Fabrication and Characterization of Tantalum–Iron Composites for Photocatalytic Hydrogen Evolution
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Chemicals
2.2. The Preparation Method of the Ta2O5/FeTaO4
2.3. Photocatalytic Hydrogen Evolution Measurement
2.4. Stability Study of Produced Samples for Photocatalytic Hydrogen Evolution
2.5. Photoelectrochemical Performance
2.6. Characterizations
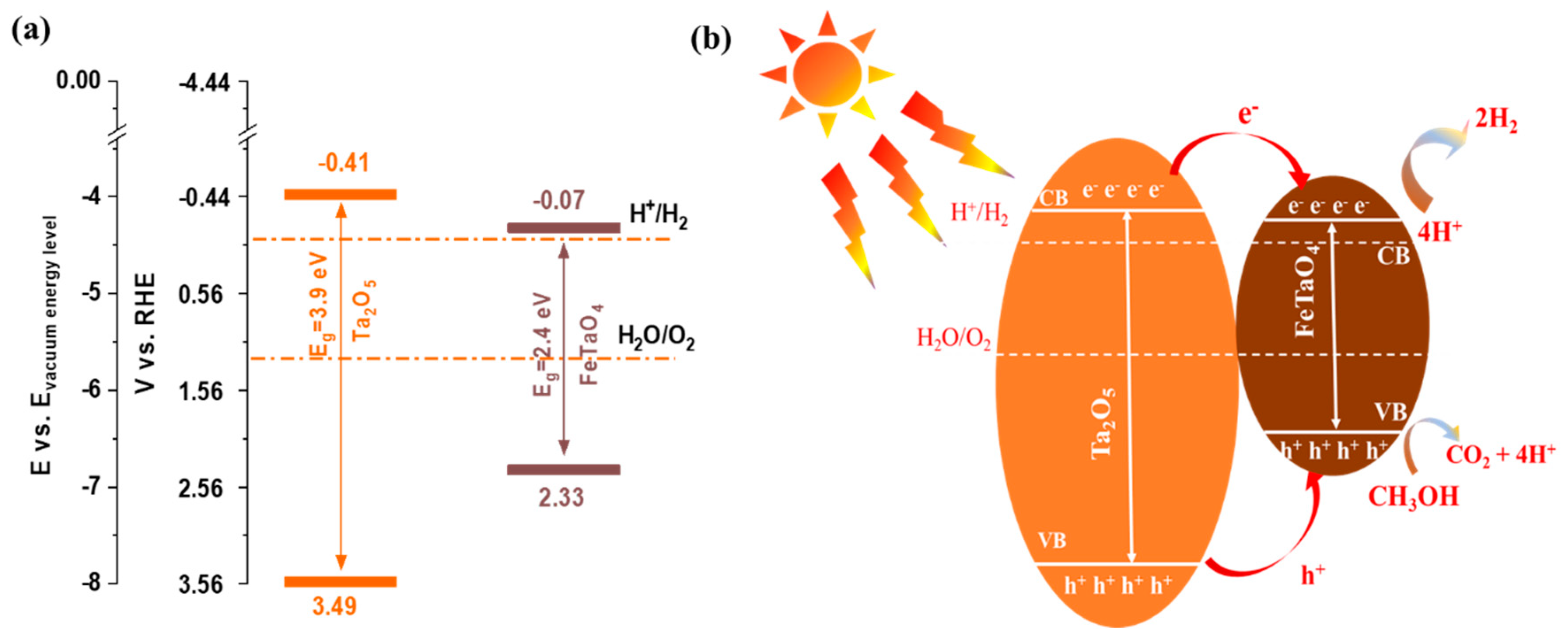
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, H.; Liu, Q.; Yan, C.; Wu, Q.; Gong, D.; He, W.; Liu, H.; Wang, J.; Mei, X. Mitigation of greenhouse gas emissions and improved yield by plastic mulching in rice production. Sci. Total Environ. 2023, 880, 162984. [Google Scholar] [CrossRef] [PubMed]
- Meda, U.S.; Rajyaguru, Y.V.; Pandey, A. Generation of green hydrogen using self-sustained regenerative fuel cells: Opportunities and challenges. Int. J. Hydrogen Energy 2023, 48, 28289–28314. [Google Scholar] [CrossRef]
- Viteri, J.P.; Viteri, S.; Alvarez-Vasco, C.; Henao, F. A systematic review on green hydrogen for off-grid communities –technologies, advantages, and limitations. Int. J. Hydrogen Energy 2023, 48, 19751–19771. [Google Scholar] [CrossRef]
- Kumar, A.; Khosla, A.; Sharma, S.K.; Dhiman, P.; Sharma, G.; Gnanasekaran, L.; Naushad, M.; Stadler, F.J. A review on S-scheme and dual S-scheme heterojunctions for photocatalytic hydrogen evolution, water detoxification and CO2 reduction. Fuel 2023, 333, 126267. [Google Scholar] [CrossRef]
