Surface Oxygen Species in Metal Oxide Photoanodes for Solar Energy Conversion
Abstract
1. Introduction
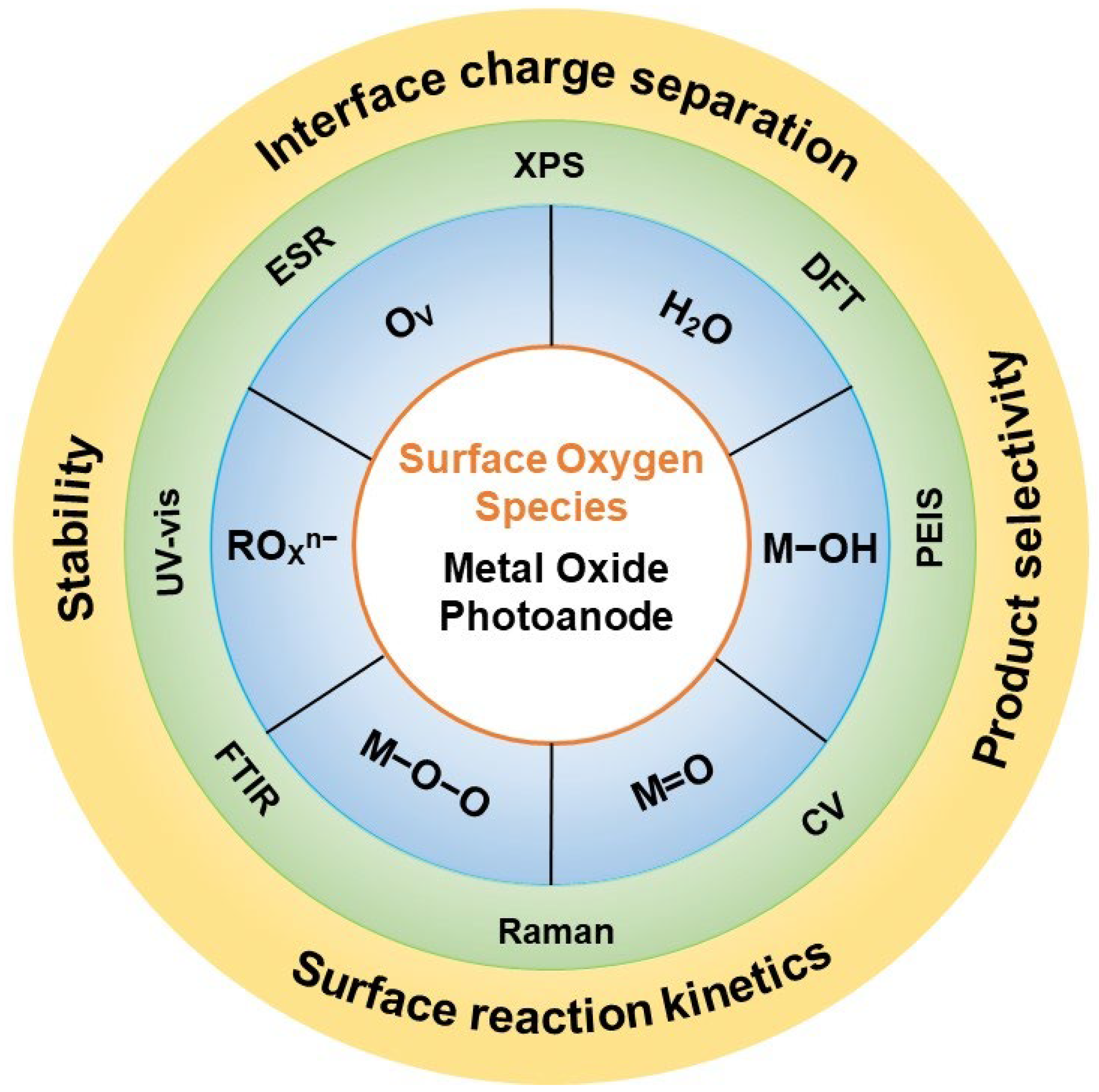
2. Surface Oxygen Species
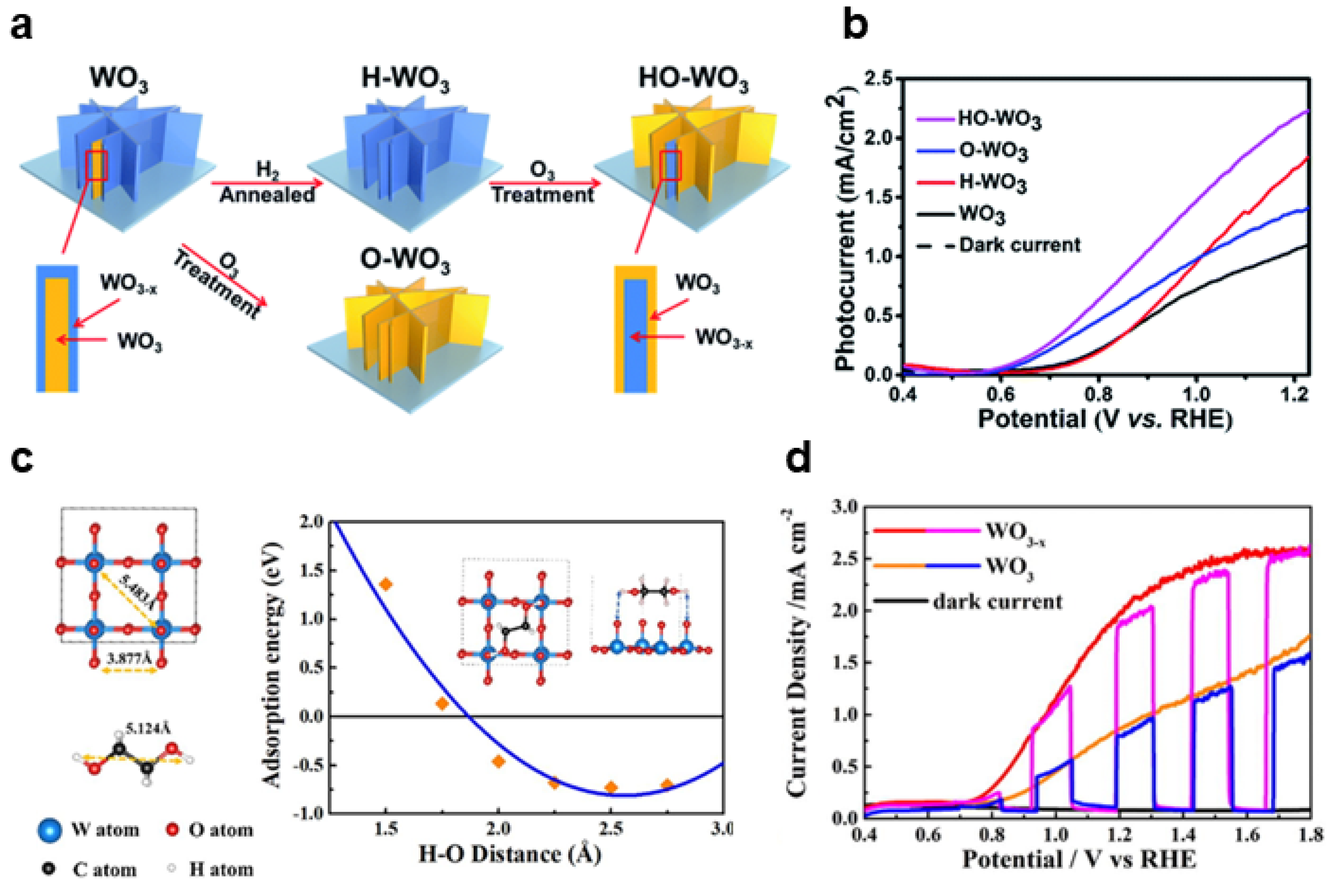
2.1. Surface Oxygen Vacancies
2.1.1. Modulation Strategies
Heat Treatment
Chemical Reduction
Photo/Electrochemical Treatment
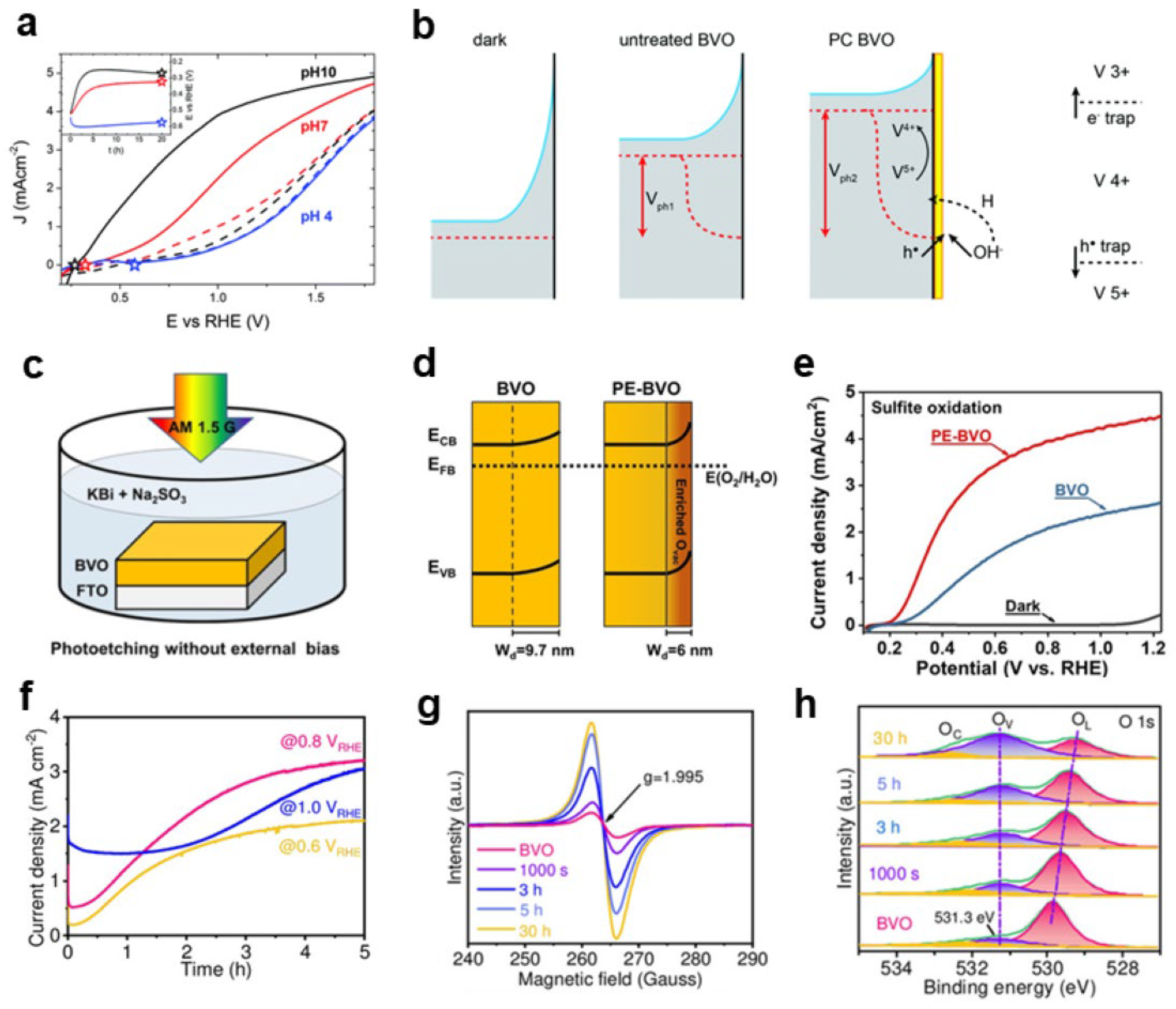
2.1.2. The Role of Surface Oxygen Vacancies
Carrier Separation
Surface Oxidation Reaction
2.2. Surface Oxygenated Species
2.2.1. Water Molecules (H2O)
2.2.2. Hydroxyl Groups (M–OH)
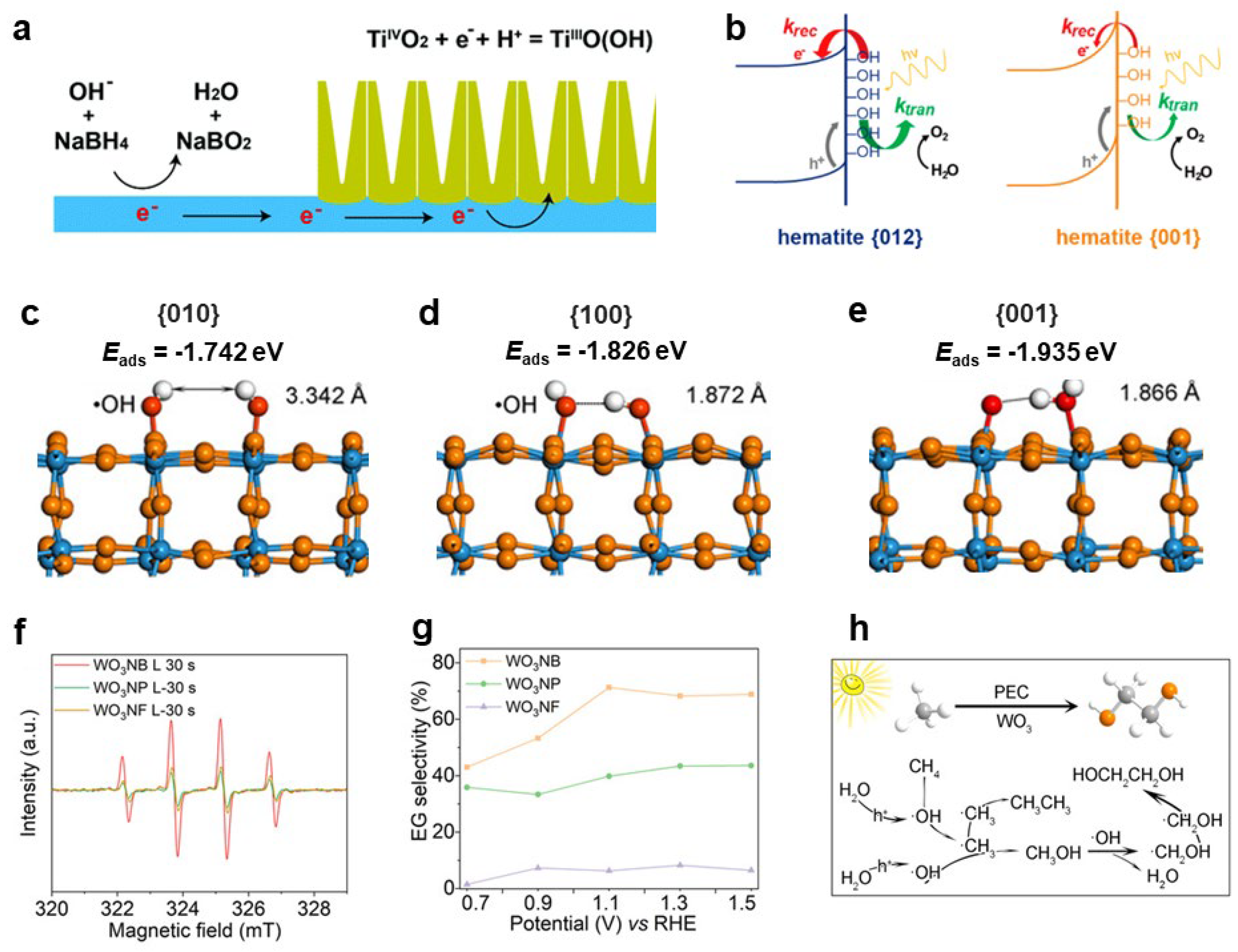
2.2.3. Adsorbed Oxygen Species (M=O)
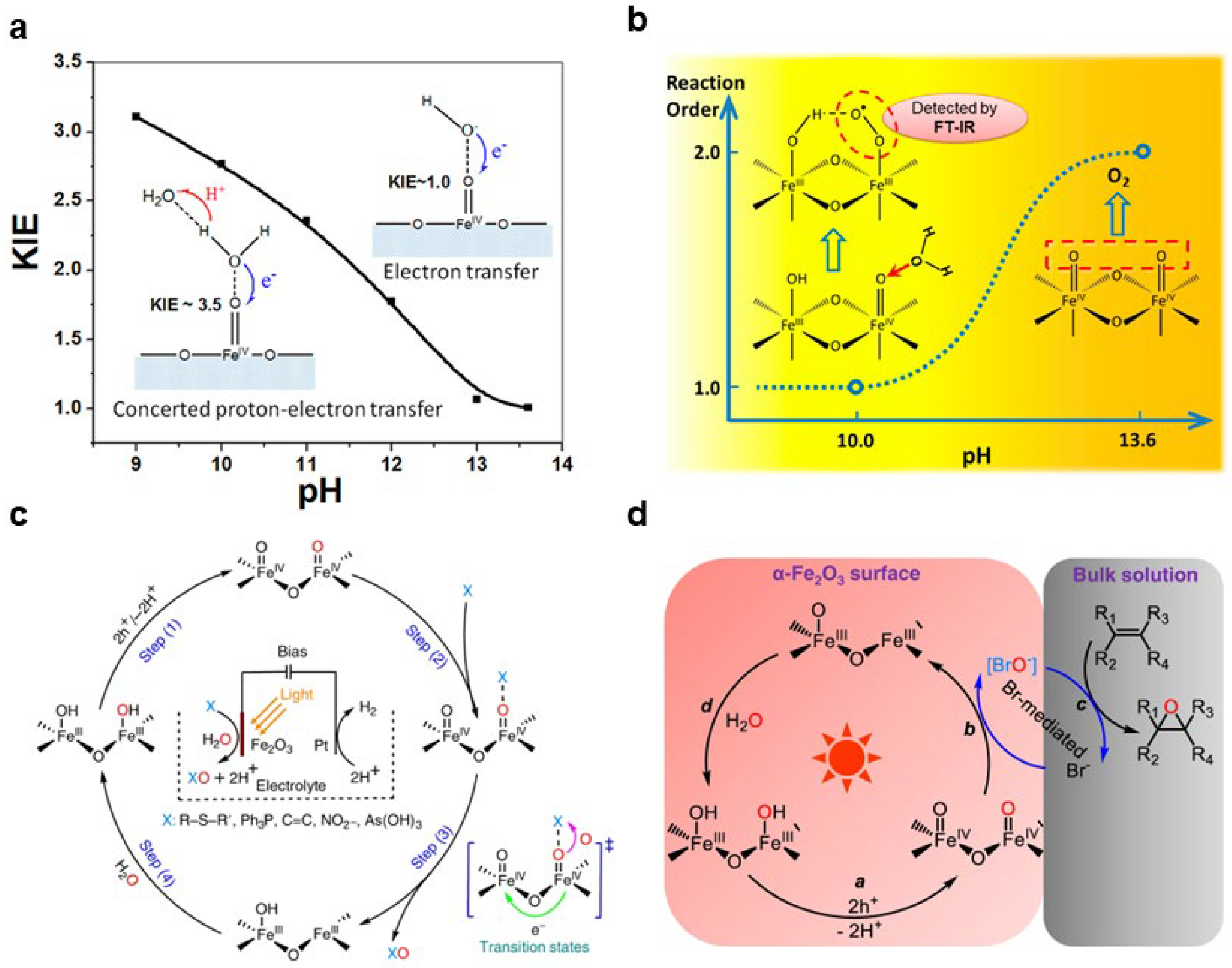
2.2.4. Peroxygen Species (M–O–O)
2.2.5. Oxyanion Species (ROXn−)
3. Characterization Techniques
3.1. Spectroscopy Techniques
3.1.1. X-ray Photoelectron Spectroscopy (XPS)
3.1.2. Electron Spin Resonance (ESR)
3.1.3. Ultraviolet–Visible Spectroscopy (UV–Vis)
3.1.4. Fourier Transform Infrared Spectrometer (FTIR)
3.1.5. Raman Spectroscopy
3.2. Electrochemical Techniques
3.2.1. Cyclic Voltammetry (CV)
3.2.2. Photoelectrochemical Impedance Spectroscopy (PEIS)
3.3. Other Methods
3.3.1. Isotope-Labeling Experiments
3.3.2. Density Functional Theory (DFT)
4. Summary and Outlook
- (1)
- Regulation and evolution: The regulation of surface oxygen vacancies and oxygen-containing species often relies on post-processing strategies involving specific atmospheres or solvents, coupled with external stimuli such as heat, electricity, or light. However, the underlying mechanisms governing surface changes during the regulation process remain unclear, and achieving precise control over the spatial distribution and concentration of these surface species remains challenging. Therefore, a deeper understanding of the regulation principles is needed, along with the development of simpler methods to achieve specific control, such as selectively introducing oxygen vacancies and oxygen-containing species on specific exposed crystal faces. Additionally, the stability and differences of surface oxygen vacancies and oxygen species generated by different regulation strategies are rarely investigated. Attention should also be directed toward their evolution during the reaction process, as this may impact their long-term activity. Protection strategies for preserving surface oxygen vacancies and oxygen species should be explored, along with efforts to achieve interface dynamic equilibrium or faster recovery strategies. The dynamic study of surface oxygen vacancies and oxygen species will provide insights into their specific roles in photoelectrocatalytic processes. The evolution study of surface oxygen species will also clarify their specific roles in the photoelectrocatalytic process.
- (2)
