Abstract
Formaldehyde, as a harmful gas produced by materials used for decorative purposes, has a serious impact on human health, and is also the focus and difficulty of indoor environmental polution prevention; hence, designing and developing gas sensors for the selective measurement of formaldehyde at room temperature is an urgent task. Herein, a series of SnS2/SnO2 composites with hollow spherical structures were prepared by a facile hydrothermal approach for the purpose of formaldehyde sensing at room temperature. These novel hierarchical structured SnS2/SnO2 composites−based gas sensors demonstrate remarkable selectivity towards formaldehyde within the concentration range of sub-ppm (0.1 ppm) to ppm (10 ppm) at room temperature. Notably, the SnS2/SnO2−2 sensor exhibits an exceptional formaldehyde-sensing performance, featuring an ultra-high response (1.93, 0.1 ppm and 17.51, 10 ppm), as well as good repeatability, long-term stability, and an outstanding theoretical detection limit. The superior sensing capabilities of the SnS2/SnO2 composites can be attributed to multiple factors, including enhanced formaldehyde adsorption, larger specific surface area and porosity of the hollow structure, as well as the synergistic interfacial incorporation of the SnS2/SnO2 heterojunction. Overall, the excellent gas sensing performance of SnS2/SnO2 hollow spheres has opened up a new way for their detection of trace formaldehyde at room temperature.
1. Introduction
Formaldehyde in indoor environments mainly comes from plywood, density board, paint, etc., in decoration materials. Due to the large use of urea formaldehyde resin adhesive materials, formaldehyde pollution in indoor air is aggravated [1], which causes serious health concerns. Even low concentrations of formaldehyde can cause breathing difficulties and headaches in humans [2]. More importantly, high concentrations of formaldehyde can disrupt the central nervous system and immune system and cause respiratory diseases and blindness [3]. Notably, multiple medical organizations and institutions around the world have proposed that formaldehyde’s maximum permissible occupational exposure concentration is 0.75 ppm over 8 h and 2 ppm over 15 min [4]; therefore, accurate detection of trance formaldehyde is highly desirable for human health.
Currently, many analytical technologies, e.g., electrochemistry [5], chromatography [6], fluorescence [7], and spectrophotometry [8], are available for detecting formaldehyde; however, they need to overcome the drawbacks of large equipment size and expensive and complicated operation, which limit their application in daily life. Under these circumstances, metal oxide semiconductors (MOS) have attracted great attention as gas sensors because of their simple operation, easy fabrication, and real-time monitoring [9,10], which have become a very effective means of formaldehyde gas detection. In the past five years, different types of MOS materials, such as ZnO [11], SnO2 [12], NiO [13], In2O3 [14], and CuO [15], have been used to fabricate gas sensors and offer good sensitivity to formaldehyde. Among them, n-type tin dioxide (SnO2) has attracted widespread attention for monitoring various reduced or oxide gases due to its excellent thermal stability [16], compatibility, and multiple morphologies [17,18]. Research has shown that SnO2 could be a promising formaldehyde gas-sensing material. Nonetheless, the commonly used SnO2 sensor shows low sensitivity and poor selectivity and usually works under high-power consumption limited to fulfilling the demands of integration and intelligence; therefore, room-temperature operating sensing materials with the capability to detect formaldehyde are being intensively explored to meet the requirement for portable or Internet of Things applications [19].
In recent years, many published studies have demonstrated that the three-dimensional (3D) hierarchical micro-nano structures of SnO2 possess numerous active sites and abundant mesopores. These structural features facilitate rapid gas diffusion and adsorption of gas molecules on the surface, thereby imparting excellent gas sensing properties [20,21,22]; however, characterizing the gas-sensing capabilities of these structures at room temperature presents a significant challenge. Recently, researchers have made considerable efforts to address this issue by constructing heterostructures using one or more different materials. These heterojunctions have been shown to have a noticeable impact on adjusting the intrinsic electronic properties of SnO2, thereby achieving an outstanding gas-sensing performance [23,24,25,26]. Particularly, tin disulfide (SnS2), as a representative Sn-based materials, has attracted much attention from researchers as an important platform for heterostructure-sensing applications owing to its layered structure, appropriate mid-bandgap, high surface activity, easy surface functionalization, and large electronegativity [27]. The SnO2 band is more positive than the SnS2 band, resulting in the photoexcitation of electrons and their movement from the SnS2 conduction band to SnO2 until the equilibrium of the Fermi level is reached. This process forms n–n heterojunctions. Since the chemical and physical properties of SnO2 and SnS2 are very similar, they can interact well at the interface. The high electron mobility through the phases promotes the adsorption of chemical species on the material’s surface, including the chemisorption of oxygen species, thereby improving the sensing properties. Along this line, some previous works have recently reported constructing SnO2/SnS2 heterojunctions for detecting toxic gas. For example, hollow core-shell-structured SnO2@SnS2 [28] and SnS2/SnO2 heterojunctions [29] both showed a superior response to NO2. Meanwhile, the response value of a flexible NH3 gas sensor based on SnO2/SnS2 is twice that of pure SnO2 at room temperature [30]. Inspired by the studies above, it is proposed that the construction of SnS2/SnO2 heterojunctions on SnO2 micro-nanostructures presents a promising strategy to overcome the bottleneck of insensitivity at room temperature. Nevertheless, to date, there has been a lack of research on the formaldehyde-sensing performances of SnS2/SnO2 composites at room temperature. Additionally, it remains a significant challenge to achieve well-defined SnS2/SnO2 composites with consistent micro-nanostructures. Furthermore, further investigation is needed to understand the sensing mechanism of SnS2/SnO2 composites, which holds more relevance in this field.
In this study, well-designed SnS2/SnO2 composites with hollow spherical structures were constructed for selective sensing of formaldehyde at room temperature, and they exhibit an ultra-high sensing response, good repeatability and stability, and ppb detection limit at room temperature. More interestingly, the SnS2/SnO2 composites have extraordinary formaldehyde selectivity, demonstrating almost no response for several common interfering gases. Density functional theory (DFT) simulations showed that the SnS2/SnO2 composites could effectively enhance the adsorption energy toward formaldehyde, contributing to its excellent selectivity. Moreover, the n–n heterojunction formed between SnO2 and SnS2 boosts the potential barrier at the interface, and the unique hollow structures bring more active sites along the material’s surface, thereby improving the sensing response. This work demonstrates that constructing SnS2/SnO2 heterojunctions on the SnO2 hierarchical structure efficiently develops next-generation room-temperature gas sensors with an improved sensing performance.
2. Experimental
2.1. Materials and Chemicals
The chemicals used in the experiment, including tin (IV) chloride pentahydrate (SnCl4·5H2O), sodium hydroxide (NaOH), acetic acid (C2H4O2), thiourea (CH4N2S), formaldehyde (HCHO), ethanol (CH3CH2OH), acetone (CH3COCH3), methanol (CH3OH), trimethylamine (C3H9N), benzene (C6H6), and toluene (C7H8), were analytical-grade reagents.
