Insights into the Sensing Mechanism of a Metal-Oxide Solid Solution via Operando Diffuse Reflectance Infrared Fourier Transform Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Syntheses and Morphological, Structural and Chemical Properties
2.2. Sensor Fabrication
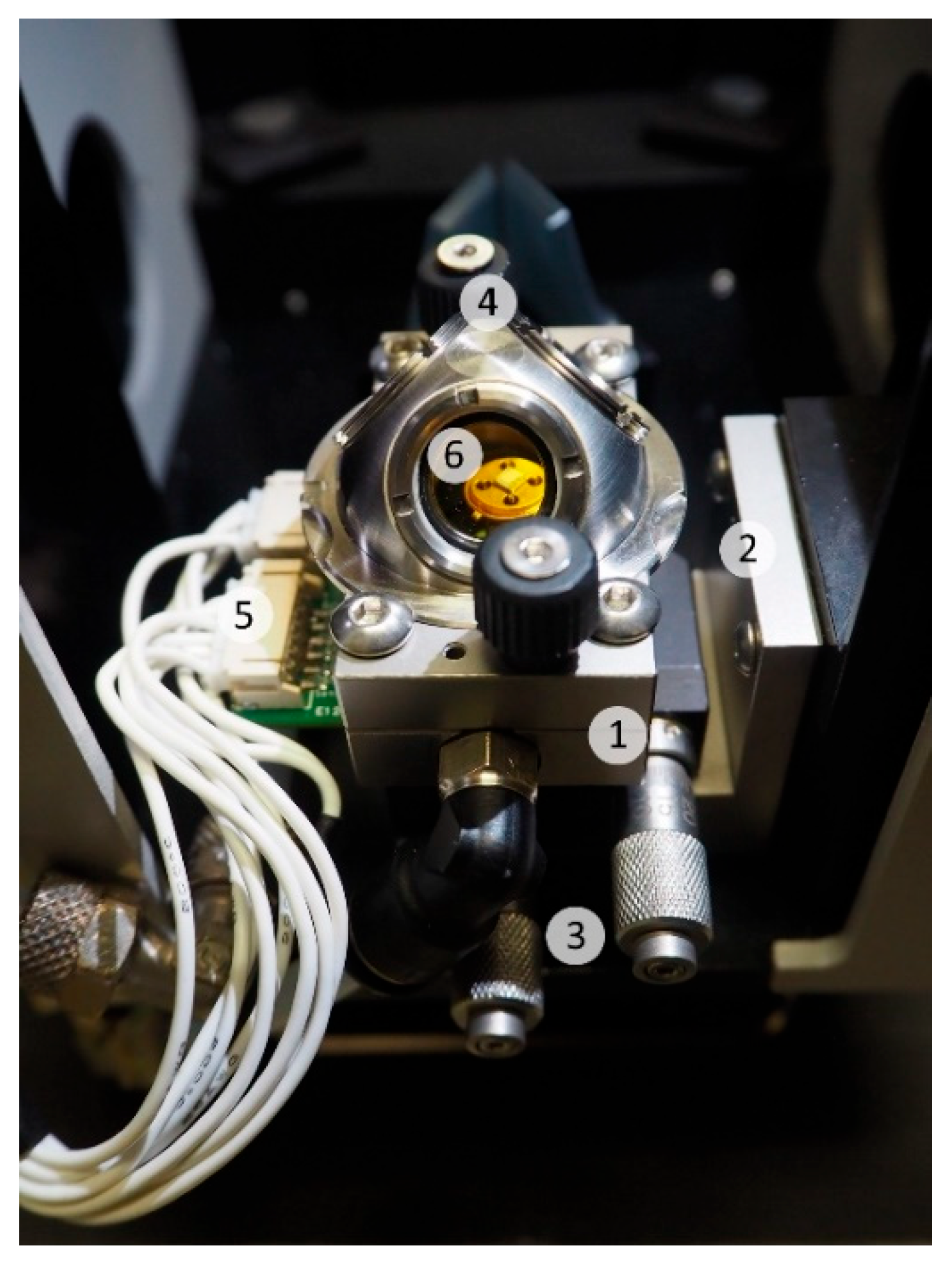
2.3. DRIFT Spectroscopy and Electrical Characterization Setup
2.4. Experimental Details and Working Conditions
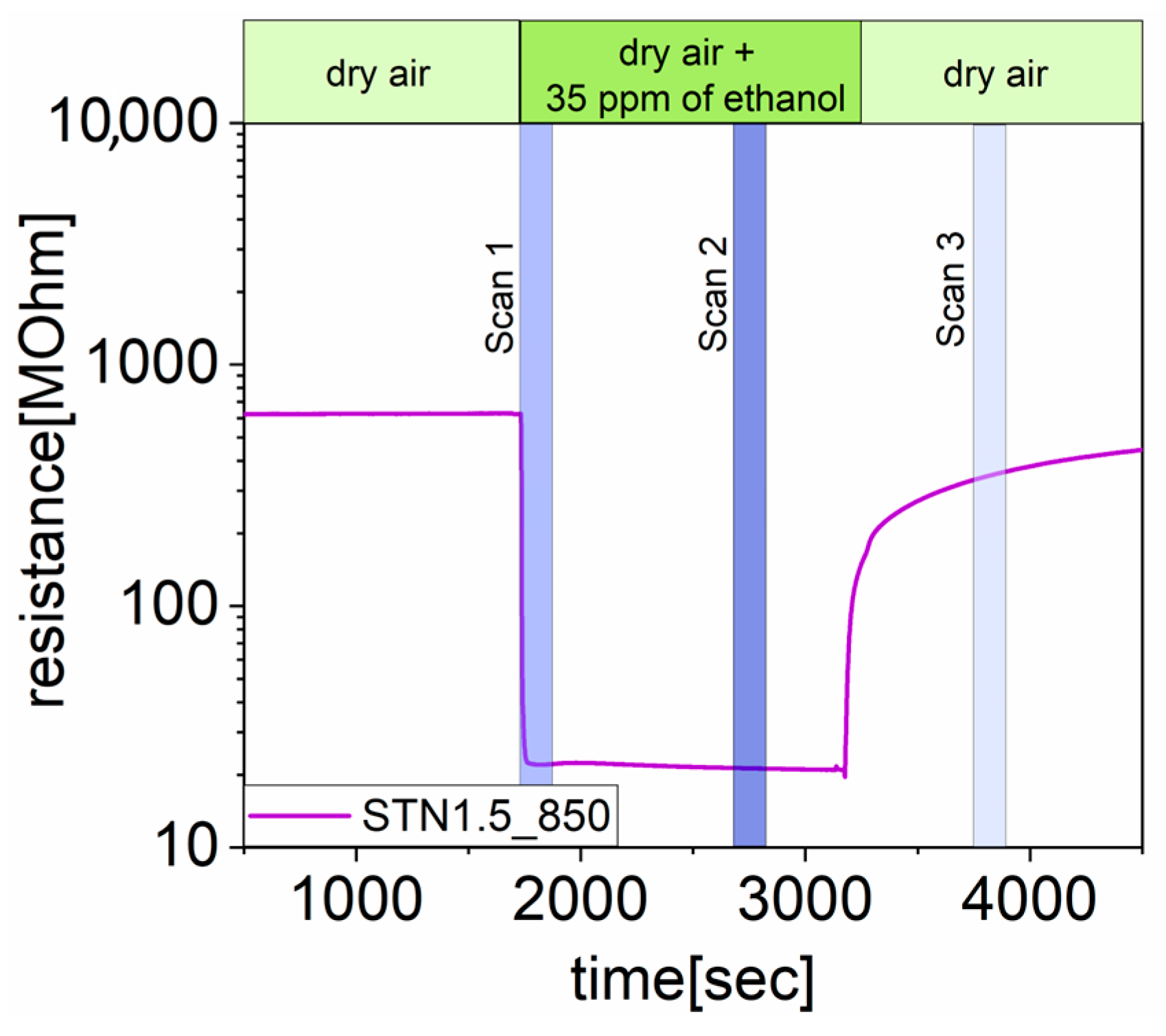
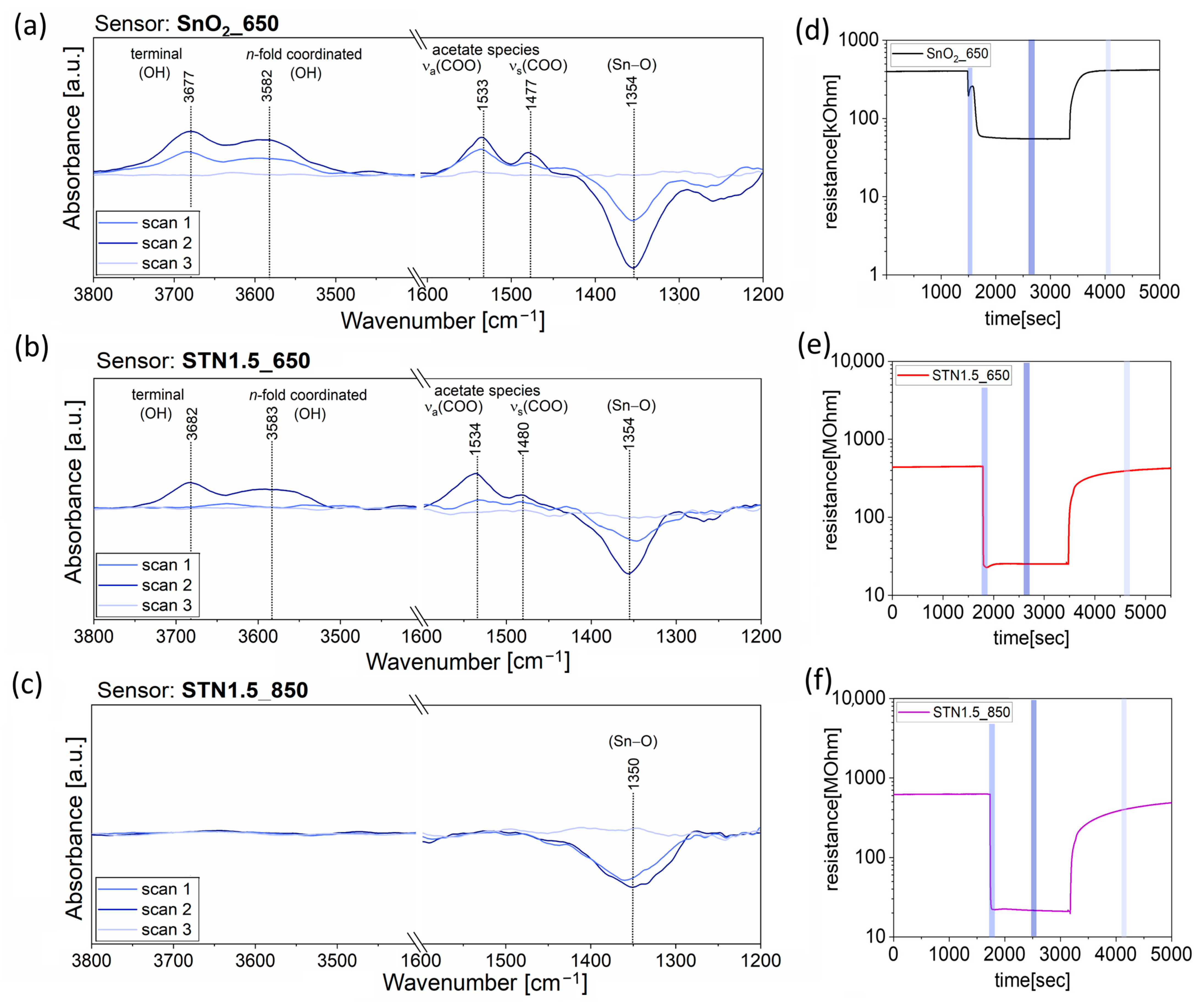
2.4.1. Study under Exposure of Ethanol
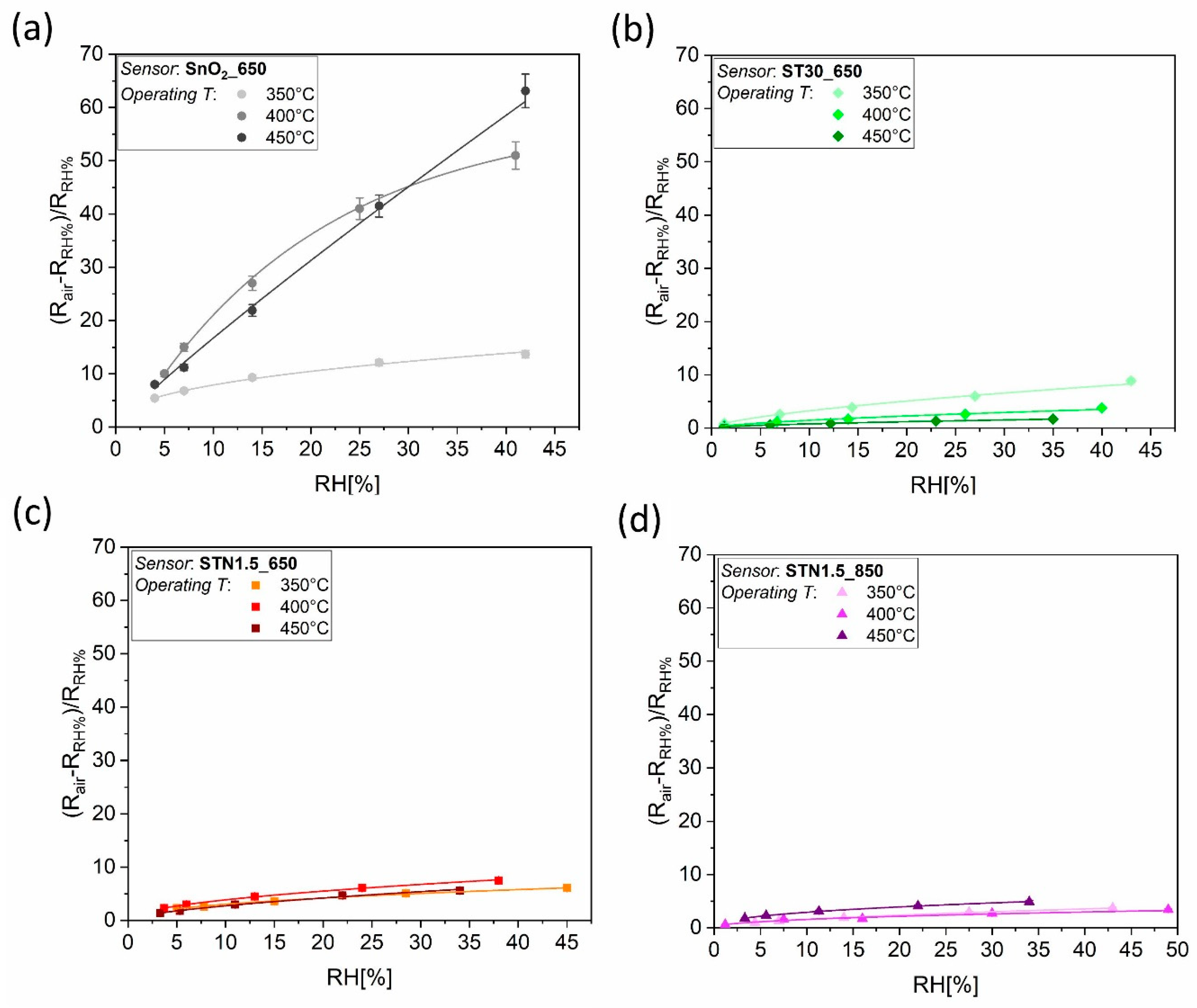
2.4.2. Study in Humid Environment
3. Results
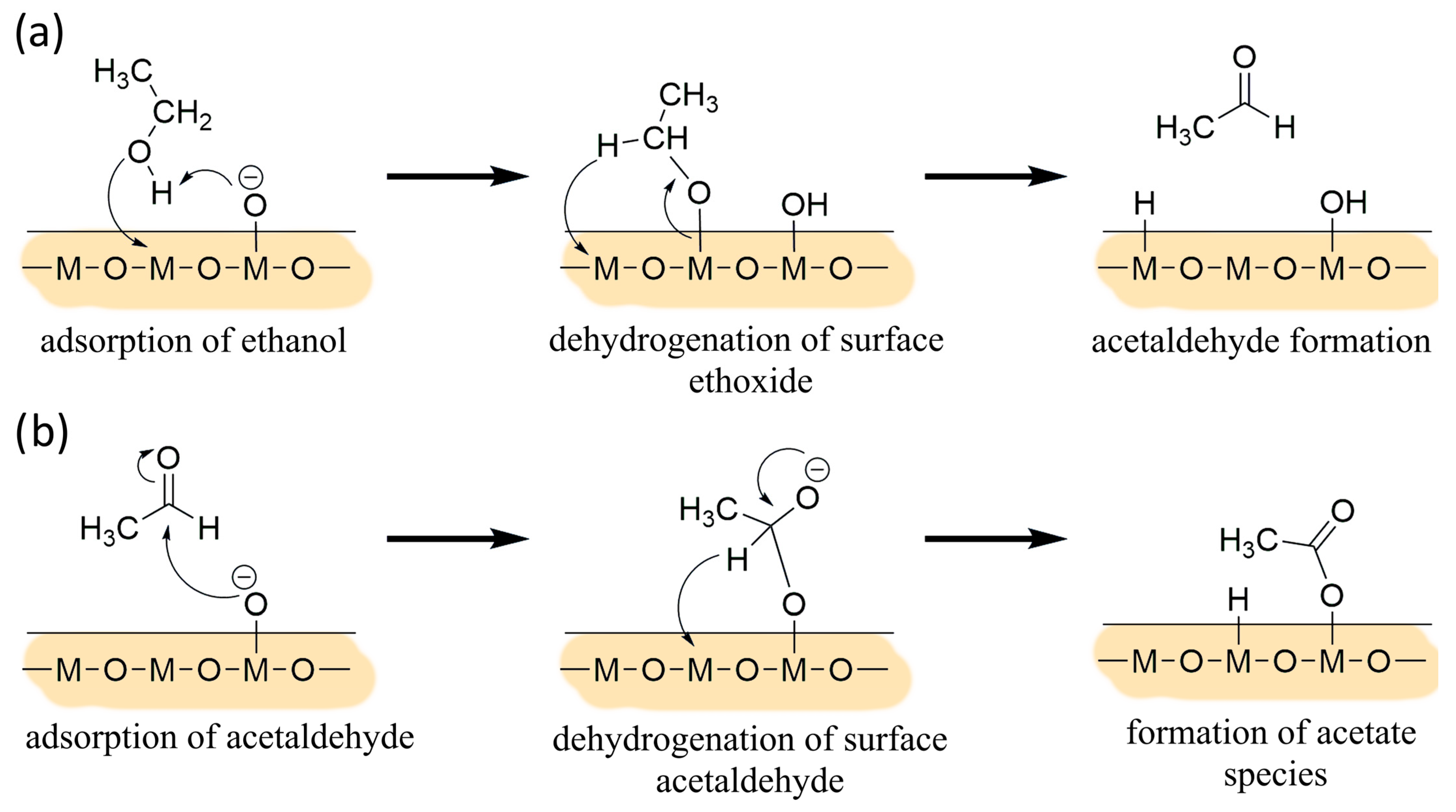
3.1. Results under Exposure of Ethanol
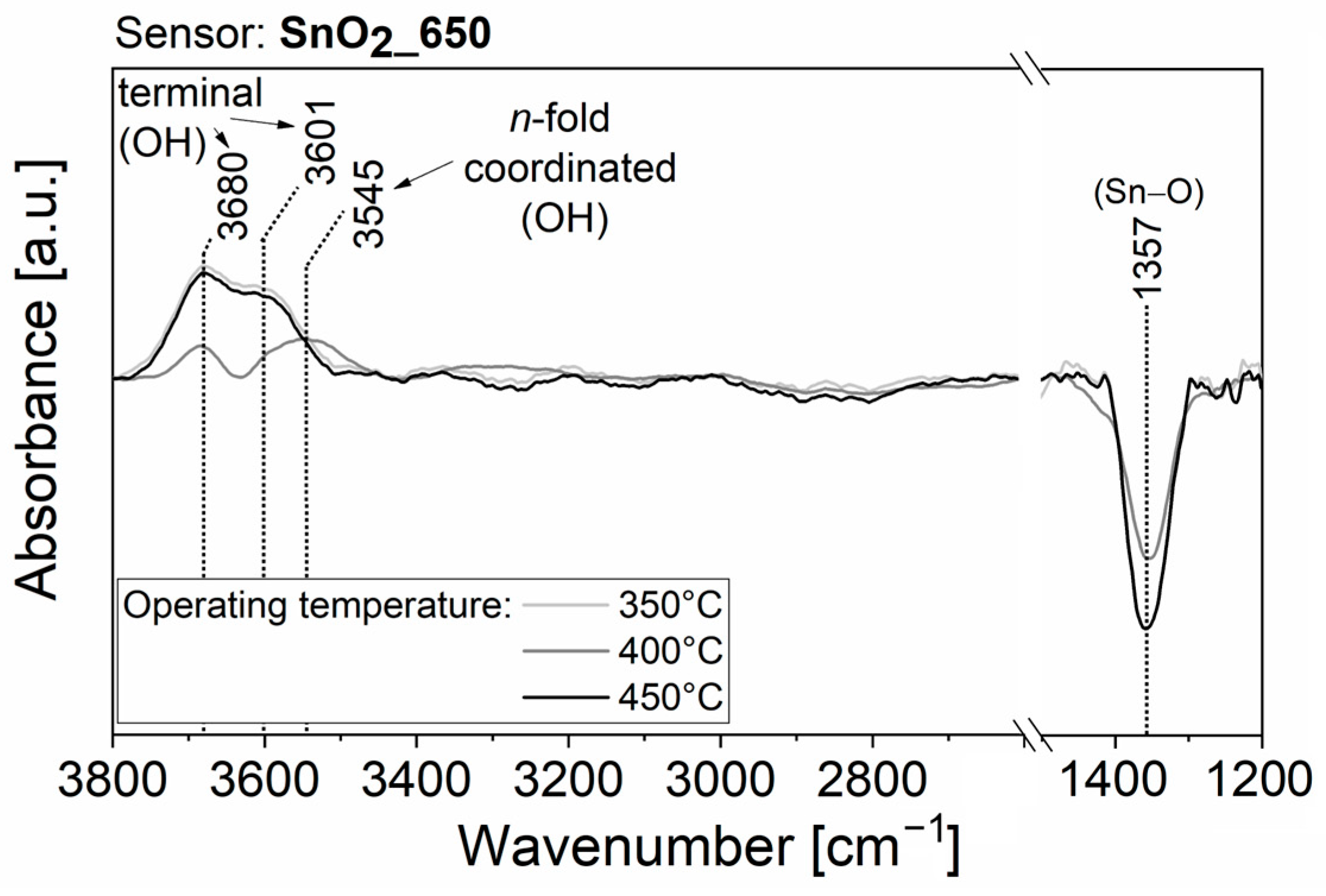
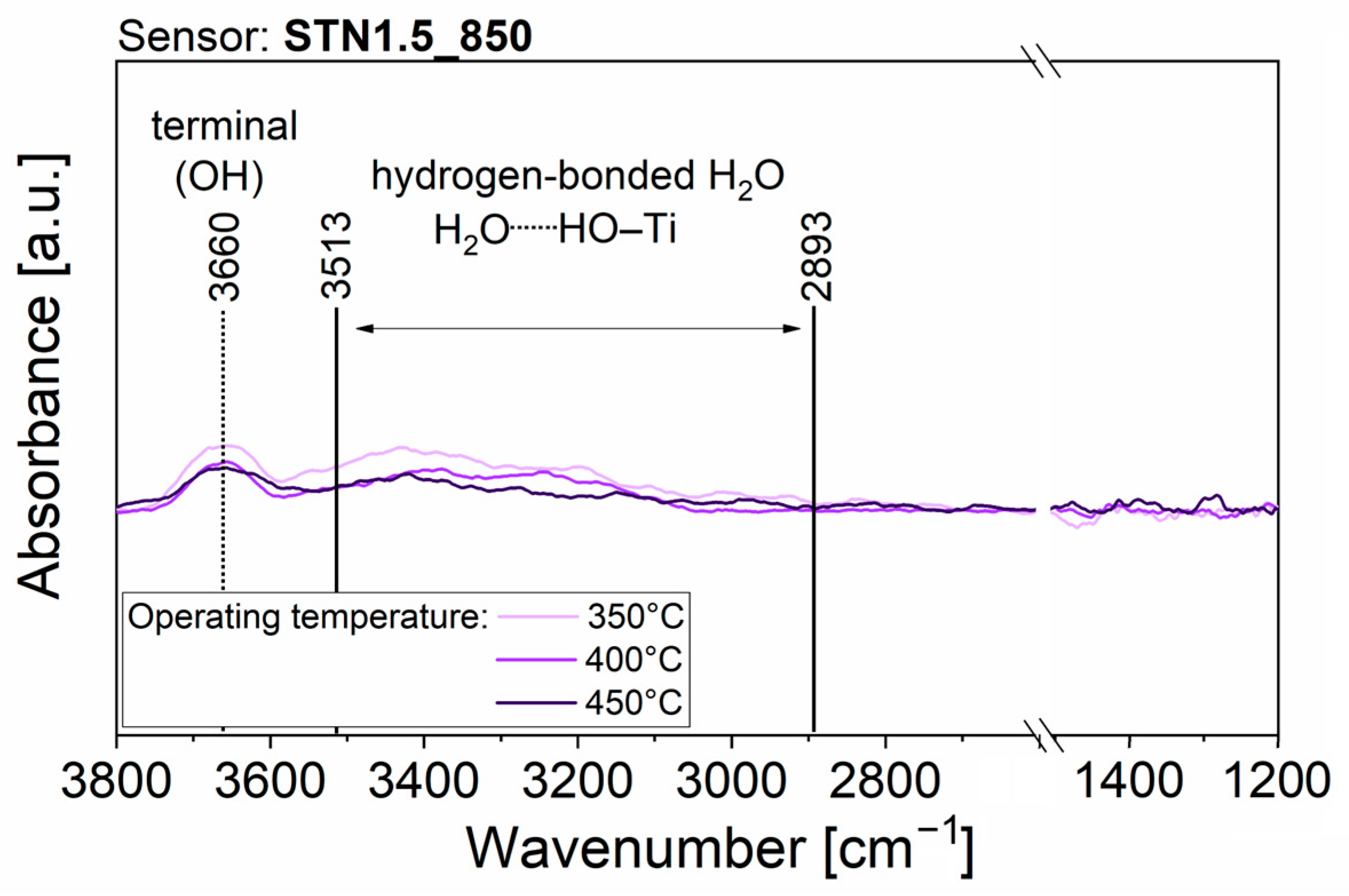
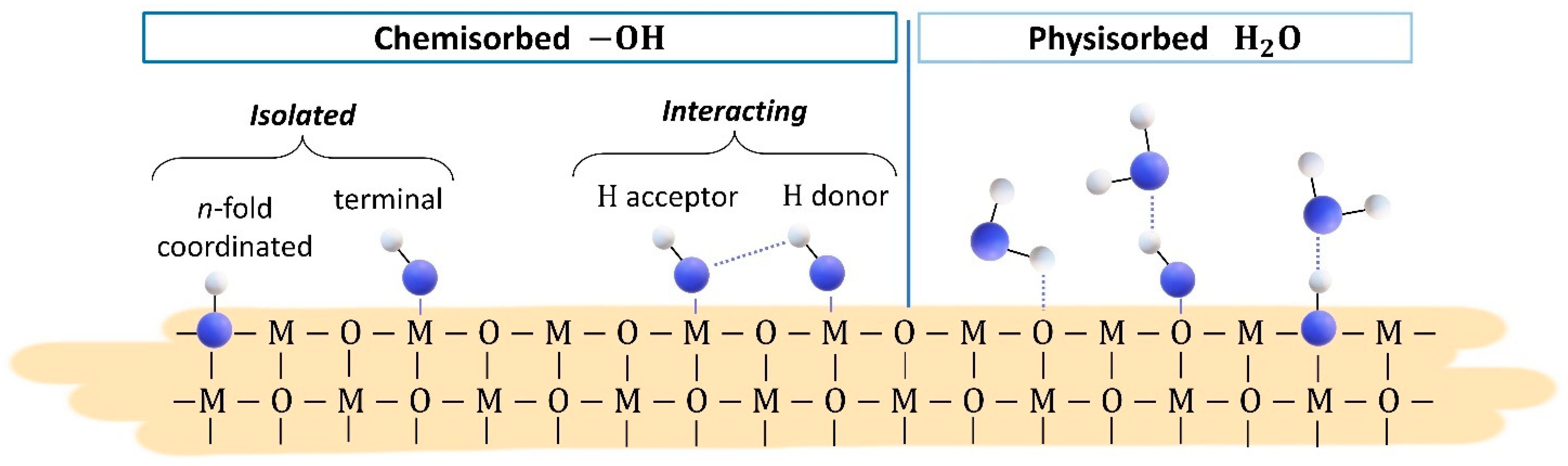
3.2. Results in Humid Environment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Staerz, A.; Weimar, U.; Barsan, N. Current State of Knowledge on the Metal Oxide Based Gas Sensing Mechanism. Sens. Actuators B Chem. 2022, 358, 131531. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal Oxide-Based Gas Sensor Research: How To? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Ciana, M.D.; Valt, M.; Fabbri, B.; Gaiardo, A.; Spagnoli, E.; Krik, S.; Bernardoni, P.; Gilli, N.; Migliori, A.; Quaranta, A.; et al. SO2 Sensing Mechanism of Nanostructured SiC-SiOxC Core Shell: An Operando DRIFT Investigation. Sens. Actuators B Chem. 2022, 371, 132497. [Google Scholar] [CrossRef]
- Benítez, J.J.; Centeno, M.A.; Merdrignac, O.M.; Guyader, J.; Laurent, Y.; Odriozola, J.A. DRIFTS Chamber for in Situ and Simultaneous Study of Infrared and Electrical Response of Sensors. Appl. Spectrosc. 1995, 49, 1094–1096. [Google Scholar] [CrossRef]
- Großmann, K.; Wicker, S.; Weimar, U.; Barsan, N. Impact of Pt Additives on the Surface Reactions between SnO2, Water Vapour, CO and H2; an Operando Investigation. Phys. Chem. Chem. Phys. 2013, 15, 19151. [Google Scholar] [CrossRef]
