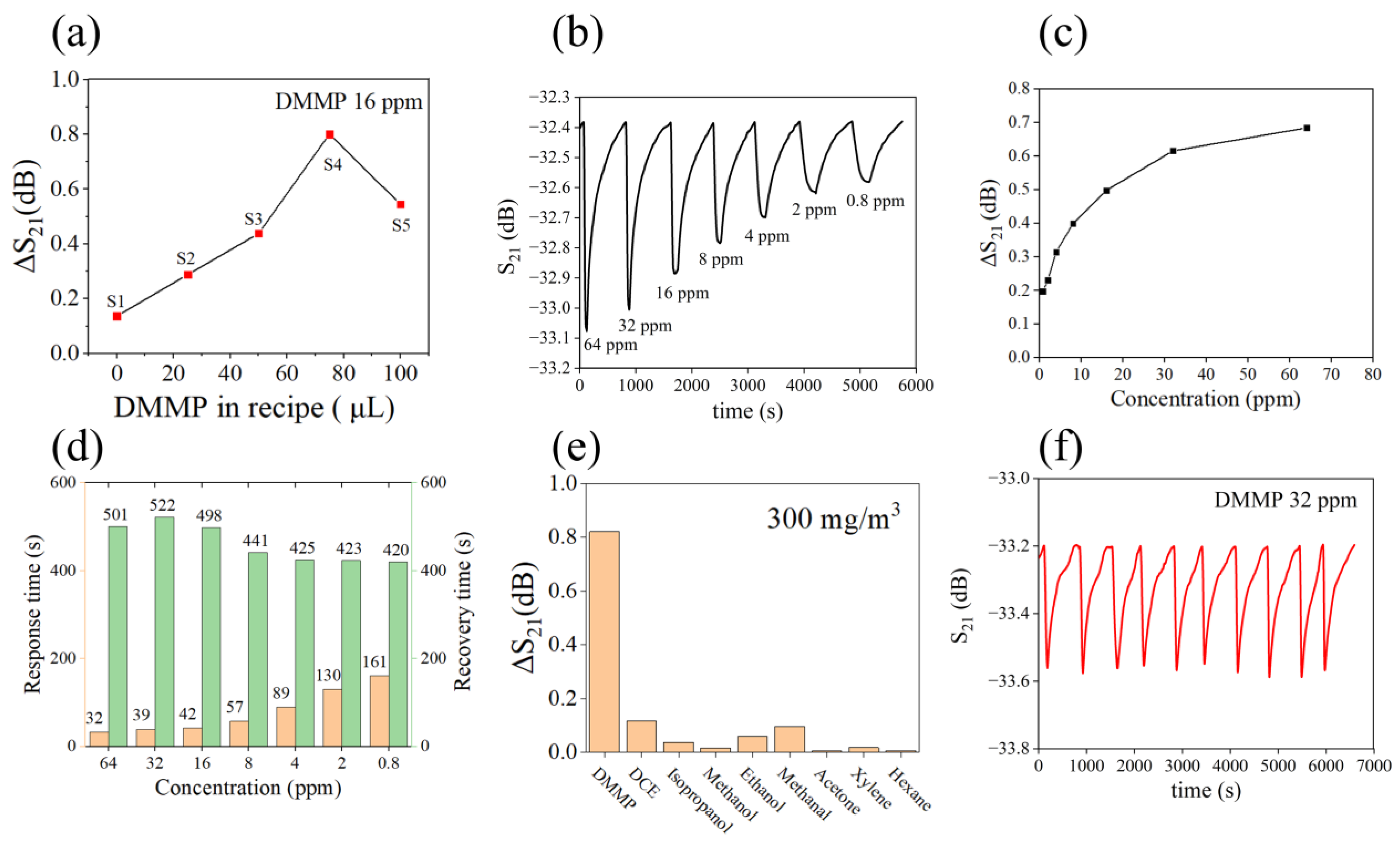
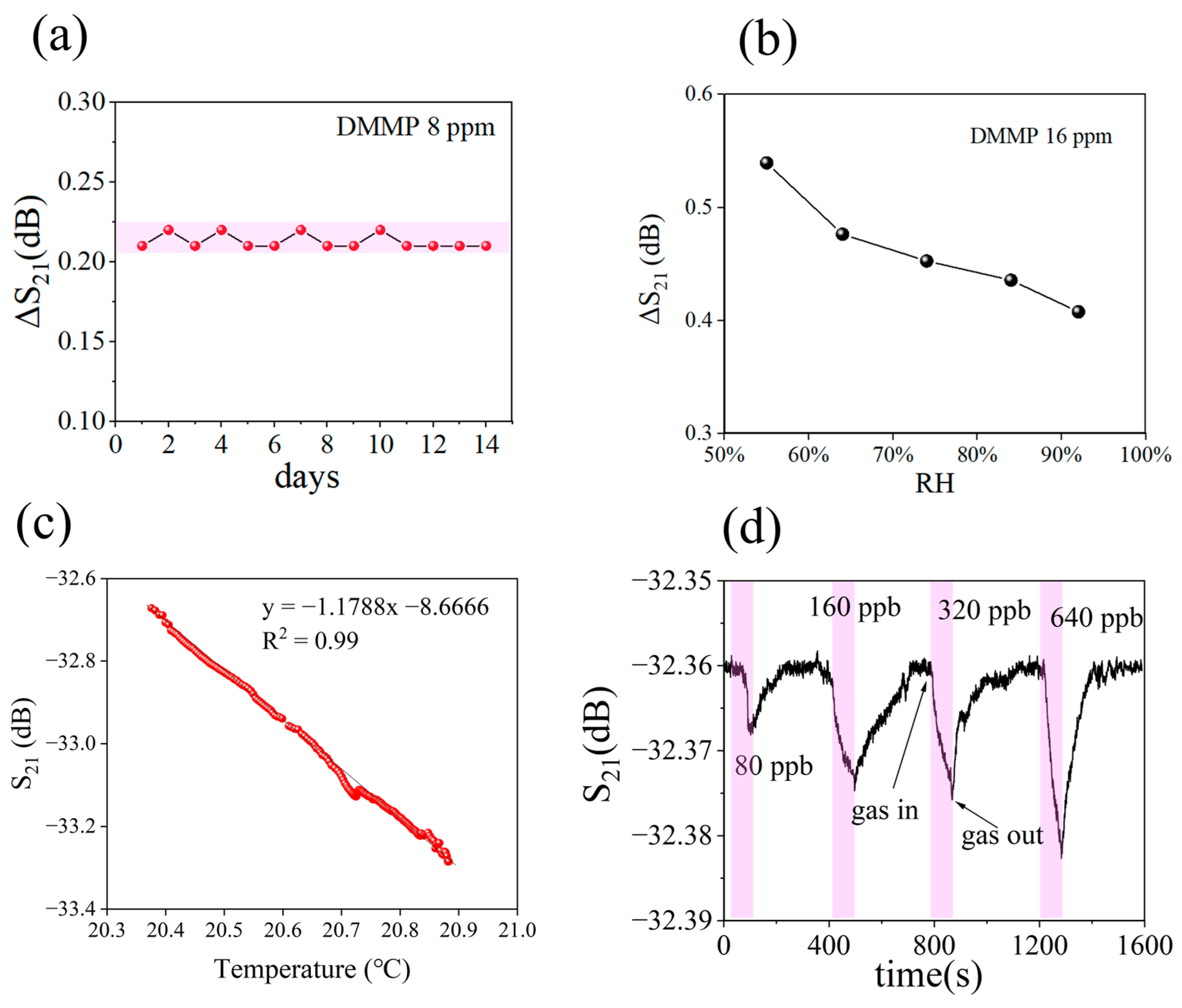

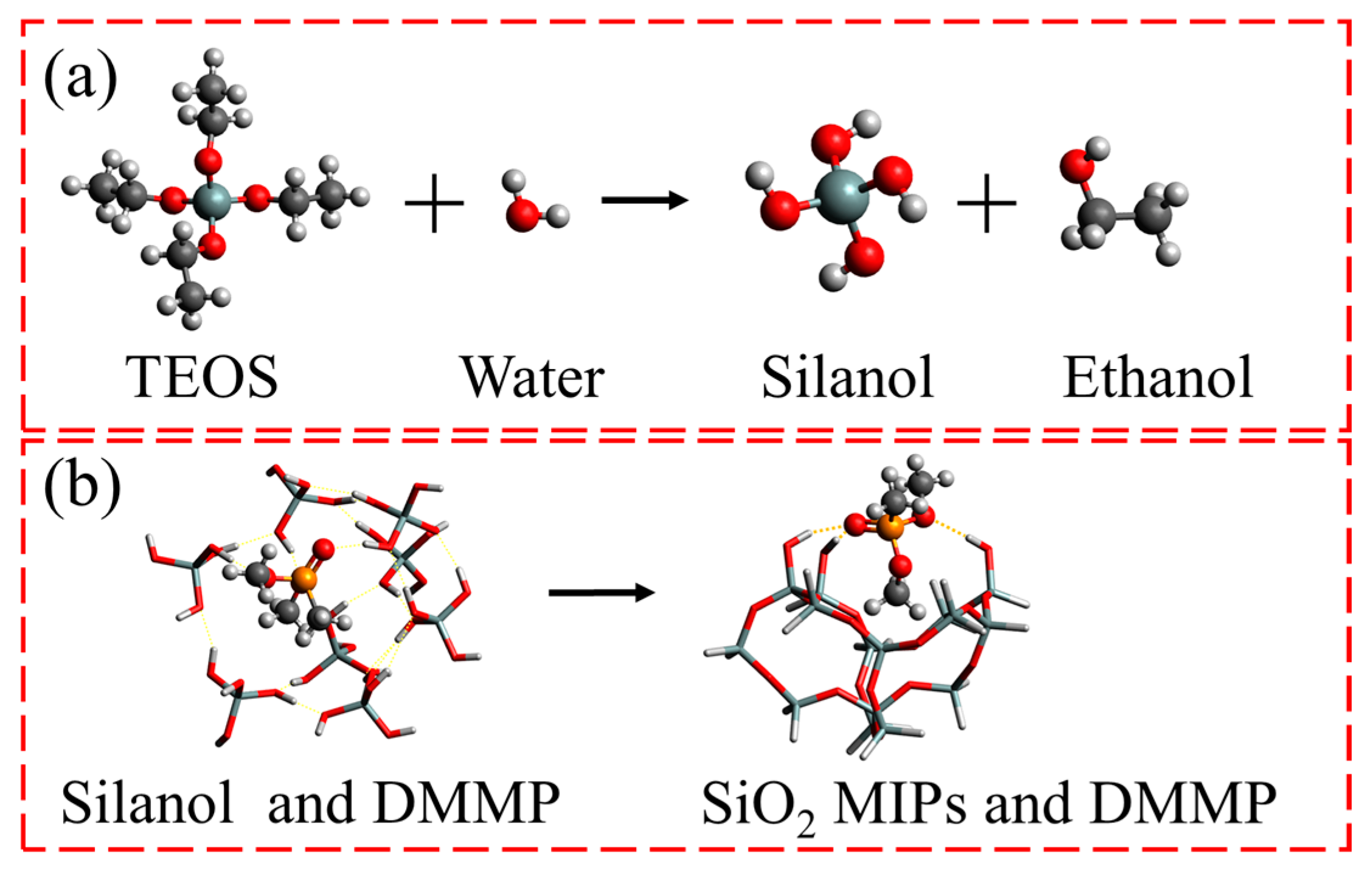
| Recipe | S1 | S2 | S3 | S4 | S5 |
|---|---|---|---|---|---|
| DMMP (μL) | 0 | 25 | 50 | 75 | 100 |
| Ethanol (μL) | 655 | ||||
| TEOS (μL) | 1260 | ||||
| Water (μL) | 205 | ||||
| HCl (μL) | 5.62 | ||||
| Frequency | Sensitive Material | Limit of Detection (DMMP) | Response Time | Ref. |
|---|---|---|---|---|
| 300 MHz | molecularly imprinted polymer | 0.1 ppm | 300 s | [28] |
| 434 MHz | polymethyl[3-(2-hydroxy) phenyl] siloxane | 5 ppm | 30 s | [29] |
| 163 MHz | carbowax, polyethyleneimine, polyepichlorohydrin. etc. | 0.04 ppm | 1800 s | [30] |
