Construction of a Two-Dimensional GO/Ti3C2TX Composite Membrane and Investigation of Mg2+/Li+ Separation Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of GO
2.3. Preparation of Ti3C2TX Nanosheets
2.4. Construction of GO/Ti3C2TX Composite Membranes
2.5. Characterization of Membranes
2.6. Membrane Performance Testing
3. Results and Discussion
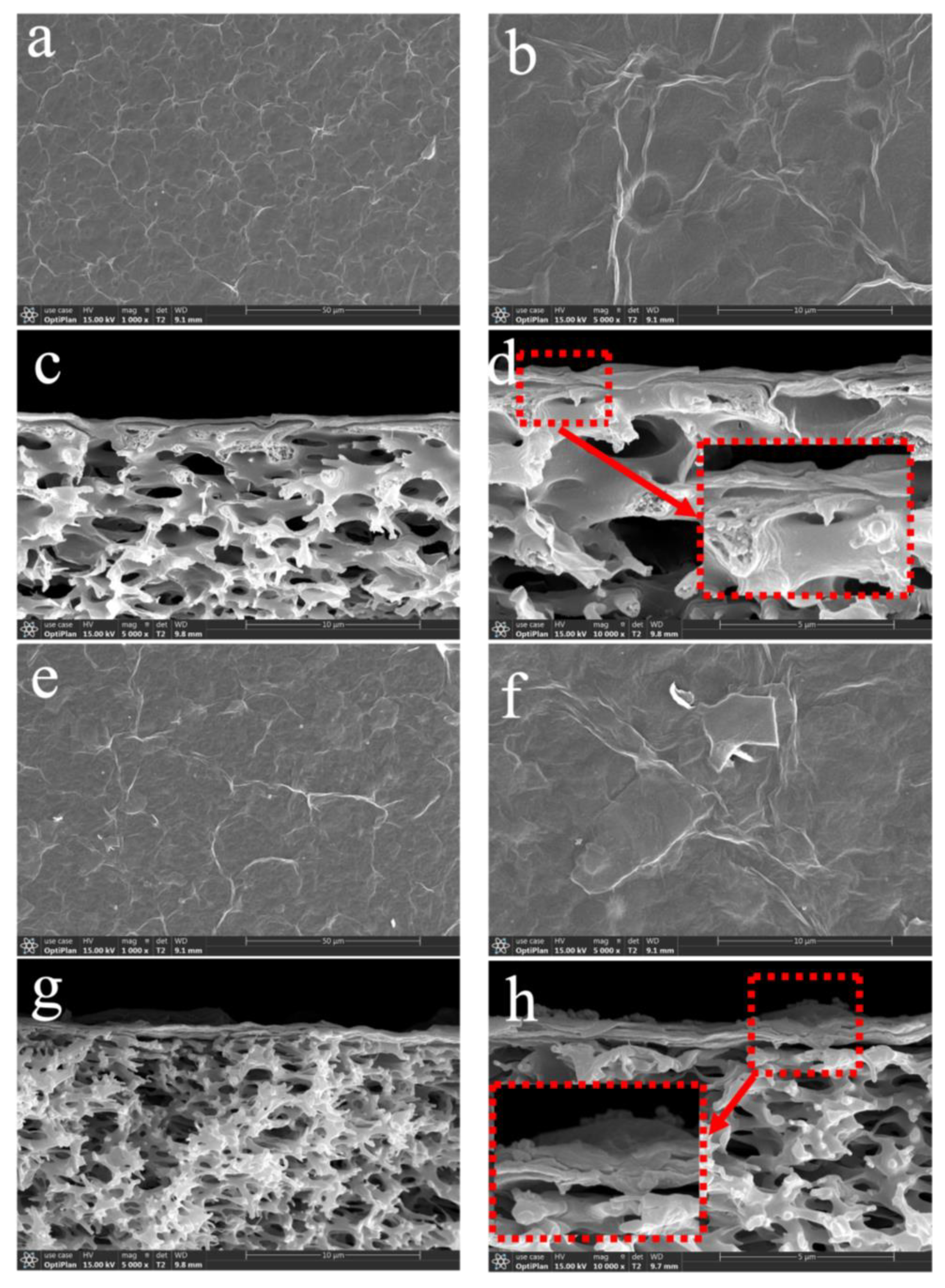
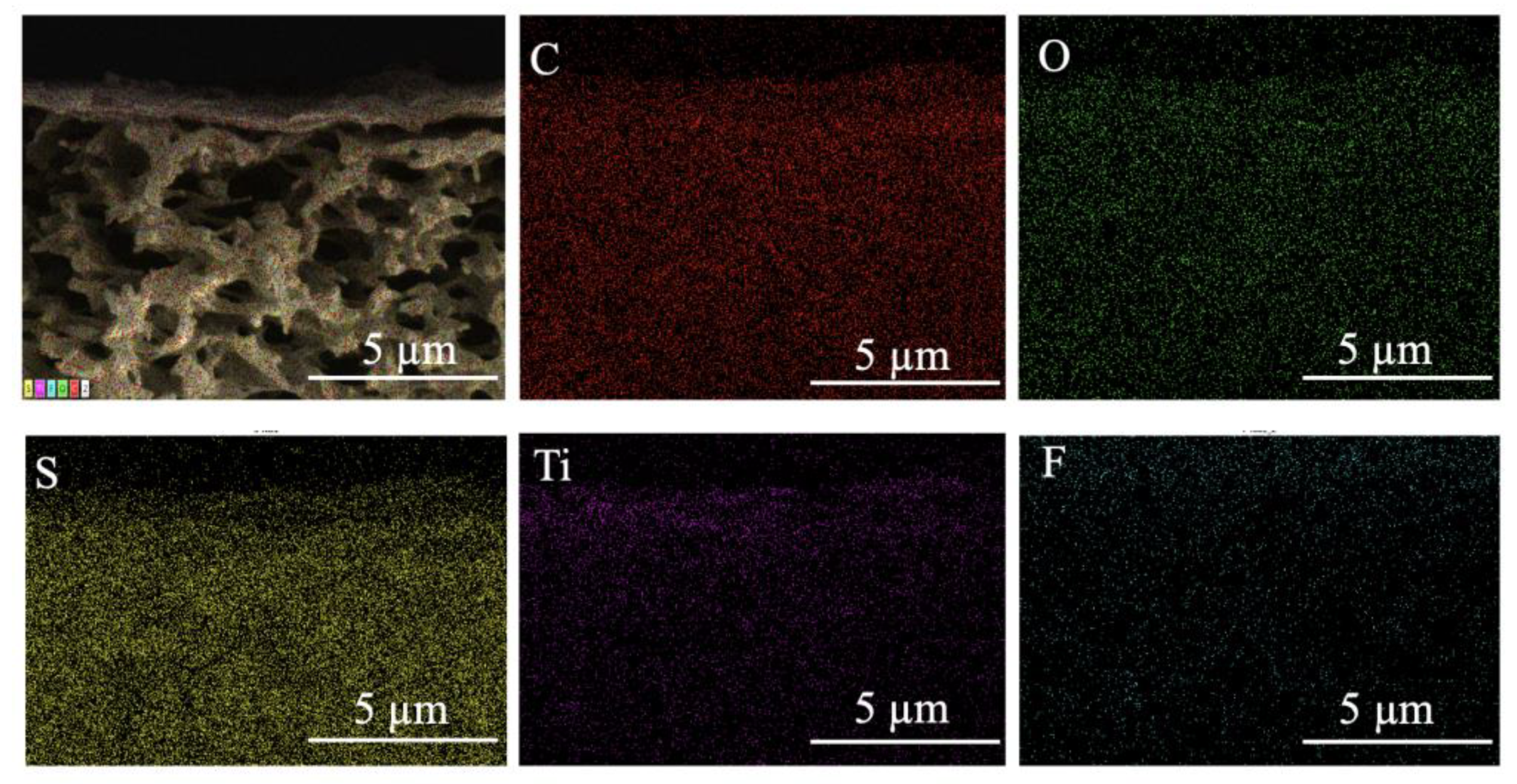
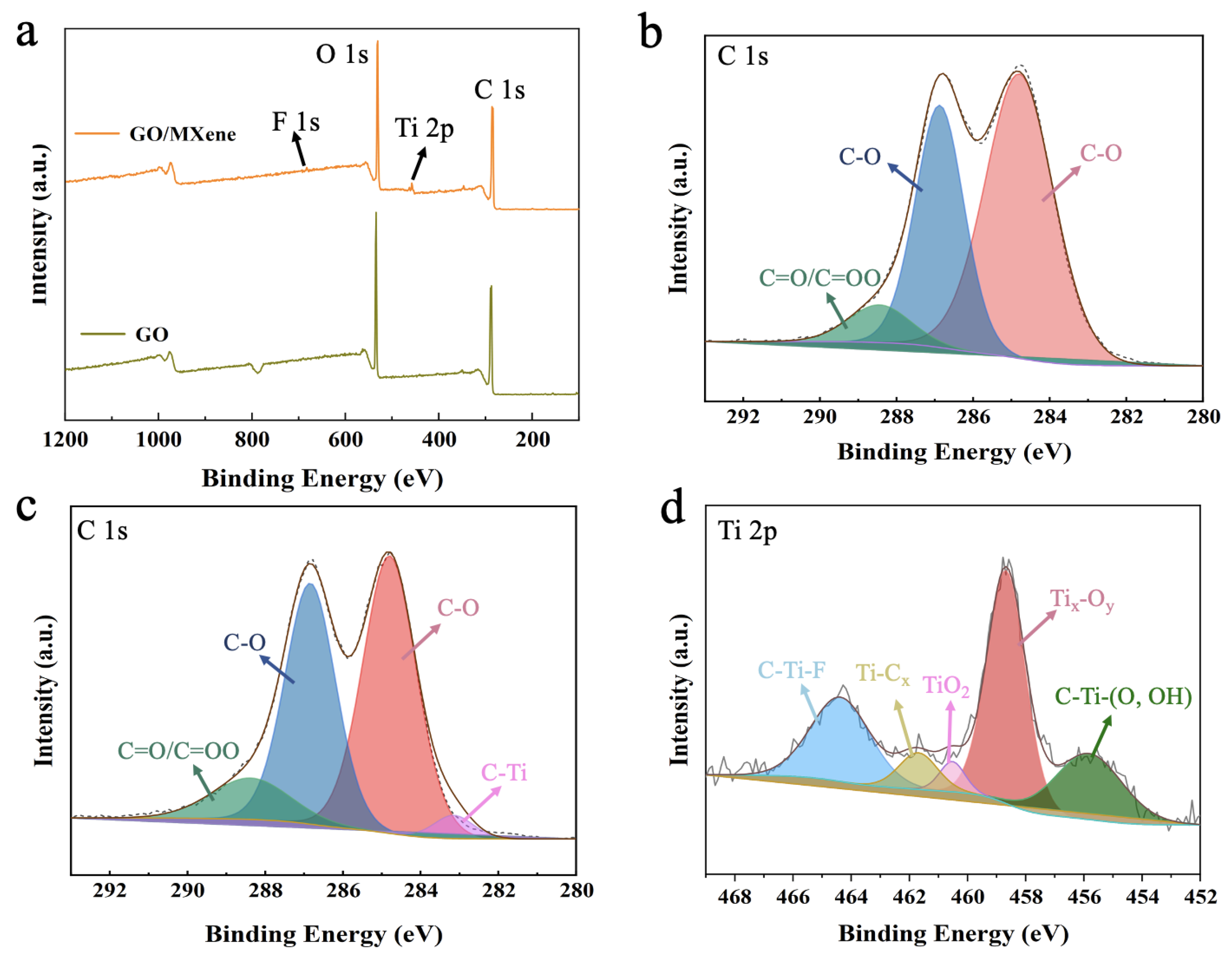
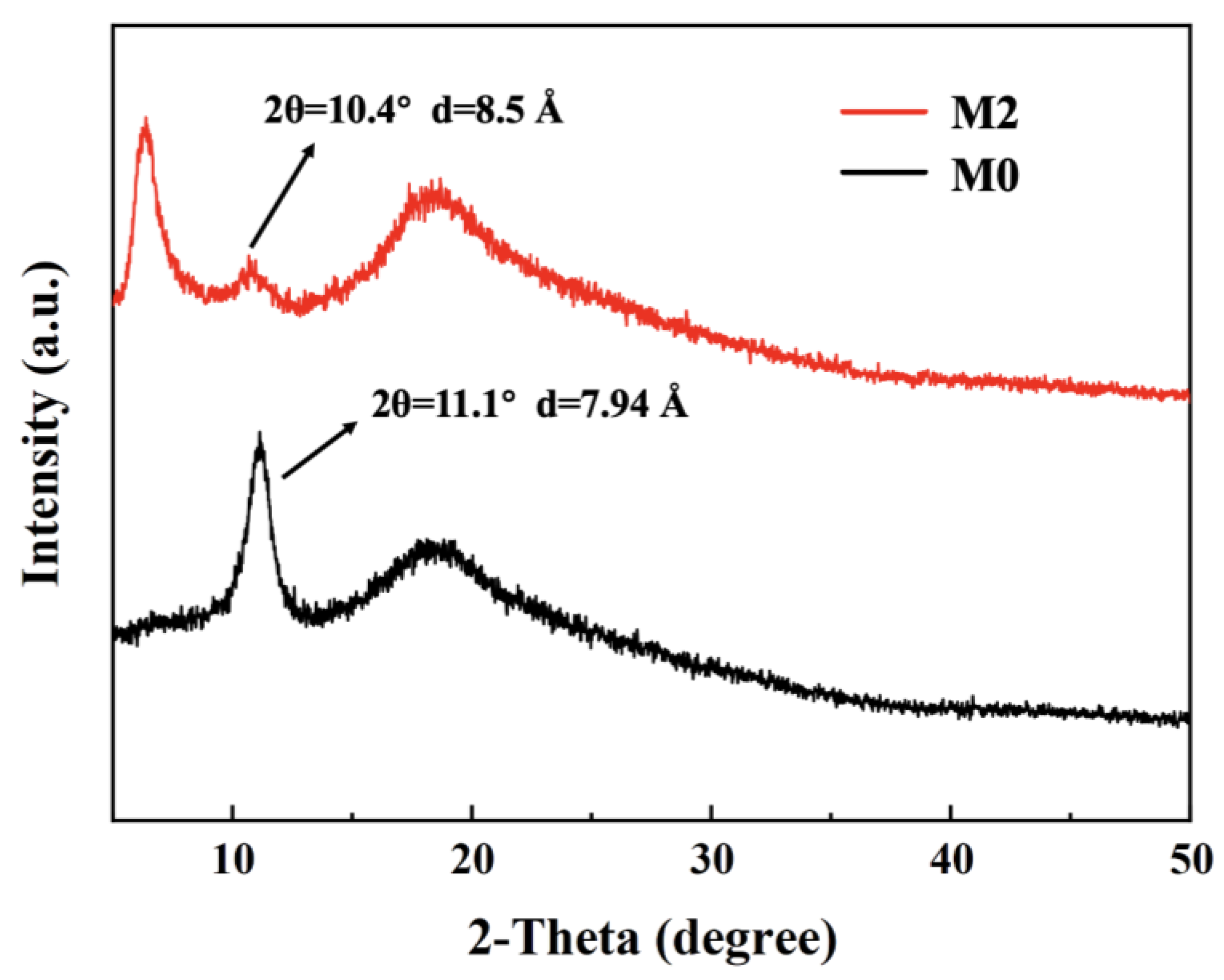
3.1. Characterization of GO and Ti3C2TX Nanosheets
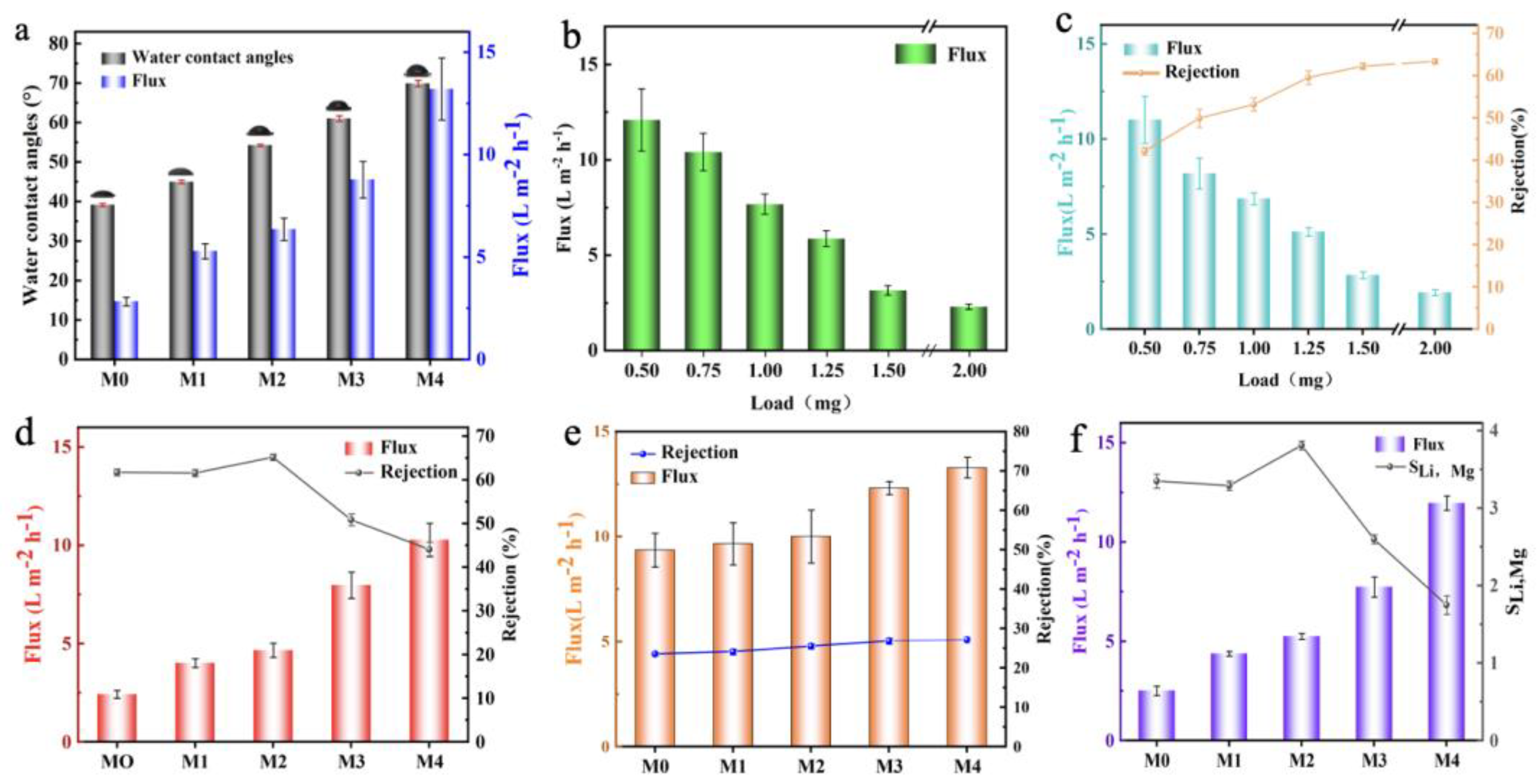
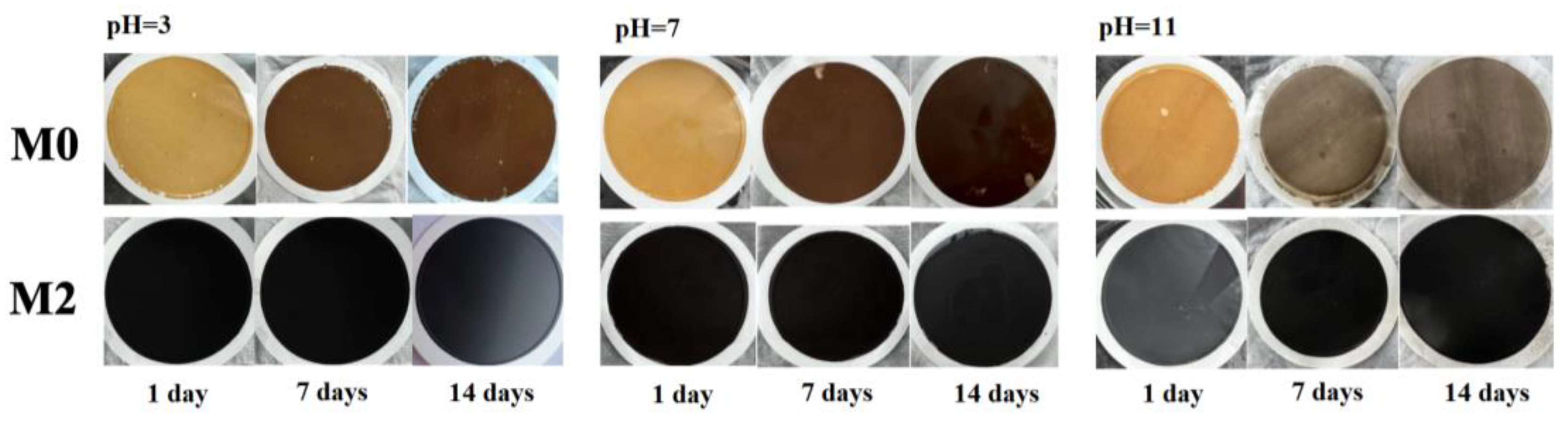
3.2. The Performance of GO/Ti3C2TX Composite Membrane
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, Z.; Huang, J.; Li, M.; Lu, Y. The Dynamic Evolution of the Material Flow of Lithium Resources in China. Sustainability 2022, 14, 16928. [Google Scholar] [CrossRef]
- Murodjon, S.; Tianlong Deng, M.S.; Tianlong, D. Lithium Recovery from Water Resources by Ion Exchange and Sorption Method. J. Chem. Soc. Pak. 2021, 43, 406. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, B.; Lu, Y.; Wang, C.; Chen, Y. A review of lithium extraction from natural resources. Int. J. Miner. Metall. Mater. 2023, 30, 209–224. [Google Scholar] [CrossRef]
- Li, J.; Zou, T.; Liu, X.; Wang, D.; Ding, X. The Metallogenetic Regularities of Lithium Deposits in China. Acta Geol. Sin.-Engl. Ed. 2015, 89, 652–670. [Google Scholar] [CrossRef]
- Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Kesler, S.E.; Everson, M.P.; Wallington, T.J. Global Lithium Availability A Constraint for Electric Vehicles? J. Ind. Ecol. 2011, 15, 760–775. [Google Scholar] [CrossRef]
- Dessemond, C.; Lajoie-Leroux, F.; Soucy, G.; Laroche, N.; Magnan, J.-F. Spodumene: The Lithium Market, Resources and Processes. Minerals 2019, 9, 334. [Google Scholar] [CrossRef]
- Ambrose, H.; Kendall, A. Understanding the future of lithium: Part 1, resource model. J. Ind. Ecol. 2020, 24, 80–89. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W. Pervaporation membrane materials: Recent trends and perspectives. J. Membr. Sci. 2021, 636, 119557. [Google Scholar] [CrossRef]
