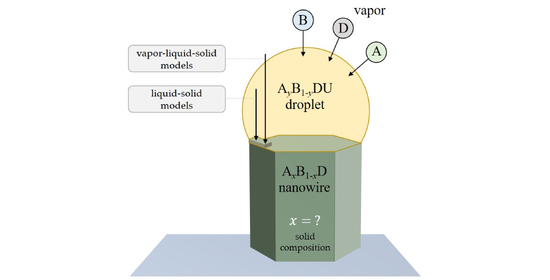
An Overview of Modeling Approaches for Compositional Control in III–V Ternary Nanowires
Abstract
1. Introduction
2. Experimental Works
- −
- Material system which defines the difference in the chemical potentials for pure elements and the shape of liquid–solid composition dependence in the case of VLS nanowires. For example, the composition of VLS InxGa1−xAs nanowires cannot be understood without accounting for the predominance of liquid in in the catalyst droplet [68].
- −
- Growth method and equipment that determine the transfer of the precursors (or atoms).
- −
- −
- −
- Surface temperature during growth [46,76,77] is one of the most complex parameters because it simultaneously influences the pyrolysis efficiencies in VPE techniques [45], surface diffusion for VS nanowires [46], binary and ternary interactions in the droplet for VLS nanowires [78], evaporation rates from the substrate surface or droplets [70], and attachment and detachment rates of a ternary island.
- −
- The flux ratio of A to B atoms in vapor is the main control parameter that influences the composition of a ternary nanowire. Higher vapor flux of one of the elements is expected to yield its higher content in solids. The flux ratio influences the nanowire growth kinetics, shadowing effect [79], and elementary processes, such as the direct impingement, diffusion from the substrate and nanowire sidewalls to its top, and evaporation [70]. In the case of growth on the reflecting masked substrates such as SiOx/Si, the situation becomes even more complex. In the initial growth, a nanowire ensemble consumes only a part of the reflected flux [71,72,73]. Long enough nanowires consume the entire group III fluxes sent from the vapor. However, the saturation lengths may be different for A and B species and depend on the A/B flux ratio.
- −
- The total III/V flux ratio may enhance or suppress the incorporation of one of the elements (A or B) into solid nanowires even at a fixed A/B ratio. For example, the content of GaSb in InxGa1−xSb nanowires decreases with an increase in the TMSb molar fraction [80].
- −
- The type of growth catalyst for VLS NWs generally influences the binary supersaturation values [78,81]. Furthermore, the composition of different growth constituencies in liquid is generally different from their vapor contents, particularly for highly volatile group V molecules, such as As2, P2, or N2. Au remains one of the most common catalysts for VLS NW growth [82,83]. However, it might lead to unwanted nanowire contamination [84,85]. This issue is safely avoided in self-catalyzed VLS growth [86,87], where the foreign Au catalyst is replaced by one of the nanowire constituents (a group III element, such as Ga or In). This growth technique is very promising for the fine tuning of the nanowire morphology by changing the droplet volume under a varying III/V flux ratio, radius self-equilibration effect, sharpening the nanowire tips, etc. [15,88,89].
- −
- Group V concentration in the catalyst droplets can be changed by varying the group V flux or III/V flux ratio. Unfortunately, its typical values (on the order of 1%) are lower than the detection limit of any of the characterization techniques, including energy-dispersive X-ray spectroscopy. On the other hand, the group V concentration is known to have a tremendous effect on the supersaturation [81,90].
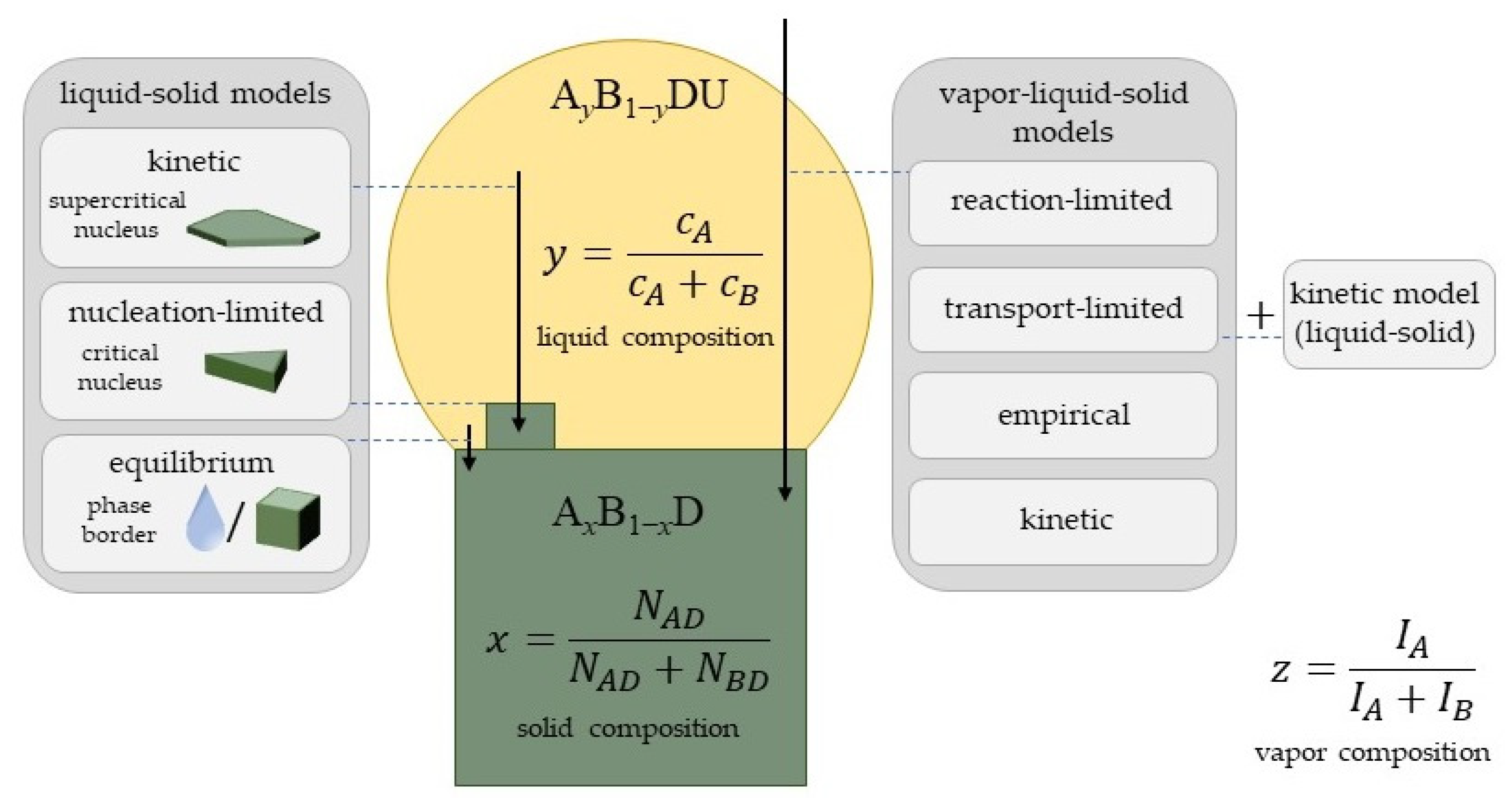
3. General Remarks and Definitions
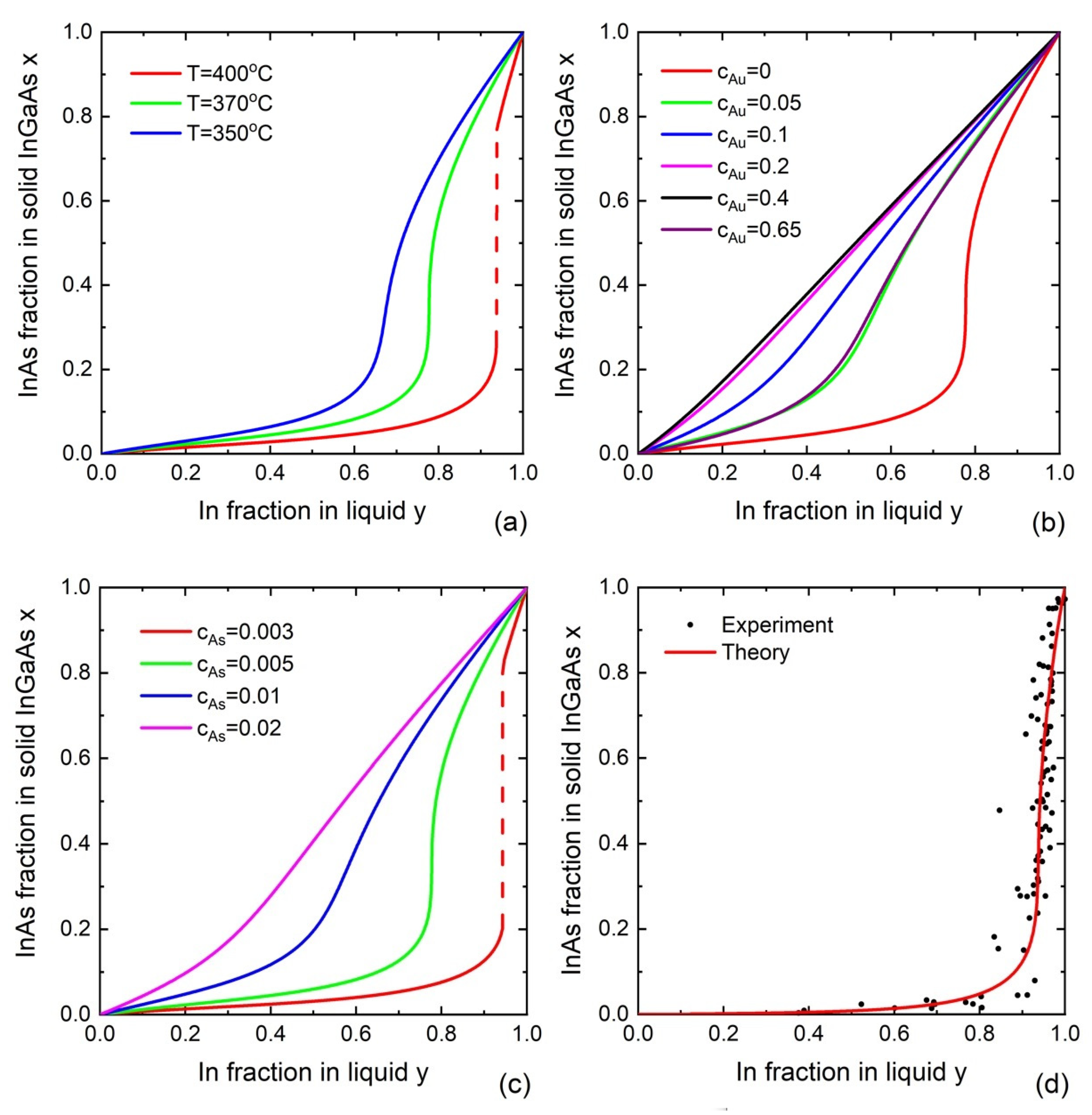
4. Liquid–Solid Incorporation Models
4.1. Equilibrium Models
4.2. Nucleation-Limited Model
4.3. Kinetic Models for Liquid–Solid Incorporation
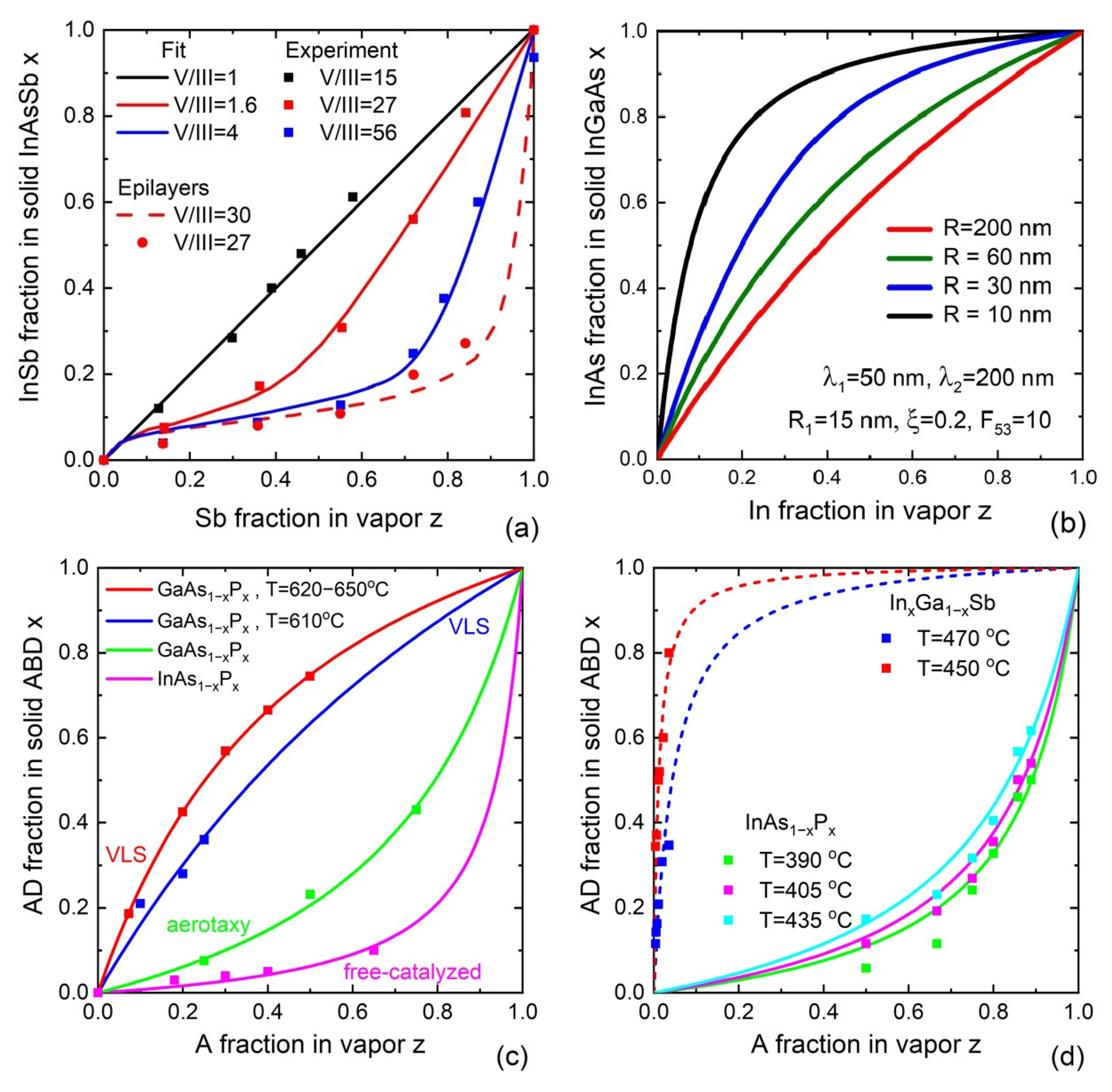
5. Vapor–Solid Incorporation Models
5.1. Reaction-Limited Models
5.2. Transport-Limited Models
5.3. Empirical Models
5.4. Kinetic Model for Vapor–Solid Growth
6. Model Comparison for VLS Ternary Nanowires
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Leong, T.G.; Zarafshar, A.M.; Gracias, D.H. Three-dimensional fabrication at small size scales. Small 2010, 6, 792–806. [Google Scholar] [CrossRef] [PubMed]
- Gates, B.D.; Xu, Q.; Stewart, M.; Ryan, D.; Willson, C.G.; Whitesides, G.M. New approaches to nanofabrication: Molding, printing, and other techniques. Chem. Rev. 2005, 105, 1171–1196. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, P.C.; Fontcuberta i Morral, A. Semiconductor nanowires: To grow or not to grow? Mater. Today Nano 2020, 9, 100058. [Google Scholar] [CrossRef]
- Kolasinski, K. Catalytic growth of nanowires: Vapor–liquid–solid, vapor–solid–solid, solution–liquid–solid and solid–liquid–solid growth. Curr. Opin. Solid State Mater. Sci. 2006, 10, 182–191. [Google Scholar] [CrossRef]
- Wang, N.; Cai, Y.; Zhang, R.Q. Growth of nanowires. Mater. Sci. Eng. R Rep. 2008, 60, 1–51. [Google Scholar] [CrossRef]
