Eu3+ Complex-Based Superhydrophobic Fluorescence Sensor for Cr(VI) Detection in Water
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Eu3+ Complex
2.2. Synthesis of Silica Particles
2.3. Electrospinning Nanofibrous Membranes
3. Characterizations
Cr(VI) Sensing with Aqueous Droplets
4. Results and Discussion
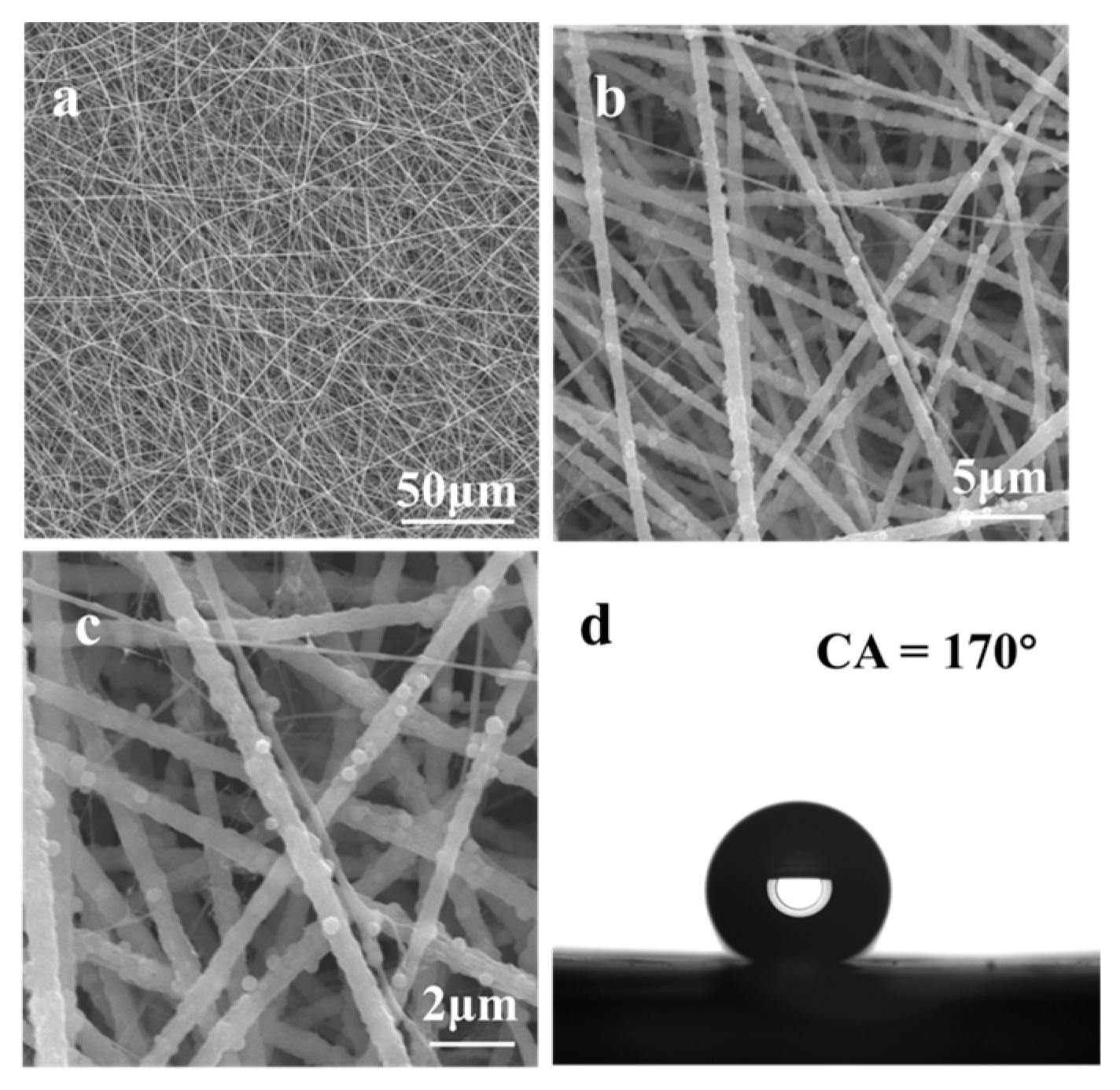
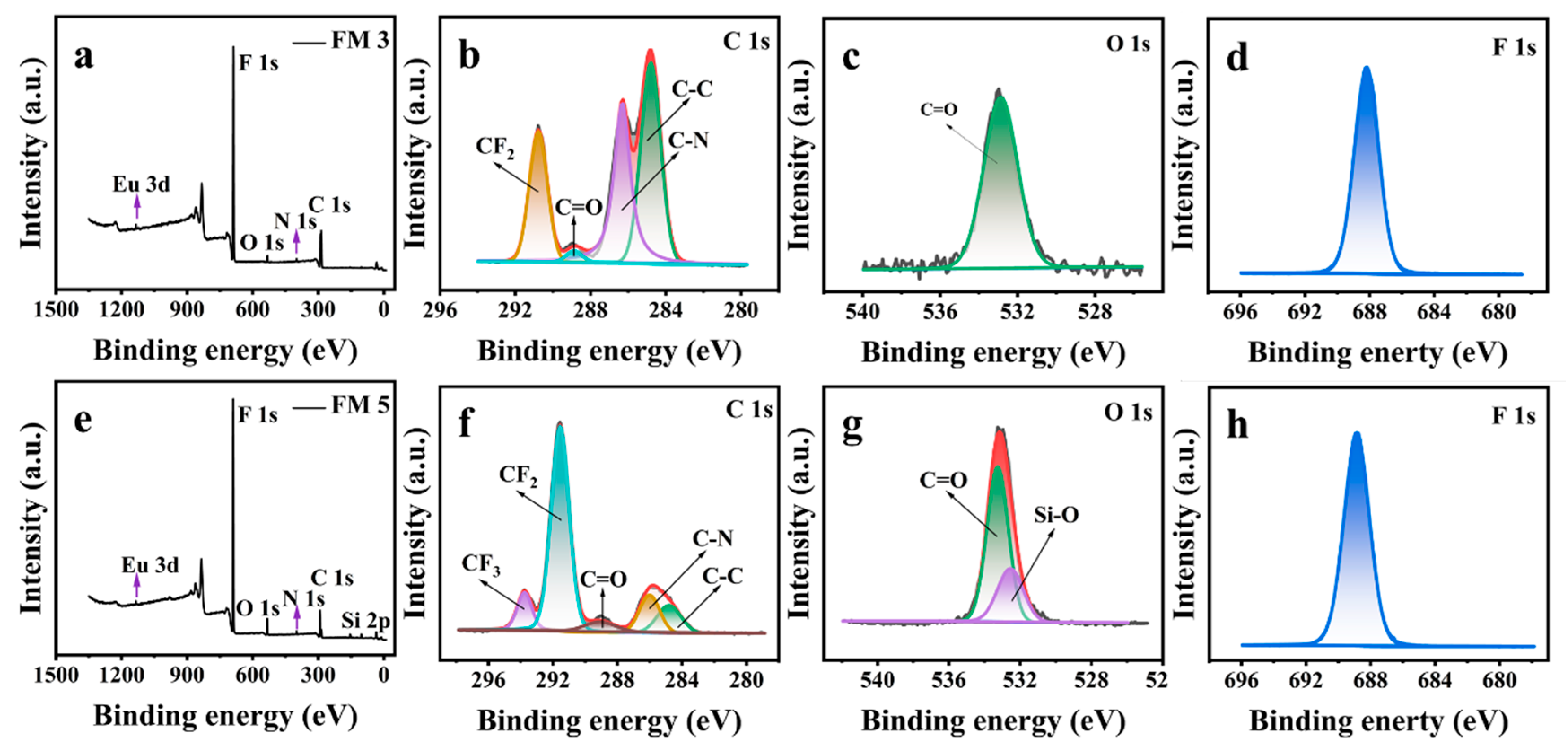
4.1. Surface Morphology, Surface Chemistry, and Water Repellency of Cr(VI) Sensor
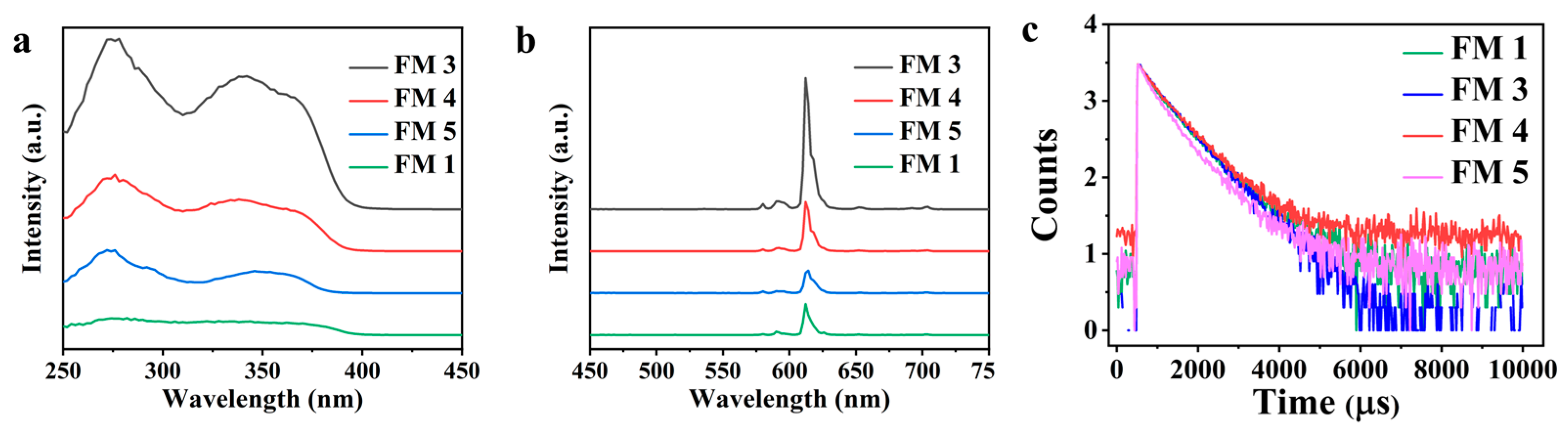
4.2. Photophysical Properties of Cr(VI) Sensor
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, Z.; Yang, M.; Ma, Y.; Li, L. Multi-Responsive Luminescent Sensors Based on Two-Dimensional