- Spagnoli, E.; Fabbri, B.; Gaiardo, A.; Valt, M.; Ardit, M.; Krik, S.; Cruciani, G.; Della Ciana, M.; Vanzetti, L.; Vola, G.; et al. Design of a Metal-Oxide Solid Solution for Selective Detection of Ethanol with Marginal Influence by Humidity. Sens. Actuators B Chem. 2022, 370, 132426. [Google Scholar] [CrossRef]
- Peris, M.; Escuder-Gilabert, L. On-Line Monitoring of Food Fermentation Processes Using Electronic Noses and Electronic Tongues: A Review. Anal. Chim. Acta 2013, 804, 29–36. [Google Scholar] [CrossRef]
- Liewhiran, C.; Camenzind, A.; Teleki, A.; Pratsinis, S.E.; Phanichphant, S. High Performance Ethanol Sensor for Control Drunken Driving Based on Flame-Made ZnO Nanoparticles. In Proceedings of the 2007 2nd IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Bangkok, Thailand, 16–19 January 2007; IEEE: Piscataway, NJ, USA, 2007; pp. 672–677. [Google Scholar]
- Tricoli, A.; Righettoni, M.; Pratsinis, S.E. Minimal Cross-Sensitivity to Humidity during Ethanol Detection by SnO2–TiO2 Solid Solutions. Nanotechnology 2009, 20, 315502. [Google Scholar] [CrossRef]
- Li, R.; Chen, S.; Lou, Z.; Li, L.; Huang, T.; Song, Y.; Chen, D.; Shen, G. Fabrication of Porous SnO2 Nanowires Gas Sensors with Enhanced Sensitivity. Sens. Actuators B Chem. 2017, 252, 79–85. [Google Scholar] [CrossRef]
- Zhang, L.; Tong, R.; Ge, W.; Guo, R.; Shirsath, S.E.; Zhu, J. Facile One-Step Hydrothermal Synthesis of SnO2 Microspheres with Oxygen Vacancies for Superior Ethanol Sensor. J. Alloys Compd. 2020, 814, 152266. [Google Scholar] [CrossRef]
- Spagnoli, E.; Krik, S.; Fabbri, B.; Valt, M.; Ardit, M.; Gaiardo, A.; Vanzetti, L.; Della Ciana, M.; Cristino, V.; Vola, G.; et al. Development and Characterization of WO3 Nanoflakes for Selective Ethanol Sensing. Sens. Actuators B Chem. 2021, 347, 130593. [Google Scholar] [CrossRef]
- Liu, T.; Liu, J.; Hao, Q.; Liu, Q.; Jing, X.; Zhang, H.; Huang, G.; Wang, J. Porous Tungsten Trioxide Nanolamellae with Uniform Structures for High-Performance Ethanol Sensing. CrystEngComm 2016, 18, 8411–8418. [Google Scholar] [CrossRef]