- Johansson, J.; Dick, K.A. Recent advances in semiconductor nanowire heterostructures. CrystEngComm 2011, 13, 7175. [Google Scholar] [CrossRef]
- Gudiksen, M.S.; Lauhon, L.J.; Wang, J.; Smith, D.C.; Lieber, C.M. Growth of nanowire superlattice structures for nanoscale photonics and electronics. Nature 2002, 415, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.K.; Zhang, S.; Lauhon, L.J. Nanowire Heterostructures. Annu. Rev. Mater. Res. 2013, 43, 451–479. [Google Scholar] [CrossRef]
- Lauhon, L.J.; Gudiksen, M.S.; Wang, D.; Lieber, C.M. Epitaxial core-shell and core-multishell nanowire heterostructures. Nature 2002, 420, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Barrigon, E.; Heurlin, M.; Bi, Z.; Monemar, B.; Samuelson, L. Synthesis and Applications of III-V Nanowires. Chem. Rev. 2019, 119, 9170–9220. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, J.; Aagesen, M.; Liu, H. III–V nanowires and nanowire optoelectronic devices. J. Phys. D Appl. Phys. 2015, 48, 463001. [Google Scholar] [CrossRef]
- Arjmand, T.; Legallais, M.; Nguyen, T.T.T.; Serre, P.; Vallejo-Perez, M.; Morisot, F.; Salem, B.; Ternon, C. Functional Devices from Bottom-Up Silicon Nanowires: A Review. Nanomaterials 2022, 12, 1043. [Google Scholar] [CrossRef] [PubMed]
- Vukajlovic-Plestina, J.; Kim, W.; Dubrovski, V.G.; Tutuncuoglu, G.; Lagier, M.; Potts, H.; Friedl, M.; Fontcuberta, I.M.A. Engineering the Size Distributions of Ordered GaAs Nanowires on Silicon. Nano Lett. 2017, 17, 4101–4108. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, E.S.; Hakkarainen, T.V.; Guina, M.D.; Dubrovskii, V.G. Sub-Poissonian Narrowing of Length Distributions Realized in Ga-Catalyzed GaAs Nanowires. Nano Lett. 2017, 17, 5350–5355. [Google Scholar] [CrossRef]
- Dubrovskii, V.G.; Xu, T.; Alvarez, A.D.; Plissard, S.R.; Caroff, P.; Glas, F.; Grandidier, B. Self-Equilibration of the Diameter of Ga-Catalyzed GaAs Nanowires. Nano Lett. 2015, 15, 5580–5584. [Google Scholar] [CrossRef]
- Wu, J.; Borg, B.M.; Jacobsson, D.; Dick, K.A.; Wernersson, L.-E. Control of composition and morphology in InGaAs nanowires grown by metalorganic vapor phase epitaxy. J. Cryst. Growth 2013, 383, 158–165. [Google Scholar] [CrossRef]
- Yuan, X.; Pan, D.; Zhou, Y.; Zhang, X.; Peng, K.; Zhao, B.; Deng, M.; He, J.; Tan, H.H.; Jagadish, C. Selective area epitaxy of III–V nanostructure arrays and networks: Growth, applications, and future directions. Appl. Phys. Rev. 2021, 8, 021302. [Google Scholar] [CrossRef]
- Dubrovskii, V.G. Nucleation Theory and Growth of Nanostructures, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2014; p. 601. [Google Scholar]
- Caroff, P.; Dick, K.A.; Johansson, J.; Messing, M.E.; Deppert, K.; Samuelson, L. Controlled polytypic and twin-plane superlattices in iii-v nanowires. Nat. Nanotechnol. 2009, 4, 50–55. [Google Scholar] [CrossRef]
- Ameruddin, A.S.; Fonseka, H.A.; Caroff, P.; Wong-Leung, J.; Op het Veld, R.L.; Boland, J.L.; Johnston, M.B.; Tan, H.H.; Jagadish, C. In(x)Ga(1-x)As nanowires with uniform composition, pure wurtzite crystal phase and taper-free morphology. Nanotechnology 2015, 26, 205604. [Google Scholar] [CrossRef]
- Priante, G.; Glas, F.; Patriarche, G.; Pantzas, K.; Oehler, F.; Harmand, J.-C. Sharpening the Interfaces of Axial Heterostructures in Self-Catalyzed AlGaAs Nanowires: Experiment and Theory. Nano Lett. 2016, 16, 1917–1924. [Google Scholar] [CrossRef]
- Wallentin, J.; Borgström, M.T. Doping of semiconductor nanowires. J. Mater. Res. 2011, 26, 2142–2156. [Google Scholar] [CrossRef]
- Wagner, R.S.; Ellis, W.C. Vapor-Liquid-Solid Mechanism of Single Crystal Growth. Appl. Phys. Lett. 1964, 4, 89–90. [Google Scholar] [CrossRef]
- Givargizov, E.I. Highly Anisotropic Crystals, 1st ed.; Springer: Dordrecht, The Netherlands, 1987; p. 394. [Google Scholar]
- Givargizov, E.I. Fundamental aspects of VLS growth. J. Cryst. Growth 1975, 31, 20–30. [Google Scholar] [CrossRef]
- Sakaki, H. Scattering Suppression and High-Mobility Effect of Size-Quantized Electrons in Ultrafine Semiconductor Wire Structures. Jpn. J. Appl. Phys. 1980, 19, L735–L738. [Google Scholar] [CrossRef]
- Lieber, C.M. Nanoscale Science and Technology: Building a Big Future from Small Things. MRS Bull. 2011, 28, 486–491. [Google Scholar] [CrossRef]
- Samuelson, L. Self-forming nanoscale devices. Mater. Today 2003, 6, 22–31. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-Dimensional Nanostructures: Synthesis, Characterization, and Applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Johnson, R.; Watkinson, A.; Mabe, M. The STM Report: An Overview of Scientific and Scholarly Journal Publishing; STM: The Hague, The Netherlands, 2018. [Google Scholar]