Lanthanide–Metal Organic Frameworks for Highly Selective and Sensitive Detection of Cr(III) and Cr(VI) Ions and Benzaldehyde. Cryst. Growth Des. 2017, 17, 4326–4335. [Google Scholar] [CrossRef]
- Zhou, A.-M.; Wei, H.; Gao, W.; Liu, J.-P.; Zhang, X.-M. Two 2D multiresponsive luminescence coordination polymers for selective sensing of Fe3+, CrVI anions and TNP in aqueous medium. CrystEngComm 2019, 21, 5185–5194. [Google Scholar] [CrossRef]
- Dutta, A.; Mahapatra, M.; Deb, M.; Mitra, M.; Dutta, S.; Chattopadhyay, P.K.; Banerjee, S.; Sil, P.C.; Maiti, D.K.; Singha, N.R. Fluorescent Terpolymers Using Two Non-Emissive Monomers for Cr(III) Sensors, Removal, and Bio-Imaging. ACS Biomater. Sci. Eng. 2020, 6, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.Y.; Dai, M.; Young, D.J.; Ren, Z.G.; Lang, J.P. Luminescent Zn(II) Coordination Polymers for Highly Selective Sensing of Cr(III) and Cr(VI) in Water. Inorg. Chem. 2017, 56, 4669–4679. [Google Scholar] [PubMed]
- Singh, M.; Senthilkumar, S.; Rajput, S.; Neogi, S. Pore-Functionalized and Hydrolytically Robust Cd(II)-Metal-Organic Framework for Highly Selective, Multicyclic CO2 Adsorption and Fast-Responsive Luminescent Monitoring of Fe(III) and Cr(VI) Ions with Notable Sensitivity and Reusability. Inorg. Chem. 2020, 59, 3012–3025. [Google Scholar] [CrossRef]
- World Health Organization. Chromium in Drinking-Water; World Health Organization: Geneva, Switzerland, 2020.
