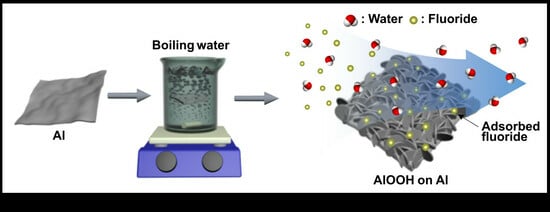
Efficient Fluoride Wastewater Treatment Using Eco-Friendly Synthesized AlOOH
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
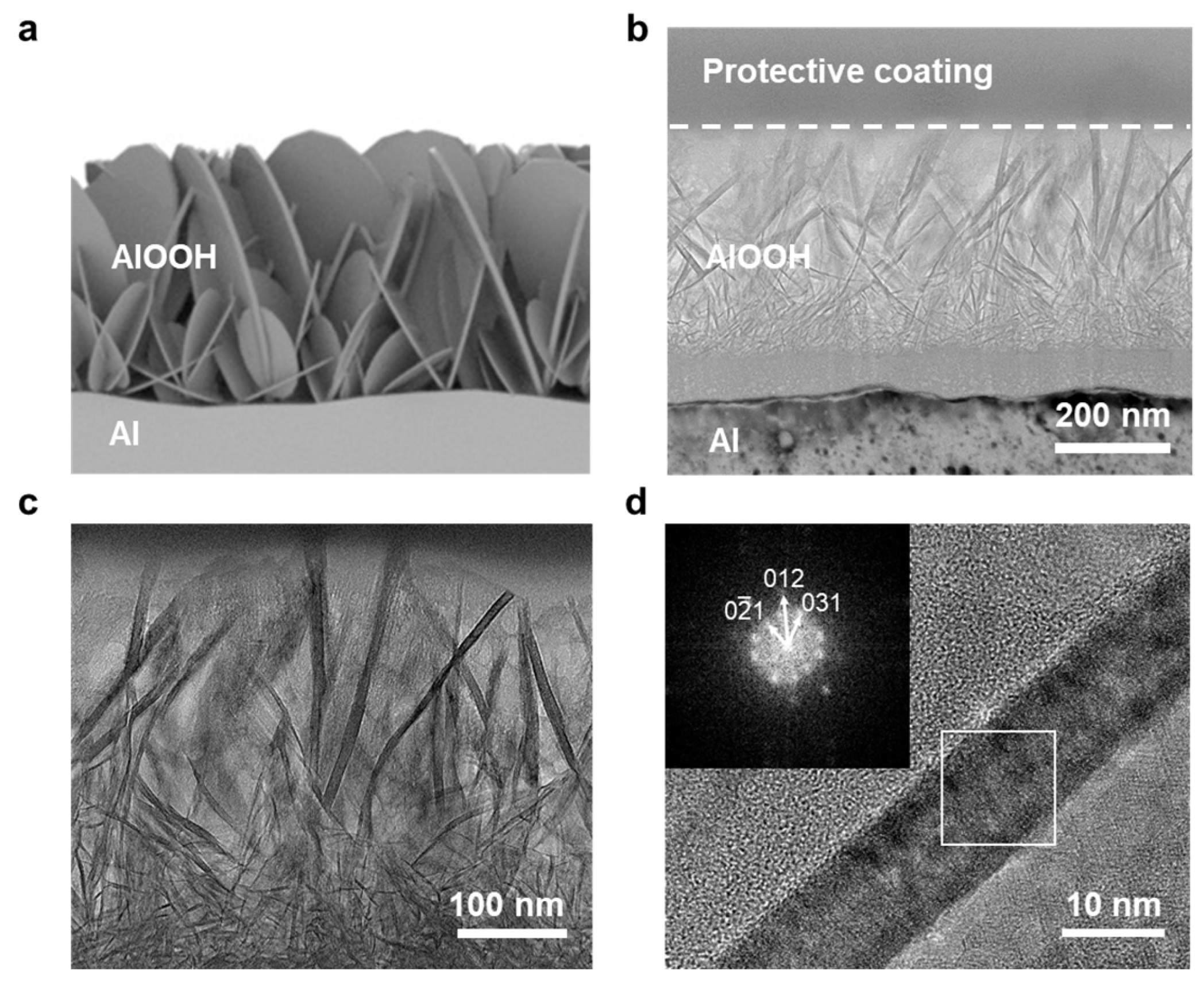
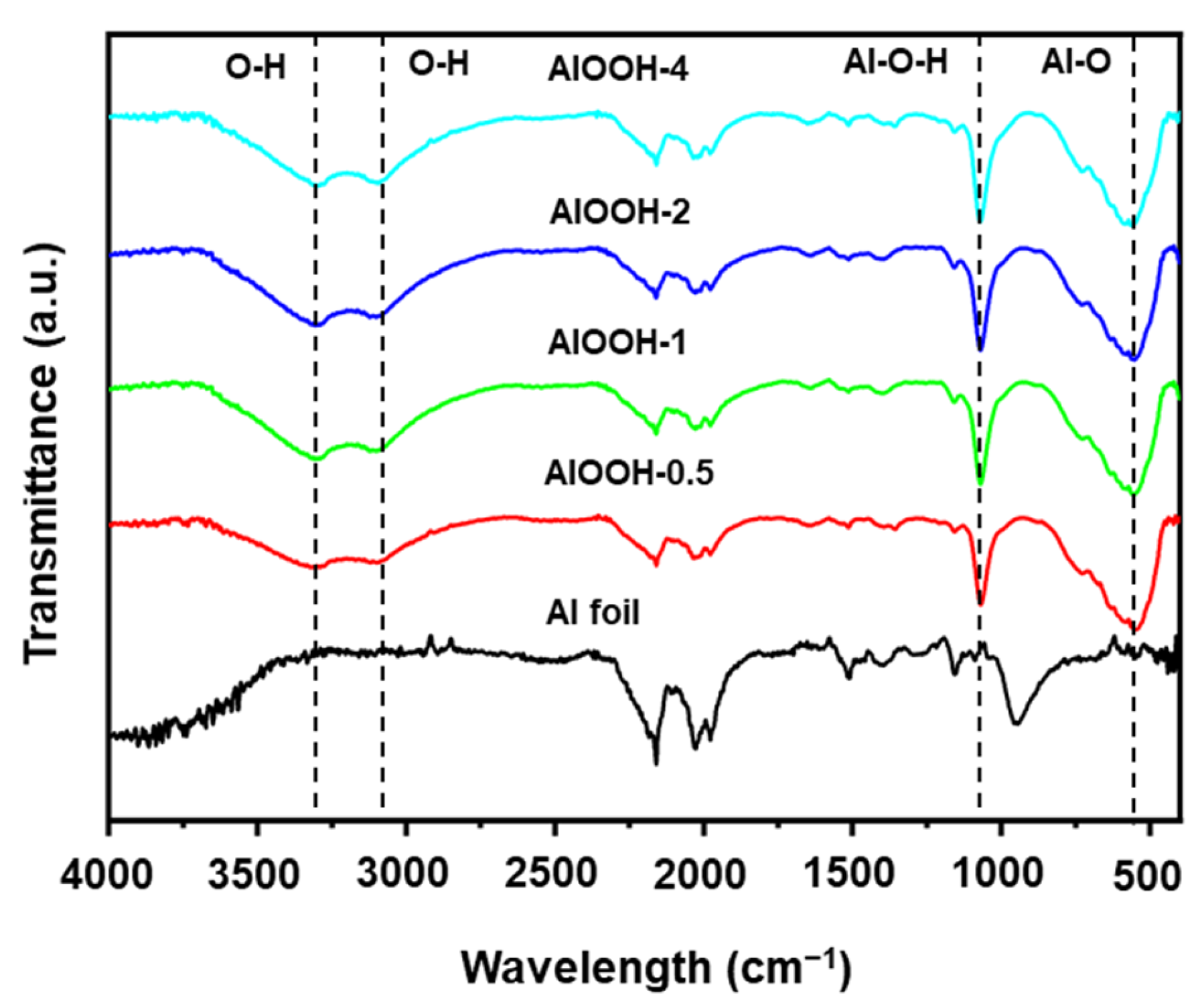
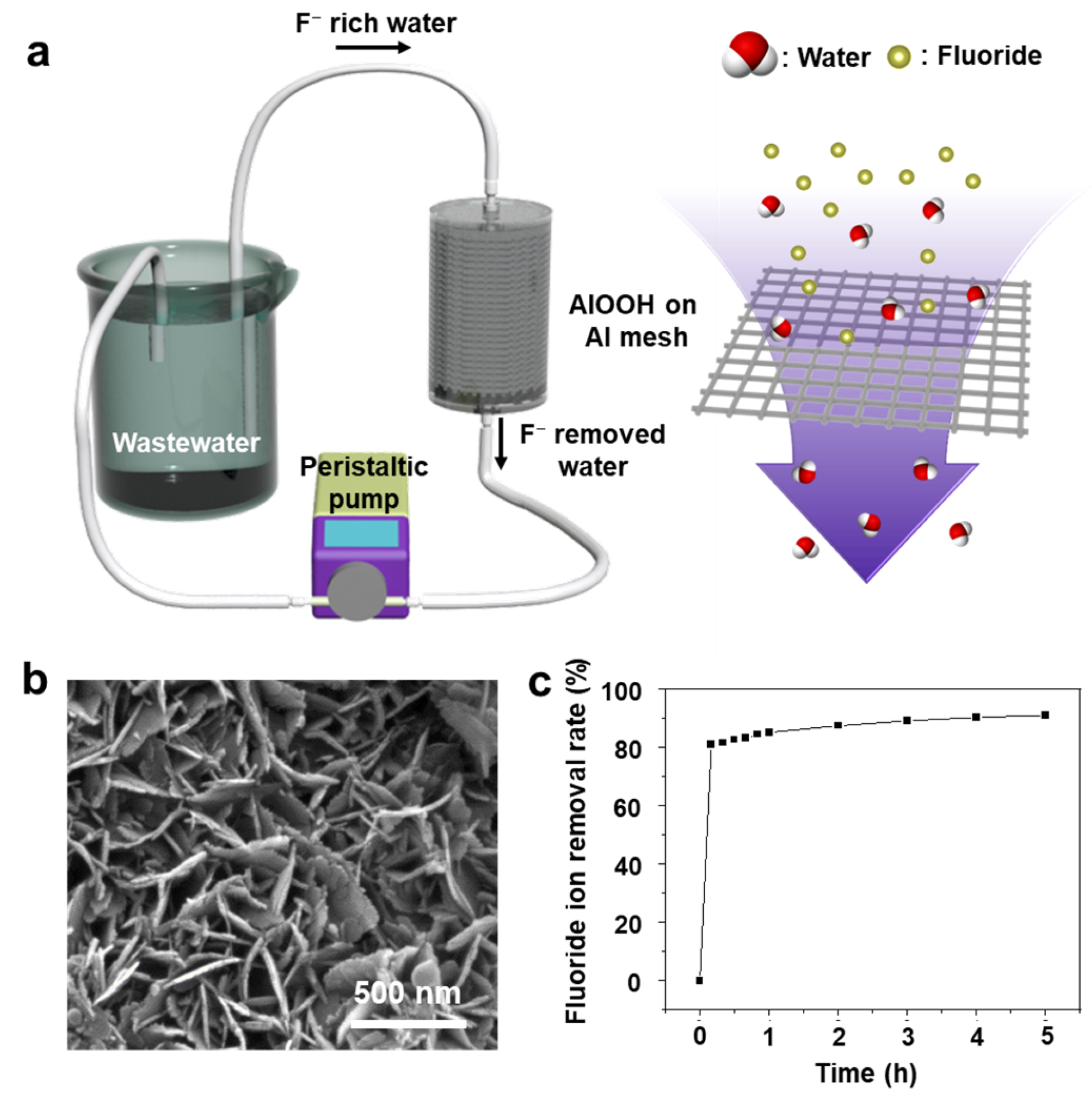
3.1. Synthesis and Characterization of AlOOH Adsorbents on Al Substrate
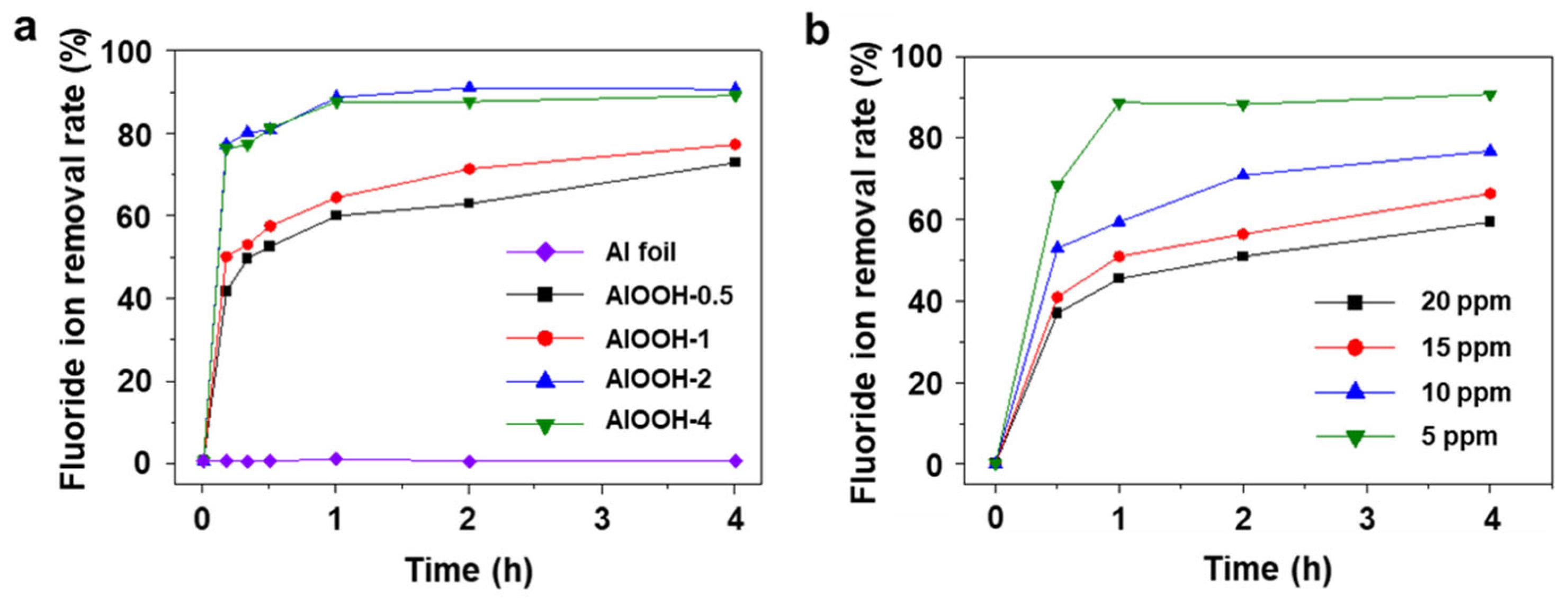
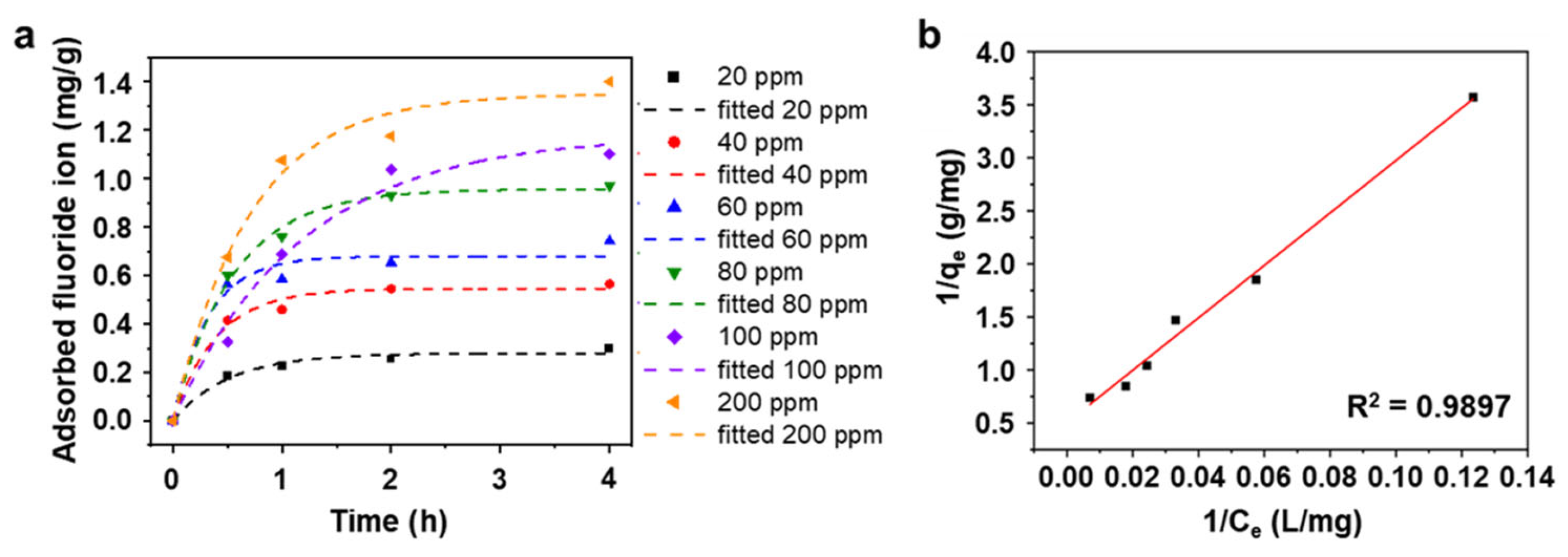
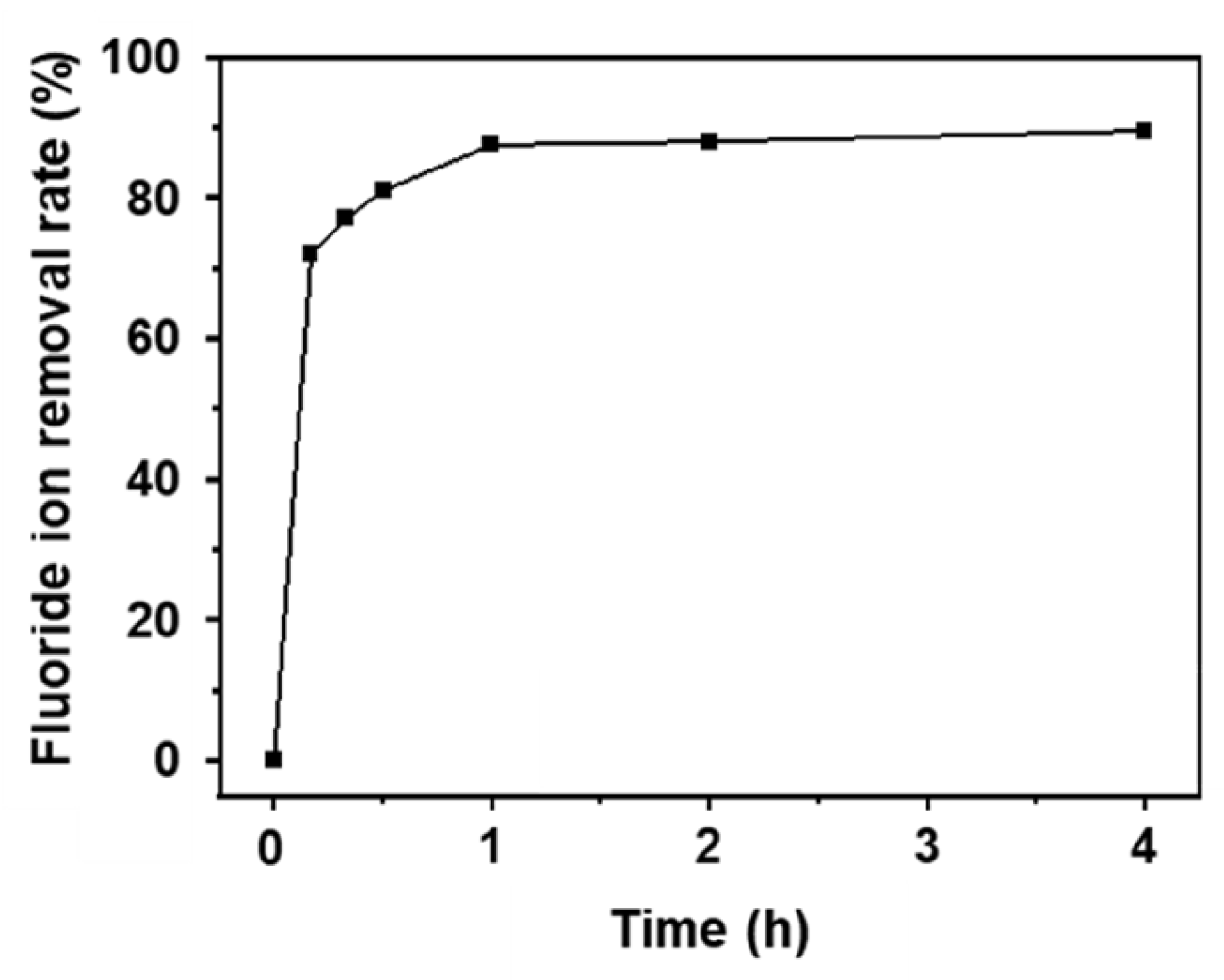
3.2. Fluoride Ion Removal Capacity of AlOOH Adsorbents on Al Substrate
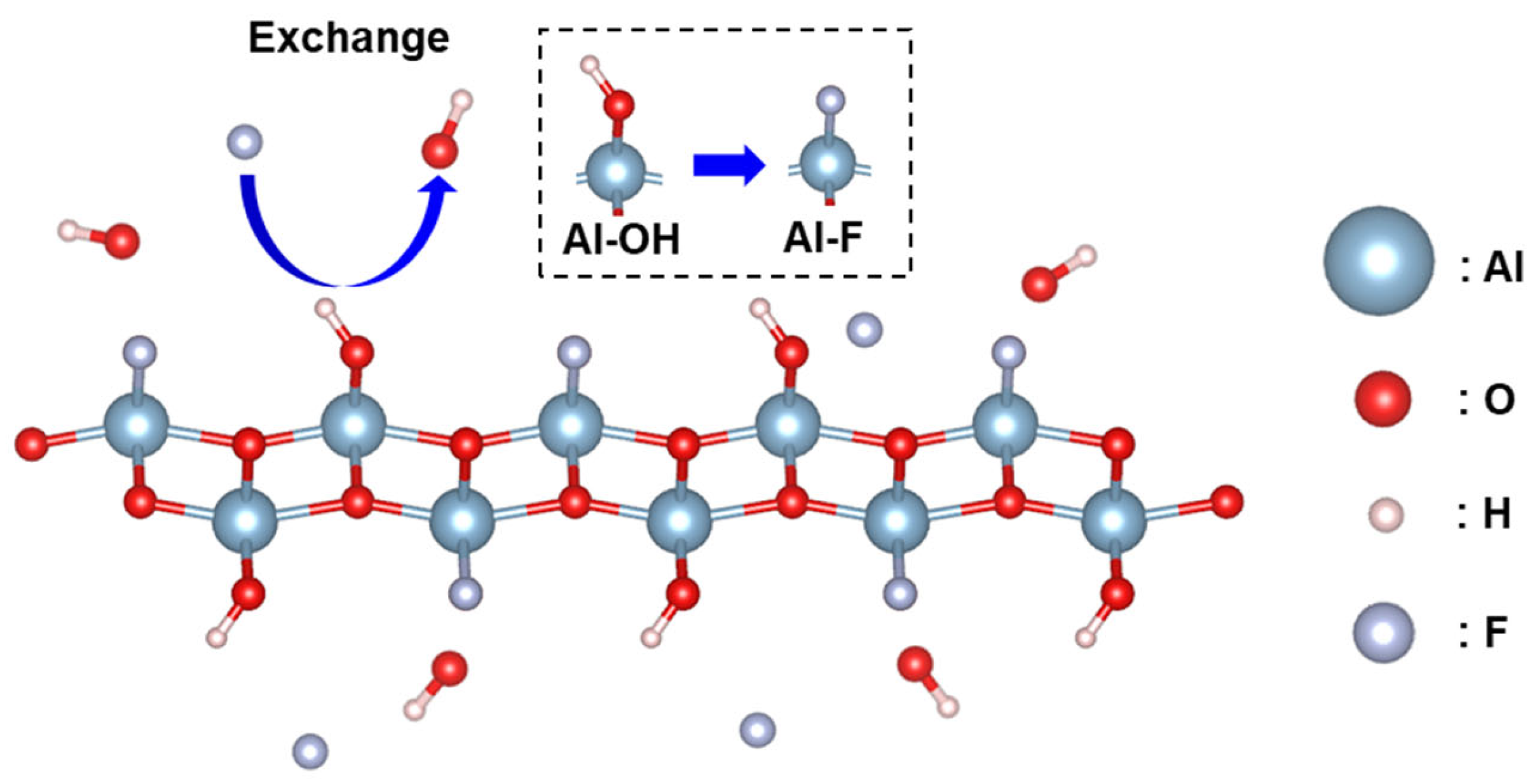
3.3. Fluoride Adsorption Mechanism of AlOOH Adsorbents on an Al Substrate
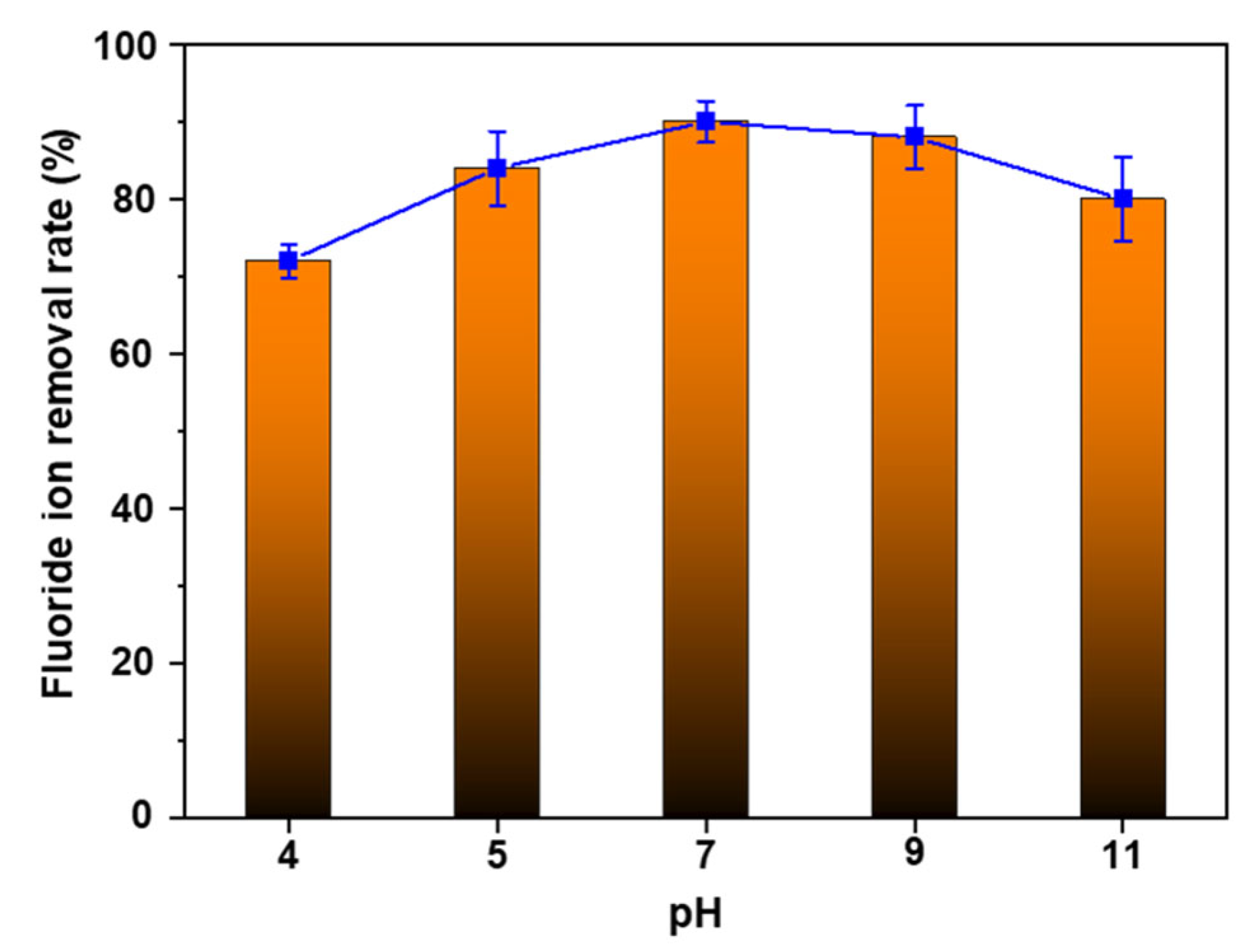
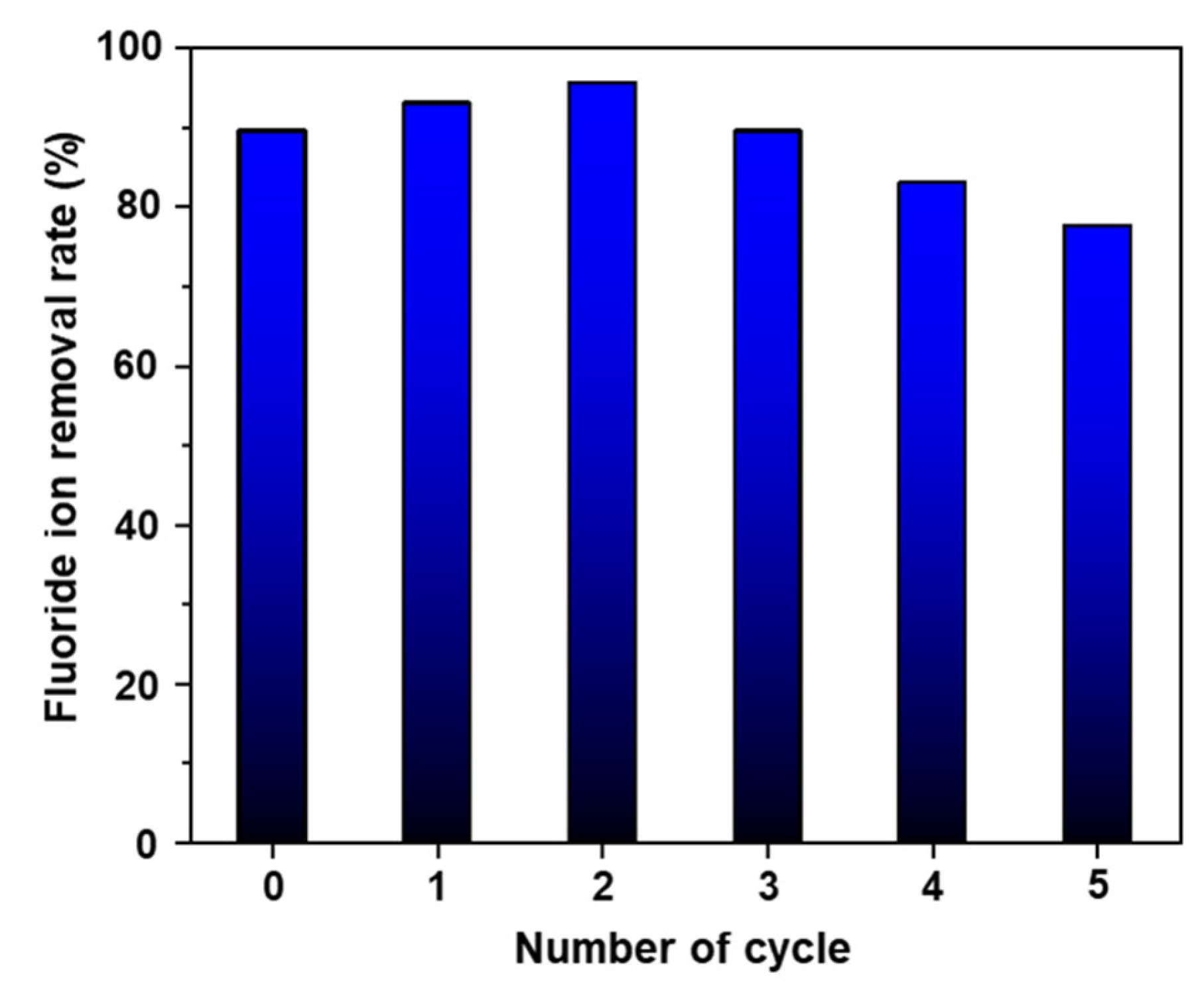
3.4. pH Variance, Reusability, and Industrial Wastewater Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wan, K.; Huang, L.; Yan, J.; Ma, B.; Huang, X.; Luo, Z.; Zhang, H.; Xiao, T. Removal of fluoride from industrial wastewater by using different adsorbents: A review. Sci. Total Environ. 2021, 773, 145535. [Google Scholar] [CrossRef] [PubMed]
- Choubisa, S.L.; Choubisa, D. Status of industrial fluoride pollution and its diverse adverse health effects in man and domestic animals in India. Environ. Sci. Pollut. Res. 2016, 23, 7244–7254. [Google Scholar] [CrossRef] [PubMed]
- Gai, W.Z.; Zhang, S.H.; Yang, Y.; Zhang, X.; Deng, Z.Y. Separation of Excess Fluoride from Water Using Amorphous and Crystalline AlOOH Adsorbents. ACS Omega 2021, 6, 16488–16497. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, H.-B.; Qu, J.; Yao, H.; Yao, J.; Yu, Z.-Z. Supercritical carbon dioxide fluid assisted synthesis of hierarchical AlOOH@reduced graphene oxide hybrids for efficient removal of fluoride ions. Chem. Eng. J. 2016, 292, 174–182. [Google Scholar] [CrossRef]
