Impact of Ce/Zr Ratio in the Nanostructured Ceria and Zirconia Composites on the Selective CO2 Adsorption
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of ZrO2, CeO2 and CexZry Adsorbents
2.2.1. Synthesis of CeO2 and ZrO2 Adsorbents
2.2.2. Synthesis of Ce0.5Zr0.5, Ce0.67Zr0.33, and Ce0.33Zr0.67 Adsorbents
2.3. Characterization
2.4. Dynamic and Static CO2 Adsorption
3. Results and Discussion
3.1. Textural Characterization
3.2. TEM Analysis
3.3. X-ray Photoelectron Spectroscopy (XPS)
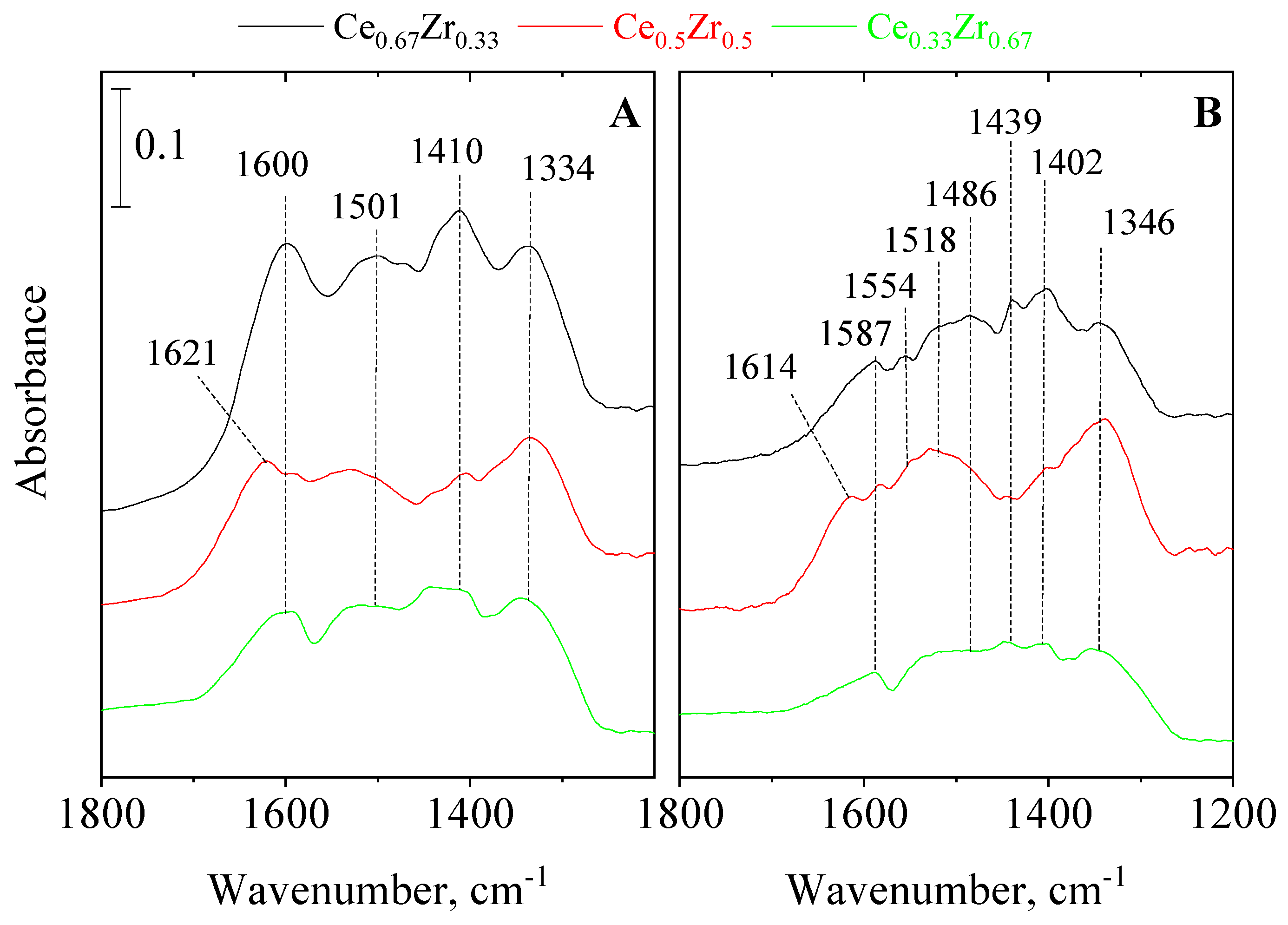
3.4. In Situ FT-IR Spectroscopy of Adsorbed Pyridine and CO2
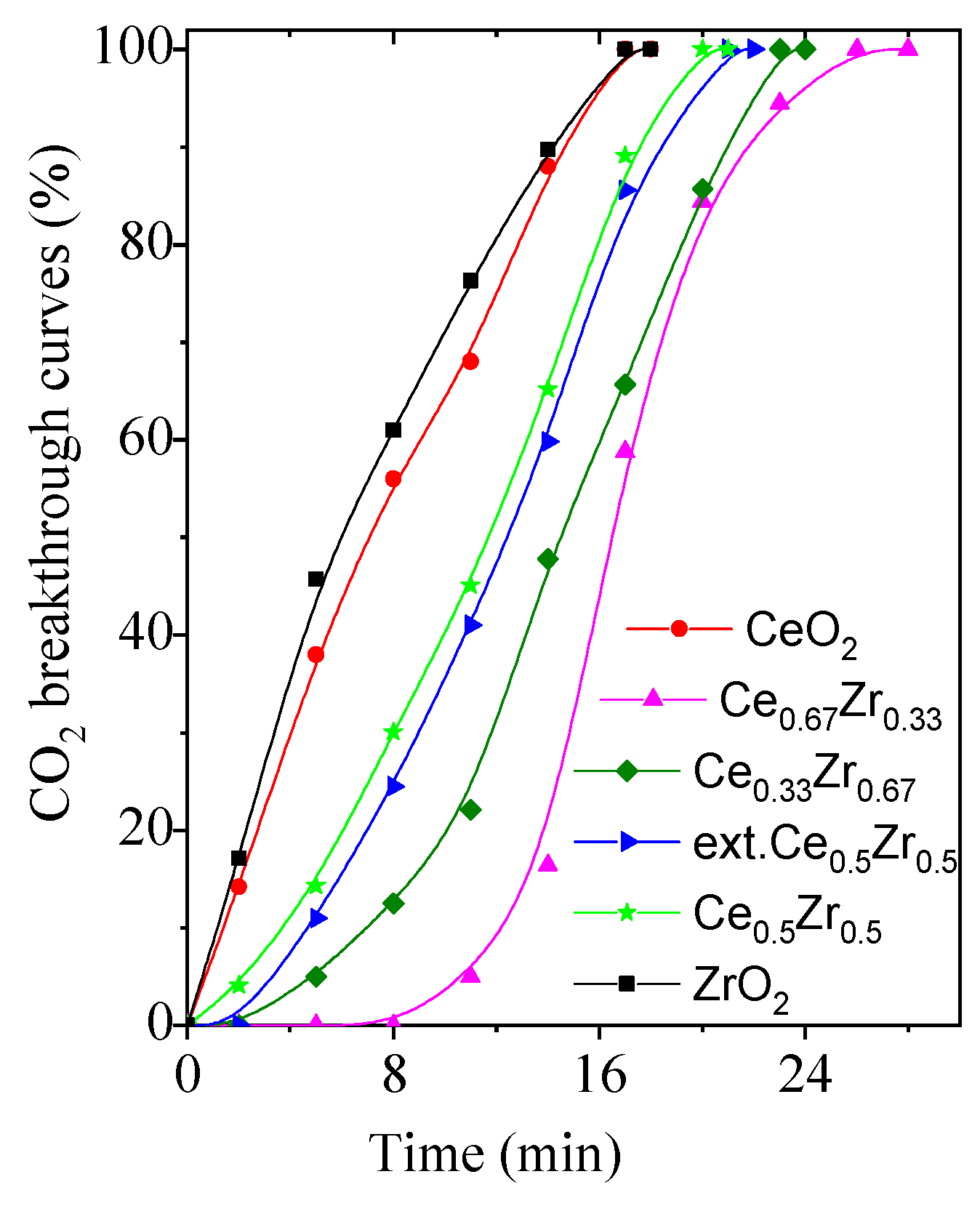
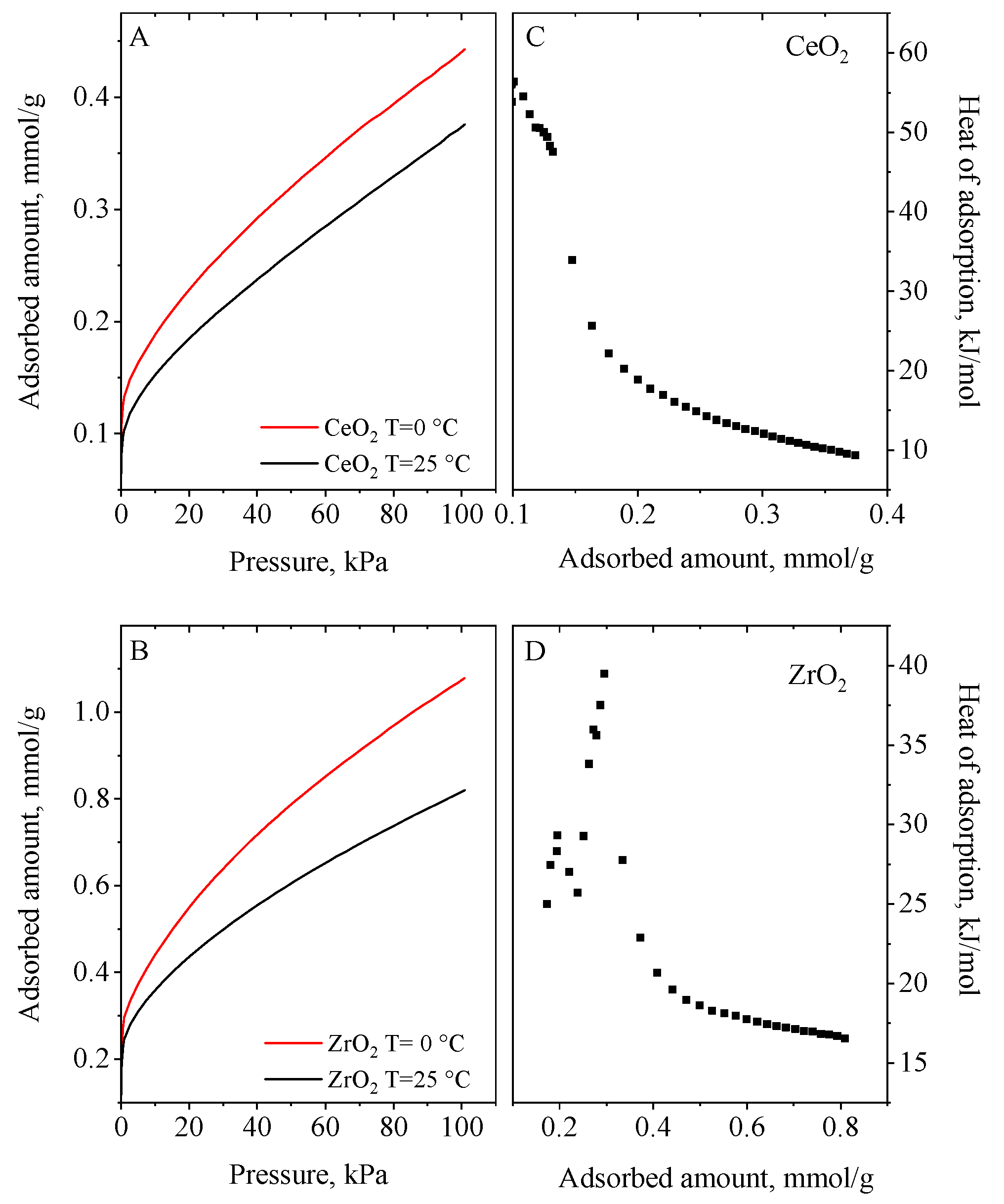
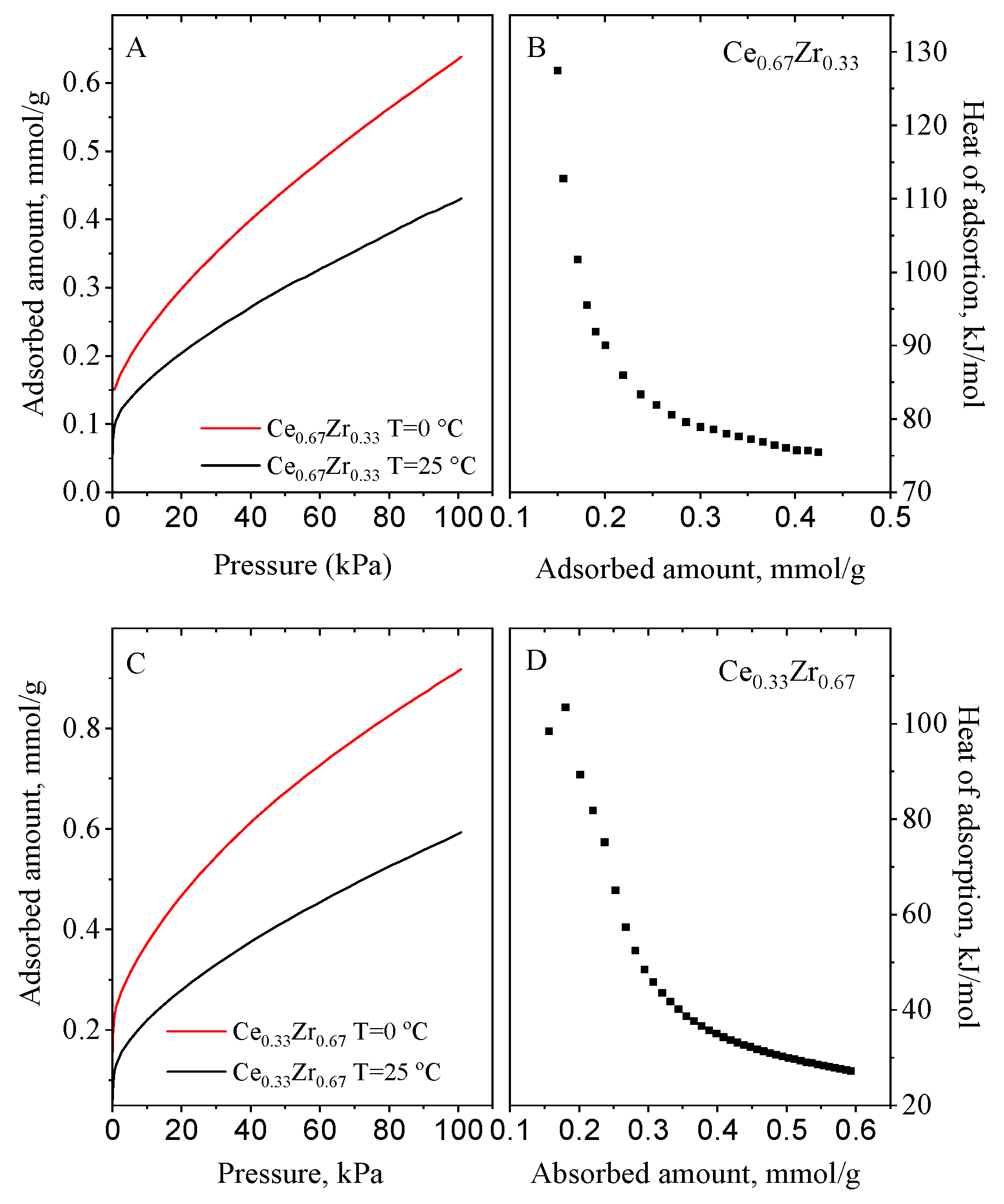
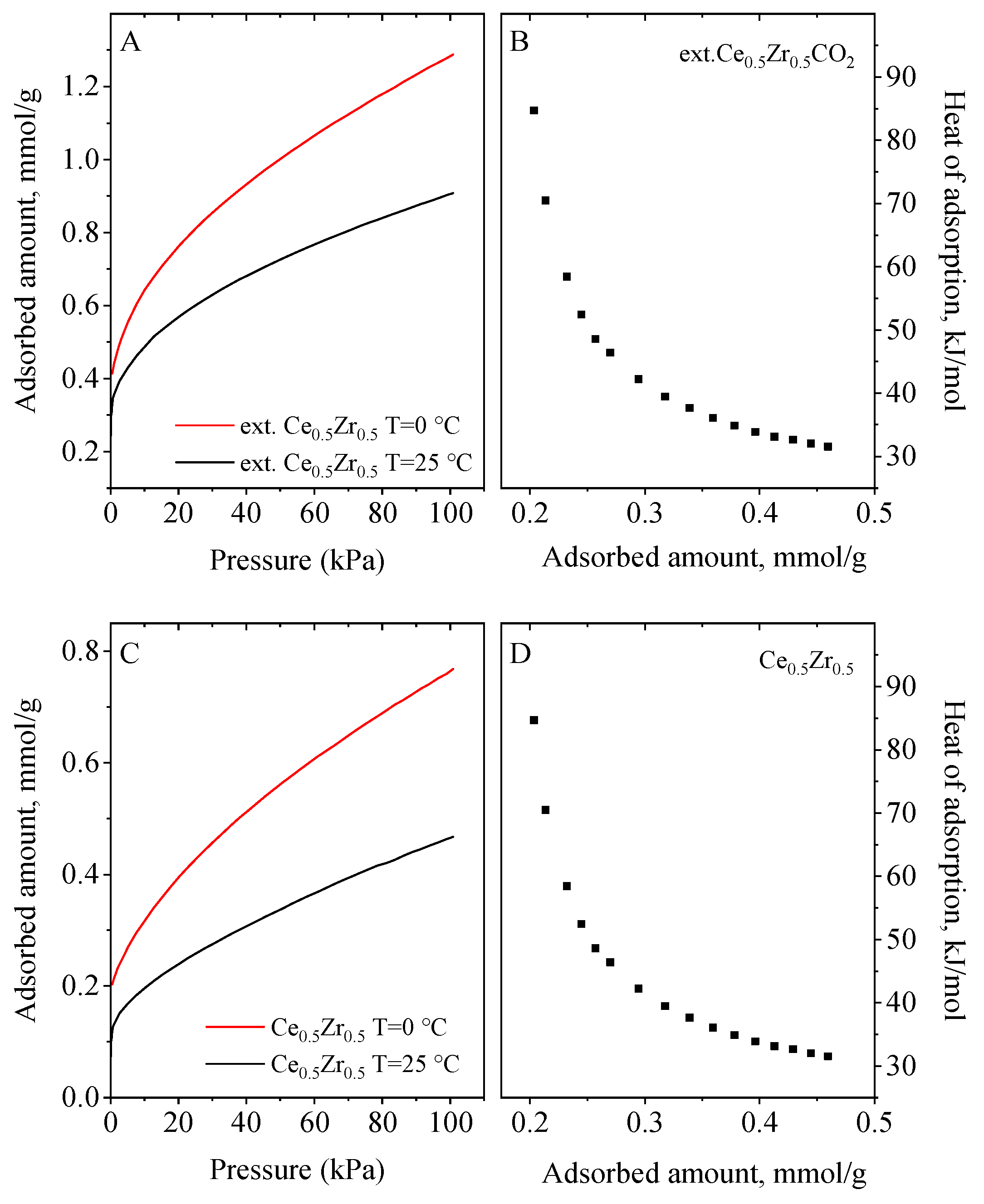
3.5. CO2 Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Azmi, A.A.; Aziz, M.A.A. Mesoporous adsorbent for CO2 capture application under mild condition: A review. J. Environ. Chem. Eng. 2019, 7, 103022. [Google Scholar] [CrossRef]
- Belmabkhout, Y.; Guillerm, V.; Eddaoudi, M. Low concentration CO2 capture using physical adsorbents: Are metal–organic frameworks becoming the new benchmark materials? Chem. Eng. J. 2016, 296, 386–397. [Google Scholar] [CrossRef]
- Davis, S.J.; Caldeira, K.; Matthews, H.D. Future CO2 emissions and climate change from existing energy infrastructure. Science 2010, 329, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Satterthwaite, D. Cities’ contribution to global warming: Notes on the allocation of greenhouse gas emissions. Environ. Urban. 2008, 20, 539–549. [Google Scholar] [CrossRef]
- Bhave, A.; Taylor, R.H.; Fennell, P.; Livingston, W.R.; Shah, N.; Mac Dowell, N.; Dennis, J.; Kraft, M.; Pourkashanian, M.; Insa, M.; et al. Screening and techno-economic assessment of biomass-based power generation with CCS technologies to meet 2050 CO2 targets. Appl. Energy 2017, 190, 481–489. [Google Scholar] [CrossRef]
