Efficient One-Step Synthesis of a Pt-Free Zn0.76Co0.24S Counter Electrode for Dye-Sensitized Solar Cells and Its Versatile Application in Photoelectrochromic Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
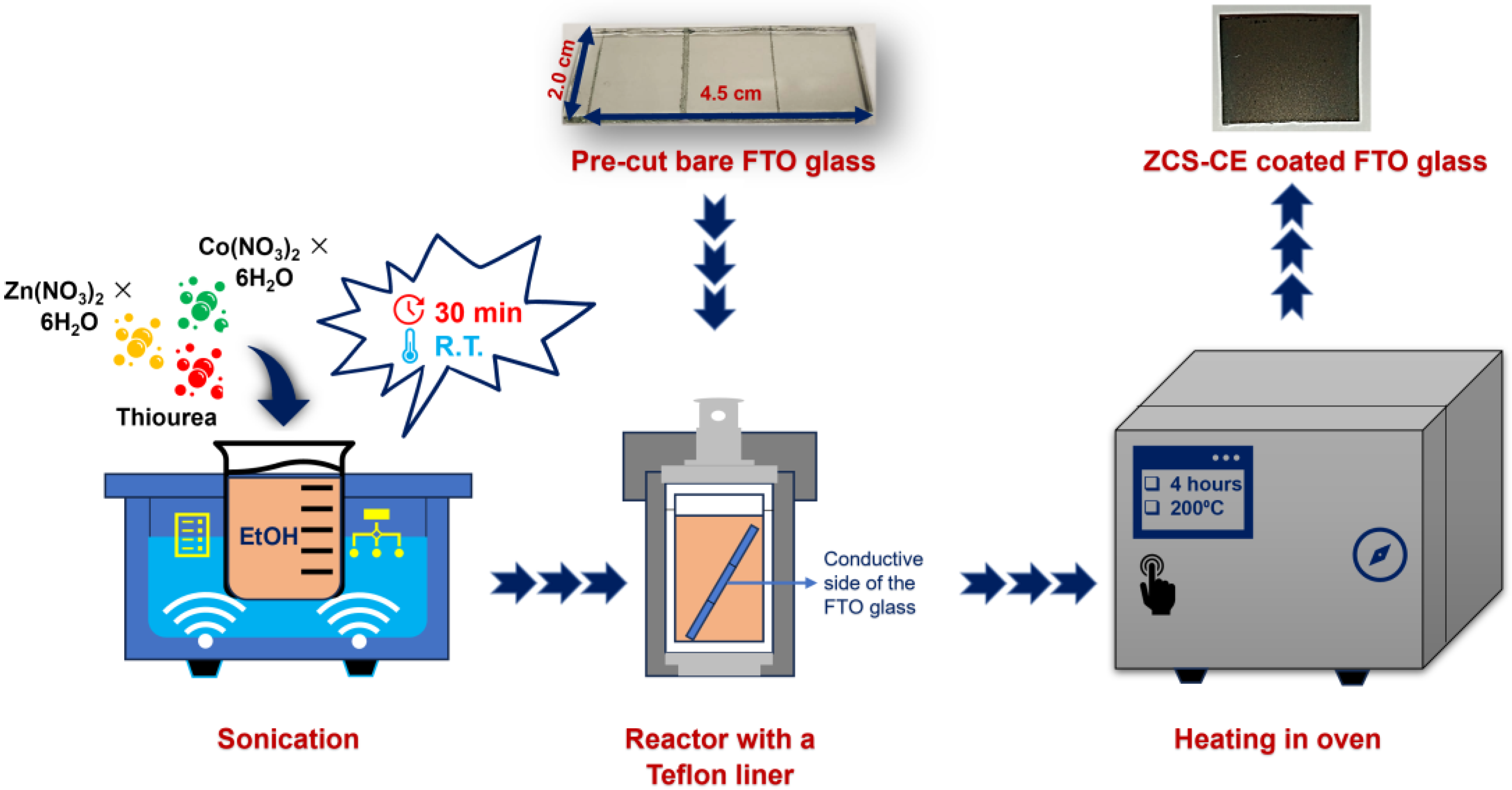
2.2. Synthesis of ZCS-CE
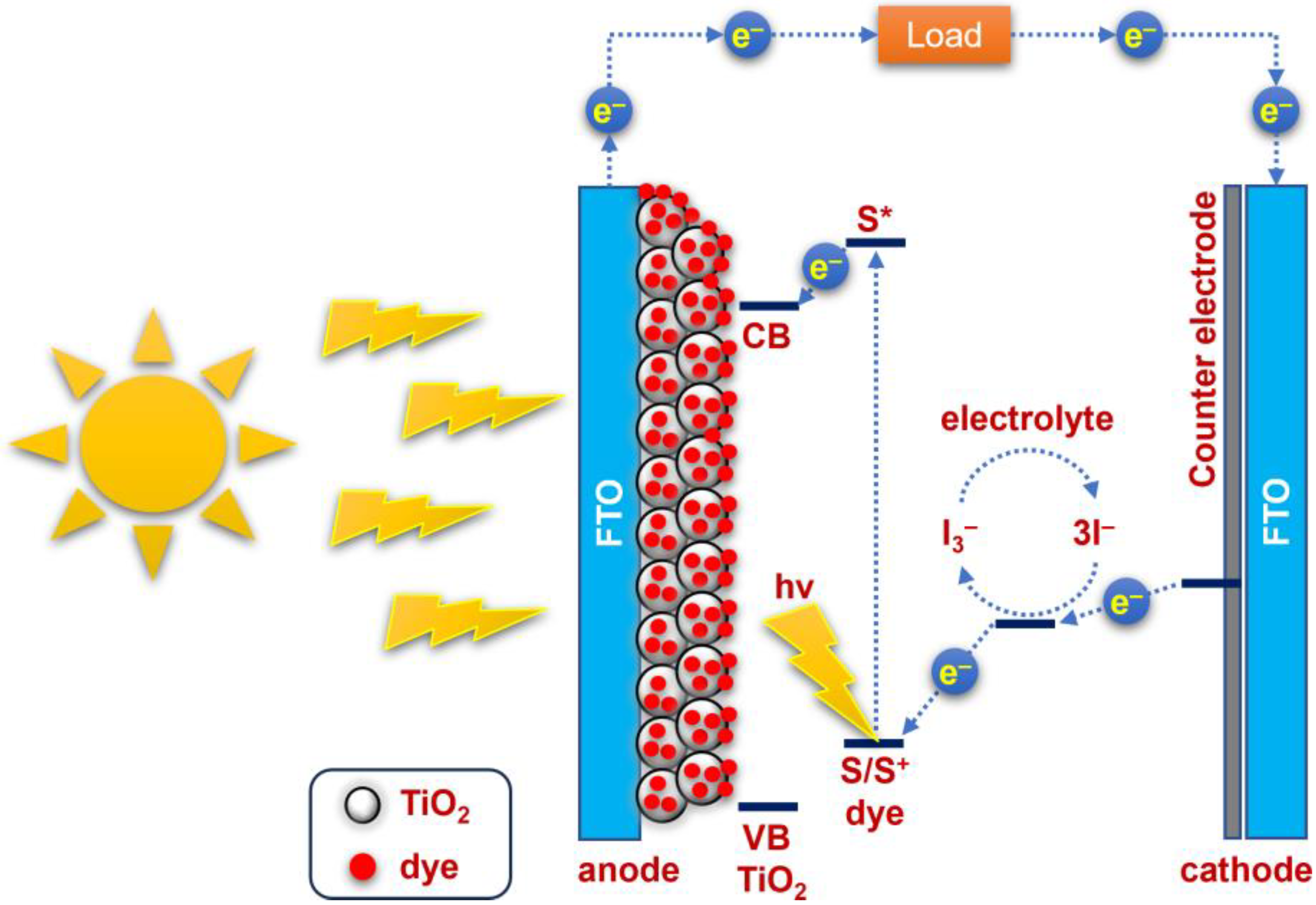
2.3. Fabrication of Dye-Sensitized Solar Cells
2.4. Deposition of Prussian Blue and Fabrication of Photoelectrochromic Device
2.5. Characterization
3. Results and Discussion
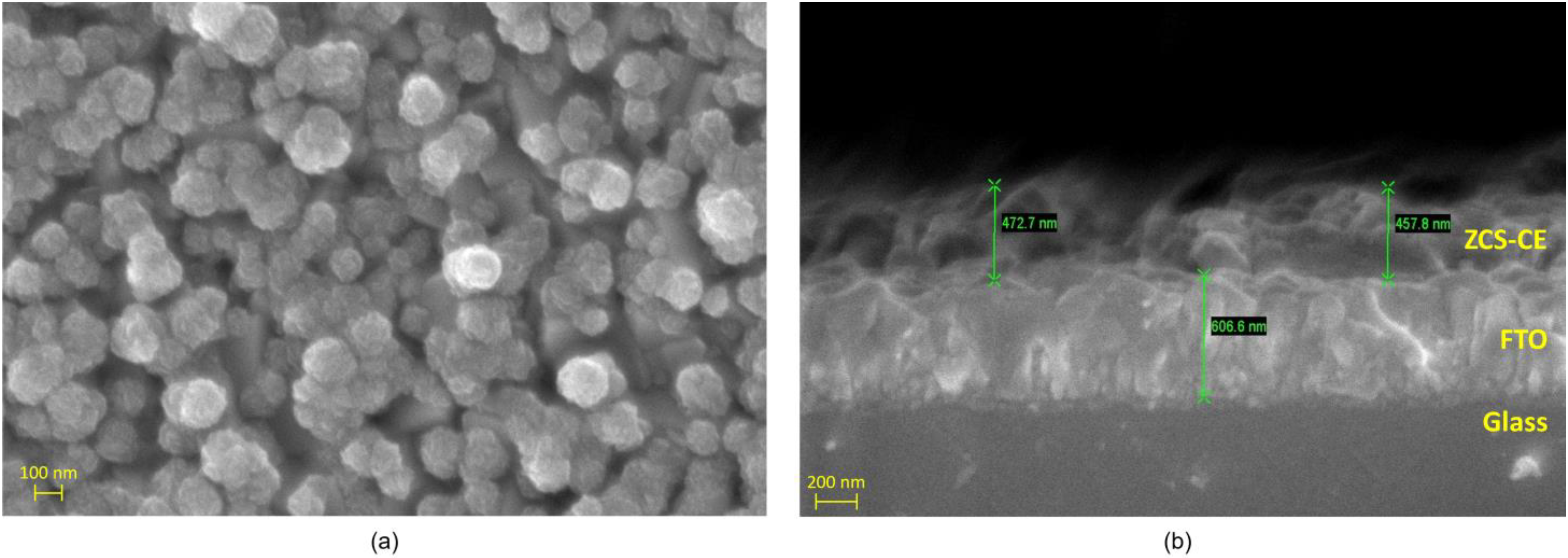
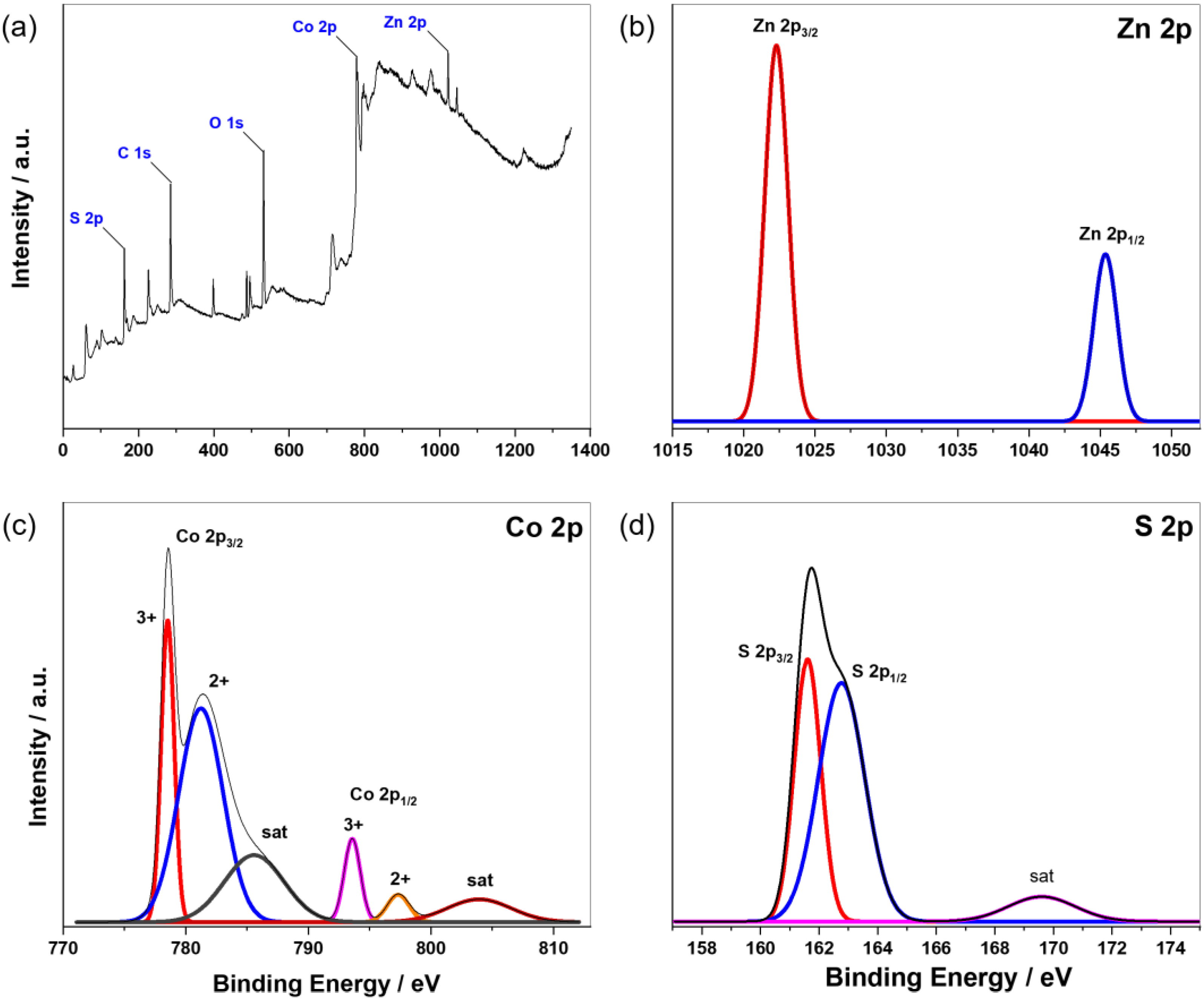
3.1. Characterization of ZCS-CE
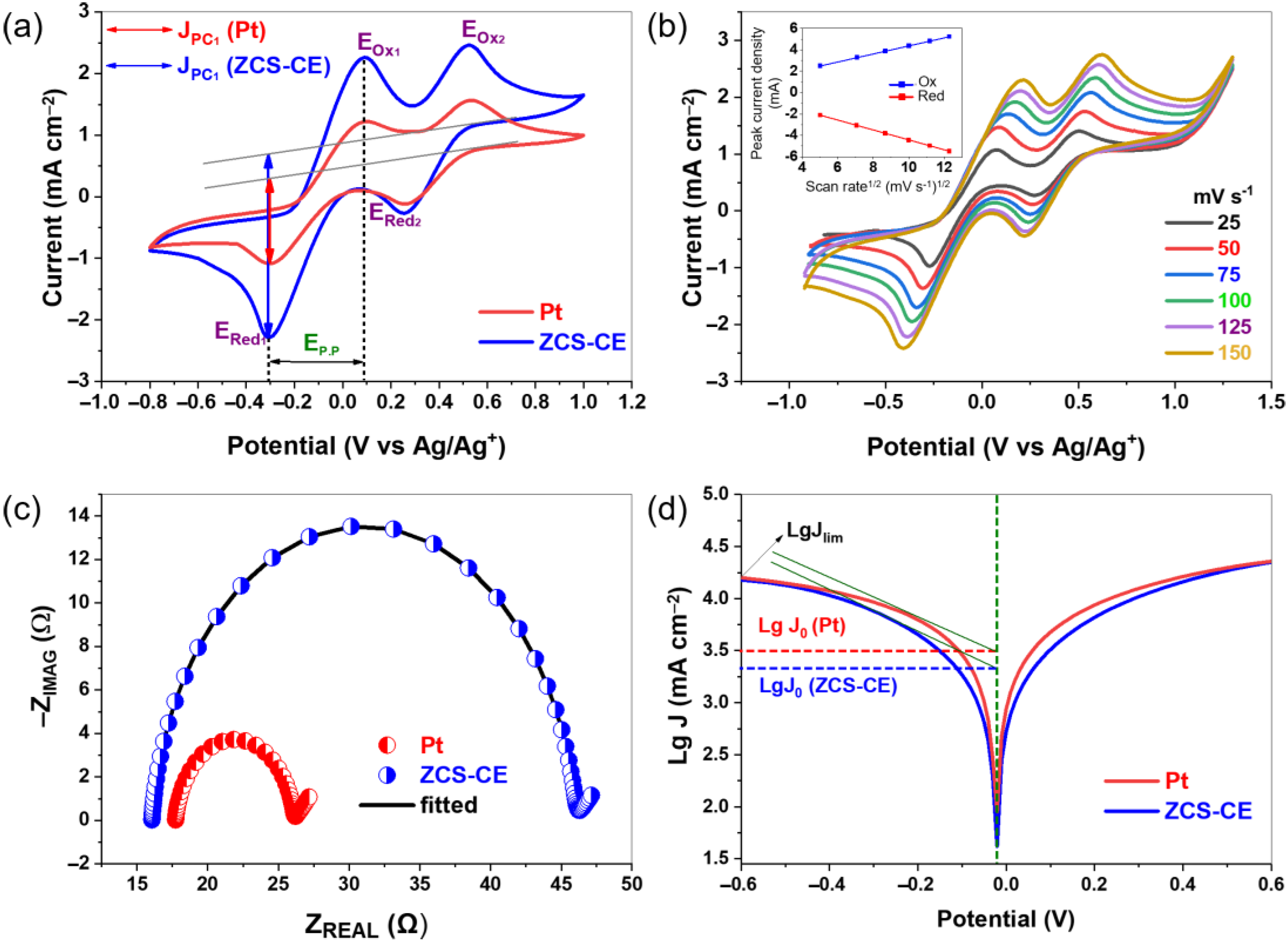
3.2. Electrochemical Analysis of ZCS-CE
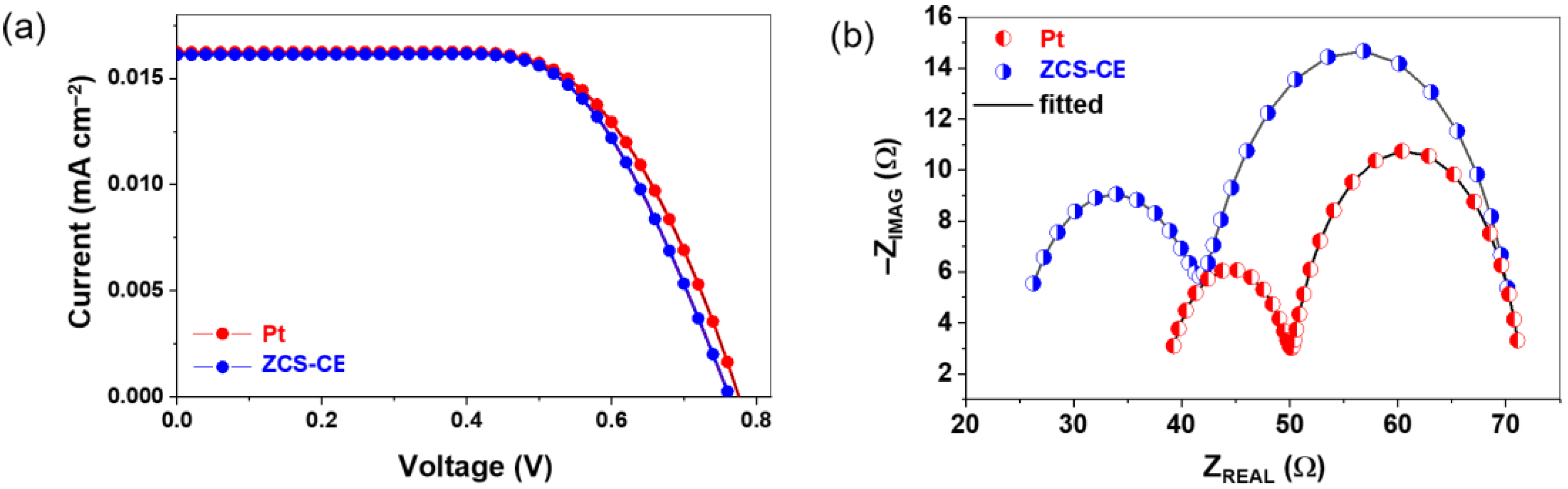
3.3. Photovoltaic Performance of ZCS-CE
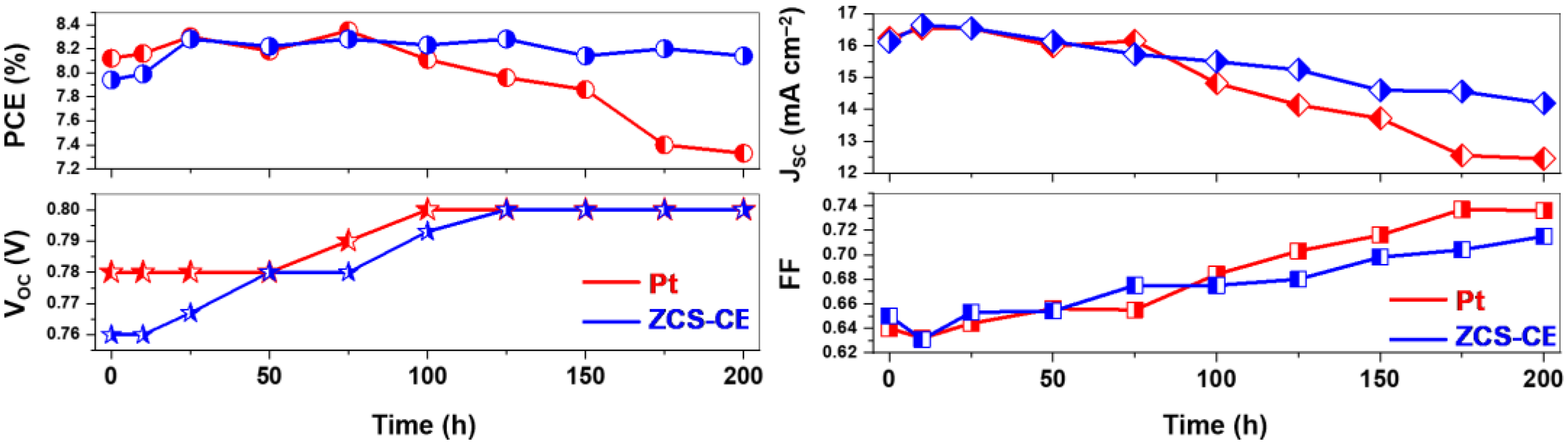
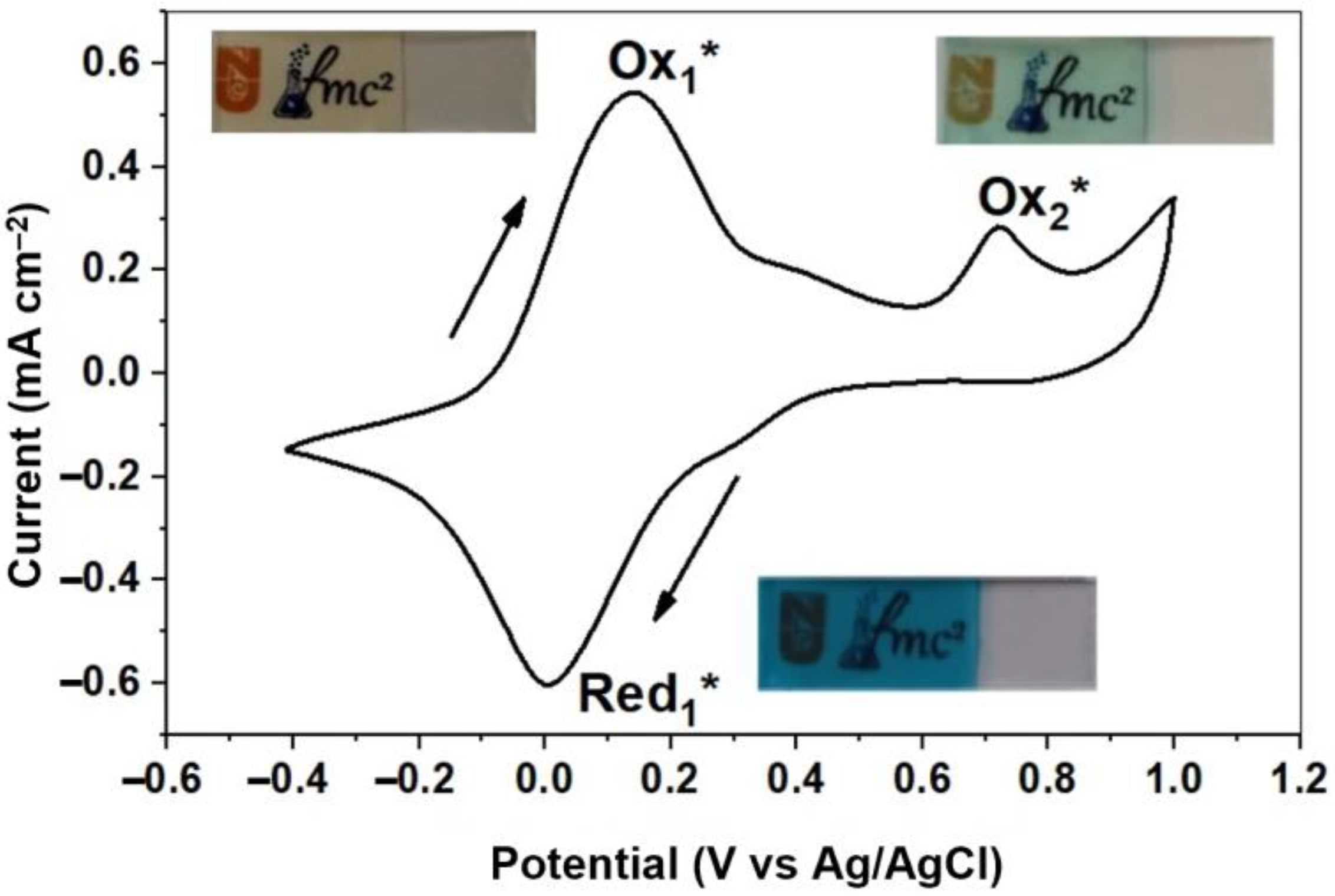
3.4. Fabrication and Characterization of Photoelectrochromic Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Noorasid, N.S.; Arith, F.; Mustafa, A.N.; Azam, M.A.; Mahalingam, S.; Chelvanathan, P.; Amin, N. Current advancement of flexible dye sensitized solar cell: A review. Optik 2022, 254, 168089. [Google Scholar] [CrossRef]
- Błaszczyk, A.; Joachimiak-Lechman, K.; Sady, S.; Tański, T.; Szindler, M.; Drygała, A. Environmental performance of dye-sensitized solar cells based on natural dyes. Sol. Energy 2021, 215, 346–355. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, D.; Suo, J.; Cao, Y.; Eickemeyer, F.T.; Vlachopoulos, N.; Zakeeruddin, S.M.; Hagfeldt, A.; Gratzel, M. Hydroxamic acid pre-adsorption raises the efficiency of cosensitized solar cells. Nature 2023, 613, 60–65. [Google Scholar] [CrossRef]
- Michaels, H.; Rinderle, M.; Freitag, R.; Benesperi, I.; Edvinsson, T.; Socher, R.; Gagliardi, A.; Freitag, M. Dye-sensitized solar cells under ambient light powering machine learning: Towards autonomous smart sensors for the internet of things. Chem. Sci. 2020, 11, 2895–2906. [Google Scholar] [CrossRef]
- Aslam, A.; Mehmood, U.; Arshad, M.H.; Ishfaq, A.; Zaheer, J.; Ul Haq Khan, A.; Sufyan, M. Dye-sensitized solar cells (DSSCs) as a potential photovoltaic technology for the self-powered internet of things (IoTs) applications. Sol. Energy 2020, 207, 874–892. [Google Scholar] [CrossRef]
- Li, G.; Sheng, L.; Li, T.; Hu, J.; Li, P.; Wang, K. Engineering flexible dye-sensitized solar cells for portable electronics. Sol. Energy 2019, 177, 80–98. [Google Scholar] [CrossRef]
- Shakeel Ahmad, M.; Pandey, A.K.; Abd Rahim, N. Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications. A review. Renew. Sust. Energ. Rev. 2017, 77, 89–108. [Google Scholar] [CrossRef]
- Najm, A.S.; Alwash, S.A.; Sulaiman, N.H.; Chowdhury, M.S.; Techato, K. N719 dye as a sensitizer for dye-sensitized solar cells (DSSCs): A review of its functions and certain rudimentary principles. Environ. Prog. Sustain. Energy 2022, 42, e13955. [Google Scholar] [CrossRef]