- Meenakshi; Maheshwari, R.C. Fluoride in drinking water and its removal. J. Hazard. Mater. 2006, 137, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Kumar, E.; Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. Interaction of anionic pollutants with Al-based adsorbents in aqueous media—A review. Chem. Eng. J. 2014, 241, 443–456. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2004; Volume 1. [Google Scholar]
- Saltworks Technologies Inc. Fluoride Removal from Industrial Wastewater Using Advanced Chemical Precipitation and Filtration; Saltworks Technologies Inc.: Richmond, BC, Canada, 2019. [Google Scholar]
- Bagastyo, A.Y.; Anggrainy, A.D.; Nindita, C.S.; Warmadewanthi. Electrodialytic removal of fluoride and calcium ions to recover phosphate from fertilizer industry wastewater. Sustain. Environ. Res. 2017, 27, 230–237. [Google Scholar] [CrossRef]
- Chen, J.; Yang, R.; Zhang, Z.; Wu, D. Removal of fluoride from water using aluminum hydroxide-loaded zeolite synthesized from coal fly ash. J. Hazard. Mater. 2022, 421, 126817. [Google Scholar] [CrossRef]
- Wambu, E.; Frau, F.; Machunda, R.; Pasape, L.; Barasa, S.; Ghiglieri, G. Water Defluoridation Methods Applied in Rural Areas over the World. 2022. Available online: https://dspace.nm-aist.ac.tz/handle/20.500.12479/1483 (accessed on 5 October 2023).
- Reardon, E.J.; Wang, Y. A Limestone Reactor for Fluoride Removal from Wastewaters. Environ. Sci. Technol. 2000, 34, 3247–3253. [Google Scholar] [CrossRef]
- Meenakshi, S.; Viswanathan, N. Identification of selective ion-exchange resin for fluoride sorption. J. Colloid Interface Sci. 2007, 308, 438–450. [Google Scholar] [CrossRef]
- Amor, Z.; Bariou, B.; Mameri, N.; Taky, M.; Nicolas, S.; Elmidaoui, A. Fluoride removal from brackish water by electrodialysis. Desalination 2001, 133, 215–223. [Google Scholar] [CrossRef]