2.2. Synthesis of SnO2 Hollow Spheres
The SnO2 hollow spheres were prepared via a facile hydrothermal route. Briefly, 1.73 g SnCl4·5H2O, 1.25 g NaOH, and 30 mL of deionized water were added to a glass beaker and vigorously stirred for 30 min. Then, the clarified solution was poured into a 50 mL Teflon-lined stainless-steel autoclave and reacted at 200 °C for 24 h. After natural cooling to room temperature, the precipitate was collected, washed with deionized water and ethanol for 6 cycles, and then dried overnight at 60 °C to obtain the SnO2 hollow spheres.
2.3. Synthesis of SnS2/SnO2 Composites with Hollow Spherical Structures
Similarly, a series of SnS2/SnO2 composites with hollow spherical structures were also synthesized using a facile hydrothermal method. After introducing thiourea into the synthesis system, a portion of SnO2 was converted into SnS2; the detailed synthesis process can be found in the Supplementary Materials.
2.4. Synthesis of SnS2/SnO2 Composites with Hollow Spherical Structures
The detailed characterization of the crystal structure, morphology characteristics, elemental analysis, surface valence states, specific surface area, electrochemical testing, and electron paramagnetic resonance can be found in the Supplementary Materials.
2.5. Fabrication and Measurement of Sensors
The gas sensor was fabricated as follows. Firstly, a homogeneous slurry of either SnO2 or SnS2/SnO2 was prepared by dispersing the synthesized products (40 mg) in ethanol. This slurry was then coated onto the surface of an Al2O3 ceramic tube (4 mm in length, 1.2 mm in external diameter, and 0.8 mm in internal diameter). At each end of the Al2O3 ceramic tube, there were two Au electrodes, which were connected to two Pt lead wires. After natural drying, a Ni–Cr alloy coil was inserted through the central tunnel of the ceramic tube to act as a heater and regulate the operating temperature of the gas sensor. The Pt lead wires and the Ni–Cr heating wire were soldered on a four-corner base to fabricate the gas sensor. To enhance the stability and repeatability of the fabricated gas sensor, it was aged on a TS60 desktop (Winsen Electronics Co., Ltd., Zhengzhou, China) at 200 °C for 48 h. The gas-sensing tests were carried out in a static system (Weisheng Tech Co., Ltd., Zhengzhou, China) at room temperature. This work defines the sensor’s response as the resistance ratio, S = Ra/Rg (Rg: resistance in testing gas, Ra: resistance in the air). The schematic diagram of the synthesis process of the SnS2/SnO2 composites and the fabricated gas sensor is illustrated in Figure 1.
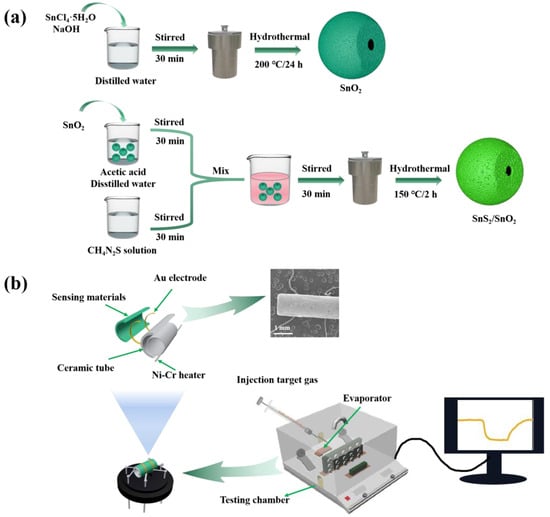
Figure 1.
(a) Schematic illustration of the synthesis process of the SnS2/SnO2 composites. (b) Schematic illustration and corresponding SEM image of the gas sensor, and schematic illustration of measurement system.
3. Results and Discussion
3.1. Structural and Morphological Characteristics
The appearance and morphology of the SnO2 and SnS2/SnO2 samples are shown in Figure 2. Obviously, pure SnO2 is composed of numerous spherical architectures with a diameter of approximately 2~4 μm (Figure 2a), and it can be seen that the spherical architecture is hierarchically constructed by many primary particles with a size of a few dozen nanometers in the amplifying FE−SEM images (Figure 2b), whose more detailed structure can be seen clearly in a broken SnO2 sphere. Interestingly, after the sulfurization of SnO2 (Figure 2c–h), the sample still maintains a hollow spherical structure with almost no change in size; however, compared to the SnO2 sample, the hollow sphere surface of SnS2/SnO2 becomes rough, which may be due to the formation of SnS2 nanoparticles on the SnO2 hollow sphere surface. The FE–SEM images exhibit porous, rough surface structures, which can absorb more gas and accelerate gas transmission, resulting in an improvement in the gas-sensing properties. According to the EDS mapping images of SnS2/SnO2−2 in Figure 2i–l, it can be seen that the three elements, namely, Sn, S, and O, are uniformly distributed on the surface, confirming the existence of SnS2 and SnO2.
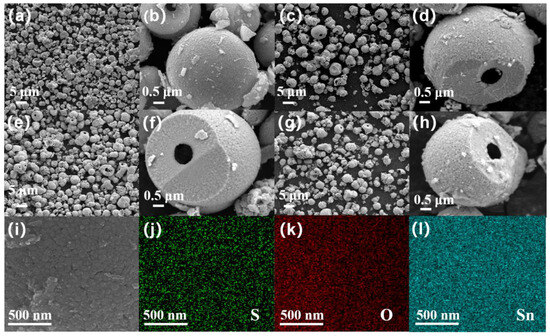
Figure 2.
The FE–SEM images of (a,b) SnO2, (c,d) SnS2/SnO2−1, (e,f) SnS2/SnO2−2, and (g,h) SnS2/SnO2−3. (i–l) EDS elemental mapping images of SnS2/SnO2−2.
Furthermore, the crystal structures of the SnO2 and SnS2/SnO2 samples were analyzed using XRD. As shown in Figure 3, the diffraction peaks of the SnO2 hollow spheres at 2θ of 26.6°, 33.9°, 38.9°, 51.8°, and 54.7° correspond to the (110), (101), (111), (211), and (220) crystal planes of SnO2, respectively, which is in good agreement with the tetragonal SnO2 phase (JCPDS No. 41–1445). After the sulfurization of SnO2, all the crystal phases belong to SnO2 and SnS2, confirming the formation of SnS2/SnO2 composites. Apart from the peaks belonging to pure SnO2, the weak diffraction peaks at about 29.3, 32.1, and 50.1° are assigned to the (101), (102), and (110) planes of SnS2 (JCPDS no. 21–1231). Additionally, no excess impurity peaks indicate the high purity of the as-synthesized samples.