- Sun, T.; Wei, J.; Zhou, C.; Wang, Y.; Shu, Z.; Zhou, J.; Chen, J. Facile preparation and enhanced photocatalytic hydrogen evolution of cation-exchanged zeolite LTA supported TiO2 photocatalysts. Int. J. Hydrogen Energy 2023, 48, 13851–13863. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.W.; Lim, S.J.; Kim, H. Visible light activated MoS2/ZnO composites for photocatalytic degradation of ciprofloxacin antibiotic and hydrogen production. J. Photochem. Photobiol. A Chem. 2023, 434, 114250. [Google Scholar] [CrossRef]
- Hunge, Y.; Yadav, A.; Kang, S.-W.; Kim, H. Facile synthesis of multitasking composite of Silver nanoparticle with Zinc oxide for 4-nitrophenol reduction, photocatalytic hydrogen production, and 4-chlorophenol degradation. J. Alloys Compd. 2022, 928, 167133. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Lee, K.; Hahn, R.; Schmuki, P. Anodic growth of hierarchically structured nanotubular ZnO architectures on zinc surfaces using a sulfide based electrolyte. Electrochem. Commun. 2013, 34, 9–13. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Lee, K.; Kirchgeorg, R.; Hahn, R.; Schmuki, P. Self-organization and zinc doping of Ga2O3 nanoporous architecture: A potential nano-photogenerator for hydrogen. Electrochem. Commun. 2013, 35, 112–115. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Bui, H.T.; Lee, T.; Noh, Y.-Y. Interfacial Engineering of Nanoporous Architectures in Ga2O3 Film toward Self-Aligned Tubular Nanostructure with an Enhanced Photocatalytic Activity on Water Splitting. Langmuir 2018, 34, 4575–4583. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Z.; Yang, M.; Li, Y.; Lin, X.; Li, M.; Tang, S.; Teng, Y.; Kuang, D.-B. Constructing the Sulfur-Doped CdO@In2O3 Nanofibers Ternary Heterojunction for Efficient Photocatalytic Hydrogen Production. Nanomaterials 2023, 13, 401. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Li, T.; Jin, Z. Synergistic Effect of Bimetallic Sulfide Enhances the Performance of CdS Photocatalytic Hydrogen Evolution. Adv. Sustain. Syst. 2022, 7, 2200139. [Google Scholar] [CrossRef]