- Li, P.; Yin, X.-M.; Gao, L.-L.; Yang, S.-L.; Sui, Q.; Gong, T.; Gao, E.-Q. Modulating Excitation Energy of Luminescent Metal–Organic Frameworks for Detection of Cr(VI) in Water. ACS Appl. Nano Mater. 2019, 2, 4646–4654. [Google Scholar] [CrossRef]
- Lv, R.; Wang, J.; Zhang, Y.; Li, H.; Yang, L.; Liao, S.; Gu, W.; Liu, X. An amino-decorated dual-functional metal–organic framework for highly selective sensing of Cr(iii) and Cr(vi) ions and detection of nitroaromatic explosives. J. Mater. Chem. A 2016, 4, 15494–15500. [Google Scholar] [CrossRef]
- Aleem, A.R.; Ding, W.; Liu, J.; Li, T.; Guo, Y.; Wang, Q.; Wang, Y.; Wang, Y.; Rehman, F.U.L.; Kipper, M.J.; et al. Visible-light excitable Eu(3+)-induced hyaluronic acid-chitosan aggregates with heterocyclic ligands for sensitive and fast recognition of hazardous ions. Int. J. Biol. Macromol. 2021, 184, 188–199. [Google Scholar] [CrossRef]
- Lin, D.; Wu, J.; Wang, M.; Yan, F.; Ju, H. Triple signal amplification of graphene film, polybead carried gold nanoparticles as tracing tag and silver deposition for ultrasensitive electrochemical immunosensing. Anal. Chem. 2012, 84, 3662–3668. [Google Scholar] [CrossRef]
- Prakash, A.; Chandra, S.; Bahadur, D. Structural, magnetic, and textural properties of iron oxide-reduced graphene oxide hybrids and their use for the electrochemical detection of chromium. Carbon 2012, 50, 4209–4219. [Google Scholar] [CrossRef]
- Wang, C.-X.; Xia, Y.-P.; Yao, Z.-Q.; Xu, J.; Chang, Z.; Bu, X.-H. Two luminescent coordination polymers as highly selective and sensitive chemosensors for Cr VI-anions in aqueous medium. Dalton Trans. 2019, 48, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Aleem, A.R.; Liu, J.; Wang, J.; Wang, J.; Zhao, Y.; Wang, Y.; Wang, Y.; Wang, W.; Rehman, F.U.L.; Kipper, M.J.; et al. Selective Sensing of Cu2+ and Fe3+ Ions with Vis-Excitation using Fluorescent Eu3+-Induced Aggregates of Polysaccharides (EIAP) in Mammalian Cells and Aqueous Systems. J. Hazard. Mater. 2020, 399, 122991. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; Wang, J.; Wang, Y.; Cao, J.; Hou, L.; Ge, R.; Chi, J.; Huang, L.; Guo, J.; et al. Smart sensing of Cu2+ in living cells by water-soluble and nontoxic Tb3+/Eu3+-induced aggregates of polysaccharides through fluorescence imaging. J. Mater. Chem. C 2020, 8, 8171–8182. [Google Scholar] [CrossRef]
- Alqarni, S.A. A Review on Conducting Polymers for Colorimetric and Fluorescent Detection of Noble Metal Ions (Ag+, Pd2+, Pt2+/4+, and Au3+). Crit. Rev. Anal. Chem. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Das, D.D. Recent developments in fluorescent sensors for trace-level determination of toxic-metal ions. TrAC Trends Anal. Chem. 2012, 32, 113–132. [Google Scholar]
- Chang, H.C.; Ho, J.A. Gold Nanocluster-Assisted Fluorescent Detection for Hydrogen Peroxide and Cholesterol Based on the Inner Filter Effect of Gold Nanoparticles. Anal. Chem. 2015, 87, 10362–10367. [Google Scholar] [CrossRef]
- Zheng, M.; Xie, Z.; Qu, D.; Li, D.; Du, P.; Jing, X.; Sun, Z. On-off-on fluorescent carbon dot nanosensor for recognition of chromium(VI) and ascorbic acid based on the inner filter effect. ACS Appl. Mater. Interfaces 2013, 5, 13242–13247. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Lian, X.; Yan, B. A dual-functional bimetallic-organic framework nanosensor for detection and decontamination of lachrymator in drinking water. Sens. Actuators B Chem. 2019, 281, 168–174. [Google Scholar] [CrossRef]