- Li, W.; Cao, C.Y.; Wu, L.Y.; Ge, M.F.; Song, W.G. Superb fluoride and arsenic removal performance of highly ordered mesoporous aluminas. J. Hazard. Mater. 2011, 198, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, A.M.; Asgedom, A.G.; Mekonnen, K.N.; Ashebir, M.E.; Gebremikael, Z.H.; Mesfin, K.A. Defluoridation of Water Using Aluminum Hydroxide Activated Carbon Biosorbents. Adv. Mater. Sci. Eng. 2022, 2022, 4038444. [Google Scholar] [CrossRef]
- Kuang, L.; Liu, Y.; Fu, D.; Zhao, Y. FeOOH-graphene oxide nanocomposites for fluoride removal from water: Acetate mediated nano FeOOH growth and adsorption mechanism. J. Colloid Interface Sci. 2017, 490, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Chiavola, A.; D’Amato, E.; Di Marcantonio, C. Comparison of Adsorptive Removal of Fluoride from Water by Different Adsorbents under Laboratory and Real Conditions. Water 2022, 14, 1423. [Google Scholar] [CrossRef]
- Salifu, A. Fluoride Removal from Groundwater by Adsorption Technology; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Hu, H.; Yang, L.; Lin, Z.; Zhao, Y.; Jiang, X.; Hou, L. A low-cost and environment friendly chitosan/aluminum hydroxide bead adsorbent for fluoride removal from aqueous solutions. Iran. Polym. J. 2018, 27, 253–261. [Google Scholar] [CrossRef]
- Burton, D.J.; Briney, G.C. Disproportionation of 3, 3-difluorotetrachloropropene. Application of the hard and soft acids and bases principle to organic halogen compounds. J. Org. Chem. 1970, 35, 3036–3045. [Google Scholar] [CrossRef]
- Wang, S.-G.; Ma, Y.; Shi, Y.-J.; Gong, W.-X. Defluoridation performance and mechanism of nano-scale aluminum oxide hydroxide in aqueous solution. J. Chem. Technol. Biotechnol. 2009, 84, 1043–1050. [Google Scholar] [CrossRef]
- Gai, W.-Z.; Zhang, S.-H.; Yang, Y.; Sun, K.; Jia, H.; Deng, Z.-Y. Defluoridation performance comparison of aluminum hydroxides with different crystalline phases. Water Supply 2022, 22, 3673–3684. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Z.; He, Y.; Chai, L.; Yang, W.; Deng, H.; Wang, H.; Chen, Y.; Crittenden, J. Adsorption mechanism for removing different species of fluoride by designing of core-shell boehmite. J. Hazard. Mater. 2020, 394, 122555. [Google Scholar] [CrossRef]
