Design, Synthesis, and Characterization of a Novel Blue-Green Long Afterglow BaYAl3O7:Eu2+, Nd3+ Phosphor and Its Anti-Counterfeiting Application
Abstract
:1. Introduction
2. Experimental Section
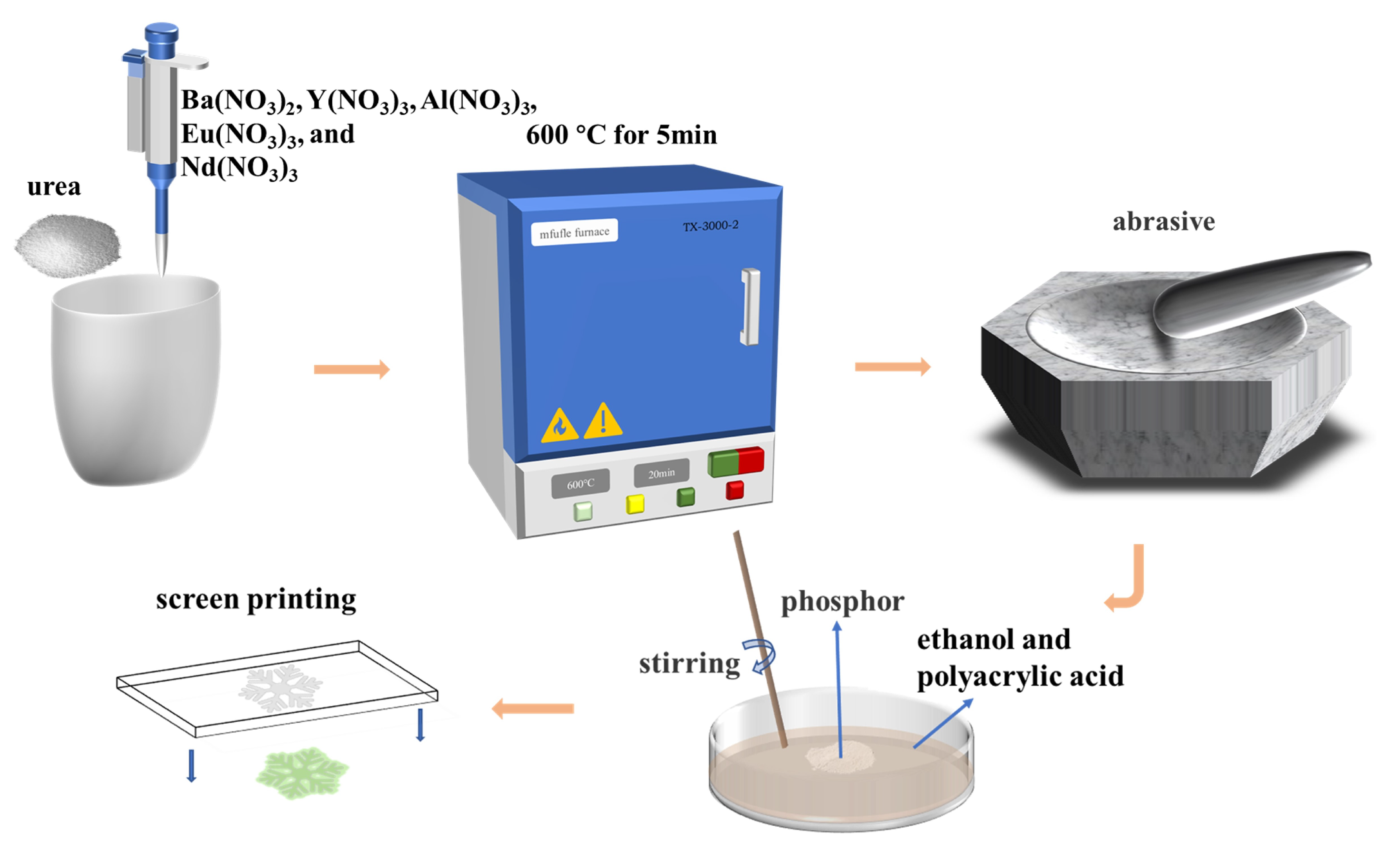
2.1. Materials and Method
2.2. Synthesis of Nanomaterials
2.3. Preparation of Anti-Counterfeit Ink
2.4. Characterizations
3. Results and Discussion
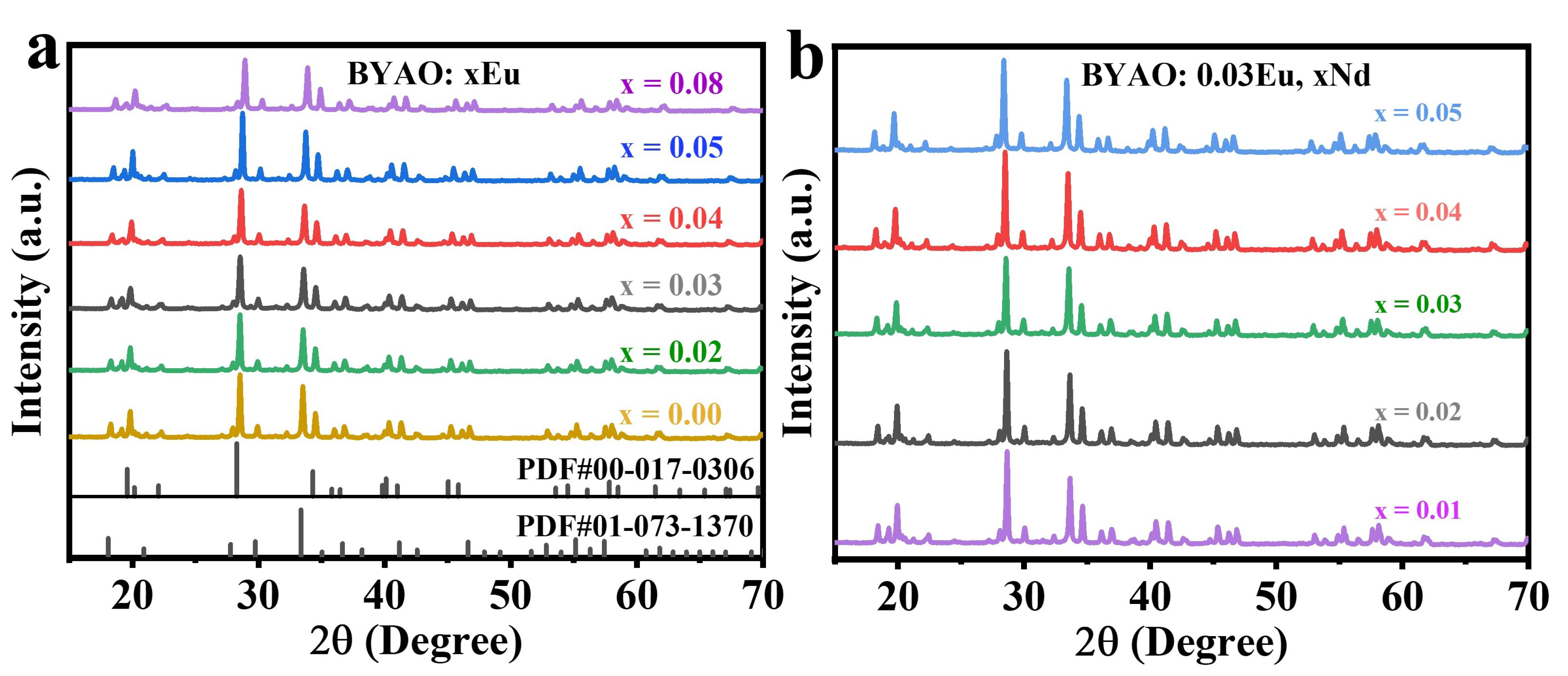
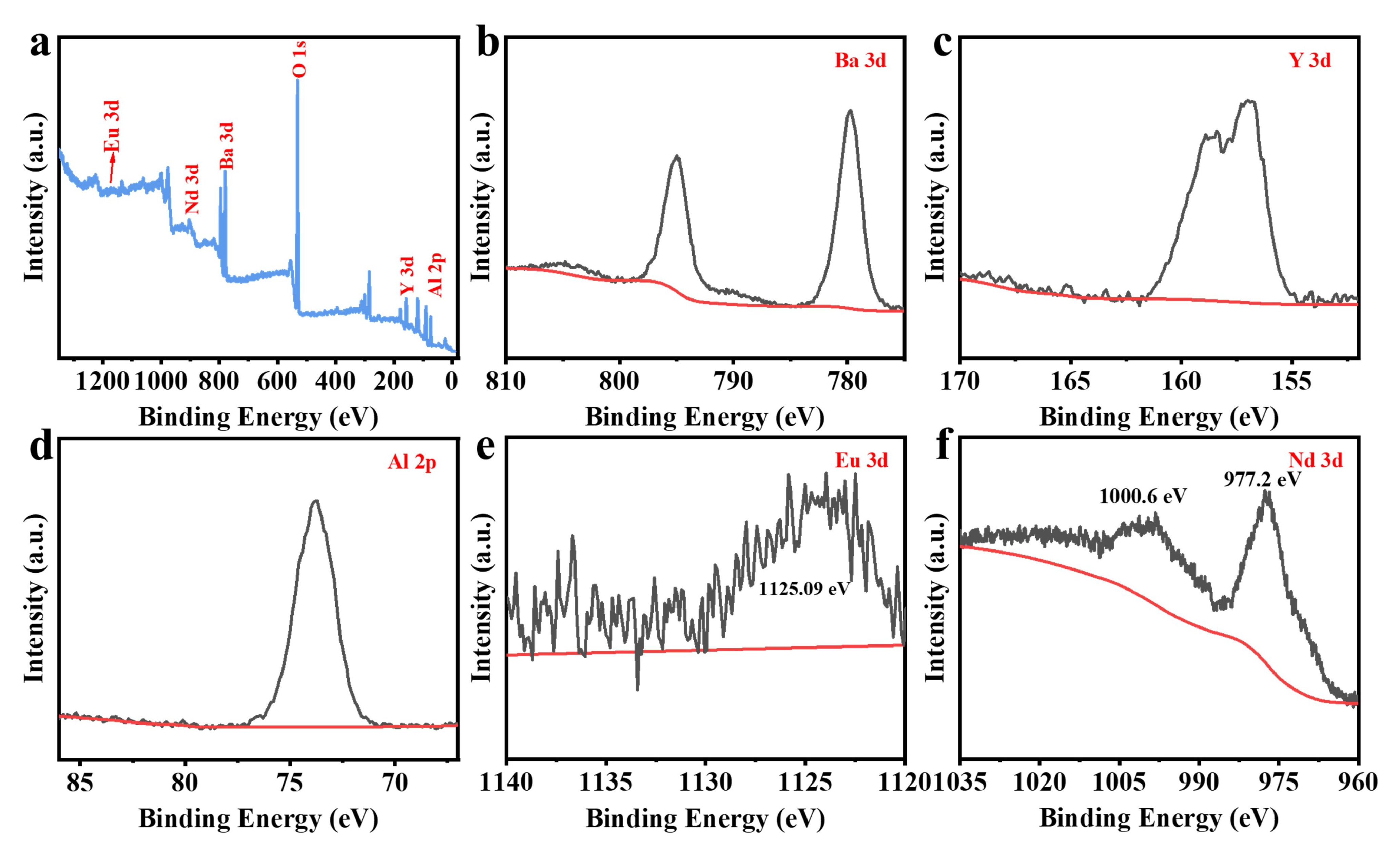
3.1. XRD Patterns and Morphology Analysis
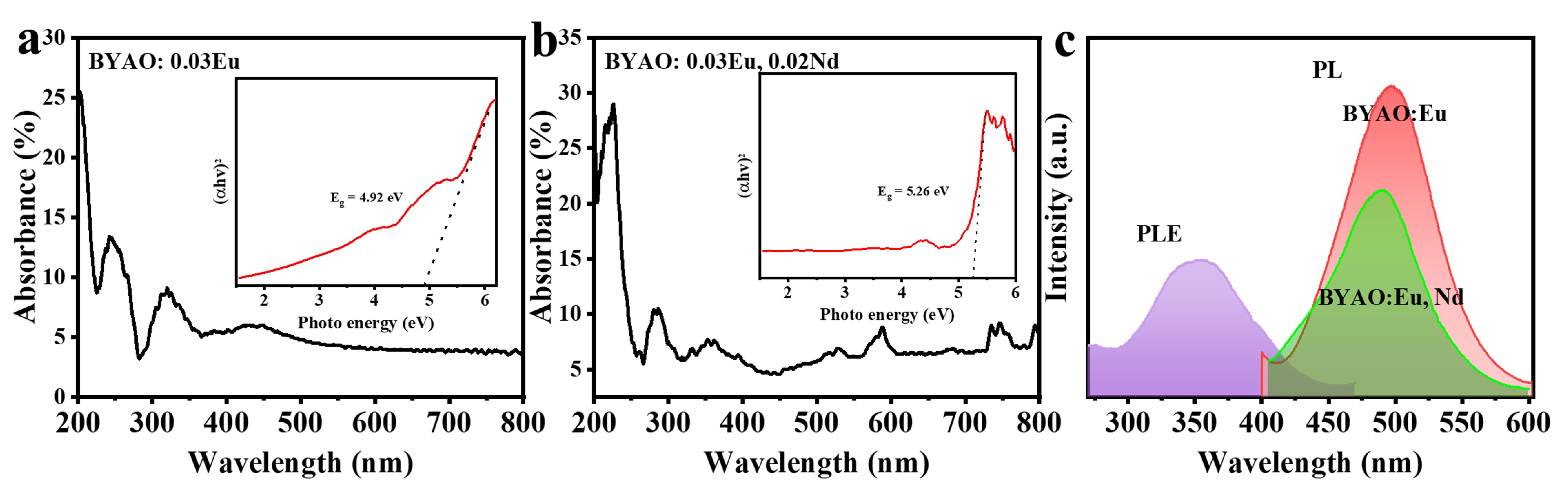
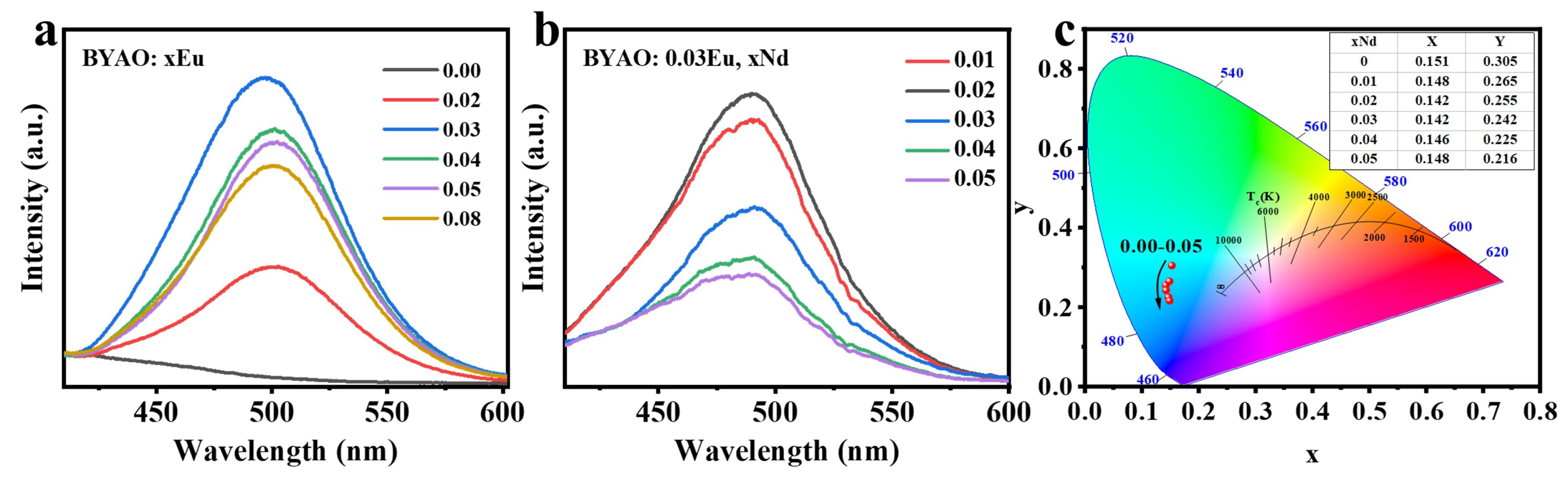
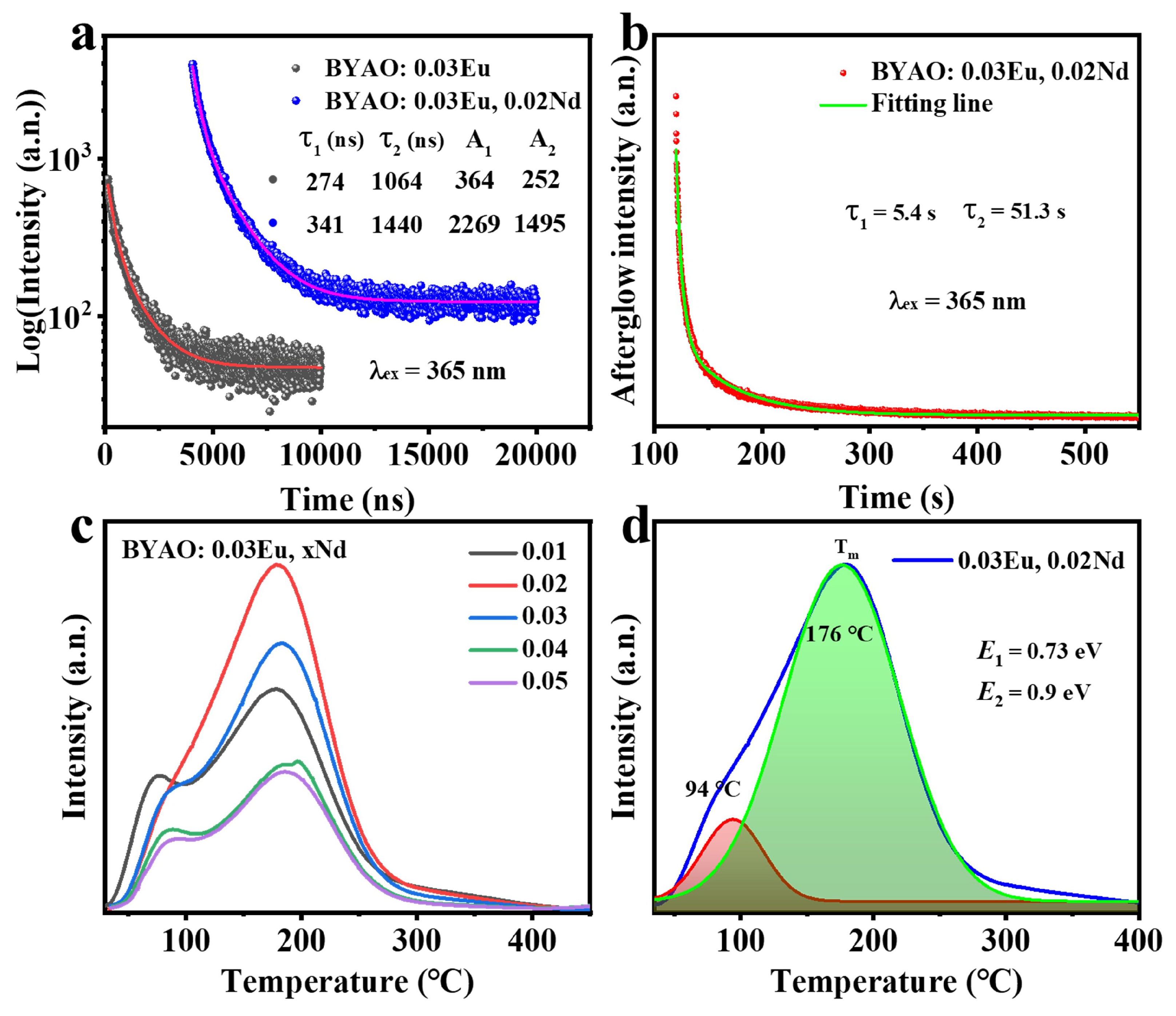
3.2. Photoluminescence and Afterglow Properties
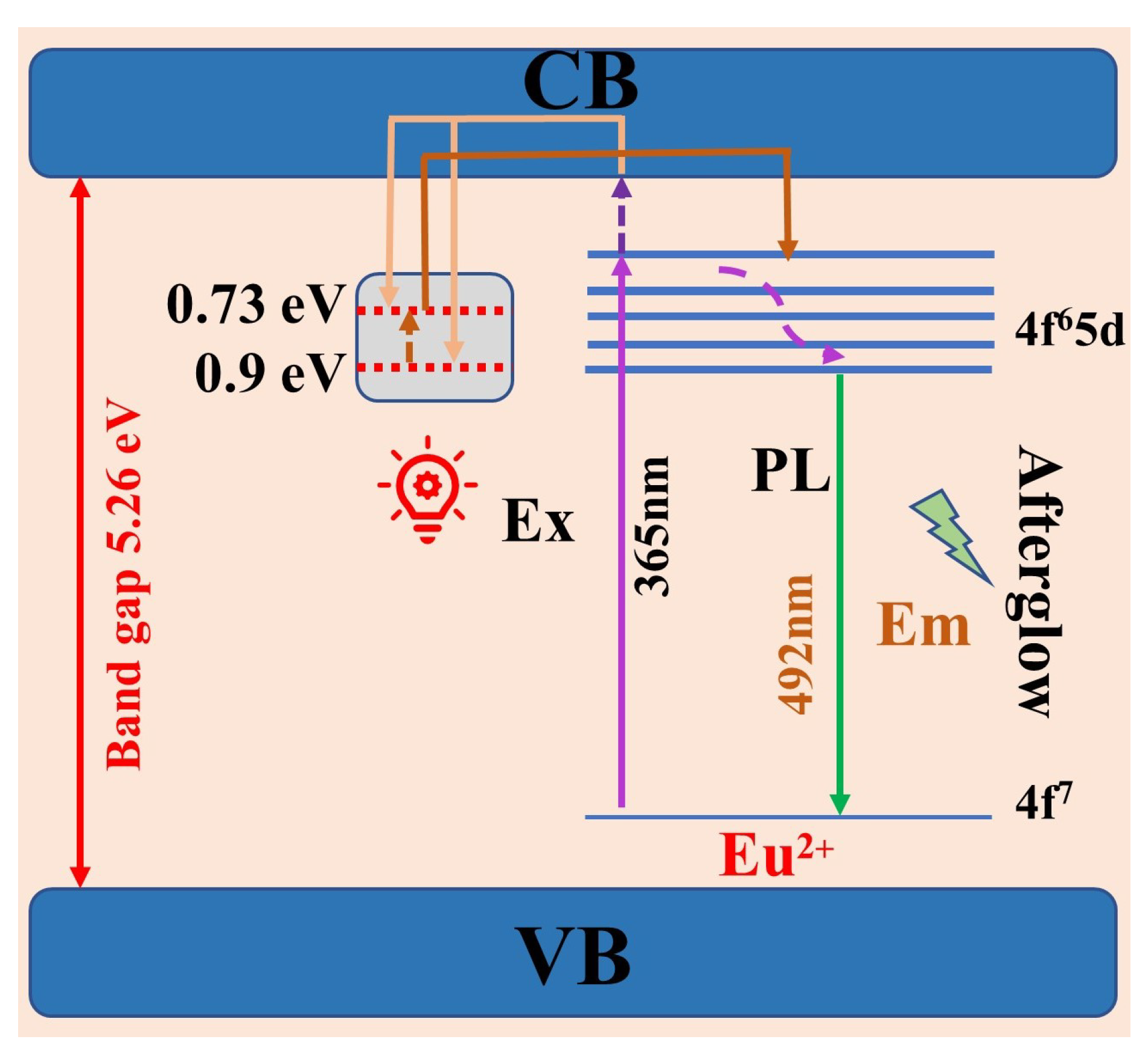
3.3. Mechanism for the Afterglow of the BYAO:Eu2+, Nd3+ Phosphor
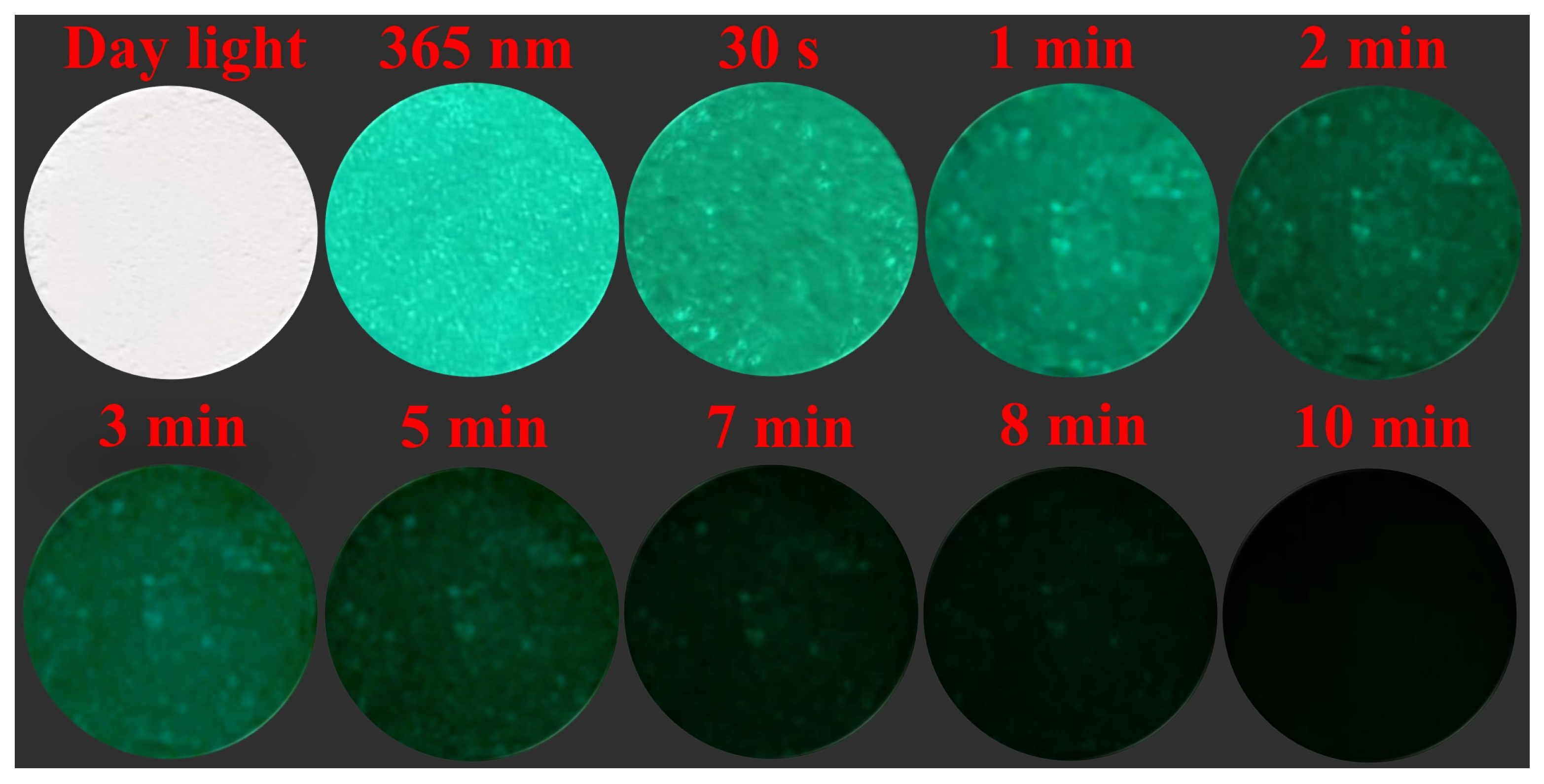
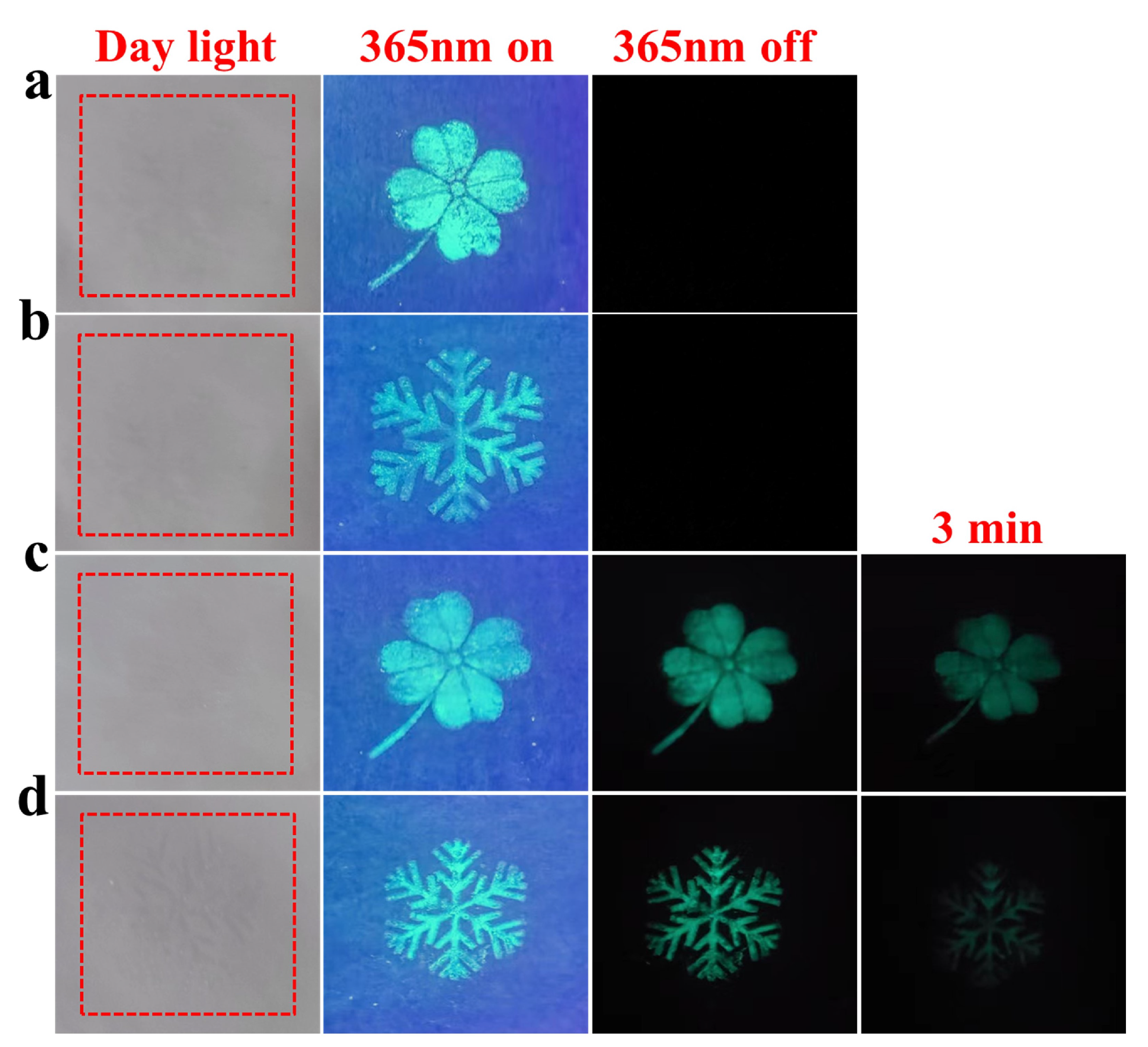
3.4. Anti-Counterfeiting Application
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, Z.; Lu, Y.Y.; Liu, F. Sunlight-activated long-persistent luminescence in the near-infrared from Cr3+-doped zinc gallogermanates. Nat. Mater. 2012, 11, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, S. Recent Progress on Lanthanide-based Long Persistent Phosphors: An Overview. J. Mater. Chem. C 2023, 11, 8649–8687. [Google Scholar] [CrossRef]
- Wang, J.; Su, Q.; Wang, S. Blue and red long lasting phosphorescence (LLP) in β-Zn3(PO4)2:Mn2+, Zr4+. J. Phys. Chem. Solids 2005, 66, 1171–1176. [Google Scholar] [CrossRef]
- Liang, L.; Chen, N.; Jia, Y.; Ma, Q.; Wang, J.; Yuan, Q.; Tan, W. Recent progress in engineering near-infrared persistent luminescence nanoprobes for time-resolved biosensing/bioimaging. Nano Res. 2019, 12, 1279–1292. [Google Scholar] [CrossRef]
- Li, H.; Pang, R.; Sun, W.; Li, H.; Ma, T.; Jia, Y.; Li, D.; Jiang, L.; Zhang, S.; Li, C. Sr1.7Zn0.3CeO4F0.2:Eu3+: Novel dual-emission temperature sensors for remote, noncontact thermometric application. RSC Adv. 2017, 7, 9645–9652. [Google Scholar] [CrossRef]