- Zeng, W.; Miao, B.; Li, T.; Zhang, H.; Hussain, S.; Li, Y.; Yu, W. Hydrothermal Synthesis, Characterization of h-WO3 Nanowires and Gas Sensing of Thin Film Sensor Based on This Powder. Thin Solid Films 2015, 584, 294–299. [Google Scholar] [CrossRef]
- Xu, W.; Qiu, C.; Zhou, J.; Chen, Y. Regulation of Specific Surface Area of 3D Flower-like WO3 Hierarchical Structures for Gas Sensing Application. Ceram. Int. 2020, 46, 11372–11378. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Wang, Y.; Li, X.; Cheng, P.; Zhao, Y.; Dang, F.; Zhang, Y. Hydrothermal Synthesis of Au@SnO2 Hierarchical Hollow Microspheres for Ethanol Detection. Sens. Actuators B Chem. 2020, 319, 128299. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Huang, D.; Wang, X.; Cai, L.; Chen, Y.; Wang, W.; Song, Y.; Han, G.; Zhen, B. A High-Performance Ethanol Gas Sensor Based on Ce-Doped SnO2 Nanomaterials Prepared by the Pechini Method. Mater. Sci. Semicond. Process. 2022, 137, 106188. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Li, T.; Li, Z.; Wu, W.; Zhou, C.; Sun, P.; Liu, F.; Yan, X.; Gao, Y.; et al. Highly Efficient Ethanol Gas Sensor Based on Hierarchical SnO2/Zn2SnO4 Porous Spheres. Sens. Actuators B Chem. 2019, 282, 339–346. [Google Scholar] [CrossRef]
- Liu, J.; Wang, T.; Wang, B.; Sun, P.; Yang, Q.; Liang, X.; Song, H.; Lu, G. Highly Sensitive and Low Detection Limit of Ethanol Gas Sensor Based on Hollow ZnO/SnO2 Spheres Composite Material. Sens. Actuators B Chem. 2017, 245, 551–559. [Google Scholar] [CrossRef]
- Fan, H.; Zheng, X.; Shen, Q.; Wang, W.; Dong, W. Hydrothermal Synthesis and Their Ethanol Gas Sensing Performance of 3-Dimensional Hierarchical Nano Pt/SnO2. J. Alloys Compd. 2022, 909, 164693. [Google Scholar] [CrossRef]
