Abstract
The advancement in nanotechnology has enabled a significant expansion in agricultural production. Agri-nanotechnology is an emerging discipline where nanotechnological methods provide diverse nanomaterials (NMs) such as nanopesticides, nanoherbicides, nanofertilizers and different nanoforms of agrochemicals for agricultural management. Applications of nanofabricated products can potentially improve the shelf life, stability, bioavailability, safety and environmental sustainability of active ingredients for sustained release. Nanoscale modification of bulk or surface properties bears tremendous potential for effective enhancement of agricultural productivity. As NMs improve the tolerance mechanisms of the plants under stressful conditions, they are considered as effective and promising tools to overcome the constraints in sustainable agricultural production. For their exceptional qualities and usages, nano-enabled products are developed and enforced, along with agriculture, in diverse sectors. The rampant usage of NMs increases their release into the environment. Once incorporated into the environment, NMs may threaten the stability and function of biological systems. Nanotechnology is a newly emerging technology, so the evaluation of the associated environmental risk is pivotal. This review emphasizes the current approach to NMs synthesis, their application in agriculture, interaction with plant-soil microbes and environmental challenges to address future applications in maintaining a sustainable environment.
1. Introduction
Nanotechnology is a novel approach with potential to manipulate the physicochemical properties of substances at the molecular level for the development of innovative products. A boom in nanotechnology research has been experienced over recent years. It is impacting our everyday lives and leaving influential footprints in our society [1]. It has wide usage in all industrial sectors, be it pharmaceuticals, food, animal feeds, cosmetics, electronics or agricultural production [2,3,4].
Agriculture supports the economy of developing nations by providing food, fabric, wood and raw materials for several industries. The Food and Agricultural Organization (FAO), in 2019, estimated that global demand for agricultural production must increase by 25–70% by 2050 to address the food crisis in the growing human population. Several challenges such as climate change, rampant usage of chemical fertilizers, soil contaminants, exploitation and deterioration of soil and water resources plague agricultural productivity. Current advancements in nanotechnology research are showing substantial impact on agriculture development and precision farming. The application of nano-enabled products in agriculture maximizes agriculture output (i.e., yield) while minimizing agrochemical input (herbicides, pesticides and fertilizers) by administering controlled and targeted actions [2].
The implementation of nano-based tools and techniques can immensely improve agricultural products. The nanosized materials (1–100 nm in at least one dimension) have diverse physicochemical properties, high catalytic reactivity, solubility and biochemical activity, due to their high surface-to-volume ratio [5]. NMs used in crop improvement and sustainable agriculture can be of natural origin (i.e., naturally formed in the final product) or intentionally added. Intentionally added NMs can be synthesized by physical, chemical and biological methods [6,7,8]. Synthesized NMs can be categorized into organic NMs, metals, metal oxides and carbon-based nanostructures [9]. Each NM has its own set of characteristics and applications. NMs are potentially used as nanofertilizers, nanoherbicides and nanopesticides with targeted action and delivery of active components, as nanosensors to detect environmental changes and nutrient requirements and also as catalysts in soil and ground water remediation [10,11,12]. Application of zinc, titanium, iron, silicon and selenium nanoparticles (NPs) has been reported to induce a tolerance response against stress and improve crop productivity [13,14,15]. As the world is grappling with environmental degradation and global food security issues, qualitative and quantitative expansion of crop productivity is pivotal. It is believed that NMs can be better tools for sustainable agriculture.
NMs have well-known applications in various industries. Despite the potential benefit of nanotechnology in the agriculture sector, very few nano-enabled agro inputs have made their way to the market [16]. The primary causes of the lack of commercialized nano-agricultural products in the market are limited knowledge concerning nanomaterial biosafety, regulatory guidelines, adverse effects, fate and interaction with the biological system once they are disseminated into the environment. The potential risks of NMs are still inconclusive and are under active research. This review briefly covers recent advances from the agricultural nanotechnological aspect and provides insight into toxicological fundamentals and risk assessment of NMs for legislation, as well as public awareness and acceptance.
2. Methods of NMs Synthesis
There are three different approaches, physical, chemical and biological, adopted for the synthesis of NMs. The selection of synthesis method is crucial for the properties of NMs as these significantly control their size, surface coating and their interaction with living cells. The desired characteristics of NMs can be achieved by using suitable reducing agents and synthesis methods. The different techniques used in NMs synthesis are briefly described in this section.
Two basic approaches for manufacturing nanosized materials, a top-down approach and a bottom-up approach, are discussed as follows.
2.1. Top-Down Method
It is a destructive method where bulk materials are pressed or crushed down into the nanometer size range through a mechanical approach. This approach includes techniques such as laser ablation, electro-sputtering, ball milling, lithography, thermal evaporation and sputtering [17]. The top-down method generally includes physical methods.
