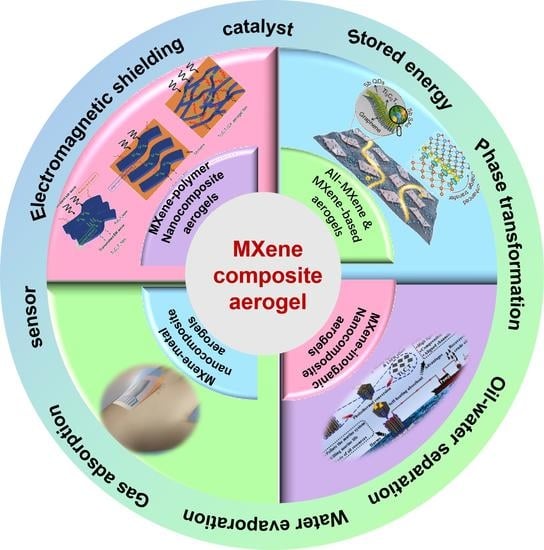
Fabrication, Performance, and Potential Applications of MXene Composite Aerogels
Abstract
1. Introduction
2. Scope and Progress
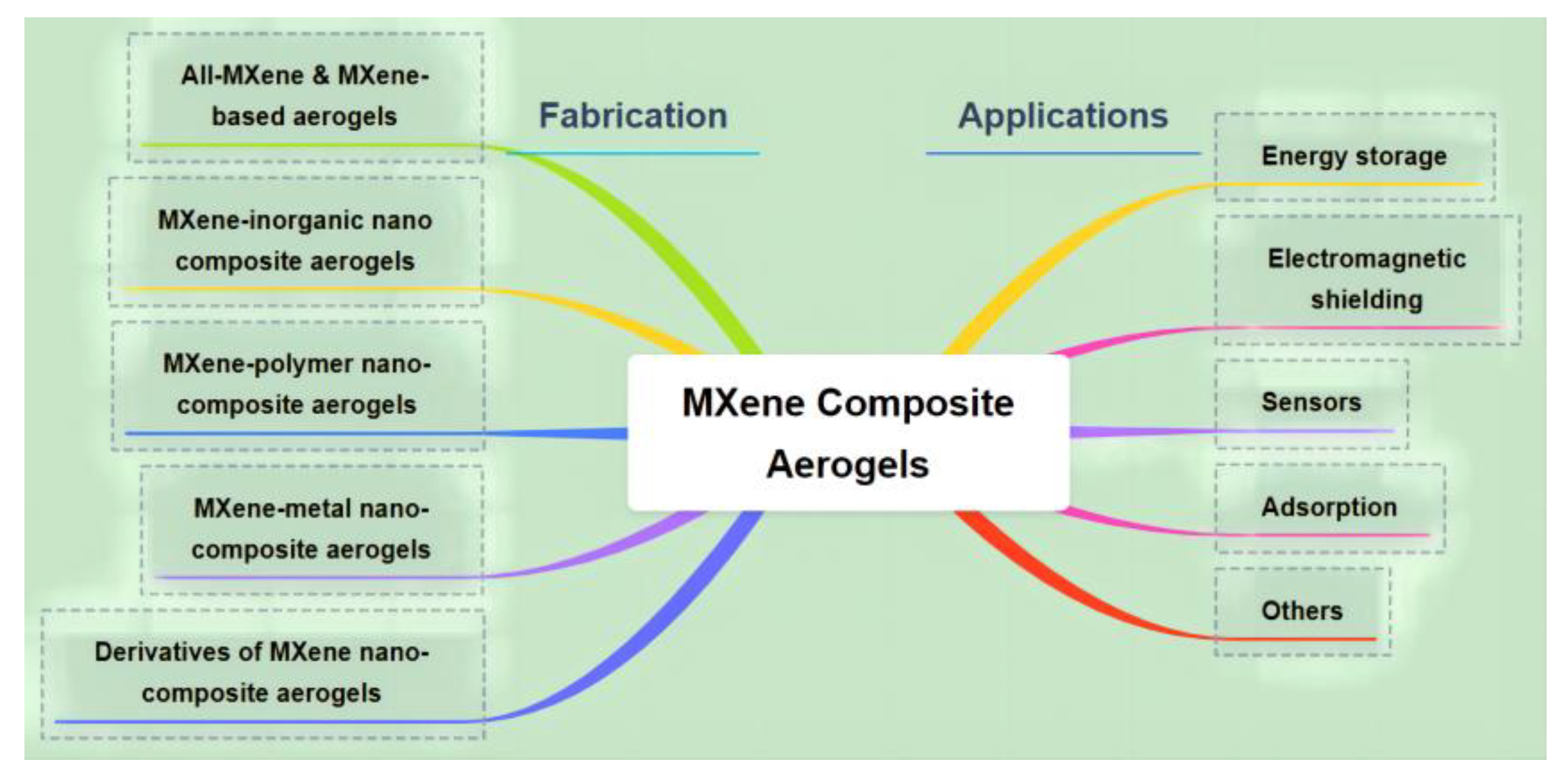
3. Design and Construction of MXene Aerogel Composites
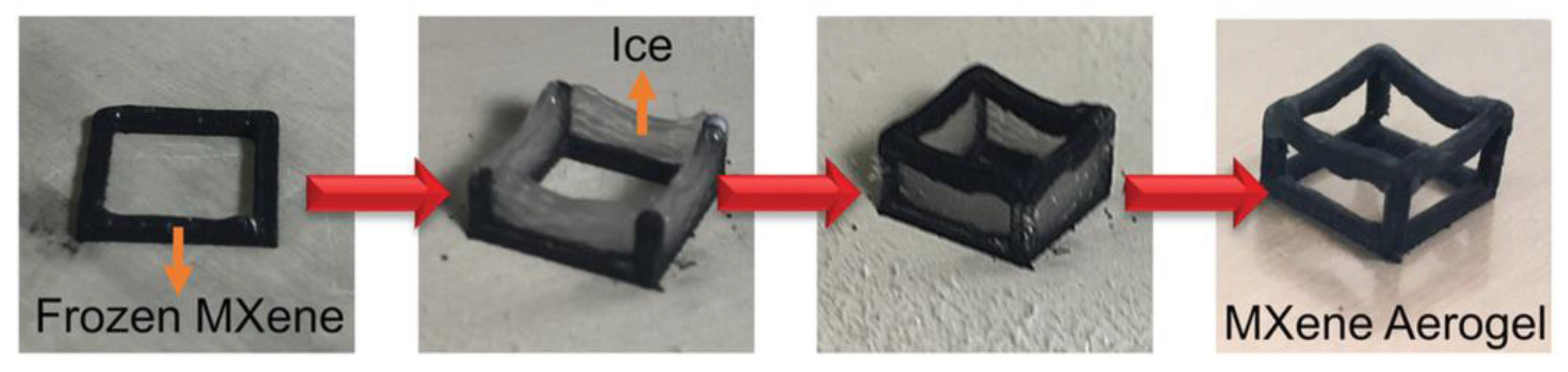
3.1. All-MXene Aerogels & MXene-Based Aerogels
3.2. MXene-Inorganic Nanocomposite Aerogels
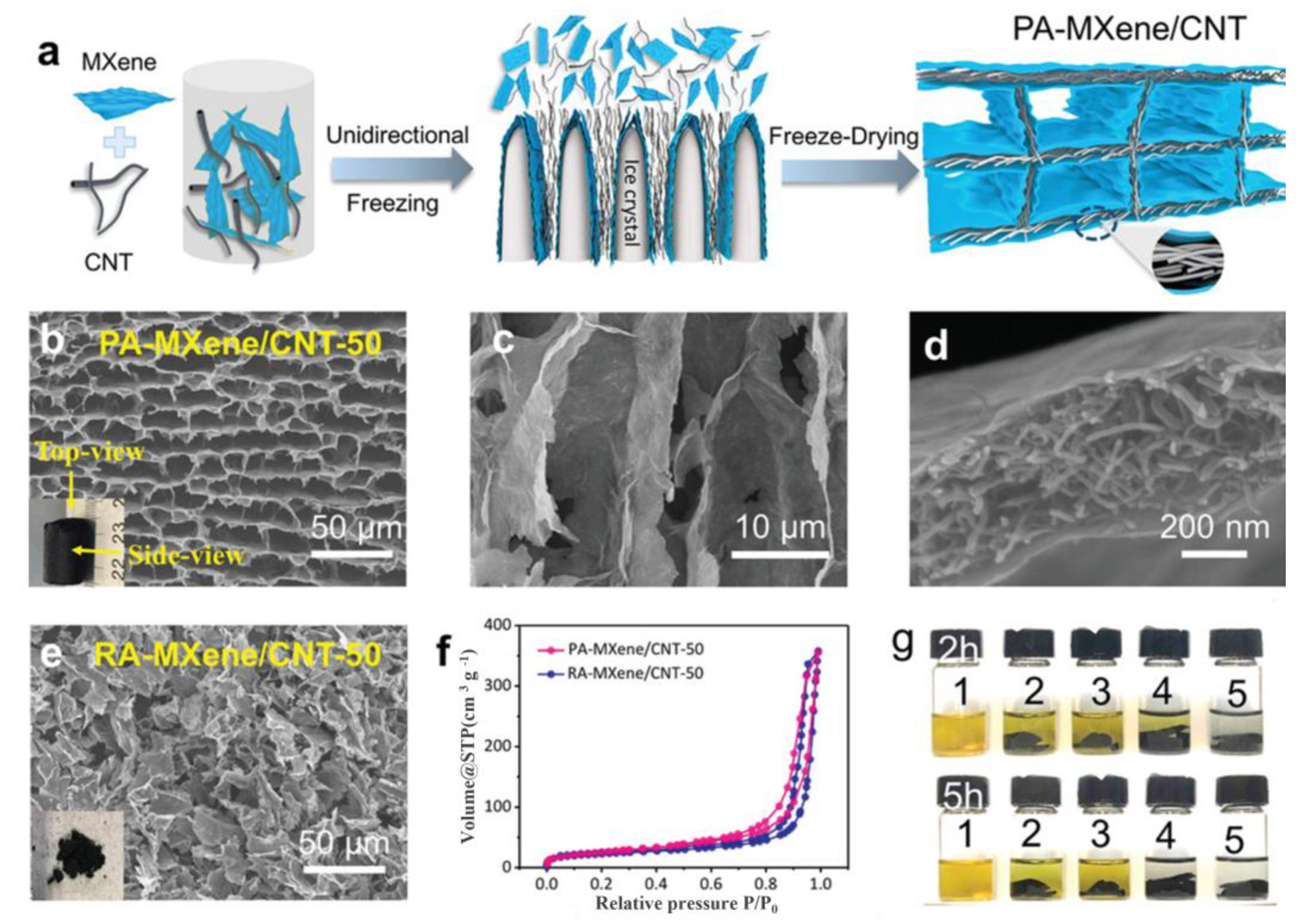
3.3. MXene-Polymer Nanocomposite Aerogels
3.4. MXene-Metal Nanocomposite Aerogel
3.5. Derivatives of MXene Composite Aerogels
4. Application of MXene Aerogel Composites
4.1. Energy Storage
4.1.1. Supercapacitor
4.1.2. Battery
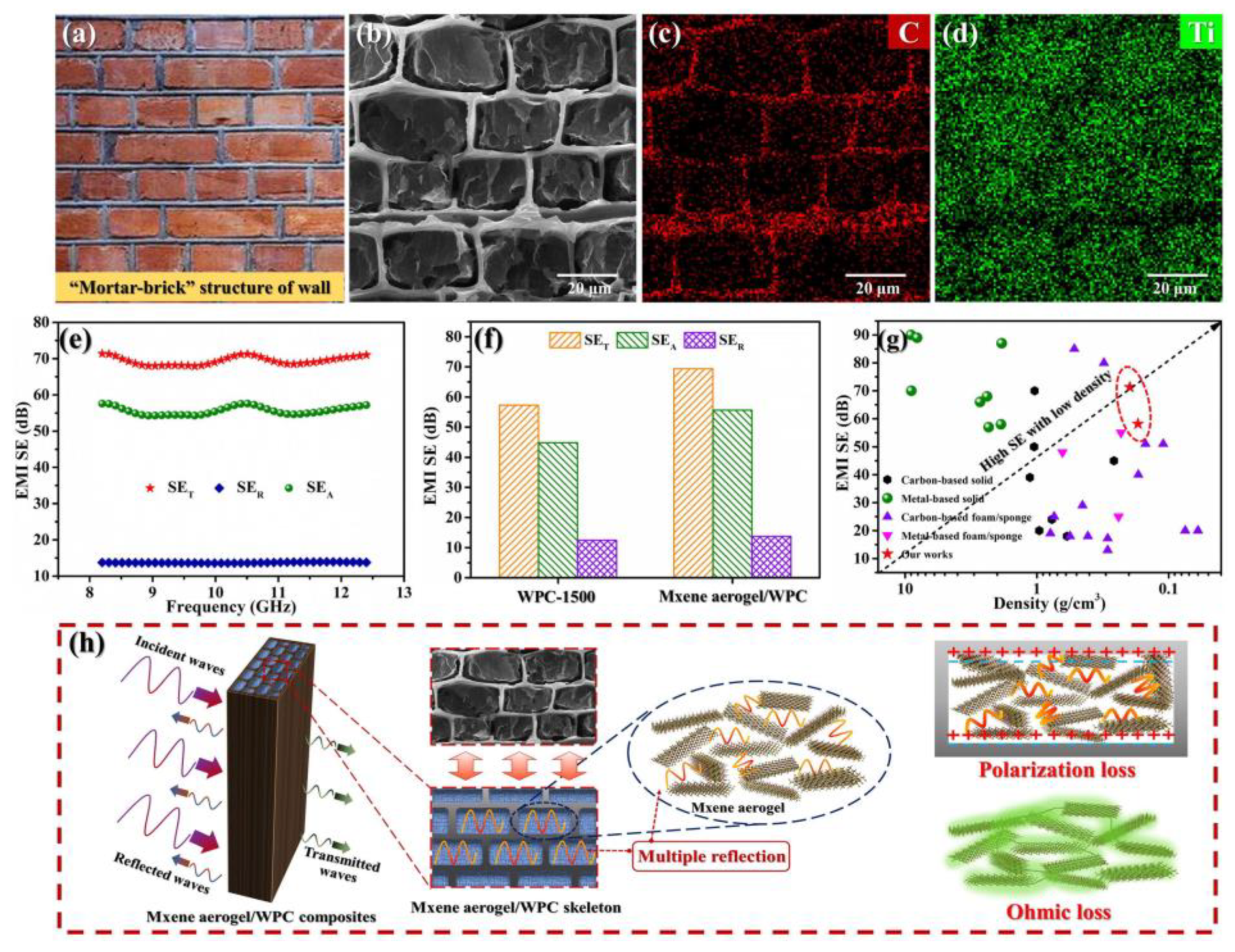
4.2. Electromagnetic Shielding
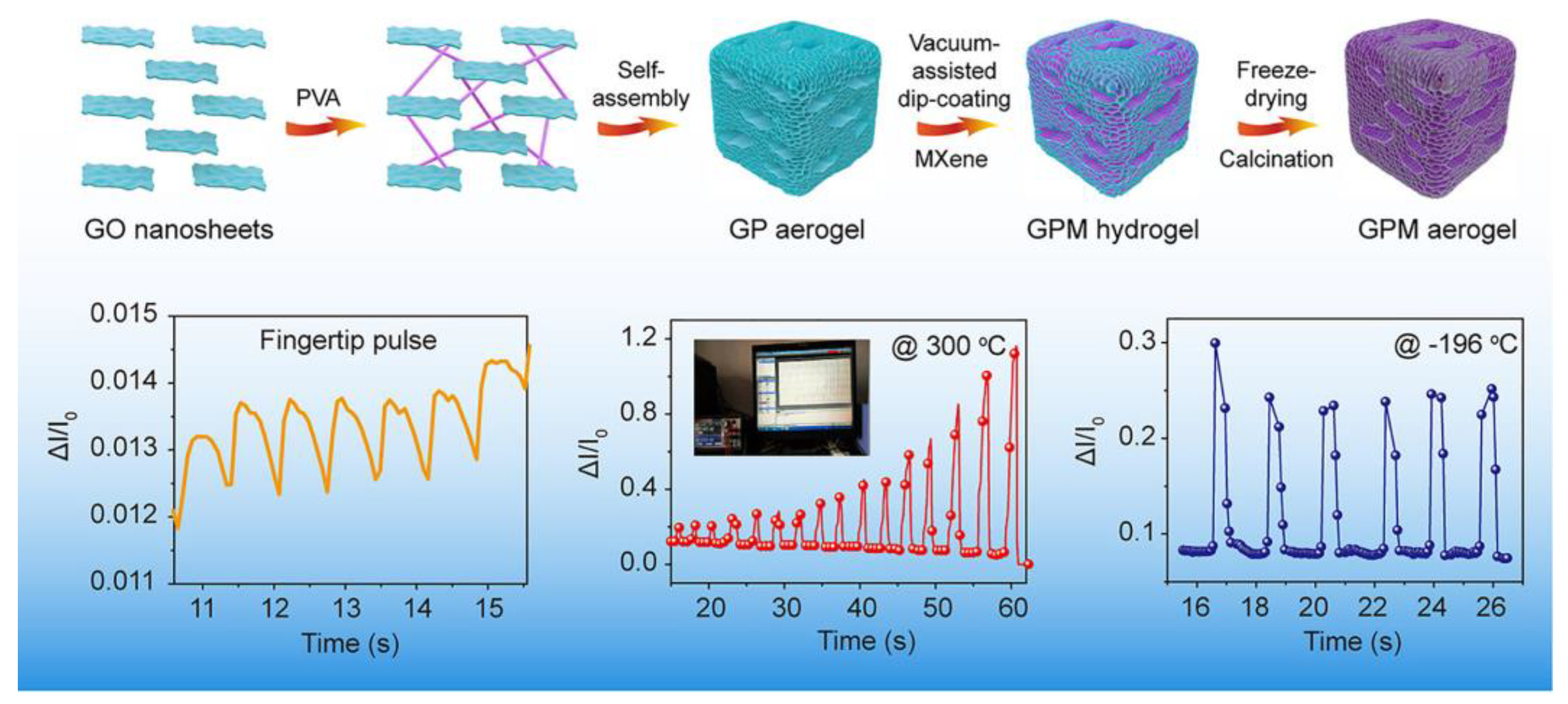
4.3. Sensors
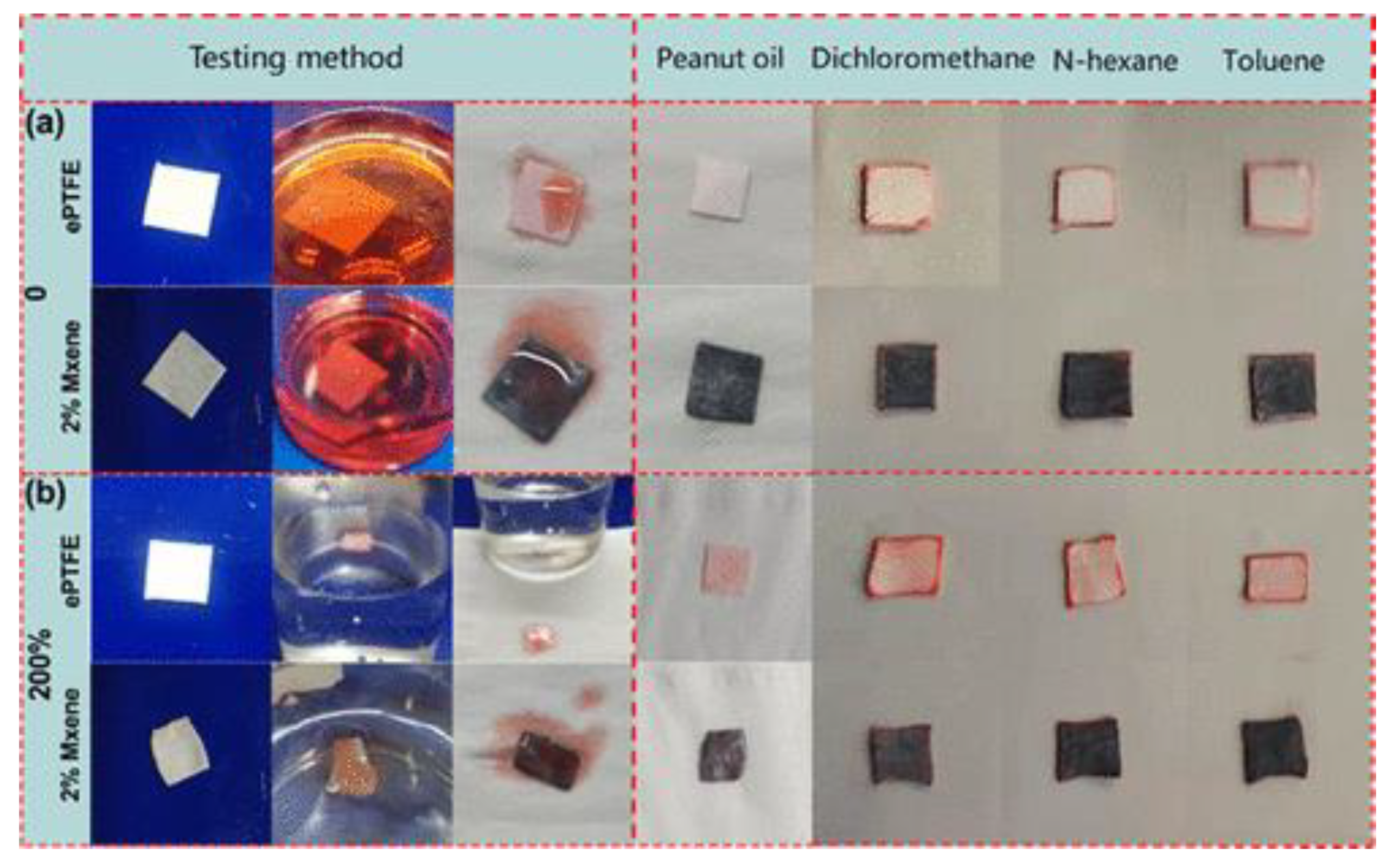
4.4. Adsorption of Oil
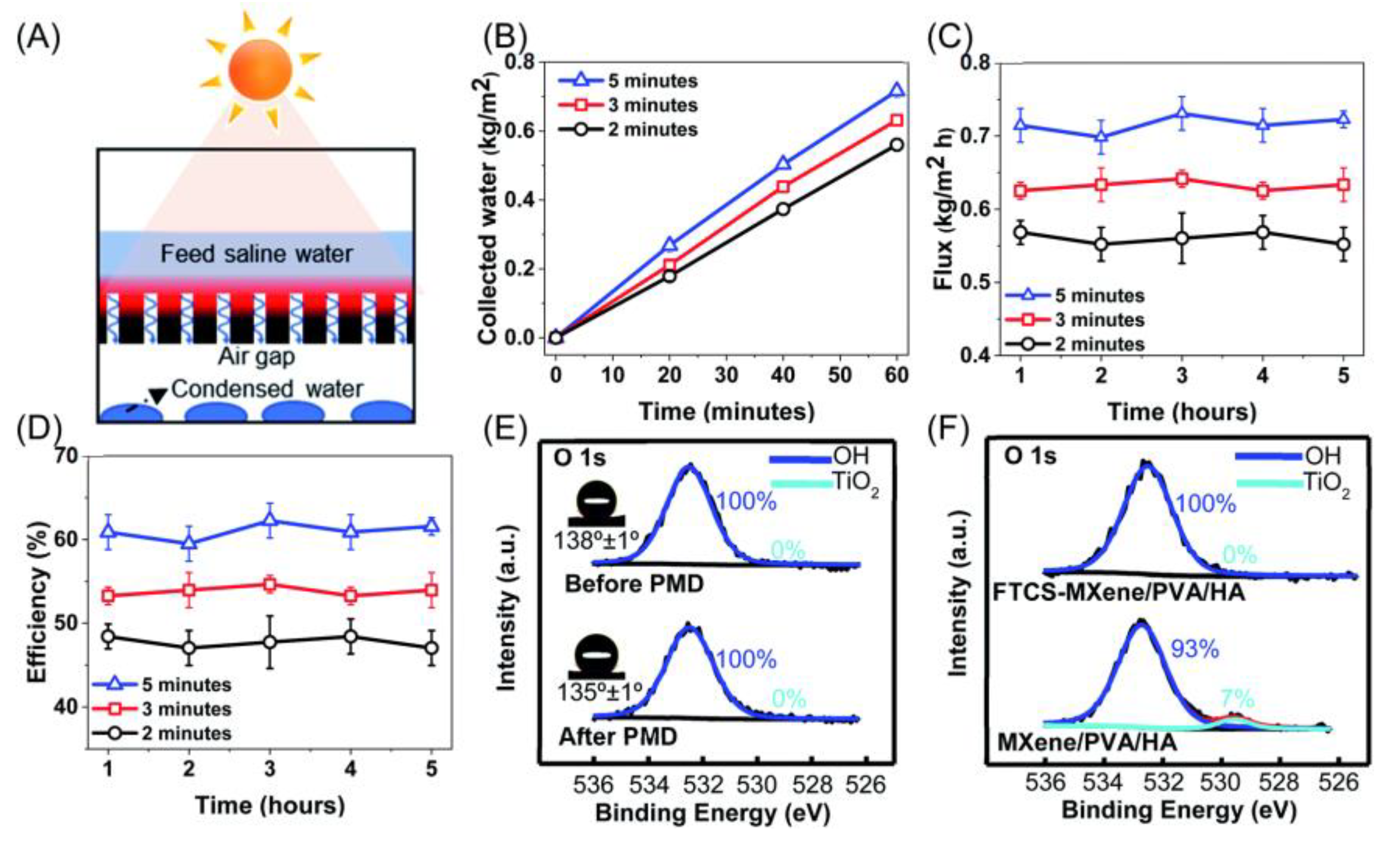
4.5. Others
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Linhares, T.; Pessoa de Amorim, M.T.; Durães, L. Silica Aerogel Composites with Embedded Fibres: A Review on Their Preparation, Properties and Applications. J. Mater. Chem. A 2019, 7, 22768–22802. [Google Scholar] [CrossRef]
- Basak, S.; Singhal, R.S. The Potential of Supercritical Drying as a “green” Method for the Production of Food-grade Bioaerogels: A comprehensive Critical Review. Food Hydrocoll. 2023, 141, 108738. [Google Scholar] [CrossRef]
- Baldino, L.; Zuppolini, S.; Cardea, S.; Diodato, L.; Borriello, A.; Reverchon, E.; Nicolais, L. Production of Biodegradable Superabsorbent Aerogels using a Supercritical CO2 Assisted Drying. J. Supercrit. Fluids 2020, 156, 104681. [Google Scholar] [CrossRef]
- Phalippou, J.; Woignier, T.; Prassas, M. Glasses from aerogels. J. Mater. Sci. 1990, 25, 3111–3117. [Google Scholar] [CrossRef]
- Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar]
- Si, Y.; Fu, Q.; Wang, X.; Zhu, J.; Yu, J.; Sun, G.; Ding, B. Superelastic and Superhydrophobic Nanofiber-assembled Cellular Aerogels for Effective Separation of Oil/water Emulsions. ACS Nano 2015, 94, 3791–3799. [Google Scholar] [CrossRef] [PubMed]
- Peri, J.B. Infrared Study of OH and NH2 Groups on the Surface of a Dry Silica Aerogel. J. Phys. Chem. 1966, 70, 2937–2945. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Guerrero-Alburquerque, N.; Koebel, M.M.; Nystrom, G. Biopolymer Aerogels and Foams: Chemistry, Properties, and Applications. Angew. Chem.-Int. Ed. 2018, 57, 7580–7608. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.M. Progress in Research and Development on MAX Phases: A Family of Layered Ternary Compounds. Int. Mater. Rev. 2011, 56, 143–166. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341, 1502. [Google Scholar] [CrossRef] [PubMed]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D Metal Carbides and Nitrides (MXenes) for Energy Storage. Nat. Rev. Mater. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, H.; Zhang, P.; Bao, Z.; Zheng, W.; Tian, W.B.; Zhang, W.; Zhou, M.; Sun, Z. Self-assembled Sandwich Hollow Porous Carbon Sphere @ MXene Composites as Superior LiS battery Cathode Hosts. 2d Mater. 2020, 7, 025–049. [Google Scholar] [CrossRef]
- Hantanasirisakul, K.; Gogotsi, Y. Electronic and Optical Properties of 2D Transition Metal Carbides and Nitrides (MXenes). Adv. Mater. 2018, 30, e1804779. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, J.; Peng, W.; Zhu, Y.; Zhao, Y.; Jiang, K.; Peng, M.; Tan, Y. Highly Stable 3D Ti3C3Tx MXene-Based Foam Architectures toward High-Performance Terahertz Radiation Shielding. ACS Nano 2020, 14, 2109–2117. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, K.H.; Anjum, D.H.; Sougrat, R.; Jiang, Q.; Kim, H.; Alshareef, H.N. MXenes Stretch Hydrogel Sensor Performance to New Limits. Sci. Adv. 2018, 4, eaat0098. [Google Scholar] [CrossRef]