- Tan, J.; Dun, M.; Li, L.; Zhao, J.; Li, X.; Hu, Y.; Huang, G.; Tan, W.; Huang, X. Self-Template Derived CuO Nanowires Assembled Microspheres and Its Gas Sensing Properties. Sens. Actuators B Chem. 2017, 252, 1–8. [Google Scholar] [CrossRef]
- Ferroni, M.; Carotta, M.; Guidi, V.; Martinelli, G.; Ronconi, F.; Richard, O.; Van Dyck, D.; Van Landuyt, J. Structural Characterization of Nb–TiO2 Nanosized Thick-Films for Gas Sensing Application. Sens. Actuators B Chem. 2000, 68, 140–145. [Google Scholar] [CrossRef]
- Spagnoli, E.; Gaiardo, A.; Fabbri, B.; Valt, M.; Krik, S.; Ardit, M.; Cruciani, G.; Della Ciana, M.; Vanzetti, L.; Vola, G.; et al. Design of a Metal-Oxide Solid Solution for Sub-Ppm H2 Detection. ACS Sens. 2022, 7, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Chiorino, A.; Ghiotti, G.; Prinetto, F.; Carotta, M.C.; Malagù, C.; Martinelli, G. Preparation and Characterization of SnO2 and WOx–SnO2 Nanosized Powders and Thick Films for Gas Sensing. Sens. Actuators B Chem. 2001, 78, 89–97. [Google Scholar] [CrossRef]
- Valt, M.; Ciana, M.D.; Fabbri, B.; Sali, D.; Gaiardo, A.; Guidi, V. Design and Validation of a Novel Operando Spectroscopy Reaction Chamber for Chemoresistive Gas Sensors. Sens. Actuators B Chem. 2021, 341, 130012. [Google Scholar] [CrossRef]
- Carotta, M.C.; Gherardi, S.; Guidi, V.; Malagù, C.; Martinelli, G.; Vendemiati, B.; Sacerdoti, M.; Ghiotti, G.; Morandi, S. Electrical and Spectroscopic Properties of Ti0.2Sn0.8O2 Solid Solution for Gas Sensing. Thin Solid Films 2009, 517, 6176–6183. [Google Scholar] [CrossRef]
- Howard, C.J.; Sabine, T.M.; Dickson, F. Structural and Thermal Parameters for Rutile and Anatase. Acta Crystallogr. Sect. B Struct. Sci. 1991, 47, 462–468. [Google Scholar] [CrossRef]
- Yamanaka, T.; Kurashima, R.; Mimaki, J. X-Ray Diffraction Study of Bond Character of Rutile-Type SiO2, GeO2 and SnO2. Z. Krist. Cryst. Mater. 2000, 215, 424–428. [Google Scholar] [CrossRef]
- Rossi, A.; Spagnoli, E.; Tralli, F.; Marzocchi, M.; Guidi, V.; Fabbri, B. New Approach for the Detection of Sub-Ppm Limonene: An Investigation through Chemoresistive Metal-Oxide Semiconductors. Sensors 2023, 23, 6291. [Google Scholar] [CrossRef]
