Interface Coordination Engineering of P-Fe3O4/Fe@C Derived from an Iron-Based Metal Organic Framework for pH-Universal Water Splitting
Abstract
1. Introduction
2. Experimental
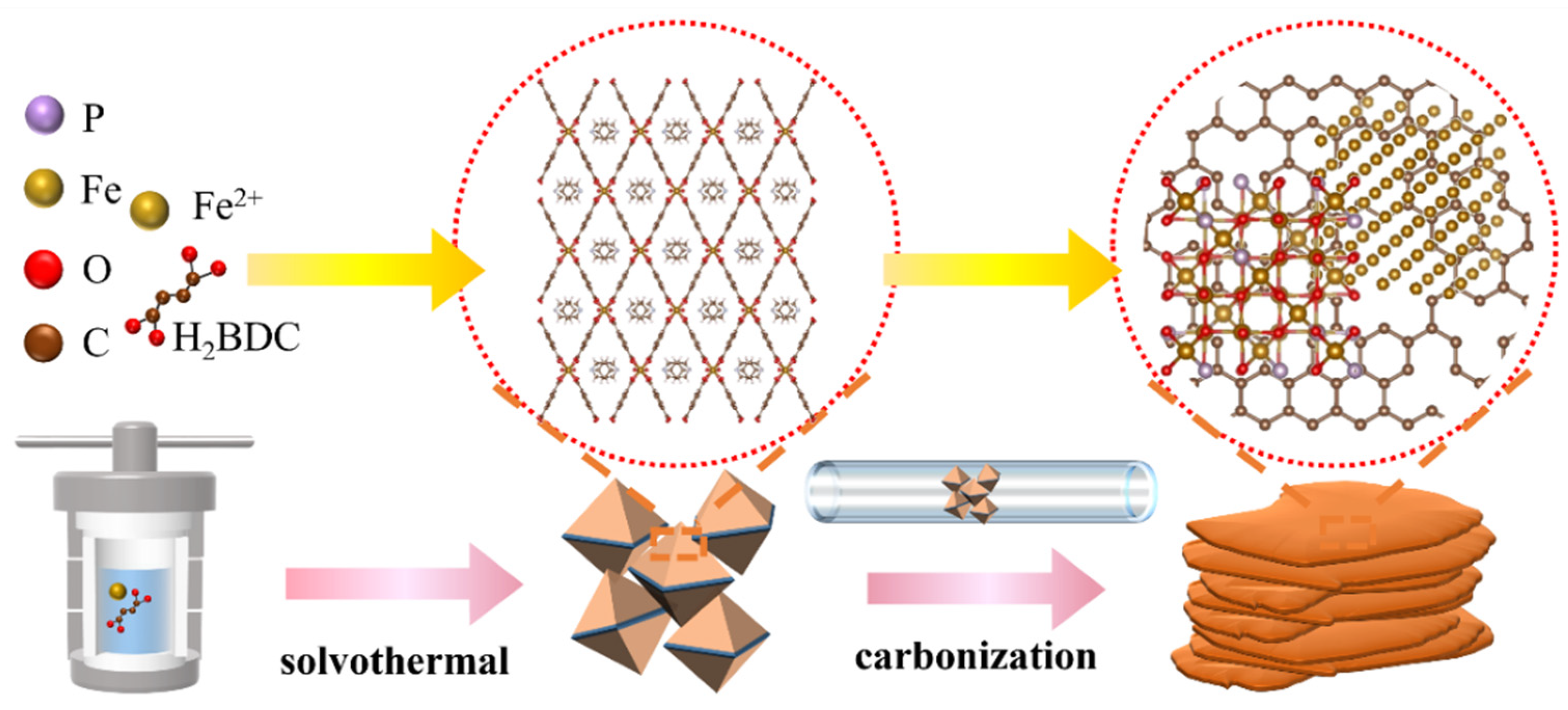
2.1. Synthesis of MIL-53(Fe)
2.2. Fabrication of P-Fe3O4/Fe@C Catalysts
3. Results and Discussion
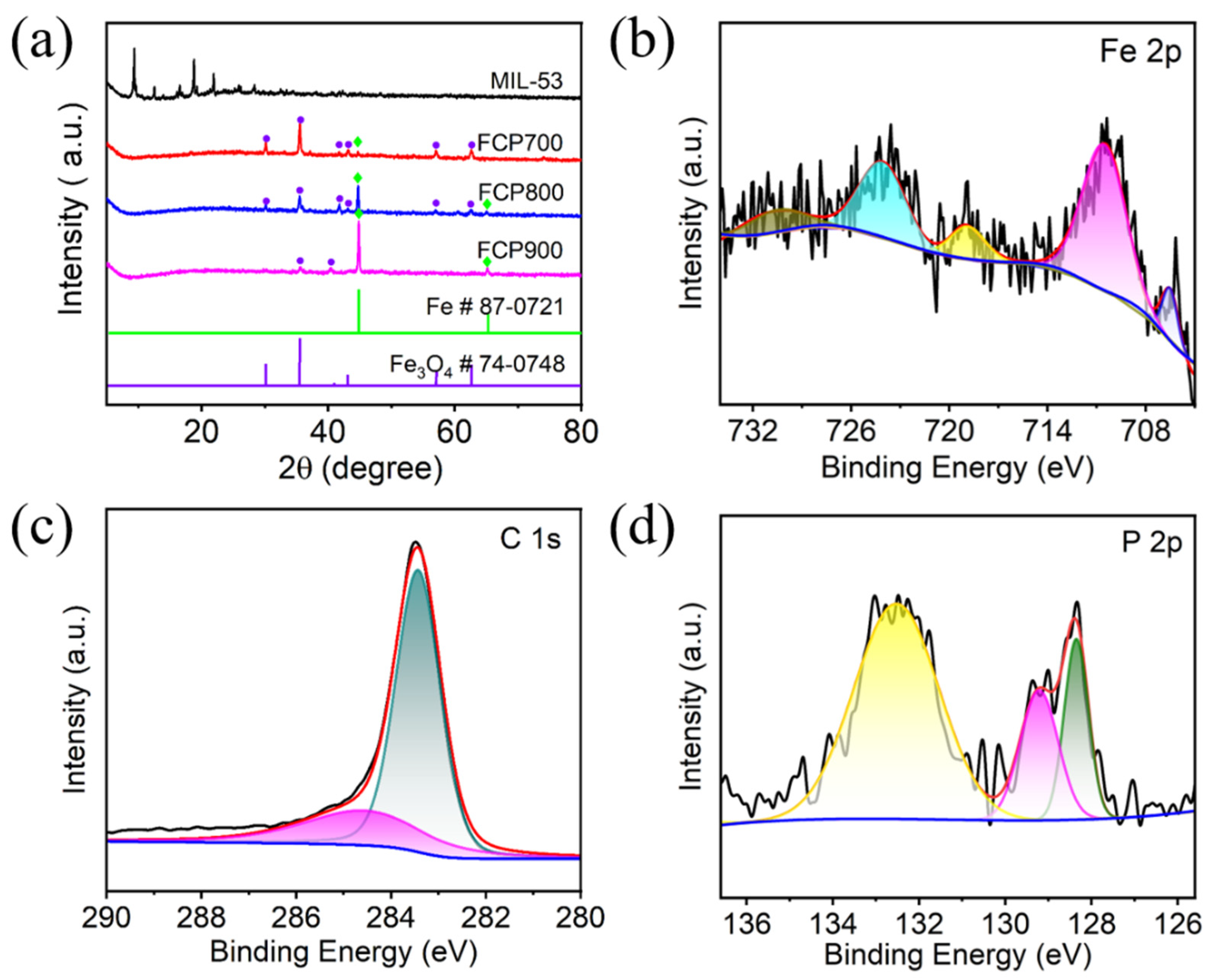
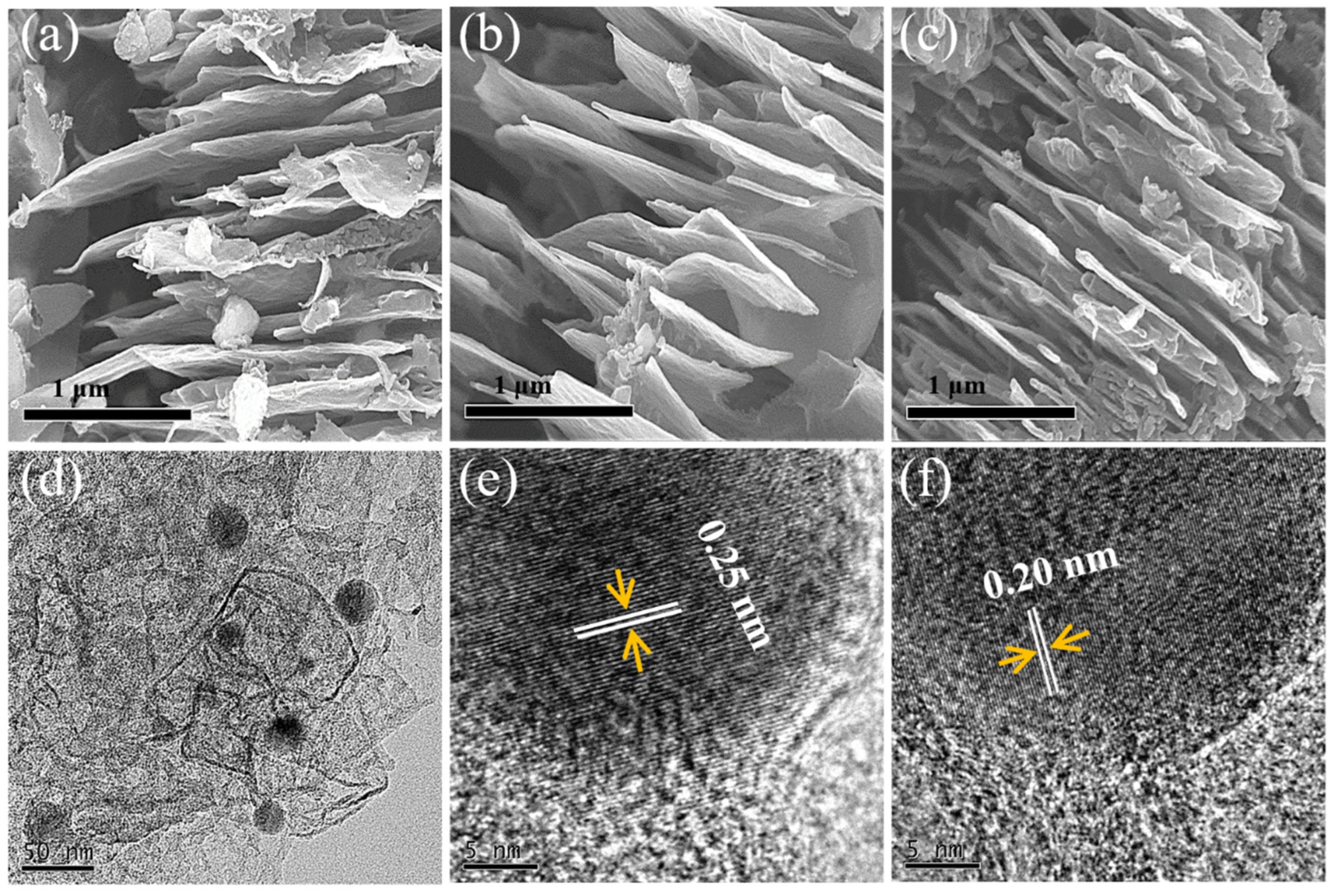
3.1. Characterization
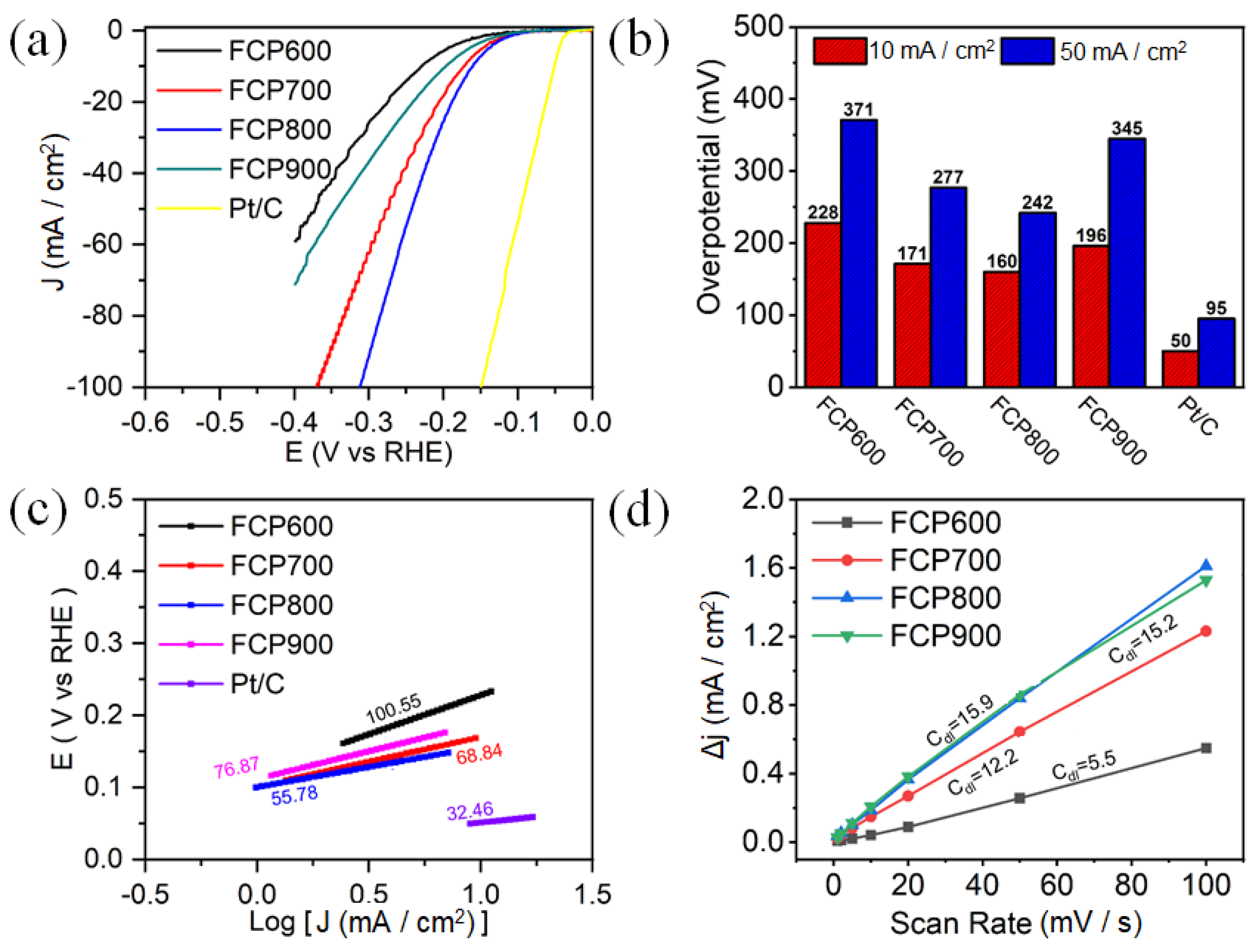
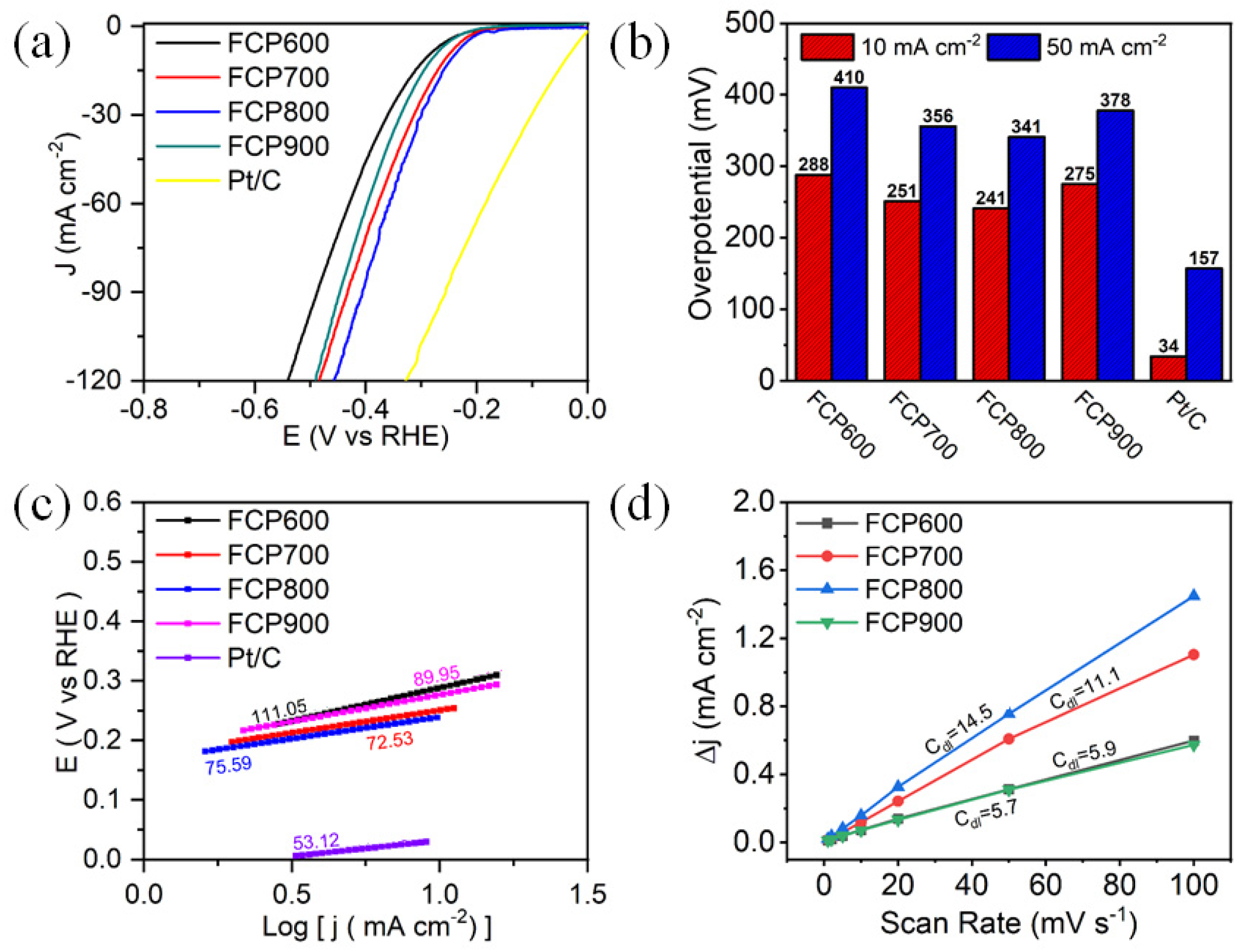
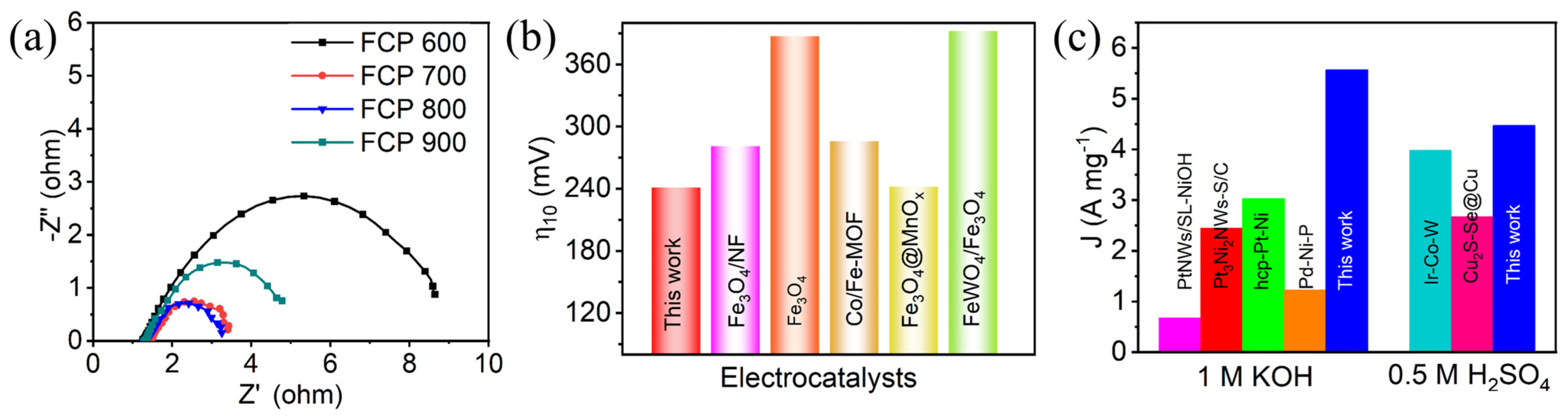
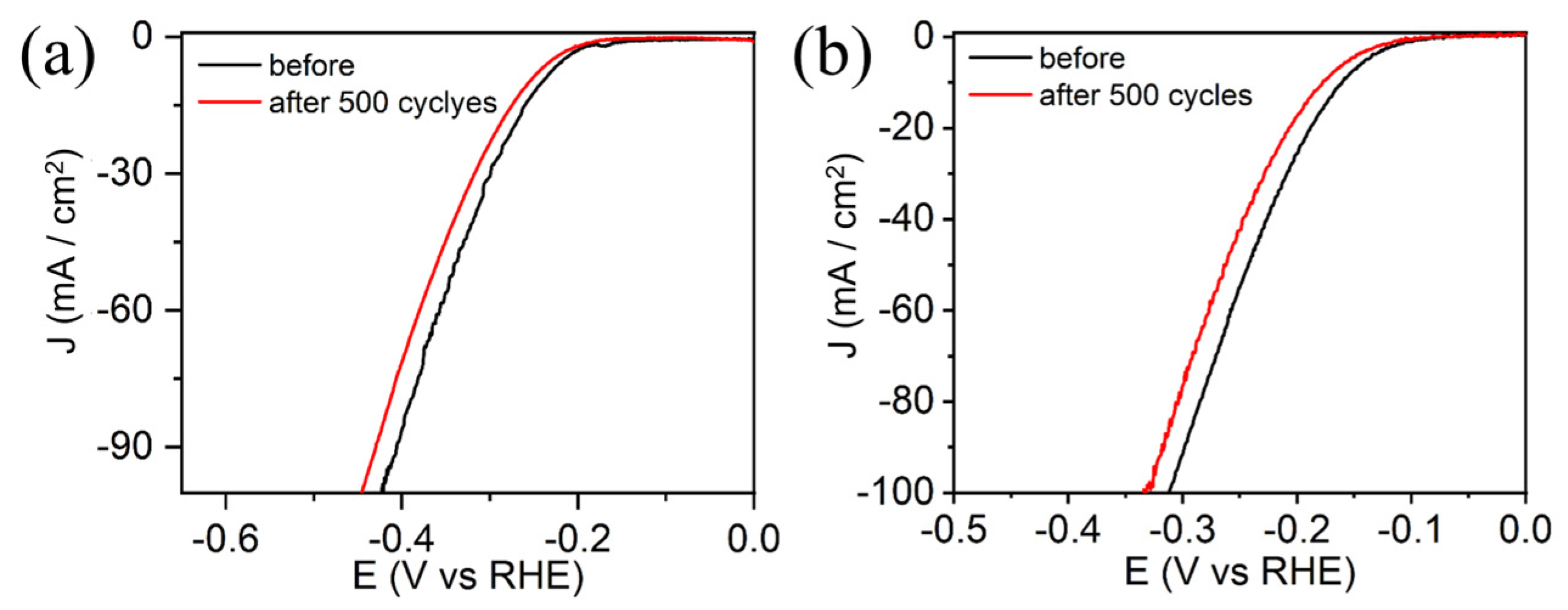
3.2. Electrocatalytic Activity of HER
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, F.; Guo, X.X.; Wang, Q.H.; Lu, S.W.; Wang, J.L.; Hu, Y.B.; Hao, G.Z.; Li, Q.L.; Yang, M.Q.; Jiang, W. A hybrid of MIL-53(Fe) and conductive sulfide as a synergistic electrocatalyst for the oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 14574–14582. [Google Scholar] [CrossRef]
- Fu, C.Y.; Hao, W.J.; Fan, J.L.; Zhang, Q.; Guo, Y.H.; Fan, J.C.; Chen, Z.L.; Li, G.S. Fabrication of Ultra-Durable and Flexible NiPx-Based Electrode toward High-Efficient Alkaline Seawater Splitting at Industrial Grade Current Density. Small 2023, 19, 2205689. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.L.; Gao, P.J.; Chen, H.; Tang, Z.H.; Li, J.; Xiu, H.X.; Yang, J.H. Graphited carbon black curled nanoribbons simultaneously boosted stability and electrocatalytic activity of 1T-MoS2/MoO3 toward hydrogen evolution. J. Alloys Compd. 2023, 949, 169831. [Google Scholar] [CrossRef]