- Carrier separation: Surface oxygen species can be either favorable or unfavorable for carrier separation. They can act as charge recombination centers, accelerating charge recombination, or serve as electron donors, leading to Fermi level pinning and inhibiting hole migration, among other undesirable effects. Conversely, they can function as electron or hole capture acceptors, enhancing carrier lifetime and facilitating hole migration. Exploring the fundamental aspects of charge transport and providing a more comprehensive explanation for how surface structure influences electron and vacancy behavior are necessary. For instance, the role of polarons in charge transport and how the introduction of surface oxygen species affects them should be investigated. Furthermore, the influence of surface oxygen species on other material properties should be considered to avoid drawing erroneous conclusions regarding the relationship between catalyst surface structure and charge separation.
- (3)
- Control of product selectivity: The selectivity of oxidation reaction products is affected by various factors, including the adsorption configuration or strength of the substrate on the surface, as well as the energy barriers of different steps in the oxidation process such as dehydrogenation or hydration. Therefore, as the understanding of substrate oxidation reaction mechanisms advances, the role of surface oxygen species in this context should be further explored. Additionally, a clearer understanding of how oxygen species on metal oxide surfaces affect reaction intermediates and products is necessary. Attention should also be given to the influence of different oxygen-containing species on competing oxidation reactions and whether different reaction pathways share common oxygen-containing intermediates. When studying the effect of surface oxygen species on product selectivity, the effect of different metal atoms should be further explored, considering the synergy between single atoms or clusters and surface oxygen species.
- (4)
- For research methods and characterization strategies: To investigate the specific role of surface oxygen vacancies and oxygen-containing species in photocatalytic processes, the development of more comprehensive research methods is essential. These methods should aim to explore their influence on the behavior of molecules and charges. For example, experimental designs can be employed to examine the adsorption performance of surface oxygen species on different substrates or to investigate the adsorption strength of specific functional groups on the catalyst surface. As surface characterization technology advances, finer analyses of surface compositions may become possible. However, the detection of intermediate species for different metal oxides under varying electrolyte or substrate conditions is still lacking. Therefore, it is crucial to employ in situ characterization techniques to detect oxidation reaction intermediates under relevant conditions.