- Rossi, A.; Fabbri, B.; Spagnoli, E.; Gaiardo, A.; Valt, M.; Ferroni, M.; Ardit, M.; Krik, S.; Pedrielli, A.; Vanzetti, L.; et al. Functionalization of Indium Oxide for Empowered Detection of CO2 over an Extra-Wide Range of Concentrations. ACS Appl. Mater. Interfaces 2023. [Google Scholar] [CrossRef]
- Della Ciana, M.; Valt, M.; Fabbri, B.; Bernardoni, P.; Guidi, V.; Morandi, V. Development of a Dedicated Instrumentation for Electrical and Thermal Characterization of Chemiresistive Gas Sensors. Rev. Sci. Instrum. 2021, 92, 74702. [Google Scholar] [CrossRef] [PubMed]
- Hakkarainen, T.; Mikkola, E.; Laperre, J.; Gensous, F.; Fardell, P.; Le Tallec, Y.; Baiocchi, C.; Paul, K.; Simonson, M.; Deleu, C.; et al. Smoke Gas Analysis by Fourier Transform Infrared Spectroscopy-Summary of the SAFIR Project Results. Fire Mater. 2000, 24, 101–112. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Hess, C. Application of Transient Infrared Spectroscopy To Investigate the Role of Gold in Ethanol Gas Sensing over Au/SnO2. J. Phys. Chem. C 2022, 126, 3980–3992. [Google Scholar] [CrossRef]
- Boehme, I.; Weimar, U.; Barsan, N. Unraveling the Surface Chemistry of CO Sensing with In2O3 Based Gas Sensors. Sensors Actuators B Chem. 2021, 326, 129004. [Google Scholar] [CrossRef]
- Degler, D.; Wicker, S.; Weimar, U.; Barsan, N. Identifying the Active Oxygen Species in SnO2 Based Gas Sensing Materials: An Operando IR Spectrsocopy Study. J. Phys. Chem. C 2015, 119, 11792–11799. [Google Scholar] [CrossRef]
- Wicker, S.; Guiltat, M.; Weimar, U.; Hémeryck, A.; Barsan, N. Ambient Humidity Influence on CO Detection with SnO2 Gas Sensing Materials. A Combined DRIFTS/DFT Investigation. J. Phys. Chem. C 2017, 121, 25064–25073. [Google Scholar] [CrossRef]
- Panayotov, D.A.; Yates, J.T. Depletion of Conduction Band Electrons in TiO2 by Water Chemisorption-IR Spectroscopic Studies of the Independence of Ti-OH Frequencies on Electron Concentration. Chem. Phys. Lett. 2005, 410, 11–17. [Google Scholar] [CrossRef]