- Bodzek, M.; Konieczny, K.; Kwiecinska-Mydlak, A. Application of nanotechnology and nanomaterials in water and wastewater treatment: Membranes, photocatalysis and disinfection. Desalination Water Treat. 2020, 186, 88–106. [Google Scholar] [CrossRef]
- Peng, H.; Zhao, Q. A Nano-Heterogeneous Membrane for Efficient Separation of Lithium from High Magnesium/Lithium Ratio Brine. Adv. Funct. Mater. 2021, 31, 2009430. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, J.; Zhang, X.; Chen, L.; Zhang, Q.; Ma, L. Recent advances in membrane-based materials for desalination and gas separation. J. Clean. Prod. 2023, 387, 135845. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Wu, H.; Tian, Z.; Xin, Q.; He, G.; Peng, D.; Chen, S.; Yin, Y.; Jiang, Z.; et al. Advances in high permeability polymer-based membrane materials for CO2 separations. Energy Environ. Sci. 2016, 9, 1863–1890. [Google Scholar] [CrossRef]
- Prasetya, N.; Himma, N.F.; Sutrisna, P.D.; Wenten, I.G.; Ladewig, B.P. A review on emerging organic-containing microporous material membranes for carbon capture and separation. Chem. Eng. J. 2020, 391, 123575. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, Y.; Yang, Z.; He, Z.; Wang, H.; Zhang, L.; Belle Marie Yap Ang, M.; Zeng, G. Construction and application of two-dimensional MXene-based membranes for water treatment: A mini-review. Results Eng. 2022, 15, 100494. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Two-Dimensional-Material Membranes: A New Family of High-Performance Separation Membranes. Angew. Chem.-Int. Ed. 2016, 55, 13384–13397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Guan, K.; Ji, Y.; Liu, G.; Jin, W.; Xu, N. Controllable ion transport by surface-charged graphene oxide membrane. Nat. Commun. 2019, 10, 1253. [Google Scholar] [CrossRef]
- Chong, J.Y.; Wang, B.; Mattevi, C.; Li, K. Dynamic microstructure of graphene oxide membranes and the permeation flux. J. Membr. Sci. 2018, 549, 385–392. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, J.; Mao, Y.; Liu, G.; Jin, W. Effect of substrate on formation and nanofiltration performance of graphene oxide membranes. J. Membr. Sci. 2019, 574, 196–204. [Google Scholar] [CrossRef]