Funding
Data Availability Statement
Conflicts of Interest
References
- Lv, J.; Xie, J.; Mohamed, A.G.A.; Zhang, X.; Feng, Y.; Jiao, L.; Zhou, E.; Yuan, D.; Wang, Y. Solar utilization beyond photosynthesis. Nat. Rev. Chem. 2023, 7, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Romano, V.; D’Angelo, G.; Perathoner, S.; Centi, G. Current density in solar fuel technologies. Energy Environ. Sci. 2021, 14, 5760–5787. [Google Scholar] [CrossRef]
- Ta, X.M.C.; Daiyan, R.; Thi Kim Anh, N.; Amal, R.; Thanh, T.-P.; Tricoli, A. Alternatives to water photooxidation for photoelectrochemical solar energy conversion and green H2 production. Adv. Energy Mater. 2022, 12, 2201358. [Google Scholar] [CrossRef]
- Putri, L.K.; Ng, B.-J.; Ong, W.-J.; Chai, S.-P.; Mohamed, A.R. Toward excellence in photocathode engineering for photoelectrochemical CO2 reduction: Design rationales and current progress. Adv. Energy Mater. 2022, 12, 2201093. [Google Scholar] [CrossRef]
- Andrei, V.; Ucoski, G.M.; Pornrungroj, C.; Uswachoke, C.; Wang, Q.; Achilleos, D.S.; Kasap, H.; Sokol, K.P.; Jagt, R.A.; Lu, H.; et al. Floating perovskite-BiVO4 devices for scalable solar fuel production. Nature 2022, 608, 518–522. [Google Scholar] [CrossRef]
- Pan, J.-B.; Shen, S.; Chen, L.; Au, C.-T.; Yin, S.-F. Core-shell photoanodes for photoelectrochemical water oxidation. Adv. Funct. Mater. 2021, 31, 2104269. [Google Scholar] [CrossRef]
- Shi, Q.; Duan, H. Recent progress in photoelectrocatalysis beyond water oxidation. Chem. Catalysis. 2022, 2, 3471–3496. [Google Scholar] [CrossRef]
- Corby, S.; Rao, R.R.; Steier, L.; Durrant, J.R. The kinetics of metal oxide photoanodes from charge generation to catalysis. Nat. Rev. Mater. 2021, 6, 1136–1155. [Google Scholar] [CrossRef]
- Xiao, M.; Luo, B.; Wang, Z.; Wang, S.; Wang, L. Recent advances of metal-oxide photoanodes: Engineering of charge separation and transportation toward efficient solar water splitting. Solar Rrl. 2020, 4, 1900509. [Google Scholar] [CrossRef]
- Song, R.; Chi, H.; Ma, Q.; Li, D.; Wang, X.; Gao, W.; Wang, H.; Wang, X.; Li, Z.; Li, C. Highly efficient degradation of persistent pollutants with 3D nanocone TiO2-based photoelectrocatalysis. J. Am. Chem. Soc. 2021, 143, 13664–13674. [Google Scholar] [CrossRef]
- Ouyang, J.; Liu, X.; Wang, B.-H.; Pan, J.-B.; Shen, S.; Chen, L.; Au, C.-T.; Yin, S.-F. WO photoanode with predominant exposure of {202} facets for enhanced selective oxidation of glycerol to glyceraldehyde. ACS Appl. Mater. Interfaces 2022, 14, 23536–23545. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Zhou, H.; Chen, Z.; Dou, Z.; Wang, M. Photoelectrocatalytic reforming of polyol-based biomass into CO and H2 over nitrogen-doped WO3 with built-in electric fields. Angew. Chem. Int. Ed. 2022, 61, e202210745. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, L.; Du, A.; Irani, R.; van de Krol, R.; Abdi, F.F.; Ng, Y.H. Low-bias photoelectrochemical water splitting via mediating trap states and small polaron hopping. Nat. Commun. 2022, 13, 6231. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, Y.; Fan, X.; Li, Y.; Zhou, D.; Cai, B.; Wang, L.; Fan, K.; Zhang, K. Boosting charge transport in BiVO4 photoanode for solar water oxidation. Adv. Mater. 2022, 34, 2108178. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, X.; Zhang, Y.; Zhai, P.; Li, Z.; Jin, D.; Cao, J.; Wang, C.; Zhang, B.; Gao, J.; et al. Engineering MoOx/Mxene hole transfer layers for unexpected boosting photoelectrochemical water oxidation. Angew. Chem. Int. Ed. 2022, 61, e202200946. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Liu, B.; Guo, Z.; Ostrikov, K.; Wang, L.; Huang, W. Vacancy defect engineering of BiVO4 photoanodes for photoelectrochemical water splitting. Nanoscale 2021, 13, 17989–18009. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L. Role of oxygen vacancy in metal oxide based photoelectrochemical water splitting. Ecomat 2021, 3, e12075. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Balogun, M.S.; Tong, Y.; Huang, Y. Oxygen vacancy-based metal oxides photoanodes in photoelectrochemical water splitting. Mater. Today Sustain. 2022, 18, 100118. [Google Scholar] [CrossRef]
- Li, Z.; Luo, L.; Li, M.; Chen, W.; Liu, Y.; Yang, J.; Xu, S.-M.; Zhou, H.; Ma, L.; Xu, M.; et al. Photoelectrocatalytic C-H halogenation over an oxygen vacancy-rich TiO2 photoanode. Nat. Commun. 2021, 12, 6698. [Google Scholar] [CrossRef]
- Wang, G.; Ling, Y.; Wang, H.; Yang, X.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated WO3 nanoflakes show enhanced photostability. Energy Environ. Sci. 2012, 5, 6180–6187. [Google Scholar] [CrossRef]
- Zhang, J.; Chang, X.; Li, C.; Li, A.; Liu, S.; Wang, T.; Gong, J. WO3 photoanodes with controllable bulk and surface oxygen vacancies for photoelectrochemical water oxidation. J. Mater. Chem. A 2018, 6, 3350–3354. [Google Scholar] [CrossRef]
- Shao, C.Y.; Malik, A.S.; Han, J.F.; Li, D.; Dupuis, M.; Zong, X.; Li, C. Oxygen vacancy engineering with flame heating approach towards enhanced photoelectrochemical water oxidation on wo3 photoanode. Nano Energy 2020, 77, 105190. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-treated TiO2 nanowire arrays for photoelectrochemical water splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