- Kim, M.G.; Choi, Y.H. Electrocatalytic Properties of Co3O4 Prepared on Carbon Fibers by Thermal Metal-Organic Deposition for the Oxygen Evolution Reaction in Alkaline Water Electrolysis. Nanomaterials 2023, 13, 1021. [Google Scholar] [CrossRef] [PubMed]
- Salvo, D.; Mosconi, D.; Neyman, A.; Bar-Sadan, M.; Calvillo, L.; Granozzi, G.; Cattelan, M.; Agnoli, S. Nanoneedles of Mixed Transition Metal Phosphides as Bifunctional Catalysts for Electrocatalytic Water Splitting in Alkaline Media. Nanomaterials 2023, 13, 683. [Google Scholar] [CrossRef]
- Liang, R.K.; Fan, J.L.; Lei, F.J.; Li, P.; Fu, C.Y.; Lu, Z.K.; Hao, W.J. Fabrication of ultra-stable and high-efficient CoP-based electrode toward seawater splitting at industrial-grade current density. J. Colloid Interface Sci. 2023, 645, 227–240. [Google Scholar] [CrossRef]
- Cebollada, J.; Sebastian, D.; Lazaro, M.J.; Martinez-Huerta, M.V. Carbonized Polydopamine-Based Nanocomposites: The Effect of Transition Metals on the Oxygen Electrocatalytic Activity. Nanomaterials 2023, 13, 1549. [Google Scholar] [CrossRef]
- Zeng, X.S.; Tu, Z.X.; Yuan, Y.L.; Liao, L.L.; Xiao, C.C.; Wen, Y.F.; Xiong, K. Two-Dimensional Transition Metal-Hexaaminobenzene Monolayer Single-Atom Catalyst for Electrocatalytic Carbon Dioxide Reduction. Nanomaterials 2022, 12, 4005. [Google Scholar] [CrossRef]
- Zhang, X.J.; Ou-Yang, W.; Zhu, G.; Lu, T.; Pan, L.K. Shuttle-like carbon-coated FeP derived from metal-organic frameworks for lithium-ion batteries with superior rate capability and long-life cycling performance. Carbon 2019, 143, 116–124. [Google Scholar] [CrossRef]