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Li, H.; Yan, D.; Zhang, Z.; Lichtfouse, E. Prediction of CO2 absorption by physical solvents using a chemoinformatics-based machine learning model. Environ. Chem. Lett. 2019, 17, 1397–1404. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z. Mining the intrinsic trends of CO2 solubility in blended solutions. J. CO2 Util. 2018, 26, 496–502. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Yeh, J.T.; Resnik, K.P.; Rygle, K.; Pennline, H.W. Semi-batch absorption and regeneration studies for CO2 capture by aqueous ammonia. Fuel Process. Technol. 2005, 86, 1533–1546. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, M.; Fu, Y.; Huang, Y.; Tao, Z.; Li, W. Using 13X, LiX, and LiPdAgX zeolites for CO2 capture from post-combustion flue gas. Appl. Energy 2017, 191, 87–98. [Google Scholar] [CrossRef]
- Lakhi, K.S.; Cha, W.S.; Joseph, S.; Wood, B.J.; Aldeyab, S.S.; Lawrence, G.; Choy, J.-H.; Vinu, A. Cage type mesoporous carbon nitride with large mesopores for CO2 capture. Catal. Today 2015, 243, 209–217. [Google Scholar] [CrossRef]
- Hiremath, V.; Shavi, R.; Seo, J.G. Controlled oxidation state of Ti in MgO-TiO2 composite for CO2 capture. Chem. Eng. J. 2017, 308, 177–183. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Kaneeda, M.; Nakamura, H. Development of Novel CeO2-based CO2 adsorbent and analysis on its CO2 adsorption and desorption mechanism. Energy Procedia 2017, 114, 2481–2487. [Google Scholar] [CrossRef]
- Xu, H.; Hou, X. Synergistic effect of CeO2 modified Pt/C electrocatalysts on the performance of PEM fuel cells. Int. J. Hydrog. 2007, 32, 4397–4401. [Google Scholar] [CrossRef]
- Kozerozhets, V.; Panasyuk, G.P.; Semenov, E.A.; Voroshilov, I.L.; Avdeeva, V.V.; Buzanov, G.A.; Danchevskaya, M.N.; Kolmakova, A.A.; Malinina, E.A. A new approach to the synthesis of nanosized powder CaO and its application as precursor for the synthesis of calcium borates. Ceram. Int. 2022, 48, 7522–7532. [Google Scholar] [CrossRef]