- Rioult, M.; Stanescu, D.; Fonda, E.; Barbier, A.; Magnan, H. Oxygen vacancies engineering of iron oxides films for solar water splitting. J. Phys. Chem. C 2016, 120, 7482–7490. [Google Scholar] [CrossRef]
- Li, H.-M.; Wang, Z.-Y.; Jing, H.-J.; Yi, S.-S.; Zhang, S.-X.; Yue, X.-Z.; Zhang, Z.-T.; Lu, H.-X.; Chen, D.-L. Synergetic integration of passivation layer and oxygen vacancy on hematite nanoarrays for boosted photoelectrochemical water oxidation. Appl. Catal. B-Environ. 2021, 284, 119760. [Google Scholar] [CrossRef]
- Wang, G.; Ling, Y.; Lu, X.; Qian, F.; Tong, Y.; Zhang, J.Z.; Lordi, V.; Leao, C.R.; Li, Y. Computational and photoelectrochemical study of hydrogenated bismuth vanadate. J. Phys. Chem. C 2013, 117, 10957–10964. [Google Scholar] [CrossRef]
- Pan, J.-B.; Wang, B.-H.; Wang, J.-B.; Ding, H.-Z.; Zhou, W.; Liu, X.; Zhang, J.-R.; Shen, S.; Guo, J.-K.; Chen, L.; et al. Activity and stability boosting of an oxygen-vacancy-rich BiVO4 photoanode by NiFe-MOFs thin layer for water oxidation. Angew. Chem. Int. Ed. 2021, 60, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Zhou, T.; Chen, S.; Zhu, H.; Bai, J.; Li, J.; Zhou, B. Efficient WO3-x nanoplates photoanode based on bidentate hydrogen bonds and thermal reduction of ethylene glycol. Chem. Eng. J. 2021, 404, 127089. [Google Scholar] [CrossRef]
- Zhang, L.J.; Xue, X.X.; Guo, T.; Bi, L.L.; Hu, T.; Tan, L.Q.; Zhang, X.J.; Jiang, J.L.; Hong, K.; Zhang, Q.H. Creation of oxygen vacancies to activate Fe2O3 photoanode by simple solvothermal method for highly efficient photoelectrochemical water oxidation. Int. J. Hydrog. Energy 2021, 46, 12897–12905. [Google Scholar] [CrossRef]
- Ma, M.; Zhang, K.; Li, P.; Jung, M.S.; Jeong, M.J.; Park, J.H. Dual oxygen and tungsten vacancies on a WO3 photoanode for enhanced water oxidation. Angew. Chem. Int. Ed. 2016, 55, 11819–11823. [Google Scholar] [CrossRef]
- Li, J.; Guo, L.; Lei, N.; Song, Q.; Liang, Z. Metallic bi nanocrystal-modified defective BiVO4 photoanodes with exposed (040) facets for photoelectrochemical water splitting. Chemelectrochem 2017, 4, 2852–2861. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, L.; Zhang, Z.; Cheng, J.; Chen, R.; Liu, Y.; Luo, J. Elucidating the role of hypophosphite treatment in enhancing the performance of BiVO4 photoanode for photoelectrochemical water oxidation. ACS Appl. Mater. Interfaces 2022, 14, 26642–26652. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Chen, P.; Yun, J.H.; Hu, Y.X.; Wang, L.Z. An electrochemically treated BiVO4 photoanode for efficient photoelectrochemical water splitting. Angew. Chem. Int. Ed. 2017, 56, 8500–8504. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tao, Y.; Zhang, C.; Zhu, Q.; Zhang, D.; Li, G. Activation of chloride by oxygen vacancies-enriched TiO2 photoanode for efficient photoelectrochemical treatment of persistent organic pollutants and simultaneous H2 generation. J. Hazard. Mater. 2023, 443, 130363. [Google Scholar] [CrossRef] [PubMed]
- Trzesniewski, B.J.; Smith, W.A. Photocharged BiVO4 photoanodes for improved solar water splitting. J. Mater. Chem. A 2016, 4, 2919–2926. [Google Scholar] [CrossRef]
- Trzesniewski, B.J.; Digdaya, I.A.; Nagaki, T.; Ravishankar, S.; Herraiz-Cardona, I.; Vermaas, D.A.; Longo, A.; Gimenez, S.; Smith, W.A. Near-complete suppression of surface losses and total internal quantum efficiency in BiVO4 photoanodes. Energy Environ. Sci. 2017, 10, 1517–1529. [Google Scholar] [CrossRef]
- Feng, S.; Wang, T.; Liu, B.; Hu, C.; Li, L.; Zhao, Z.-J.; Gong, J. Enriched surface oxygen vacancies of photoanodes by photoetching with enhanced charge separation. Angew. Chem. Int. Ed. 2020, 59, 2044–2048. [Google Scholar] [CrossRef]
- Kong, H.; Yang, H.; Park, J.-S.; Chae, W.-S.; Kim, H.Y.; Park, J.; Lee, J.H.; Choi, S.Y.; Park, M.; Kim, H.; et al. Spatial control of oxygen vacancy concentration in monoclinic WO3 photoanodes for enhanced solar water splitting. Adv. Funct. Mater. 2022, 32, 2204106. [Google Scholar] [CrossRef]
- Gao, R.-T.; Wang, L. Stable cocatalyst-free photoanodes with passivated surface states for photocorrosion inhibition. Angew. Chem. Int. Ed. 2020, 59, 23094–23099. [Google Scholar] [CrossRef]
- Sun, M.; Gao, R.-T.; He, J.; Liu, X.; Nakajima, T.; Zhang, X.; Wang, L. Photo-driven oxygen vacancies extends charge carrier lifetime for efficient solar water splitting. Angew. Chem. Int. Ed. 2021, 60, 17601–17607. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Nagashima, H.; Tachikawa, T. Ultra-narrow depletion layers in a hematite mesocrystal-based photoanode for boosting multihole water oxidation. Angew. Chem. Int. Ed. 2020, 59, 9047–9054. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, C.-W.; Choung, S.; Cho, Y.; Kim, S.; Oh, C.; Lee, K.-S.; Lee, C.-L.; Zhang, K.; Han, J.W.; et al. Continuous oxygen vacancy gradient in TiO2 photoelectrodes by a photoelectrochemical-driven “self-purification” process. Adv. Energy Mater. 2022, 12, 2103495. [Google Scholar] [CrossRef]