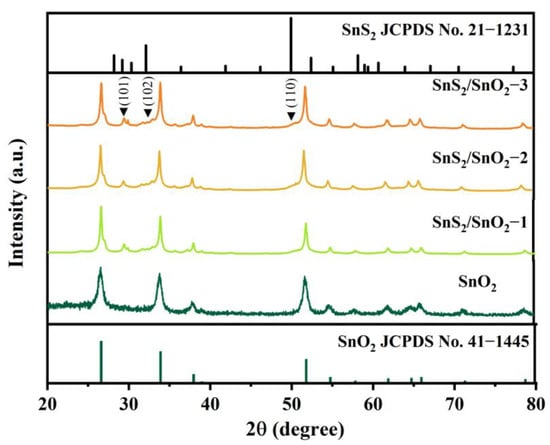
Figure 3.
XRD patterns of SnO2 and SnS2/SnO2 samples.
In order to further investigate the internal microstructure and crystal structure of the SnS2/SnO2 composites, TEM and HRTEM analyses were conducted (Figure 4), which revealed that the material has a hollow spherical structure observed from the broken material (Figure 4a). Moreover, a loose and porous surface can be observed in Figure 4b, which is in accordance with the results of the FE–SEM analyses. Figure 4c–g illustrates well-defined lattice stripes of the SnS2/SnO2 composites. The crystallographic distances of 0.34, 0.18, and 0.28 nm correspond to the (110) crystallographic planes of SnO2, and the (110) and (102) crystallographic planes of SnS2, respectively. These values align with the dominant peaks observed in the XRD patterns. Furthermore, the lattice stripes observed between SnS2 and SnO2 exhibit a seamless continuity, indicating the successful formation of an n–n heterojunction.
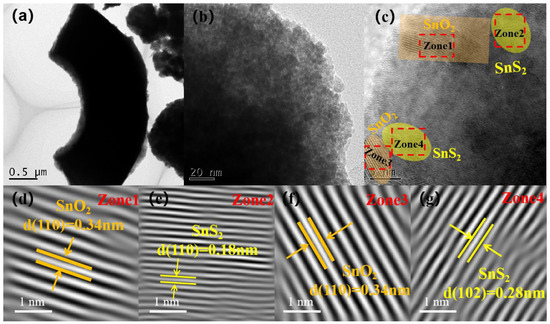
Figure 4.
TEM image (a) and (b–g) corresponding HR–TEM image of SnS2/SnO2−2 composites.
The elemental composition and chemical state of the SnO2 and SnS2/SnO2−2 composites were characterized by XPS analysis, and the pure SnO2 comprises Sn and O (Figure 5a). In contrast, the SnS2/SnO2 composites contain Sn, O, and S. Notably, the signal peak of C is caused by contaminated carbon or corrected carbon. The Sn 3d spectrum of both pure SnO2 and the SnS2/SnO2 composites (Figure 5b) exhibits two peaks at 486.1 and 494.5 eV (SnO2) and 486.6 and 494.9 eV (SnS2/SnO2), conforming with Sn 3d5/2 and Sn 3d3/2, respectively. This confirms the presence of Sn in the samples as a Sn4+ form [29,31]. Additionally, the Sn 3d peaks of the SnS2/SnO2 composites exhibit a slight shift towards a higher binding energy compared to pure SnO2, which suggests that there is some interaction between SnO2 and SnS2 in the composites [32]. In Figure 5c, the O 1s asymmetric peak of pure SnO2 can be divided into three peaks at 530.0 eV, 531.0 eV, and 531.9 eV, which are attributed to lattice oxygen (OL), oxygen vacancies (OV), and surface-absorbed oxygen (OC), respectively [33]. The O 1s peaks of the SnS2/SnO2 composites are slightly shifted towards the high energy direction at 530.4 eV, 531.3 eV, and 532.4 eV, corresponding to OL, OV, and OC, respectively. Generally, the OV can promote gas absorption and reaction by providing abundant active sites, and OC directly affects the chemical adsorption and ionized oxygen state on the surface of sensing materials; hence, a high ratio of OV and OC is favorable for achieving good gas-sensing properties [34]. Through calculation, it was found that the concentration of OV (25.1%) and OC (11.6%) in SnS2/SnO2 are higher than those of pure SnO2 OV (17.2%) and OC (10.4%), indicating its good sensing performance. In addition, the S 2p spectrum can be divided into two peaks at 161.7 eV and 163.1 eV (Figure 5d), corresponding to S 2p3/2 and S 2p1/2, respectively [35]. The results further indicate that the as-synthesized samples are SnS2/SnO2 composites.
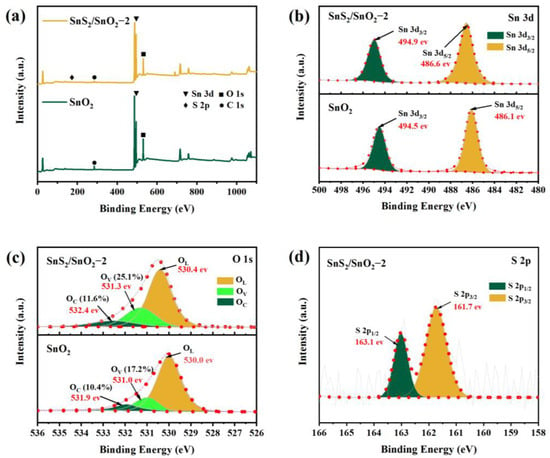
Figure 5.
XPS spectra of SnO2 and SnS2/SnO2−2 samples. (a) Survey spectrum, (b) Sn 3d spectrum, (c) O 1s spectrum, and (d) S 2p spectrum.
To further confirm the presence of oxygen vacancies in the samples, electron paramagnetic resonance (EPR) spectra of the SnO2 and SnS2/SnO2−2 were developed, as shown in Figure 6. The EPR spectra were measured at a temperature of 25 °C using a Bruker EMXPLUS spectrometer operating at the X band with a magnetic field modulation of 100.00 kHz. The microwave power was set to 3.170 mW and the modulation amplitude was 1.000 G [36]. Both samples revealed a strong signal corresponding to g(I) = 2.003, suggesting the presence of oxygen vacancies. The g value was determined by a comparison with a DPPH standard [37]. In addition, the signal intensity of SnS2/SnO2−2 is higher than that of SnO2, supporting the XPS results that the oxygen vacancy content increases after partial sulfurization of SnO2 to SnS2.
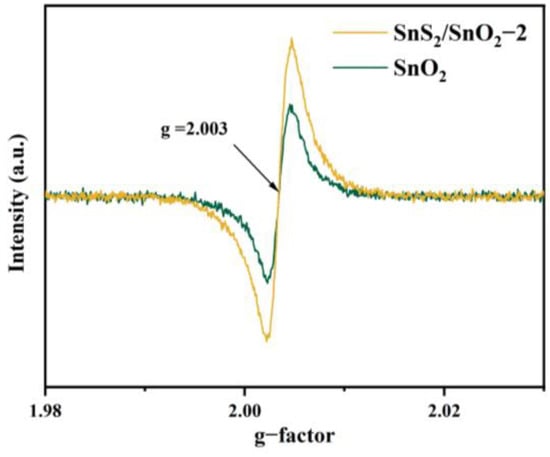
Figure 6.