- Boschloo, G.; Hagfeldt, A. Characteristics of the iodide/triiodide redox mediator in dye-sensitized solar cells. Acc. Chem. Res. 2009, 42, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, H.; Sonai, G.G.; Hashmi, S.G.; Nogueira, A.F.; Lund, P.D. Progress on electrolytes development in dye-sensitized solar cells. Materials 2019, 12, 1998. [Google Scholar] [CrossRef]
- Ding, S.; Yang, C.; Yuan, J.; Li, H.; Yuan, X.; Li, M. An overview of the preparation and application of counter electrodes for DSSCs. RSC Adv. 2023, 13, 12309–12319. [Google Scholar] [CrossRef]
- Olsen, E.; Hagen, G.; Eric Lindquist, S. Dissolution of platinum in methoxy propionitrile containing LiI/I2. Sol. Energy Mater. Sol. Cells 2000, 63, 267–273. [Google Scholar] [CrossRef]
- Huo, J.; Wu, J.; Zheng, M.; Tu, Y.; Lan, Z. Effect of ammonia on electrodeposition of cobalt sulfide and nickel sulfide counter electrodes for dye-sensitized solar cells. Electrochim. Acta 2015, 180, 574–580. [Google Scholar] [CrossRef]
- Vikraman, D.; Patil, S.A.; Hussain, S.; Mengal, N.; Kim, H.-S.; Jeong, S.H.; Jung, J.; Kim, H.-S.; Park, H.J. Facile and cost-effective methodology to fabricate MoS2 counter electrode for efficient dye-sensitized solar cells. Dyes Pigm. 2018, 151, 7–14. [Google Scholar] [CrossRef]
- Liu, X.; Yue, G.; Zheng, H. A promising vanadium sulfide counter electrode for efficient dye-sensitized solar cells. RSC Adv. 2017, 7, 12474–12478. [Google Scholar] [CrossRef]