Physical Methods
The physical method includes different approaches. Ball milling is a very effective method for synthesizing carbon NMs which provides solutions for environmental remediation, energy storage and conservation demands. Mechanical milling methods are generally used for the preparation of metal nanoalloys (nickel, aluminium, copper, etc.) and other nanocomposites. This method is very cost effective for producing very small (2–20 nm) NMs [17,18]. The electrospinning method is the simplest method for the production of micro- and nanofibers. Modified electrospinning includes coaxial electrospinning that can be used to form long, ultra-thin fibers. This is a simple and versatile method used for the manufacturing of core–shell, polymer (inorganic, organic and hybrid) and hybrid NMs [19]. Lithography is another valuable technique for the production of nano architectures through the use of a focused electron or light beam. The two most common types of lithographic techniques are masked and unmasked lithography. Soft, nanoimprint and photolithography are examples of masked lithography. The unmasked lithography method includes focused ion beam, electron beam and scanning probe lithography [20,21]. Sputtering is a unique process for creating thin films of NMs by bombarding the surface of the material with high-energy particles such as plasma or gaseous ions. Sputtering is very special because the composition of the sputtered material remains the same as the target material with fewer contaminations, and it is more economical than electron beam lithography. The advanced laser ablation technique utilizes a high-energy laser beam to vaporize precursor material. This method is considered a green technique for producing a wide range of high-purity NPs in the quantum size range (< 10 nm) [22].
2.2. Bottom-Up Approach
Bottom-up manufacturing involves the building up of the atom or molecules to form NPs. This technique encompasses chemical reactions to assemble the basic units (atoms or molecules with nuclei) at the nanoscale. The bottom-up approach is a better technique, resulting in good surface properties and particle size because of the self-assembly of the materials used.
2.2.1. Chemical Method
The chemical reduction process is the most common chemical method for NMs synthesis. The chemical approach includes processes such as microemulsion, sol–gel methods, hydrothermal reduction, co-precipitation and thermal reduction. For the preparation of carbon-based NMs, chemical vapor deposition (CVD) methods are crucial. The ideal precursor for chemical vapor depositions must have adequate volatility and good stability, should be non-hazardous, cheap and chemically pure and have a good shelf life. In CVD, a thin film of NMs is formed via the deposition of the gaseous precursor at a very high temperature. It is an effective approach for the production of high-quality two-dimensional NMs [23]. The sol–gel method is a wet chemical approach that is extensively utilized for the manufacturing of NMs (MNMs). In this method, a liquid precursor is first transformed into the sol, which is later converted into a gel network. This method is very effective for manufacturing high-quality metal-based NMs. The method is more economical and has several advantages, such as the low processing temperature, homogeneity of the generated material and the ease with which it can be used to produce high-quality nanocomposite and nanostructures. The hydrothermal method is also widely employed for the manufacturing of a variety of NMs, such as nanorods, nanosheets, nanowires and nanospheres [24]. A hydrothermal method utilizes the aqueous phase at high pressure and at a critical temperature in a closed vessel. Hydrothermal methods combined with microwaves have gained significant attention due to the benefits yielded by microwaves. Solvothermal and hydrothermal processes are identical; the only difference being the occurrence of non-aqueous phase in solvothermal process [25]. The reverse micelle approach includes water in an oil emulsion system, where the water core acts as a nanoreactor for the synthesis of NPs. The water and oil ratio is the size-controlling factor in the reverse micelle approach. In this method, water concentration primarily affects the size of synthesized NPs; therefore, tiny water droplets form smaller NPs [26]. In comparison to physical methods, chemical methods have several benefits, such as high yield and low cost. However, the use of toxic and hazardous chemical-reducing agents can pose harm to the environment.
2.2.2. Biological Methods
Biological methods have emerged as a viable solution to overcome the limitations of traditional physical and chemical synthesis methods. Biological approaches are simpler, cost effective and eco-friendly. Biogenically synthesized NPs are easy to characterize and superior in quality to traditionally synthesized ones [27]. Even though physical and chemical methods can form a large quantity of NMs in a short time, they are cumbersome, expensive and release toxic chemicals detrimental to the biotic as well as abiotic components in the environment. In the last 10 years, a spurt in academic articles on the green synthesis of NPs has been observed every year. The biological synthesis of NPs can be achieved by using various biological systems, including plants, plant products, bacteria, fungi, yeast and viruses (Figure 1) [28,29,30].
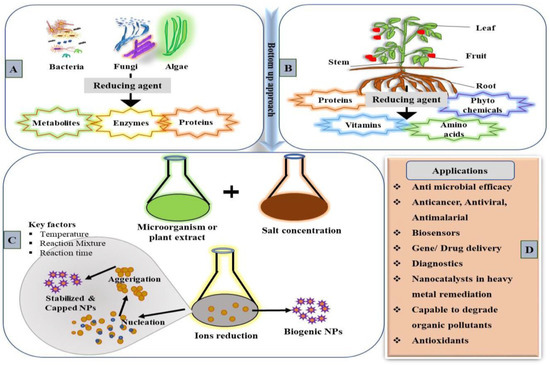
Figure 1.