- Poelman, D.; Van der Heggen, D.; Du, J.; Cosaert, E.; Smet, P.F. Persistent phosphors for the future: Fit for the right application. J. Appl. Phys. 2020, 128, 240903. [Google Scholar] [CrossRef]
- Gupta, I.; Singh, S.; Bhagwan, S.; Singh, D. Rare earth (RE) doped phosphors and their emerging applications: A review. Ceram. Int. 2021, 47, 19282–19303. [Google Scholar] [CrossRef]
- Lian, S.; Qi, Y.; Rong, C.; Yu, L.; Zhu, A.; Yin, D.; Liu, S. Effectively leveraging solar energy through persistent dual red phosphorescence: Preparation, characterization, and density functional theory study of Ca2Zn4Ti16O38:Pr3+. J. Phys. Chem. C 2010, 114, 7196–7204. [Google Scholar] [CrossRef]
- Matsuzawa, T.; Aoki, Y.; Takeuchi, N.; Murayama, Y. A new long phosphorescent phosphor with high brightness, SrAl2O4:Eu2+, Dy3+. J. Electrochem. Soc. 1996, 143, 2670. [Google Scholar] [CrossRef]
- Kong, J.; Meijerink, A. Identification and Quantification of Charge Transfer in CaAl2O4:Eu2+, Nd3+ Persistent Phosphor. Adv. Opt. Mater. 2023, 11, 2203004. [Google Scholar] [CrossRef]
- Kadyan, S.; Singh, S.; Simantilleke, A.P.; Singh, D. Synthesis and optical studies of nanocrystalline Eu2+-doped and RE3+ (Nd3+, Dy3+)-codoped Ba4Al14O25 materials for UV-LEDs. Optik 2020, 212, 164671. [Google Scholar] [CrossRef]
- Palilla, F.C.; Levine, A.K.; Tomkus, M.R. Fluorescent properties of alkaline earth aluminates of the type MAl2O4 activated by divalent europium. J. Electrochem. Soc. 1968, 115, 642. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, Y.; Han, S.; Chen, W.; Li, G. Investigation on long-persistent luminescence of Ca2BO3Cl:Eu2+, Ln3+ (Ln = Nd, Dy, Er). Opt. Mater. 2014, 36, 1819–1821. [Google Scholar] [CrossRef]
- Yang, Y.M.; Li, Z.Y.; Zhang, J.Y.; Lu, Y.; Guo, S.Q.; Zhao, Q.; Wang, X.; Yong, Z.J.; Li, H.; Ma, J.P.; et al. X-ray-activated long persistent phosphors featuring strong UVC afterglow emissions. Light. Sci. Appl. 2018, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Lv, Y.; Li, Y.; Zhou, T.; Xu, J.; Ueda, J.; Tanabe, S.; Xie, R.J. Study on trap levels in SrSi2AlO2N3:Eu2+, Ln3+ persistent phosphors based on host-referred binding energy scheme and thermoluminescence analysis. Inorg. Chem. 2016, 55, 11890–11897. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Matsuzawa, T. Mechanism of long phosphorescence of SrAl2O4:Eu2+, Dy3+ and CaAl2O4:Eu2+, Nd3+. J. Lumin. 1997, 72, 287–289. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, Z.; Zhang, Z.; Wang, X.; Zhang, J. Preparation of a new long afterglow blue-emitting Sr2MgSi2O7-based photoluminescent phosphor. J. Mater. Sci. Lett. 2001, 20, 1505–1506. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, Y.; Han, S.; Chen, W.; Li, G.; Wang, Y.; Wen, Y. Design, synthesis and characterization of a novel yellow long-persistent phosphor:Ca2BO3Cl:Eu2+, Dy3+. J. Mater. Chem. C 2013, 1, 3004–3011. [Google Scholar] [CrossRef]
- Ueda, J.; Maki, R.; Tanabe, S. Vacuum referred binding energy (VRBE)-guided design of orange persistent Ca3Si2O7:Eu2+ phosphors. Inorg. Chem. 2017, 56, 10353–10360. [Google Scholar] [CrossRef]
- Suda, Y.; Tamura, Y.; Yamaguchi, S.; Nanai, Y.; Okuno, T. Red afterglow and luminescence arising from defects in CaS:Eu2+, Tm3+. J. Phys. D Appl. Phys. 2021, 54, 415103. [Google Scholar] [CrossRef]
- Talewar, R.A.; Mahamuda, S.; Rao, A.; Gaikwad, V.M.; Belsare, P.; Moharil, S. Broadband excited Nd3+ NIR emission in Sr5(PO4)3Cl:Eu2+, Nd3+ phosphor for solar spectral modification. J. Lumin. 2020, 222, 117118. [Google Scholar] [CrossRef]
- Peng, T.; Huajun, L.; Yang, H.; Yan, C. Synthesis of SrAl2O4:Eu, Dy phosphor nanometer powders by sol–gel processes and its optical properties. Mater. Chem. Phys. 2004, 85, 68–72. [Google Scholar] [CrossRef]
- Zand, S.K.; Baghshahi, S.; Rajabi, M. The effect of Eu and Dy dopants on the luminescence properties of Sr4Al14O25:Eu2+, Dy3+ phosphorescent nano-pigments prepared by the solution combustion method. J. Mater. Sci. Mater. Electron. 2016, 27, 12533–12538. [Google Scholar] [CrossRef]
