Interfacial Charge Transfer Effects of MoS2/α-Fe2O3 Nano-Heterojunction and Efficient Photocatalytic Hydrogen Evolution under Visible-Light Irradiation
Abstract
:1. Introduction
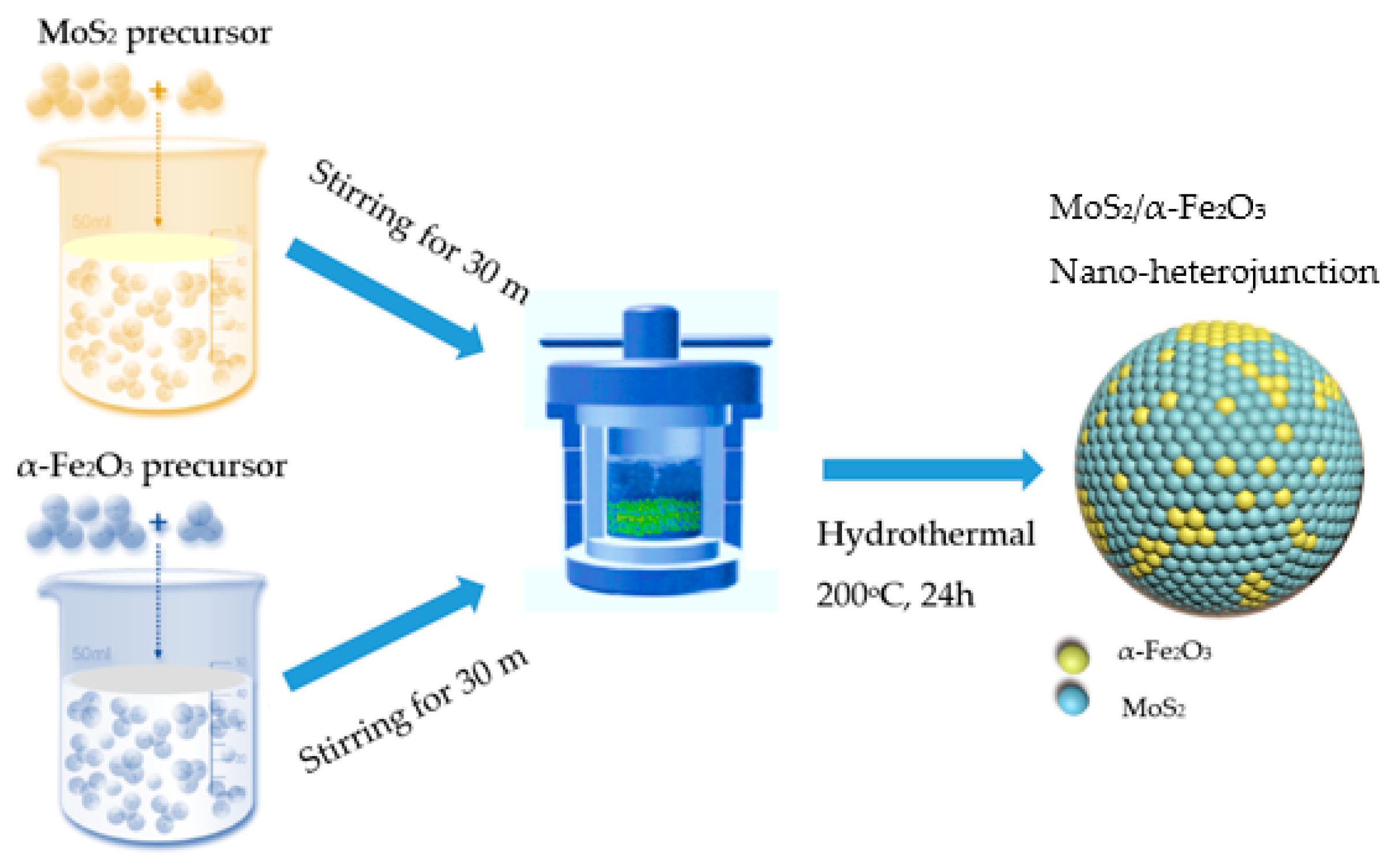
2. Materials and Methods
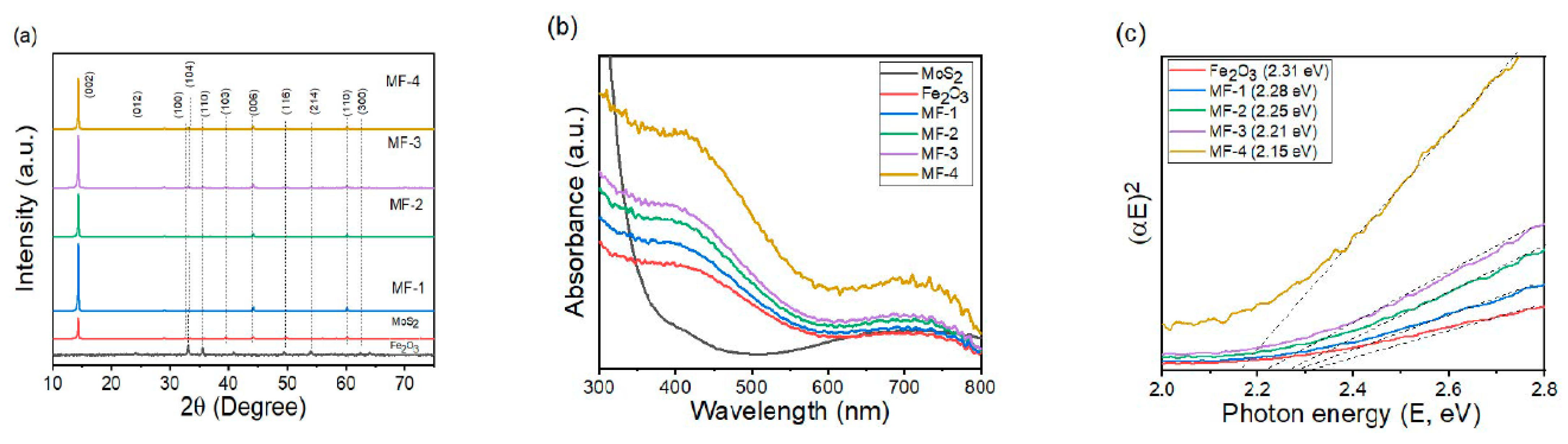
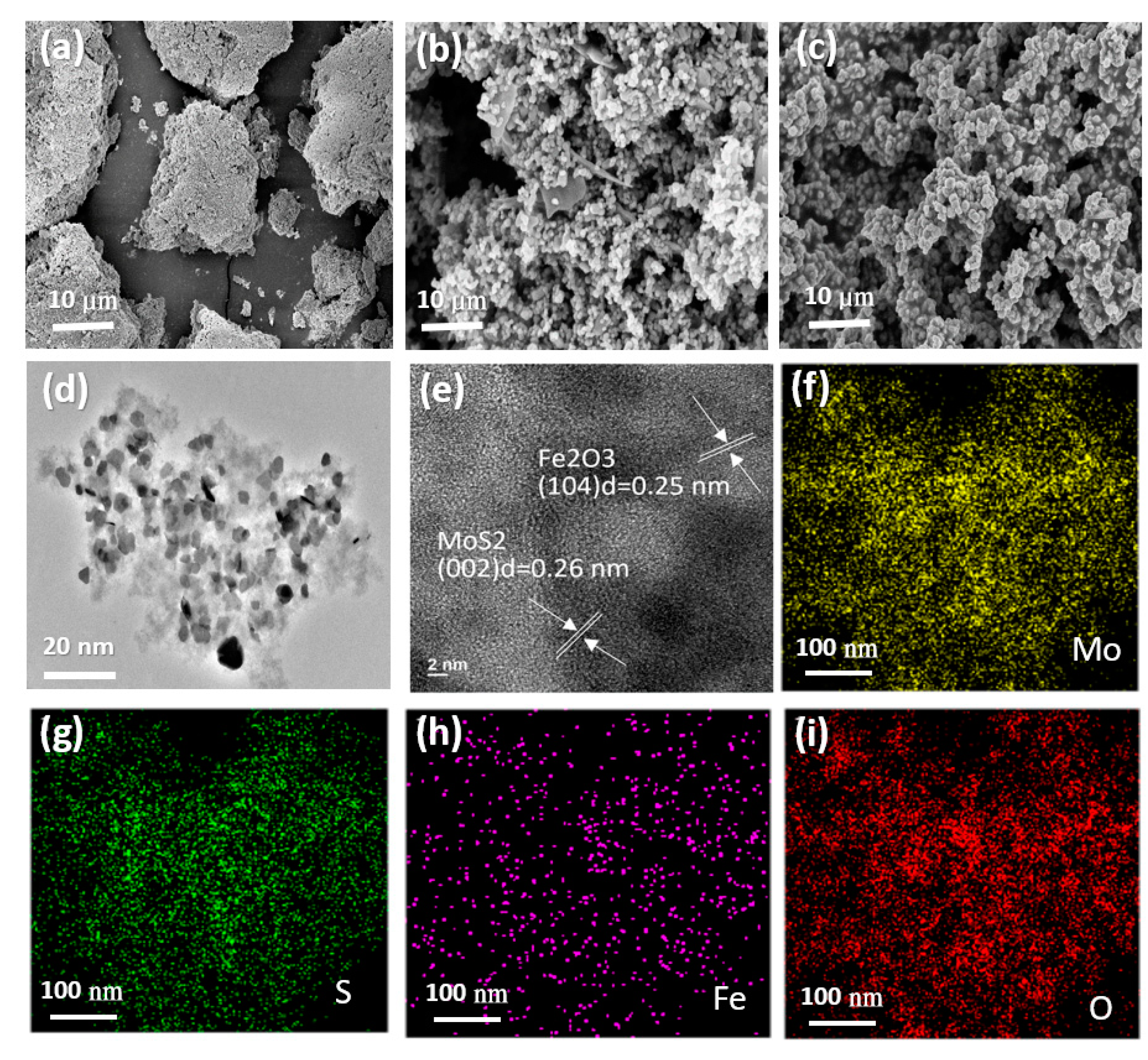
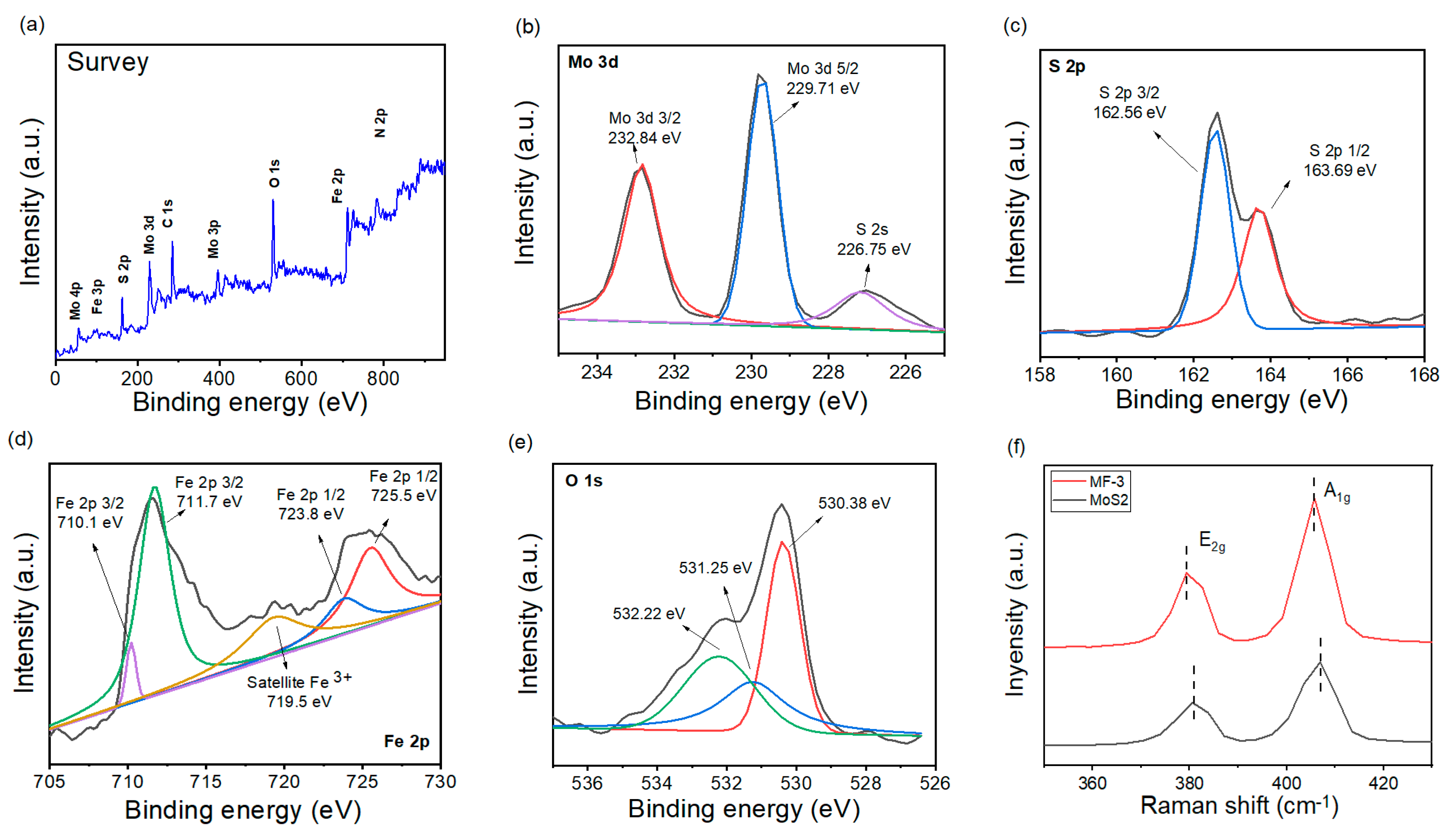
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Roy, A.; Alhabradi, M.; Alruwaili, M.; Chang, H.; Tahir, A.A. Fabrication and Characterization of Tantalum–Iron Composites for Photocatalytic Hydrogen Evolution. Nanomaterials 2023, 13, 2464. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Lee, C.R.; Chuang, Y.J.; Wu, Z.H.; Chen, C. Synthesis and Optical Properties of Lead-Free Cesium Tin Halide Perovskite Quantum Rods with High-Performance Solar Cell Application. J. Phys. Chem. Lett. 2016, 7, 5028–5035. [Google Scholar] [CrossRef]
- Meda, U.S.; Rajyaguru, Y.V.; Pandey, A. Generation of green hydrogen using self-sustained regenerative fuel cells: Opportunities and challenges. Int. J. Hydrogen Energy 2023, 48, 28289–28314. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.W.; Lim, S.J.; Kim, H. Visible light activated MoS2/ZnO composites for photocatalytic degradation of ciprofloxacin antibiotic and hydrogen production. J. Photochem. Photobiol. A Chem. 2023, 434, 114250. [Google Scholar] [CrossRef]
- Chen, L.J. Tunable photoluminescence emission from Cadmium Tellurium nanorods with ethylenediamine template-assistance at a low temperature. Mater. Lett. 2013, 101, 83–85. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Lee, K.; Kirchgeorg, R.; Hahn, R.; Schmuki, P. Self-organization and zinc doping of Ga2O3 nanoporous architecture: A potential nano-photogenerator for hydrogen. Electrochem. Commun. 2013, 35, 112–115. [Google Scholar] [CrossRef]