- Arifutzzaman, A.; Musa, I.N.; Aroua, M.K.; Saidur, R.M. Xene based activated carbon novel nano-sandwich for efficient CO2 adsorption in fixed-bed column. J. CO2 Util. 2023, 68, 102353. [Google Scholar] [CrossRef]
- Ligero, A.; Calero, M.; Martín-Lara, M.Á.; Blázquez, G.; Solís, R.R.; Pérez, A. Fixed-bed CO2 adsorption onto activated char from the pyrolysis of a non-recyclable plastic mixture from real urban residues. J. CO2 Util. 2023, 73, 102517. [Google Scholar] [CrossRef]
- Kozerozhets, I.V.; Panasyuk, G.P.; Semenov, E.A.; Buzanov, G.A.; Avdeeva, V.V.; Danchevskaya, M.N.; Tsvetov, N.S.; Shapovalov, S.S.; Vasil’ev, M.G. Function of the Non-Crystalline X-Ray Amorphous Phase of Highly Dispersed Powder of CaO in Adsorption of CO2 and H2O. Russ. J. Gen. Chem. 2021, 91, S98–S105. [Google Scholar] [CrossRef]
- Buckeridge, J.; Scanlon, D.O.; Walsh, A.; Catlow, C.R.A.; Sokol, A.A. Dynamical response and instability in ceria under lattice expansion. Phys. Rev. B 2013, 87, 214304. [Google Scholar] [CrossRef]
- Sharan, R.; Dutta, A. Structural analysis of Zr4+ doped ceria, a possible material for ammonia detection in ppm level. J. Alloys Compd. 2017, 693, 936–944. [Google Scholar] [CrossRef]
- Chuang, C.-C.; Hsiang, H.-I.; Yen, F.-S.; Chen, C.-C.; Yang, S.-J. Phase evolution and reduction behavior of Ce0.6Zr0.4O2 powders prepared using the chemical co-precipitation method. Ceram. Int. 2013, 39, 1717–1722. [Google Scholar] [CrossRef]
- Trovarelli, A.; Zamar, F.; Llorca, J.; Leitenburg, C.; Dolcetti, G.; Kiss, J.T. Nanophase Fluorite-Structured CeO2–ZrO2 Catalysts Prepared by High-Energy Mechanical Milling. J. Catal. 1997, 169, 490–502. [Google Scholar] [CrossRef]
- Rumruangwong, M.; Wongkasemjit, S. Synthesis of ceria–zirconia mixed oxide from cerium and zirconium glycolates via sol–gel process and its reduction property. Appl. Organomet. Chem. 2006, 20, 615–625. [Google Scholar] [CrossRef]