- Cai, Z.; Hao, X.; Sun, X.-b.; Du, P.; Liu, W.; Fu, J. Highly Active WO3@anatase-SiO2 Aerogel for Solar-light-driven Phenanthrene Degradation: Mechanism Insight and Toxicity Assessment. Water Res. 2019, 162, 369–382. [Google Scholar] [CrossRef]
- Venkateshalu, S.; Grace, A.N. MXenes—A New Class of 2D Layered Materials: Synthesis, Properties, Applications as Supercapacitor Electrode and Beyond. Appl. Mater. Today 2020, 18, 100509. [Google Scholar] [CrossRef]
- Qin, L.; Yang, D.; Zhang, M.; Zhao, T.; Luo, Z.; Yu, Z.Z. Superelastic and Ultralight Electrospun Carbon Nanofiber/MXene Hybrid Aerogels with Anisotropic Microchannels for Pressure Sensing and Energy Storage. J. Colloid Interface Sci. 2021, 589, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, K.; Wang, Y.; Wu, Z.; Russell, T.P.; Shi, S. MXene-Based Porous Monoliths. Nanomaterials 2022, 12, 3792. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Zhang, J.; Qin, S.; Wang, Z.; Usman, K.A.S.; Hegh, D.; Liu, J.; Lei, W.; Razal, J.M. Superelastic Ti3C2Tx MXene-Based Hybrid Aerogels for Compression-Resilient Devices. ACS Nano 2021, 15, 5000–5010. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, W.; Qiu, Z.; Yang, T.; Wang, J.; Ji, X.; Huang, Y. Manipulation of Impedance Matching toward 3D-Printed Lightweight and Stiff MXene-Based Aerogels for Consecutive Multiband Tunable Electromagnetic Wave Absorption. ACS Nano 2023, 17, 8420–8432. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xie, M.; Wu, F.; Mei, Y.; Hao, Y.; Li, L.; Chen, R. Encapsulation of Metallic Zn in a Hybrid MXene/Graphene Aerogel as a Stable Zn Anode for Foldable Zn-Ion Batteries. Adv. Mater. 2022, 34, 2106897. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Zhang, A.; Zheng, K.; Liu, R.; Cheng, Y.; Wang, L.; Li, A.; Liu, J. Fabrication of Cobaltous Sulfide Nanoparticle-modified 3D MXene/carbon Foam Hybrid Aerogels for All-solid-state Supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 28222–28230. [Google Scholar] [CrossRef]
- Abdinejad, M.; Subramanian, S.; Motlagh, M.K.; Noroozifar, M.; Duangdangchote, S.; Neporozhnii, I.; Ripepi, D.; Pinto, D.; Li, M.; Tang, K.; et al. Insertion of MXene-Based Materials into Cu–Pd 3D Aerogels for Electroreduction of CO2 to Formate. Adv. Energy Mater. 2023, 13, 2300402. [Google Scholar] [CrossRef]
- Song, F.; Hu, J.; Li, G.; Wang, J.; Chen, S.; Xie, X.; Wu, Z.; Zhang, N. Room-Temperature Assembled MXene-Based Aerogels for High Mass-Loading Sodium-Ion Storage. Nano-Micro Lett. 2021, 14, 37. [Google Scholar] [CrossRef]
- Fan, Q.; Yi, M.; Chai, C.; Li, W.; Qi, P.; Wang, J.; Hao, J. Oxidation Stability Enhanced MXene-based Porous Materials Derived from Water-in-ionic Liquid Pickering Emulsions for Wearable Piezoresistive Sensor and Oil/Water Separation Applications. J. Colloid Interface Sci. 2022, 618, 311–321. [Google Scholar] [CrossRef]
- Lu, X.; Arduini-Schuster, M.C.; Kuhn, J.; Nilsson, O.; Fricke, J.; Pekala, R.W. Thermal Conductivity of Monolithic Organic Aerogels. Science 1992, 255, 971–972. [Google Scholar] [CrossRef]
- Mayer, S.T.; Pekala, R.W.; Kaschmitter, J.L. The Aerocapacitor: An Electrochemical Double-Layer Energy-Storage Device. J. Electrochem. Soc. 1993, 140, 446–451. [Google Scholar] [CrossRef]
- Kim, J.-h.; Suh, D.J.; Park, T.-J.; Kim, K.-L. Effect of Metal Particle Size on Coking during CO2 Reforming of CH4 over Ni–alumina Aerogel Catalysts. Appl. Catal. A-Gen. 2000, 197, 191–200. [Google Scholar] [CrossRef]