EPR spectra of SnO2 and SnS2/SnO2−2 hollow spheres.
3.2. Gas-Sensing Properties
The formaldehyde-sensing properties of SnO2, SnS2, and SnS2/SnO2 hollow spheres were studied at room temperature. Figure 7a shows the transient resistance curve of SnO2, SnS2, SnS2/SnO2−1, SnS2/SnO2−2, and SnS2/SnO2−3 sensors to 0.1 ppm formaldehyde. Obviously, it is hard to obtain the sensing behavior of the SnO2 and SnS2 sensor at this low concentration. As for the SnS2/SnO2 sensors, an obvious response and recovery performance towards formaldehyde was observed, where the resistance decreased upon exposure to formaldehyde and recovered to its initial value after exposure to clean air, exhibiting typical n-type semiconductor characteristics. In addition, their response to formaldehyde is determined by the amount of S (Figure 7a). Notably, the SnS2/SnO2−2 sensor shows the highest response value of 1.93, meaning it has an excellent sensing ability for detecting trance formaldehyde in the atmosphere. Figure 7b shows the transient resistance curves and response value of five gas sensors to 10 ppm formaldehyde at room temperature, wherein the SnO2 and SnS2 sensor still had no response to formaldehyde at a relatively high concentration. In contrast, the SnS2/SnO2 sensor showed a significant increase in the response and recovery amplitudes, indicating that sulfurization functionalized SnO2 forming SnS2/SnO2 heterostructures can significantly improve the sensing ability of SnO2 hollow spheres. The selectivity of four sensors was investigated by comparing the sensing response to various reducing gases (formaldehyde, acetone, methanol, TMA, benzene, and toluene) with concentrations of 0.1 ppm and 10 ppm at room temperature (Figure 7c,d). Obviously, the SnO2 sensor had no response to any of the test gases at low or high concentrations (0.1 or 10 ppm), suggesting its poor sensing activity at room temperature. In contrast, all the SnS2/SnO2 sensors responded well to formaldehyde and were not very sensitive to other interfering gases. Specifically, the SnS2/SnO2−2 sensor was more sensitive to formaldehyde than the other sensors, demonstrating that it is more suitable for trace formaldehyde detection, and the following sensing performance was focused on the SnS2/SnO2−2 sensor.
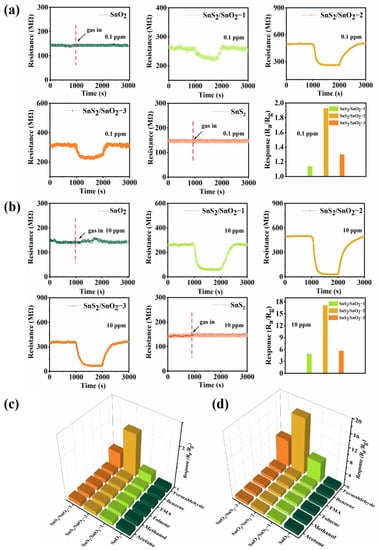
Figure 7.
Gas-sensing properties of the sensors at room temperature: dynamic response recovery curve and corresponding response of SnO2, SnS2, and SnS2/SnO2 hollow spheres at room temperature to (a) 0.1 ppm formaldehyde and (b) 10 ppm formaldehyde; selectivity of SnO2 and SnS2/SnO2 sensors to six different gases at room temperature at (c) 0.1 ppm and (d) 10 ppm.
The transient resistance curve of the SnS2/SnO2−2 sensor to a low formaldehyde concentration (0.1–1 ppm) and high formaldehyde concentration (10–100 ppm) is shown in Figure 8a,c. The corresponding response is plotted against the formaldehyde concentration, and it is apparent that the resistance decreased rapidly and reached a certain level after some time (Figure 8b,d). In contrast, upon removing formaldehyde from the chamber, the resistance gradually recovered to its original baseline value, confirming its good reproducibility. As the formaldehyde concentration increased, the response improved significantly (0.1–1 ppm), but when the formaldehyde concentration was above 10 ppm, the magnitude of the rise in response gradually slowed down due to the high surface coverage of formaldehyde [38]. In addition, the response versus the formaldehyde concentration (0.1–1 ppm) presents a good linear relationship with a fitting slope of 8.44408 ppm−1 (Figure 8b) [39]. The theoretical detection limit is calculated to be approximately 5.81 ppb based on the signal-to-noise ratio (see the Supplementary Materials for details). Such results suggest that the SnS2/SnO2−2 sensor in this work can potentially monitor ppb-level formaldehyde in the environment.
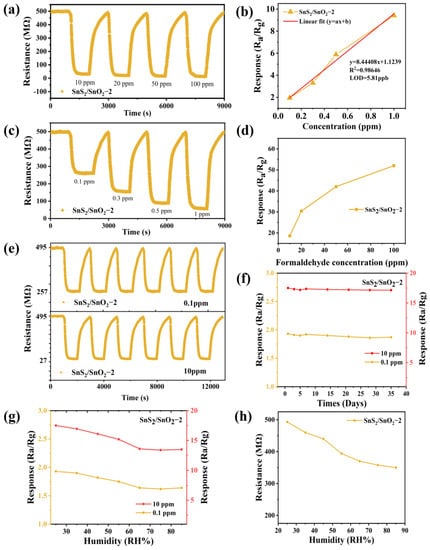
Figure 8.
Gas-sensing properties of the SnS2/SnO2−2 sensor at room temperature: (a) dynamic response recovery curve and (b) correlation curve of the response with formaldehyde concentration (0.1–1 ppm); (c) dynamic response recovery curve and (d) corresponding response with formaldehyde concentration (10–100 ppm); (e) repeatability, (f) long-term stability, (g) response changes under varying humidity to formaldehyde (0.1 ppm or 10 ppm), and (h) the variation of baseline resistance versus relative humidity.
