Characterization of Tunneled Wide Band Gap Mixed Conductors: The Na2O-Ga2O3-TiO2 System
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, X.; Marks, T.J.; Facchetti, A. Metal oxides for optoelectronic applications. Nat. Mater. 2016, 15, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, J.; Yang, L.; Qu, M.; Qi, D.-C.; Zhang, K.H.L. Wide Bandgap Oxide Semiconductors: From Materials Physics to Optoelectronic Devices. Adv. Mater. 2021, 33, 2006230. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Zheng, W.; Zhang, D.; Zhang, Z.; Liao, Q.; Yang, L.; Huang, F. High-Performance Graphene/β-Ga2O3 Heterojunction Deep-Ultraviolet Photodetector with Hot-Electron Excited Carrier Multiplication. ACS Appl. Mater. Interfaces 2018, 10, 22419–22426. [Google Scholar] [CrossRef] [PubMed]
- Chikoidze, E.; Sartel, C.; Madaci, I.; Mohamed, H.; Vilar, C.; Ballesteros, B.; Belarre, F.; Del Corro, E.; Vales-Castro, P.; Sauthier, G.; et al. p-Type Ultrawide-Band-Gap Spinel ZnGa2O4: New Perspectives for Energy Electronics. Cryst. Growth Des. 2020, 20, 2535–2546. [Google Scholar] [CrossRef]
- Rickert, K.; Huq, A.; Lapidus, S.H.; Wustrow, A.; Ellis, D.E.; Poeppelmeier, K.R. Site Dependency of the High Conductivity of Ga2In6Sn2O16: The Role of the 7-Coordinate Site. Chem. Mater. 2015, 27, 8084–8809. [Google Scholar] [CrossRef]