- Tewari, P.H.; Hunt, A.J.; Lofftus, K.D. Ambient-temperature Supercritical Drying of Transparent Silica Aerogels. Mater. Lett. 1985, 3, 363–367. [Google Scholar] [CrossRef]
- Pajonk, G. Transparent Silica Aerogels. J. Non-Cryst. Solids 1998, 225, 307–314. [Google Scholar] [CrossRef]
- Shimizu, T.; Kanamori, K.; Nakanishi, K. Silicone-based Organic–inorganic Hybrid Aerogels and Xerogels. Chem.–A Eur. J. 2017, 23, 5176–5187. [Google Scholar] [CrossRef]
- Cao, W.-T.; Chen, F.-F.; Zhu, Y.; Zhang, Y.-G.; Jiang, Y.; Ma, M.; Chen, F. Binary Strengthening and Toughening of MXene/Cellulose Nanofiber Composite Paper with Nacre-Inspired Structure and Superior Electromagnetic Interference Shielding Properties. ACS Nano 2018, 125, 4583–4593. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, X.; Xin, X.; Tang, Z.-R.; Xu, Y.J. Ti3C2Tx-Based Three-Dimensional Hydrogel by a Graphene Oxide-Assisted Self-Convergence Process for Enhanced Photoredox Catalysis. ACS Nano 2018, 131, 295–304. [Google Scholar] [CrossRef]
- Shang, T.; Lin, Z.; Qi, C.; Liu, X.; Li, P.; Tao, Y.; Wu, Z.; Li, D.; Simon, P.; Yang, Q.H. 3D Macroscopic Architectures from Self-Assembled MXene Hydrogels. Adv. Funct. Mater. 2019, 29, 1903960. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, X.S.; Chen, Y.; Liu, Y.; Yang, R.; Sui, G. Hierarchically Structured Cellulose Aerogels with Interconnected MXene Networks and Their Enhanced Microwave Absorption Properties. J. Mater. Chem. C 2018, 6, 8679–8687. [Google Scholar] [CrossRef]
- Tetik, H.; Orangi, J.; Yang, G.; Zhao, K.; Mujib, S.B.; Singh, G.; Beidaghi, M.; Lin, D. 3D Printed MXene Aerogels with Truly 3D Macrostructure and Highly Engineered Microstructure for Enhanced Electrical and Electrochemical Performance. Adv. Mater. 2022, 34, e2104980. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X. Electrically Conductive, Optically Responsive, and Highly Orientated Ti3C2Tx MXene Aerogel Fibers. Adv. Funct. Mater. 2022, 32, 2107767. [Google Scholar] [CrossRef]
- Deng, Y.; Shang, T.; Wu, Z.; Tao, Y.; Luo, C.; Liang, J.; Han, D.; Lyu, R.; Qi, C.; Lv, W.; et al. Fast Gelation of Ti3C2Tx MXene Initiated by Metal Ions. Adv. Mater. 2019, 31, e1902432. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Ding, Y.; Huang, M.; Li, J.; Han, Q.; Yang, M.; Li, W. MXene/CNTs/Aramid Aerogels for Electromagnetic Interference Shielding and Joule Heating. ACS Appl. Nano Mater. 2023, 6, 6141–6150. [Google Scholar] [CrossRef]
- Ma, Y.; Yue, Y.; Zhang, H.; Cheng, F.; Zhao, W.; Rao, J.; Luo, S.; Wang, J.; Jiang, X.; Liu, Z.; et al. 3D Synergistical MXene/Reduced Graphene Oxide Aerogel for a Piezoresistive Sensor. ACS Nano 2018, 12, 3209–3216. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, C.; Zhou, G.; Pan, Z.Z.; Ma, J.; Nishihara, H.; He, Y.B.; Kang, F.; Lv, W.; Yang, Q.H. Lamellar MXene Composite Aerogels with Sandwiched Carbon Nanotubes Enable Stable Lithium–Sulfur Batteries with a High Sulfur Loading. Adv. Funct. Mater. 2021, 31, 2100793. [Google Scholar] [CrossRef]
- Li, Y.; Meng, F.; Mei, Y.; Wang, H.; Guo, Y.; Wang, Y.; Peng, F.; Huang, F.; Zhou, Z. Electrospun Generation of Ti3C2Tx MXene@graphene Oxide Hybrid Aerogel Microspheres for Tunable High-performance Microwave Absorption. Chem. Eng. J. 2020, 391, 123512. [Google Scholar] [CrossRef]
- Wu, N.; Yang, Y.; Wang, C.; Wu, Q.; Pan, F.; Zhang, R.; Liu, J.; Zeng, Z. Ultrathin Cellulose Nanofiber Assisted Ambient-Pressure-Dried, Ultralight, Mechanically Robust, Multifunctional MXene Aerogels. Adv. Mater. 2023, 35, 2207969. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, W.; Xiao, Y.; Du, B.; Zhang, X.; Wen, M.; Lai, C.; Huang, Y.; Sheng, L. Multifunctional, Robust, and Porous PHBV—GO/MXene Composite Membranes with Good Hydrophilicity, Antibacterial Activity, and Platelet Adsorption. Polymers 2021, 13, 3748. [Google Scholar] [CrossRef]