- Degler, D.; Junker, B.; Allmendinger, F.; Weimar, U.; Barsan, N. Investigations on the Temperature-Dependent Interaction of Water Vapor with Tin Dioxide and Its Implications on Gas Sensing. ACS Sens. 2020, 5, 3207–3216. [Google Scholar] [CrossRef]
- Khamfoo, K.; Staerz, A.; Boepple, M.; Wisitsoraat, A.; Liewhiran, C.; Weimar, U.; Barsan, N. Operando DRIFT Measurements on Flame-Spray-Made Zn2SnO4 Nanoparticles Based Environmental Sensors. Sensors Actuators B Chem. 2022, 371, 132495. [Google Scholar] [CrossRef]
- Tan, T.H.; Scott, J.; Ng, Y.H.; Taylor, R.A.; Aguey-Zinsou, K.F.; Amal, R. C-C Cleavage by Au/TiO2 during Ethanol Oxidation: Understanding Bandgap Photoexcitation and Plasmonically Mediated Charge Transfer via Quantitative in situ DRIFTS. ACS Catal. 2016, 6, 8021–8029. [Google Scholar] [CrossRef]
- Holz, M.C.; Tölle, K.; Muhler, M. Gas-Phase Oxidation of Ethanol over Au/TiO2 Catalysts to Probe Metal–Support Interactions. Catal. Sci. Technol. 2014, 4, 3495–3504. [Google Scholar] [CrossRef]
- Takeuchi, M.; Bertinetti, L.; Martra, G.; Coluccia, S.; Anpo, M. States of H2O Adsorbed on Oxides: An Investigation by near and Mid Infrared Spectroscopy. Appl. Catal. A Gen. 2006, 307, 13–20. [Google Scholar] [CrossRef]
- Anderson, J.H.; Wickersheim, K.A. Near Infrared Characterization of Water and Hydroxyl Groups on Silica Surfaces. Surf. Sci. 1964, 2, 252–260. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Nayak, A.K.; Ghosh, R.; Santra, S.; Guha, P.K.; Pradhan, D. Hierarchical Nanostructured WO3–SnO2 for Selective Sensing of Volatile Organic Compounds. Nanoscale 2015, 7, 12460–12473. [Google Scholar] [CrossRef]
- Jinkawa, T.; Sakai, G.; Tamaki, J.; Miura, N.; Yamazoe, N. Relationship between Ethanol Gas Sensitivity and Surface Catalytic Property of Tin Oxide Sensors Modified with Acidic or Basic Oxides. J. Mol. Catal. A Chem. 2000, 155, 193–200. [Google Scholar] [CrossRef]