- Wallentin, J.; Anttu, N.; Asoli, D.; Huffman, M.; Aberg, I.; Magnusson, M.H.; Siefer, G.; Fuss-Kailuweit, P.; Dimroth, F.; Witzigmann, B.; et al. InP nanowire array solar cells achieving 13.8% efficiency by exceeding the ray optics limit. Science 2013, 339, 1057–1060. [Google Scholar] [CrossRef]
- Kayes, B.M.; Atwater, H.A.; Lewis, N.S. Comparison of the device physics principles of planar and radial p-n junction nanorod solar cells. J. Appl. Phys. 2005, 97, 114302. [Google Scholar] [CrossRef]
- Karimi, M.; Jain, V.; Heurlin, M.; Nowzari, A.; Hussain, L.; Lindgren, D.; Stehr, J.E.; Buyanova, I.A.; Gustafsson, A.; Samuelson, L.; et al. Room-temperature InP/InAsP Quantum Discs-in-Nanowire Infrared Photodetectors. Nano Lett. 2017, 17, 3356–3362. [Google Scholar] [CrossRef]
- Patolsky, F.; Zheng, G.; Hayden, O.; Lakadamyali, M.; Zhuang, X.; Lieber, C.M. Electrical detection of single viruses. Proc. Natl. Acad. Sci. USA 2004, 101, 14017–14022. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Lu, W.; Jin, S.; Lieber, C.M. Synthesis and Fabrication of High-Performance n-Type Silicon Nanowire Transistors. Adv. Mater. 2004, 16, 1890–1893. [Google Scholar] [CrossRef]
- Björk, M.T.; Ohlsson, B.J.; Thelander, C.; Persson, A.I.; Deppert, K.; Wallenberg, L.R.; Samuelson, L. Nanowire resonant tunneling diodes. Appl. Phys. Lett. 2002, 81, 4458–4460. [Google Scholar] [CrossRef]
- Huang, M.H.; Mao, S.; Feick, H.; Yan, H.; Wu, Y.; Kind, H.; Weber, E.; Russo, R.; Yang, P. Room-temperature ultraviolet nanowire nanolasers. Science 2001, 292, 1897–1899. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, T.; Zhu, L.; Li, W.; Wang, S.; Hou, X.; Mao, X.; Wang, Z.L. Piezoelectric nanogenerators with high performance against harsh conditions based on tunable N doped 4H-SiC nanowire arrays. Nano Energy 2021, 83, 105826. [Google Scholar] [CrossRef]
- Dick, K.A. A review of nanowire growth promoted by alloys and non-alloying elements with emphasis on Au-assisted III–V nanowires. Prog. Cryst. Growth Charact. Mater. 2008, 54, 138–173. [Google Scholar] [CrossRef]
- Ning, C.-Z.; Dou, L.; Yang, P. Bandgap engineering in semiconductor alloy nanomaterials with widely tunable compositions. Nat. Rev. Mater. 2017, 2, 17070. [Google Scholar] [CrossRef]
- Vurgaftman, I.; Meyer, J.R.; Ram-Mohan, L.R. Band parameters for III–V compound semiconductors and their alloys. J. Appl. Phys. 2001, 89, 5815–5875. [Google Scholar] [CrossRef]
- Hiruma, K.; Yazawa, M.; Katsuyama, T.; Ogawa, K.; Haraguchi, K.; Koguchi, M.; Kakibayashi, H. Growth and optical properties of nanometer-scale GaAs and InAs whiskers. J. Appl. Phys. 1995, 77, 447–462. [Google Scholar] [CrossRef]
- Duan, X.; Lieber, C.M. Laser-Assisted Catalytic Growth of Single Crystal GaN Nanowires. J. Am. Chem. Soc. 1999, 122, 188–189. [Google Scholar] [CrossRef]
- Borg, B.M.; Dick, K.A.; Eymery, J.; Wernersson, L.-E. Enhanced Sb incorporation in InAsSb nanowires grown by metalorganic vapor phase epitaxy. Appl. Phys. Lett. 2011, 98, 113104. [Google Scholar] [CrossRef]
- Stringfellow, G.B. Organometallic Vapor-Phase Epitaxy, 2nd ed.; Academic Press: Cambridge, MA, USA, 1999; p. 572. [Google Scholar]
- Ameruddin, A.S.; Caroff, P.; Tan, H.H.; Jagadish, C.; Dubrovskii, V.G. Understanding the growth and composition evolution of gold-seeded ternary InGaAs nanowires. Nanoscale 2015, 7, 16266–16272. [Google Scholar] [CrossRef] [PubMed]
- Tchernycheva, M.; Travers, L.; Patriarche, G.; Glas, F.; Harmand, J.-C.; Cirlin, G.E.; Dubrovskii, V.G. Au-assisted molecular beam epitaxy of InAs nanowires: Growth and theoretical analysis. J. Appl. Phys. 2007, 102, 094313. [Google Scholar] [CrossRef]
- Ghasemi, M.; Leshchenko, E.D.; Johansson, J. Assembling your nanowire: An overview of composition tuning in ternary III-V nanowires. Nanotechnology 2021, 32, 072001. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, J.; Yang, W.; Zhang, X.; Jiang, X.; Bando, Y. Semiconductor Solid-Solution Nanostructures: Synthesis, Property Tailoring, and Applications. Small 2017, 13, 1701998. [Google Scholar] [CrossRef] [PubMed]
- Anabestani, H.; Shazzad, R.; Fattah, M.F.A.; Therrien, J.; Ban, D. Review on GaAsSb nanowire potentials for future 1D heterostructures: Properties and applications. Mater. Today Commun. 2021, 28, 102542. [Google Scholar] [CrossRef]
- Jacobsson, D.; Persson, J.M.; Kriegner, D.; Etzelstorfer, T.; Wallentin, J.; Wagner, J.B.; Stangl, J.; Samuelson, L.; Deppert, K.; Borgstrom, M.T. Particle-assisted Ga(x)In(1-x)P nanowire growth for designed bandgap structures. Nanotechnology 2012, 23, 245601. [Google Scholar] [CrossRef]
- Wu, Z.H.; Sun, M.; Mei, X.Y.; Ruda, H.E. Growth and photoluminescence characteristics of AlGaAs nanowires. Appl. Phys. Lett. 2004, 85, 657–659. [Google Scholar] [CrossRef]
- Kuykendall, T.; Ulrich, P.; Aloni, S.; Yang, P. Complete composition tunability of InGaN nanowires using a combinatorial approach. Nat. Mater. 2007, 6, 951–956. [Google Scholar] [CrossRef]