- Wang, J.; Ni, G.; Liao, W.; Liu, K.; Chen, J.; Liu, F.; Zhang, Z.; Jia, M.; Li, J.; Fu, J.; et al. Subsurface engineering induced fermi level de-pinning in metal oxide semiconductors for photoelectrochemical water splitting. Angew. Chem. Int. Ed. 2023, 62, e202217026. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.-T.; Liu, S.; Guo, X.; Zhang, R.; He, J.; Liu, X.; Nakajima, T.; Zhang, X.; Wang, L. Pt-induced defects curing on BiVO4 photoanodes for near-threshold charge separation. Adv. Energy Mater. 2021, 11, 2102384. [Google Scholar] [CrossRef]
- Zhang, Z.; Karimata, I.; Nagashima, H.; Muto, S.; Ohara, K.; Sugimoto, K.; Tachikawa, T. Interfacial oxygen vacancies yielding long-lived holes in hematite mesocrystal-based photoanodes. Nat. Commun. 2019, 10, 4832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, L.; Zhang, Y.; Ding, Y.; Bi, Y. Ultrathin FeOOH nanolayers with abundant oxygen vacancies on BiVO4 photoanodes for efficient water oxidation. Angew. Chem. Int. Ed. 2018, 57, 2248–2252. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, T.; Yun, J.-H.; Hu, Y.; Xiao, M.; Du, A.; Wang, L. New iron-cobalt oxide catalysts promoting BiVO4 films for photoelectrochemical water splitting. Adv. Funct. Mater. 2018, 28, 1802685. [Google Scholar] [CrossRef]
- Zhou, T.; Li, L.; Li, J.; Wang, J.; Bai, J.; Xia, L.; Xu, Q.; Zhou, B. Electrochemically reduced TiO2 photoanode coupled with oxygen vacancy-rich carbon quantum dots for synergistically improving photoelectrochemical performance. Chem. Eng. J. 2021, 425, 131770. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, D.; Xie, H.; Liu, Y.; Meng, C.; Zhang, P.; Fan, F.; Li, R.; Li, C. Modulating oxygen vacancies in lead chromate for photoelectrocatalytic water splitting. Adv. Mater. 2023, e2300914. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, X.; Chen, P.; Xiao, M.; Monny, S.A.; Wang, S.; Konarova, M.; Du, A.; Wang, L. Understanding the roles of oxygen vacancies in hematite-based photoelectrochemical processes. Angew. Chem. Int. Ed. 2019, 58, 1030–1034. [Google Scholar] [CrossRef]
- Hu, Z.; Shen, Z.; Yu, J.C. Covalent fixation of surface oxygen atoms on hematite photoanode for enhanced water oxidation. Chem. Mater. 2016, 28, 564–572. [Google Scholar] [CrossRef]
- Li, C.; Wang, D.; Gu, J.; Liu, Y.; Zhang, X. Promoting photoelectrochemical water oxidation on Ti-doped Fe2O3 nanowires photoanode by O2 plasma treatment. Catalysts 2021, 11, 82. [Google Scholar] [CrossRef]
- Zhang, H.; Park, J.H.; Byun, W.; Song, M.H.; Lee, J.S. Activating the surface and bulk of hematite photoanodes to improve solar water splitting. Chem. Sci. 2019, 10, 10436–10444. [Google Scholar] [CrossRef]
- Ai, M.H.; Li, X.D.; Pan, L.; Xu, X.T.; Yang, J.; Zou, J.J.; Zhang, X.W. Surface states modulation of hematite photoanodes for enhancing photoelectrochemical catalysis. Chem. Eng. Sci. 2022, 250, 117397. [Google Scholar] [CrossRef]
- Guo, W.; Wang, Y.; Lian, X.; Nie, Y.; Tian, S.; Wang, S.; Zhou, Y.; Henkelman, G. Insights into the multiple effects of oxygen vacancies on CuWO4 for photoelectrochemical water oxidation. Catal. Sci. Technol. 2020, 10, 7344–7351. [Google Scholar] [CrossRef]
- Zhang, H.; Li, P.; Zhou, H.C.; Xu, J.M.; Jiang, Q.F.; Hadden, J.H.L.; Wang, Y.Y.; Wang, M.N.; Chen, S.L.; Xie, F.; et al. Unravelling the synergy of oxygen vacancies and gold nanostars in hematite for the electrochemical and photoelectrochemical oxygen evolution reaction. Nano Energy 2022, 94, 106968. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, J.; Yao, X.; Chen, S.; Chen, Z. Clarifying the roles of oxygen vacancy in W-doped BiVO4 for solar water splitting. ACS Appl. Energy Mater. 2018, 1, 3410–3419. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Zhang, Y.; Xu, L.; Wang, T.; Xiao, X.; Wang, S.; Wang, L.; Huang, W. A BiVO4 photoanode with a VOx layer bearing oxygen vacancies offers improved charge transfer and oxygen evolution kinetics in photoelectrochemical water splitting. Angew. Chem. Int. Ed. 2023, 135, e202217346. [Google Scholar]
- Yang, Q.; Du, J.; Li, J.; Wu, Y.; Zhou, Y.; Yang, Y.; Yang, D.; He, H. Thermodynamic and kinetic influence of oxygen vacancies on the solar water oxidation reaction of alpha-Fe2O3 photoanodes. ACS Appl. Mater. Interfaces 2020, 12, 11625–11634. [Google Scholar] [CrossRef]
- Yang, M.; He, H.; Du, J.; Peng, H.; Ke, G.; Zhou, Y. Insight into the kinetic influence of oxygen vacancies on the WO3 photoanodes for solar water oxidation. J. Phys. Chem. Lett. 2019, 10, 6159–6165. [Google Scholar] [CrossRef]
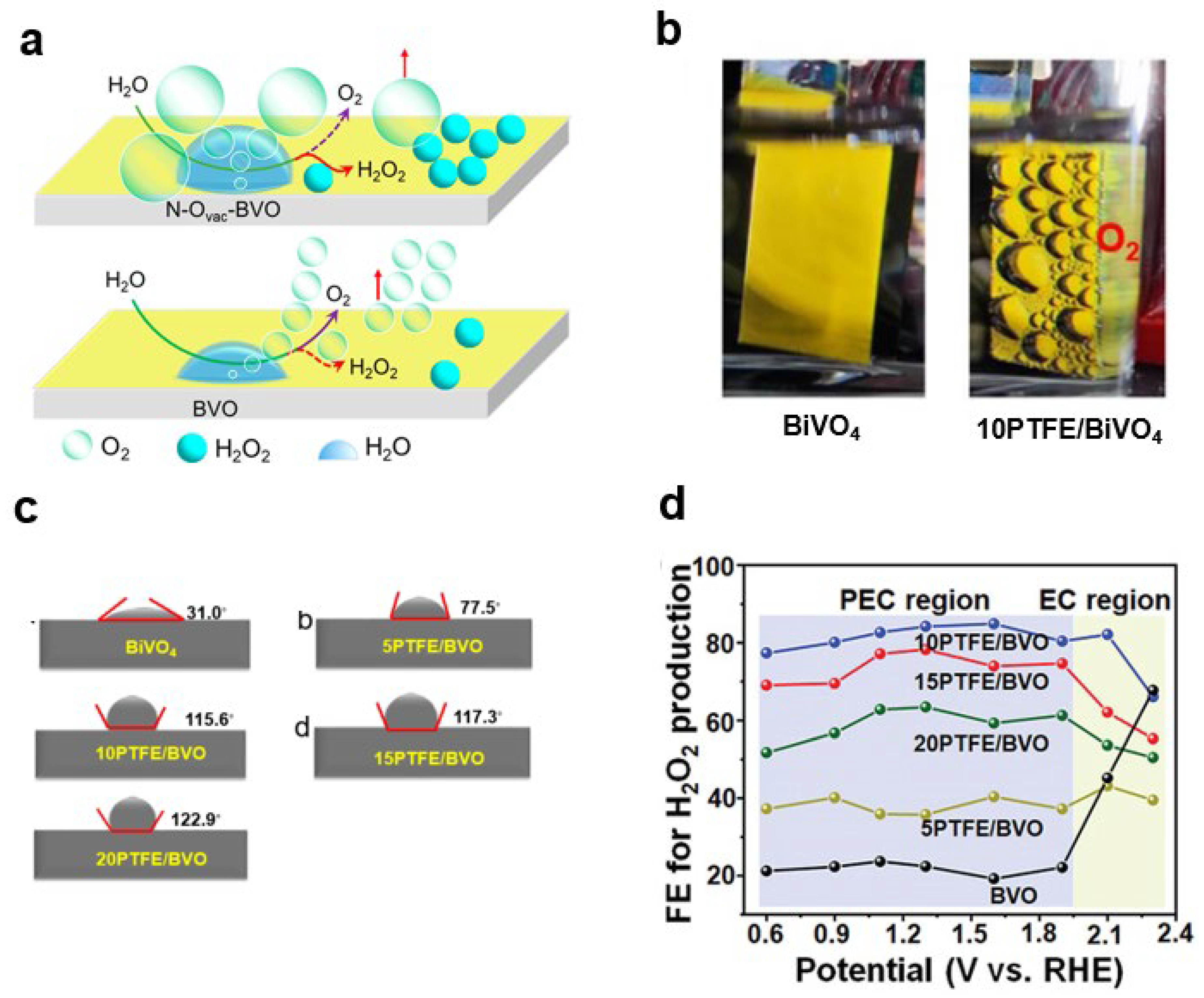
- Wan, S.; Dong, C.; Jin, J.; Li, J.; Zhong, Q.; Zhang, K.; Park, J.H. Tuning the surface wettability of a BiVO4 photoanode for kinetically modulating water oxidative H2O2 accumulation. ACS Energy Lett. 2022, 7, 3024–3031. [Google Scholar] [CrossRef]
- Nikacevic, P.; Hegner, F.S.; Ramon Galan-Mascaros, J.; Lopez, N. Influence of oxygen vacancies and surface facets on water oxidation selectivity toward oxygen or hydrogen peroxide with BiVO4. ACS Catal. 2021, 11, 13416–13422. [Google Scholar] [CrossRef]