- Balan, B.; Xavier, M.M.; Mathew, S. MoS2-Based Nanocomposites for Photocatalytic Hydrogen Evolution and Carbon Dioxide Reduction. ACS Omega 2023, 8, 25649–25673. [Google Scholar] [CrossRef]
- Yang, S.; Wang, K.; Yu, H.; Huang, Y.; Guo, P.; Ye, C.; Wen, H.; Zhang, G.; Luo, D.; Jiang, F.; et al. Carbon fibers derived from spent cigarette filters for supporting ZnIn2S4/g-C3N4 heterojunction toward enhanced photocatalytic hydrogen evolution. Mater. Sci. Eng. B 2023, 288, 116214. [Google Scholar] [CrossRef]
- Zhong, X.; Li, Y.; Wu, H.; Xie, R. Recent progress in BiVO4-based heterojunction nanomaterials for photocatalytic applications. Mater. Sci. Eng. B 2023, 289, 116278. [Google Scholar] [CrossRef]
- An, L.; Han, X.; Li, Y.; Wang, H.; Hou, C.; Zhang, Q. One step synthesis of self-doped F–Ta2O5 nanoshuttles photocatalyst and enhanced photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2020, 46, 3996–4006. [Google Scholar] [CrossRef]
- Alhabradi, M.; Nundy, S.; Ghosh, A.; Tahir, A.A. Vertically Aligned CdO-Decked α-Fe2O3 Nanorod Arrays by a Radio Frequency Sputtering Method for Enhanced Photocatalytic Applications. ACS Omega 2022, 7, 28396–28407. [Google Scholar] [CrossRef]
- Ren, R.; Zhao, H.; Sui, X.; Guo, X.; Huang, X.; Wang, Y.; Dong, Q.; Chen, J. Exfoliated Molybdenum Disulfide Encapsulated in a Metal Organic Framework for Enhanced Photocatalytic Hydrogen Evolution. Catalysts 2019, 9, 89. [Google Scholar] [CrossRef]
- Romero, N.; Bofill, R.; Francàs, L.; García-Antón, J.; Sala, X. Light-Driven Hydrogen Evolution Assisted by Covalent Organic Frameworks. Catalysts 2021, 11, 754. [Google Scholar] [CrossRef]