- Wu, S.; Chen, D.; Han, W.; Xie, Y.; Zhao, G.; Dong, S.; Tan, M.; Huang, H.; Xu, S.; Chen, G. Ultralight and hydrophobic MXene/chitosan-derived Hybrid Carbon Aerogel with Hierarchical Pore Structure for Durable Electromagnetic Interference Shielding and Thermal Insulation. Chem. Eng. J. 2022, 446, 137093. [Google Scholar] [CrossRef]
- de Luna, M.S.; Ascione, C.; Santillo, C.; Verdolotti, L.; Lavorgna, M.; Buonocore, G.; Castaldo, R.; Filippone, G.; Xia, H.; Ambrosio, L. Optimization of Dye Adsorption Capacity and Mechanical Strength of Chitosan Aerogels through Crosslinking Strategy and Graphene Oxide Addition. Carbohydr. Polym. 2019, 211, 195–203. [Google Scholar] [CrossRef]
- Zhuo, H.; Hu, Y.; Chen, Z.; Peng, X.; Liu, L.; Luo, Q.; Yi, J.; Liu, C.; Zhong, L. A carbon Aerogel with Super Mechanical and Sensing Performances for Wearable Piezoresistive Sensors. J. Mater. Chem. A 2019, 7, 8092–8100. [Google Scholar] [CrossRef]
- Liang, C.; Qiu, H.; Song, P.; Shi, X.; Kong, J.; Gu, J. Ultra-light MXene aerogel/wood-derived Porous Carbon Composites with Wall-like “Mortar/Brick” Structures for Electromagnetic Interference Shielding. Sci. Bull. 2020, 65, 616–622. [Google Scholar] [CrossRef]
- Liang, L.; Li, Q.; Yan, X.; Feng, Y.; Wang, Y.; Zhang, H.-B.; Zhou, X.; Liu, C.; Shen, C.; Xie, X. Multifunctional Magnetic Ti3C2Tx MXene/graphene Aerogel with Superior Electromagnetic Wave Absorption Performance. ACS Nano 2021, 15, 6622–6632. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, A.; Tang, J.; Tian, J.; Huang, W.; Cai, J.; Barrow, C.; Yang, W.; Liu, J. Fabrication of Cobaltosic Oxide Nanoparticle-Doped 3 D MXene/Graphene Hybrid Porous Aerogels for All-Solid-State Supercapacitors. Chem.-A Eur. J. 2019, 25, 5547–5554. [Google Scholar] [CrossRef]
- Li, Q.; Song, T.; Wang, Z.; Wang, X.; Zhou, X.; Wang, Q.; Yang, Y. A General Strategy toward Metal Sulfide Nanoparticles Confined in a Sulfur-Doped Ti3C2Tx MXene 3D Porous Aerogel for Efficient Ambient N2 Electroreduction. Small 2021, 17, 2103305. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Gu, Q.; Yu, X. Three-Dimensional MOFs@MXene Aerogel Composite Derived MXene Threaded Hollow Carbon Confined CoS Nanoparticles toward Advanced Alkali-Ion Batteries. ACS Nano 2021, 15, 3228–3240. [Google Scholar] [CrossRef] [PubMed]
- Le Goff, R.; Mahé, O.; Le Coz-Botrel, R.; Malo, S.; Goupil, J.M.; Brière, J.F.; Dez, I. Insight in Chitosan Aerogels Derivatives -Application in Catalysis. React. Funct. Polym. 2020, 146, 104393. [Google Scholar] [CrossRef]
- Lim, M.B.; Ganas, A.S.; Hanson, J.L.; Zhou, X.; Hellner, B.; Manandhar, S.; Gariepy, R.E.; Baneyx, F.o.; Wilbur, D.S.; Pauzauskie, P.J. Crystalline Loading of Lipophilic Coenzyme Q10 Pharmaceuticals within Conjugated Carbon Aerogel Derivatives. Carbon 2020, 164, 451–458. [Google Scholar] [CrossRef]
- Mitropoulos, A.N.; Burpo, F.J.; Nguyen, C.K.; Nagelli, E.A.; Ryu, M.Y.; Wang, J.; Sims, R.K.; Woronowicz, K.; Wickiser, J.K. Noble Metal Composite Porous Silk Fibroin Aerogel Fibers. Materials 2019, 12, 894. [Google Scholar] [CrossRef]
- Zuo, X.; Fan, T.; Qu, L.; Zhang, X.; Miao, J. Smart Multi-responsive Aramid Aerogel Fiber Enabled Self-powered Fabrics. Nano Energy 2022, 101, 107559. [Google Scholar] [CrossRef]
- Li, X.; Wen, C.; Li, H.; Sun, G. In Situ Decoration of Nanosized Metal Oxide on Highly Conductive MXene Nanosheets as Efficient Catalyst for Li-O2 Battery. J. Energy Chem. 2020, 47, 272–280. [Google Scholar] [CrossRef]