- Idriss, H.; Seebauer, E.G. Reactions of Ethanol over Metal Oxides. J. Mol. Catal. A Chem. 2000, 152, 201–212. [Google Scholar] [CrossRef]
- Huang, R.; Fung, V.; Wu, Z.; Jiang, D. Understanding the Conversion of Ethanol to Propene on In2O3 from First Principles. Catal. Today 2020, 350, 19–24. [Google Scholar] [CrossRef]
- Mattos, L.V.; Jacobs, G.; Davis, B.H.; Noronha, F.B. Production of Hydrogen from Ethanol: Review of Reaction Mechanism and Catalyst Deactivation. Chem. Rev. 2012, 112, 4094–4123. [Google Scholar] [CrossRef]
- Guo, Y.; He, X.; Huang, W.; Wang, M. Microstructure Effects on Effective Gas Diffusion Coefficient of Nanoporous Materials. Transp. Porous Media 2019, 126, 431–453. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Aboul-Enein, H.Y. Vibrational Spectroscopy Applications in Drugs Analysis. In Encyclopedia of Spectroscopy and Spectrometry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 575–581. [Google Scholar]
- Egashira, M.; Nakashima, M.; Kawasumi, S.; Selyama, T. Temperature Programmed Desorption Study of Water Adsorbed on Metal Oxides. 2. Tin Oxide Surfaces. J. Phys. Chem. 1981, 85, 4125–4130. [Google Scholar] [CrossRef]
- Bandura, A.V.; Kubicki, J.D.; Sofo, J.O. Comparisons of Multilayer H2O Adsorption onto the (110) Surfaces of α-TiO2 and SnO2 as Calculated with Density Functional Theory. J. Phys. Chem. B 2008, 112, 11616–11624. [Google Scholar] [CrossRef]
- Carotta, M.C.; Cervi, A.; Gherardi, S.; Guidi, V.; Malagu’, C.; Martinelli, G.; Vendemiati, B.; Sacerdoti, M.; Ghiotti, G.; Morandi, S.; et al. (Ti, Sn)O2 Solid Solutions for Gas Sensing: A Systematic Approach by Different Techniques for Different Calcination Temperature and Molar Composition. Sens. Actuators B Chem. 2009, 139, 329–339. [Google Scholar] [CrossRef]
- Carotta, M.C.; Fioravanti, A.; Gherardi, S.; Malagù, C.; Sacerdoti, M.; Ghiotti, G.; Morandi, S. (Ti,Sn) Solid Solutions as Functional Materials for Gas Sensing. Sens. Actuators B Chem. 2014, 194, 195–205. [Google Scholar] [CrossRef]