- Cao, X.; Wen, P.; Ma, R.; Liu, Y.; Sun, S.; Ma, Q.; Zhang, P.; Qiu, Y. Ni2P nanocrystals modification on Ta:α-Fe2O3 photoanode for efficient photoelectrochemical water splitting: In situ formation and synergistic catalysis of Ni2P@NiOOH cocatalyst. Chem. Eng. J. 2022, 449, 137792. [Google Scholar] [CrossRef]
- Chang, H.-W.; Fu, Y.; Lee, W.-Y.; Lu, Y.-R.; Huang, Y.-C.; Chen, J.-L.; Chen, C.-L.; Chou, W.C.; Chen, J.-M.; Lee, J.-F.; et al. Visible light-induced electronic structure modulation of Nb- and Ta-doped α-Fe2O3 nanorods for effective photoelectrochemical water splitting. Nanotechnology 2018, 29, 064002. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Chen, Z.; Wei, X.; Xiao, W.; Ding, J. Economical Fe-doped Ta2O5 electrocatalyst toward efficient oxygen evolution: A combined experimental and first-principles study. MRS Commun. 2017, 7, 563–569. [Google Scholar] [CrossRef]
- Jing, D.; Guo, L. Hydrogen production over Fe-doped tantalum oxide from an aqueous methanol solution under the light irradiation. J. Phys. Chem. Solids 2007, 68, 2363–2369. [Google Scholar] [CrossRef]
- Kong, F.; Jiao, G.; Wang, J.; Tao, S.; Han, Z.; Fang, Y.; Zhang, L.; Qian, B.; Jiang, X. Preparation and characterization of nano-sized FeTaO4/graphite for lithium-ion batteries. Solid State Ionics 2017, 313, 45–51. [Google Scholar] [CrossRef]
- Zhang, H.; Noh, W.Y.; Li, F.; Kim, J.H.; Jeong, H.Y.; Lee, J.S. Three Birds, One-Stone Strategy for Hybrid Microwave Synthesis of Ta and Sn Codoped Fe2O3 @FeTaO4 Nanorods for Photo-Electrochemical Water Oxidation. Adv. Funct. Mater. 2019, 29, 1805737. [Google Scholar] [CrossRef]