- Russell, H.B.; Andriotis, A.N.; Menon, M.; Jasinski, J.B.; Martinez-Garcia, A.; Sunkara, M.K. Direct Band Gap Gallium Antimony Phosphide (GaSbxP(1-x)) Alloys. Sci. Rep. 2016, 6, 20822. [Google Scholar] [CrossRef]
- Ngo, C.; Zhou, H.; Mecklenburg, M.; Pozuelo, M.; Regan, B.C.; Xiao, Q.F.; Shenoy, V.B.; Hicks, R.F.; Kodambaka, S. Effect of precursor flux on compositional evolution in InP1−xSbx nanowires grown via self-catalyzed vapor–liquid–solid process. J. Cryst. Growth 2011, 336, 14–19. [Google Scholar] [CrossRef]
- Gagliano, L.; Kruijsse, M.; Schefold, J.D.D.; Belabbes, A.; Verheijen, M.A.; Meuret, S.; Koelling, S.; Polman, A.; Bechstedt, F.; Haverkort, J.E.M.; et al. Efficient Green Emission from Wurtzite AlxIn1−xP Nanowires. Nano Lett. 2018, 18, 3543–3549. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Heurlin, M.; Tsopanidis, S.; Pistol, M.E.; Borgstrom, M.T. Growth of wurtzite AlxGa1−xP nanowire shells and characterization by Raman spectroscopy. Nanotechnology 2017, 28, 035706. [Google Scholar] [CrossRef] [PubMed]
- DeHoff, R. Thermodynamics in Materials Science, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006; p. 620. [Google Scholar]
- Zhuang, Q.D.; Anyebe, E.A.; Chen, R.; Liu, H.; Sanchez, A.M.; Rajpalke, M.K.; Veal, T.D.; Wang, Z.M.; Huang, Y.Z.; Sun, H.D. Sb-induced phase control of InAsSb nanowires grown by molecular beam epitaxy. Nano Lett. 2015, 15, 1109–1116. [Google Scholar] [CrossRef]
- Li, L.; Pan, D.; Xue, Y.; Wang, X.; Lin, M.; Su, D.; Zhang, Q.; Yu, X.; So, H.; Wei, D.; et al. Near Full-Composition-Range High-Quality GaAs(1-x)Sb(x) Nanowires Grown by Molecular-Beam Epitaxy. Nano Lett. 2017, 17, 622–630. [Google Scholar] [CrossRef]
- Ji, X.; Yang, X.; Du, W.; Pan, H.; Yang, T. Selective-Area MOCVD Growth and Carrier-Transport-Type Control of InAs(Sb)/GaSb Core-Shell Nanowires. Nano Lett. 2016, 16, 7580–7587. [Google Scholar] [CrossRef]
- Glas, F.; Harmand, J.C.; Patriarche, G. Why does wurtzite form in nanowires of III-V zinc blende semiconductors? Phys. Rev. Lett. 2007, 99, 146101. [Google Scholar] [CrossRef]
- Johansson, J.; Leshchenko, E.D. Zinc blende and wurtzite crystal structure formation in gold catalyzed InGaAs nanowires. J. Cryst. Growth 2019, 509, 118–123. [Google Scholar] [CrossRef]
- Bauer, J.; Gottschalch, V.; Paetzelt, H.; Wagner, G.; Fuhrmann, B.; Leipner, H.S. MOVPE growth and real structure of vertical-aligned GaAs nanowires. J. Cryst. Growth 2007, 298, 625–630. [Google Scholar] [CrossRef]
- Ikejiri, K.; Noborisaka, J.; Hara, S.; Motohisa, J.; Fukui, T. Mechanism of catalyst-free growth of GaAs nanowires by selective area MOVPE. J. Cryst. Growth 2007, 298, 616–619. [Google Scholar] [CrossRef]
- Gil, E.; André, Y.; Cadoret, R.; Trassoudaine, A. Hydride Vapor Phase Epitaxy for Current III–V and Nitride Semiconductor Compound Issues. In Handbook of Crystal Growth; Elsevier: Amsterdam, The Netherlands, 2015; pp. 51–93. [Google Scholar]
- Wu, Z.H.; Mei, X.Y.; Kim, D.; Blumin, M.; Ruda, H.E. Growth of Au-catalyzed ordered GaAs nanowire arrays by molecular-beam epitaxy. Appl. Phys. Lett. 2002, 81, 5177–5179. [Google Scholar] [CrossRef]
- Sjokvist, R.; Jacobsson, D.; Tornberg, M.; Wallenberg, R.; Leshchenko, E.D.; Johansson, J.; Dick, K.A. Compositional Correlation between the Nanoparticle and the Growing Au-Assisted InxGa1−xAs Nanowire. J. Phys. Chem. Lett. 2021, 12, 7590–7595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sanchez, A.M.; Sun, Y.; Wu, J.; Aagesen, M.; Huo, S.; Kim, D.; Jurczak, P.; Xu, X.; Liu, H. Influence of Droplet Size on the Growth of Self-Catalyzed Ternary GaAsP Nanowires. Nano Lett. 2016, 16, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Glas, F.; Ramdani, M.R.; Patriarche, G.; Harmand, J.-C. Predictive modeling of self-catalyzed III-V nanowire growth. Phys. Rev. B 2013, 88, 195304. [Google Scholar] [CrossRef]
- Dubrovskii, V.G.; Leshchenko, E.D. Modeling the Radial Growth of Self-Catalyzed III-V Nanowires. Nanomaterials 2022, 12, 1698. [Google Scholar] [CrossRef]
- Dubrovskii, V.G. Theory of MBE Growth of Nanowires on Reflecting Substrates. Nanomaterials 2022, 12, 253. [Google Scholar] [CrossRef]
- Oehler, F.; Cattoni, A.; Scaccabarozzi, A.; Patriarche, G.; Glas, F.; Harmand, J.C. Measuring and Modeling the Growth Dynamics of Self-Catalyzed GaP Nanowire Arrays. Nano Lett. 2018, 18, 701–708. [Google Scholar] [CrossRef]
- Ren, D.; Huh, J.; Dheeraj, D.L.; Weman, H.; Fimland, B.-O. Influence of pitch on the morphology and luminescence properties of self-catalyzed GaAsSb nanowire arrays. Appl. Phys. Lett. 2016, 109, 243102. [Google Scholar] [CrossRef]
- Leshchenko, E.D.; Kuyanov, P.; LaPierre, R.R.; Dubrovskii, V.G. Tuning the morphology of self-assisted GaP nanowires. Nanotechnology 2018, 29, 225603. [Google Scholar] [CrossRef]