- Sajeevan, A.C.; Sajith, V. Synthesis of stable cerium zirconium oxide nanoparticle—Diesel suspension and investigation of its effects on diesel properties and smoke. Fuel 2016, 183, 155–163. [Google Scholar] [CrossRef]
- Reddy, G.K.; Gunugunuri, K.R.; Ibram, G.; Ferreira, J. Single step synthesis of nanosized CeO2–MxOy mixed oxides (MxOy = SiO2, TiO2, ZrO2, and Al2O3) by microwave induced solution combustion synthesis: Characterization and CO oxidation. J. Mater. Sci. 2009, 44, 2743–2751. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Xu, C.; Liu, J. Structure, synthesis, and catalytic properties of nanosize cerium-zirconium-based solid solutions in environmental catalysis. Chin. J. Catal. 2019, 40, 1438–1487. [Google Scholar] [CrossRef]
- Mukherjee, D.; Rao, B.G.; Reddy, B.M. CO and soot oxidation activity of doped ceria: Influence of dopants. Appl. Catal. B 2016, 197, 105–115. [Google Scholar] [CrossRef]
- Campos, P.T.A.; Oliveira, C.F.; Lima, J.P.V.; Queiroz Silva, D.R.; Dias, S.C.L.; Dias, J.A. Cerium–zirconium mixed oxide synthesized by sol-gel method and its effect on the oxygen vacancy and specific surface area. J. Solid State Chem. 2022, 307, 122752. [Google Scholar] [CrossRef]
- Reddy, B.M.; Bharali, P.; Saikia, P.; Park, S.E.; van den Berg, M.W.E.; Muhler, M.; Grünert, W. Structural Characterization and Catalytic Activity of Nanosized CexM1-xO2 (M = Zr and Hf) Mixed Oxides. J. Phys. Chem. C 2008, 112, 11729–11737. [Google Scholar] [CrossRef]
- Shih, S.-J.; Wu, Y.-Y.; Chou, Y.-J.; Borisenko, K.B. Nanoscale control of composition in cerium and zirconium mixed oxide nanoparticles. Mater. Chem. Phys. 2012, 135, 749–754. [Google Scholar] [CrossRef]
- Nagai, Y.; Yamamoto, T.; Tanaka, T.; Yoshida, S.; Nonaka, T.; Okamoto, T.; Suda, A.; Sugiura, M. X-ray absorption fine structure analysis of local structure of CeO2–ZrO2 mixed oxides with the same composition ratio (Ce/Zr = 1). Catal. Today 2002, 74, 225–234. [Google Scholar] [CrossRef]
- Yoshioka, T.; Dosaka, K.; Sato, T.; Okuwaki, A.; Tanno, S.; Miura, T. Preparation of spherical ceria-doped tetragonal zirconia by the spray-pyrolysis method. J. Mater. Sci. 1992, 11, 51–55. [Google Scholar] [CrossRef]