- Lucas, S.; Moskovkin, P. Simulation at high temperature of atomic deposition, islands coalescence, Ostwald and inverse Ostwald ripening with a general simple kinetic Monte Carlo code. Thin Solid Films 2010, 518, 5355–5361. [Google Scholar] [CrossRef]
- Kharatyan, T.; Gopireddy, S.R.; Ogawa, T.; Kodama, T.; Nishimoto, N.; Osada, S.; Scherließ, R.; Urbanetz, N.A. Quantitative Analysis of Glassy State Relaxation and Ostwald Ripening during Annealing Using Freeze-Drying Microscopy. Pharmaceutics 2022, 14, 1176. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.; Xin, Y.; Liu, F.; Xuan, J.; Guo, M.; Duan, T. IrO2-Ta2O5 Anode for Oxygen Evolution with TaOx Interlayer Prepared by Thermal Decomposition in Inert Atmosphere. J. Electrochem. Soc. 2022, 169, 046516. [Google Scholar] [CrossRef]
- Mathi, S.; Jayabharathi, J. Enhanced stability and ultrahigh activity of amorphous ripple nanostructured Ni-doped Fe oxyhydroxide electrode toward synergetic electrocatalytic water splitting. RSC Adv. 2020, 10, 26364–26373. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, N.; Guan, J. Accelerative oxygen evolution by Cu-doping into Fe-Co oxides. Sustain. Energy Fuels 2020, 4, 143–148. [Google Scholar] [CrossRef]
- Li, Z.; Chen, H.; Liu, W. Full-Spectrum Photocatalytic Activity of ZnO/CuO/ZnFe2O4 Nanocomposite as a PhotoFenton-Like Catalyst. Catalysts 2018, 8, 557. [Google Scholar] [CrossRef]
- Abdelkader, E.; Nadjia, L.; Naceur, B.; Favier-Teodorescu, L. Thermal, structural and optical properties of magnetic BiFeO3 micron-particles synthesized by coprecipitation method: Heterogeneous photocatalysis study under white LED irradiation. Cerâmica 2022, 68, 84–96. [Google Scholar] [CrossRef]