- Yadav, S.; Ibrar, I.; Altaee, A.; Samal, A.K.; Ghobadi, R.; Zhou, J. Feasibility of brackish water and landfill leachate treatment by GO/MoS2-PVA composite membranes. Sci. Total Environ. 2020, 745, 141088. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Graphene-based membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Zhang, B.; Liu, J.; Zhang, H.; Song, C. Preparation and characterization of HPEI-GO/PES ultrafiltration membrane with antifouling and antibacterial properties. J. Membr. Sci. 2013, 447, 452–462. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, M.; Yu, S.; Zhao, Y.; Gao, C.; Shen, J. Ultrathin and stable graphene oxide film via intercalation polymerization of polydopamine for preparation of digital inkjet printing dye. J. Membr. Sci. 2019, 586, 15–22. [Google Scholar] [CrossRef]
- Zhang, M.; Mao, Y.; Liu, G.; Liu, G.; Fan, Y.; Jin, W. Molecular Bridges Stabilize Graphene Oxide Membranes in Water. Angew. Chem.-Int. Ed. 2020, 59, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Liu, G.; Shen, J.; Chu, Z.; Zhou, H.; Gu, X.; Jin, W.; Xu, N. High-Efficiency Water-Transport Channels using the Synergistic Effect of a Hydrophilic Polymer and Graphene Oxide Laminates. Adv. Funct. Mater. 2015, 25, 5809–5815. [Google Scholar] [CrossRef]
- Ahmadi, H.; Zakertabrizi, M.; Hosseini, E.; Cha-Umpong, W.; Abdollahzadeh, M.; Korayem, A.H.; Chen, V.; Shon, H.K.; Asadnia, M.; Razmjou, A. Heterogeneous asymmetric passable cavities within graphene oxide nanochannels for highly efficient lithium sieving. Desalination 2022, 538, 115888. [Google Scholar] [CrossRef]
- Zeng, G.; Lin, Q.; Wei, K.; Liu, Y.; Zheng, S.; Zhan, Y.; He, S.; Patra, T.; Chiao, Y.-H. High-performing composite membrane based on dopamine-functionalized graphene oxide incorporated two-dimensional MXene nanosheets for water purification. J. Mater. Sci. 2021, 56, 6814–6829. [Google Scholar] [CrossRef]