- Onyango, M.S.; Matsuda, H. Fluoride removal from water using adsorption technique. Adv. Fluor. Sci. 2006, 2, 1–48. [Google Scholar] [CrossRef]
- Krasovskiĭ, G.; Vasukovich, L.; Chariev, O. Experimental study of biological effects of leads and aluminum following oral administration. Environ. Health Persp. 1979, 30, 47–51. [Google Scholar] [CrossRef][Green Version]
- Nieboer, E.; Gibson, B.L.; Oxman, A.D.; Kramer, J.R. Health effects of aluminum: A critical review with emphasis on aluminum in drinking water. Environ. Rev. 1995, 3, 29–81. [Google Scholar] [CrossRef]
- Wang, X.; Pan, S.; Zhang, M.; Qi, J.; Sun, X.; Gu, C.; Wang, L.; Li, J. Modified hydrous zirconium oxide/PAN nanofibers for efficient defluoridation from groundwater. Sci. Total Environ. 2019, 685, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Parashar, K.; Ballav, N.; Debnath, S.; Pillay, K.; Maity, A. Hydrous ZrO2 decorated polyaniline nanofibres: Synthesis, characterization and application as an efficient adsorbent for water defluoridation. J. Colloid Interface Sci. 2017, 508, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Chigondo, M.; Kamdem Paumo, H.; Bhaumik, M.; Pillay, K.; Maity, A. Hydrous CeO2-Fe3O4 decorated polyaniline fibers nanocomposite for effective defluoridation of drinking water. J. Colloid Interface Sci. 2018, 532, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Sankararamakrishnan, N.; Srivastava, I.; Mishra, S. Studies on novel nano-bimetal doped cellulose nanofibers derived from agrowaste towards deflouridation. Int. J. Biol. Macromol. 2019, 128, 556–565. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, G.; Eckhoff, R.K. Kinetics study of hydration reaction between aluminum powder and water based on an improved multi-stage shrinking core model. Int. J. Hydrogen Energy 2021, 46, 33635–33655. [Google Scholar] [CrossRef]