- Maia DL, S.; Pepe, I.; da Silva, A.F.; Silva, L.A. Visible-light-driven photocatalytic hydrogen production over dye-sensitized β-BiTaO4. J. Photochem. Photobiol. A Chem. 2012, 243, 61–64. [Google Scholar] [CrossRef]
- Montoya, A.T.; Gillan, E.G. Enhanced Photocatalytic Hydrogen Evolution from Transition-Metal Surface-Modified TiO2. ACS Omega 2018, 3, 2947–2955. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Diwu, J.; Zhang, Q.; Xu, H.; Chou, X.; Tang, J.; Xue, C. A Green and Facile Synthesis of Carbon-Incorporated Co3O4 Nanoparticles and Their Photocatalytic Activity for Hydrogen Evolution. J. Nanomater. 2015, 2015, 618492. [Google Scholar] [CrossRef]
- Ye, J.; Zou, Z.; Oshikiri, M.; Matsushita, A.; Shimoda, M.; Imai, M.; Shishido, T. A novel hydrogen-evolving photocatalyst InVO4 active under visible light irradiation. Chem. Phys. Lett. 2002, 356, 221–226. [Google Scholar] [CrossRef]
- Zou, Z.; Ye, J.; Sayama, K.; Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature 2001, 414, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Ye, J.; Sayama, K.; Arakawa, H. Photocatalytic and photophysical properties of a novel series of solid photocatalysts, BiTa1−xNbxO4 (0 ≤ x ≤ 1). Chem. Phys. Lett. 2001, 343, 303–308. [Google Scholar] [CrossRef]
- Yang, M.; Huang, X.; Yan, S.; Li, Z.; Yu, T.; Zou, Z. Improved hydrogen evolution activities under visible light irradiation over NaTaO3 codoped with lanthanum and chromium. Mater. Chem. Phys. 2010, 121, 506–510. [Google Scholar] [CrossRef]
- Hu, C.-C.; Nian, J.-N.; Teng, H. Electrodeposited p-type Cu2O as photocatalyst for H2 evolution from water reduction in the presence of WO3. Sol. Energy Mater. Sol. Cells 2008, 92, 1071–1076. [Google Scholar] [CrossRef]