- Baptayev, B.; Mustazheb, D.; Abilova, Z.; Balanay, M.P. Nanostructured flower-shaped CuCo2S4 as a Pt-free counter-electrode for dye-sensitized solar cells. Chem. Commun. 2020, 56, 12190–12193. [Google Scholar] [CrossRef] [PubMed]
- Baptayev, B.; Tashenov, Y.; Adilov, S.; Balanay, M.P. Facile fabrication of ZnCo2S4@MWCNT as Pt-free counter electrode for high performance dye-sensitized solar cells. Surf. Interfaces 2023, 37, 102699. [Google Scholar] [CrossRef]
- Baptayev, B.; Tashenov, Y.; Aliakbarova, A.; Adilov, S.; Balanay, M. Ternary NiCuS electrocatalyst for iodide/triiodide reduction in dye-sensitized solar cells. Mater. Today Proc. 2022, 71, 94–99. [Google Scholar] [CrossRef]
- Baptayev, B.; Mustazheb, D.; Balanay, M.P. Binary transition metal sulfides as an economical Pt-free counter electrodes for dye-sensitized solar cells. Mater. Today Proc. 2020, 25, 24–27. [Google Scholar] [CrossRef]
- Kharboot, L.H.; Fadil, N.A.; Bakar, T.A.A.; Najib, A.S.M.; Nordin, N.H.; Ghazali, H. A review of transition metal sulfides as counter electrodes for dye-sensitized and quantum dot-sensitized solar cells. Materials 2023, 16, 2881. [Google Scholar] [CrossRef]
- Amin, B.G.; Swesi, A.T.; Masud, J.; Nath, M. CoNi2Se4 as an efficient bifunctional electrocatalyst for overall water splitting. Chem. Commun. 2017, 53, 5412–5415. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yu, A.; Zou, Z.; Xu, C. One-pot synthesis of iron–nickel–selenide nanorods for efficient and durable electrochemical oxygen evolution. Inorg. Chem. Front. 2018, 5, 814–818. [Google Scholar] [CrossRef]