Systematic representation illustrating the various biological systems used for the biogenic synthesis of NMs. (A) Different microorganism sources for NP synthesis and their reducing factors [27]. (B) Plant parts and the phytochemicals used in NPs synthesis. (C) Catalytic activities and factors involved in biogenic synthesis. (D) Biogenically synthesized NPs’ diverse application in various fields.
Microbe-Mediated Synthesis
An account of their ubiquity in the environment, fast growth, easier cultivation and ability to adapt to ambient pH, temperature, pressure and environmental conditions showed that microorganisms are suitable machinery for the biosynthesis of NPs. The mechanism of NPs synthesis through biological agents varies from organism to organism [31,32,33]. Microbial synthesis can be extracellular or intracellular depending on the type of reaction condition [33,34]. Table 1 summarizes various NPs synthesized by microbes as biological agents. Fungal synthesis of NPs has been explored by exploiting bioactive compounds and metabolites from fungi. These microorganisms are an attractive agent for the synthesis of silver NPs due to their heavy metal tolerance, biomineralization and metal accumulation abilities. Several different fungal strains have been used to synthesize silver NPs, such as Aspergillus flavus [35], Guignardia mangiferae [36], Aspergillus versicolor [37], Cladosporium cladosporioides [38], Beauveria bassiana [39], Penicillium oxalicum [40], Bjerkandera sp. [41], Aspergillus sp. [42], Aspergillus oryzae [43] and Aspergillus terreus [44]. Intracellular synthesis involves exposure of metal precursors to a fungal mycelia culture that results in metal internalization and reduction inside the cell. After synthesis, additional treatment is required to release the NPs [44,45]. While extracellular synthesis is more economic, as NPs are synthesized using only cell-free fungal filtrate containing bioactive compounds, this method is more appropriate because no post-treatments are required to harvest NPs from the cell. Dispersed NPs can be easily purified using simple processes such as filtration, dialysis and gel filtration [46,47]. Many enzymes can initiate the process of NPs synthesis, but nicotinamide adenine dinucleotide (NADH) and NADH-dependent nitrate reductase enzymes are accounted for the microbial based synthesis of metal NPs. Recently, Hietzschold et al. reported that NADP can be solely responsible for the reduction of silver nitrate to form silver NPs [48].
Owing to the biosorption properties, intracellular sequestration, extracellular precipitation and efflux pump for metal tolerance, bacteria are the suitable candidates for metal ion reduction and NPs formation [7,49]. Researchers have successfully synthesized different NPs from the bacterial strains Pseudomonas deceptionensis [50], Pseudoduganella eburnean [51], Bacillus subtilis [52] and Cuprividus spp. [53].
Actinomycetes, the filamentous soil bacteria, are famed for producing bioactive compounds to survive in harsh environmental conditions. These bioactive compounds have received more attention due to their high stability, commercial value, unique antimicrobial properties and uncommon substrate specificity. The genus Streptomyces is known for its significant contribution to secondary metabolite production with high commercial value [54]. Many researchers explored this property of the Streptomyces genus and used biomass filtrate as a reducing agent for the green synthesis of silver NPs. The synthesized NPs were crystalline, spherical and had an average size of less than 100 nm with a surface plasmon resonance absorption band at 400–450 nm [55]. In another study, silver NPs were synthesized by using haloalkaliphilic Streptomyces spp. Ag NPs synthesized through Streptomyces spp. were spherical in shape with an average diameter of 16.4 ± 2.2 nm and had significant phytopathogenic activity against Fusarium verticillioides and Ustilago maydis [56]. Extracellular production of zinc and gold NPs through Streptomyces spp. was also reported [57,58]. The NPs produced by green synthesis were found to be more stable because of the presence of natural biomolecules that act as capping and stabilizing agents.
Several studies demonstrated that viruses can serve as a versatile platform for nanoscale product formation [59,60]. Viral biosynthesis provides a broad range of shapes, sizes, compositions and physicochemical properties of NPs. Material scientists use virus capsids as bio-templates for the development of novel nanohybrid materials. Plant viral capsid proteins are suitable biofactories for the fabrication of a wide range of NMs. They offer desired properties with inorganic and organic moieties integrated in a very precise and controlled manner [60]. The advantages of using viral proteins for NMs synthesis include ease of chemical and genetic manipulation, degradability, nontoxicity for humans and a well-known atomic structure with a possible ligand-attaching site [61,62]. In a study by Ahiwale et al., gold nanoparticles were prepared by using a rare bacteriophage of the podoviridae family. Viral-inspired Au NPs were found to be in the 20–100 nm size range. They showed antibiofilm activity against the human pathogen Pseudomonas aeruginosa at a very low concentration of 0.2 mM [63]. Recently, nanotechnologists exploited the plant virus squash leaf curl China virus (SLCCNV) for gold and silver NPs fabrication. A virus–metallic nanohybrid (Au and Ag) was synthesized using the pH-activated capsid of SLCCNV, and its electrical conductivity was also determined for biomedical applications [64]. Several studies illustrated the viral-mediated synthesis of different nanostructures such as platinum nanotube [65] and viral-like NPs (VLN) [66] and cadmium sulfide (CdS) nanocrystal [67], gold and iron oxide NPs [68]. A large number of viral-mediated NPs studies are available, but their application in agricultural practices or against plant pathogens is yet to be explored.