- El-Bery, H.M.; Salah, M.R.; Ahmed, S.M.; Soliman, S.A. Efficient non-metal based conducting polymers for photocatalytic hydrogen production: Comparative study between polyaniline, polypyrrole and PEDOT. RSC Adv. 2021, 11, 13229–13244. [Google Scholar] [CrossRef]
- Yin, X.-T.; Dastan, D.; Wu, F.-Y.; Li, J. Facile Synthesis of SnO2/LaFeO3−XNX Composite: Photocatalytic Activity and Gas Sensing Performance. Nanomaterials 2019, 9, 1163. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, Y.; Mao, S.; Hao, X.; Duan, X.; Wen, Y. Different Morphology MoS2 Over the g-C3N4 as a Boosted Photo-Catalyst for Pollutant Removal Under Visible-Light. J. Inorg. Organomet. Polym. Mater. 2020, 31, 32–42. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, X.; Li, Z.; Meng, X. Photocatalytic Reforming for Hydrogen Evolution: A Review. Catalysts 2020, 10, 335. [Google Scholar] [CrossRef]
- Liu, D.; Zi, W.; Sajjad, S.D.; Hsu, C.; Shen, Y.; Wei, M.; Liu, F. Reversible Electron Storage in an All-Vanadium Photoelectrochemical Storage Cell: Synergy between Vanadium Redox and Hybrid Photocatalyst. ACS Catal. 2015, 5, 2632–2639. [Google Scholar] [CrossRef]
- Park, G.-S.; Kim, Y.B.; Park, S.Y.; Li, X.S.; Heo, S.; Lee, M.-J.; Chang, M.; Kwon, J.H.; Kim, M.; Chung, U.-I.; et al. In situ observation of filamentary conducting channels in an asymmetric Ta2O5−x/TaO2−x bilayer structure. Nat. Commun. 2013, 4, 2382. [Google Scholar] [CrossRef] [PubMed]
- Chun, W.-J.; Ishikawa, A.; Fujisawa, H.; Takata, T.; Kondo, J.N.; Hara, M.; Kawai, M.; Matsumoto, Y.; Domen, K. Conduction and Valence Band Positions of Ta2O5, TaON, and Ta3N5 by UPS and Electrochemical Methods. J. Phys. Chem. B 2003, 107, 1798–1803. [Google Scholar] [CrossRef]
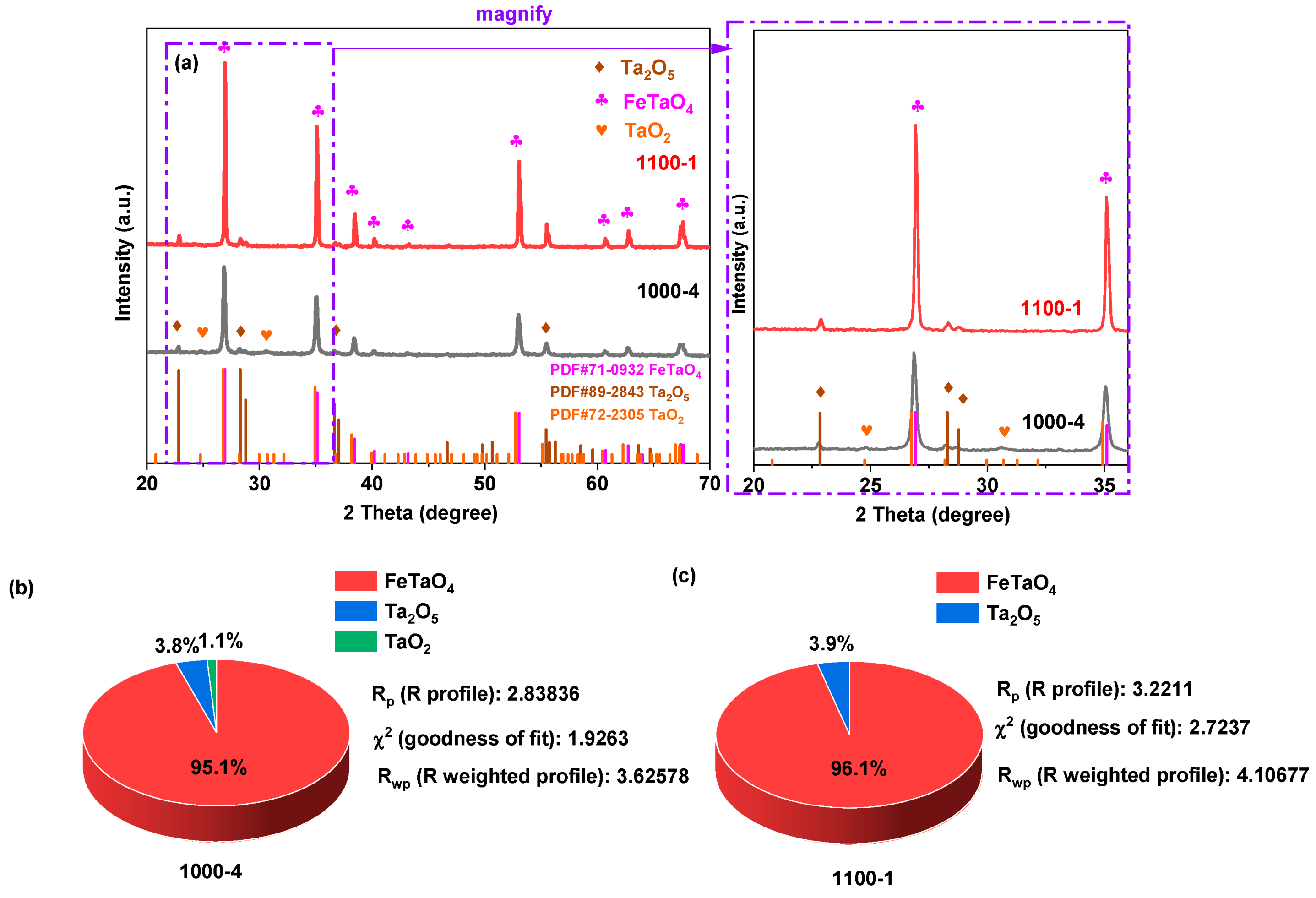
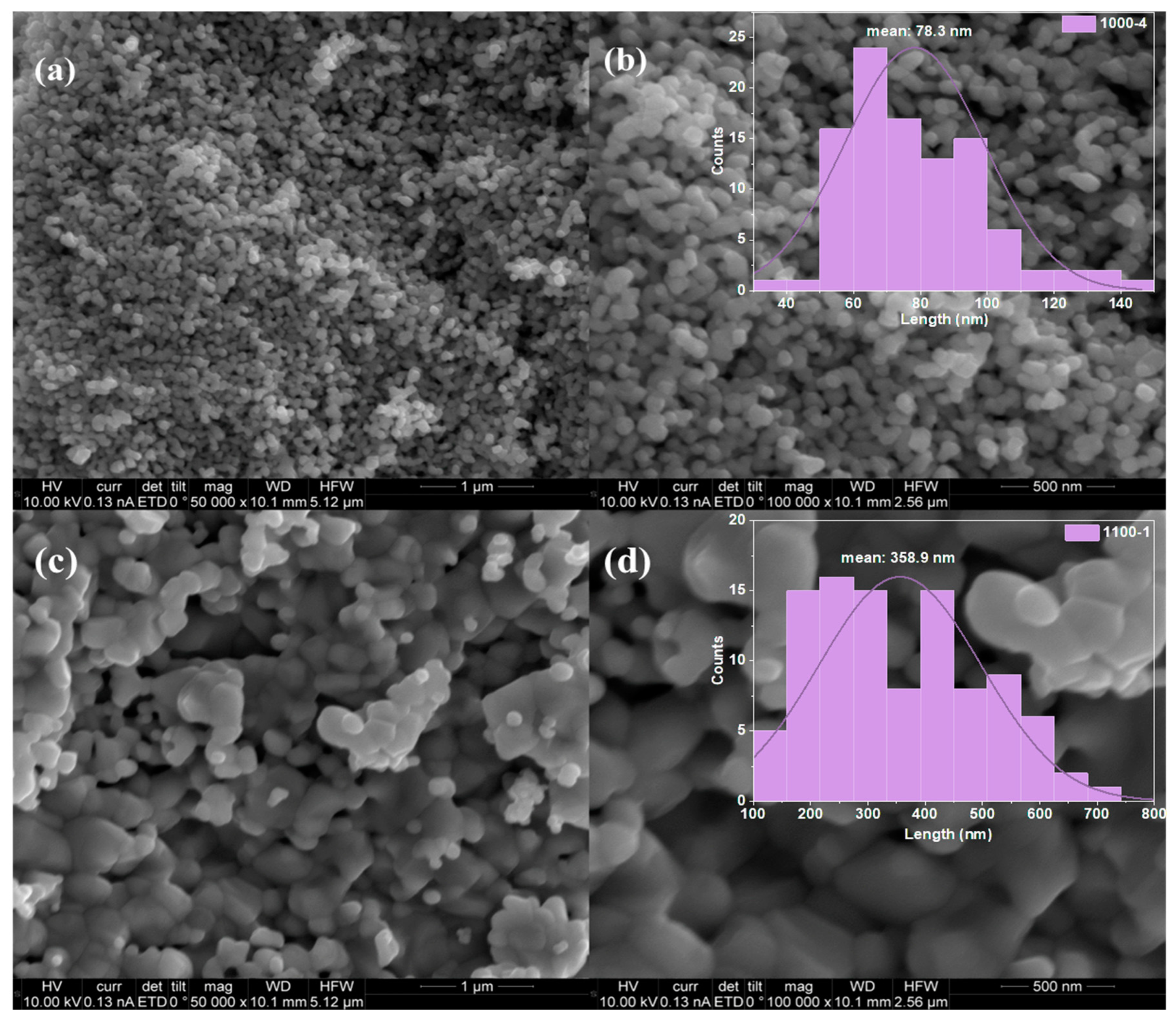