- Ran, J.; Chu, C.; Pan, T.; Ding, L.; Cui, P.; Fu, C.-F.; Zhang, C.-L.; Xu, T. Non-covalent cross-linking to boost the stability and permeability of graphene-oxide-based membranes. J. Mater. Chem. A 2019, 7, 8085–8091. [Google Scholar] [CrossRef]
- Song, N.; Gao, X.; Ma, Z.; Wang, X.; Wei, Y.; Gao, C. A review of graphene-based separation membrane: Materials, characteristics, preparation and applications. Desalination 2018, 437, 59–72. [Google Scholar] [CrossRef]
- Wei, S.; Xie, Y.; Xing, Y.; Wang, L.; Ye, H.; Xiong, X.; Wang, S.; Han, K. Two-dimensional graphene Oxide/MXene composite lamellar membranes for efficient solvent permeation and molecular separation. J. Membr. Sci. 2019, 582, 414–422. [Google Scholar] [CrossRef]
- Xi, Y.-H.; Liu, Z.; Ji, J.; Wang, Y.; Faraj, Y.; Zhu, Y.; Xie, R.; Ju, X.-J.; Wang, W.; Lu, X.; et al. Graphene-based membranes with uniform 2D nanochannels for precise sieving of mono-/multi-valent metal ions. J. Membr. Sci. 2018, 550, 208–218. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, Q.; Zeng, G.; Zhao, S.; Yan, G.; Ang, M.B.M.Y.; Chiao, Y.-H.; Pu, S. Ternary hetero-structured BiOBr/Bi2MoO6@MXene composite membrane: Construction and enhanced removal of antibiotics and dyes from water. J. Membr. Sci. 2022, 669, 121329. [Google Scholar] [CrossRef]
- Cheng, X.; Liao, J.; Xue, Y.; Lin, Q.; Yang, Z.; Yan, G.; Zeng, G.; Sengupta, A. Ultrahigh-flux and self-cleaning composite membrane based on BiOCl-PPy modified MXene nanosheets for contaminants removal from wastewater. J. Membr. Sci. 2022, 644, 120188. [Google Scholar] [CrossRef]
- Zhao, Q.-N.; Zhang, Y.-J.; Duan, Z.-H.; Wang, S.; Liu, C.; Jiang, Y.-D.; Tai, H.-L. A review on Ti3C2Tx-based nanomaterials: Synthesis and applications in gas and humidity sensors. Rare Met. 2020, 40, 1459–1476. [Google Scholar] [CrossRef]
- Chen, X.; Kong, Z.; Li, N.; Zhao, X.; Sun, C. Proposing the prospects of Ti3CN transition metal carbides (MXenes) as anodes of Li-ion batteries: A DFT study. Phys. Chem. Chem. Phys. 2016, 18, 32937–32943. [Google Scholar] [CrossRef]