- Kumar, D.; Muthukumar, K. An overview on activation of aluminium-water reaction for enhanced hydrogen production. J. Alloys Compd. 2020, 835, 155189. [Google Scholar] [CrossRef]
- Etminanbakhsh, M.; Allahkaram, S.R. Reaction of aluminum particles with superheated steam to generate hydrogen gas as a readily usable clean fuel. Fuel 2023, 332, 126011. [Google Scholar] [CrossRef]
- Wang, Z.; Du, H.; Gong, J.; Yang, S.; Ma, J.; Xu, J. Facile synthesis of hierarchical flower-like γ-AlOOH films via hydrothermal route on quartz surface. Colloids Surf. A 2014, 450, 76–82. [Google Scholar] [CrossRef]
- Han, D.; Lee, D. Morphology Controlled Synthesis of gamma-Al(2)O(3) Nano-Crystallites in Al@Al(2)O(3) Core-Shell Micro-Architectures by Interfacial Hydrothermal Reactions of Al Metal Substrates. Nanomaterials 2021, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Strålin, A.; Hjertberg, T. Influence of surface composition on initial hydration of aluminium in boiling water. Appl. Surf. Sci. 1994, 74, 263–275. [Google Scholar] [CrossRef]
- Christensen, A.N.; Lehmann, M.; Convert, P. Deuteration of crystalline hydroxides. Hydrogen bonds of g-AlOO (H, D) and g-FeOO (H, D). Acta Chem. Scand. 1982, 36, 303–308. [Google Scholar] [CrossRef]
- Scott, J.R.; Groenewold, G.S.; Gianotto, A.K.; Benson, M.T.; Wright, J.B. Experimental and Computational Study of Hydration Reactions of Aluminum Oxide Anion Clusters. J. Phys. Chem. A 2000, 104, 7079–7090. [Google Scholar] [CrossRef]
- Alwitt, R.S. The Growth of Hydrous Oxide Films on Aluminum. J. Electrochem. Soc. 1974, 121, 1322. [Google Scholar] [CrossRef]
- Clayton, G.D.; Clayton, F.E. Patty’s Industrial Hygiene and Toxicology. Vol. 2A. Toxicology; John Wiley & Sons, Inc.: Chichester, UK, 1981. [Google Scholar]