- John Peter, I.; Rajamanickam, N.; Ragavendran, V.; Mayandi, J.; Nithiananthi, P. M1−xSb1−ySδ (M = Ni, Cu, Co) ternary metal sulfides: Emerging candidates for I3− reduction in bifacial dye-sensitized solar cells. Mater. Sci. Eng. B 2023, 287, 116142. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Fu, Y.; Han, X.; Wang, Y.; Zhang, Y.; Zhang, W.; Li, L. NiCo2S4 nanotube arrays grown in situ on Ni foam as a low-cost counter electrode for dye-sensitized solar cells. Sol. Energy 2021, 225, 297–304. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Senthil, R.A.; Buraidah, M.H.; Madhavan, J.; Arof, A.K.; Ashokkumar, M. One-step electrochemical deposition of Ni1−xMoxS ternary sulfides as an efficient counter electrode for dye-sensitized solar cells. J. Mater. Chem. A 2016, 4, 16119–16127. [Google Scholar] [CrossRef]
- Tamilarasan, S.; Sundararaj, S.B.; Bhojanaa, K.B.; Pandikumar, A.; Basha, S.J.S.; Thangavelu, S. Unraveling the enhanced electrochemical performances of nickel aluminum sulfide lattices for overall water splitting and as counter electrode materials for dye-sensitized solar cells. J. Phys. Chem. C 2023, 127, 11921–11931. [Google Scholar] [CrossRef]