- Schulz, H.; Stark, W.J.; Maciejewski, M.; Pratsinis, S.E.; Baiker, A. Flame-made nanocrystalline ceria/zirconia doped with alumina or silica: Structural properties and enhanced oxygen exchange capacity. J. Mater. Chem. 2003, 13, 2979–2984. [Google Scholar] [CrossRef]
- Aguirre, S.B.; Vargas, L.; Rodriguez, J.R.; Estrada, M.; Castillon, F.; Lopez, M.; Simakova, I.; Simakov, A. One-pot synthesis of uniform hollow nanospheres of Ce–Zr–O mixed oxides by spray pyrolysis. Micropor. Mesopor. Mater. 2020, 294, 109886. [Google Scholar] [CrossRef]
- Alifanti, B.B.M.; Blangenois, N.; Naud, J.; Grange, P.; Delmon, B. Characterization of CeO2−ZrO2 Mixed Oxides. Comparison of the Citrate and Sol−Gel Preparation Methods. Chem. Mater. 2003, 15, 395–403. [Google Scholar] [CrossRef]
- Sugiura, M.; Ozawa, M.; Suda, A.; Suzuki, T.; Kanazawa, T. Development of Innovative Three-Way Catalysts Containing Ceria–Zirconia Solid Solutions with High Oxygen Storage/Release Capacity. Bull. Chem. Soc. Jpn. 2005, 78, 752–767. [Google Scholar] [CrossRef]
- Boaro, M.; Colussi, S.; Trovarelli, A. Ceria-Based Materials in Hydrogenation and Reforming Reactions for CO2 Valorization. Front. Chem. Sec. Phys. Chem. Chem. Phys. 2019, 7, 28. [Google Scholar] [CrossRef]
- Chen, W.; Fan, L.; Jiang, X.; Guo, J.; Liu, H.; Tian, M. Preparation of CexZr1–xO2 by Different Methods and Its Catalytic Oxidation Activity for Diesel Soot. ACS Omega 2022, 7, 16352–16360. [Google Scholar] [CrossRef]
- Hori, C.E.; Permana, H.; Ng, K.Y.S.; Brenner, A.; More, K.; Rahmoeller, K.M.; Belton, D. Thermal stability of oxygen storage properties in a mixed CeO2-ZrO2 system. Appl. Catal. B Environ. 1998, 16, 105–117. [Google Scholar] [CrossRef]
- Ai, C.; Zhang, Y.; Wang, P.; Wang, W. Catalytic Combustion of Diesel Soot on Ce/Zr Series Catalysts Prepared by Sol-Gel Method. Catalysts 2019, 9, 646. [Google Scholar] [CrossRef]