- Balan, B.; Xavier, M.M.; Mathew, S. MoS2-Based Nanocomposites for Photocatalytic Hydrogen Evolution and Carbon Dioxide Reduction. ACS Omega 2023, 8, 25649–25673. [Google Scholar] [CrossRef]
- Ren, R.; Zhao, H.; Sui, X.; Guo, X.; Huang, X.; Wang, Y.; Dong, Q.; Chen, J. Exfoliated Molybdenum Disulfide Encapsulated in a Metal Organic Framework for Enhanced Photocatalytic Hydrogen Evolution. Catalysts 2019, 9, 89. [Google Scholar] [CrossRef]
- Chen, L.J.; Chuang, Y.J.; Chen, C. Surface passivation assisted lasing emission in the quantum dots doped cholesteric liquid crystal resonating cavity with polymer template. RSC Adv. 2014, 4, 18600. [Google Scholar] [CrossRef]
- Lee, C.R.; Chen, L.J. Polyvinylbutyral assisted synthesis and characterization of kesterite quaternary semiconductor Cu2ZnSnSe4 nanofibers by electrospinning route. Sol. Energy Mater. Sol. Cells 2016, 151, 24–29. [Google Scholar] [CrossRef]
- Geng, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Z-Scheme 2D/2D α-Fe2O3/g-C3N4 heterojunction for photocatalytic oxidation of nitric oxide. Appl. Catal. B Environ. 2021, 280, 119409. [Google Scholar] [CrossRef]
- Alp, E.; İmamoǧlu, R.; Savac, U.; Turan, S.; Kazmanl, M.K.; Genc, A. Plasmon-enhanced photocatalytic and antibacterial activity of gold nanoparticles-decorated hematite nanostructures. J. Alloys Compd. 2021, 852, 157021. [Google Scholar] [CrossRef]
- Arzaee, N.A.; Noh, M.F.M.; Halim, A.A.; Rahim, M.A.F.A.; Ita, N.S.H.M.; Mohamed, N.A.; Nasir, S.N.F.M.; Ismail, A.F.; Teridi, M.A.M. Cyclic voltammetry-A promising approach towards improving photoelectrochemical activity of hematite. J. Alloys Compd. 2021, 852, 156757. [Google Scholar] [CrossRef]