- Mohapatra, M.; Anand, S.; Mishra, B.K.; Giles, D.E.; Singh, P. Review of fluoride removal from drinking water. J. Environ. Manag. 2009, 91, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Adeno, F.; Mulugeta, E.; Zewge, F.; Chebude, Y. Adsorptive removal of fluoride from water using nanoscale aluminium oxide hydroxide (AlOOH). Bull. Chem. Soc. Ethiop. 2014, 28, 215–227. [Google Scholar] [CrossRef]
- Tao, W.; Zhong, H.; Pan, X.; Wang, P.; Wang, H.; Huang, L. Removal of fluoride from wastewater solution using Ce-AlOOH with oxalic acid as modification. J. Hazard. Mater. 2020, 384, 121373. [Google Scholar] [CrossRef]
- Barathi, M.; Kumar, A.S.K.; Rajesh, N. Aluminium hydroxide impregnated macroreticular aromatic polymeric resin as a sustainable option for defluoridation. J. Environ. Chem. Eng. 2015, 3, 630–641. [Google Scholar] [CrossRef]
- Barathi, M.; Kumar, A.S.K.; Kodali, J.; Mittal, S.; Samhith, G.D.; Rajesh, N. Probing the Interaction between Fluoride and the Polysaccharides in Al (III)-and Zr (IV)-Modified Tea Waste by Using Diverse Analytical Characterization Techniques. ChemistrySelect 2017, 2, 10123–10135. [Google Scholar] [CrossRef]
- Pommerenk, P.; Schafran, G.C. Adsorption of inorganic and organic ligands onto hydrous aluminum oxide: Evaluation of surface charge and the impacts on particle and NOM removal during water treatment. Environ. Sci. Technol. 2005, 39, 6429–6434. [Google Scholar] [CrossRef] [PubMed]
- Omar, F.M.; Rahman, N.N.N.A.; Ahmad, A. COD reduction in semiconductor wastewater by natural and commercialized coagulants using response surface methodology. Water Air Soil Pollut. 2008, 195, 345–352. [Google Scholar] [CrossRef]
- He, Z.; Liu, R.; Xu, J.; Liu, H.; Qu, J. Defluoridation by Al-based coagulation and adsorption: Species transformation of aluminum and fluoride. Sep. Purif. Technol. 2015, 148, 68–75. [Google Scholar] [CrossRef]