Table 1.
Biological synthesis of MNPs using a diverse group of microbes.
Table 1.
Biological synthesis of MNPs using a diverse group of microbes.
| Nanoparticle Synthesized | Source | Size | Application | Ref. |
|---|---|---|---|---|
| Ag NPs | Bacteria | |||
| Bacillus endophyticus | 5 nm | Antimicrobial activity against Candida albicans, Escherichia coli, Staphylococcus aureus | [69] | |
| Sinomonas mesophile | 4–50 nm | Antimicrobial activity against multi-drug-resistant S. aureus | [70] | |
| Pantoea ananatis | 8.06–91.31 nm | Antimicrobial activity against multidrug-resistant bacteria and efficient against some pathogenic microbes as well | [71] | |
| Pseudomonas strain | 20–70 nm | Showed highest antibacterial activity | [72] | |
| Fungi | ||||
| Aspergillus terreus | 16–57 nm | Efficacy in antibacterial activity | [73] | |
| Penicillium aculeatum | 4–55 nm | Antimicrobial agent, drug delivery vehicle (anticancer drug) | [74] | |
| Fusarium oxysporum | 5–13 nm | Antibacterial and antitumor activities | [75] | |
| Metarhizium anisopliae | 28–38 nm | Antimalarial activity | [76] | |
| Algae | ||||
| Portieria hornemannii (Red algae) | 60–70 nm | Alternative to antibiotics which are commercially available against fish pathogens | [77] | |
| Padina sp. (marine algae) | ~25–60 nm | Antibacterial and antioxidant activities | [78] | |
| Au-Ag/ Ag NPs | Bacteria | |||
| Stenotrophomonas | Silver (40–60 nm) and Gold (10–50 nm) | - | [79] | |
| Bacillus subtilis | 20–25 nm | Dye degradation | [80] | |
| Mycobacterium sp. | 5–55 nm | Anticancerous activity | [81] | |
| Fungi | ||||
| Cladosporium cladosporioides | 60 nm | Antibacterial and antioxidant activities | [82] | |
| Aspergillus sp. | 2.5–6.7 nm | Biocatalysis of nitrophenol compounds | [83] | |
| Rhizopus oryzae | 16–43 nm | Hemocompatible activity | [84] | |
| Algae | ||||
| Gelidiella acerosa (Marine algae) | 58–117.6 nm | Antidiabetic, antibacterial and antioxidant activity | [85] | |
| Cystoseira baccata (Brown algae) | 8.4 nm | Cancer therapies | [86] | |
| Pithophora oedogonia | 32.06 nm | Electrocatalytic activity by determining the presence of carbendazim molecules in soil | [87] | |
| Cu NPs | Bacteria | |||
| Shewanella loihica | 10–16 nm | Antimicrobial activity | [88] | |
| Shewanella oneidensiS | 20–40 nm | Biocatalysts | [89] | |
| Se NPS | Bacteria | |||
| Lysinibacillus sp. | 100–200 nm | Photocatalytic activity | [90] | |
| Bacillus subtilis | 50–400 nm | H2O2 sensoristic device | [91] | |
| CdS NPs | Bacteria | |||
| Escherichia coli | 2–5 nm | – | [92] | |
| Pseudomonas aeruginos | 20–40 nm | Removal of heavy metasl as cadmium | [93] | |
| TiO2 NPs | Bacteria | |||
| Bacillus mycoides | 40–60 nm | Qantum dot sensitized solar cells | [94] | |
| Aeromonas hydrophila | 28–54 nm | Antibacterial activity | [95] | |
| ZnO NPs | Bacteria | |||
| Bacillus licheniformis | 40–400 nm | Photocatalytic activity, dye degradation and bioremediation | [96] | |
| Serratia nematodiphila | 10–50 nm | Antimicrobial as well as antifungal activity | [97] | |
| Fungi | ||||
| Candida albicans | 25 nm | Synthesis of steroidal pyrazolines | [98] | |
| Aspergillus terreus | 10–45 nm | Antibacterial, cytotoxic activity, UV protection | [99] | |
| Algae | ||||
| Chlorella extract (Microalgae) | 20 nm | Showed photocatalytic activity | [100] | |
| Sargassum muticum | 30–57 nm | Beneficial cytotoxic effect on human liver cancer cells | [101] |
Plant-Mediated Synthesis
The green synthesis approach for NMs synthesis aims to develop a method, reagent and process that substitute remediation or decreases the release of toxic chemicals to ensure the safety of the environment. Plants are the richest source of a diverse group of chemicals, such as terpenoids, proteins, amino acids, tannins, phenols, flavones, saponins, alkaloids and polysaccharides, that is actively involved in the reduction of metals. Because of the presence of these phytochemicals, the harnessing of plant materials for NPs synthesis has been appraised as more reliable as well as eco-friendly [102,103,104]. Different plant parts, including stem [105], root [106], seeds [107], leaves [108], fruits [109], bark [110] and flowers [111], have been explored for NPs synthesis (Table 2).