- Yang, M.; Hu, W.H.; Li, M.X.; Cao, Y.N.; Dong, B.; Ma, Y.; Zhao, H.Y.; Wang, F.G.; Huang, J.E.; Chai, Y.M. Controlled high-density interface engineering of Fe3O4-FeS nanoarray for efficient hydrogen evolution. J. Energy Chem. 2022, 68, 96–103. [Google Scholar] [CrossRef]
- Liu, Y.F.; Li, Y.; Wu, Q.; Su, Z.; Wang, B.; Chen, Y.F.; Wang, S.F. Hollow CoP/FeP4 Heterostructural Nanorods Interwoven by CNT as a Highly Efficient Electrocatalyst for Oxygen Evolution Reactions. Nanomaterials 2021, 11, 1450. [Google Scholar] [CrossRef]
- Ma, F.X.; Xu, C.Y.; Lyu, F.C.; Song, B.; Sun, S.C.; Li, Y.Y.; Lu, J.; Zhen, L. Construction of FeP Hollow Nanoparticles Densely Encapsulated in Carbon Nanosheet Frameworks for Efficient and Durable Electrocatalytic Hydrogen Production. Adv. Sci. 2019, 6, 1801490. [Google Scholar] [CrossRef]
- Pan, X.; Wang, W.J.; Chen, Y.; Wen, Q.; Li, X.Q.; Lin, C.H.; Wang, J.H.; Xu, H.T.; Yang, L.Q.Y. Bio-electrocatalyst Fe3O4/Fe@C derived from MOF as a high-performance bioanode in single-chamber microbial fuel cell. Biochem. Eng. J. 2022, 187, 108611. [Google Scholar] [CrossRef]
- Ramachandra, S.K.; Nagaraju, D.H.; Marappa, S.; Kapse, S.; Thapa, R. Highly efficient catalysts of ruthenium clusters on Fe3O4/MWCNTs for the hydrogen evolution reaction. New J. Chem. 2022, 46, 7014–7023. [Google Scholar] [CrossRef]
- Saleh, M.R.; Thabet, S.M.; El-Gendy, R.A.; Saleh, M.; El-Bery, H.M. MIL-53 (Fe) for constructing hydrogenated Fe3O4@C@TiO2 double core-shell nanocrystals as superior bifunctional photocatalyst. J. Photochem. Photobiol. A Chem. 2022, 432, 114125. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, F.T.; Fan, R.Y.; Zhao, J.; Dong, B.; Wang, F.L.; Liu, H.J.; Yu, J.F.; Liu, C.G.; Chai, Y.M. F, P double-doped Fe3O4 with abundant defect sites for efficient hydrogen evolution at high current density. J. Mater. Chem. A 2021, 9, 15836–15845. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Shang, X.; Ren, H.; Chi, J.Q.; Fu, H.; Dong, B.; Liu, C.G.; Chai, Y.M. Modulation of Inverse Spinel Fe3O4 by Phosphorus Doping as an Industrially Promising Electrocatalyst for Hydrogen Evolution. Adv. Mater. 2019, 31, 1905107. [Google Scholar] [CrossRef]
- Xu, W.L.; Zhong, W.D.; Yang, C.F.; Zhao, R.; Wu, J.; Li, X.K.; Yang, N.J. Tailoring interfacial electron redistribution of Ni/Fe3O4 electrocatalysts for superior overall water splitting. J. Energy Chem. 2022, 73, 330–338. [Google Scholar] [CrossRef]
- Adamson, W.; Bo, X.; Li, Y.B.; Suryanto, B.H.R.; Chen, X.J.; Zhao, C. Co-Fe binary metal oxide electrocatalyst with synergistic interface structures for efficient overall water splitting. Catal. Today 2020, 351, 44–49. [Google Scholar] [CrossRef]
- Srinivas, K.; Lu, Y.J.; Chen, Y.F.; Zhang, W.L.; Yang, D.X. FeNi3-Fe3O4 Heterogeneous Nanoparticles Anchored on 2D MOF Nanosheets/1D CNT Matrix as Highly Efficient Bifunctional Electrocatalysts for Water Splitting. ACS Sustain. Chem. Eng. 2020, 8, 3820–3831. [Google Scholar] [CrossRef]
- Mirabella, F.; Müllner, M.; Touzalin, T.; Riva, M.; Jakub, Z.; Kraushofer, F.; Schmid, M.; Koper, M.T.M.; Parkinson, G.S.; Diebold, U. Ni-modified Fe3O4 (001) surface as a simple model system for understanding the oxygen evolution reaction. Electrochim. Acta 2021, 389, 138638. [Google Scholar] [CrossRef]
- Meng, S.C.; Sun, S.C.; Liu, Y.; Lu, Y.K.; Chen, M. Synergistic modulation of inverse spinel Fe3O4 by doping with chromium and nitrogen for efficient electrocatalytic water splitting. J. Colloid Interface Sci. 2022, 624, 433–442. [Google Scholar] [CrossRef]