- Yan, J.; Ren, C.E.; Maleski, K.; Hatter, C.B.; Anasori, B.; Urbankowski, P.; Sarycheva, A.; Gogotsi, Y. Flexible MXene/Graphene Films for Ultrafast Supercapacitors with Outstanding Volumetric Capacitance. Adv. Funct. Mater. 2017, 27, 1701264. [Google Scholar] [CrossRef]
- Mashtalir, O.; Cook, K.M.; Mochalin, V.N.; Crowe, M.; Barsoum, M.W.; Gogotsi, Y. Dye adsorption and decomposition on two-dimensional titanium carbide in aqueous media. J. Mater. Chem. A 2014, 2, 14334–14338. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Thomas, A.M.; Vidyasagar, D.; Rao, V.N.; Yoon, S.-G.; Kim, Y.-H.; Kim, S.-G.; Kim, M.-D. ZnO@Ti3C2Tx MXene Hybrid Composite-Based Schottky-Barrier-Coated SAW Sensor for Effective Detection of Sub-ppb-Level NH3 at Room Temperature under UV Illumination. ACS Mater. Lett. 2023, 5, 2739–2746. [Google Scholar] [CrossRef]
- Qin, M.; Merzougui, C.; Su, Y.-m.; Li, Y.-f.; Chen, W.-y.; Huang, D. Recent developments in MXene and MXene/carbon composites for use in biomedical applications. New Carbon Mater. 2023, 38, 496–506. [Google Scholar] [CrossRef]
- Karahan, H.E.; Goh, K.; Zhang, C.; Yang, E.; Yıldırım, C.; Chuah, C.Y.; Ahunbay, M.G.; Lee, J.; Tantekin-Ersolmaz, Ş.B.; Chen, Y.; et al. MXene Materials for Designing Advanced Separation Membranes. Adv. Mater. 2020, 32, 1906697. [Google Scholar] [CrossRef]
- Ihsanullah, I. Potential of MXenes in Water Desalination: Current Status and Perspectives. Nano-Micro Lett. 2020, 12, 72. [Google Scholar] [CrossRef]
- Li, Z.-K.; Liu, Y.; Li, L.; Wei, Y.; Caro, J.; Wang, H. Ultra-thin titanium carbide (MXene) sheet membranes for high-efficient oil/water emulsions separation. J. Membr. Sci. 2019, 592, 117361. [Google Scholar] [CrossRef]
- Sagita, F.; Radiman, C.L.; Ledyastuti, M.; Khalil, M.; Kadja, G.T.M. Salt-modified MXene membrane for ultrafast and efficient cationic and anionic dyes removal. J. Water Process Eng. 2022, 49, 103133. [Google Scholar] [CrossRef]