- Hu, C.-C.; Nian, J.-N.; Teng, H. Electrodeposited p-type Cu2O as photocatalyst for H2 evolution from water reduction in the presence of WO3. Sol. Energy Mater. Sol. Cells 2008, 92, 1071–1076. [Google Scholar] [CrossRef]
- Chen, L.J.; Chuang, Y.J. Directly electrospinning growth of single crystal Cu2ZnSnS4 nanowires film for high performance thin film solar cell. J. Power Sources 2013, 241, 259–265. [Google Scholar] [CrossRef]
- Vamvasakis, I.; Andreou, E.K.; Armatas, G.S. Mesoporous Dual-Semiconductor ZnS/CdS Nanocomposites as Efficient Visible Light Photocatalysts for Hydrogen Generation. Nanomaterials 2023, 13, 2426. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, M.; Ishaq, T.; Bhatti, I.A.; Maryam, M.; Jilani, A.; Melaibari, A.A.; Abu-Hamdeh, N.H. Semiconductor Nanomaterial Photocatalysts for Water-Splitting Hydrogen Production: The Holy Grail of Converting Solar Energy to Fuel. Nanomaterials 2023, 13, 546. [Google Scholar] [CrossRef]
- Tien, T.M.; Chuang, Y.; Chen, E.L. Z-scheme driven of MoS2/Co3O4 nano-heterojunction for efficient photocatalysis hydrogen evolution and Rhodamine B degradation. J. Photochem. Photobiol. A Chem. 2023, 444, 114986. [Google Scholar] [CrossRef]
- Guo, C.; Chu, L.; Zhang, Q.; Li, Z.; Yang, G.; Peng, F. The Zinc Vacancy Induced CdS/ZnS Z-Scheme Structure as a Highly Stable Photocatalyst for Hydrogen Production. J. Alloys Compd. 2021, 888, 161620. [Google Scholar] [CrossRef]
- Chen, L.J.; Chuang, Y.J. Quaternary Semiconductor Derived and formation mechanism by non-vacuum Route from Solvothermal Nanostructures for High-Performance application. Mater. Lett. 2013, 91, 372–375. [Google Scholar] [CrossRef]
- Abd-Elkader, O.H.; Abdelsalam, H.; Sakr, M.A.S.; Shaltout, A.A.; Zhang, Q. First-Principles Study of MoS2, WS2, and NbS2 Quantum Dots: Electronic Properties and Hydrogen Evolution Reaction. Crystals 2023, 13, 994. [Google Scholar] [CrossRef]