- García-Fernández, J.; Bartolomé, J.; Torres-Pardo, A.; Peche-Herrero, A.; Moreno, J.; Ramírez-Castellanos, J.; Cremades, A.; González-Calbet, J.M.; Piqueras, J. Structural characterization at the atomic level and optical properties of the ZnkIn2Ok+3 (3 ≤ k ≤ 13) system. J. Mater. Chem. C 2017, 5, 10176–10184. [Google Scholar] [CrossRef]
- Guo, D.; Guo, Q.; Chen, Z.; Wu, Z.; Li, P.; Tang, W. Review of Ga2O3-based optoelectronic devices. Mater. Today Phys. 2019, 11, 100157. [Google Scholar] [CrossRef]
- Pearton, S.J.; Yang, J.; Cary, P.H.; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A. A review of Ga2O3 materials, processing, and devices. Appl. Phys. Rev. 2018, 5, 011301. [Google Scholar] [CrossRef]
- Michiue, Y.; Mori, T.; Prytuliak, A.; Matsushita, Y.; Tanaka, M.; Kimizuka, N. Electrical, optical, and thermoelectric properties of Ga2O3(ZnO)9. RSC Adv. 2011, 1, 1788–1793. [Google Scholar] [CrossRef]
- Rickert, K.; Boullay, P.; Malo, S.; Caignaert, V.; Poeppelmeier, K. A Rutile Chevron Modulation in Delafossite-Like Ga3–xIn3TixO9+x/2. Inorg. Chem. 2016, 55, 4403–4409. [Google Scholar] [CrossRef]
- García-Fernández, J.; Torres-Pardo, A.; Bartolomé, J.; Martínez-Casado, R.; Zhang, Q.; Ramírez-Castellanos, J.; Terasaki, O.; Cremades, A.; González-Calbet, J.M. Influence of Cation Substitution on the Complex Structure and Luminescent Properties of the ZnkIn2Ok+3 System. Chem. Mater. 2020, 32, 6176–6185. [Google Scholar] [CrossRef]
- Eichhorn, S.; Schmid, H.; Assenmacher, W.; Mader, W. Homologous compounds of type ARO3(ZnO)m in the system Ga-Sn-Zn-O. J. Solid State Chem. 2017, 246, 214–220. [Google Scholar] [CrossRef]