- Nikhare, G.; Gedam, S.; Dhoble, S. Luminescence in Sr4Al14O25:Ce3+ aluminate phosphor. Luminescence 2015, 30, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Yuan, Z.; Mao, D. Eu2+ activated long persistent strontium aluminate nano scaled phosphor prepared by precipitation method. J. Alloys Compd. 2006, 415, 220–224. [Google Scholar] [CrossRef]
- Panasyuk, G.; Kozerozhets, I.; Semenov, E.; Azarova, L.; Belan, V.; Danchevskaya, M. A New Method for producing a nanosized γ-Al2O3 powder. Russ. J. Inorg. Chem. 2018, 63, 1303–1308. [Google Scholar] [CrossRef]
- Rafiaei, S.M.; Dini, G.; Bahrami, A. Synthesis, crystal structure, optical and adsorption properties of BaAl2O4:Eu2+, Eu2+/ L3+ (L = Dy, Er, Sm, Gd, Nd, and Pr) phosphors. Ceram. Int. 2020, 46, 20243–20250. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, I.; Singh, D. Sm3+-activated YAG nanocrystals: Synthesis, structural and spectroscopic analysis for orange-red emitting LEDs. Optik 2021, 238, 166482. [Google Scholar] [CrossRef]
- Zheng, B.; Fan, J.; Chen, B.; Qin, X.; Wang, J.; Wang, F.; Deng, R.; Liu, X. Rare-earth doping in nanostructured inorganic materials. Chem. Rev. 2022, 122, 5519–5603. [Google Scholar] [CrossRef]
- Wang, Y.; Seto, T.; Ishigaki, K.; Uwatoko, Y.; Xiao, G.; Zou, B.; Li, G.; Tang, Z.; Li, Z.; Wang, Y. Pressure-Driven Eu2+-Doped BaLi2Al2Si2N6: A New Color Tunable Narrow-Band Emission Phosphor for Spectroscopy and Pressure Sensor Applications. Adv. Funct. Mater. 2020, 30, 2001384. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Kang, R.; Ji, R.; Wang, Y. Sr2BN2Cl:Eu2+-Based Narrow-Band Blue-Emitting Phosphor: A Potential Color Converter for Illumination and Displays. Inorg. Chem. 2022, 61, 18245–18252. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Ni, Q.; Zhu, Q.; Zeng, Z.; Huo, J.; Long, C.; Wang, Q. Key Role Effect of Samarium in Realizing Zero Thermal Quenching and Achieving a Moisture-Resistant Reddish-Orange Emission in Ba3LaNb3O12:Sm3+. Inorg. Chem. 2022, 61, 17883–17892. [Google Scholar] [CrossRef] [PubMed]