Table 2.
Summary of biologically synthesized NPs using plant extract and their characteristics.
3. Nanomaterials for Agricultural Application
Nanotechnology offers a multifold scope for agricultural advancements by usage of NMs. Several fields such as biomedicine, food, energy, defense, textiles, paints and home goods have examined the use of and benefits presented by nanotechnology on a regular basis. Techniques such as nano-priming for rapid seed growth and increasing crop yield through the use of nanofertilizers, nanopesticides and nanoweedicides, etc., are proving to be a panacea for agriculture.
3.1. Nanomaterials in Crop Production
Nanotechnology as a broad area of research has come up with numerous applications in agronomy that have benefited agriculture through the improved yield of crops and the accelerated germination process of seeds and plant growth as well. The biological process of seed germination is complex, and it depends on the soil’s properties, genetic makeup and environmental conditions. Recent studies have demonstrated that NPs, including carbon nanotubes (CNTs), silicon dioxide (SiO2), zinc oxide (ZnO), titanium dioxide (TiO2) and gold (Au) NPs, have eased the germination of seed in crops such as wheat, pearl millet, tomato soybean, barley, rice and maize [122,123]. TiO2 NPs have been found to enhance seed germination by significantly decreasing the mean time for germination in Agropyron desertorum [124]. Furthermore, non-metallic NPs such as multiwalled carbon nanotubes (MWCNTs) can promote the germination of seeds in a variety of crops by improving the seed’s capacity to absorb water [125]. Additionally, NPs increase the tolerance of plants against abiotic stress primarily by scavenging reactive oxygen species (ROS) and increasing enzyme antioxidant activity [126]. Graphene NPs increase alfalfa’s resistance to alkaline circumstances, specifically by enhancing the activity of antioxidant enzymes and boosting seedling root length along with its fresh and dry weight [127].
Nano-priming is a seed priming method that contributes to increased seed germination, growth and yield. Many studies claimed a potential increase in the germination of seeds and seedling efficiency in crops such as wheat and tomato [127,128,129]. Yang et al. showed that phytohormones are responsive to NP treatment [130], while the content of indole acetic acid (IAA) and abscisic acid (AB) has been reported to increase in the roots of transgenic and non-transgenic rice in response to Fe2O3 [131]. Nano-priming is considerably effective under stress conditions, where it enhances the percentage of seed germination, length of root and shoots and seed vigor index [132]. Calendula officinalis seeds primed with silicon NPs exhibited improved antioxidant activity and total flavonoid content under drought stress. Thus, under the stress condition, priming with nanosilicon improves C. officinalis’s physiological and metabolic characteristics [133]. Another major role of NMs in crop production is the controlled delivery of materials (pesticides and fertilizers) via nanoencapsulation, which presents a meticulous option for crop enhancement while maintaining the surrounding environmental health. Formation of nano-agrochemicals that can regulate the nutrient release, and, as well, maintain the health and fertility of the soil by providing it with the beneficiary elements, is being studied. While external elements such as rain and wind can easily leach fertilizers from the application site, porous NPs encapsulate the fertilizer and retain it in the soil. This characteristic extends the fertilizer release period and enhances both the chemical and physical characteristics of the soil [134]. For reducing chemical pesticide doses, boosting crop productivity and fostering sustainable development, nanoformulation or nanoencapsulation of insecticides, herbicides, fungicides and bactericides with NMs hold immense potential.
3.2. Application of Nanomaterials as Herbicides, Pesticides and Nanofertilizers
In recent years, nanotechnology with smart nanoscale carriers for effective delivery of macro- and micronutrients, plant growth regulators, pesticides and fertilizers has become an efficient method for sustainable agriculture [135,136]. Nano-carriers prevent chemical discharge and solve environmental issues by securing plant roots to the organic materials and soil structure in the ecosystem. These aids in enhancing the bioavailability of active ingredients to the plant while lowering the effort and waste product [137]. Research on herbicides clearly places a higher priority on lowering the non-target toxicity of the herbicides through the use of NPs. De Oliveira et al. showed that pre-emergence application of solid lipid nanoparticles containing atrazine and simazine was more effective at eliminating the target plant Raphanus raphanistrum than post-emergence use of the herbicide alone [138]. Chidambaram et al. (2016) examined the loaded nano-sized rice husk waste particles with 2,4-dichlorophenoxyacetic acid (2,4-D). They discovered that NPs loaded with 2,4-D herbicide had superior herbicidal action to 2,4-D alone against the target plant (Brassica sp.). It was also evaluated that herbicides loading at rice husk reduced the leaching effect in soil [139]. In comparison to paraquat alone, Dos Santos Silva et al. employed paraquat loaded with alginate or chitosan, which reduced the leaching of herbicide in soil sorption experiments [140].