- García-Sánchez, J.; Solache-Ríos, M.; Martínez-Gutiérrez, J.; Arteaga-Larios, N.; Ojeda-Escamilla, M.; Rodríguez-Torres, I. Modified natural magnetite with Al and La ions for the adsorption of fluoride ions from aqueous solutions. J. Fluor. Chem. 2016, 186, 115–124. [Google Scholar] [CrossRef]
- Cai, H.-m.; Chen, G.-j.; Peng, C.-y.; Zhang, Z.-z.; Dong, Y.-y.; Shang, G.-z.; Zhu, X.-h.; Gao, H.-j.; Wan, X.-c. Removal of fluoride from drinking water using tea waste loaded with Al/Fe oxides: A novel, safe and efficient biosorbent. Appl. Surf. Sci. 2015, 328, 34–44. [Google Scholar] [CrossRef]
- Jin, H.; Ji, Z.; Yuan, J.; Li, J.; Liu, M.; Xu, C.; Dong, J.; Hou, P.; Hou, S. Research on removal of fluoride in aqueous solution by alumina-modified expanded graphite composite. J. Alloys Compd. 2015, 620, 361–367. [Google Scholar] [CrossRef]
- Wan, Z.; Chen, W.; Liu, C.; Liu, Y.; Dong, C. Preparation and characterization of γ-AlOOH@ CS magnetic nanoparticle as a novel adsorbent for removing fluoride from drinking water. J. Colloid Interface Sci. 2015, 443, 115–124. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Xiong, P.; Ma, W.; Qian, N.; Lu, W. A novel method for synthesis of Co–Al layered double hydroxides and their conversions to mesoporous CoAl2O4 nanostructures for applications in adsorption removal of fluoride ions. Microporous Mesoporous Mater. 2015, 201, 91–98. [Google Scholar] [CrossRef]
- Tangsir, S.; Hafshejani, L.D.; Lähde, A.; Maljanen, M.; Hooshmand, A.; Naseri, A.A.; Moazed, H.; Jokiniemi, J.; Bhatnagar, A. Water defluoridation using Al2O3 nanoparticles synthesized by flame spray pyrolysis (FSP) method. Chem. Eng. J. 2016, 288, 198–206. [Google Scholar] [CrossRef]
- Dayananda, D.; Sarva, V.R.; Prasad, S.V.; Arunachalam, J.; Parameswaran, P.; Ghosh, N.N. Synthesis of MgO nanoparticle loaded mesoporous Al2O3 and its defluoridation study. Appl. Surf. Sci. 2015, 329, 1–10. [Google Scholar] [CrossRef]