- Brgoch, J.; Hasz, K.; Denault, K.A.; Borg, C.K.; Mikhailovsky, A.A.; Seshadri, R. Data-driven discovery of energy materials: Efficient BaM2Si3O10:Eu2+ (M = Sc, Lu) phosphors for application in solid state white lighting. Faraday Discuss. 2014, 176, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Atuchin, V.; Kesler, V.; Kokh, A.; Pokrovsky, L. X-ray photoelectron spectroscopy study of β-BaB2O4 optical surface. Appl. Surf. Sci. 2004, 223, 352–360. [Google Scholar] [CrossRef]
- Rubio, E.; Atuchin, V.; Kruchinin, V.; Pokrovsky, L.; Prosvirin, I.; Ramana, C. Electronic structure and optical quality of nanocrystalline Y2O3 film surfaces and interfaces on silicon. J. Phys. Chem. C 2014, 118, 13644–13651. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Vinnik, D.; Gavrilova, T.; Gudkova, S.; Isaenko, L.; Jiang, X.; Pokrovsky, L.; Prosvirin, I.; Mashkovtseva, L.; Lin, Z. Flux crystal growth and the electronic structure of BaFe12O19 hexaferrite. J. Phys. Chem. C 2016, 120, 5114–5123. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, Y.; Duan, J.; Yu, J. Synthesis and luminescence properties of single-phase Ca2P2O7:Eu2+, Eu3+ phosphor with tunable red/blue emission. J. Mater. Sci. Mater. Electron. 2019, 30, 16384–16394. [Google Scholar] [CrossRef]
- Chae, K.W.; Park, T.R.; Cheon, C.I.; Cho, N.I.; Kim, J.S. The enhancement of luminescence in Co-doped cubic Eu2O3 using Li+ and Al3+ ions. J. Lumin. 2011, 131, 2597–2605. [Google Scholar] [CrossRef]
- Reshak, A.H.; Alahmed, Z.A.; Bila, J.; Atuchin, V.V.; Bazarov, B.G.; Chimitova, O.D.; Molokeev, M.S.; Prosvirin, I.P.; Yelisseyev, A.P. Exploration of the electronic structure of monoclinic α-Eu2(MoO4)3: DFT-based study and X-ray photoelectron spectroscopy. J. Phys. Chem. C 2016, 120, 10559–10568. [Google Scholar] [CrossRef]
- Venkatesan, A.; Chandar, N.R.K.; Kandasamy, A.; Chinnu, M.K.; Marimuthu, K.N.; Kumar, R.M.; Jayavel, R. Luminescence and electrochemical properties of rare earth (Gd, Nd) doped V2O5 nanostructures synthesized by a non-aqueous sol–gel route. RSC Adv. 2015, 5, 21778–21785. [Google Scholar] [CrossRef]