- Ding, M.; Xu, H.; Chen, W.; Yang, G.; Kong, Q.; Ng, D.; Lin, T.; Xie, Z. 2D laminar maleic acid-crosslinked MXene membrane with tunable nanochannels for efficient and stable pervaporation desalination. J. Membr. Sci. 2020, 600, 117871. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Chao, Y.; Zhu, L.; Luo, G.; Sun, J.; Jiang, L.; Zhu, W.; Liu, Z.; Xu, C. Highly selective separation of lithium with hierarchical porous lithium-ion sieve microsphere derived from MXene. Desalination 2022, 537, 115847. [Google Scholar] [CrossRef]
- Li, Z.K.; Wei, Y.; Gao, X.; Ding, L.; Lu, Z.; Deng, J.; Yang, X.; Caro, J.; Wang, H. Antibiotics Separation with MXene Membranes Based on Regularly Stacked High-Aspect-Ratio Nanosheets. Angew. Chem. Int. Ed. 2020, 59, 9751–9756. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.E.; Hatzell, K.B.; Alhabeb, M.; Ling, Z.; Mahmoud, K.A.; Gogotsi, Y. Charge- and Size-Selective Ion Sieving through Ti3C2Tx MXene Membranes. J. Phys. Chem. Lett. 2015, 6, 4026–4031. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, L.; Xu, Y.; Li, Q.; Jiang, J.; Wang, X. Preparation and characteristics of graphene oxide-blending PVDF nanohybrid membranes and their applications for hazardous dye adsorption and rejection. J. Colloid Interface Sci. 2017, 504, 429–439. [Google Scholar] [CrossRef]
- Zeng, G.; He, Z.; Wan, T.; Wang, T.; Yang, Z.; Liu, Y.; Lin, Q.; Wang, Y.; Sengupta, A.; Pu, S. A self-cleaning photocatalytic composite membrane based on g-C3N4@MXene nanosheets for the removal of dyes and antibiotics from wastewater. Sep. Purif. Technol. 2022, 292, 121037. [Google Scholar] [CrossRef]
- Lin, Q.; Zeng, G.; Yan, G.; Luo, J.; Cheng, X.; Zhao, Z.; Li, H. Self-cleaning photocatalytic MXene composite membrane for synergistically enhanced water treatment: Oil/water separation and dyes removal. Chem. Eng. J. 2022, 427, 119058. [Google Scholar] [CrossRef]
- Liao, F.; Xu, Z.; Fan, Z.; Meng, Q.; Lv, B.; Ye, X.; Shen, C.; Zhang, G. Confined assembly of ultrathin dual-functionalized Z-MXene nanosheet intercalated GO nanofilms with controlled structure for size-selective permeation. J. Mater. Chem. A 2021, 9, 12236–12243. [Google Scholar] [CrossRef]