- Garling, J.; Assenmacher, W.; Schmid, H.; Longo, P.; Mader, W. Real structure of (Sb1/3Zn2/3)GaO3(ZnO)3, a member of the homologous series ARO3(ZnO)m with ordered site occupation. J. Solid State Chem. 2018, 258, 809–817. [Google Scholar] [CrossRef]
- European Commission. Study on the Critical Raw Materials for the EU 2023—Final Report; Publications Office of the European Union: Luxembourg, 2023. [Google Scholar]
- Chandrashekhar, G.V.; Bednowitz, B.; Laplaca, S.J. Fast Ion Transport in Solids; Elsevier North Holland Inc.: Amsterdam, The Netherlands, 1979; pp. 447–450. [Google Scholar]
- Amoroso, J.W.; Edwards, D.D. Phase formation and stability of polycrystalline NaxGa4+xTi1−xO8 (x~0.7). Solid State Ion. 2008, 179, 878–880. [Google Scholar] [CrossRef]
- Michiue, Y.; Watanabe, M. Synthesis and structure of new sodium titanogallate NaxTi2−xGa4+xO10, containing one-dimensional channels. Solid State Ion. 1994, 70–71, 186–190. [Google Scholar] [CrossRef]
- Michiue, Y.; Sato, A. Structure refinements of Na0.8Ti1.2Ga4.8O10: X-ray diffraction analysis for the sodium ion distribution in a one-dimensional tunnel-like space. Acta Cryst. Sect. B Struct. Sci. 2004, 60, 692–697. [Google Scholar] [CrossRef]
- Michiue, Y.; Sasaki, T.; Watanabe, M.; Fujiki, Y. Synthesis and structure refinement of sodium titanogallate containing one-dimensional channels of large cross section. Mat. Res. Bull. 1993, 28, 173–178. [Google Scholar] [CrossRef]