- Felhi, H.; Smari, M.; Bajorek, A.; Nouri, K.; Dhahri, E.; Bessais, L. Controllable synthesis, XPS investigation and magnetic property of multiferroic BiMn2O5 system: The role of neodyme doping. Prog. Nat. Sci. Mater. Int. 2019, 29, 198–209. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, J.; Zhang, T.; Ren, X.; Hu, W.; Zheng, L.; Wang, J. Towards thermal barrier coating application for rare earth silicates RE2SiO5 (RE = La, Nd, Sm, Eu, and Gd). J. Eur. Ceram. Soc. 2019, 39, 1463–1476. [Google Scholar] [CrossRef]
- Atuchin, V.; Kesler, V.; Maklakova, N.Y.; Pokrovsky, L.; Sheglov, D. Core level spectroscopy and RHEED analysis of KGd0.95Nd0.05(WO4)2 surface. Eur. Phys. J. B-Condens. Matter Complex Syst. 2006, 51, 293–300. [Google Scholar] [CrossRef]
- Atuchin, V.; Gavrilova, T.; Grivel, J.C.; Kesler, V. Electronic structure of layered titanate Nd2Ti2O7. Surf. Sci. 2008, 602, 3095–3099. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Subanakov, A.; Aleksandrovsky, A.; Bazarov, B.; Bazarova, J.; Dorzhieva, S.; Gavrilova, T.; Krylov, A.; Molokeev, M.; Oreshonkov, A.; et al. Exploration of structural, thermal, vibrational and spectroscopic properties of new noncentrosymmetric double borate Rb3NdB6O12. Adv. Powder Technol. 2017, 28, 1309–1315. [Google Scholar] [CrossRef]
- Gedekar, K.; Wankhede, S.; Moharil, S.; Belekar, R. d–f luminescence of Ce3+ and Eu2+ ions in BaAl2O4, SrAl2O4 and CaAl2O4 phosphors. J. Adv. Ceram. 2017, 6, 341–350. [Google Scholar] [CrossRef]
- Atuchin, V.; Isaenko, L.; Kesler, V.; Lin, Z.; Molokeev, M.; Yelisseyev, A.; Zhurkov, S. Exploration on anion ordering, optical properties and electronic structure in K3WO3F3 elpasolite. J. Solid State Chem. 2012, 187, 159–164. [Google Scholar] [CrossRef]
- Ji, H.; Huang, Z.; Xia, Z.; Molokeev, M.S.; Jiang, X.; Lin, Z.; Atuchin, V.V. Comparative investigations of the crystal structure and photoluminescence property of eulytite-type Ba3Eu(PO4)3 and Sr3Eu(PO4)3. Dalton Trans. 2015, 44, 7679–7686. [Google Scholar] [CrossRef]