- Xiao, J.; Zhao, F.; Zhong, J.; Huang, Z.; Fan, L.; Peng, L.; Zhou, S.-F.; Zhan, G. Performance enhancement of hematite photoanode with oxygen defects for water splitting. Chem. Eng. J. 2020, 402, 126163. [Google Scholar] [CrossRef]
- Deng, J.; Li, Y.; Xiao, Y.; Feng, K.; Lu, C.; Nie, K.; Lv, X.; Xu, H.; Zhong, J. Improved water oxidation of Fe2O3/Fe2TiO5 photoanode by functionalizing with a hydrophilic organic hole storage overlayer. ACS Catal. 2022, 12, 7833–7842. [Google Scholar] [CrossRef]
- Ou, M.; Geng, M.; Fang, X.; Shao, W.; Bai, F.; Wan, S.; Ye, C.; Wu, Y.; Chen, Y. Tailored BiVO4 photoanode hydrophobic microenvironment enables water oxidative H2O2 accumulation. Adv. Sci. 2023, 10, 2300169. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, Y.-S.; Wei, D.-D.; Duan, H.-M.; Mo, L.-M.; Wang, H.-Y. Carbon quantum dots aqueous solution as electrolyte for H2O2 production based on photoelectrochemical water splitting. J. Mater. Chem. A 2023, 11, 4162–4169. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, M.; Zhou, J.; Li, W.; Wang, H.; Xu, Z.; Lu, L.; Pei, L.; Shi, Z.; Yan, S.; et al. Surface states as electron transfer pathway enhanced charge separation in TiO2 nanotube water splitting photoanodes. Appl. Catal. B-Environ. 2018, 234, 100–108. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, Y.; Zhu, K.; Yan, S.; Lu, L.; Zhao, M.; Fu, H.; Li, Z.; Zou, Z. Galvanic cell reaction driven electrochemical doping of TiO2 nanotube photoanodes for enhanced charge separation. Chem. Commun. 2018, 54, 11116–11119. [Google Scholar] [CrossRef]
- Ma, W.; Huang, K.; Wu, X.; Wang, M.; Feng, S. Surface polarization enables high charge separation in TiO2 nanorod photoanode. Nano Res. 2021, 14, 4056–4062. [Google Scholar] [CrossRef]
- Li, Y.; Liang, S.; Sun, H.; Hua, W.; Wang, J.-G. Defect engineering and surface polarization of TiO2 nanorod arrays toward efficient photoelectrochemical oxygen evolution. Catalysts 2022, 12, 1021. [Google Scholar] [CrossRef]
- Li, W.; Yang, K.R.; Yao, X.; He, Y.; Dong, Q.; Brudvig, G.W.; Batista, V.S.; Wang, D. Facet-dependent kinetics and energetics of hematite for solar water oxidation reactions. ACS Appl. Mater. Interfaces 2019, 11, 5616–5622. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, J.; Wang, L.; Jin, B.; Yang, X.; Zhang, S.; Park, J.H. Near-complete suppression of oxygen evolution for photoelectrochemical H2O oxidative H2O2 synthesis. J. Am. Chem. Soc. 2020, 142, 8641–8648. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, H. Facile fire treatment of nanostructured hematite with an enhanced photoelectrochemical water splitting performance. J. Mater. Chem. A 2016, 4, 14974–14977. [Google Scholar] [CrossRef]
- Ma, M.; Li, J.; Zhang, Z.; Ning, D.; Liu, Z.; Li, W.; Zhong, G.; Yang, X.; Lam, D.C.C.; Xing, Z. Regulation of surface oxygen species to boost charge steering for solar water oxidation. Appl. Surf. Sci. 2023, 609, 155241. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Zhang, B.; Yan, D.; Xiang, X. Mediating the oxidizing capability of surface-bound hydroxyl radicals produced by photoelectrochemical water oxidation to convert glycerol into dihydroxyacetone. ACS Catal. 2022, 12, 6946–6957. [Google Scholar] [CrossRef]
- Ma, J.; Mao, K.; Low, J.; Wang, Z.; Xi, D.; Zhang, W.; Ju, H.; Qi, Z.; Long, R.; Wu, X.; et al. Efficient photoelectrochemical conversion of methane into ethylene glycol by WO3 nanobar arrays. Angew. Chem. Int. Ed. 2021, 60, 9357–9361. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Chen, W.; Xu, S.-M.; Yang, J.; Li, M.; Zhou, H.; Xu, M.; Shao, M.; Kong, X.; Li, Z.; et al. Selective photoelectrocatalytic glycerol oxidation to dihydroxyacetone via enhanced middle hydroxyl adsorption over a Bi2O3-incorporated catalyst. J. Am. Chem. Soc. 2022, 144, 7720–7730. [Google Scholar] [CrossRef] [PubMed]
- Dang, V.D.; Annadurai, T.; Khedulkar, A.P.; Lin, J.-Y.; Adorna, J., Jr.; Yu, W.-J.; Pandit, B.; Huynh, T.V.; Doong, R.-A. S-scheme n-doped carbon dots anchored g-C3N4/Fe2O3 shell/core composite for photoelectrocatalytic trimethoprim degradation and water splitting. Appl. Catal. B-Environ. 2023, 320, 121928. [Google Scholar] [CrossRef]
- Wang, D.; He, Y.; Zhong, N.; He, Z.; Shen, Y.; Zeng, T.; Lu, X.; Ma, J.; Song, S. In situ chloride-mediated synthesis of TiO2 thin film photoanode with enhanced photoelectrochemical activity for carbamazepine oxidation coupled with simultaneous cathodic H2 production and CO2 conversion to fuels. J. Hazard. Mater. 2021, 410, 124563. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Zhang, H.N.; Ji, H.W.; Ma, W.H.; Chen, C.C.; Zhao, J.C. Pivotal role and regulation of proton transfer in water oxidation on hematite photoanodes. J. Am. Chem. Soc. 2016, 138, 2705–2711. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Zhang, H.N.; Liu, A.A.; Chen, C.C.; Song, W.J.; Zhao, J.C. Rate-limiting O-O bond formation pathways for water oxidation on hematite photoanode. J. Am. Chem. Soc. 2018, 140, 3264–3269. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, C.; Tang, D.; Ding, L.; Zhang, Y.; Sheng, H.; Ji, H.; Song, W.; Ma, W.; Chen, C.; et al. Alpha-Fe2O3 as a versatile and efficient oxygen atom transfer catalyst in combination with H2O as the oxygen source. Nat. Catal. 2021, 4, 684–691. [Google Scholar] [CrossRef]