Similar to nanoherbicides, nanopesticides are also garnering recognition for replacing traditional pesticides due to their enhanced efficacy against a variety of pests and potential for tailored action, which lowers the environmental toxicity. In a study with Drosophila melanogaster as a model organism, the anti-pest activity of chitosan loaded with permethrin and spinosad was tested at various doses. It demonstrated that the combination of spinosad and permethrin in chitosan is more effective than either compound alone, suggesting that nanoformulations may be used for pest control management [141]. Further, the association of chitosan with zinc helps enhance the plant’s immunity. According to Choudhary et al., the formulation of zinc and chitosan nanoparticles increases the antioxidants and content of lignin in maize crops to elevate disease control [142]. Red-seaweed-extract-derived silver nanoparticles are suited for use in the formulation of nanopesticides due to their antibacterial and antifungal properties [143].
As crop plants can only absorb 30–50% of chemical fertilizers, a sizable portion of the input remains in the soil, which causes soil sterility and ground water contamination. Due to saturation, fertilizer efficiency thus declines over time [144]. Nanofertilizers have efficiency to reduce the nutrient loss via controlled release and thus may minimize the amount of fertilizer application in the field [145]. According to the available literature, nanofertilizers are benefiting several crop yields [146,147,148,149]. Gatahi et al. (2015) studied the impacts of nanobiofertilizer in tomato crops affected by Ralstonia solanacearum-caused bacterial wilt disease and its pest-resistant function against wilt disease [150]. Gouda et al. examined the protective effects of nanobiofertilizers that contain PGPR (Pseudomonas sp., Bacillus subtilis and Paenibacillus elgii) on leguminous crops against several diseases in the rhizosphere [151]. Many NPs themselves work as nanofertilizers or as a nanoencapsulating agent to transform conventional fertilizer into nanofertilizers [152]. The combination of biofertilizer (Piriformospora indica, a plant-growth-promoting fungus) and copper nanoparticles (Cu NPs) on Cajanus cajan, a leguminous crop, demonstrated that this combination application of nano + biofertilizer (nanobiofertilizer) might stimulate plant growth and vitality more effectively [153]. The list of nano-enabled agricultural products which are approved and manufactured for import in different countries is summarized in Table 3. Based on the research, nanofertilizers can improve the bioavailability of nutrients by increasing the shelf life of bioactive compounds in soil, thus, having a more obvious impact on crop growth [154].

Table 3.
Manufactured and approved nanotechnology-enabled nano agro products/inputs.
3.3. Nanomaterials as Biotic and Abiotic Stress Alleviators
Plants are inevitably subjected to biotic and abiotic stress, which inhibits their growth and reduces yield, augmenting the global food crisis. NMs are regarded as effective and promising tools for overcoming the constraints in sustainable agricultural production by improving plant tolerance mechanisms under these stresses. Hojjat et al. stated in their investigation that biogenic Ag NPs improved the growth, length and weight of lentils and improved germination in Trigonella foenum during drought [155]. Various investigations reported that NMs restore plant development via increasing antioxidant activity, regulating metabolism and enhancing the quantity of photosynthetic pigments in plant systems subjected to plant stress [156]. It has been shown that plants can recover from oxidative stress after being treated with TiO2 NPs. TiO2 NPs improved the activity of antioxidant enzymes in corn tissues and additionally improved physiological functions and chlorophyll concentration by successfully minimizing Cd toxicity to the Glycine max plant [157]. Hence, the utilization of TiO2 NPs offers excellent application potential in reducing heavy-metal-induced plant oxidative stress. CeO2 NMs improved the salt tolerance of maize by maintaining Na+/K+ homeostasis, enhancing photosynthetic efficiency and reducing levels of ROS in salt-stressed Zea mays leaves [158]. The application of silicon dioxide improved plant growth parameters by lowering levels of hydrogen peroxide, electrolyte leakage and malondialdehyde. Furthermore, it decreased chlorophyll degradation while increasing stomatal conductance, net photosynthetic rate, transpiration rate and water use efficiency [159]. In a study by Rezvani et al., 10-day floodingstress reduced the root biomass, number of roots, leaf biomass and root length in the medicinal and aromatic plant species Crocus sativus [160]. Soaking the C. sativus corms in 40 or 80 ppm concentrations of nanosilver mitigated the negative effects of flooding stress and increased root development. When Al2O3 NPs of 30 to 60 nm were examined on the Glycine max under flooding conditions, their root length increased while glycolysis-related mitochondrial proteins expression repressed [161]. Iqbal et al. conducted experiments to find the effect of Ag NPs on wheat growth under heat stress. Ag NPs synthesized using a plant extract from Moringa oleifera were sprayed on Triticum aestivum at the three-leaf stage in various quantities. Exposure to heat stress alone decreased the dry biomass, but T. aestivum treated with Ag NPs at concentrations of 50 and 75 mg/L was found to be protected from heat stress and showed significant improvement in growth [122]. Petal longevity in Pelargonium zonale was increased after being treated with Ag NPs, which have been proven to mitigate the negative effects of dark-stress-induced oxidative damage [162]. They also examined reduced petal abscission in geranium cultivars subjected to nano silver and thidiazuron under dark preservation. NMs exhibit antibacterial properties against nematodes, bacteria and fungi that cause plant diseases in addition to abiotic stress. For instance, a ZnO nanoparticle made from a flower extract showed antibacterial action against R. solanacearum and decreased tomato bacterial wilt illness [163]. In contrast, a ZnO NPs made from Citrus medica peel extracts showed antimicrobial activity against Bacillus subtilis, Streptomyces sannanesis, Salmonella enterica and Pseudomonas aeruginosa [15]. According to Boxi et al., two prominent phytopathogens, Fusarium solani (which causes Fusarium wilt illnesses in potato and tomato plants) and Venturia inaequalis (which causes apple scab disease) were both inhibited by TiO2 nanoparticles at 0.75 and 0.43 mg/plate [164]. A TiO2 nanoparticle foliar spray in poinsettia and geranium (25 and 75 mM), as well as cucumber (1.6%) and poinsettia and geranium (1.6%), showed antibacterial action against pathogens Pseudomonas cubensis and Pseudomonas syringae pv. lachrymans and Xanthomonas hortorum [165,166]. All these experimental results validate the significant role of NMs in mitigating both biotic and abiotic stress and thereby enhancing plant growth and yield through numerous plant physiological mechanisms. It is clearly noticeable that NMs reduce the impact of environmental stresses on plants and help to improve the crop yields.