- Chuang, Y.J.; Liao, J.D.; Chen, L.J. Polyvinylbutyral-assisted synthesis and characterization of mesoporous silica nanofibers by electrospinning route. J. Compos. Mater. 2012, 46, 227–236. [Google Scholar] [CrossRef]
- Tien, T.M.; Chen, E.L. A Novel MoS2/TiO2/Graphene Nanohybrid for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Catalysts 2023, 13, 1152. [Google Scholar] [CrossRef]
- Lassoued, A.; Dkhil, B.; Gadri, A.; Ammar, S. Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys. 2017, 7, 3007–3015. [Google Scholar] [CrossRef]
- Chen, L.J.; Chuang, Y.J. Hydrothermal Synthesis and Characterization of Hexagonal Zinc Oxide Nanorods with a Hexamethylenetetramine (HMTA) Template-assisted at a Low Temperature. Mater. Lett. 2012, 68, 460–462. [Google Scholar] [CrossRef]
- Wang, S.; Tang, B.; Yang, W.; Wu, F.; Zhang, G.; Zhao, B.; He, X.; Yang, Y.; Jiang, J. The flower-like heterostructured Fe2O3/MoS2 coated by amorphous Si-Oxyhydroxides: An effective surface modification method for sulfide photocatalysts in photo-Fenton reaction. J. Alloys Compd. 2019, 784, 1099–1105. [Google Scholar] [CrossRef]
- Jia, L.; Wang, C.; Liu, H.; Chen, R.; Wu, K. Preparation of defect-enriched (SiO32−, Cu2+)–α-Fe2O3/MoS2 Z-scheme composites with enhanced photocatalytic-Fenton performance. J. Alloys Compd. 2022, 923, 166293. [Google Scholar] [CrossRef]
- Bagheri, F.; Chaibakhsh, N. Efficient visible-light photocatalytic ozonation for dye degradation using Fe2O3/MoS2 nanocomposite. Sep. Sci. Technol. 2021, 56, 3022–3032. [Google Scholar] [CrossRef]
- Khabiri, G.; Aboraia, A.M.; Soliman, M.; Guda, A.A.; Butova, V.V.; Yahia, I.S.; Soldatov, A.V. A novel α-Fe2O3@MoS2 QDs heterostructure for enhanced visible-light photocatalytic performance using ultrasonication approach. Ceram. Int. 2020, 46, 19600–19608. [Google Scholar] [CrossRef]