- Andrade, R.R.; Malachias, A.; Kellerman, G.; Negreiros, F.R.; Santos, N.M.; Sobolev, N.A.; Moreira, M.V.B.; de Oliveira, A.G.; González, J.C. Experimental Evidence and Modified Growth Model of Alloying in InxGa1–xAs Nanowires. J. Phys. Chem. C 2012, 116, 24777–24783. [Google Scholar] [CrossRef]
- Mandl, B.; Keplinger, M.; Messing, M.E.; Kriegner, D.; Wallenberg, R.; Samuelson, L.; Bauer, G.; Stangl, J.; Holy, V.; Deppert, K. Self-Seeded Axio-Radial InAs-InAs(1-x)P(x) Nanowire Heterostructures beyond “Common” VLS Growth. Nano Lett. 2018, 18, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Leshchenko, E.D.; Ghasemi, M.; Dubrovskii, V.G.; Johansson, J. Nucleation-limited composition of ternary III–V nanowires forming from quaternary gold based liquid alloys. CrystEngComm 2018, 20, 1649–1655. [Google Scholar] [CrossRef]
- Kelrich, A.; Calahorra, Y.; Greenberg, Y.; Gavrilov, A.; Cohen, S.; Ritter, D. Shadowing and mask opening effects during selective-area vapor-liquid-solid growth of InP nanowires by metalorganic molecular beam epitaxy. Nanotechnology 2013, 24, 475302. [Google Scholar] [CrossRef] [PubMed]
- Gorji Ghalamestani, S.; Ek, M.; Ganjipour, B.; Thelander, C.; Johansson, J.; Caroff, P.; Dick, K.A. Demonstration of defect-free and composition tunable GaxIn(1)-xSb nanowires. Nano Lett. 2012, 12, 4914–4919. [Google Scholar] [CrossRef]
- Grecenkov, J.; Dubrovskii, V.G.; Ghasemi, M.; Johansson, J. Quaternary Chemical Potentials for Gold-Catalyzed Growth of Ternary InGaAs Nanowires. Cryst. Growth Des. 2016, 16, 4526–4530. [Google Scholar] [CrossRef]
- Ihn, S.-G.; Song, J.-I.; Kim, Y.-H.; Lee, J.Y. GaAs nanowires on Si substrates grown by a solid source molecular beam epitaxy. Appl. Phys. Lett. 2006, 89, 053106. [Google Scholar] [CrossRef]
- Messing, M.E.; Hillerich, K.; Bolinsson, J.; Storm, K.; Johansson, J.; Dick, K.A.; Deppert, K. A comparative study of the effect of gold seed particle preparation method on nanowire growth. Nano Res. 2010, 3, 506–519. [Google Scholar] [CrossRef]
- Krogstrup, P.; Popovitz-Biro, R.; Johnson, E.; Madsen, M.H.; Nygard, J.; Shtrikman, H. Structural phase control in self-catalyzed growth of GaAs nanowires on silicon (111). Nano Lett. 2010, 10, 4475–4482. [Google Scholar] [CrossRef]
- Bemski, G. Recombination Properties of Gold in Silicon. Phys. Rev. 1958, 111, 1515–1518. [Google Scholar] [CrossRef]
- Jabeen, F.; Grillo, V.; Rubini, S.; Martelli, F. Self-catalyzed growth of GaAs nanowires on cleaved Si by molecular beam epitaxy. Nanotechnology 2008, 19, 275711. [Google Scholar] [CrossRef]
- Colombo, C.; Spirkoska, D.; Frimmer, M.; Abstreiter, G.; Fontcuberta i Morral, A. Ga-assisted catalyst-free growth mechanism of GaAs nanowires by molecular beam epitaxy. Phys. Rev. B 2008, 77, 155326. [Google Scholar] [CrossRef]
- Tersoff, J. Stable Self-Catalyzed Growth of III-V Nanowires. Nano Lett. 2015, 15, 6609–6613. [Google Scholar] [CrossRef] [PubMed]
- Dubrovskii, V.G. Kinetic narrowing of size distribution. Phys. Rev. B 2016, 93, 174203. [Google Scholar] [CrossRef]
- Dubrovskii, V.G. Influence of the group V element on the chemical potential and crystal structure of Au-catalyzed III-V nanowires. Appl. Phys. Lett. 2014, 104, 053110. [Google Scholar] [CrossRef]
- Sosso, G.C.; Chen, J.; Cox, S.J.; Fitzner, M.; Pedevilla, P.; Zen, A.; Michaelides, A. Crystal Nucleation in Liquids: Open Questions and Future Challenges in Molecular Dynamics Simulations. Chem. Rev. 2016, 116, 7078–7116. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, A.; Napari, I. Breakdown of the Capillarity Approximation in Binary Nucleation: A Density Functional Study. J. Phys. Chem. B 2001, 105, 11678–11682. [Google Scholar] [CrossRef]
- Lutsko, J.F.; Duran-Olivencia, M.A. Classical nucleation theory from a dynamical approach to nucleation. J. Chem. Phys. 2013, 138, 244908. [Google Scholar] [CrossRef]
- Kashchiev, D. Toward a better description of the nucleation rate of crystals and crystalline monolayers. J. Chem. Phys. 2008, 129, 164701. [Google Scholar] [CrossRef]
- Kashchiev, D. Nucleation Basic Theory with Applications, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2000; p. 544. [Google Scholar]
- Cramer, C.J. Essentials of Computational Chemistry: Theories and Models, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004; p. 624. [Google Scholar]
- Sholl, D.; Steckel, J.A. Density Functional Theory: A Practical Introduction, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2023; p. 224. [Google Scholar]
- Atkins, P.; De Paula, J.; Friedman, R. Physical Chemistry: Quanta, Matter, and Change, 2nd ed.; Oxford University Press: Oxford, UK, 2013; p. 1008. [Google Scholar]
- Sakong, S.; Du, Y.A.; Kratzer, P. Atomistic modeling of the Au droplet–GaAs interface for size-selective nanowire growth. Phys. Rev. B 2013, 88, 155309. [Google Scholar] [CrossRef]