- Wu, Z.; Li, C.; Li, Z.; Feng, K.; Cai, M.; Zhang, D.; Wang, S.; Chu, M.; Zhang, C.; Shen, J. Niobium and Titanium Carbides (MXenes) as Superior Photothermal Supports for CO2 Photocatalysis. ACS Nano 2021, 15, 5696–5705. [Google Scholar] [CrossRef] [PubMed]
- Seidi, F.; Arabi Shamsabadi, A.; Dadashi Firouzjaei, M.; Elliott, M.; Saeb, M.R.; Huang, Y.; Li, C.; Xiao, H.; Anasori, B. MXenes Antibacterial Properties and Applications: A Review and Perspective. Small 2023, 19, 2206716. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-X.; Guan, G.-Z.; Shi, X.; Lu, L.; Fan, Y.-C.; Xu, J.; Shang, Y.-Y.; Zhang, Y.-J.; Wei, J.-Q.; Guo, F.-M. Bidirectionally aligned MXene hybrid aerogels assembled with MXene nanosheets and microgels for supercapacitors. Rare Met. 2023, 42, 1249–1260. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, Y.; Bin, X.; Xia, C.; Que, W. 3D Porous Compact 1D/2D Fe2O3/MXene Composite Aerogel Film Electrodes for All-Solid-State Supercapacitors. Small 2022, 18, 2204917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, K.; Li, Y.; Wang, Z.; Zhan, K.; Yang, J.; Zhao, B. Nanoparticles of Fe3O4 Anchored on Ti3C2Tx MXene/rGO Aerogels as Hybrid Negative Electrodes for Advanced Supercapacitors. ACS Appl. Nano Mater. 2023, 6, 482–491. [Google Scholar] [CrossRef]
- Shao, L.; Xu, J.; Ma, J.; Zhai, B.; Li, Y.; Xu, R.; Ma, Z.; Zhang, G.; Wang, C.; Qiu, J. MXene/RGO composite aerogels with light and high-strength for supercapacitor electrode materials. Compos. Commun. 2020, 19, 108–113. [Google Scholar] [CrossRef]
- Chaudhary, K.; Zulfiqar, S.; Somaily, H.H.; Aadil, M.; Warsi, M.F.; Shahid, M. Rationally designed multifunctional Ti3C2 MXene@Graphene composite aerogel integrated with bimetallic selenides for enhanced supercapacitor performance and overall water splitting. Electrochim. Acta 2022, 431, 141103. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Dong, S.; Zhang, X.; Hou, S. Synthesis of ultra-high specific surface area aerogels with nitrogen-enriched Ti3C2Tx nanosheets as high-performance supercapacitor electrodes. J. Mater. Chem. C 2022, 10, 14929–14938. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, Y.; Fan, L.; Ye, D.; Xu, W.; Xu, J. Ultralight PPy@PVA/BC/MXene Composite Aerogels for High-performance Supercapacitor Eltrodes and Pressure Sensors. Appl. Surf. Sci. 2023, 624, 157138. [Google Scholar] [CrossRef]
- Guo, X.; Gao, H.; Wang, S.; Yang, G.; Zhang, X.; Zhang, J.; Liu, H.; Wang, G. MXene-Based Aerogel Anchored with An-timony Single Atoms and Quantum Dots for High-Performance Potassium-Ion Batteries. Nano Lett. 2022, 22, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Guo, X.; Zhang, J.; Chen, Y.; Zhang, C.; Luo, L.; Wang, F.; Wang, G. Rational Design of Free-standing 3D Porous MXene/rGO Hybrid Aerogels as Polysulfide Reservoirs for High-energy Lithium–sulfur Batteries. J. Mater. Chem. A 2019, 7, 6507–6513. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Zheng, Z.-J.; Feng, Y.-Q.; Ye, H.; Cao, F.-F.; Guo, Z.-P. Topological Design of Ultrastrong MXene Paper Hosted Li Enables Ultrathin and Fully Flexible Lithium Metal Batteries. Nano Energy 2020, 74, 104817. [Google Scholar] [CrossRef]
- Ding, M.; Li, S.; Guo, L.; Jing, L.; Gao, S.P.; Yang, H.; Little, J.M.; Dissanayake, T.U.; Li, K.; Yang, J. Metal Ion-Induced Assembly of MXene Aerogels via Biomimetic Microtextures for Electromagnetic Interference Shielding, Capacitive Deionization, and Microsupercapacitors. Adv. Energy Mater. 2021, 11, 2101494. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, W.; Lu, X.; Wang, C. Lightweight and Flexible MXene/carboxymethyl Cellulose Aerogel for Electromagnetic Shielding, Energy Harvest and Self-powered Sensing. Nano Energy 2022, 98, 107229. [Google Scholar] [CrossRef]