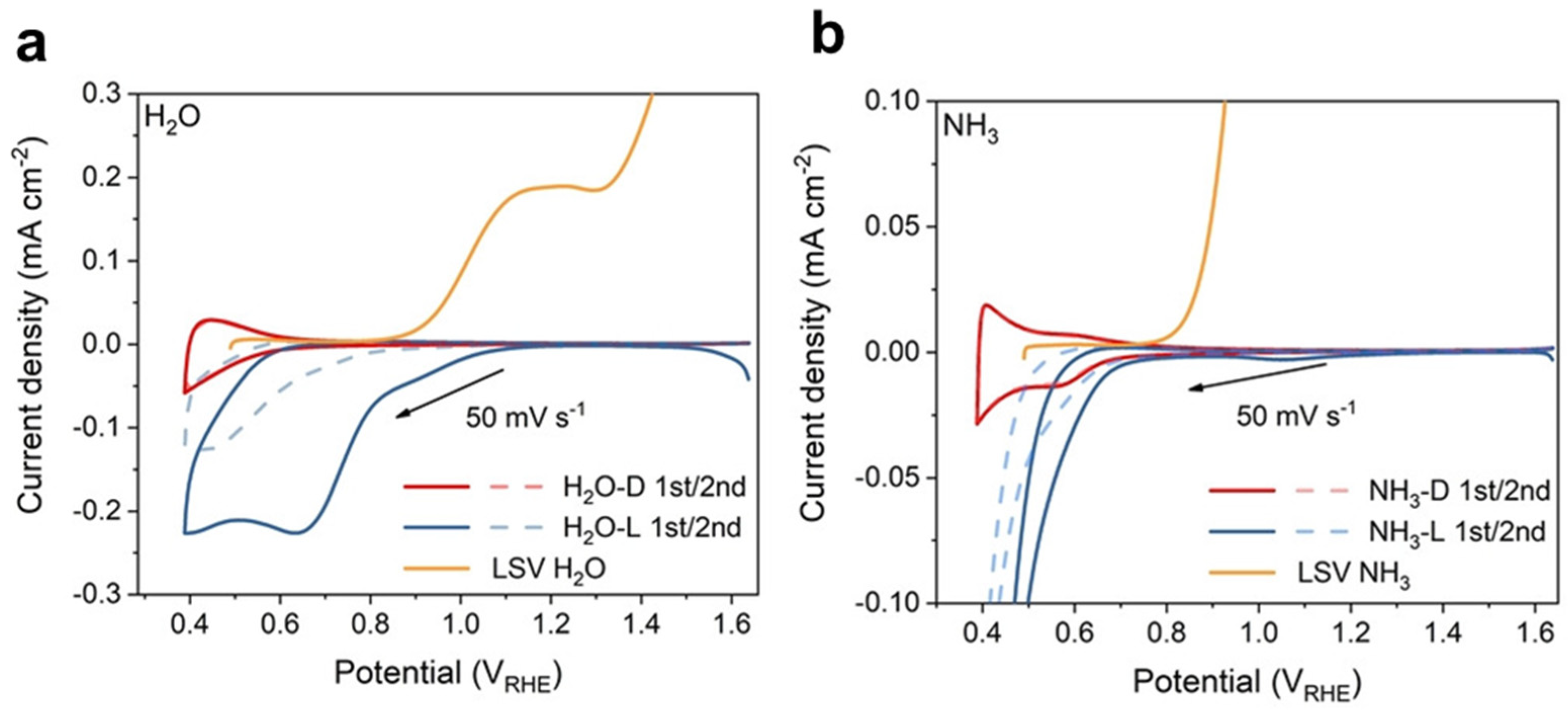
- Wu, L.; Tang, D.J.; Xue, J.; Liu, S.Q.; Wang, J.M.; Ji, H.W.; Chen, C.C.; Zhang, Y.C.; Zhao, J.C. Competitive non-radical nucleophilic attack pathways for NH3 oxidation and H2O oxidation on hematite photoanodes. Angew. Chem. Int. Ed. 2022, 61, e202214580. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Duan, M.; Deng, C.; Yang, J.; Yang, S.; Zhang, Y.; Sheng, H.; Li, Y.; Chen, C.; Zhao, J. Br−/BrO−-mediated highly efficient photoelectrochemical epoxidation of alkenes on alpha-Fe2O3. Nat. Commun. 2023, 14, 1943. [Google Scholar] [CrossRef] [PubMed]
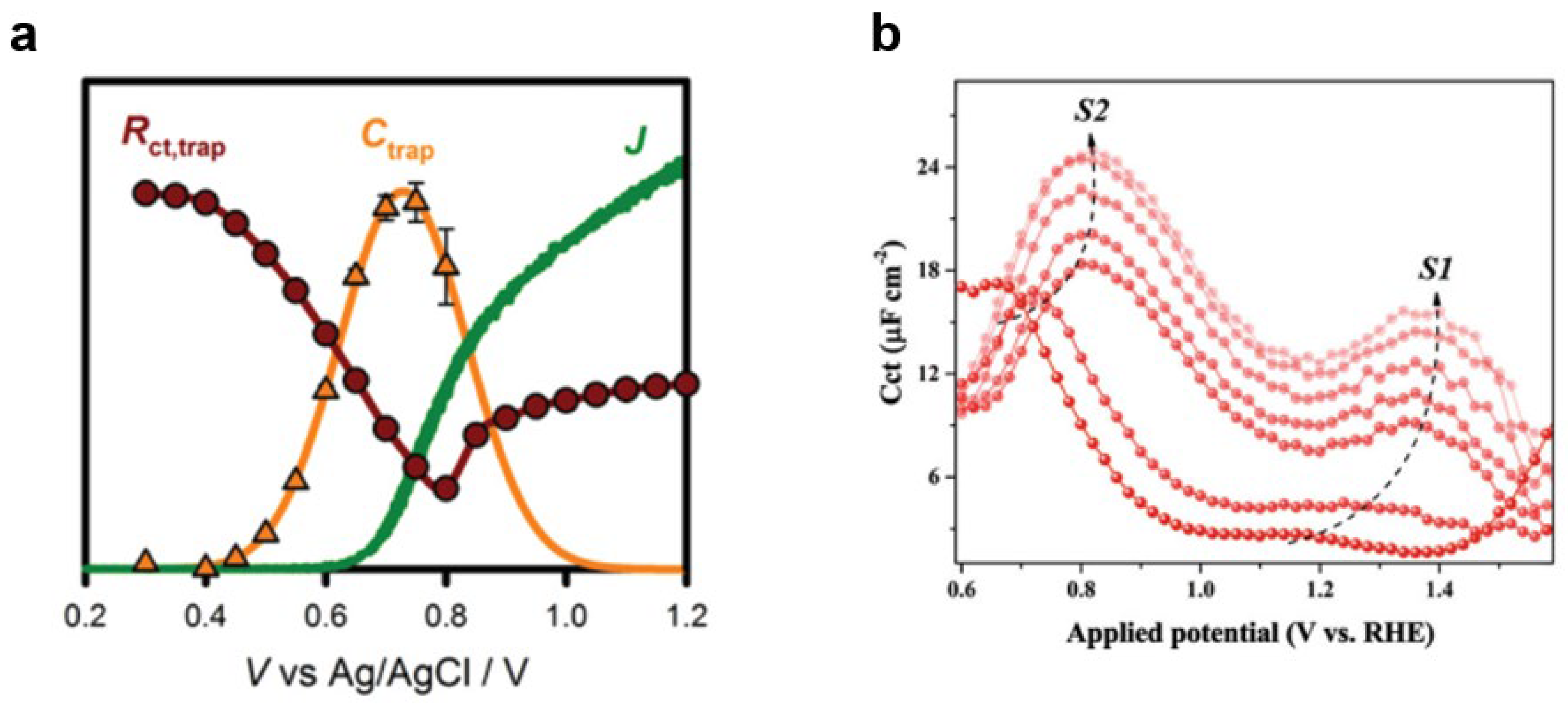
- Li, J.; Wan, W.; Triana, C.A.; Chen, H.; Zhao, Y.; Mavrokefalos, C.K.; Patzke, G.R. Reaction kinetics and interplay of two different surface states on hematite photoanodes for water oxidation. Nat. Commun. 2021, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, J.; Ke, G.; Liu, B.; Dong, F.; Yang, L.; He, H.; Zhou, Y. WO3 homojunction photoanode: Integrating the advantages of WO3 different facets for efficient water oxidation. J. Energy Chem. 2021, 56, 37–45. [Google Scholar] [CrossRef]
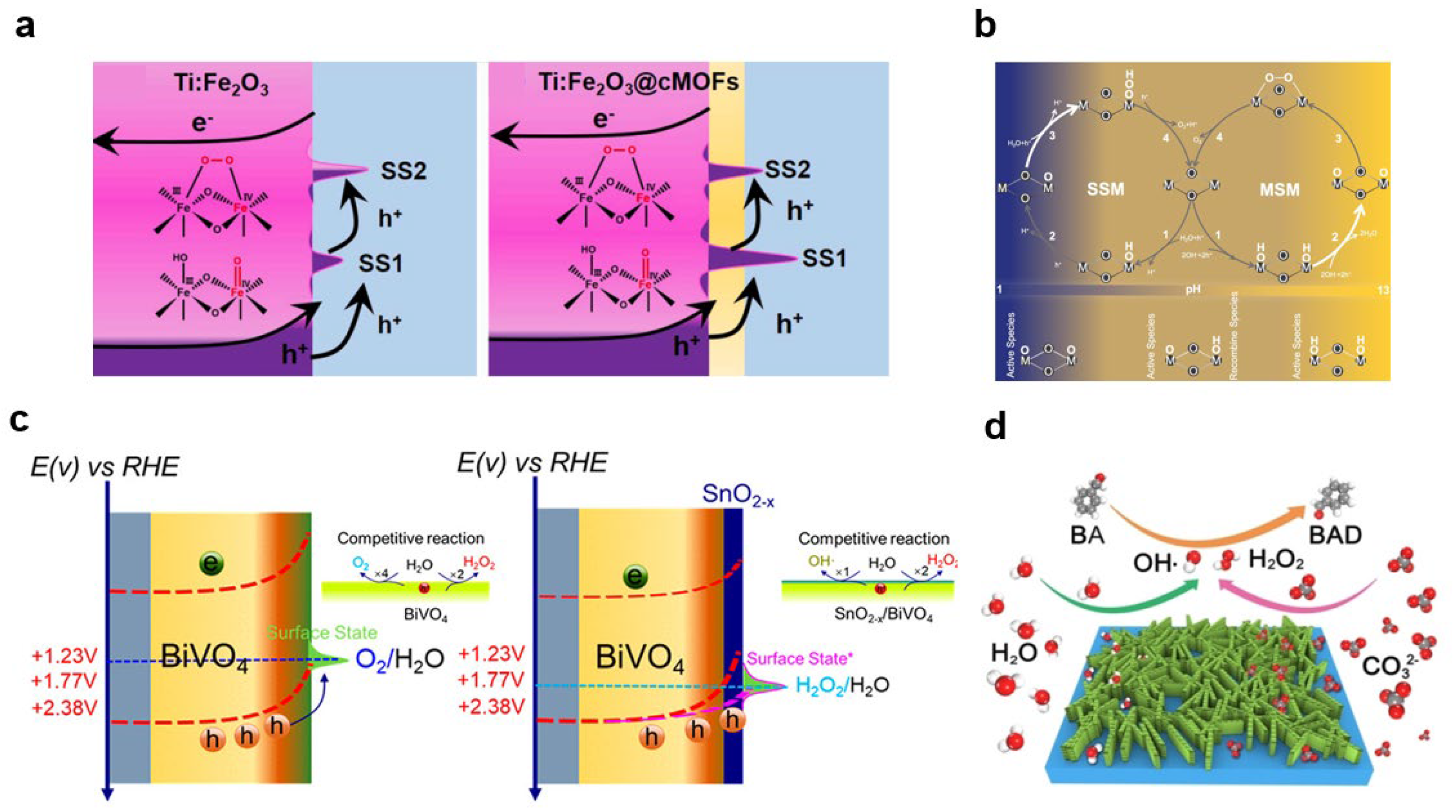
- Pan, J.-B.; Liu, X.; Wang, B.-H.; Chen, Y.-A.; Tan, H.-Y.; Ouyang, J.; Zhou, W.; Shen, S.; Chen, L.; Au, C.-T.; et al. Conductive MOFs coating on hematite photoanode for activity boost via surface state regulation. Appl. Catal. B-Environ. 2022, 315, 121526. [Google Scholar] [CrossRef]
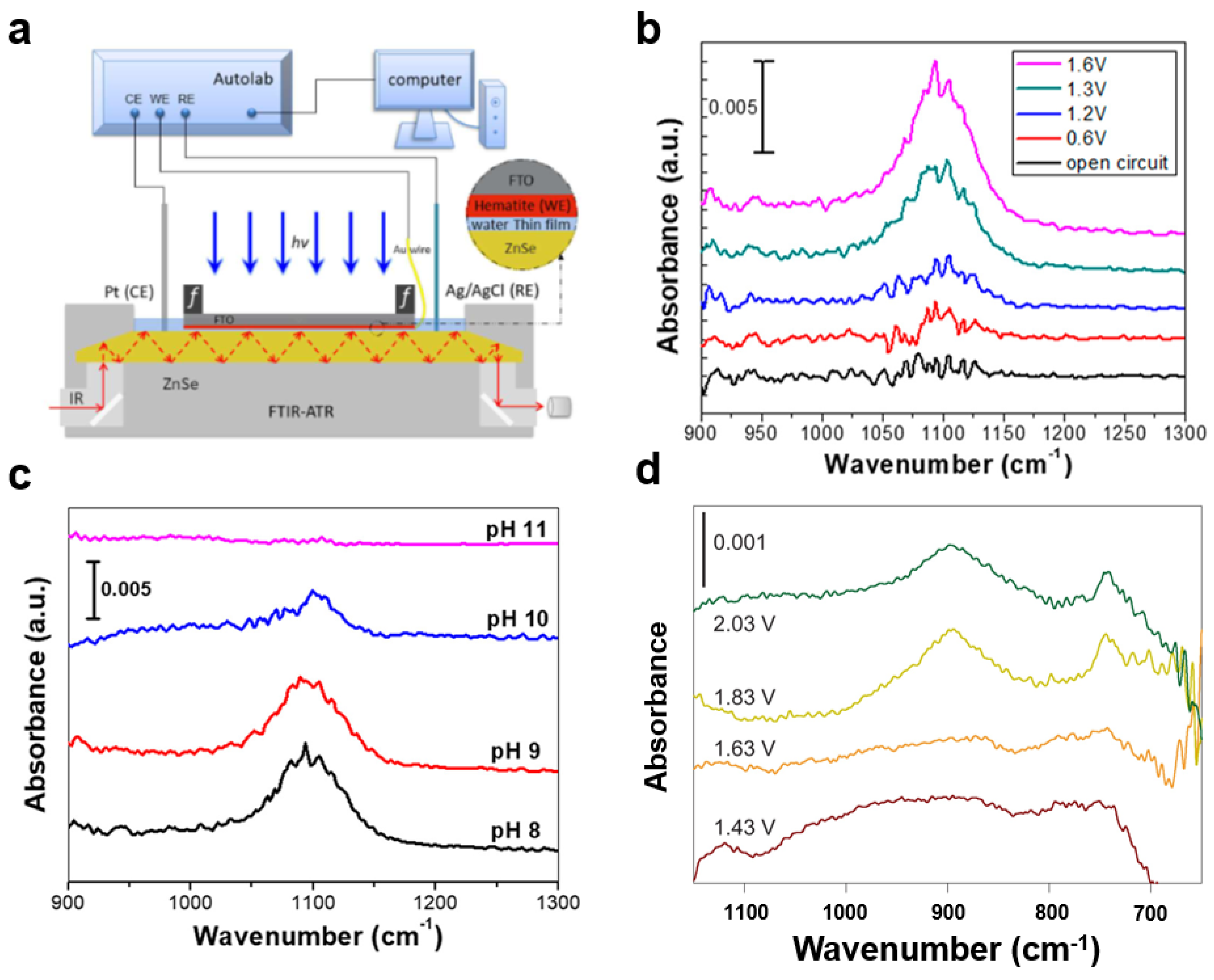
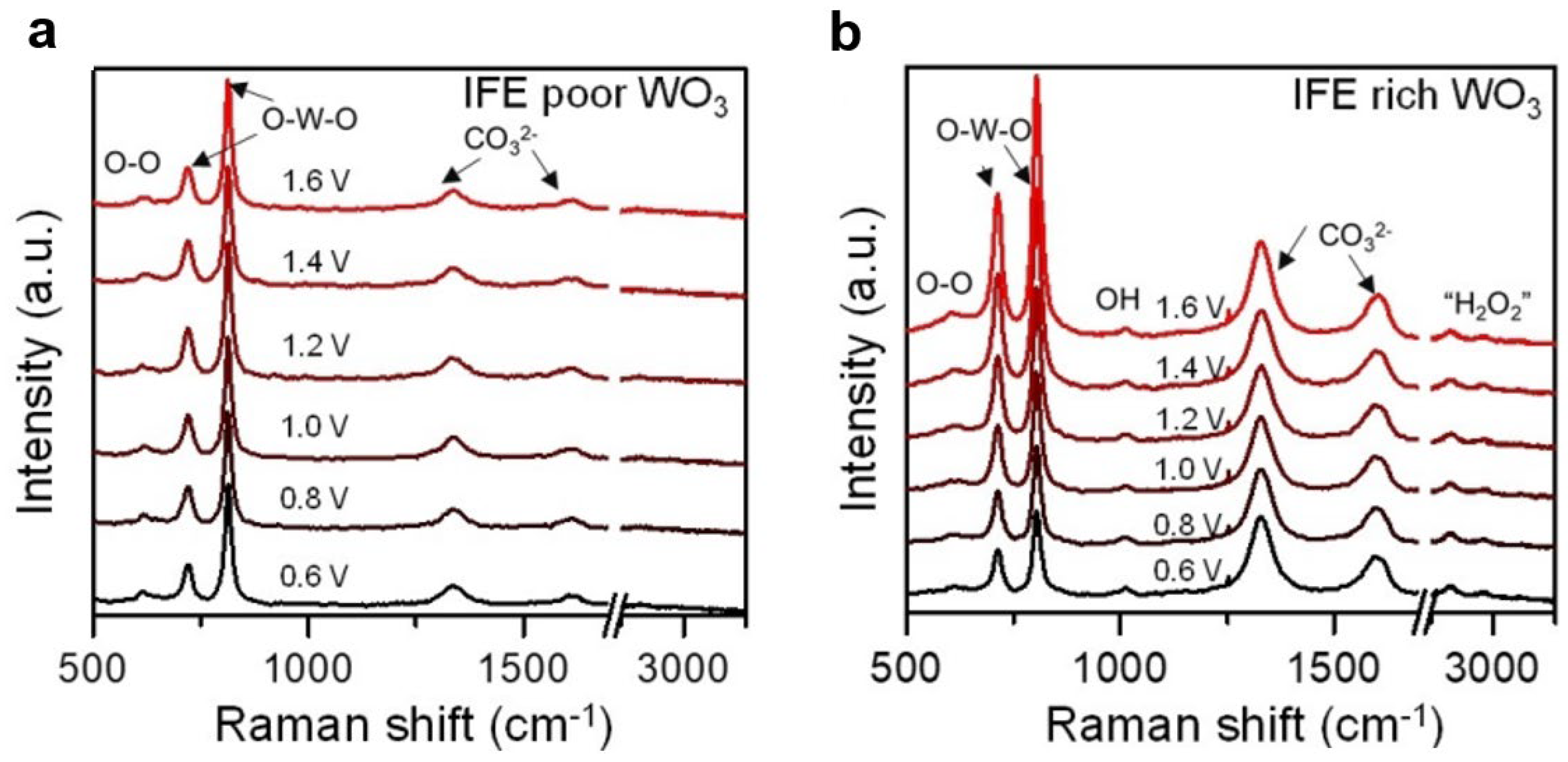
- Ji, X.; Ou, Y.; Huang, L.; Gan, L.; Zhang, Y.; Xiao, P. Evolution of intermediates on metal oxide photoanodes in a full pH range. ACS Catal. 2023, 13, 5841–5849. [Google Scholar] [CrossRef]
- Schanz, T.; Burek, B.O.; Bloh, J.Z. Fate and reactivity of peroxides formed over BiVO4 anodes in bicarbonate electrolytes. ACS Energy Lett. 2023, 8, 1463–1467. [Google Scholar] [CrossRef]
- Zhang, K.; Lu, Y.; Zou, Q.; Jin, J.; Cho, Y.; Wang, Y.; Zhang, Y.; Park, J.H. Tuning selectivity of photoelectrochemical water oxidation via facet-engineered interfacial energetics. ACS Energy Lett. 2021, 6, 4071–4078. [Google Scholar] [CrossRef]
- Jeon, T.H.; Kim, H.; Kim, H.I.; Choi, W. Highly durable photoelectrochemical H2O2 productionviadual photoanode and cathode processes under solar simulating and external bias-free conditions. Energy Environ. Sci. 2020, 13, 1730–1742. [Google Scholar] [CrossRef]
- Baek, J.H.; Gill, T.M.; Abroshan, H.; Park, S.; Shi, X.; Norskoy, J.; Jung, H.S.; Siahrostami, S.; Zheng, X. Selective and efficient Gd-doped BiVO4 photoanode for two-electron water oxidation to H2O2. ACS Energy Lett. 2019, 4, 720–728. [Google Scholar] [CrossRef]
- Qu, S.; Wu, H.; Ng, Y.H. Thin zinc oxide layer passivating bismuth vanadate for selective photoelectrochemical water oxidation to hydrogen peroxide. Small 2023, 2300347. [Google Scholar] [CrossRef]
- Li, H.; Lin, C.; Yang, Y.; Dong, C.; Min, Y.; Shi, X.; Wang, L.; Lu, S.; Zhang, K. Boosting reactive oxygen species generation using inter-facet edge rich WO3 arrays for photoelectrochemical conversion. Angew. Chem. Int. Ed. 2023, 62, e202210804. [Google Scholar]
- Yang, L.; Chen, H.; Xu, Y.; Qian, R.; Chen, Q.; Fang, Y. Synergetic effects by Co2+ and PO43− on Mo-doped BiVO4 for an improved photoanodic H2O2 evolution. Chem. Eng. Sci. 2022, 251, 117435. [Google Scholar] [CrossRef]
- Fuku, K.; Sayama, K. Efficient oxidative hydrogen peroxide production and accumulation in photoelectrochemical water splitting using a tungsten trioxide/bismuth vanadate photoanode. Chem. Commun. 2016, 52, 5406–5409. [Google Scholar] [CrossRef]
- Chen, F.; Xia, L.; Zhang, Y.; Bai, J.; Wang, J.; Li, J.; Rahim, M.; Xu, Q.; Zhu, X.; Zhou, B. Efficient degradation of refractory organics for carbonate-containing wastewater via generation carbonate radical based on a photoelectrocatalytic TNA-MCF system. Appl. Catal. B-Environ. 2019, 259, 118071. [Google Scholar] [CrossRef]
- Hill, J.C.; Choi, K.-S. Effect of electrolytes on the selectivity and stability of n-type WO3 photoelectrodes for use in solar water oxidation. J. Phys. Chem. C 2012, 116, 7612–7620. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, B.; Fan, L.; Liu, H.; Valvo, M.; Edstrom, K.; Cuartero, M.; de Marco, R.; Crespo, G.A.; Sun, L. Efficient BiVO4 photoanodes by postsynthetic treatment: Remarkable improvements in photoelectrochemical performance from facile borate modification. Angew. Chem. Int. Ed. 2019, 58, 19027–19033. [Google Scholar] [CrossRef]
- Lee, D.K.; Choi, K.-S. Enhancing long-term photostability of BiVO4 photoanodes for solar water splitting by tuning electrolyte composition. Nat. Energy 2018, 3, 53–60. [Google Scholar] [CrossRef]