Repeatability and stability are essential parameters for practical applications. The repeatability of the SnS2/SnO2−2 sensor was investigated by exposing it to 0.1 ppm or 10 ppm formaldehyde in five cycles of response-recovery at room temperature (Figure 8e). Each cycle exhibited similar adsorption-desorption trends, and the initial resistance value did not change significantly. Additionally, the response also showed slight fluctuations in each trial, implying its good repeatability. Furthermore, the formaldehyde-sensing response of the SnS2/SnO2−2 sensor was monitored for 35 days to identify its long-term stability (Figure 8f). Interestingly, there was no significant decrease in the response over the testing period; even 35 days later, the response remained at 96.89% (0.1 ppm) and 97.94% (10 ppm), indicating its good long-term stability. At room temperature, humidity is also an essential factor affecting the sensor’s practical application. The effect of relative humidity (RH) on the formaldehyde-sensing response (0.1 ppm or 10 ppm) at room temperature was explored, and the result is shown in Figure 8g. It was found that in the range of RH from 25% to 55%, the response to 0.1 ppm or 10 ppm formaldehyde decreased slightly with increasing RH levels. In contrast, the decrease in response was relatively higher when the RH value was above 55%, which could be originated from the adsorption competition between oxygen species and water molecules on the sensor surface, hindering the redox reaction of the target gas [40]. A reduction of the baseline resistance as the RH levels increased was also observed (Figure 8h), implying the occurrence of surface reactions between water molecules and the adsorbed oxygen species [41]. In addition, Table 1 compares the results of this work and others reported in the literature for formaldehyde detection. Compared to the previously described sensing material, the SnS2/SnO2−2 composites offer more significant potential for a formaldehyde-sensing ability at room temperature due to their high responses and low detection limit.

Table 1.
Comparison of sensing ability of gas sensors based on different sensing materials toward formaldehyde.
3.3. Gas-Sensing Mechanism
Generally, the sensing mechanism of SnO2 sensors is related to the resistance modulation caused by the chemical interaction between the absorbed target gas and ionized oxygen species on the SnO2 [51]. In this work, the SnO2 and SnS2/SnO2 composites showed a typical n-type sensing feature, meaning that electrons act as the major charge carriers for sensing reactions. This is also confirmed by the Mott–Schottky analyses, and both SnO2 and SnS2/SnO2−2 hollow spheres show a positive slope in the M–S plots (Figure 9a), indicating that electrons act as the leading carriers and exhibit n-type semiconductor conduction characteristics. Subsequently, the flat band potential (UFB) of the composite was ascertained by calculating the intercept of the linear segment of the Mott–Schottky curve of the SnS2/SnO2−2 composite with the potential axis [52]. The UFB of the composite is −0.43 V (standard hydrogen electrode, SHE). For the pure SnO2 hollow spheres, electrons from the conduction band can react with the oxygen molecules in the ambient atmosphere to form oxygen anions (O2−, O−, and O2−), depending on the operational temperatures. This process forms an electron depletion layer on the SnO2 surface, thereby increasing the resistance (Ra) of the SnO2 sensor in the air. Usually, at room temperature, the surface oxygen species were mainly O2−. Upon exposure to reducing gas (formaldehyde), the ionized oxygen species could react with the formaldehyde molecules to release the trapped electrons into the SnO2 conduction band, thereby narrowing the thickness of the electron depletion layer and reducing the resistance value of the sensor (Rg). The chemical reactions are as follows [53,54,55]:
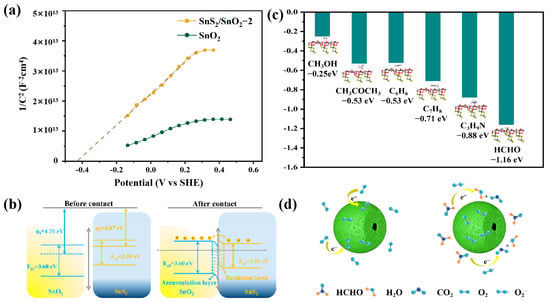
Figure 9.
(a) Mott–Schottky plots of the SnO2 and SnS2/SnO2−2 hollow spheres; (b) energy band structure diagram of the SnS2/SnO2 heterojunctions; (c) DFT-calculated adsorption energies of the SnS2/SnO2 heterojunction surface; (d) schematic diagram of sensing mechanism of SnS2/SnO2 hollow spheres in air and formaldehyde.
The reasons for the improved gas-sensing performance of the SnS2/SnO2 composite materials, unlike single-type SnO2, are as follows.
- (1)
- The existence of an n–n heterojunction plays a crucial role in enhancing the sensing performance. The difference between the electronic work function of SnO2 and SnS2 makes the electrons in SnS2 flow to SnO2 until the Fermi level reaches equilibrium [28] when they contact each other (Figure 9b). The transfer of electrons and the significant difference in electron work functions result in band bending within the material, further causing the accumulation of electrons at the surface of SnO2 and the depletion of electrons at the surface of SnS2. Meanwhile, a potential barrier is generated between the heterojunction architecture. As the SnS2/SnO2 is exposed to the air environment, more ionized oxygen species are absorbed on the surface of the sensing materials, leading to a more significant initial resistance state immediately. When it is exposed to formaldehyde, the sensing reaction of the oxygen species with formaldehyde releases more electrons back to the conduction band. This process narrows the electron depletion layer, dramatically decreasing the sensor resistance with the reduced heterojunction potential barrier height; thus, the SnS2/SnO2 heterojunction configuration significantly enhances the sensor’s capabilities.
- (2)
- The oxygen/formaldehyde adsorption capacity has an important impact on the gas-sensing performance of the materials. The XPS and EPR analyses show that the SnS2/SnO2 composites process more oxygen vacancies, implying their high oxygen adsorption capacity. Upon exposure to formaldehyde, more oxygen species means more formaldehyde molecules can react, consequently leading to a high gas-sensing ability. At the same time, the enhanced adsorption ability of the SnS2/SnO2 composites was revealed through DFT calculations, which were performed employing the CASTEP module in Materials Studio software (see the Supporting Information and Figure S2 for details). As shown in Figure 9c, when the sensor is in contact with the tested gas molecules, the adsorption energy of formaldehyde on the (110)/(101) plane of SnS2/SnO2 is −1.11 eV, which is much larger than that of the other tested gases (acetone: −0.53 eV, methanol: −0.25 eV, toluene: −0.71 eV, benzene: −0.53 eV, TMA: −0.88 eV, formaldehyde: −1.16 eV). This indicates a strong interaction between formaldehyde and the SnS2/SnO2 surface, directly proving the improved sensing performance to formaldehyde from the energy point of view.
- (3)
- The unique structural merits, including the hollow and porous structure, are also essential in improving the gas-sensing performance. The hollow, mesoporous structure of the SnS2/SnO2 composites significantly contributes to the specific surface area (Figure 9d). The BET measurements (see the Supporting Information and Figure S1 for details) revealed that both SnO2 and SnS2/SnO2 hollow spheres possess a high specific surface area, and the SnS2/SnO2-2 hollow spheres (92.5 m2 g−1) have a higher specific surface area than that of SnO2 (87.4 m2 g−1). This indicates that the SnS2/SnO2−2 hollow spheres can provide many active sites for the adsorption of oxygen species and formaldehyde gas, booting the resistance modulation. In addition, the porous channel structure can essentially promote the penetration efficiency of air/target molecules in the sensing interaction, boosting the reaction of formaldehyde and oxygen species.