- Wei, S.; Xing, Y.; Li, Y.; Zhao, Y.; Du, W.; Zhou, M. Preparation and Gas Sensing Properties of Flower-like WO3 Hierarchical Architecture. Vacuum 2016, 129, 13–19. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Yan, X.; Zhou, P.; Yin, Y.; Lu, R.; Han, C.; Cui, B.; Wei, D. Complex-Surfactant-Assisted Hydrothermal Synthesis of One-Dimensional ZnO Nanorods for High-Performance Ethanol Gas Sensor. Sens. Actuators B Chem. 2019, 286, 501–511. [Google Scholar] [CrossRef]


| Sensor Label | Sn:Ti:Nb Molar Ratio | Calcination Temperature |
|---|---|---|
| SnO2_650 | 100:0:0 | 650 °C |
| ST30_650 | 70:30:0 | 650 °C |
| STN1.5_650 | 69.0:29.5:1.5 | 650 °C |
| STN1.5_850 | 69.0:29.5:1.5 | 850 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spagnoli, E.; Valt, M.; Gaiardo, A.; Fabbri, B.; Guidi, V. Insights into the Sensing Mechanism of a Metal-Oxide Solid Solution via Operando Diffuse Reflectance Infrared Fourier Transform Spectroscopy. Nanomaterials 2023, 13, 2708. https://doi.org/10.3390/nano13192708
Spagnoli E, Valt M, Gaiardo A, Fabbri B, Guidi V. Insights into the Sensing Mechanism of a Metal-Oxide Solid Solution via Operando Diffuse Reflectance Infrared Fourier Transform Spectroscopy. Nanomaterials. 2023; 13(19):2708. https://doi.org/10.3390/nano13192708
Chicago/Turabian StyleSpagnoli, Elena, Matteo Valt, Andrea Gaiardo, Barbara Fabbri, and Vincenzo Guidi. 2023. "Insights into the Sensing Mechanism of a Metal-Oxide Solid Solution via Operando Diffuse Reflectance Infrared Fourier Transform Spectroscopy" Nanomaterials 13, no. 19: 2708. https://doi.org/10.3390/nano13192708
APA StyleSpagnoli, E., Valt, M., Gaiardo, A., Fabbri, B., & Guidi, V. (2023). Insights into the Sensing Mechanism of a Metal-Oxide Solid Solution via Operando Diffuse Reflectance Infrared Fourier Transform Spectroscopy. Nanomaterials, 13(19), 2708. https://doi.org/10.3390/nano13192708