- Kim, W.; Dubrovskii, V.G.; Vukajlovic-Plestina, J.; Tutuncuoglu, G.; Francaviglia, L.; Guniat, L.; Potts, H.; Friedl, M.; Leran, J.B.; Fontcuberta, I.M.A. Bistability of Contact Angle and Its Role in Achieving Quantum-Thin Self-Assisted GaAs nanowires. Nano Lett. 2018, 18, 49–57. [Google Scholar] [CrossRef]
- Kratzer, P.; Sakong, S.; Pankoke, V. Catalytic role of gold nanoparticle in GaAs nanowire growth: A density functional theory study. Nano Lett. 2012, 12, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Glas, F. Vapor fluxes on the apical droplet during nanowire growth by molecular beam epitaxy. Phys. Status Solidi B 2010, 247, 254–258. [Google Scholar] [CrossRef]
- Hansen, P.L.; Wagner, J.B.; Helveg, S.; Rostrup-Nielsen, J.R.; Clausen, B.S.; Topsoe, H. Atom-resolved imaging of dynamic shape changes in supported copper nanocrystals. Science 2002, 295, 2053–2055. [Google Scholar] [CrossRef] [PubMed]
- Ross, F.M. Controlling nanowire structures through real time growth studies. Rep. Prog. Phys. 2010, 73, 114501. [Google Scholar] [CrossRef]
- Jacobsson, D.; Panciera, F.; Tersoff, J.; Reuter, M.C.; Lehmann, S.; Hofmann, S.; Dick, K.A.; Ross, F.M. Interface dynamics and crystal phase switching in GaAs nanowires. Nature 2016, 531, 317–322. [Google Scholar] [CrossRef]
- Sibirev, N.V.; Fedorov, V.; Shtrom, I.V.; Bolshakov, A.D.; Berdnikov, Y. Control of (Al,Ga)P composition in self-catalyzed nanowire growth. Mater. Phys. Mech. 2020, 44, 316–323. [Google Scholar]
- Hildebrand, J.H. Solubility. Xii. Regular Solutions1. J. Am. Chem. Soc. 2002, 51, 66–80. [Google Scholar] [CrossRef]
- Redlich, O.; Kister, A.T. Algebraic Representation of Thermodynamic Properties and the Classification of Solutions. Ind. Eng. Chem. 2002, 40, 345–348. [Google Scholar] [CrossRef]
- Lukas, H.; Fries, S.G.; Sundman, B. Computational Thermodynamics; Cambridge University Press: Cambridge, UK, 2007; p. 324. [Google Scholar]
- Weinberg, F. Grain boundaries in metals. Prog. Met. Phys. 1959, 8, 105–146. [Google Scholar] [CrossRef]
- Glas, F. Comparison of Modeling Strategies for the Growth of Heterostructures in III–V Nanowires. Cryst. Growth Des. 2017, 17, 4785–4794. [Google Scholar] [CrossRef]
- Harmand, J.C.; Patriarche, G.; Glas, F.; Panciera, F.; Florea, I.; Maurice, J.L.; Travers, L.; Ollivier, Y. Atomic Step Flow on a Nanofacet. Phys. Rev. Lett. 2018, 121, 166101. [Google Scholar] [CrossRef] [PubMed]
- Marnauza, M.; Tornberg, M.; Martensson, E.K.; Jacobsson, D.; Dick, K.A. In situ observations of size effects in GaAs nanowire growth. Nanoscale Horiz. 2023, 8, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.; Ghasemi, M. Composition of Gold Alloy Seeded InGaAs Nanowires in the Nucleation Limited Regime. Cryst. Growth Des. 2017, 17, 1630–1635. [Google Scholar] [CrossRef]
- Dubrovskii, V.G.; Koryakin, A.A.; Sibirev, N.V. Understanding the composition of ternary III-V nanowires and axial nanowire heterostructures in nucleation-limited regime. Mater. Des. 2017, 132, 400–408. [Google Scholar] [CrossRef]
- Leshchenko, E.D.; Johansson, J. Interfacial profile of axial nanowire heterostructures in the nucleation limited regime. CrystEngComm 2022, 24, 8052–8059. [Google Scholar] [CrossRef]
- Leshchenko, E.D.; Johansson, J. Surface energy driven miscibility gap suppression during nucleation of III–V ternary alloys. CrystEngComm 2021, 23, 5284–5292. [Google Scholar] [CrossRef]
- Shin, J.C.; Kim, K.H.; Yu, K.J.; Hu, H.; Yin, L.; Ning, C.Z.; Rogers, J.A.; Zuo, J.M.; Li, X. InxGa1−xAs nanowires on silicon: One-dimensional heterogeneous epitaxy, bandgap engineering, and photovoltaics. Nano Lett. 2011, 11, 4831–4838. [Google Scholar] [CrossRef]
- Stauffer, D. Kinetic theory of two-component (“hetero-molecular”) nucleation and condensation. J. Aerosol Sci. 1976, 7, 319–333. [Google Scholar] [CrossRef]
- Johansson, J.; Ghasemi, M. Kinetically limited composition of ternary III-V nanowires. Phys. Rev. Mater. 2017, 1, 040401. [Google Scholar] [CrossRef]
- Leshchenko, E.D.; Johansson, J. Role of Thermodynamics and Kinetics in the Composition of Ternary III-V Nanowires. Nanomaterials 2020, 10, 2553. [Google Scholar] [CrossRef]
- Ghalamestani, S.G.; Ek, M.; Ghasemi, M.; Caroff, P.; Johansson, J.; Dick, K.A. Morphology and composition controlled GaxIn1−x)Sb nanowires: Understanding ternary antimonide growth. Nanoscale 2014, 6, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Zeghouane, M.; Avit, G.; Andre, Y.; Bougerol, C.; Robin, Y.; Ferret, P.; Castelluci, D.; Gil, E.; Dubrovskii, V.G.; Amano, H.; et al. Compositional control of homogeneous InGaN nanowires with the In content up to 90. Nanotechnology 2019, 30, 044001. [Google Scholar] [CrossRef] [PubMed]
- Roche, E.; Andre, Y.; Avit, G.; Bougerol, C.; Castelluci, D.; Reveret, F.; Gil, E.; Medard, F.; Leymarie, J.; Jean, T.; et al. Circumventing the miscibility gap in InGaN nanowires emitting from blue to red. Nanotechnology 2018, 29, 465602. [Google Scholar] [CrossRef]