Overall, the synergistic effect of the n–n heterojunctions between SnO2 and SnS2, the strong oxygen/formaldehyde adsorption capacity, and the unique structural merits, including the hollow and porous structure, enhance the formaldehyde-sensing performance in the SnS2/SnO2 composites; however, excessive SnS2 can decrease the sensor response, as it can hide the SnO2 surface and become the dominant conductive path, hindering the desired sensing function. Moreover, increasing the SnS2 content further degrades the quality of the n–n heterojunction, weakening its effect. Based on the results, the SnS2/SnO2−2 sensor with optimized decoration significantly improves the formaldehyde-sensing properties of the material, making it a more effective gas sensor for detecting this harmful gas.
4. Conclusions
In summary, SnS2/SnO2 heterostructures with hollow spherical structures were synthesized by a simple hydrothermal method for highly sensitive and selective formaldehyde detection. Benefiting from the synergistic effect of the n–n heterojunction between SnO2 and SnS2, strong oxygen/formaldehyde adsorption capacity, and unique structural features, including the hollow and porous structure, the SnS2/SnO2 sensor presents a superior formaldehyde-sensing performance at room temperature. Remarkably, the SnS2/SnO2−2 sensor demonstrates exceptional selectivity, detecting formaldehyde in concentrations as low as 0.1 ppm, with the highest sensing response of 1.93. Furthermore, the sensor exhibits good repeatability, long-term stability, and an outstanding theoretical detection limit. To gain further insight into the enhanced gas-sensing performance, we conducted DFT calculations, which shed light on the underlying mechanism. The excellent comprehensive gas-sensing properties enable SnS2/SnO2 hollow spheres to position themselves as highly selective gas-sensing platforms for the detection of trace amounts of formaldehyde. This technology is well-suited for energy-saving and portable detection systems, meeting the demands of various applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/2079-4991/13/17/2493/s1, Figure S1: N2 adsorption–desorption isotherms and corresponding BJH pore size distribution curves of (a) SnO2 and (b) SnS2/SnO2−2 hollow spheres. Figure S2. Optimized adsorption configurations of (a) formaldehyde, (b) ethanol, (c) methanol, (d) benzene, (e) toluene, and (f) trimethylamine on the SnO2/SnS2 surface.
Author Contributions
Conceptualization, Z.X.; data curation, Z.X.; formal analysis, Z.X., M.W., G.W., and Y.Z.; funding acquisition, D.M.; investigation, Z.X.; methodology, D.M., Z.X., J.Q., and Q.J.; project administration, D.M., X.S., and J.Q.; resources, G.W.; supervision, X.S., J.Q., and Q.J.; validation, D.M. and J.X.; visualization, Z.X. and Y.Z.; writing—original draft, Z.X.; writing—review and editing, D.M., J.Q., and Q.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 61973223; the Liao Ning Revitalization Talents Program, grant number XLYC2007051; the Education Department Foundation of Jilin Province, grant number JJKH20220997KJ; the Support Plan for Innovative Talents in Colleges and Universities in Liaoning Province, grant number 2020389; the Liaoning Educational Department Foundation, grant number LJKMZ20220762; the Natural Science Foundation of Liaoning Province, grant number 2021-MS-257; and the Young and Middle-aged Scientific and Technological Innovation Talents of Shenyang Science and Technology Bureau, grant number RC200352.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Das, T.; Mojumder, S.; Chakraborty, S.; Saha, D.; Pal, M. Beneficial effect of Sn doping on bismuth ferrite nanoparticle-based sensor for enhanced and highly selective detection of trace formaldehyde. Appl. Surf. Sci. 2022, 602, 154340. [Google Scholar] [CrossRef]
- Wang, D.; Pu, X.X.; Yu, X.; Bao, L.P.; Cheng, Y.; Xu, J.C.; Han, S.C.; Ma, Q.X.; Wang, X.Y. Controlled preparation and gas sensitive properties of two-dimensional and cubic structure ZnSnO3. J. Colloid Interface Sci. 2022, 608, 1074–1085. [Google Scholar] [CrossRef]
- Rong, Q.; Li, Y.; Hao, S.Q.; Cai, S.T.; Wolverton, C.; Dravid, V.P.; Zhai, T.Y.; Liu, Q.J. Raspberry-like mesoporous Co-doped TiO2 nanospheres for a high-performance formaldehyde gas sensor. J. Mater. Chem. A 2021, 9, 6529–6537. [Google Scholar] [CrossRef]
- Yu, H.M.; Li, J.Z.; Luo, W.B.; Li, Z.Y.; Tian, Y.W.; Yang, Z.D.; Gao, X.W.; Liu, H. Hetero-structure La2O3-modified SnO2–Sn3O4 from tin anode slime for highly sensitive and ppb-Level formaldehyde detection. Appl. Surf. Sci. 2020, 513, 145825. [Google Scholar] [CrossRef]
- Xi, H.T.; Chen, X.G.; Cao, Y.; Xu, J.J.; Ye, C.Z.; Deng, D.W.; Zhang, J.S.; Huang, G.H. Electrochemical determination of formaldehyde via reduced AuNPs@PPy composites modified electrode. Microchem. J. 2020, 156, 104846. [Google Scholar] [CrossRef]
- Melo Cardozo, I.M.; Sousa, E.T.; da Rocha, G.O.; Pereira dos Anjos, J.; de Andrade, J.B. Determination of free- and bound-carbonyl compounds in airborne particles by ultra-fast liquid chromatography coupled to mass spectrometry. Talanta 2020, 217, 121033. [Google Scholar] [CrossRef]
- Xu, L.; Ge, M.Y.; Zhang, F.; Huang, H.J.; Sun, Y.; He, D.N. Nanostructured of SnO2/NiO composite as a highly selective formaldehyde gas sensor. J. Mater. Res. 2020, 35, 3079–3090. [Google Scholar] [CrossRef]