- Cui, Q.; Yang, Q.; Wang, W.; Yao, J.; Liu, Z.; Zuo, X.; Zhu, K.; Li, G.; Jin, S. Controllable synthesize core-shelled Zn0.76Co0.24S nanospheres as the counter-electrode in dye-sensitized solar cells and its enhanced electrocatalytic performance. J. Mater. Sci Mater. Electron. 2019, 31, 1797–1807. [Google Scholar] [CrossRef]
- Isfahani, V.B.; Memarian, N.; Dizaji, H.R.; Arab, A.; Silva, M.M. The physical and electrochromic properties of Prussian Blue thin films electrodeposited on ITO electrodes. Electrochim. Acta 2019, 304, 282–291. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, J.; Shi, L.; Cong, G.; Zhu, C.; Xu, J. Combining Zn0.76Co0.24S with S-doped graphene as high-performance anode materials for lithium- and sodium-ion batteries. Nanotechnol. Rev. 2020, 9, 1227–1236. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, W.; Li, S.; Ci, L.; Si, P. Surfactant-dependent flower- and grass-like Zn0.76Co0.24S/Co3S4 for high-performance all-solid-state asymmetric supercapacitors. J. Mater. Chem. A 2018, 6, 22830–22839. [Google Scholar] [CrossRef]
- Gao, X.; Chang, Q.; Hong, J.; Long, D.; Jin, G.; Xiao, X. Zinc Cobalt Sulfide Microspheres as a High-Performance Electrode Material for Supercapacitors. ChemistrySelect 2018, 3, 13751–13758. [Google Scholar] [CrossRef]
- Mohamed, S.G.; Hussain, I.; Shim, J.J. One-step synthesis of hollow C-NiCo2S4 nanostructures for high-performance supercapacitor electrodes. Nanoscale 2018, 10, 6620–6628. [Google Scholar] [CrossRef]