- Yin, Z.; Lu, Z.; Xu, Y.; Zhang, Y.; He, L.; Li, P.; Xiong, L.; Ding, L.; Wei, Y.; Wang, H. Supported MXene/GO Composite Membranes with Suppressed Swelling for Metal Ion Sieving. Membranes 2021, 11, 621. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Zeng, G.; Pu, S.; Yan, G.; Luo, J.; Wan, Y.; Zhao, Z. A dual regulation strategy for MXene-based composite membrane to achieve photocatalytic self-cleaning properties and multi-functional applications. Chem. Eng. J. 2022, 443, 136335. [Google Scholar] [CrossRef]
- Liu, T.; Liu, X.; Graham, N.; Yu, W.; Sun, K. Two-dimensional MXene incorporated graphene oxide composite membrane with enhanced water purification performance. J. Membr. Sci. 2020, 593, 117431. [Google Scholar] [CrossRef]
- Kang, K.M.; Kim, D.W.; Ren, C.E.; Cho, K.M.; Kim, S.J.; Choi, J.H.; Nam, Y.T.; Gogotsi, Y.; Jung, H.T. Selective Molecular Separation on Ti3C2Tx-Graphene Oxide Membranes during Pressure-Driven Filtration: Comparison with Graphene Oxide and MXenes. ACS Appl. Mater. Interfaces 2017, 9, 44687–44694. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Liu, Y.; Lin, Q.; Pu, S.; Zheng, S.; Ang, M.B.M.Y.; Chiao, Y.-H. Constructing composite membranes from functionalized metal organic frameworks integrated MXene intended for ultrafast oil/water emulsion separation. Sep. Purif. Technol. 2022, 293, 121052. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Q.; Zeng, G.; Zhang, L.; Zhou, Y.; Sengupta, A. Nature-inspired green method decorated MXene-based composite membrane for high-efficiency oil/water separation. Sep. Purif. Technol. 2022, 283, 120218. [Google Scholar] [CrossRef]
- Zhao, X.; Che, Y.; Mo, Y.; Huang, W.; Wang, C. Fabrication of PEI modified GO/MXene composite membrane and its application in removing metal cations from water. J. Membr. Sci. 2021, 640, 119847. [Google Scholar] [CrossRef]
- Ma, J.; Tang, X.; He, Y.; Fan, Y.; Chen, J.; Yu, H. Robust stable MoS2/GO filtration membrane for effective removal of dyes and salts from water with enhanced permeability. Desalination 2020, 480, 114328. [Google Scholar] [CrossRef]
- Xu, P.; Wang, W.; Qian, X.; Wang, H.; Guo, C.; Li, N.; Xu, Z.; Teng, K.; Wang, Z. Positive charged PEI-TMC composite nanofiltration membrane for separation of Li+ and Mg2+ from brine with high Mg2+/Li+ ratio. Desalination 2019, 449, 57–68. [Google Scholar] [CrossRef]