- Dubrovskii, V.G.; Grecenkov, J. Zeldovich Nucleation Rate, Self-Consistency Renormalization, and Crystal Phase of Au-Catalyzed GaAs Nanowires. Cryst. Growth Des. 2014, 15, 340–347. [Google Scholar] [CrossRef]
- Dubrovskii, V.G. Fully Analytical Description for the Composition of Ternary Vapor–Liquid–Solid Nanowires. Cryst. Growth Des. 2015, 15, 5738–5743. [Google Scholar] [CrossRef]
- Dubrovskii, V.G.; Shtrom, I.V.; Reznik, R.R.; Samsonenko, Y.B.; Khrebtov, A.I.; Soshnikov, I.P.; Rouvimov, S.; Akopian, N.; Kasama, T.; Cirlin, G.E. Origin of Spontaneous Core–Shell AlGaAs Nanowires Grown by Molecular Beam Epitaxy. Cryst. Growth Des. 2016, 16, 7251–7255. [Google Scholar] [CrossRef]
- Ahmad, E.; Karim, M.R.; Hafiz, S.B.; Reynolds, C.L.; Liu, Y.; Iyer, S. A Two-Step Growth Pathway for High Sb Incorporation in GaAsSb Nanowires in the Telecommunication Wavelength Range. Sci. Rep. 2017, 7, 10111. [Google Scholar] [CrossRef]
- Biefeld, R.M. The preparation of InSb and InAs1−x Sbx by metalorganic chemical vapor deposition. J. Cryst. Growth 1986, 75, 255–263. [Google Scholar] [CrossRef]
- Tomasini, P. Vapor—Solid distribution of silicon germanium chemical vapor deposition determined with classical thermodynamics. J. Cryst. Growth 2021, 563, 126106. [Google Scholar] [CrossRef]
- Tomasini, P. Mapping vapor—Solid distributions of silicon germanium chemical vapor depositions. Mater. Sci. Semicond. Process. 2021, 123, 105516. [Google Scholar] [CrossRef]
- Egorov, A.Y.; Kovsh, A.R.; Ustinov, V.M.; Zhukov, A.E.; Kop’ev, P.S.; Tu, C.W. A thermodynamic analysis of the growth of III–V compounds with two volatile group V elements by molecular-beam epitaxy. J. Cryst. Growth 1998, 188, 69–74. [Google Scholar] [CrossRef]
- Fukui, T.; Horikoshi, Y. Organometallic VPE Growth of InAs1-xSbxon InAs. Jpn. J. Appl. Phys. 1980, 19, L53–L56. [Google Scholar] [CrossRef]
- Kajiyama, K. Vapor Pressure Dependence of the Relative Composition of III–V Mixed Crystals in Vapor Phase Epitaxy. J. Electrochem. Soc. 1976, 123, 423–425. [Google Scholar] [CrossRef]
- Farrell, A.C.; Lee, W.J.; Senanayake, P.; Haddad, M.A.; Prikhodko, S.V.; Huffaker, D.L. High-Quality InAsSb Nanowires Grown by Catalyst-Free Selective-Area Metal-Organic Chemical Vapor Deposition. Nano Lett. 2015, 15, 6614–6619. [Google Scholar] [CrossRef] [PubMed]
- Sokolovskii, A.S.; Robson, M.T.; LaPierre, R.R.; Dubrovskii, V.G. Modeling selective-area growth of InAsSb nanowires. Nanotechnology 2019, 30, 285601. [Google Scholar] [CrossRef] [PubMed]
- Himwas, C.; Collin, S.; Rale, P.; Chauvin, N.; Patriarche, G.; Oehler, F.; Julien, F.H.; Travers, L.; Harmand, J.C.; Tchernycheva, M. In situ passivation of GaAsP nanowires. Nanotechnology 2017, 28, 495707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Aagesen, M.; Holm, J.V.; Jorgensen, H.I.; Wu, J.; Liu, H. Self-catalyzed GaAsP nanowires grown on silicon substrates by solid-source molecular beam epitaxy. Nano Lett. 2013, 13, 3897–3902. [Google Scholar] [CrossRef] [PubMed]
- Isakov, I.; Panfilova, M.; Sourribes, M.J.; Tileli, V.; Porter, A.E.; Warburton, P.A. InAs(1-x)P(x) nanowires grown by catalyst-free molecular-beam epitaxy. Nanotechnology 2013, 24, 085707. [Google Scholar] [CrossRef] [PubMed]
- Metaferia, W.; Persson, A.R.; Mergenthaler, K.; Yang, F.; Zhang, W.; Yartsev, A.; Wallenberg, R.; Pistol, M.E.; Deppert, K.; Samuelson, L.; et al. GaAsP Nanowires Grown by Aerotaxy. Nano Lett. 2016, 16, 5701–5707. [Google Scholar] [CrossRef]
- Persson, A.I.; Bjork, M.T.; Jeppesen, S.; Wagner, J.B.; Wallenberg, L.R.; Samuelson, L. InAs1-xPx nanowires for device engineering. Nano Lett. 2006, 6, 403–407. [Google Scholar] [CrossRef]
- Dubrovskii, V.G. Understanding the vapor–liquid–solid growth and composition of ternary III–V nanowires and nanowire heterostructures. J. Phys. D Appl. Phys. 2017, 50, 453001. [Google Scholar]
- Samuelson, L.; Omling, P.; Grimmeiss, H.G. Alloying mechanisms in MOVPE GaAs1-xPx. J. Cryst. Growth 1983, 61, 425–426. [Google Scholar] [CrossRef]
- Fukui, T.; Horikoshi, Y. InAsSbP-InAs Superlattice Grown by Organometallic VPE Method. Jpn. J. Appl. Phys. 1980, 19, L551. [Google Scholar]
- Shu-Dong, W.; Li-Wei, G.; Wen-Xin, W.; Zhi-Hua, L.; Ping-Juan, N.; Qi, H.; Jun-Ming, Z. Incorporation Behaviour of Arsenic and Phosphorus in GaAsP/GaAs Grown by Solid Source Molecular Beam Epitaxy with a GaP Decomposition Source. Chin. Phys. Lett. 2005, 22, 960–962. [Google Scholar] [CrossRef]
- Saitoh, T.; Minagawa, S. Epitaxial Growth and Characterization of Pyrolytic-Grown GaAs[sub 1−x]P[sub x] for Electroluminescent Diodes. J. Electrochem. Soc. 1973, 120, 656. [Google Scholar] [CrossRef]
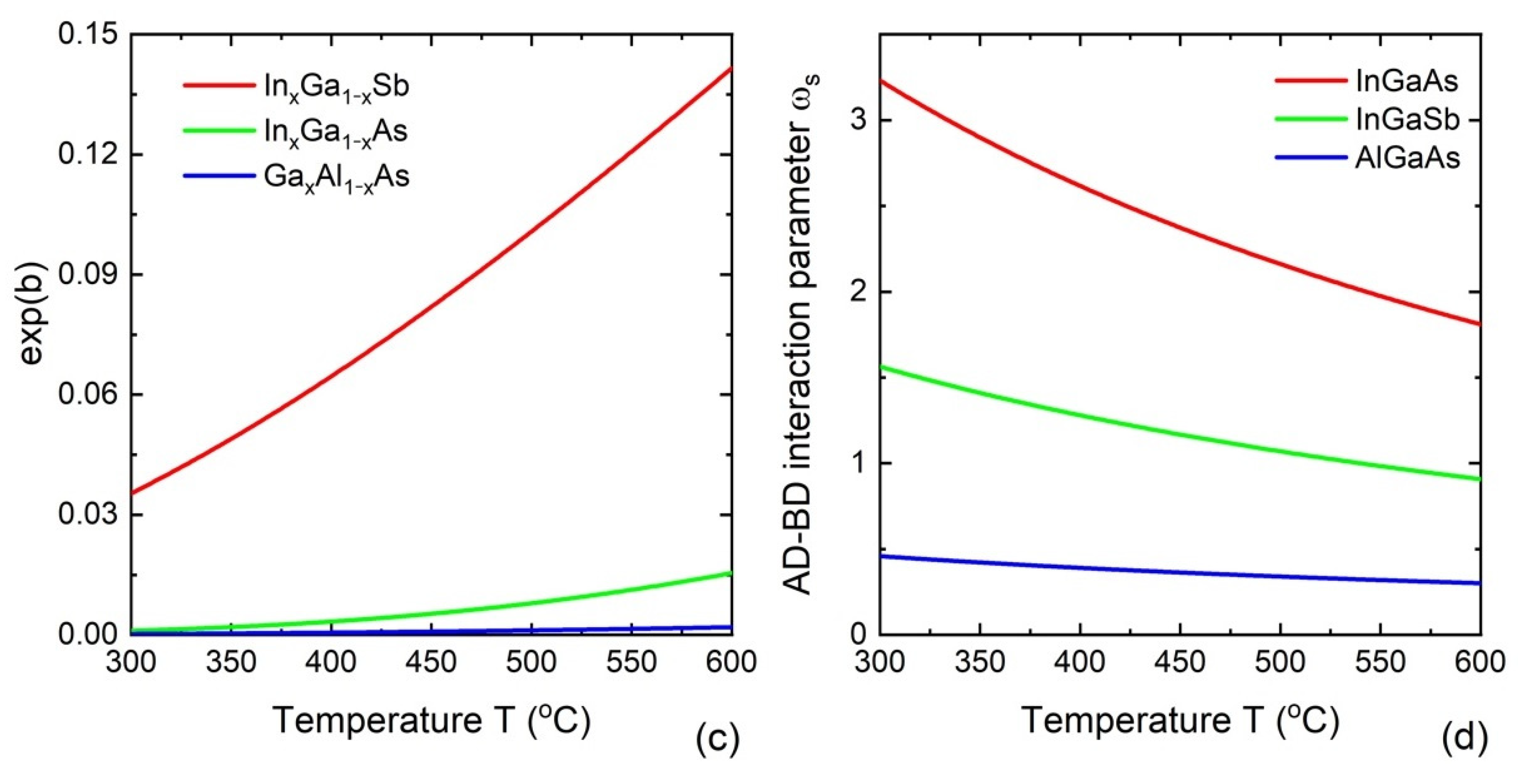
- Dubrovskii, V.G.; Leshchenko, E.D. Kinetically controlled composition of III-V ternary nanostructures. Phys. Rev. Mater. 2023, submitted.