- García-Fernández, J.; García-Carrión, M.; Torres-Pardo, A.; Martínez-Casado, R.; Ramírez-Castellanos, J.; Nogales, E.; González-Calbet, J.M.; Méndez, B. New insights into the luminescence properties of a Na stabilized Ga–Ti oxide homologous series. J. Mater. Chem. C 2020, 8, 2725–2731. [Google Scholar] [CrossRef]
- Goikolea, E.; Palomares, V.; Wang, S.; Ruiz de Larramendi, I.; Guo, X.; Wang, G.; Teofilo Rojo, T. Na-Ion Batteries—Approaching Old and New Challenges. Adv. Energy Mater. 2020, 10, 2002055. [Google Scholar] [CrossRef]
- Wang, Y.X.; Lim, Y.G.; Park, M.S.; Chou, S.L.; Kim, J.H.; Liu, H.K.; Doub, S.X.; Kim, Y.J. Ultrafine SnO2 nanoparticle loading onto reduced graphene oxide as anodes for sodium-ion batteries with superior rate and cycling performances. J. Mater. Chem. A 2014, 2, 529. [Google Scholar] [CrossRef]
- Gan, Q.; He, H.; Zhu, Y.; Wang, Z.; Qin, N.; Gu, S.; Li, Z.; Luo, W.; Lu, Z. Defect-Assisted Selective Surface Phosphorus Doping to Enhance Rate Capability of Titanium Dioxide for Sodium Ion Batteries. ACS Nano 2019, 13, 9247–9258. [Google Scholar] [CrossRef] [PubMed]
- Sanford, S.; Misture, S.T.; Edwards, D.D. A comparison of the photocatalytic activity of six tunneled titanates. J. Solid State Chem. 2013, 200, 189–196. [Google Scholar] [CrossRef]
- Empie, N.; Edwards, D. Atomic Force Microscopy Study of the Interaction of DNA and Nanostructured β-Gallia Rutile. Langmuir 2006, 22, 7658–7663. [Google Scholar] [CrossRef]