- Liu, X.J.; Zhang, X.; Bo, M.L.; Li, L.; Tian, H.W.; Nie, Y.G.; Sun, Y.; Xu, S.Q.; Wang, Y.; Zheng, W.T.; et al. Coordination-Resolved Electron Spectrometrics. Chem. Rev. 2015, 115, 6746–6810. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.J.; Xu, X.T.; Wang, M.; Lu, T.; Sun, C.Q.; Pan, L.K. Synergistic conversion and removal of total Cr from aqueous solution by photocatalysis and capacitive deionization. Chem. Eng. J. 2018, 337, 398–404. [Google Scholar] [CrossRef]
- Qiang, T.T.; Chen, L.; Qin, X.T. Biomass-based 0D/3D N-CQD/MIL-53(Fe) photocatalyst for the simultaneous remediation of multiple hazardous pollutants in sewage. Catal. Sci. Technol. 2021, 11, 4931–4943. [Google Scholar] [CrossRef]
- Sun, C.Q. The BOLS-NEP theory reconciling the attributes of undercoordinated adatoms, defects, surfaces and nanostructures. Nano Mater. Sci. 2020, 2, 333–345. [Google Scholar] [CrossRef]
- Sun, C.Q. Perspective: Bonding and electronic origin of Au atomic-undercoordination-derivacy and nanoscale-size-dependency. Vacuum 2021, 186, 110061. [Google Scholar] [CrossRef]
- Peng, W.W.; Pan, X.R.; Liu, X.J.; Gao, Y.; Lu, T.; Li, J.B.; Xu, M.; Pan, L.K. A moisture self-regenerative, ultra-low temperature anti-freezing and self-adhesive polyvinyl alcohol/polyacrylamide/CaCl2/MXene ionotronics hydrogel for bionic skin strain sensor. J. Colloid Interface Sci. 2023, 634, 782–792. [Google Scholar] [CrossRef]