3.4. Biodegradable Nanoencapsulated System and Its Application in Agriculture
In order to enhance agricultural productivity, the use of agrochemicals has become increasingly prevalent. Among the many nanotechnological processes applied in the agricultural industry, micro-/macro- and nanoencapsulation are particularly noteworthy. These techniques involve the entrapment of bioactive compounds which can then be released in a controlled manner under specific conditions. This approach offers enormous potential for the development of agrochemicals with targeted chemical compositions and enhanced efficiency. Additionally, the agricultural sector benefits from a wide range of nano-based products, such as nanofertilizers, nanopesticides, nanofungicides, food and nutraceuticals, which are being used to promote sustainable farming practices, improve crop yields and fortify food supplies. By utilizing nanoencapsulation techniques, fertilizers can be modified to improve their efficiency and ensure a delayed release of entrapped bioactive compounds.
Encapsulation addresses the challenges of agronomical practices and minimizes the agrochemical load in practices [167]. One of the key benefits of encapsulating active ingredients is the gradual release of these substances to the crop. The sustained release intensifies the usage of resources in the best possible way, along with enhancing overall environmental safety [168]. Yet, it is worth noting that the primary focus of encapsulating active compounds has predominantly been on pesticides [169] and fertilizers [170]. In a study, it was demonstrated that chitosan is a great carrier for essential plant microbes [171]. Azospirillum brasiliense and Pseudomonas fluorescens were encapsulated in a chitosan–starch formulation, and this was applied to generate a controlled-release fertilizer [172]. The starch was added as filler to a chitosan-based formulation and employed as the crosslinking agent. The synthesized bacteria maintained a high vitality (109 colony-forming units (CFUs) of A. brasilense/g and 108 CFUs of P. fluorescens/g) for at least 12 months at room temperature and humidity. With their application to soil, bacteria gradually multiplied over the first 20 days before declining. Because of encapsulation, many targeted risks can be tackled [172].
Due to their adaptability, compatibility, nontoxicity and permeability, polymers have long served as encapsulants [173]. For the specific, controlled release of micromolecules, natural molecules such as chitosan, alginate, cellulose, starch, modified polysaccharides, carboxymethyl cellulose (CMC), Xanthan gum and gum Arabic have been employed as encapsulators. According to Tesfay et al., covering avocado fruits with 1% CMC and moringa leaf extract dramatically enhanced fruit quality and slowed the process of ripening [173]. The biological synthesis of ZnO NPs was performed by Saekow et al., who also evaluated the impact of ZnONP-loaded CMC on tomato quality characteristics and the capability of this mixture to combat Alternaria alternatives [174]. Some artificial polymers such as polyvinyl alcohol (PVA), polystyrene and polyalkylene glycol (PAG) have also been linked to similar uses. These polymers contain growth regulators such as metals, amino acids and other macro- and micronutrients, as well as pesticides, insecticides and herbicides [138].
Several studies have been performed to validate the properties of encapsulating an active ingredient with polymers. Alginate is employed in the agricultural sector as an absorbent polymer for the purpose of seed and fruit coating, a transporter of microbes and an enhancer of microbial bacterial activity for promoting plant growth. Agrochemical formulations use alginate encapsulation particularly to regulate the release of active chemicals [175] and the release of pesticides [176,177] or fungicides. Improved growth conditions for wheat plants were observed in stress conditions when encapsulated formulations of Burkholderia cepacia and Pseudomonas fluorescens were administered [178].