- Maria, H.G.; Teresa, F.D.M.; Javier, G.F.; Maria, G.C.J.; Julio, R.-C. Structural Characterization of Luminescent Complex Oxides: AxGa4+xTin−4−xO2n−2 (A = Na, Li; N = 5, 6 and 7 and X = 0.7); Institut Laue-Langevin (ILL): Grenoble, France, 2019. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffr. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272. [Google Scholar] [CrossRef]
- Watanabe, M.; Fujiki, Y.; Yoshikado, S.; Ohachi, T. Structural features of a new compound K1−xTi2+xGa5−xO12 which exhibits one-dimensional ionic conduction. Solid State Ion. 1989, 35, 369–375. [Google Scholar] [CrossRef]
- Onoda, Y.; Michiue, Y.; Watanabe, M.; Yoshikado, S.; Ohachi, T. NMR study of one-dimensional ionic conductor, sodium titano-gallates (I). Solid State Ion. 1995, 79, 45–50. [Google Scholar] [CrossRef]
- Hasegawa, R.; Okabe, M.; Asaka, T.; Ishizawa, N.; Fukuda, K. Structure and ionic conductivity of well-aligned polycrystalline sodium titanogallate grown by reactive diffusion. J. Solid State Chem. 2015, 229, 252–259. [Google Scholar] [CrossRef]
- Baies, R.; Pérez, O.; Caignaert, V.; Pralong, V.; Raveau, B. A new sodium cobaltophosphate with a tunnel structure, ionic conductor. J. Mater. Chem. 2006, 16, 2434–2438. [Google Scholar] [CrossRef]
- Dridi, N.; Boukhari, A.; Réau, J.M.; Holt, E.M. Structure and Na+ ion conductivity inside double phosphates of Na8-2xM2+4+x (P2O7)4 formulation (M=Mn, Co and Ni). Solid State Ion. 1998, 107, 25–30. [Google Scholar] [CrossRef]