| 392 MHz | polyethyleneimine, polyepichlorhydrine, polyisobutylene | 2.5 ppm | 60 s | [31] |
| 150 MHz | fluoroalcoholpolysiloxane (SXFA) | 0.025 ppm | [32] | |
| 152 MHz | hexafluoroisopropanol (HFIP) modified carbon nanotubes | 0.1 ppm | 3 s | [33] |
| 152 MHz | molecularly imprinted polymer (SiO2 MIPs) | 0.08 ppm | 60 s | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, X.; Wu, Q.; Yuan, Y.; Liu, W.; Han, C.; Wang, X. Detection of Dimethyl Methyl Phosphonate by Silica Molecularly Imprinted Materials. Nanomaterials 2023, 13, 2871. https://doi.org/10.3390/nano13212871
Wang X, Li X, Wu Q, Yuan Y, Liu W, Han C, Wang X. Detection of Dimethyl Methyl Phosphonate by Silica Molecularly Imprinted Materials. Nanomaterials. 2023; 13(21):2871. https://doi.org/10.3390/nano13212871
Chicago/Turabian StyleWang, Xuming, Xin Li, Qiang Wu, Yubin Yuan, Weihua Liu, Chuanyu Han, and Xiaoli Wang. 2023. "Detection of Dimethyl Methyl Phosphonate by Silica Molecularly Imprinted Materials" Nanomaterials 13, no. 21: 2871. https://doi.org/10.3390/nano13212871
APA StyleWang, X., Li, X., Wu, Q., Yuan, Y., Liu, W., Han, C., & Wang, X. (2023). Detection of Dimethyl Methyl Phosphonate by Silica Molecularly Imprinted Materials. Nanomaterials, 13(21), 2871. https://doi.org/10.3390/nano13212871