- Chen, L.J.; Chuang, Y.; Yang, W.D.; Tsai, K.C.; Chen, C.W.; Dong, C.D. All-inorganic perovskite CsPbX3 electrospun nanofibers with color-tunable photoluminescence and high performance optoelectronic applications. J. Alloys Compd. 2021, 856, 157426. [Google Scholar] [CrossRef]
- Chen, L.J.; Chen, C.W.; Dong, C.D. Direct Z-Scheme Heterostructures Based on MoSSe Quantum Dots for Visible Light-Driven Photocatalytic Tetracycline Degradation. ACS Appl. Nano Mater. 2021, 4, 1038–1047. [Google Scholar] [CrossRef]
- Chen, L.J.; Arshad, M.; Chuang, Y.; Nguyen, T.B.; Wu, C.H.; Chen, C.W.; Dong, C.D. A novel nano-heterojunction MoS2/α-Fe2O3 photocatalysts with high photocatalytic and photoelectrochemical performance under visible light irradiation. J. Alloys Compd. 2023, 947, 169577. [Google Scholar] [CrossRef]
- Cho, Y.; Huh, Y. Preparation of Hyperbranched Structures of α-Fe2O3. Bull. Korean Chem. Soc. 2009, 30, 1413–1415. [Google Scholar]
- Chen, L.J. Synthesis and optical properties of lead-free cesium germanium halide perovskite quantum rods. RSC Adv. 2018, 18, 18396–18399. [Google Scholar] [CrossRef] [PubMed]
- Hinnemann, B.; Moses, P.G.; Bonde, J.; Jørgensen, K.P.; Nielsen, J.H.; Horch, S.; Chorkendorff, I.; Nørskov, J.K. Biomimetic hydrogen evolution: MoS2 nanoparticles as catalyst for hydrogen evolution. J. Am. Chem. Soc. 2005, 127, 5308–5309. [Google Scholar] [CrossRef]
- Khasawneh, O.; Palaniandy, P. Removal of organic pollutants from water by Fe2O3/TiO2 based photocatalytic degradation: A review. Environ. Technol. Innov. 2021, 21, 101230. [Google Scholar] [CrossRef]
- Chen, L.J.; Chuang, Y.J. Diethylenetriamine assisted synthesis and characterization of stannite quaternary semiconductor Cu2ZnSnSe4 nanorods by self-assembly. J. Cryst. Growth 2013, 376, 11–16. [Google Scholar] [CrossRef]