- Ortiz-Mosquera, J.F.; Nieto-Muñoz, A.M.; Rodrigues, A.C.M. Precursor glass stability, microstructure and ionic conductivity of glass-ceramics from the Na1+xAlxGe2–x(PO4)3 NASICON series. J. Non-Cryst. Solids 2019, 513, 36–43. [Google Scholar] [CrossRef]
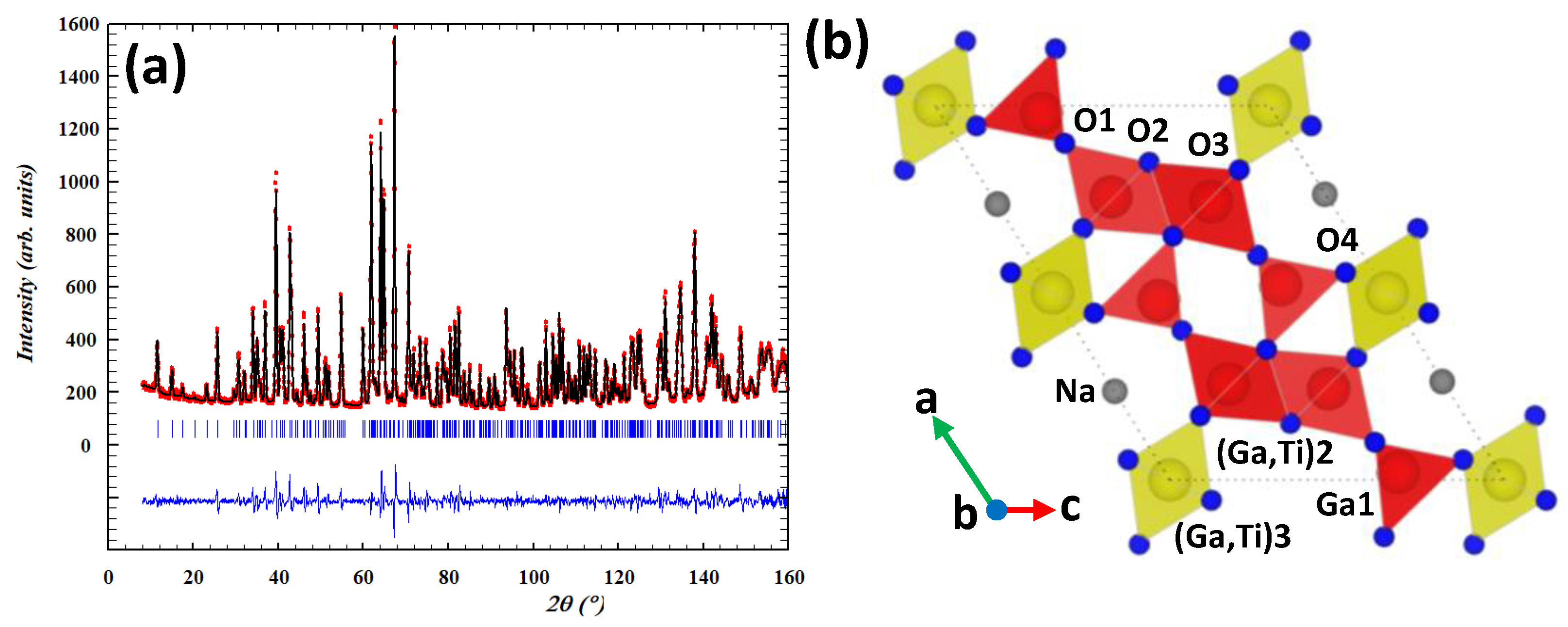
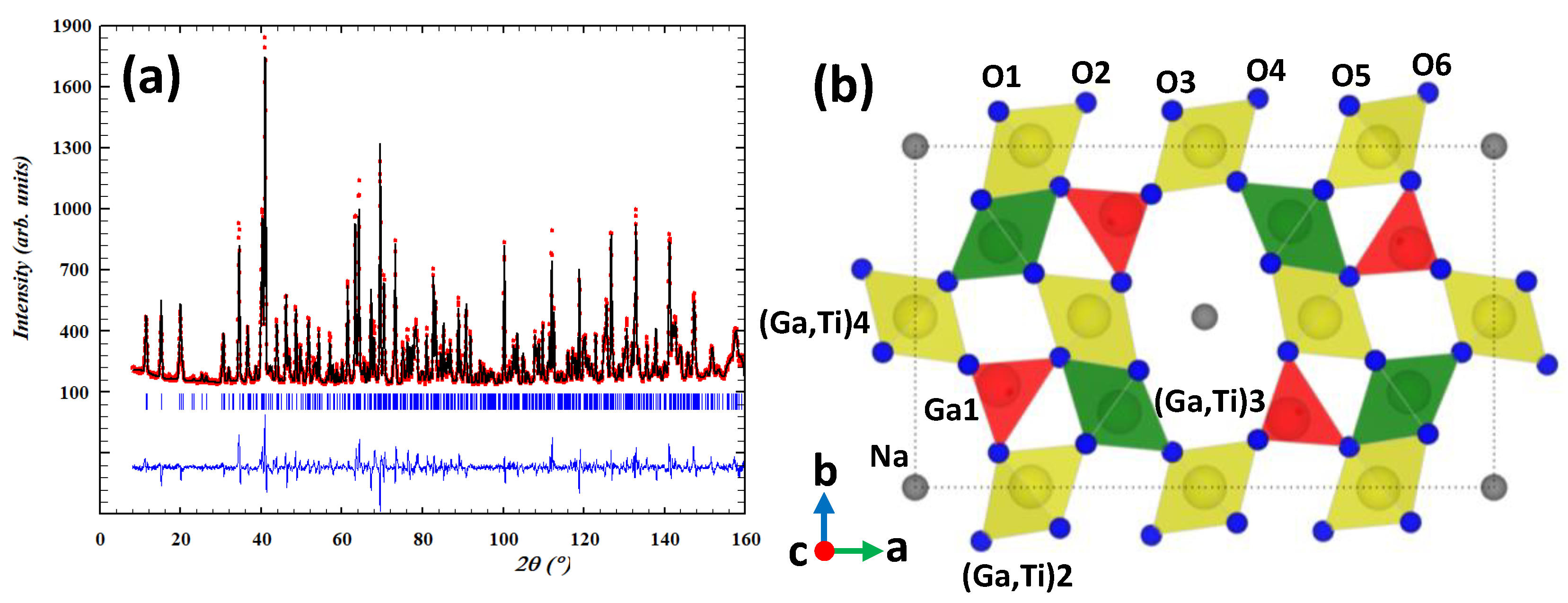

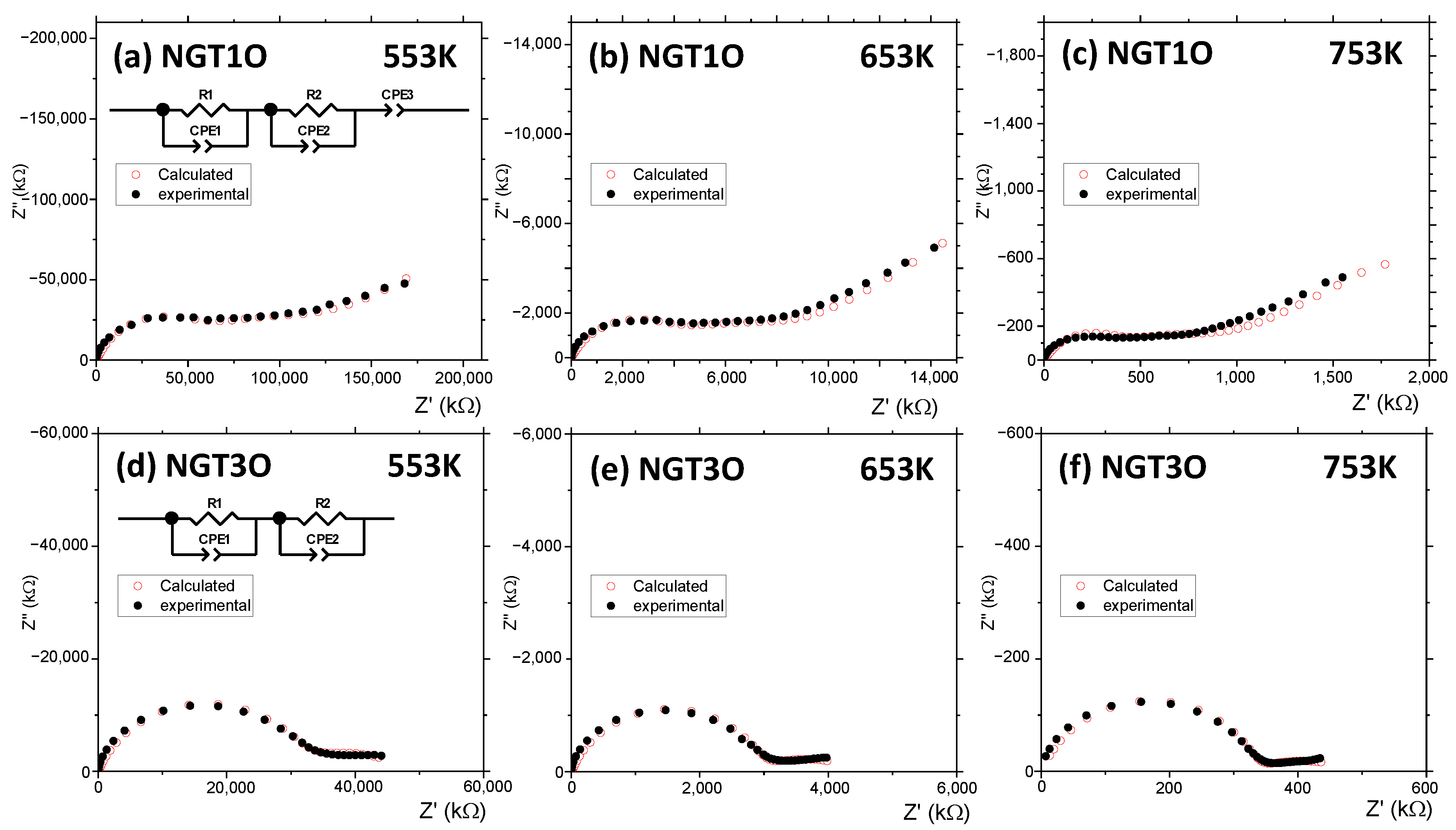
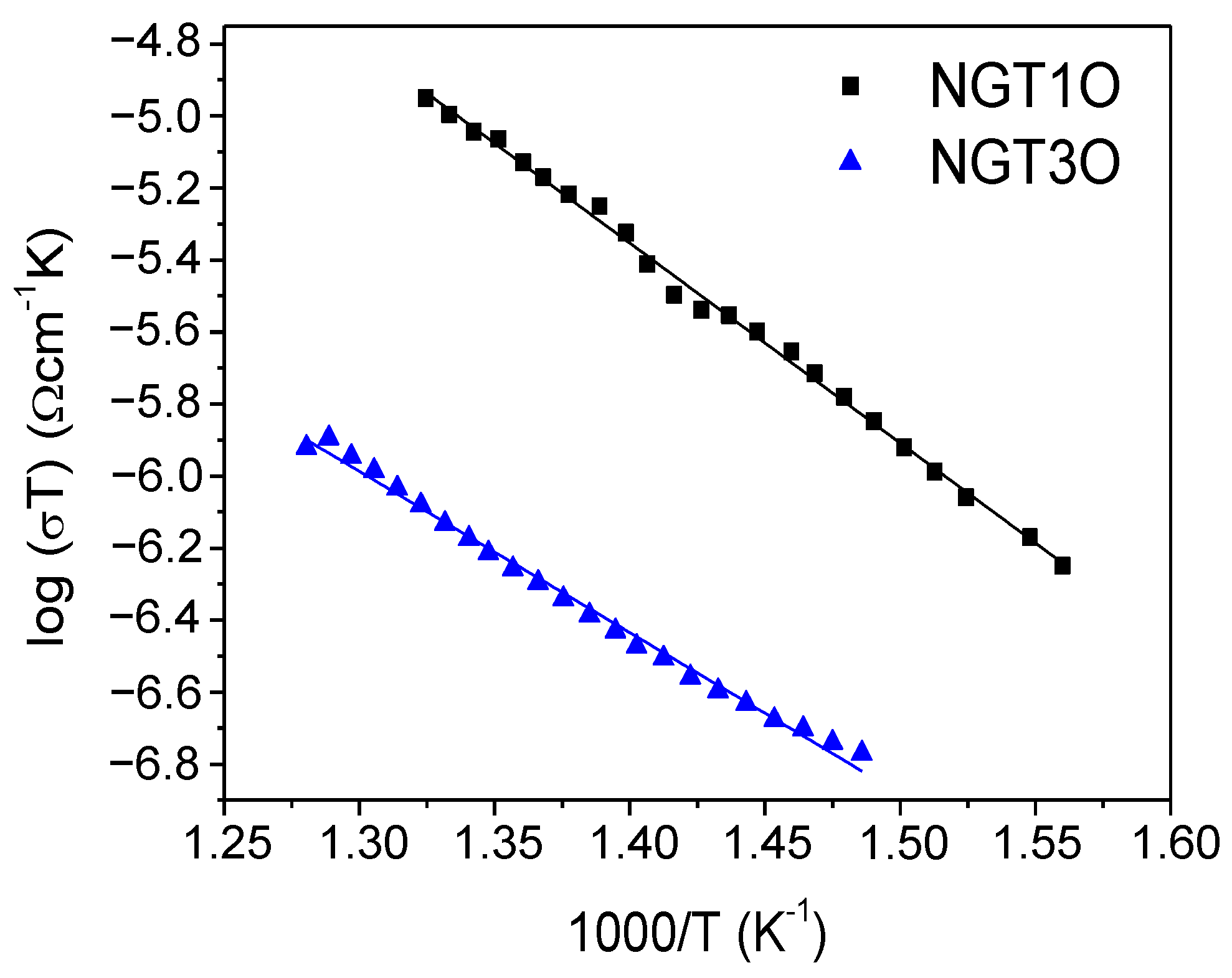
| Atom | Wyckoff | x | y | z | Biso (Å2) | Occ. |
|---|---|---|---|---|---|---|
| Ga1 | 4i | 0.4794(2) | 0 | 0.3030(3) | 0.49(3) | 1 |
| (Ga/Ti)2 | 4i | 0.2432(2) | 0 | 0.6443(3) | 0.29(4) | 0.930(4)/0.070(4) |
| (Ga/Ti)3 | 2a | 0 | 0 | 0 | 0.68(8) | 0.800(6)/0.200(6) |
| Na | 4i | 0.2368(14) | 0 | 0.006(2) | * | 0.400(14) |
| O1 | 4i | 0.1027(3) | 0 | 0.6847(3) | 0.61(4) | 1 |
| O2 | 4i | 0.3501(3) | 0 | 0.5358(3) | 0.38(4) | 1 |
| O3 | 4i | 0.1721(3) | 0 | 0.2134(3) | 0.64(4) | 1 |
| O4 | 4i | 0.4451(3) | 0 | 0.0864(3) | 0.63(4) | 1 |
| Atom | Wyckoff | x | y | z | Biso (Å2) | Occ. |
|---|---|---|---|---|---|---|
| Ga1 | 4g | 0.1447(2) | 0.3000 (4) | 0 | 0.30(1) | 1 |
| (Ga/Ti)2 | 4i | 0.1985(3) | 0.9979(5) | 0.5 | 0.44(1) | 0.83(6)/0.17(6) |
| (Ga/Ti)3 | 4g | 0.3545(8) | 0.2261(12) | 0 | 0.98(20) | 0.11(1)/0.89(1) |
| (Ga/Ti)4 | 2d | 0 | 0.5 | 0.5 | 0.47(1) | 0.85(1)/0.15(1) |
| Na | 4e | 0 | 0 | 0.108(5) | * | 0.35(3) |
| O1 | 4g | 0.1439(3) | 0.1004(4) | 0 | 0.48(7) | 1 |
| O2 | 4h | 0.2945(3) | 0.1283(5) | 0.5 | 0.31(6) | 1 |
| O3 | 4g | 0.4445(3) | 0.1011(4) | 0 | 0.35(7) | 1 |
| O4 | 4h | 0.0872(3) | 0.3534(5) | 0.5 | 0.41(6) | 1 |