- Xu, L.M.; Ding, Z.B.; Chen, Y.Y.; Xu, X.T.; Liu, Y.; Li, J.B.; Lu, T.; Pan, L.K. Carbon nanotube bridged nickel hexacyanoferrate architecture for high-performance hybrid capacitive deionization. J. Colloid Interface Sci. 2023, 630, 372–381. [Google Scholar] [CrossRef]
- Zhang, S.H.; Wu, M.F.; Tang, T.T.; Xing, Q.J.; Peng, C.Q.; Li, F.; Liu, H.; Luo, X.B.; Zou, J.P.; Min, X.B.; et al. Mechanism investigation of anoxic Cr(VI) removal by nano zero-valent iron based on XPS analysis in time scale. Chem. Eng. J. 2018, 335, 945–953. [Google Scholar] [CrossRef]
- Liang, R.W.; Jing, F.F.; Shen, L.J.; Qin, N.; Wu, L. MIL-53(Fe) as a highly efficient bifunctional photocatalyst for the simultaneous reduction of Cr(VI) and oxidation of dyes. J. Hazard. Mater. 2015, 287, 364–372. [Google Scholar] [CrossRef]
- Meng, F.Y.; Ding, Z.B.; Chen, Z.Q.; Wang, K.; Liu, X.J.; Li, J.F.; Lu, T.; Xu, X.T.; Pan, L.K. N-doped carbon@Cu core-shell nanostructure with nearly full solar spectrum absorption and enhanced solar evaporation efficiency. J. Mater. Chem. A 2022, 10, 9575–9581. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Xu, X.T.; Jiao, Y.; Pan, L.K. In situ synthesis of ultrasmall NaTi2(PO4)3 nanocube decorated carbon nanofiber network enables ultrafast and superstable rocking-chair capacitive deionization. Chem. Eng. J. 2023, 463, 142394. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.L.; Zhao, C.Y.; Zhao, Q.; Niu, T.Q.; Pan, L.K.; Xu, P.W.; Zhang, F.Q.; Wu, W.D.; Ni, T.J. Facile synthesis of three-dimensional hollow porous carbon doped polymeric carbon nitride with highly efficient photocatalytic performance. Chem. Eng. J. 2022, 438, 135623. [Google Scholar] [CrossRef]