- Sun, Y.J.; Yang, H.Y.; Zhao, Z.T.; Suematsu, K.; Li, P.W.; Yu, Z.C.; Zhang, W.D.; Hu, J. Fabrication of ZnO quantum dots@SnO2 hollow nanospheres hybrid hierarchical structures for effectively detecting formaldehyde. Sens. Actuators B 2020, 318, 128222. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sens. Actuators A 2022, 341, 113578. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Reddeppa, M.; Chougule, S.S.; Bak, N.-H.; Nam, D.-J.; Jung, N.; Cho, H.D.; Kim, S.-G.; Kim, M.-D. High performance langasite based SAW NO2 gas sensor using 2D g-C3N4@TiO2 hybrid nanocomposite. J. Hazard. Mater. 2022, 427, 128174. [Google Scholar] [CrossRef]
- Huang, J.Y.; Liang, H.; Ye, J.X.; Jiang, D.T.; Sun, Y.L.; Li, X.J.; Geng, Y.F.; Wang, J.Q.; Qian, Z.F.; Du, Y. Ultrasensitive formaldehyde gas sensor based on Au-loaded ZnO nanorod arrays at low temperature. Sens. Actuators B 2021, 346, 130568. [Google Scholar] [CrossRef]
- Kang, Z.J.; Zhang, D.Z.; Li, T.T.; Liu, X.H.; Song, X.S. Polydopamine-modified SnO2 nanofiber composite coated QCM gas sensor for high-performance formaldehyde sensing. Sens. Actuators B 2021, 345, 130299. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, T.T.; Huo, L.H.; Gao, S.; Li, B.S.; Guo, C.Y.; Yu, H.X.; Major, Z.; Zhang, X.F.; Cheng, X.L.; et al. Small size porous NiO/NiFe2O4 nanocubes derived from Ni–Fe bimetallic metal-organic frameworks for fast volatile organic compounds detection. Appl. Surf. Sci. 2023, 623, 157075. [Google Scholar] [CrossRef]
- Im, D.; Kim, D.; Jeong, D.; Park, W.I.; Chun, M.; Park, J.-S.; Kim, H.; Jung, H. Improved formaldehyde gas sensing properties of well-controlled Au nanoparticle-decorated In2O3 nanofibers integrated on low power MEMS platform. J. Mater. Sci. Technol. 2020, 38, 56–63. [Google Scholar] [CrossRef]
- Lu, Z.C.; Ma, Z.R.; Song, P.; Wang, Q. Facile synthesis of CuO nanoribbons/rGO nanocomposites for high-performance formaldehyde gas sensor at low temperature. J. Mater. Sci. Mater. Electron. 2021, 32, 19297–19308. [Google Scholar] [CrossRef]
- Devabharathi, N.; Umarji, A.M.; Dasgupta, S. Fully Inkjet-Printed Mesoporous SnO2-Based Ultrasensitive Gas Sensors for Trace Amount NO2 Detection. ACS Appl. Mater. Interfaces 2020, 12, 57207–57217. [Google Scholar] [CrossRef]
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Meng, F.L.; Ji, H.Y.; Yuan, Z.Y.; Chen, Y.R.; Zhang, H.; Qin, W.B.; Gao, H.L. Dynamic Measurement and Recognition Methods of SnO2 Sensor to VOCs Under Zigzag-Rectangular Wave Temperature Modulation. IEEE Sens. J. 2021, 21, 10915–10922. [Google Scholar] [CrossRef]
- Meng, X.; Bi, M.; Xiao, Q.; Gao, W. Ultrasensitive gas sensor based on Pd/SnS2/SnO2 nanocomposites for rapid detection of H2. Sens. Actuators B 2022, 359, 131612. [Google Scholar] [CrossRef]
- Manikandan, D.; Murugan, R. Genesis and tuning of ferromagnetism in SnO2 semiconductor nanostructures: Comprehensive review on size, morphology, magnetic properties and DFT investigations. Prog. Mater. Sci. 2022, 130, 100970. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Zuo, K.Y.; Meng, F.L.; Ma, Z.W.; Xu, W.X.; Dong, H. Microscale analysis and gas sensing characteristics based on SnO2 hollow spheres. Microelectron. Eng. 2020, 231, 111372. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Y.; Dong, L.H.; Wang, J.X. Fabricating pod-like SnO2 hierarchical micro-nanostructures for enhanced acetone gas detection. Mater. Sci. Semicond. Process. 2021, 121, 105451. [Google Scholar] [CrossRef]
- Pandit, N.A.; Ahmad, T. Tin Oxide Based Hybrid Nanostructures for Efficient Gas Sensing. Molecules 2022, 27, 7038. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.S.; Meng, S.J.; Ma, Z.Y.; Yang, M.X.; Ma, D.W.; Yang, Z.X.; Talib, S.H. Electronic and catalytic properties of Ti single atoms@SnO2 and its implications on sensing mechanism for CO. Appl. Surf. Sci. 2022, 594, 153500. [Google Scholar] [CrossRef]
- Nascimento, E.P.; Firmino, H.C.T.; Neves, G.A.; Menezes, R.R. A review of recent developments in tin dioxide nanostructured materials for gas sensors. Ceram. Int. 2022, 48, 7405–7440. [Google Scholar] [CrossRef]
- Chen, Z.L.; Wang, D.; Wang, X.Y.; Yang, J.H. Enhanced formaldehyde sensitivity of two-dimensional mesoporous SnO2 by nitrogen-doped graphene quantum dots. Rare Met. 2021, 40, 1561–1570. [Google Scholar] [CrossRef]
- Li, Y.X.; Leonardi, S.G.; Bonavita, A.; Neri, G.; Wlodarski, W. Two-Dimensional (2D) SnS2-based Oxygen Sensor. Procedia Eng. 2016, 168, 1102–1105. [Google Scholar] [CrossRef]
- Liu, D.; Tang, Z.L.; Zhang, Z.T. Visible light assisted room-temperature NO2 gas sensor based on hollow SnO2@SnS2 nanostructures. Sens. Actuators B 2020, 324, 128754. [Google Scholar] [CrossRef]
- Gu, D.; Li, X.G.; Zhao, Y.Y.; Wang, J. Enhanced NO2 sensing of SnO2/SnS2 heterojunction based sensor. Sens. Actuators B 2017, 244, 67–76. [Google Scholar] [CrossRef]
- Bai, J.Z.; Shen, Y.b.; Zhao, S.K.; Li, A.; Kang, Z.k.; Cui, B.Y.; Wei, D.Z.; Yuan, Z.Y.; Meng, F.L. Room-Temperature NH3 Sensor Based on SnO2 Quantum Dots Functionalized SnS2 Nanosheets. Adv. Mater. Technol. 2023, 8, 2201671. [Google Scholar] [CrossRef]
- Kou, X.Y.; Meng, F.Q.; Chen, K.; Wang, T.S.; Sun, P.; Liu, F.M.; Yan, X.; Sun, Y.F.; Liu, F.M.; Shimanoe, K.; et al. High-performance acetone gas sensor based on Ru-doped SnO2 nanofibers. Sens. Actuators B 2020, 320, 128292. [Google Scholar] [CrossRef]
- Zhao, W.; He, M.; Chen, F.; Jin, X.; Duan, H.; Long, M.; Wu, Z.; Cao, B.; Yu, Y. One-pot synthesis of flower-like SnS2/SnO2 heterojunction with enhanced visible light photocatalytic performance. Opt. Mater. 2022, 123, 111934. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wang, F.; Hermawan, A.; Asakura, Y.; Hasegawa, T.; Kumagai, H.; Kato, H.; Kakihana, M.; Zhu, J.F.; Yin, S. SnO-SnO2 modified two-dimensional MXene Ti3C2Tx for acetone gas sensor working at room temperature. J. Mater. Sci. Technol. 2021, 73, 128–138. [Google Scholar] [CrossRef]