| O5 | 4g | 0.2529(3) | 0.3784(4) | 0 | 0.19(6) | 1 |
| O6 | 4h | 0.3905(2) | 0.3485(4) | 0.5 | 0.34(6) | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Fernández, J.; Hernando, M.; Torres-Pardo, A.; López, M.L.; Fernández-Díaz, M.T.; Zhang, Q.; Terasaki, O.; Ramírez-Castellanos, J.; González-Calbet, J.M. Characterization of Tunneled Wide Band Gap Mixed Conductors: The Na2O-Ga2O3-TiO2 System. Nanomaterials 2023, 13, 2054. https://doi.org/10.3390/nano13142054
García-Fernández J, Hernando M, Torres-Pardo A, López ML, Fernández-Díaz MT, Zhang Q, Terasaki O, Ramírez-Castellanos J, González-Calbet JM. Characterization of Tunneled Wide Band Gap Mixed Conductors: The Na2O-Ga2O3-TiO2 System. Nanomaterials. 2023; 13(14):2054. https://doi.org/10.3390/nano13142054
Chicago/Turabian StyleGarcía-Fernández, Javier, María Hernando, Almudena Torres-Pardo, María Luisa López, María Teresa Fernández-Díaz, Qing Zhang, Osamu Terasaki, Julio Ramírez-Castellanos, and José M. González-Calbet. 2023. "Characterization of Tunneled Wide Band Gap Mixed Conductors: The Na2O-Ga2O3-TiO2 System" Nanomaterials 13, no. 14: 2054. https://doi.org/10.3390/nano13142054
APA StyleGarcía-Fernández, J., Hernando, M., Torres-Pardo, A., López, M. L., Fernández-Díaz, M. T., Zhang, Q., Terasaki, O., Ramírez-Castellanos, J., & González-Calbet, J. M. (2023). Characterization of Tunneled Wide Band Gap Mixed Conductors: The Na2O-Ga2O3-TiO2 System. Nanomaterials, 13(14), 2054. https://doi.org/10.3390/nano13142054