- Liu, X.H.; Xu, X.T.; Xuan, X.X.; Xia, W.; Feng, G.L.; Zhang, S.H.; Wu, Z.G.; Zhong, B.H.; Guo, X.D.; Xie, K.Y.; et al. Unlocking Enhanced Capacitive Deionization of NaTi2(PO4)3/Carbon Materials by the Yolk-Shell Design. J. Am. Chem. Soc. 2023, 145, 9242–9253. [Google Scholar] [CrossRef]
- Ding, Z.B.; Yu, H.Z.; Liu, X.J.; He, N.N.; Chen, X.H.; Li, H.B.; Wang, M.; Yamauchi, Y.; Xu, X.T.; Amin, M.A.; et al. Prussian blue analogue derived cobalt-nickel phosphide/carbon nanotube composite as electrocatalyst for efficient and stable hydrogen evolution reaction in wide-pH environment. J. Colloid Interface Sci. 2022, 616, 210–220. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, W.; Cao, R. Porous Materials as Highly Efficient Electrocatalysts for the Oxygen Evolution Reaction. ChemCatChem 2018, 10, 1206–1220. [Google Scholar] [CrossRef]
- Jin, M.T.; Zhang, X.; Niu, S.Z.; Wang, Q.; Huang, R.Q.; Ling, R.H.; Huang, J.Q.; Shi, R.; Amini, A.; Cheng, C. Strategies for Designing High-Performance Hydrogen Evolution Reaction Electrocatalysts at Large Current Densities above 1000 mA cm–2. ACS Nano. 2022, 16, 11577–11597. [Google Scholar] [CrossRef]
- Bertran-Serra, E.; Musheghyan-Avetisyan, A.; Chaitoglou, S.; Amade-Rovira, R.; Alshaikh, I.; Pantoja-Suarez, F.; Andujar-Bella, J.; Jawhari, T.; Perez-del-Pino, A.; Gyorgy, E. Temperature-modulated synthesis of vertically oriented atomic bilayer graphene nanowalls grown on stainless steel by inductively coupled plasma chemical vapour deposition. Appl. Surf. Sci. 2023, 610, 155530. [Google Scholar] [CrossRef]
- Chaitoglou, S.; Amade, R.; Bertran, E. Insights into the inherent properties of vertical graphene flakes towards hydrogen evolution reaction. Appl. Surf. Sci. 2022, 592, 153327. [Google Scholar] [CrossRef]