- Dorenbos, P. Energy of the first 4f7→4f65d transition of Eu2+ in inorganic compounds. J. Lumin. 2003, 104, 239–260. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, Y.; Molokeev, M.S.; Atuchin, V.V. Structural and luminescence properties of yellow-emitting NaScSi2O6:Eu2+ phosphors:Eu2+ site preference analysis and generation of red emission by codoping Mn2+ for white-light-emitting diode applications. J. Phys. Chem. C 2013, 117, 20847–20854. [Google Scholar] [CrossRef]
- Ji, H.; Huang, Z.; Xia, Z.; Molokeev, M.S.; Atuchin, V.V.; Fang, M.; Huang, S. New yellow-emitting whitlockite-type structure Sr1.75Ca1.25(PO4)2:Eu2+ phosphor for near-UV pumped white light-emitting devices. Inorg. Chem. 2014, 53, 5129–5135. [Google Scholar] [CrossRef]
- Adachi, S. Review–Photoluminescence Spectroscopy of Eu2+-Activated Phosphors: From Near-UV to Deep Red Luminescence. ECS J. Solid State Sci. Technol. 2023, 12, 016002. [Google Scholar] [CrossRef]
- Ji, H.; Huang, Z.; Xia, Z.; Molokeev, M.S.; Atuchin, V.V.; Huang, S. Cation substitution dependent bimodal photoluminescence in whitlockite structural Ca3-xSrx(PO4)2:Eu2+ (0⩽x⩽2) solid solution phosphors. Inorg. Chem. 2014, 53, 11119–11124. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xia, Z.; Molokeev, M.S.; Atuchin, V.V.; Liu, Q. Blue-shift of Eu2+ emission in (Ba, Sr)3Lu(PO4)3:Eu2+ eulytite solid-solution phosphors resulting from release of neighbouring-cation-induced stress. Dalton Trans. 2014, 43, 16800–16804. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Liu, K.; Ju, Z.; Wei, R.; Liu, W. Achieving mechano-upconversion-downshifting-afterglow multimodal luminescence in Pr3+/Er3+ coactivated Ba2Ga2GeO7 for multidimensional anticounterfeiting. J. Mater. Chem. C 2022, 10, 5240–5248. [Google Scholar] [CrossRef]
- Sawada, K.; Nakamura, T.; Adachi, S. Synthesis and properties of Ca3Ga2Ge3O12:Tb3+ garnet phosphor. Ceram. Int. 2017, 43, 14225–14232. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Su, Q. Synthesis, photoluminescence and thermostimulated-luminescence properties of novel red long-lasting phosphorescent materials β-Zn3(PO4)2:Mn2+, M3+ (M = Al and Ga). J. Mater. Chem. 2004, 14, 2569–2574. [Google Scholar] [CrossRef]