- Bêche, E.; Charvin, P.; Perarnau, D.; Abanades, S.; Flamant, G. Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz). Surf. Interface Anal. 2008, 40, 264–267. [Google Scholar] [CrossRef]
- Jampaiah, D.; Ippolito, S.J.; Sabri, Y.M.; Tardio, J.; Selvakannan, P.R.; Nafady, A.; Reddy, B.M.; Bhargava, S.K. Ceria–zirconia modified MnOx catalysts for gaseous elemental mercury oxidation and adsorption. Catal. Sci. Technol. 2016, 6, 1792–1803. [Google Scholar] [CrossRef]
- Rao, M.V.R.; Shripathi, T. Photoelectron spectroscopic study of X-ray induced reduction of CeO2. J. Electron Spectrosc. Relat. Phenom. 1997, 87, 121–126. [Google Scholar] [CrossRef]
- Galtayries, A.; Sporken, R.; Riga, J.; Blanchard, G.; Caudano, R. XPS comparative study of ceria/zirconia mixed oxides: Powders and thin film characterisation. J. Electron Spectrosc. Relat. Phenom. 1998, 88–91, 951–956. [Google Scholar] [CrossRef]
- Shan, W.; Liu, F.; He, H.; Shi, X.; Zhang, C. An environmentally-benign CeO2-TiO2 catalyst for the selective catalytic reduction of NOx with NH3 in simulated diesel exhaust. Catal. Today 2012, 184, 160–165. [Google Scholar] [CrossRef]
- Zhang, R.; Zhong, Q.; Zhao, W.; Yu, L.; Qu, H. Promotional effect of fluorine on the selective catalytic reduction of NO with NH3 over CeO2-TiO2 catalyst at low temperature. Appl. Surf. Sci. 2014, 289, 237–244. [Google Scholar] [CrossRef]
- Tsunekawa, S.; Asami, K.; Ito, S.; Yashima, M.; Sugimoto, T. XPS study of the phase transition in pure zirconium oxide nanocrystallites. Appl. Surf. Sci. 2005, 252, 1651–1656. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Ivanova, R.; Henych, J.; Dimitrov, M.; Kormunda, M.; Kovacheva, D.; Scotti, N.; Dal Santo, V. Effect of preparation procedure on the formation of nanostructured ceria–zirconia mixed oxide catalysts for ethyl acetate oxidation:Homogeneous precipitation with urea vs template-assisted hydrothermal synthesis. Appl. Catal. A 2015, 502, 418–432. [Google Scholar] [CrossRef]