- Qu, X.-L.; Yan, B. Stable Tb (III)-based metal–organic framework: Structure, photoluminescence, and chemical sensing of 2-thiazolidinethione-4-carboxylic acid as a biomarker of CS2. Inorg. Chem. 2018, 58, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, B. A portable self-calibrating logic detector for gradient detection of formaldehyde based on luminescent metal organic frameworks. J. Mater. Chem. C 2019, 7, 5652–5657. [Google Scholar] [CrossRef]
- Weng, G.; Thanneeru, S.; He, J. Dynamic coordination of Eu–iminodiacetate to control fluorochromic response of polymer hydrogels to multistimuli. Adv. Mater. 2018, 30, 1706526. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef]
- Gao, E.; Wu, S.; Wang, J.; Zhu, M.; Zhang, Y.; Fedin, V.P. Water-Stable Lanthanide Coordination Polymers with Triple Luminescent Centers for Tunable Emission and Efficient Self-Calibration Sensing Wastewater Pollutants. Adv. Opt. Mater. 2020, 8, 1901659. [Google Scholar] [CrossRef]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water stability and adsorption in metal-organic frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Keser Demir, N.; Chen, J.P.; Li, K. Applications of water stable metal-organic frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, Q.; Li, C.; Sun, L.; Li, F. Iridium(III) complex-coated nanosystem for ratiometric upconversion luminescence bioimaging of cyanide anions. J. Am. Chem. Soc. 2011, 133, 15276–15279. [Google Scholar] [CrossRef]
- Tan, L.; Li, Y.; Wu, X.; Liu, W.; Peng, Z.; Dong, Y.; Huang, Z.; Zhang, L.; Liang, Y. Fluorescent sensor array based on Janus silica nanoflakes to realize pattern recognition of multiple aminoglycoside antibiotics and heavy metal ions. Sens. Actuators B Chem. 2023, 378, 133154. [Google Scholar] [CrossRef]
- Melnikov, A.G.; Bykov, D.A.; Varezhnikov, A.S.; Sysoev, V.V.; Melnikov, G.V. Toward a Selective Analysis of Heavy Metal Salts in Aqueous Media with a Fluorescent Probe Array. Sensors 2022, 22, 1465. [Google Scholar] [CrossRef]
- Amin, Z.; Rauf, T.; Jan, Q.; Kuchey, M.Y.; Sofi, F.A.; Ismail, T.; Rashid, A.; Bhat, B.A.; Sidiq, N.; Bhat, M.A. Synthesis of a Novel Hydrazone Functionality based Spectrophotometric Probe for Selective and Sensitive Estimation of Toxic Heavy Metal Ions. ChemistrySelect 2023, 8, e202202632. [Google Scholar] [CrossRef]
- Spanu, D.; Monticelli, D.; Binda, G.; Dossi, C.; Rampazzi, L.; Recchia, S. One-minute highly selective Cr(VI) determination at ultra-trace levels: An ICP-MS method based on the on-line trapping of Cr(III). J. Hazard. Mater. 2021, 412, 125280. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shang, M.; Wei, H.; Zhang, M.; Zou, W.; Meng, X.; Chen, W.; Shao, H.; Lai, Y. Specific and sensitive on-site detection of Cr(VI) by surface-enhanced Raman spectroscopy. Sens. Actuators B Chem. 2021, 346, 130594. [Google Scholar] [CrossRef]
- Ezebuiro, P.; Gandhi, J.; Zhang, C.L.; Mathew, J.; Ritter, M.; Humphrey, M. Optimal Sample Preservation and Analysis of Cr(VI) in Drinking Water Samples by High Resolution Ion Chromatography Followed by Post Column Reaction and UV/Vis Detection. J. Anal. Sci. Methods Instrum. 2012, 2, 74–80. [Google Scholar] [CrossRef]
- Wang, W.; Sun, J.; Vallabhuneni, S.; Pawlowski, B.; Vahabi, H.; Nellenbach, K.; Brown, A.C.; Scholle, F.; Zhao, J.; Kota, A.K. On-demand, remote and lossless manipulation of biofluid droplets. Mater. Horiz. 2022, 9, 2863–2871. [Google Scholar] [CrossRef]