- Guo, Z.; Li, Y.; Jin, P.; Zhang, T.; Zhao, Y.; Ai, Y.; Xiu, H.; Zhang, Q.; Fu, Q. Poly (vinyl alcohol)/MXene Biomimetic Aerogels with Tunable Mechanical Properties and Electromagnetic Interference Shielding Performance Controlled by Core Structure. Polymer 2021, 230, 124101. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, C.; Siqueira, G.; Han, D.; Huch, A.; Abdolhosseinzadeh, S.; Heier, J.; Nüesch, F.; Zhang, C.; Nyström, G. Nanocellulose-MXene Biomimetic Aerogels with Orientation-Tunable Electromagnetic Interference Shielding Performance. Adv. Sci. 2020, 7, 2000979. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, Q.; Yin, G.; Wang, W.; Yu, D. Flexible, Ultralight, and Mechanically Robust Waterborne Polyurethane/Ti3C2Tx MXene/Nickel Ferrite Hybrid Aerogels for High-Performance Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2021, 13, 21831–21843. [Google Scholar] [CrossRef]
- Deng, Z.; Tang, P.; Wu, X.; Zhang, H.B.; Yu, Z.Z. Superelastic, Ultralight, and Conductive Ti3C2Tx MXene/Acidified Carbon Nanotube Anisotropic Aerogels for Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2021, 13, 20539–20547. [Google Scholar] [CrossRef]
- Han, M.; Yin, X.; Hantanasirisakul, K.; Li, X.; Iqbal, A.; Hatter, C.B.; Anasori, B.; Koo, C.M.; Torita, T.; Soda, Y.; et al. Anisotropic MXene Aerogels with a Mechanically Tunable Ratio of Electromagnetic Wave Reflection to Absorption. Adv. Opt. Mater. 2019, 7, 1900267. [Google Scholar] [CrossRef]
- Cai, Z.; Ma, Y.; Zhao, K.; Yun, M.; Wang, X.; Tong, Z.; Wang, M.; Suhr, J.; Xiao, L.; Jia, S.; et al. Ti3C2Tx MXene/Graphene Oxide/Co3O4 Nanorods Aerogels with Tunable and Broadband electromagnetic Wave Absorption. Chem. Eng. J. 2023, 462, 142042. [Google Scholar] [CrossRef]
- Xu, T.; Song, Q.; Liu, K.; Liu, H.; Pan, J.; Liu, W.; Dai, L.; Zhang, M.; Wang, Y.; Si, C.; et al. Nanocellulose-Assisted Construction of Multifunctional MXene-Based Aerogels with Engineering Biomimetic Texture for Pressure Sensor and Compressible Electrode. Nano-Micro Lett. 2023, 15, 98. [Google Scholar] [CrossRef]
- Li, L.; Cheng, Y.; Cao, H.; Liang, Z.; Liu, Z.; Yan, S.; Li, L.; Jia, S.; Wang, J.; Gao, Y. MXene/rGO/PS spheres multiple physical networks as high-performance pressure sensor. Nano Energy 2022, 95, 106986. [Google Scholar] [CrossRef]
- Chen, T.; Yang, G.; Li, Y.; Li, Z.; Ma, L.; Yang, S.; Wang, J. Temperature-adaptable Pressure Sensors Based on MXene-coated GO Hierarchical Aerogels with Superb Detection Capability. Carbon 2022, 200, 47–55. [Google Scholar] [CrossRef]
- Xu, W.; Wu, Q.; Gwon, J.; Choi, J.W. Ice-Crystal-Templated “Accordion-Like” Cellulose Nanofiber/MXene Composite Aerogels for Sensitive Wearable Pressure Sensors. ACS Sustain. Chem. Eng. 2023, 11, 3208–3218. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wu, X.; Liu, Z.; Zhang, H.B.; Yu, Z.Z. Highly sensitive, robust and anisotropic MXene aerogels for efficient broadband microwave absorption. Compos. Part B Eng. 2020, 200, 108263. [Google Scholar] [CrossRef]
- Zhang, W.; Pan, Z.; Ma, J.; Wei, L.; Chen, Z.; Wang, J. Degradable Cross-Linked Collagen Fiber/MXene Composite Aerogels as a High-Performing Sensitive Pressure Sensor. ACS Sustain. Chem. Eng. 2022, 10, 1408–1418. [Google Scholar] [CrossRef]
- Niu, F.; Qin, Z.; Min, L.; Zhao, B.; Lv, Y.; Fang, X.; Pan, K. Ultralight and Hyperelastic Nanofiber-Reinforced MXene-Graphene Aerogel for High-Performance Piezoresistive Sensor. Adv. Mater. Technol. 2021, 6, 2100394. [Google Scholar] [CrossRef]