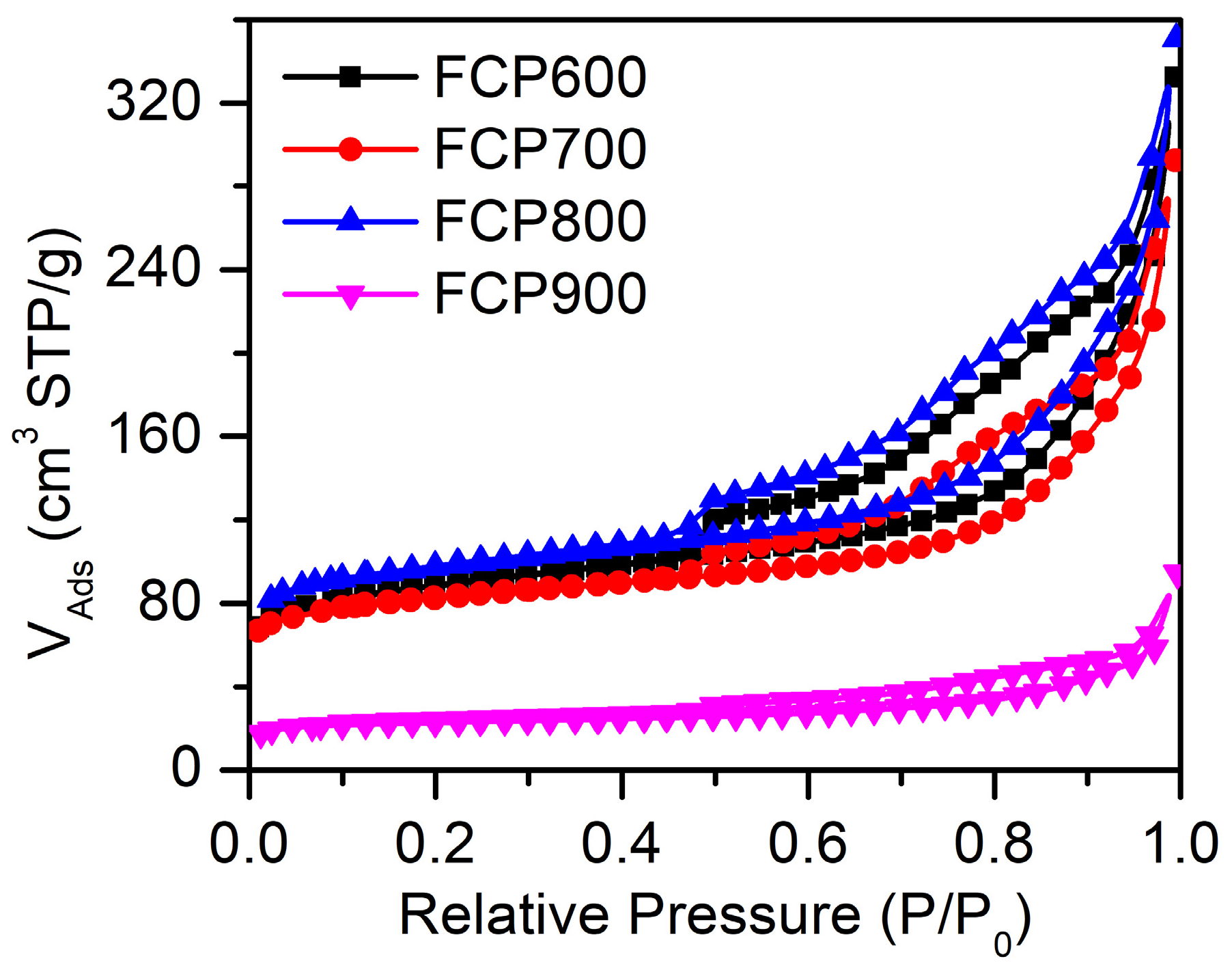
| Sample | Specific Surface Areas (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Diameters (nm) |
|---|---|---|---|
| FCP600 | 322.073 | 0.5141 | 3.911 |
| FCP700 | 302.940 | 0.4521 | 3.915 |
| FCP800 | 363.318 | 0.5427 | 3.913 |
| FCP900 | 87.306 | 0.146 | 3.911 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, M.; Li, P.; Liu, B.; Gong, Y.; Luo, C.; Yang, K.; Liu, X.; Fan, J.; Xue, Y. Interface Coordination Engineering of P-Fe3O4/Fe@C Derived from an Iron-Based Metal Organic Framework for pH-Universal Water Splitting. Nanomaterials 2023, 13, 1909. https://doi.org/10.3390/nano13131909
Fan M, Li P, Liu B, Gong Y, Luo C, Yang K, Liu X, Fan J, Xue Y. Interface Coordination Engineering of P-Fe3O4/Fe@C Derived from an Iron-Based Metal Organic Framework for pH-Universal Water Splitting. Nanomaterials. 2023; 13(13):1909. https://doi.org/10.3390/nano13131909
Chicago/Turabian StyleFan, Minmin, Peixiao Li, Baibai Liu, Yun Gong, Chengling Luo, Kun Yang, Xinjuan Liu, Jinchen Fan, and Yuhua Xue. 2023. "Interface Coordination Engineering of P-Fe3O4/Fe@C Derived from an Iron-Based Metal Organic Framework for pH-Universal Water Splitting" Nanomaterials 13, no. 13: 1909. https://doi.org/10.3390/nano13131909
APA StyleFan, M., Li, P., Liu, B., Gong, Y., Luo, C., Yang, K., Liu, X., Fan, J., & Xue, Y. (2023). Interface Coordination Engineering of P-Fe3O4/Fe@C Derived from an Iron-Based Metal Organic Framework for pH-Universal Water Splitting. Nanomaterials, 13(13), 1909. https://doi.org/10.3390/nano13131909