- Daturi, M.; Binet, C.; Lavalley, J.C.; Blanchard, G. Surface FTIR investigations on CexZr1-xO2 system. Surf. Interface Anal. 2000, 30, 273–277. [Google Scholar] [CrossRef]
- Li, M.; Tumuluri, U.; Wu, Z.; Dai, S. Effect of Dopants on the Adsorption of Carbon Dioxide on Ceria Surfaces. Chem. Sus. Chem. 2015, 8, 3651–3660. [Google Scholar] [CrossRef] [PubMed]
- Daturi, M.; Binet, C.; Lavalley, J.-C.; Galtayries, A.; Sporken, R. Surface investigation on CexZr1-xO2 compounds. Phys. Chem. Chem. Phys. 1999, 1, 5717–5724. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Sato, H.; Kaneeda, M.; Kondo, J.N. Synthesis and analysis of CO2 adsorbents based on cerium oxide. J. CO2 Util. 2014, 8, 34–38. [Google Scholar] [CrossRef]
- Bhatta, L.K.G.; Subramanyam, S.; Chengala, M.D.; Bhatta, U.M.; Pandita, N.; Venkatesh, K. Investigation of CO2 adsorption on carbon material derived from Mesua ferrea L. seed cake. J. Environ. Chem. Eng. 2015, 3, 2957–2965. [Google Scholar] [CrossRef]
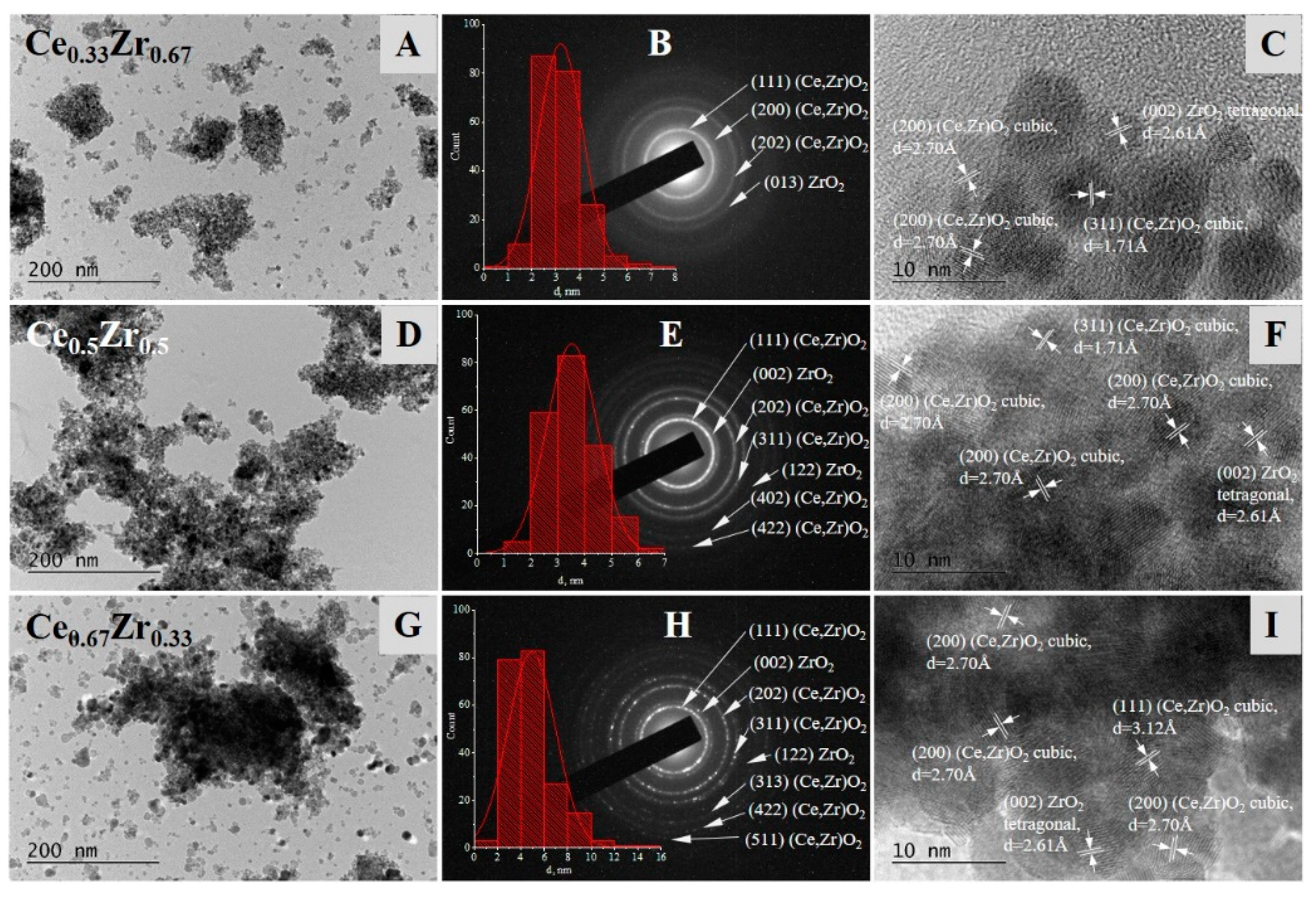
| Samples | SBET, m2/g | Pore Volume, cm3/g |
|---|---|---|
| ZrO2 | 271 | 0.41 |
| Ce0.33Zr0.67 | 185 | 0.18 |
| ext.Ce0.5Zr0.5 | 181 | 0.15 |
| Ce0.5Zr0.5 | 153 | 0.17 |
| Ce0.67Zr0.33 | 119 | 0.13 |
| CeO2 | 55 | 0.10 |
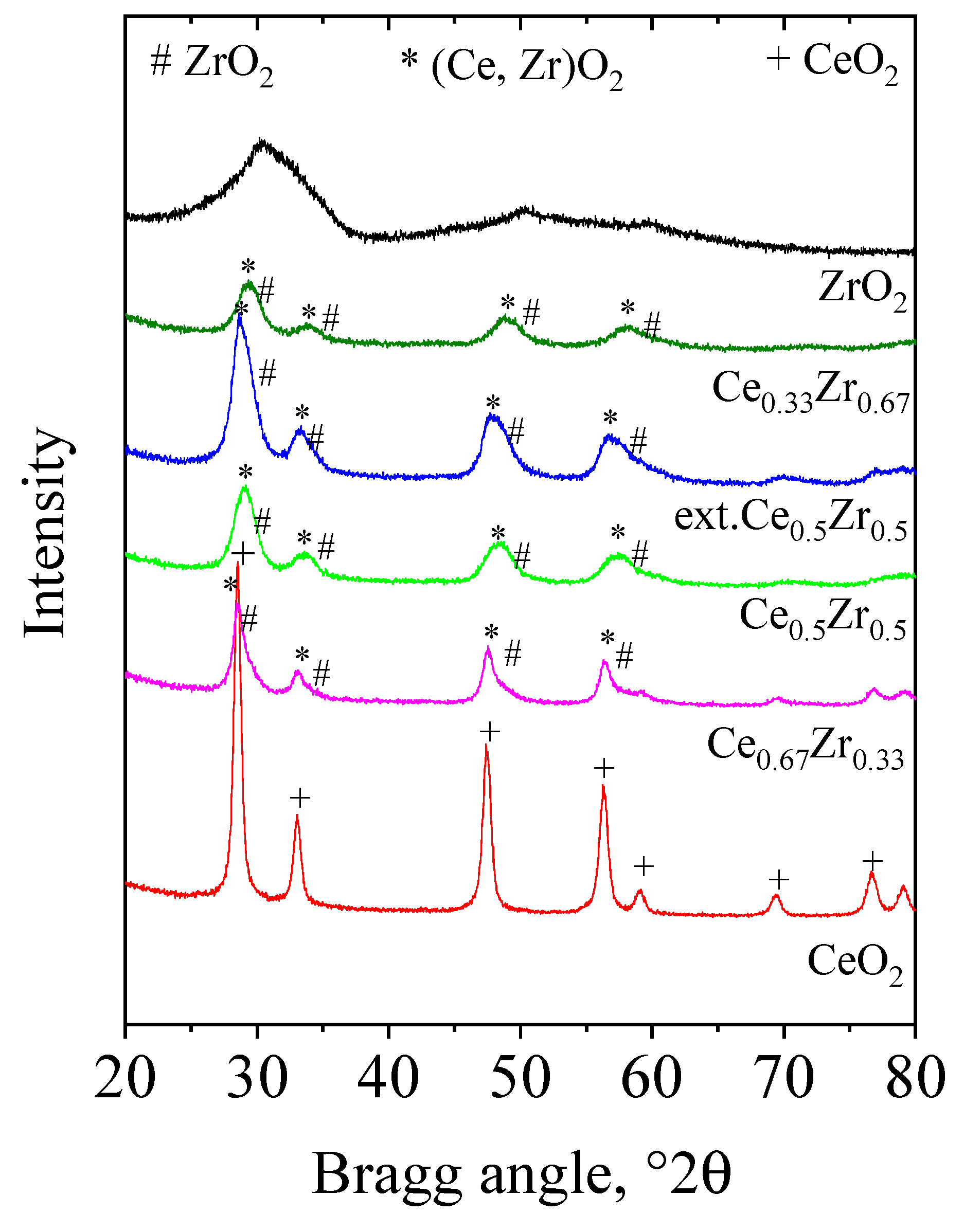
| Samples | Phase Composition (Space Group) | Unit Cell Parameters (Å) | Crystallite Size, nm (±0.5–1 nm) |
|---|---|---|---|
| CeO2 | CeO2 (Fm-3m) | a = 5.4146(3) | 16 |
| Ce0.67Zr0.33 | (Ce,Zr)O2 (Fm-3m) ZrO2 (P42/nmc) | a = 5.406(1) a = 3.709(5) c = 5.33(1) | 12 5 |
| Ce0.5Zr0.5 | (Ce,Zr)O2 (Fm-3m) ZrO2 (P42/nmc) | a = 5.382(5) a = 3.710(5) c = 5.313(8) | 8 6 |
| ext.Ce0.5Zr0.5 | (Ce,Zr)O2 (Fm-3m) ZrO2 (P42/nmc) | a = 5.394(2) a = 3.709(3) c = 5.339(5) | 10 5 |
| Ce0.33Zr0.67 | (Ce,Zr)O2 (Fm-3m) ZrO2 (P42/nmc) | a = 5.36(1) a = 3.70(1) c = 5.31(2) | 6 5 |
| ZrO2 | n.d. | n.d. | n.d. |
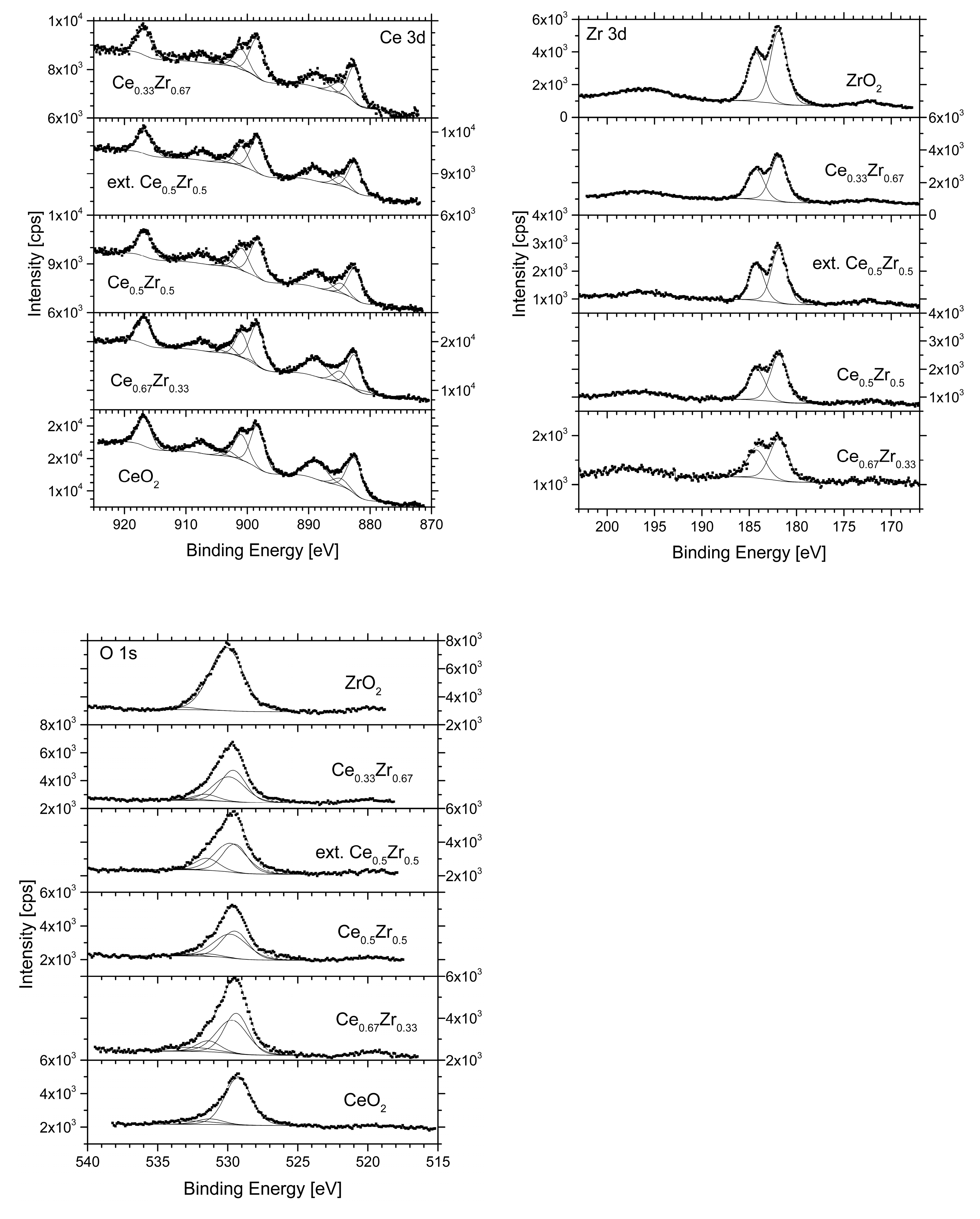
| Samples | Concentration, at. % | Ratio of Oxidation States, % | Binding Energy, eV | |||