- Cheng, P.; Chen, Y.; Gu, Y.-H.; Yan, X.; Lang, W.-Z. Hybrid 2D WS2/GO nanofiltration membranes for finely molecular sieving. J. Membr. Sci. 2019, 591, 117308. [Google Scholar] [CrossRef]
- Zhang, P.; Gong, J.-L.; Zeng, G.-M.; Song, B.; Cao, W.; Liu, H.-Y.; Huan, S.-Y.; Peng, P. Novel “loose” GO/MoS2 composites membranes with enhanced permeability for effective salts and dyes rejection at low pressure. J. Membr. Sci. 2019, 574, 112–123. [Google Scholar] [CrossRef]


| Membrane | GO (mg) | Ti3C2TX (mg) |
|---|---|---|
| M0 | 1.5 | 0 |
| M1 | 1.5 | 0.5 |
| M2 | 1.5 | 1 |
| M3 | 1.5 | 1.5 |
| M4 | 1.5 | 2 |
| Membrane | C (%) | O (%) | Ti (%) | F (%) |
|---|---|---|---|---|
| M0 | 72.1 | 27.9 | 0 | 0 |
| M2 | 69.74 | 28.24 | 0.97 | 1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Z.; Liu, C.; Tang, B.; Yang, X.; Jiang, W.; Wang, P.; Tang, X.; Wang, H.; Zeng, X.; Zeng, G. Construction of a Two-Dimensional GO/Ti3C2TX Composite Membrane and Investigation of Mg2+/Li+ Separation Performance. Nanomaterials 2023, 13, 2777. https://doi.org/10.3390/nano13202777
Feng Z, Liu C, Tang B, Yang X, Jiang W, Wang P, Tang X, Wang H, Zeng X, Zeng G. Construction of a Two-Dimensional GO/Ti3C2TX Composite Membrane and Investigation of Mg2+/Li+ Separation Performance. Nanomaterials. 2023; 13(20):2777. https://doi.org/10.3390/nano13202777
Chicago/Turabian StyleFeng, Zhenhua, Chengwen Liu, Binbin Tang, Xiaojun Yang, Wenjie Jiang, Peng Wang, Xianjun Tang, Hongshan Wang, Xiangdong Zeng, and Guangyong Zeng. 2023. "Construction of a Two-Dimensional GO/Ti3C2TX Composite Membrane and Investigation of Mg2+/Li+ Separation Performance" Nanomaterials 13, no. 20: 2777. https://doi.org/10.3390/nano13202777
APA StyleFeng, Z., Liu, C., Tang, B., Yang, X., Jiang, W., Wang, P., Tang, X., Wang, H., Zeng, X., & Zeng, G. (2023). Construction of a Two-Dimensional GO/Ti3C2TX Composite Membrane and Investigation of Mg2+/Li+ Separation Performance. Nanomaterials, 13(20), 2777. https://doi.org/10.3390/nano13202777