- Leshchenko, E.D.; Dubrovskii, V.G. Kinetic modeling of interfacial abruptness in axial nanowire heterostructures. Nanotechnology 2022, 34, 065602. [Google Scholar] [CrossRef]
- Glas, F.; Harmand, J.C.; Patriarche, G. Nucleation antibunching in catalyst-assisted nanowire growth. Phys. Rev. Lett. 2010, 104, 135501. [Google Scholar] [CrossRef]
- Martensson, E.K.; Lehmann, S.; Dick, K.A.; Johansson, J. Simulation of GaAs Nanowire Growth and Crystal Structure. Nano Lett. 2019, 19, 1197–1203. [Google Scholar]
- Martensson, E.K.; Johansson, J.; Dick, K.A. Simulating Vapor-Liquid-Solid Growth of Au-Seeded InGaAs Nanowires. ACS Nanosci. Au 2022, 2, 239–249. [Google Scholar] [CrossRef]
- Li, C.; Li, J.B.; Du, Z.; Lu, L.; Zhang, W. A thermodynamic reassessment of the Al-As-Ga system. J. Phase Equilibria 2001, 22, 26–33. [Google Scholar] [CrossRef]
- Yang, J.; Watson, A. An assessment of phase diagram and thermodynamic properties of the gallium-1ndium-antimony system. Calphad 1994, 18, 165–175. [Google Scholar] [CrossRef]
- Shen, J.-Y.; Chatillon, C.; Ansara, I.; Watson, A.; Rugg, B.; Chart, T. Optimisation of the thermodynamic and phase diagram data in the ternary As-Ga-In system. Calphad 1995, 19, 215–226. [Google Scholar] [CrossRef]
- Ghasemi, M.; Sundman, B.; Fries, S.G.; Johansson, J. The thermodynamic assessment of the Au–In–Ga system. J. Alloys Compd. 2014, 600, 178–185. [Google Scholar] [CrossRef]
- Gomidzelovic, L.; Zivkovic, D.; Mihajlovic, I.; Trujic, V. Predicting of thermodynamics properties of ternary Au-In-Sb system. Arch. Metall. Mater. 2006, 51, 355–363. [Google Scholar]
- Ansara, I.; Chatillon, C.; Lukas, H.L.; Nishizawa, T.; Ohtani, H.; Ishida, K.; Hillert, M.; Sundman, B.; Argent, B.B.; Watson, A.; et al. A binary database for III–V compound semiconductor systems. Calphad 1994, 18, 177–222. [Google Scholar] [CrossRef]
- Anderson, T.J.; Ansara, I. The Ga-In (Gallium-Indium) System. J. Phase Equilibria 2007, 12, 64–72. [Google Scholar] [CrossRef]
- Li, M.; Li, C.; Wang, F.; Luo, D.; Zhang, W. Thermodynamic assessment of the Al–Au system. J. Alloys Compd. 2004, 385, 199–206. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, S.W.; Lee, H.M. A thermodynamic study of phase equilibria in the Au-Sb-Sn solder system. J. Electron. Mater. 2002, 31, 557–563. [Google Scholar] [CrossRef]
- Ghasemi, M.; Johansson, J. Phase diagrams for understanding gold-seeded growth of GaAs and InAs nanowires. J. Phys. D Appl. Phys. 2017, 50, 134002. [Google Scholar] [CrossRef]
- Liu, H.S.; Cui, Y.; Ishida, K.; Jin, Z.P. Thermodynamic reassessment of the AuIn binary system. Calphad 2003, 27, 27–37. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.J.; Liu, L.B.; Zhou, H.Y.; Jin, Z.P. Thermodynamic assessment of the Au–Ga binary system. Calphad 2011, 35, 242–248. [Google Scholar] [CrossRef]
- Dinsdale, A.T. SGTE data for pure elements. Calphad 1991, 15, 317–425. [Google Scholar] [CrossRef]



| Ref. | System | (°C) | ||||
|---|---|---|---|---|---|---|
| [134] | Planar InxGa1−xAs layers | 750 | 1.4119 | 0.7 | 0 | 0.12 |
| [16] | Dense Au-catalyzed VLS InxGa1−xAs nanowires on InAs(111)B | 470 | 2.2846 | 0.25 | 0 | 0.98 |
| [16] | Sparse Au-catalyzed VLS InxGa1−xAs nanowires on InAs(111)B | 470 | 2.2846 | 0.25 | 0 | 0.85 |
| [16] | Tops of Au-catalyzed VLS InxGa1−xAs nanowires on InAs(111)B | 450 | 2.3728 | 0.1 | 0 | 0.95 |
| [16] | Bottoms of Au-catalyzed VLS InxGa1−xAs nanowires on InAs(111)B | 450 | 2.3728 | 0.1 | 0 | 0.35 |
| Models | Equilibrium and Nucleation-Limited | Kinetically Controlled 2 |
|---|---|---|
| Temperature effect | AD content increases | BD content increases |
| Catalyst concentration effect | AD content increases 1 | AD content increases at low decreases at high |
| Effect of group V total concentration | Almost no effect | AD content increases |
| Equilibrium Model | Nucleation Model | Kinetic Model | ||||
|---|---|---|---|---|---|---|
| General Case | Decoupling Similar Results as → | General Case | General Case | |||
| Describes | border between the liquid and solid phases | critical island | fractional monolayer (supercritical island) | |||
| Required supersaturation | zero | low | high | |||
| Governing equation | ||||||
| Analytic formula | (if , and ) | (if and ) | ||||
| Supression of the miscibility gap 1 | no | no | no | yes | yes | no miscibility gap |
| Advantages | • gives the fundamental limit | • no free parameters | • almost no effect of • closed form approximation | • realistic description | • flexibility • capable to fit experimental data [68] | • one-parameter model |
| Drawbacks | • infinite nucleation time | • inflexibility • no simple formula • infinite nucleation time | • nanowire composition should repeat the composition of the critical nucleus | • no simple formula • uncertainty in the surface energy values | • high sensitivity of to • unknown temperature effect on and | • disregards thermodynamics |
| Heterostructures | Not studied | [21,111] | [115,116] | Not studied | [148] | [127] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leshchenko, E.D.; Dubrovskii, V.G. An Overview of Modeling Approaches for Compositional Control in III–V Ternary Nanowires. Nanomaterials 2023, 13, 1659. https://doi.org/10.3390/nano13101659
Leshchenko ED, Dubrovskii VG. An Overview of Modeling Approaches for Compositional Control in III–V Ternary Nanowires. Nanomaterials. 2023; 13(10):1659. https://doi.org/10.3390/nano13101659
Chicago/Turabian StyleLeshchenko, Egor D., and Vladimir G. Dubrovskii. 2023. "An Overview of Modeling Approaches for Compositional Control in III–V Ternary Nanowires" Nanomaterials 13, no. 10: 1659. https://doi.org/10.3390/nano13101659
APA StyleLeshchenko, E. D., & Dubrovskii, V. G. (2023). An Overview of Modeling Approaches for Compositional Control in III–V Ternary Nanowires. Nanomaterials, 13(10), 1659. https://doi.org/10.3390/nano13101659