- Sun, Z.; He, J.; Yuan, M.; Lin, L.; Zhang, Z.; Kang, Z.; Liao, Q.; Li, H.; Sun, G.; Yang, X. Li+-clipping for edge S-vacancy MoS2 quantum dots as an efficient bifunctional electrocatalyst enabling discharge growth of amorphous Li2O2 film. Nano Energy 2019, 65, 103996. [Google Scholar] [CrossRef]
- Ramakrishna Matte, H.S.S.; Gomathi, A.; Manna, A.K.; Late, D.J.; Datta, R.; Pati, S.K.; Rao, C.N.R. MoS2 and WS2 Analogues of Graphene. Angew. Chem. Int. Ed. 2010, 49, 4059–4062. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi1, R.; Wang, F.; Wang, S.; Fu, G.; Zou, X.; Li, L.; Yu, L.; Tian, Y.; Luo, F. Separable magnetic MoS2@Fe3O4 nanocomposites with multi-exposed active edge facets toward enhanced adsorption and catalytic activities. J. Mater. Sci. 2021, 56, 5015–5030. [Google Scholar] [CrossRef]
- Yu, J.; Yin, W.; Zheng, X.; Tian, G.; Zhang, X.; Bao, T.; Dong, X.; Wang, Z.; Gu, Z.; Ma, X.; et al. Smart MoS2/Fe3O4 Nanotheranostic for Magnetically Targeted Photothermal Therapy Guided by Magnetic Resonance/Photoacoustic Imaging. Theranostics 2015, 5, 931. [Google Scholar] [CrossRef]
- Shi, F.; Xing, C.; Wang, X. Preparation of TiO2/MoSe2 heterostructure composites by a solvothermal method and their photocatalytic hydrogen production performance. Int. J. Hydrogen Energy 2021, 46, 38636–38644. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, X.; Huang, J.; Zhang, S.; Xu, X. Noble metal-free core-shell CdS/iron phthalocyanine Z-scheme photocatalyst for enhancing photocatalytic hydrogen evolution. J. Mater. Sci. Technol. 2022, 115, 199–207. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, Y.; Liu, Y.; Wang, H.; Yang, D. Ternary red phosphorus/CoP2/SiO2 microsphere boosts visible-light-driven photocatalytic hydrogen evolution from pure water splitting. J. Mater. Sci. Technol. 2022, 125, 59–66. [Google Scholar] [CrossRef]