|---|---|---|---|---|---|---|
| Ce (Ce 3d) | Zr (Zr 3d) | O (O 1s) | Ce4+:Ce3+ | O1s Ce4+ | O1s Zr | |
| CeO2 | 27 | - | 73 | 93:7 | 529.27 | - |
| Ce0.67Zr0.33 | 17 | 12 | 71 | 87:13 | 529.39 | 529.68 |
| Ce0.5Zr0.5 | 11 | 23 | 67 | 91:9 | 529.49 | 529.79 |
| ext.Ce0.5Zr0.5 | 10 | 21 | 69 | 90:10 | 529.49 | 529.79 |
| Ce0.33Zr0.67 | 5 | 28 | 67 | 87:13 | 529.61 | 529.90 |
| ZrO2 | - | 37 | 63 | - | - | 530.09 |
| Samples | CO2 ads. CO2/N2, mmol/g | CO2 ads. CO2/H2O/N2 1, mmol/g | Repeated CO2 ads. CO2/H2O/N2 2, mmol/g |
|---|---|---|---|
| ZrO2 | 2.0 | 2.1 | 1.9 |
| Ce0.33Zr0.67 | 3.2 | 3.4 | 3.2 |
| Ce0.5Zr0.5 | 2.9 | 3.1 | 3.0 |
| ext.Ce0.5Zr0.5 | 3.0 | 3.5 | 3.4 |
| Ce0.67Zr0.33 | 3.5 | 3.7 | 3.6 |
| CeO2 | 2.3 | 2.4 | 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Issa, G.; Kormunda, M.; Tumurbaatar, O.; Szegedi, Á.; Kovacheva, D.; Karashanova, D.; Popova, M. Impact of Ce/Zr Ratio in the Nanostructured Ceria and Zirconia Composites on the Selective CO2 Adsorption. Nanomaterials 2023, 13, 2428. https://doi.org/10.3390/nano13172428
Issa G, Kormunda M, Tumurbaatar O, Szegedi Á, Kovacheva D, Karashanova D, Popova M. Impact of Ce/Zr Ratio in the Nanostructured Ceria and Zirconia Composites on the Selective CO2 Adsorption. Nanomaterials. 2023; 13(17):2428. https://doi.org/10.3390/nano13172428
Chicago/Turabian StyleIssa, Gloria, Martin Kormunda, Oyundari Tumurbaatar, Ágnes Szegedi, Daniela Kovacheva, Daniela Karashanova, and Margarita Popova. 2023. "Impact of Ce/Zr Ratio in the Nanostructured Ceria and Zirconia Composites on the Selective CO2 Adsorption" Nanomaterials 13, no. 17: 2428. https://doi.org/10.3390/nano13172428
APA StyleIssa, G., Kormunda, M., Tumurbaatar, O., Szegedi, Á., Kovacheva, D., Karashanova, D., & Popova, M. (2023). Impact of Ce/Zr Ratio in the Nanostructured Ceria and Zirconia Composites on the Selective CO2 Adsorption. Nanomaterials, 13(17), 2428. https://doi.org/10.3390/nano13172428