- Lei, B.; Zhang, H.; Mai, W.; Yue, S.; Liu, Y.; Man, S.Q. Luminescent properties of orange-emitting long-lasting phosphorescence phosphor Ca2SnO4:Sm3+. Solid State Sci. 2011, 13, 525–528. [Google Scholar] [CrossRef]
- Urbach, F. Zur lumineszenz der alkalihalogenide. Sitzungsberichte Akad. Wiss. Wien 1930, 139, 363–372. [Google Scholar]
- Chen, D.; Zhou, Y.; Xu, W.; Zhong, J.; Huang, P. Persistent and photo-stimulated luminescence in Ce3+/Cr3+ activated Y3Al2Ga3O12 phosphors and transparent phosphor-in-glass. J. Mater. Chem. C 2016, 4, 11457–11464. [Google Scholar] [CrossRef]
- Shalgaonkar, C.; Narlikar, A. A review of the recent methods for determining trap depth from glow curves. J. Mater. Sci. 1972, 7, 1465–1471. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Liu, Q.; Gao, P.; Wang, J.; Qi, Y.; Li, Z.; Li, J.; Jiang, T. Design, Synthesis, and Characterization of a Novel Blue-Green Long Afterglow BaYAl3O7:Eu2+, Nd3+ Phosphor and Its Anti-Counterfeiting Application. Nanomaterials 2023, 13, 2457. https://doi.org/10.3390/nano13172457
Wu J, Liu Q, Gao P, Wang J, Qi Y, Li Z, Li J, Jiang T. Design, Synthesis, and Characterization of a Novel Blue-Green Long Afterglow BaYAl3O7:Eu2+, Nd3+ Phosphor and Its Anti-Counterfeiting Application. Nanomaterials. 2023; 13(17):2457. https://doi.org/10.3390/nano13172457
Chicago/Turabian StyleWu, Jiao, Quanxiao Liu, Peng Gao, Jigang Wang, Yuansheng Qi, Zhenjun Li, Junming Li, and Tao Jiang. 2023. "Design, Synthesis, and Characterization of a Novel Blue-Green Long Afterglow BaYAl3O7:Eu2+, Nd3+ Phosphor and Its Anti-Counterfeiting Application" Nanomaterials 13, no. 17: 2457. https://doi.org/10.3390/nano13172457
APA StyleWu, J., Liu, Q., Gao, P., Wang, J., Qi, Y., Li, Z., Li, J., & Jiang, T. (2023). Design, Synthesis, and Characterization of a Novel Blue-Green Long Afterglow BaYAl3O7:Eu2+, Nd3+ Phosphor and Its Anti-Counterfeiting Application. Nanomaterials, 13(17), 2457. https://doi.org/10.3390/nano13172457