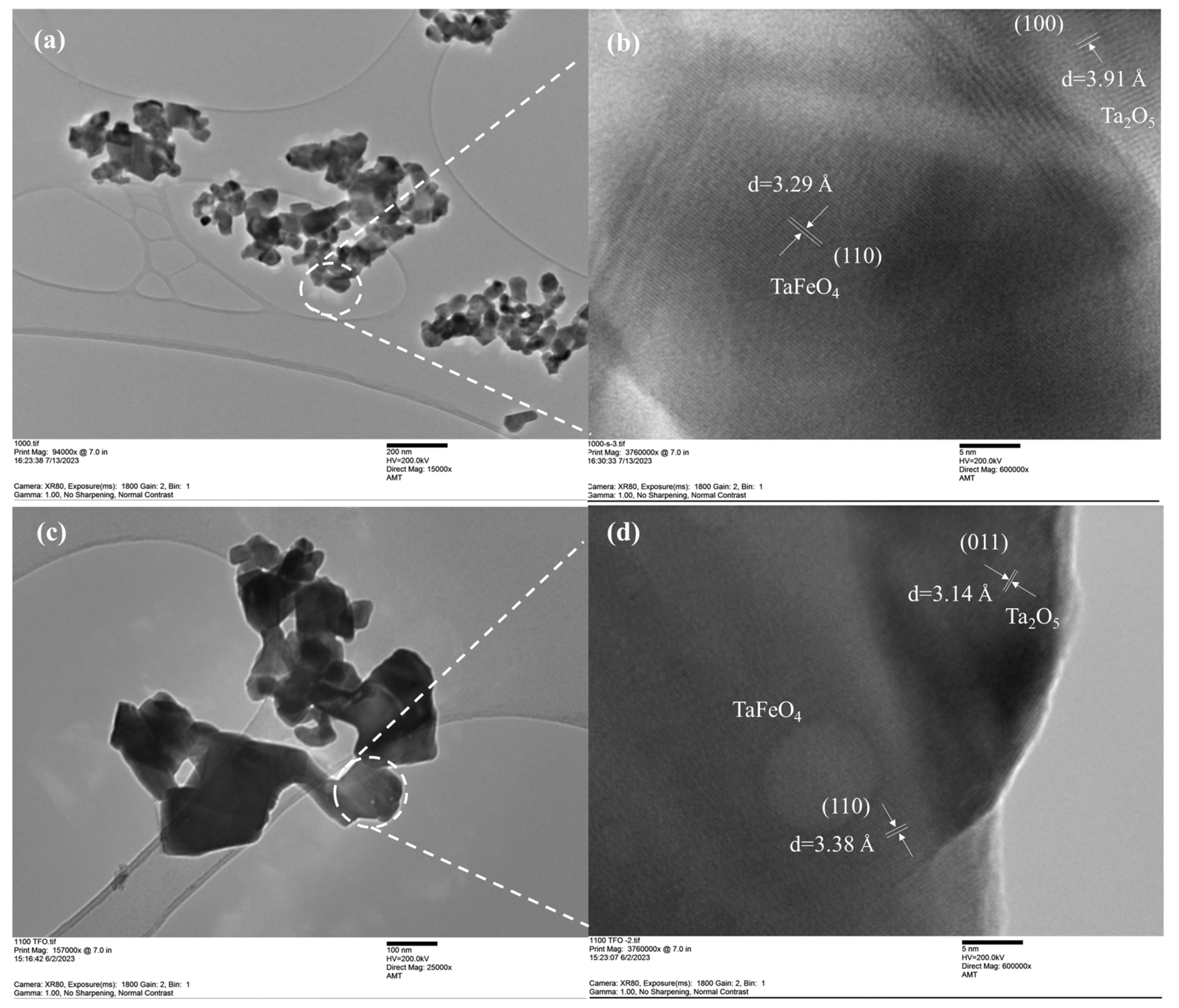
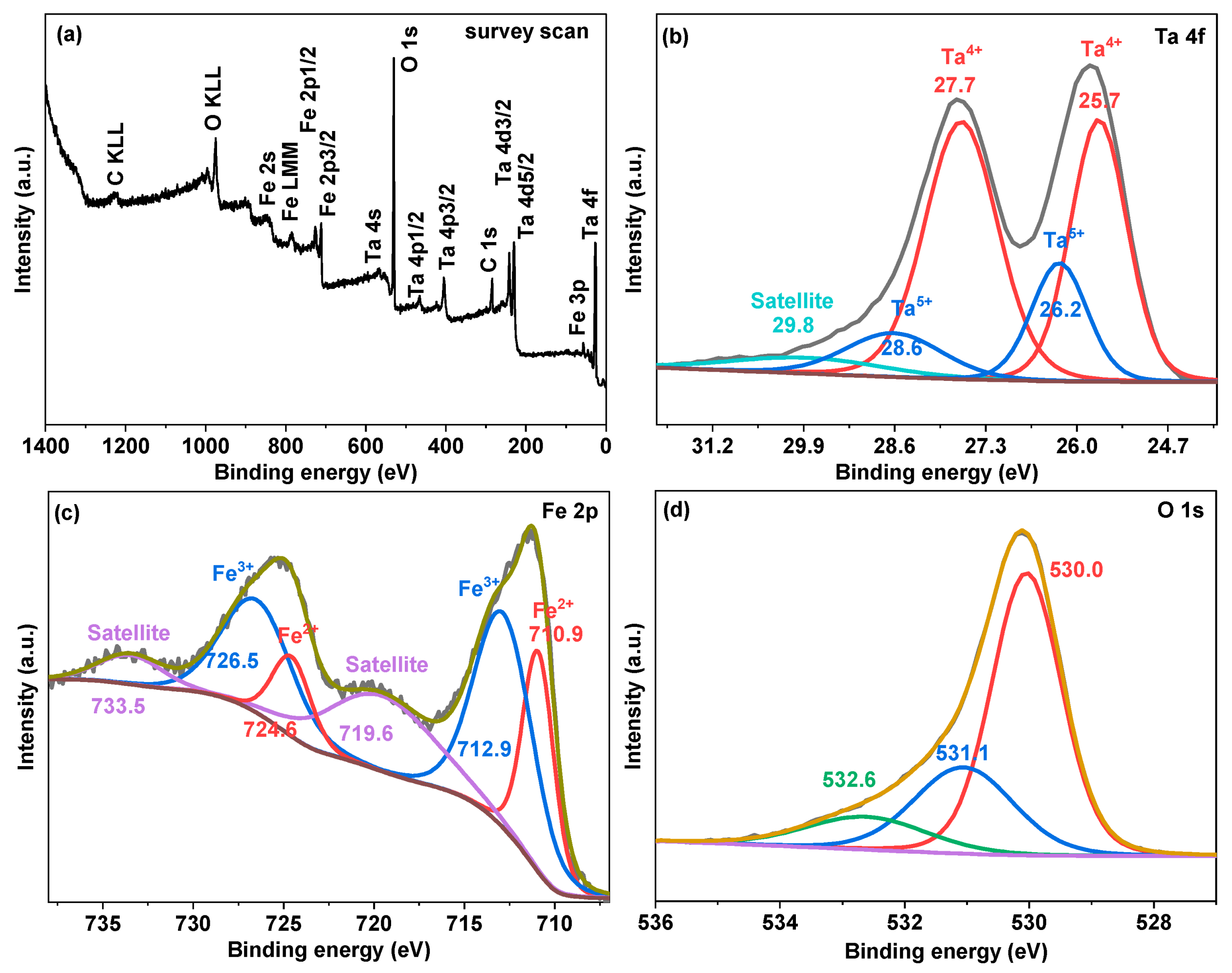
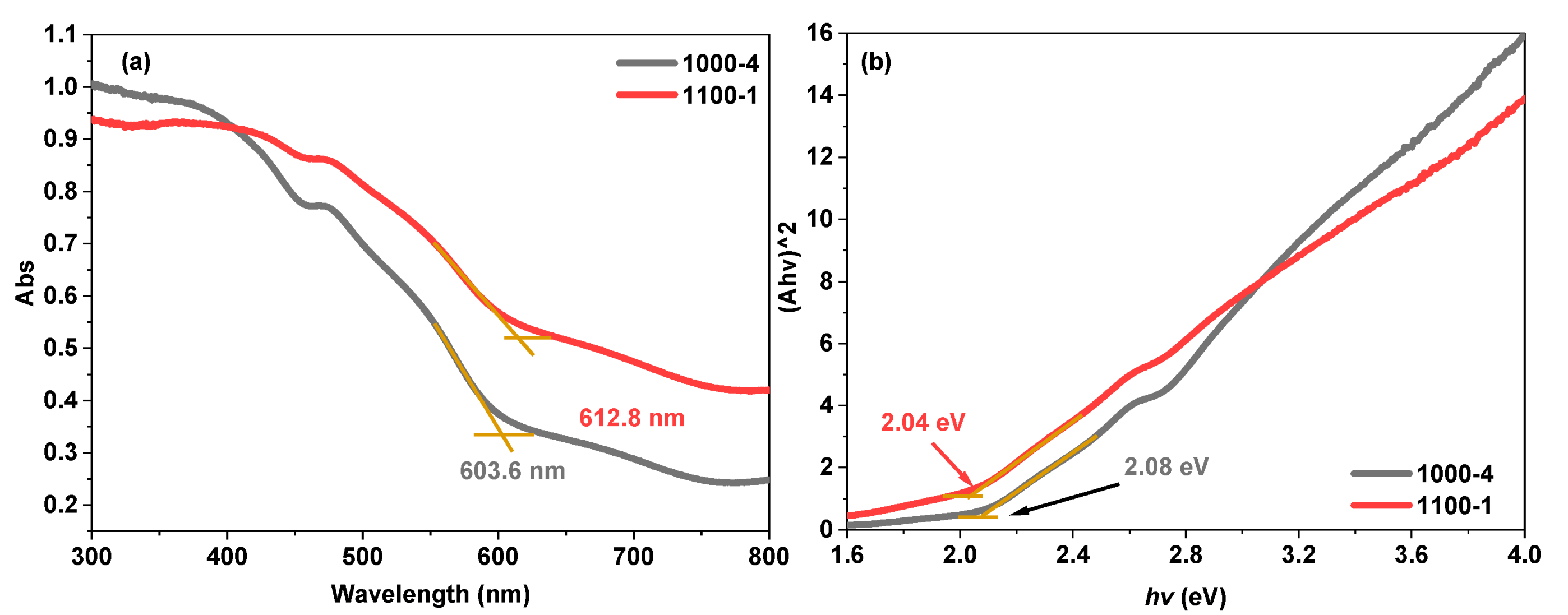
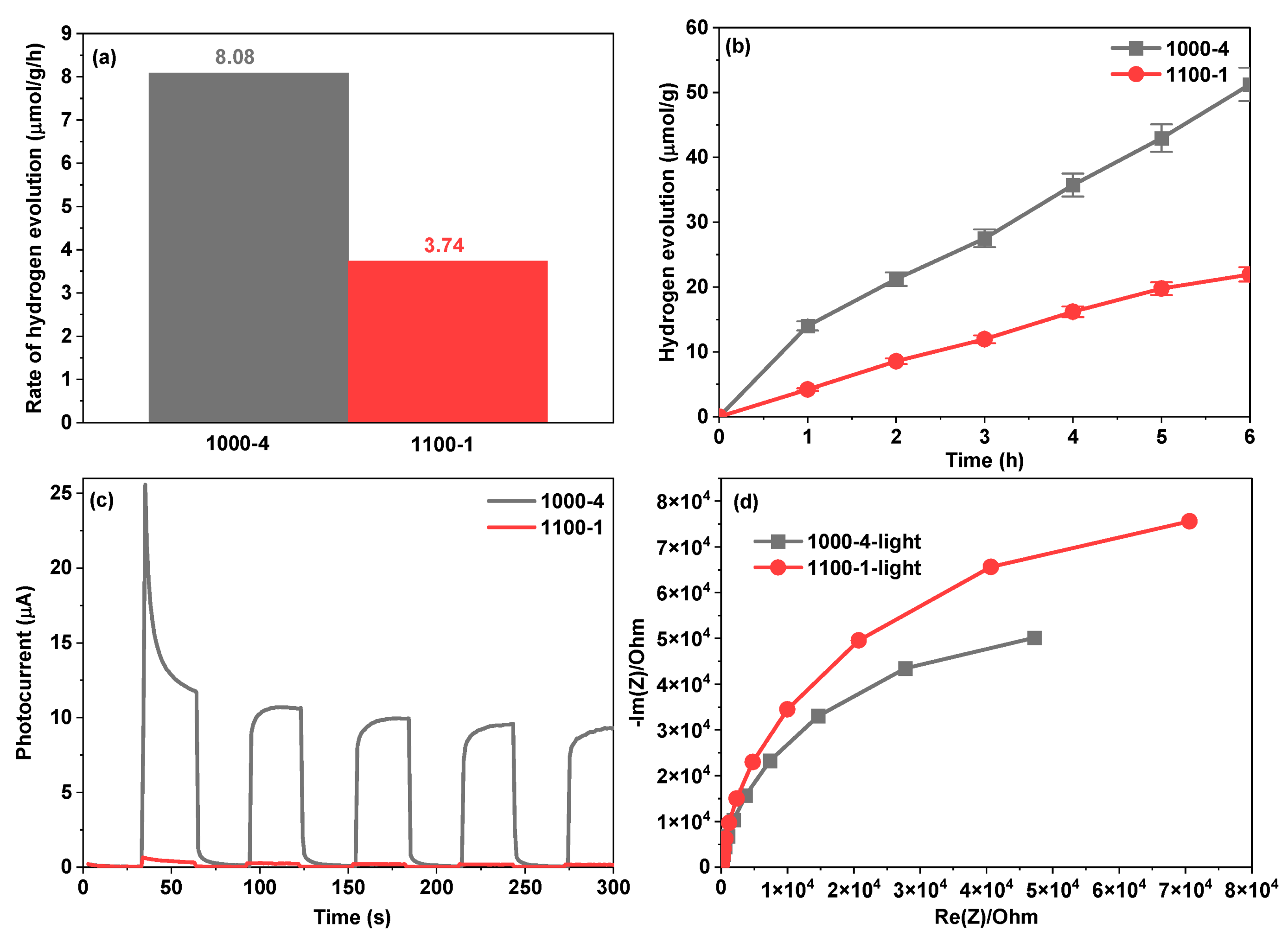
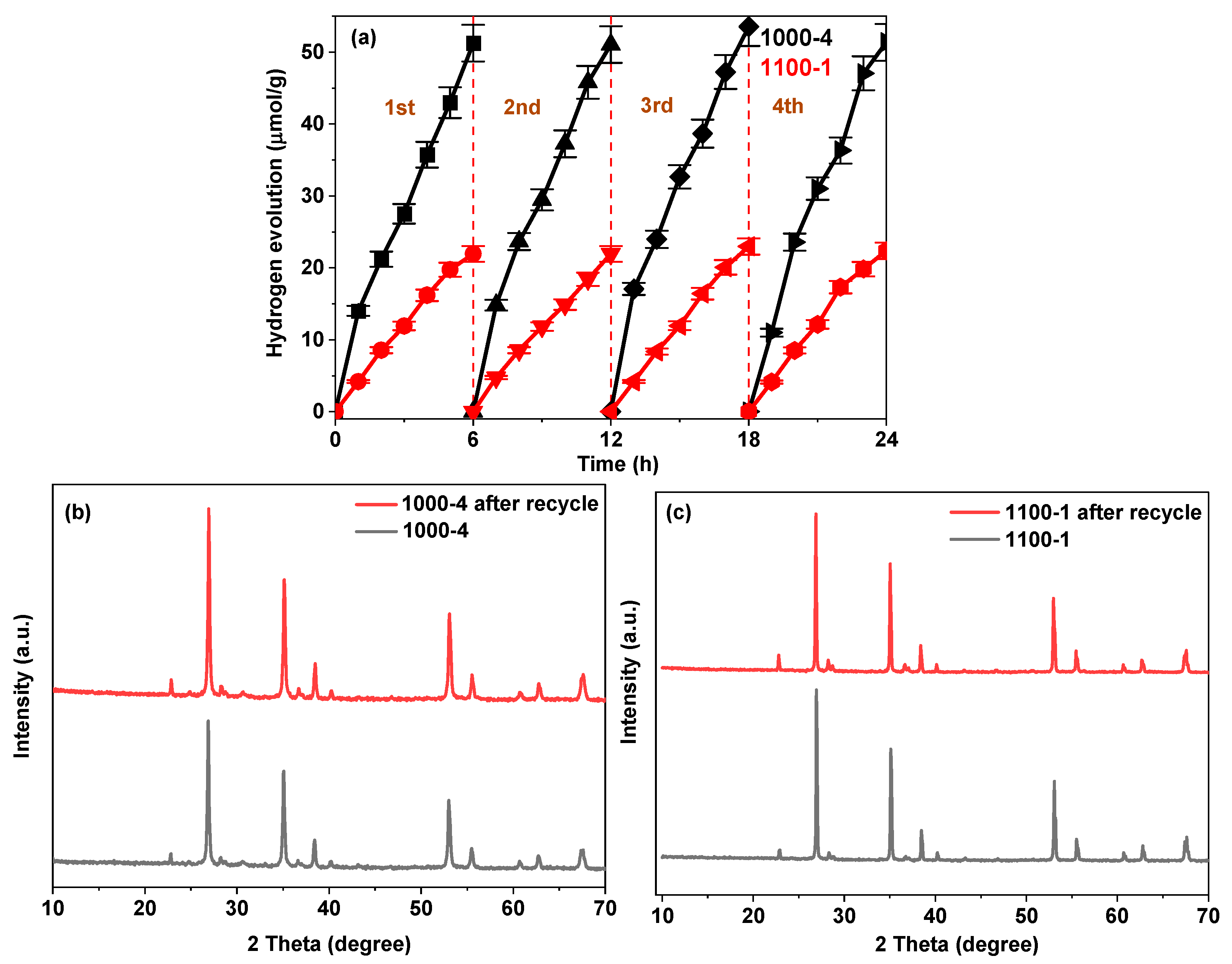
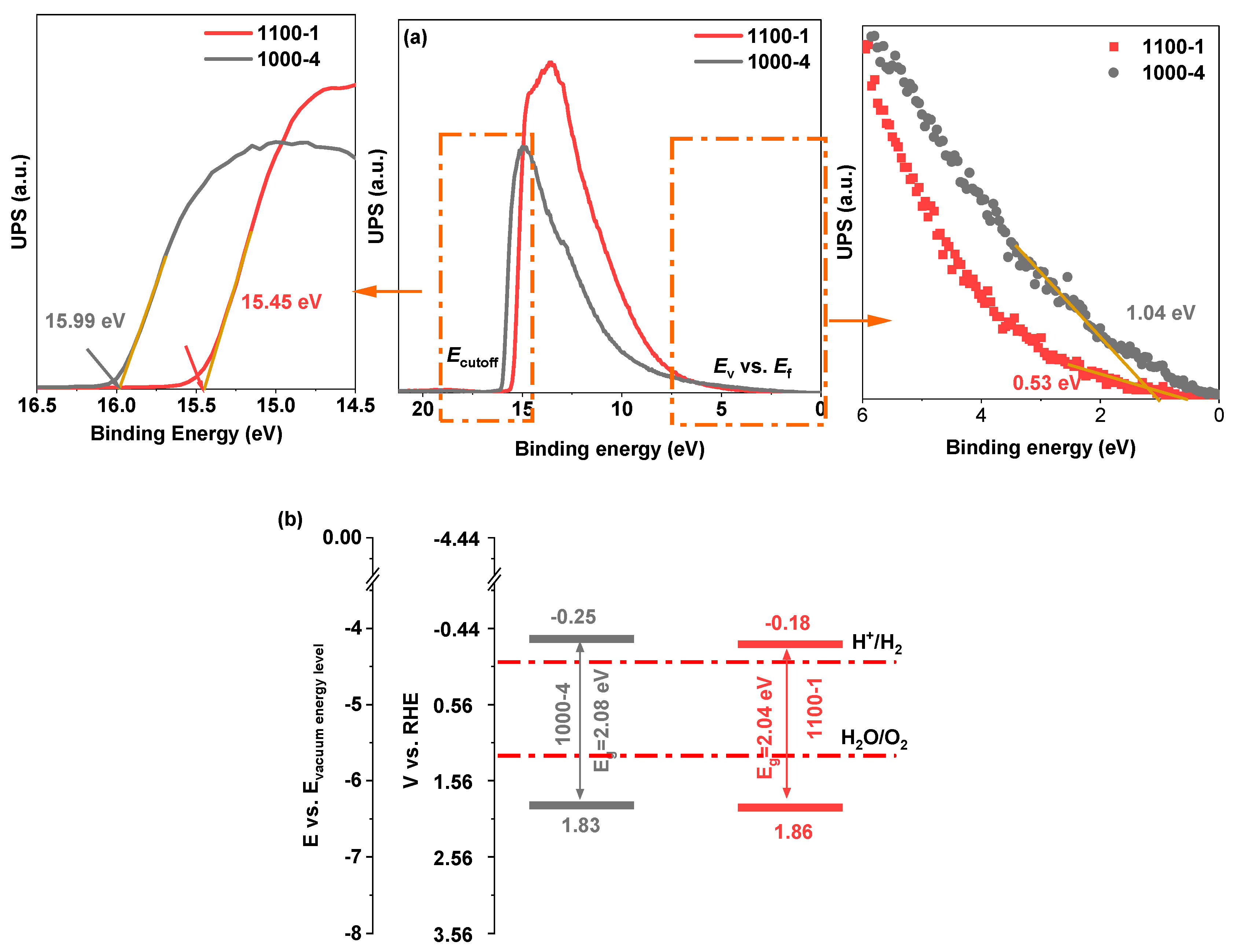
| Photocatalysts | Hydrogen Evolution Rate (µmol/h) | Light Resource | Ref. |
|---|---|---|---|
| P25 | 6.4 | Ultraviolet light | [34] |
| Carbon-incorporated Co3O4 | 0.85 | Solar light | [35] |
| InVO4 | 5 | Ultraviolet light | [36] |
| InTaO4 | 4 | Ultraviolet light | [37] |
| BiTaO4 | 4 | Ultraviolet light | [38] |
| NaTaO3: La/Cr | 4.4 | Ultraviolet light | [39] |
| Cu2O/WO3 | 1.9 | Ultraviolet light | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Roy, A.; Alhabradi, M.; Alruwaili, M.; Chang, H.; Tahir, A.A. Fabrication and Characterization of Tantalum–Iron Composites for Photocatalytic Hydrogen Evolution. Nanomaterials 2023, 13, 2464. https://doi.org/10.3390/nano13172464
Yang X, Roy A, Alhabradi M, Alruwaili M, Chang H, Tahir AA. Fabrication and Characterization of Tantalum–Iron Composites for Photocatalytic Hydrogen Evolution. Nanomaterials. 2023; 13(17):2464. https://doi.org/10.3390/nano13172464
Chicago/Turabian StyleYang, Xiuru, Anurag Roy, Mansour Alhabradi, Manal Alruwaili, Hong Chang, and Asif Ali Tahir. 2023. "Fabrication and Characterization of Tantalum–Iron Composites for Photocatalytic Hydrogen Evolution" Nanomaterials 13, no. 17: 2464. https://doi.org/10.3390/nano13172464
APA StyleYang, X., Roy, A., Alhabradi, M., Alruwaili, M., Chang, H., & Tahir, A. A. (2023). Fabrication and Characterization of Tantalum–Iron Composites for Photocatalytic Hydrogen Evolution. Nanomaterials, 13(17), 2464. https://doi.org/10.3390/nano13172464