- Jia, Y.; Zhu, B.-S.; Jin, Z.; Sun, B.; Luo, T.; Yu, X.-Y.; Kong, L.-T.; Liu, J.-H. Fluoride removal mechanism of bayerite/boehmite nanocomposites: Roles of the surface hydroxyl groups and the nitrate anions. J. Colloid Interface Sci. 2015, 440, 60–67. [Google Scholar] [CrossRef]
- Ku, Y.; Chiou, H.-M. The adsorption of fluoride ion from aqueous solution by activated alumina. Water Air Soil Pollut. 2002, 133, 349–361. [Google Scholar] [CrossRef]
- Maliyekkal, S.M.; Sharma, A.K.; Philip, L. Manganese-oxide-coated alumina: A promising sorbent for defluoridation of water. Water Res. 2006, 40, 3497–3506. [Google Scholar] [CrossRef]



| Electrons | Peak | Binding Energy (eV) | Before Adsorption | After Adsorption |
|---|---|---|---|---|
| Concentration (Atomic %) | ||||
| Al 2p | Al-O | 73.5 | 31.64 | 30.53 |
| Al-OH | 74.1 | 68.36 | 56.87 | |
| Al-F | 75.3 | - | 12.61 | |
| O 1s | Al-O-Al | 530.6 | 31.97 | 36.96 |
| Al-OH | 532.0 | 68.03 | 63.04 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.-T.; Lee, J.-W.; An, H.-E.; Cho, S.-H.; Jeong, S. Efficient Fluoride Wastewater Treatment Using Eco-Friendly Synthesized AlOOH. Nanomaterials 2023, 13, 2838. https://doi.org/10.3390/nano13212838
Kim W-T, Lee J-W, An H-E, Cho S-H, Jeong S. Efficient Fluoride Wastewater Treatment Using Eco-Friendly Synthesized AlOOH. Nanomaterials. 2023; 13(21):2838. https://doi.org/10.3390/nano13212838
Chicago/Turabian StyleKim, Wan-Tae, Joo-Won Lee, Hong-Eun An, So-Hye Cho, and Sohee Jeong. 2023. "Efficient Fluoride Wastewater Treatment Using Eco-Friendly Synthesized AlOOH" Nanomaterials 13, no. 21: 2838. https://doi.org/10.3390/nano13212838
APA StyleKim, W.-T., Lee, J.-W., An, H.-E., Cho, S.-H., & Jeong, S. (2023). Efficient Fluoride Wastewater Treatment Using Eco-Friendly Synthesized AlOOH. Nanomaterials, 13(21), 2838. https://doi.org/10.3390/nano13212838