- Meng, X.; Bi, M.; Gao, W. Shape and composition effects of PdPt bimetallic nanocrystals on hydrogen sensing properties of SnO2 based sensors. Sens. Actuators B 2023, 390, 133976. [Google Scholar] [CrossRef]
- Yang, Z.; Su, C.; Wang, S.T.; Han, Y.T.; Chen, X.W.; Xu, S.S.; Zhou, Z.H.; Hu, N.T.; Su, Y.J.; Zeng, M. Highly sensitive NO2 gas sensors based on hexagonal SnS2 nanoplates operating at room temperature. Nanotechnology 2020, 31, 075501. [Google Scholar] [CrossRef]
- Morazzoni, F.; Canevali, C.; Chiodini, N.; Mari, C.; Ruffo, R.; Scotti, R.; Armelao, L.; Tondello, E.; Depero, L.; Bontempi, E. Surface reactivity of nanostructured tin oxide and Pt-doped tin oxide as studied by EPR and XPS spectroscopies. Mater. Sci. Eng. C 2001, 15, 167–169. [Google Scholar] [CrossRef]
- Yordanov, N.D.; Christova, A. DPPH as a Primary Standard for Quantitative EPR Spectrometry. Appl. Magn. Reson. 1994, 6, 341–345. [Google Scholar] [CrossRef]
- Gao, L.P.; Fu, H.; Zhu, J.J.; Wang, J.H.; Chen, Y.P.; Liu, H.J. Synthesis of SnO2 nanoparticles for formaldehyde detection with high sensitivity and good selectivity. J. Mater. Res. 2020, 35, 2208–2217. [Google Scholar] [CrossRef]
- Xian, J.B.; Li, J.; Wang, W.J.; Zhu, J.Y.; Li, P.Z.; Leung, C.M.; Zeng, M.; Lu, X.B.; Gao, X.S.; Liu, J.M. Enhanced specific surface area of ZIF-8 derived ZnO induced by sulfuric acid modification for high-performance acetone gas sensor. Appl. Surf. Sci. 2023, 614, 156175. [Google Scholar] [CrossRef]
- Lou, C.M.; Huang, Q.X.; Li, Z.S.; Lei, G.L.; Liu, X.H.; Zhang, J. Fe2O3-sensitized SnO2 nanosheets via atomic layer deposition for sensitive formaldehyde detection. Sens. Actuators B 2021, 345, 130429. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Li, X.; Shao, C.; Han, C.; Xin, J.; Lu, D.; Niu, L.; Tang, Y.; Liu, Y. Highly permeable WO3/CuWO4 heterostructure with 3D hierarchical porous structure for high-sensitive room-temperature visible-light driven gas sensor. Sens. Actuators B 2022, 365, 131926. [Google Scholar] [CrossRef]
- Cai, H.J.; Luo, N.; Hu, Q.M.; Xue, Z.G.; Wang, X.H.; Xu, J.Q. Multishell SnO2 Hollow Microspheres Loaded with Bimetal PdPt Nanoparticles for Ultrasensitive and Rapid Formaldehyde MEMS Sensors. ACS Sens. 2022, 7, 1484–1494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, S.P.; Huang, B.Y.; Wang, N.; Li, X.G. UV-Enhanced Formaldehyde Sensor Using Hollow In2O3@TiO2 Double-Layer Nanospheres at Room Temperature. ACS Appl. Mater. Interfaces 2023, 15, 4329–4342. [Google Scholar] [CrossRef]
- Guo, L.P.; Liang, H.P.; Hu, H.Y.; Shi, S.B.; Wang, C.X.; Lv, S.T.; Yang, H.H.; Li, H.; de Rooij, N.F.; Lee, Y.-K.; et al. Large-Area and Visible-Light-Driven Heterojunctions of In2O3/Graphene Built for ppb-Level Formaldehyde Detection at Room Temperature. ACS Appl. Mater. Interfaces 2023, 15, 18205–18216. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Cao, Y.H.; Yang, Z.M.; Wu, J.F. Nanoheterostructure Construction and DFT Study of Ni-Doped In2O3 Nanocubes/WS2 Hexagon Nanosheets for Formaldehyde Sensing at Room Temperature. ACS Appl. Mater. Interfaces 2020, 12, 11979–11989. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhang, Y.J.; Wang, Y.; Zhang, Y.; Cao, J.L. Nanoporous Mixed-Phase In2O3 Nanoparticle Homojunctions for Formaldehyde Sensing. ACS Appl. Nano Mater. 2023, 6, 5635–5644. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Mi, Q.; Wang, D.Y.; Li, T.T. MXene/Co3O4 composite based formaldehyde sensor driven by ZnO/MXene nanowire arrays piezoelectric nanogenerator. Sens. Actuators B 2021, 339, 129923. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, S.Y.; Zhang, J.L.; Wang, B.J.; Pei, S.T. Enhanced formaldehyde gas sensing performance based on Bi doped Zn2SnO4/SnO2 porous nanospheres. J. Alloys Compd. 2020, 828, 154408. [Google Scholar] [CrossRef]
- Khojier, K. Preparation and investigation of Al-doped ZnO thin films as a formaldehyde sensor with extremely low detection limit and considering the effect of RH. Mater. Sci. Semicond. Process. 2021, 121, 105283. [Google Scholar] [CrossRef]
- Sun, Y.H.; Wang, J.; Du, H.Y.; Li, X.G.; Wang, C.; Hou, T.Y. Formaldehyde gas sensors based on SnO2/ZSM−5 zeolite composite nanofibers. J. Alloys Compd. 2021, 868, 159140. [Google Scholar] [CrossRef]
- Han, Z.J.; Qi, Y.; Yang, Z.Y.; Han, H.C.; Jiang, Y.Y.; Du, W.J.; Zhang, X.; Zhang, J.Z.; Dai, Z.F.; Wu, L.L.; et al. Recent advances and perspectives on constructing metal oxide semiconductor gas sensing materials for efficient formaldehyde detection. J. Mater. Chem. C 2020, 8, 13169–13188. [Google Scholar] [CrossRef]
- Hankin, A.; Bedoya-Lora, F.E.; Alexander, J.C.; Regoutz, A.; Kelsall, G.H. Flat band potential determination: Avoiding the pitfalls. J. Mater. Chem. A 2019, 7, 26162–26176. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Chougule, S.S.; Vidyasagar, D.; Bak, N.-H.; Jung, N.; Kim, Y.-H.; Lee, J.-H.; Kim, S.-G.; Kim, M.-D. UV light driven high-performance room temperature surface acoustic wave NH3 gas sensor using sulfur-doped g-C3N4 quantum dots. Nano Res. 2023, 16, 7682–7695. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Hamad, D. Low-temperature hydrogen sensor based on sputtered tin dioxide nanostructures through slow deposition rate. Appl. Surf. Sci. 2022, 598, 153857. [Google Scholar] [CrossRef]
- Liu, M.; Sun, R.Y.; Sima, Z.H.; Song, P.; Ding, Y.L.; Wang, Q. Au-decorated In2O3 nanospheres/exfoliated Ti3C2Tx MXene nanosheets for highly sensitive formaldehyde gas sensing at room temperature. Appl. Surf. Sci. 2022, 605, 154839. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).