- Mortimer, R.J.; Reynolds, J.R. In situ colorimetric and composite coloration efficiency measurements for electrochromic Prussian blue. J. Mater. Chem. 2005, 15, 2226–2233. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, J.; Leftheriotis, G.; Huang, H.; Xia, Y.; Gan, Y.; Zhang, W.; Zhang, J. A solar-powered multifunctional and multimode electrochromic smart window based on WO3/Prussian blue complementary structure. Sustain. Mater. Technol. 2022, 31, e00372. [Google Scholar] [CrossRef]
- Chang, C.-M.; Chiang, Y.-C.; Cheng, M.-H.; Lin, S.-H.; Jian, W.-B.; Chen, J.-T.; Cheng, Y.-J.; Ma, Y.-R.; Tsukagoshi, K. Fabrication of WO3 electrochromic devices using electro-exploding wire techniques and spray coating. Sol. Energy Mater. Sol. Cells 2021, 223, 110960. [Google Scholar] [CrossRef]
- Guo, X.; Xu, Z.; Huang, J.; Zhang, Y.; Liu, X.; Guo, W. Photoelectrochromic smart windows powered by flexible dye-sensitized solar cell using CuS mesh as counter electrode. Mater. Lett. 2019, 244, 92–95. [Google Scholar] [CrossRef]



| CE | PCE (%) | VOC (V) | JSC (mA/cm2) | FF | RCT (Ω cm2) | RS* (Ω) | RCT* (Ω) | (Ω) |
|---|---|---|---|---|---|---|---|---|
| ZCS-CE | 7.94 ± 0.02 | 0.760 ± 0.00 | 16.12 ± 0.07 | 0.65 ± 0.00 | 14.97 ± 0.38 | 24.32 ± 0.77 | 17.63 ± 0.31 | 29.23 ± 0.29 |
| Pt | 8.12 ± 0.09 | 0.780 ± 0.00 | 16.24 ± 0.20 | 0.64 ± 0.02 | 4.18 ± 0.69 | 38.41 ± 0.64 | 11.83 ± 0.41 | 21.35 ± 0.31 |
| λ (nm) | Tas-deposited (%) | Tcolored (%) | Tbleached (%) | ΔT (%) | CR | ΔOD |
|---|---|---|---|---|---|---|