- Wang, N.-N.; Wang, H.; Wang, Y.-Y.; Wei, Y.-H.; Si, J.-Y.; Yuen, A.C.Y.; Xie, J.-S.; Yu, B.; Zhu, S.-E.; Lu, H.-D. Robust, Lightweight, Hydrophobic, and Fire-retarded Polyimide/MXene Aerogels for Effective oil/water Separation. ACS Appl. Mater. Interfaces 2019, 11, 40512–40523. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Liu, Y.; Xu, Y.; Chang, Z.; Wang, D.; Li, Q. Expanded Polytetrafluoroethylene/Mxene Nanosheet Composites with Hydrophilicity and Lipophilicity for Purification of Oil Spills and Wastewater. ACS Appl. Nano Mater. 2022, 5, 2483–2491. [Google Scholar] [CrossRef]
- Qi, M.-Y.; Wang, P.-L.; Huang, L.-Z.; Yuan, Q.; Mai, T.; Ma, M.-G. Cellulose nanofiber/MXene/luffa aerogel for all-weather and high-efficiency cleanup of crude oil spills. Int. J. Biol. Macromol. 2023, 242, 124895. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Nan, J.; Liu, B.; Wu, F. Novel three-dimensional Ti3C2-Tx-MXene embedded zirconium alginate aerogel adsorbent for efficient phosphate removal in water. Chemosphere 2023, 319, 138016. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Zhang, G.; Shao, D.; Ding, S.; Li, L.; Li, H.; Zhou, C.; Luo, B.; Lu, L. Preparation of chitin/MXene/poly(L-arginine) Composite Aerogel Spheres for Specific Adsorption of Bilirubin. Int. J. Biol. Macromol. 2023, 243, 125140. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Wei, Z.; Huang, Y.; Fu, Y. Wood-inspired superelastic MXene Aerogels with Superior Photothermal Conversion and Durable Superhydrophobicity for Clean-up of Super-viscous Crude Oil. Chem. Eng. J. 2021, 421, 127772. [Google Scholar] [CrossRef]
- Wang, G.; Xu, Y.; Zhang, R.; Gai, S.; Zhao, Y.; Yang, F.; Cheng, K. Fire-resistant MXene Composite Aerogels for Effective Oil/Water Separation. J. Environ. Chem. Eng. 2023, 11, 109127. [Google Scholar] [CrossRef]
- Zhang, G.; Shao, D.; Yu, H.; Wan, Y.; Jiao, Y.; Li, L.; Tian, J.; Zhou, C.; Lu, L. MXene Nanosheet-Enhanced Chitin Aerogel Spheres for Bilirubin Adsorption. ACS Appl. Nano Mater. 2022, 5, 17293–17303. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, H.; Xu, J.; Du, X.; Cheng, X.; Du, Z.; Wang, H. Fabrication of MXene/PEI Functionalized Sodium Alginate Aerogel and its Excellent Adsorption Behavior for Cr(VI) and Congo Red from Aqueous Solution. J. Hazard. Mater. 2021, 416, 125777. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Xi, H.; Li, Q.; Shen, M.; Ying, G.; Zhang, J. Polydopamine Functionalized Cellulose-MXene Composite Aerogel with Superior Adsorption of Methylene blue. Cellulose 2021, 28, 4281–4293. [Google Scholar] [CrossRef]
- Shahzad, A.; Nawaz, M.; Moztahida, M.; Jang, J.; Tahir, K.; Kim, J.; Lim, Y.; Vassiliadis, V.S.; Woo, S.H.; Lee, D.S. Ti3C2Tx MXene Core-shell Spheres for Ultrahigh Removal of Mercuric ions. Chem. Eng. J. 2019, 368, 400–408. [Google Scholar] [CrossRef]
- Cao, S.; Wu, X.; Zhu, Y.; Gupta, P.; Martinez, A.; Zhang, Y.; Ghim, D.; Wang, Y.; Liu, L.; Jun, Y.-S. MXene Aerogel for Efficient Photothermally Driven Membrane Distillation with Dual-mode Antimicrobial Capability. J. Mater. Chem. A 2021, 9, 22585–22596. [Google Scholar] [CrossRef]
- Wu, F.; Qiang, S.; Zhu, X.-D.; Jiao, W.; Liu, L.; Yu, J.; Liu, Y.-T.; Ding, B. Fibrous MXene Aerogels with Tunable Pore Structures for High-Efficiency Desalination of Contaminated Seawater. Nano-Micro. Lett. 2023, 15, 71. [Google Scholar] [CrossRef] [PubMed]

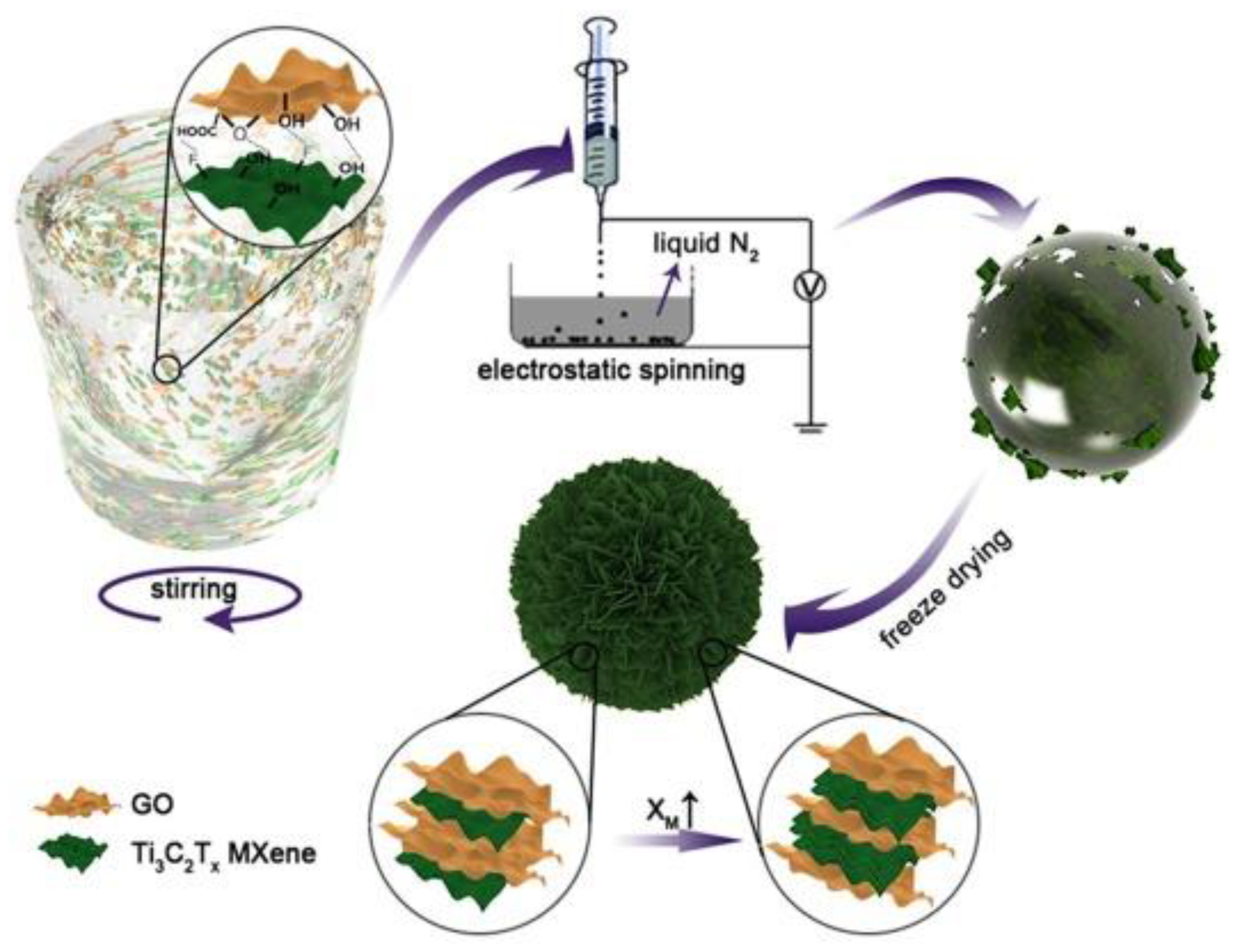

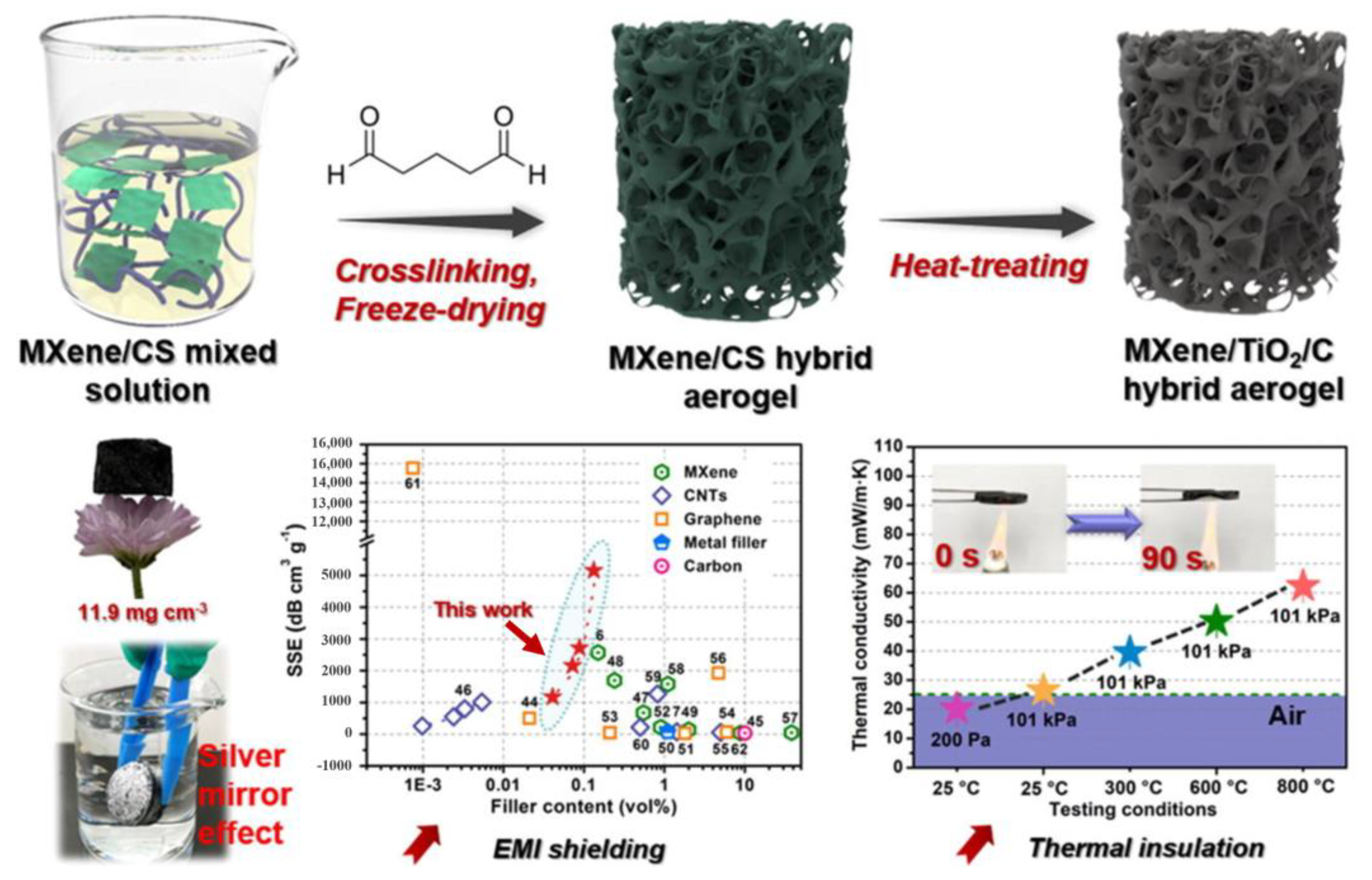
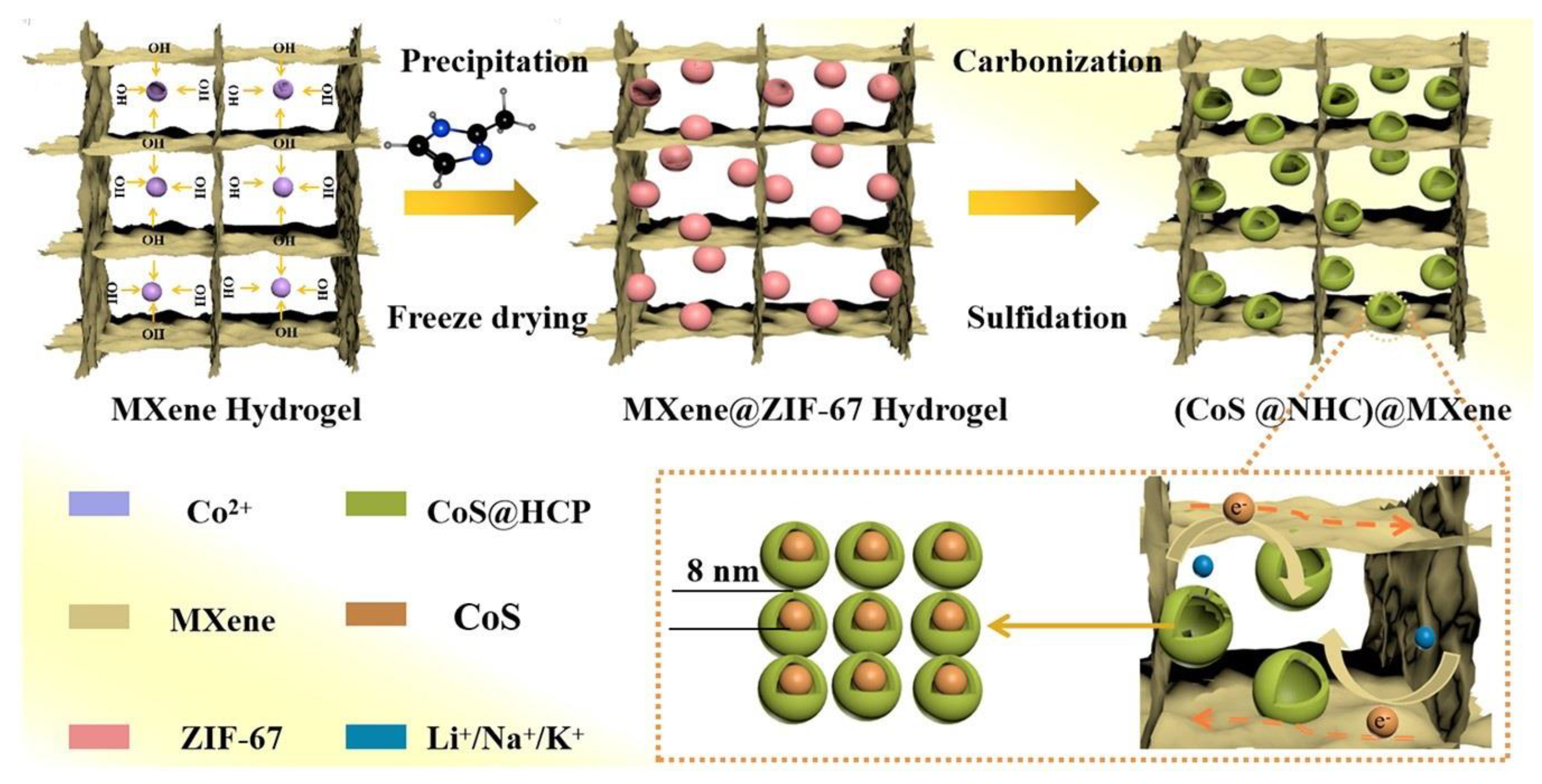
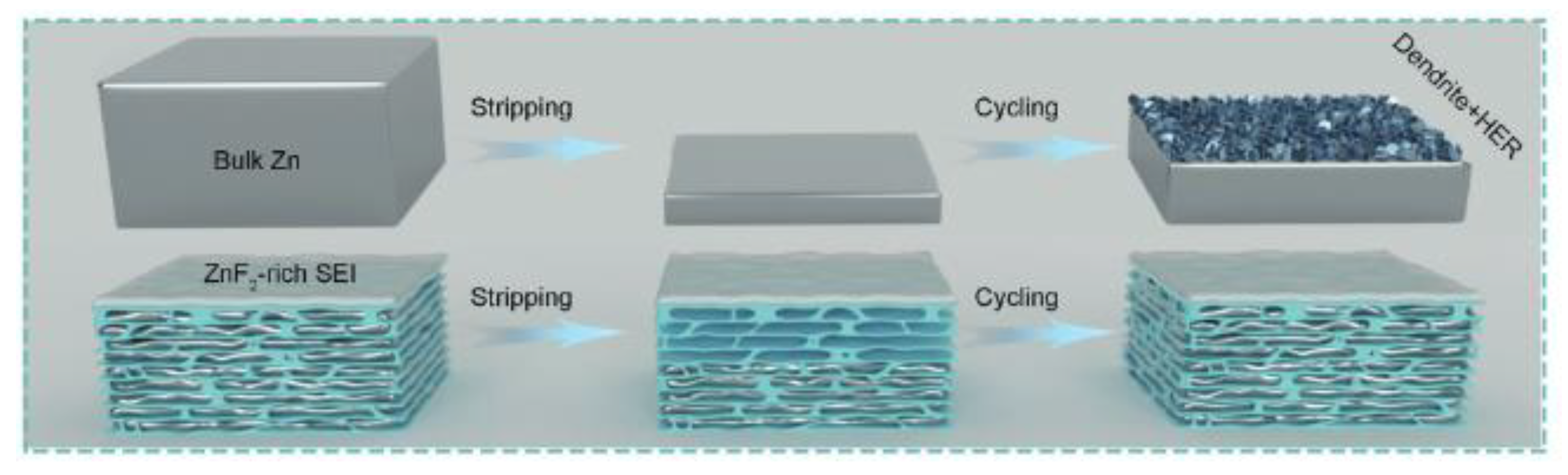
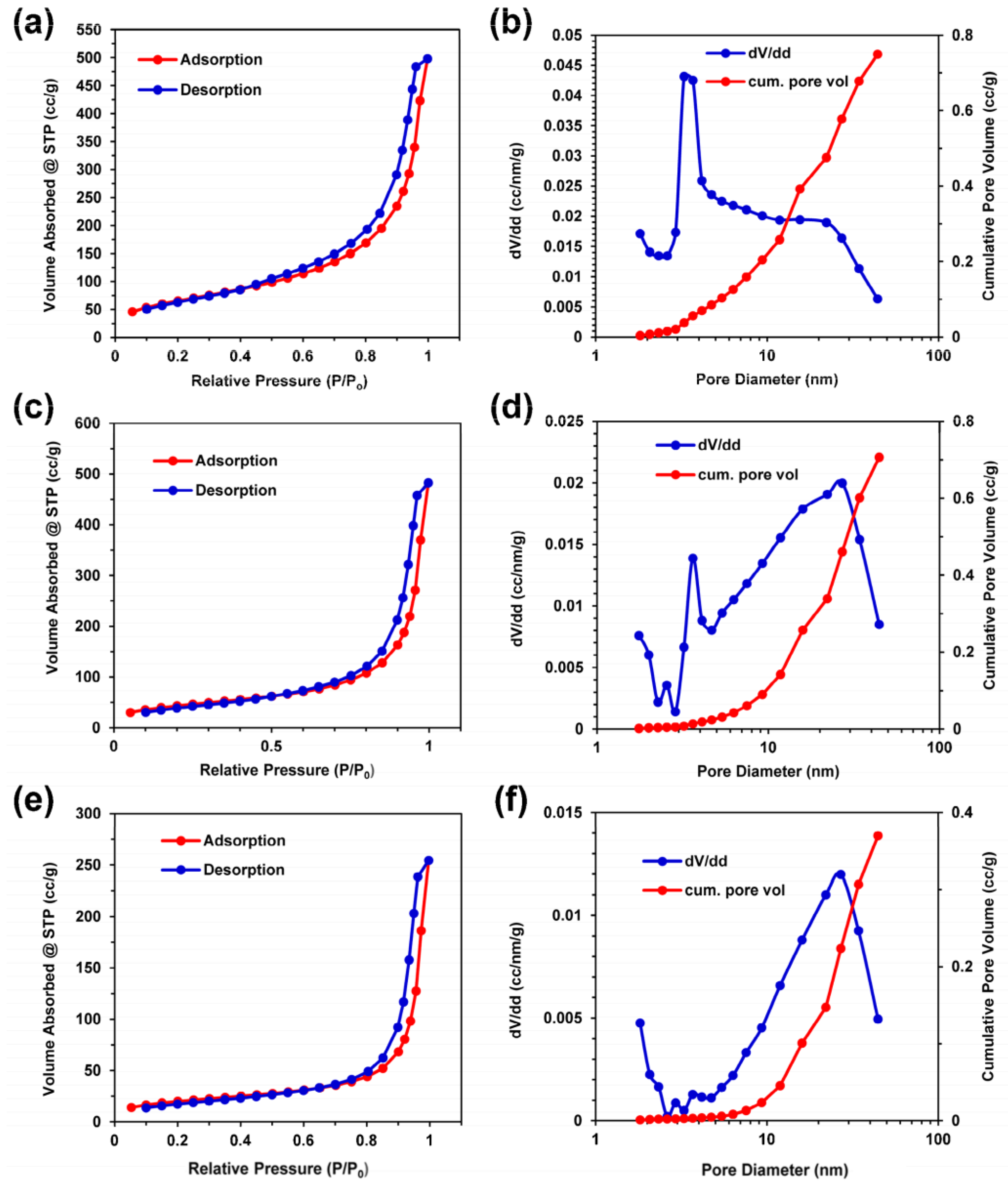
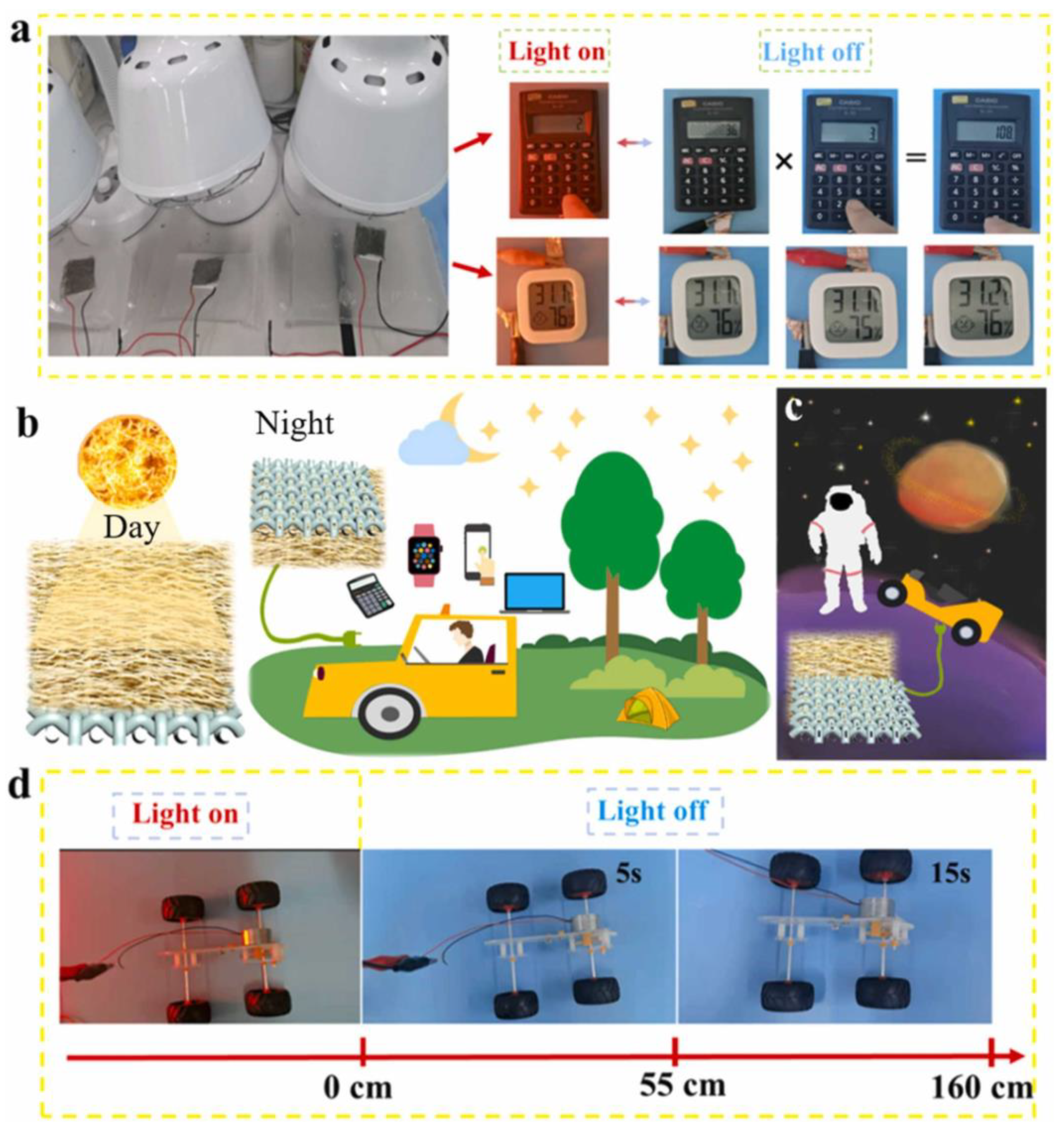