- Yang, W.; Ma, G.; Fu, Y.; Peng, K.; Yang, H.; Zhan, X.; Yang, W.; Wang, L.; Hou, H. Rationally designed Ti3C2 MXene@TiO2/CuInS2 Schottky/S-scheme integrated heterojunction for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 429, 132381. [Google Scholar] [CrossRef]
- Ge, Y.C.; Chu, H.; Chen, J.Y.; Zhuang, P.Y.; Feng, Q.Y.; Smith, W.R.; Dong, P.; Ye, M.X.; Shen, J.F. Ultrathin MoS2 Nanosheets Decorated Hollow CoP Heterostructures for Enhanced Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2019, 7, 10105–10111. [Google Scholar] [CrossRef]
- Mansour, H.; Omri, K.; Bargougui, R.; Ammar, S. Novel α-Fe2O3/TiO2 nanocomposites with enhanced photocatalytic activity. Appl. Phys. A 2020, 126, 151. [Google Scholar] [CrossRef]
- Chen, L.J.; Liao, J.D.; Chuang, Y.J.; Fu, Y.S. Synthesis and Characterization of Cu(InxB1-x)Se2 Nanocrystals for Low-Cost Thin Film Photovoltaics. J. Am. Chem. Soc. 2011, 133, 3704–3707. [Google Scholar] [CrossRef]
- Zhai, Z.; Li, C.; Zhang, L.; Wu, H.-C.; Zhang, L.; Tang, N.; Wang, W.; Gong, J. Dimensional construction and morphological tuning of heterogeneous MoS2/NiS electrocatalysts for efficient overall water splitting. J. Mater. Chem. A 2018, 6, 9833–9838. [Google Scholar] [CrossRef]
- Huang, R.; Liang, R.; Fan, H.; Ying, S.; Wu, L.; Wang, X. Enhanced Photocatalytic Fuel Denitrification over TiO2/α-Fe2O3 Nanocomposites under Visible Light irradiation. Sci. Rep. 2017, 7, 7858. [Google Scholar] [CrossRef]
- Poudel, M.B.; Awasthi, G.P.; Kim, H.J. Novel insight into the adsorption of Cr (VI) and Pb (II) ions by MOF derived Co-Al layered double hydroxide @ hematite nanorods on 3D porous carbon nanofiber network. Chem. Eng. J. 2021, 417, 129312. [Google Scholar] [CrossRef]