- Vallabhuneni, S.; Movafaghi, S.; Wang, W.; Kota, A.K. Superhydrophobic coatings for improved performance of electrical insulators. Macromol. Mater. Eng. 2018, 303, 1800313. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Liu, J.I. Strong luminescence and sharp heavy metal ion sensitivity of water-soluble hybrid polysaccharide nanoparticles with Eu 3+ and Tb 3+ inclusions. Appl. Nanosci. 2019, 9, 1833–1844. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Tang, J.; Wang, W.; Wang, J.; Belfiore, L.A. Strong enhancement effect of silver nanowires on fluorescent property of Eu3+-ligand complexes and desired fluorescent iPP composite materials. Opt. Mater. 2017, 66, 17–22. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, H. Hybrid materials based on lanthanide organic complexes: A review. Chem. Soc. Rev. 2013, 42, 387–410. [Google Scholar] [CrossRef]
- Shahi, P.K.; Singh, A.K.; Singh, S.K.; Rai, S.B.; Ullrich, B. Revelation of the Technological Versatility of the Eu(TTA)3Phen Complex by Demonstrating Energy Harvesting, Ultraviolet Light Detection, Temperature Sensing, and Laser Applications. ACS Appl. Mater. Interfaces 2015, 7, 18231–18239. [Google Scholar] [CrossRef]
- Freslon, S.; Luo, Y.; Daiguebonne, C.; Calvez, G.; Bernot, K.; Guillou, O. Brightness and Color Tuning in a Series of Lanthanide-Based Coordination Polymers with Benzene-1,2,4,5-tetracarboxylic Acid as a Ligand. Inorg. Chem. 2016, 55, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Li, H.; Mujtaba Mari, G.; Yu, X.; Yu, W.; Wen, K.; Ke, Y.; Shen, J.; Wang, Z. Fluorescence immunoassay based on the inner-filter effect of carbon dots for highly sensitive amantadine detection in foodstuffs. Food Chem. 2019, 294, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Guo, X.Y.; Dong, Z.P.; Liu, Z.L.; Wang, Y.Q. 3D Ln(III)-MOFs: Slow magnetic relaxation and highly sensitive luminescence detection of Fe(3+) and ketones. Dalton Trans. 2018, 47, 8972–8982. [Google Scholar] [CrossRef] [PubMed]


| Sample | τobs (μs) | ARAD (s−1) | ANR (s−1) | ΦEu (%) | Φtot (%) |
|---|---|---|---|---|---|
| FM 1 | 720.04 | 372.25 | 1011.5 | 26.90 | 66.02 |
| FM 3 | 700.84 | 683.55 | 743.3 | 47.91 | 45.61 |
| FM 4 | 726.32 | 628.44 | 760.4 | 45.25 | 33.74 |
| FM 5 | 626.30 | 476.27 | 952.8 | 29.83 | 27.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Vallabhuneni, S.; Liu, J.; Wang, X.; Zhao, Y.; Wang, Y.; Tang, Q.; Wang, Y.; Zhang, X.; Kota, A.K.; et al. Eu3+ Complex-Based Superhydrophobic Fluorescence Sensor for Cr(VI) Detection in Water. Nanomaterials 2023, 13, 2574. https://doi.org/10.3390/nano13182574
Ding W, Vallabhuneni S, Liu J, Wang X, Zhao Y, Wang Y, Tang Q, Wang Y, Zhang X, Kota AK, et al. Eu3+ Complex-Based Superhydrophobic Fluorescence Sensor for Cr(VI) Detection in Water. Nanomaterials. 2023; 13(18):2574. https://doi.org/10.3390/nano13182574
Chicago/Turabian StyleDing, Wei, Sravanthi Vallabhuneni, Jin Liu, Xinzhi Wang, Yue Zhao, Yao Wang, Qinglin Tang, Yanxin Wang, Xiaolin Zhang, Arun Kumar Kota, and et al. 2023. "Eu3+ Complex-Based Superhydrophobic Fluorescence Sensor for Cr(VI) Detection in Water" Nanomaterials 13, no. 18: 2574. https://doi.org/10.3390/nano13182574
APA StyleDing, W., Vallabhuneni, S., Liu, J., Wang, X., Zhao, Y., Wang, Y., Tang, Q., Wang, Y., Zhang, X., Kota, A. K., & Tang, J. (2023). Eu3+ Complex-Based Superhydrophobic Fluorescence Sensor for Cr(VI) Detection in Water. Nanomaterials, 13(18), 2574. https://doi.org/10.3390/nano13182574