- Gao, R.-T.; Nguyen, N.T.; Nakajima, T.; He, J.; Liu, X.; Zhang, X.; Wang, L.; Wu, L. Dynamic semiconductor-electrolyte interface for sustainable solar water splitting over 600 h under neutral conditions. Sci. Adv. 2023, 9, eade4589. [Google Scholar] [CrossRef] [PubMed]
- Nellist, M.R.; Laskowski, F.A.L.; Qiu, J.; Hajibabaei, H.; Sivula, K.; Hamann, T.W.; Boettcher, S.W. Potential-sensing electrochemical atomic force microscopy for in operando analysis of water-splitting catalysts and interfaces. Nat. Energy 2018, 3, 46–52. [Google Scholar] [CrossRef]
- Laskowski, F.A.L.; Oener, S.Z.; Nellist, M.R.; Gordon, A.M.; Bain, D.C.; Fehrs, J.L.; Boettcher, S.W. Nanoscale semiconductor/catalyst interfaces in photoelectrochemistry. Nat. Mater. 2020, 19, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Fu, H.J.; Mueller, T.; Brunschwig, B.S.; Lewis, N.S. Atomic force microscopy: Emerging illuminated and operando techniques for solar fuel research. J. Chem. Phys. 2020, 153, 020902. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Siretanu, I.; van den Ende, D.; Mei, B.; Mul, G.; Mugele, F. Facet-dependent surface charge and hydration of semiconducting nanoparticles at variable pH. Adv. Mater. 2021, 33, 2106229. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, M.A.; Ghorpade, U.V.; Suryawanshi, U.P.; Kumar, P.V.; Jang, S.; Jang, J.S.; Tran, L.; Lee, J.-S.; Bae, H.; Shin, S.W.; et al. Rapid synthesis of ultrathin Ni: FeOOH with in situ-induced oxygen vacancies for enhanced water oxidation activity and stability of BiVO4 photoanodes. ACS Appl. Mater. Interfaces 2023, 15, 21123–21133. [Google Scholar] [CrossRef]
- Zahidi, M.M.; Mamat, M.H.; Subki, A.; Abdullah, M.H.; Hassan, H.; Ahmad, M.K.; Bakar, S.A.; Mohamed, A.; Ohtani, B. Formation of a nanorod-assembled TiO2 actinomorphic-flower-like microsphere film via ta doping using a facile solution immersion method for humidity sensing. Nanomaterials 2023, 13, 256. [Google Scholar] [CrossRef]
- Klahr, B.; Hamann, T. Water oxidation on hematite photoelectrodes: Insight into the nature of surface states through in situ spectroelectrochernistry. J. Phys. Chem. C 2014, 118, 10393–10399. [Google Scholar] [CrossRef]
- Shi, X.; Wu, Q.; Cui, C. Modulating WO3 crystal orientation to suppress hydroxyl radicals for sustainable solar water oxidation. ACS Catal. 2023, 13, 1470–1476. [Google Scholar] [CrossRef]
- Zandi, O.; Hamann, T.W. Determination of photoelectrochemical water oxidation intermediates on haematite electrode surfaces using operando infrared spectroscopy. Nat. Chem. 2016, 8, 778–783. [Google Scholar] [CrossRef]
- Ramakrishnan, V.; Tsyganok, A.; Davydova, E.; Pavan, M.J.; Rothschild, A.; Visoly-Fisher, I. Competitive photo-oxidation of water and hole scavengers on hematite photoanodes: Photoelectrochemical and operando raman spectroelectrochemistry study. ACS Catal. 2023, 13, 540–549. [Google Scholar] [CrossRef]
- Liu, Y.T.; Smith, R.D.L. Differentiating defects and their influence on hematite photoanodes using x-ray absorption spectroscopy and raman microscopy. ACS Appl. Mater. Interfaces 2022, 14, 6615–6624. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, J.; Yang, W.; Balaghi, S.E.; Triana, C.A.; Mavrokefalos, C.K.; Patzke, G.R. The role of surface states on reduced TiO2@BiVO4 photoanodes: Enhanced water oxidation performance through improved charge transfer. ACS Catal. 2021, 11, 7637–7646. [Google Scholar] [CrossRef]
- Klahr, B.; Gimenez, S.; Fabregat-Santiago, F.; Hamann, T.; Bisquert, J. Water oxidation at hematite photoelectrodes: The role of surface states. J. Am. Chem. Soc. 2012, 134, 4294–4302. [Google Scholar] [CrossRef]
- Gao, L.; Wang, P.; Chai, H.; Li, S.; Jin, J.; Ma, J. Expediting hole transfer via surface states in hematite-based composite photoanodes. Nanoscale 2022, 14, 17044–17052. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Siahrostami, S.; Li, G.-L.; Zhang, Y.; Chakthranont, P.; Studt, F.; Jaramillo, T.F.; Zheng, X.; Norskov, J.K. Understanding activity trends in electrochemical water oxidation to form hydrogen peroxide. Nat. Commun. 2017, 8, 701. [Google Scholar] [CrossRef]

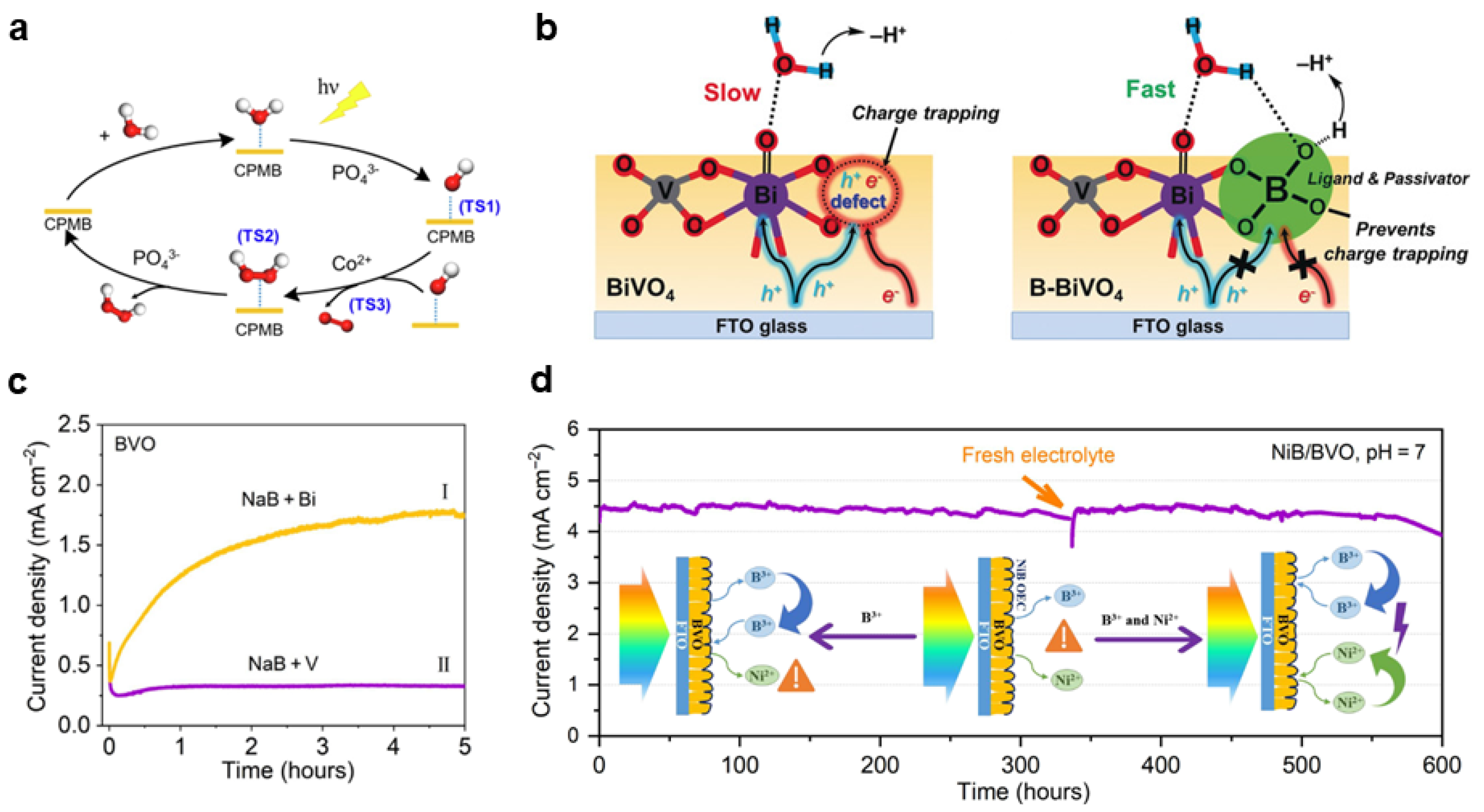
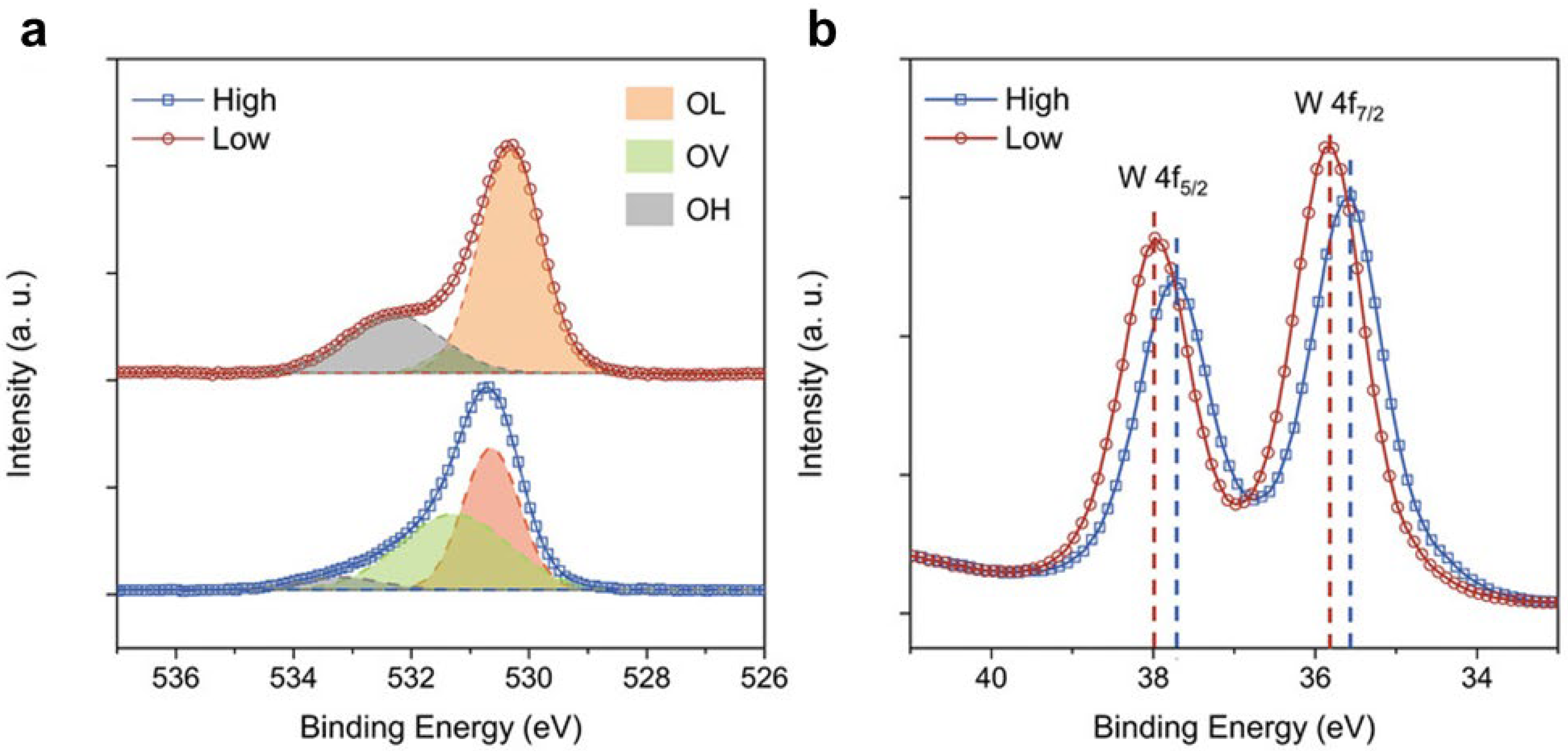
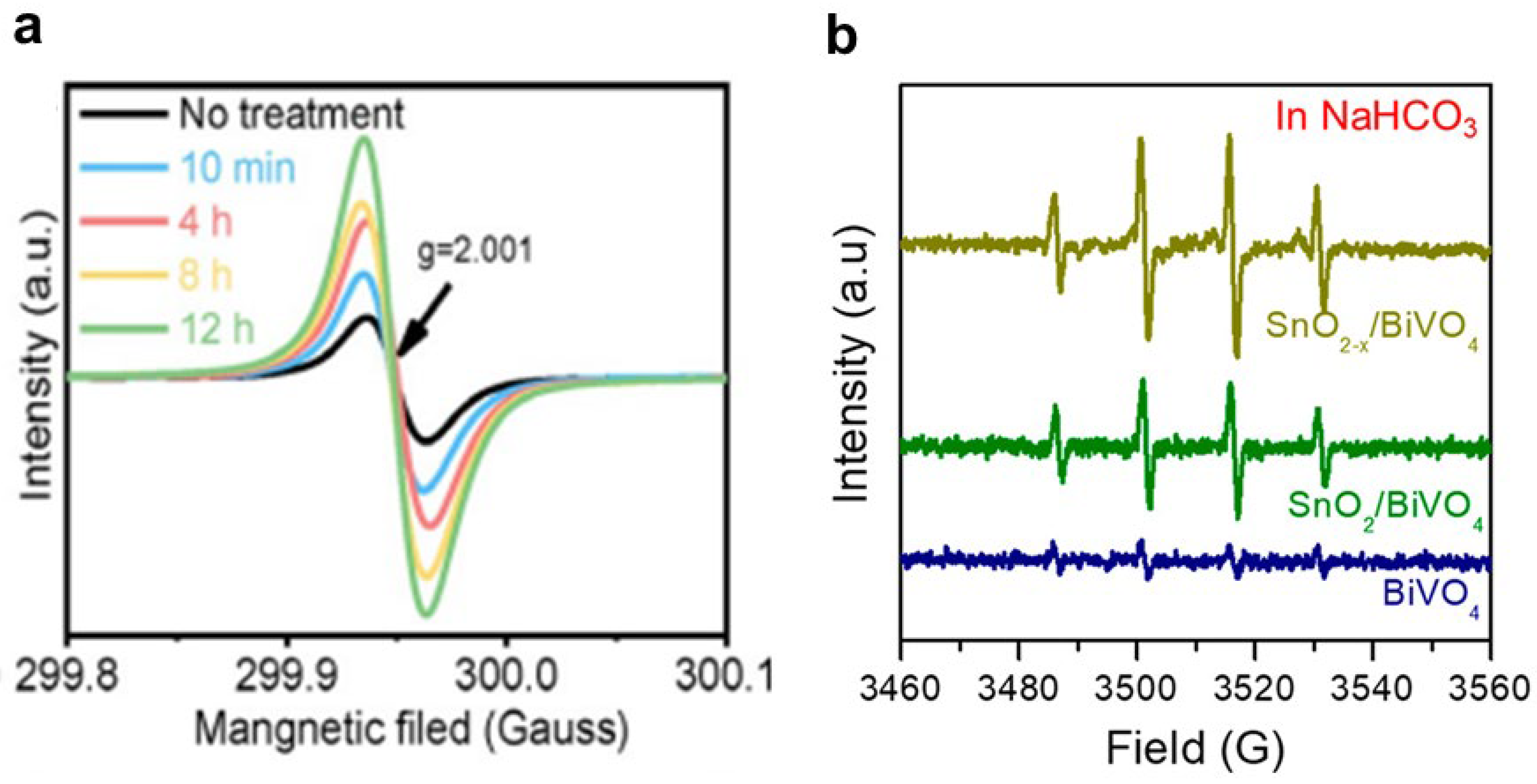
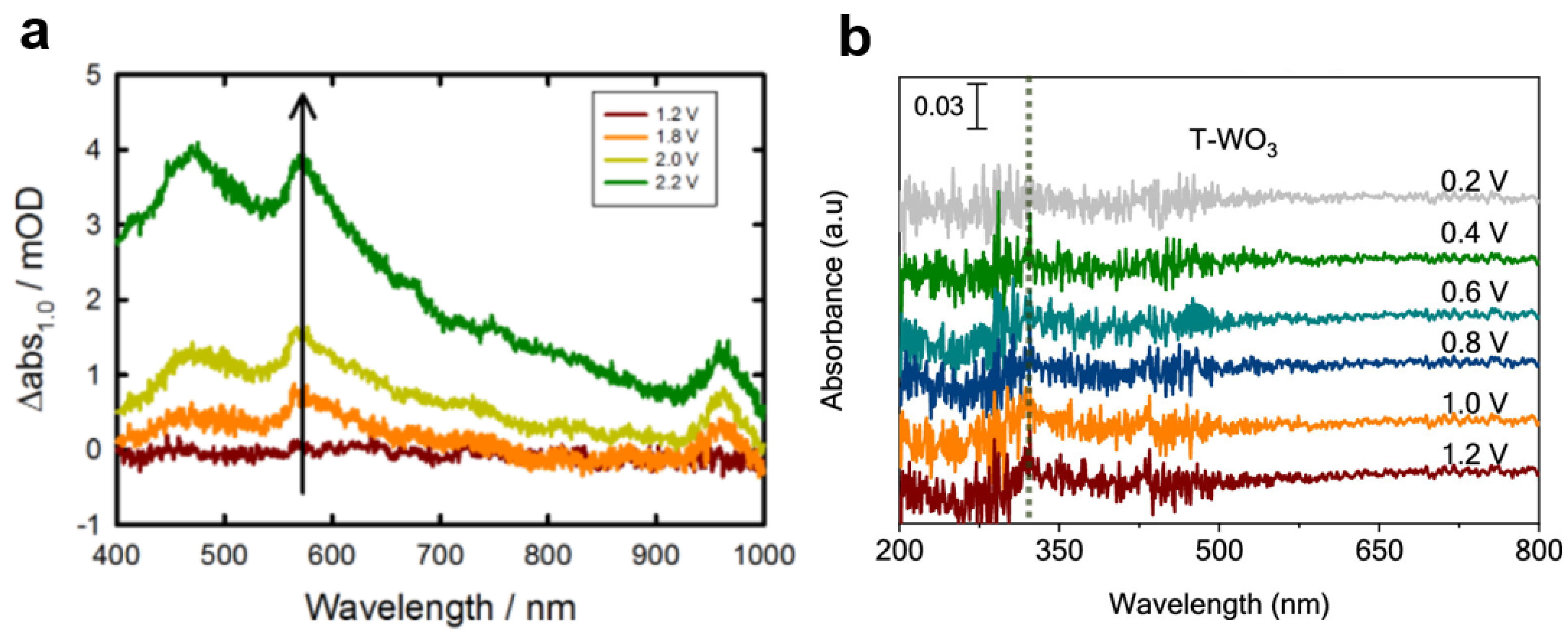

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, J.; Lu, Q.-C.; Shen, S.; Yin, S.-F. Surface Oxygen Species in Metal Oxide Photoanodes for Solar Energy Conversion. Nanomaterials 2023, 13, 1919. https://doi.org/10.3390/nano13131919
Ouyang J, Lu Q-C, Shen S, Yin S-F. Surface Oxygen Species in Metal Oxide Photoanodes for Solar Energy Conversion. Nanomaterials. 2023; 13(13):1919. https://doi.org/10.3390/nano13131919
Chicago/Turabian StyleOuyang, Jie, Qi-Chao Lu, Sheng Shen, and Shuang-Feng Yin. 2023. "Surface Oxygen Species in Metal Oxide Photoanodes for Solar Energy Conversion" Nanomaterials 13, no. 13: 1919. https://doi.org/10.3390/nano13131919
APA StyleOuyang, J., Lu, Q.-C., Shen, S., & Yin, S.-F. (2023). Surface Oxygen Species in Metal Oxide Photoanodes for Solar Energy Conversion. Nanomaterials, 13(13), 1919. https://doi.org/10.3390/nano13131919