| 488 | 74.11 | 83.03 | 90.15 | 7.12 | 1.08 | 0.03 |
| 726 | 27.18 | 30.74 | 83.23 | 52.49 | 2.71 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tashenov, Y.; Suleimenova, D.; Baptayev, B.; Adilov, S.; Balanay, M.P. Efficient One-Step Synthesis of a Pt-Free Zn0.76Co0.24S Counter Electrode for Dye-Sensitized Solar Cells and Its Versatile Application in Photoelectrochromic Devices. Nanomaterials 2023, 13, 2812. https://doi.org/10.3390/nano13202812
Tashenov Y, Suleimenova D, Baptayev B, Adilov S, Balanay MP. Efficient One-Step Synthesis of a Pt-Free Zn0.76Co0.24S Counter Electrode for Dye-Sensitized Solar Cells and Its Versatile Application in Photoelectrochromic Devices. Nanomaterials. 2023; 13(20):2812. https://doi.org/10.3390/nano13202812
Chicago/Turabian StyleTashenov, Yerbolat, Diana Suleimenova, Bakhytzhan Baptayev, Salimgerey Adilov, and Mannix P. Balanay. 2023. "Efficient One-Step Synthesis of a Pt-Free Zn0.76Co0.24S Counter Electrode for Dye-Sensitized Solar Cells and Its Versatile Application in Photoelectrochromic Devices" Nanomaterials 13, no. 20: 2812. https://doi.org/10.3390/nano13202812
APA StyleTashenov, Y., Suleimenova, D., Baptayev, B., Adilov, S., & Balanay, M. P. (2023). Efficient One-Step Synthesis of a Pt-Free Zn0.76Co0.24S Counter Electrode for Dye-Sensitized Solar Cells and Its Versatile Application in Photoelectrochromic Devices. Nanomaterials, 13(20), 2812. https://doi.org/10.3390/nano13202812