- Poudel, M.B.; Kim, H.J. Confinement of Zn-Mg-Al-layered double hydroxide and α-Fe2O3 nanorods on hollow porous carbon nanofibers: A free-standing electrode for solid-state symmetric supercapacitors. Chem. Eng. J. 2022, 429, 132345. [Google Scholar] [CrossRef]

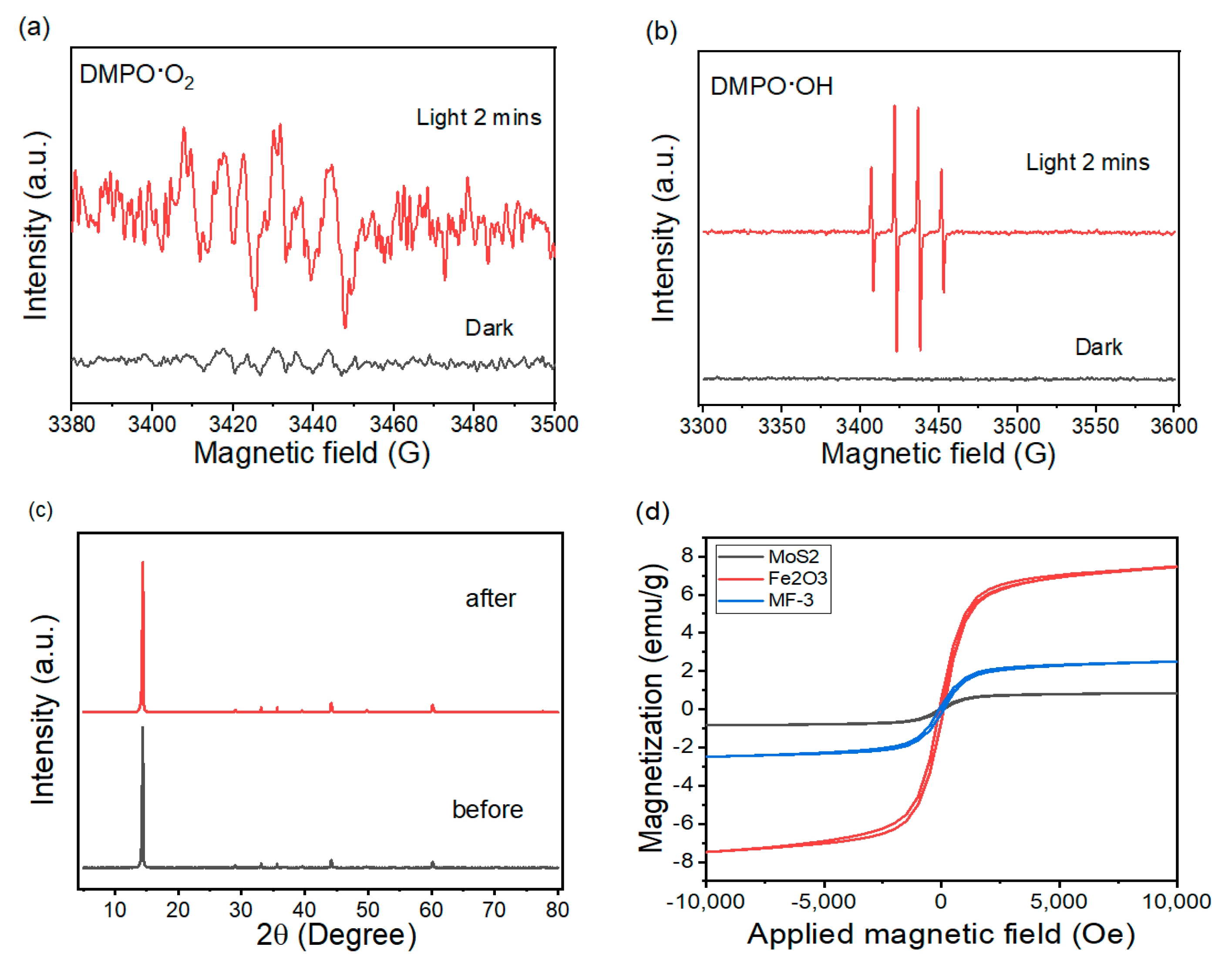

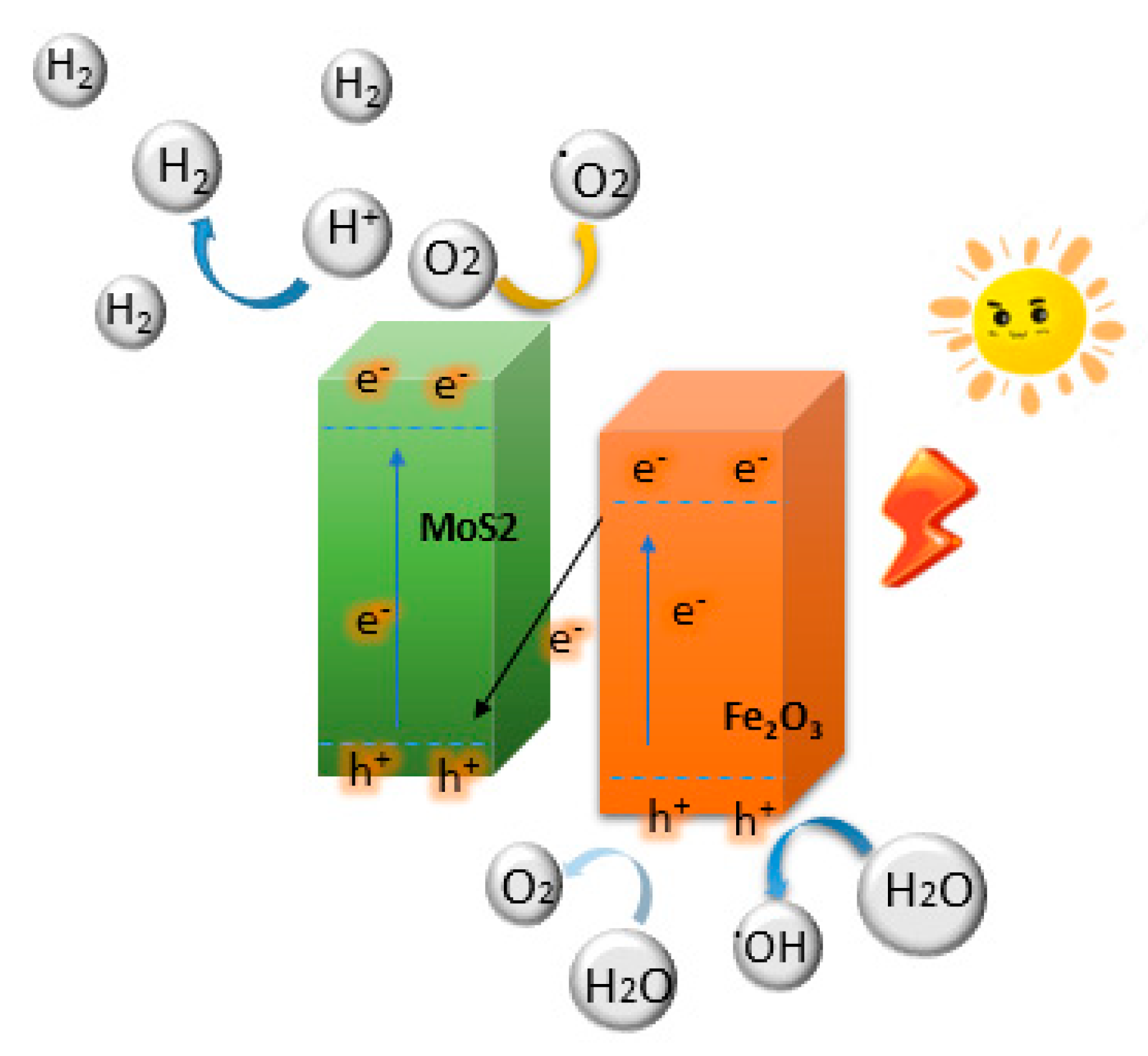
| Photocatalysts | Maximum Rates (μmol h−1 g−1) | Light Source | References |
|---|---|---|---|
| TiO2/MoSe2 | 401 | 300 W Xe lamp | [42] |
| CdS/FePc | 73.01 | 300 W Xe lamp | [43] |
| phosphorus/CoP2/SiO2 | 11.79 | 300 W Xe lamp | [44] |
| Ti3C2 MXene@TiO2/CuInS2 | 356.27 | 300 W Xe lamp | [45] |
| MoS2/α-Fe2O3 | 871.2 | 350 W Xe lamp | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tien, T.-M.; Chen, E.L. Interfacial Charge Transfer Effects of MoS2/α-Fe2O3 Nano-Heterojunction and Efficient Photocatalytic Hydrogen Evolution under Visible-Light Irradiation. Nanomaterials 2023, 13, 2763. https://doi.org/10.3390/nano13202763
Tien T-M, Chen EL. Interfacial Charge Transfer Effects of MoS2/α-Fe2O3 Nano-Heterojunction and Efficient Photocatalytic Hydrogen Evolution under Visible-Light Irradiation. Nanomaterials. 2023; 13(20):2763. https://doi.org/10.3390/nano13202763
Chicago/Turabian StyleTien, Tsung-Mo, and Edward L. Chen. 2023. "Interfacial Charge Transfer Effects of MoS2/α-Fe2O3 Nano-Heterojunction and Efficient Photocatalytic Hydrogen Evolution under Visible-Light Irradiation" Nanomaterials 13, no. 20: 2763. https://doi.org/10.3390/nano13202763
APA StyleTien, T.-M., & Chen, E. L. (2023). Interfacial Charge Transfer Effects of MoS2/α-Fe2O3 Nano-Heterojunction and Efficient Photocatalytic Hydrogen Evolution under Visible-Light Irradiation. Nanomaterials, 13(20), 2763. https://doi.org/10.3390/nano13202763