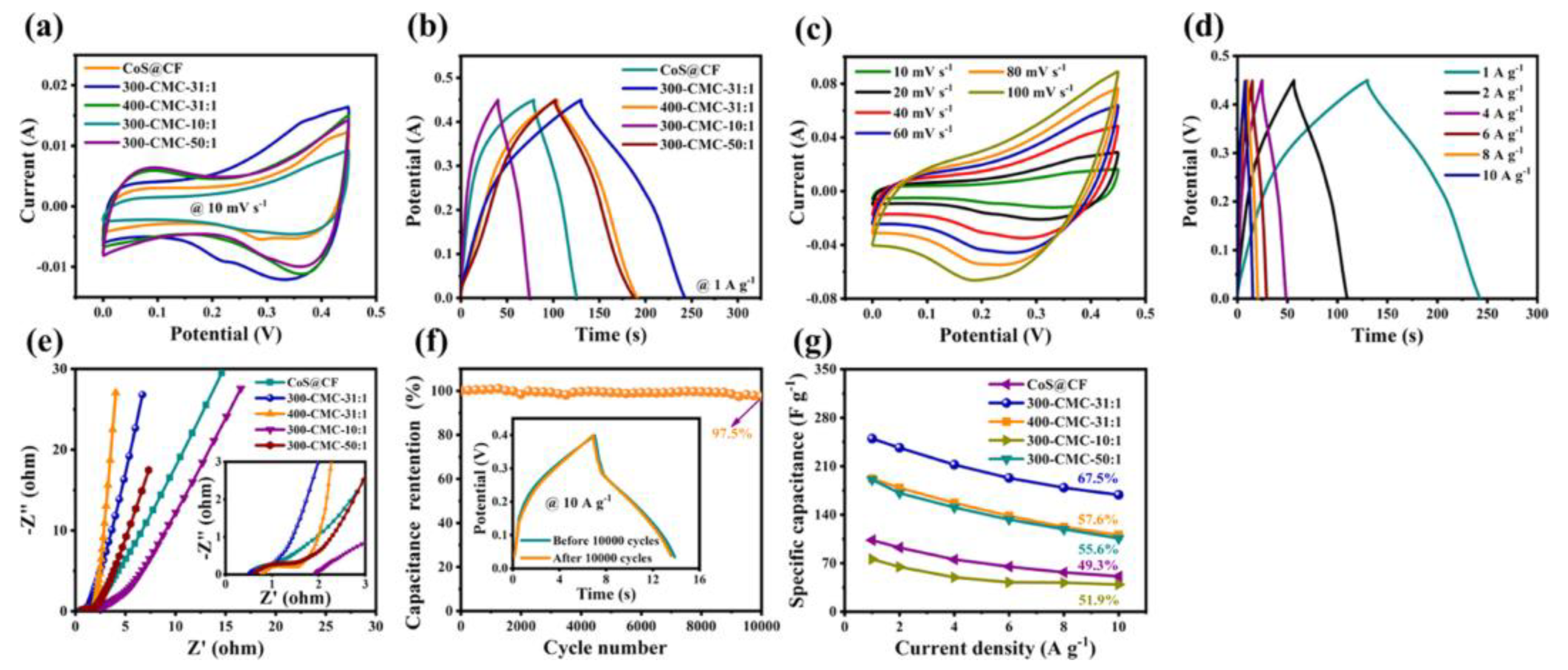
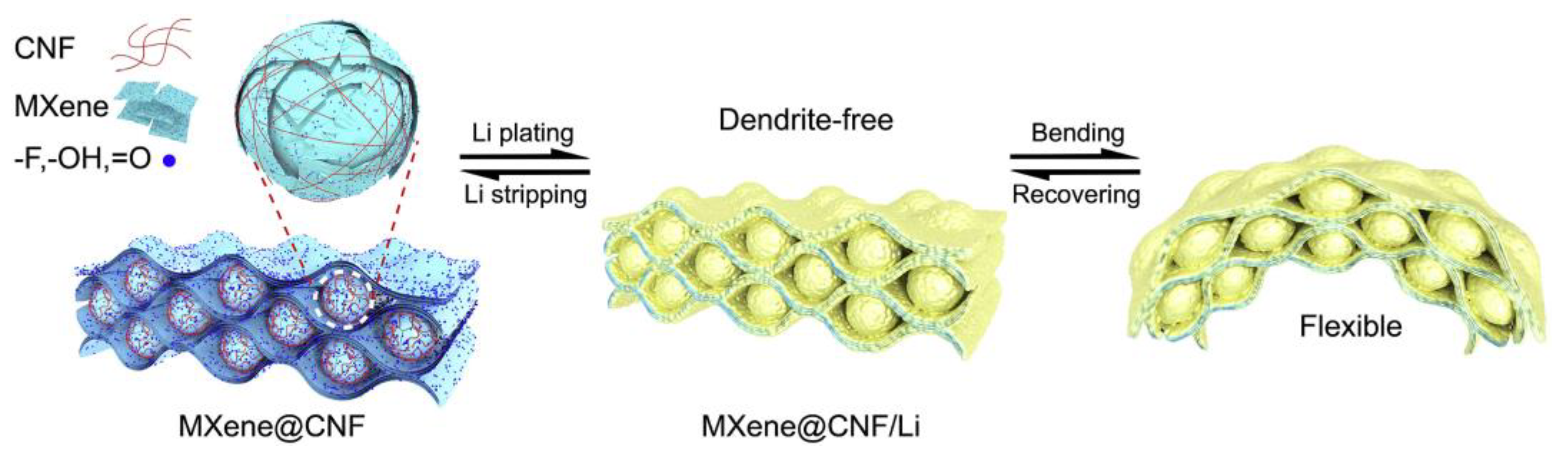
| Name and Ref. | Capacitance | Energy Density | Cycle | Electrolyte |
|---|---|---|---|---|
| A-MHA [64] | 760 F g−1 | - | 10,000 | 1 M H2SO4 |
| Fe2O3/MXene [65] | 691 mF cm−2 | 119.04 μW cm−2 | 10,000 | 3 M H2SO4 |
| Ti3C2Tx/rGO/Fe3O4 [66] | 1250.5 mF cm−2 | 802 μW cm−2 | 30,000 | 1 M KOH |
| MXene/rGO [67] | 233 F g−1 | 10,000 | 1 M H2SO4 | |
| Co3O4-MXene/rGO [68] | 345 F g−1 | 159.94 μW cm−2 | 10,000 | 1 M H2SO4 |
| CoS@MXene/CF [25] | 250 F g−1 | 10.66 W h kg−1 | 10,000 | 1 M KOH |
| NiCo2Se4@MXene/GO [54] | 352.4 mAh g−1 | - | 5000 | 1 M H2SO4 |
| Nitrogen-enriched Ti3C2Tx [69] | 410.7 mF cm−2 | - | 5000 | 1 M H2SO4 |
| PPy@PVA/BC/MXene [70] | 3948 mF cm−2 | 951 μW cm−2 | 10,000 | 1 M H2SO4 |
| Name and Ref. | Density (mg/cm3) | Thickness (mm) | RLmin (dB) | Effective Bandwidth [RL below −10 dB] (Ghz) |
|---|---|---|---|---|
| Mg2+-MXene [74] | 8 | ≈5.0 | 59.9 | 8~12 |
| Ni/MXene/rGO [53] | 6.45 | 15 | 75.2 | 2~18 |
| MXene/CNTs/Aramid [43] | 42.8 | 2 | 69 | 8~13 |
| MXene/CMC [75] | 28.2 | 2.5 | 80.36 | 8~24 |
| PVA/MXene [76] | 33 | - | 40.6 | 8~13 |
| MXene/CNF [77] | 8 | 0.2 | 76 | 8~12 |
| WPU/MXene/NiFe2O4 [78] | 7.3 | 20.2 | 64.7 | 8~13 |
| MXene/aCNTs [79] | 9.1 | 2.0 | 90 | 8~13 |
| MXene [80] | 11.0 | 1.0 | 70.5 | 8~12.5 |
| MXene/GO/Co3O4 [81] | 9 | 2~6 | 65.3 | 2~18 |
| Name and Ref. | Density (mg/cm3) | Conductivity | Linear Sensitivity (kPa−1) | Cycle |
|---|---|---|---|---|
| CNF/CNTs/MXene [82] | 7.48 | 2400 S m−1 | 817.3 | 2000% for 10,000 cycles |
| MXene/rGO/PS [83] | - | - | 224 | 50 kPa for 50,000 cycles |
| GO/PVA/MXene [84] | 10.6 | 2.84 S m−1 | 1.744 | 50% for 5000 cycles |
| MXene/CNF [85] | 50 | 180 Ω | 3.13 | 33% for 1000 cycles |
| MXene/PAA [86] | 12 | 2.4 S m−1 | 1.5 | 50% for 1000 cycles |
| CCF/MXene [87] | - | - | 61.99 | 50% for 1000 cycles |
| PPy@PVA/BC/MXene [70] | 23 | - | 313.2 | 200–3000 Pa for 3000 cycles |
| MXene/rGO [88] | 10.9 | - | 331 | 125 Pa for 17,000 cycles |
| Name and Ref. | Density (mg/cm3) | Adsorbed Objects | Adsorption Capacity | Separation Efficiency |
|---|---|---|---|---|
| MX-ZrSA [92] | - | Phosphate | 492.55 mg g−1 | - |
| Ch/MXene/PLA [93] | - | Serum albumin | 382.21 mg g−1 | - |
| MXene/PU [94] | - | Crude oil | 24.5 g/g | 76 % |
| PDMS-Fe-MXene/A-HA [95] | 22.9 | Pump oil | 78.5 g/g | 23,478 L h−1 m−2 |
| PI/MXene [89] | 23 | Pump oil | 57.78 g/g | 95.4% |
| Ch/MXene [96] | - | Bilirubin | 521.95 mg/g | - |
| APD MXene-based [47] | 18 | Crude oil | >10 g/g | - |
| MXene/PEI/SA [97] | 16.3 | Cr (IV) | 550 mg/g | - |
| PDA/CNF/MXene [98] | 35~39 | Methylene blue | 168.93 mg/g | |
| HG@MXene-SA [99] | - | Hg (II) | 932.84 mg/g | ≈100% |
| MPM [29] | 46 | Chloroform | 35.41 g/g | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Fu, X.; Liu, R.; Song, Y.; Yin, X. Fabrication, Performance, and Potential Applications of MXene Composite Aerogels. Nanomaterials 2023, 13, 2048. https://doi.org/10.3390/nano13142048
Chen Z, Fu X, Liu R, Song Y, Yin X. Fabrication, Performance, and Potential Applications of MXene Composite Aerogels. Nanomaterials. 2023; 13(14):2048. https://doi.org/10.3390/nano13142048
Chicago/Turabian StyleChen, Zhicheng, Xinming Fu, Rui Liu, Yiheng Song, and Xianze Yin. 2023. "Fabrication, Performance, and Potential Applications of MXene Composite Aerogels" Nanomaterials 13, no. 14: 2048. https://doi.org/10.3390/nano13142048
APA StyleChen, Z., Fu, X., Liu, R., Song, Y., & Yin, X. (2023). Fabrication, Performance, and Potential Applications of MXene Composite Aerogels. Nanomaterials, 13(14), 2048. https://doi.org/10.3390/nano13142048