Chitosan, a potent natural polymer, has earned an illustrious presence due to its extraordinary qualities in terms of biocompatibility, chemical resistance and biodegradability. It is a popular eco-friendly substitute in the field of agriculture which is now widely utilized in a variety of agricultural techniques, including in biopesticides, seed treatment agents, biofertilizers, soil conditioners and growth enhancers [179]. A study showed new soil conditioner systems that address both soil fertilization and water-holding capacity. These systems were created via in situ hydrogelation of chitosan with salicylaldehyde in the presence of urea fertilizer. Chitosan has additionally been used to co-encapsulate resveratrol and curcumin [180]. Nanocomposite films from chitosan can effectively control infections by Penicillium chrysogenum and Aspergillus species by preventing their proliferation [181].
There are many types of red macroalgae (Rhodophyta) that contain the hydrophilic polysaccharides known as carrageenans (CGs). These are absorbent polymers that can help the soil retain water, offering a crucial support for plants during droughts [182]. Arabic gum is also used in agriculture due to its distinct structure, which includes a low viscosity and a high solid content, which makes it a good wall material for bacterial encapsulation. In modern encapsulation technology, particularly in the spray-drying method [183], Arabic gum is combined with other polymers, such as polysaccharides. A formulation for reducing tick harm was created in 2018 by Oliveira and her coworkers. This formulation, which contains Pseudomonas spp., Arabic gum, chitosan polymer and sodium casein, was created using the encapsulation process and applied to tomato and pepper plants to control adult mites [184] (Figure 2). Another study employed P. putida and their delivery system made up with chitosan, Arabic gum and sodium alginate against Helicoverpa armigera (Lepidoptera: Noctuidae), one of the most significant pests of cotton, for counting and manufacturing [185].
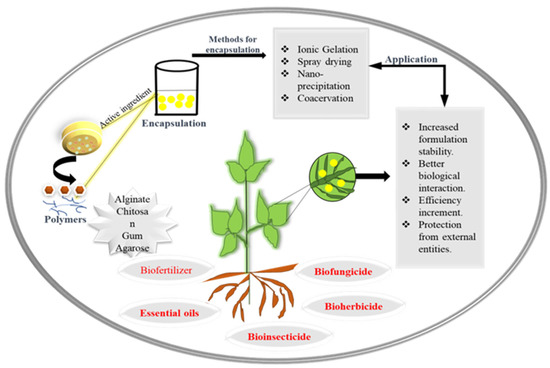
Figure 2.
Systematic representation of encapsulation of bioactive compounds and their agricultural applications.
4. Uptake and Presence of Nanoparticles in Plants
In a soil system, the bioavailability, transport, fate and toxicity of nanoparticles are governed by soil factors such as organic matter, soil type, pH and moisture contents because these factors induce a series of changes in NP chemistry such as agglomeration, aggregation, dissolution and biotransformation [186,187]. Nanoparticles adsorbed firstly by the root system can be translocated to the aerial portion where they start accumulating in cellular or subcellular organelles [188]. The adsorption of nanoparticles by plant root surfaces is the first step of bioaccumulation. Studies suggest that silver nanoparticle uptake in the root is also strengthened by the acidic ambiance of the root cap [189] and iron plaque in the plant root [190].
The right size of NP facilitates their entry via biological pores (cell walls and stomata) revealing size as the crucial determinant for adsorption in plants [191,192]. A study proved that the adsorption of 50 nm copper NPs in wheat roots caused changes in root cell morphology and observed the existence of Cu NPs adhering to the root surface through SEM-EDS analysis [193]. NP agglomeration and reactivity with plant cell surfaces are correlated with the shape of NPs [194]. The surface charge and hydrophobicity of plant cell surfaces also act as critical determinants of the NP uptake process. Barrios et al., in their study, examined the uptake behavior of Ce NPs (coated and noncoated or bare Ce NPs). They suggested that surface coating reduces the uptake efficiency of NPs, but their translocations in the aerial portion remain unaffected [195]. The above-described facts support the design of NP ecotoxicological-based studies to understand the exact toxic mechanisms of NPs in plant systems. It is reported that the root epidermal cell allows the passage of small-sized NPs (3–5 nm) either directly through the biological pores or along with capillary and osmotic force. After crossing the root cell wall, NPs follow two basic pathways in the root epidermis to reach the vascular system. The apoplastic pathway is the most studied pathway for NPs transport, where NPs diffuse into the intercellular space of the cell wall and plasma membrane (without crossing the cell membrane) until they reach the vascular system, allowing the xylem to transport NPs unidirectionally upward [196,197]. A previous study reported that lanthanum oxide (La2O3) NPs increase the essential component of apoplastic barrier lignin by 1.5-fold and drastically reduce stomatal conductance and transpiration rate, subsequently causing significant growth inhibition [198]. Recently, another study also confirmed that Ce NPs promote endodermal suberization in the root of the Sedum alfredii plant [197]. NPs can cross the casparian strip symplastically to enter the vascular cylinder. Here, this process is promoted by the endodermal cell membrane’s carrier proteins through endocytosis or via pore formation. NPs move through the xylem into the aerial portion and through the phloem back to the roots [188].
Uptake of Ag NPs may also occur through the leaf, and the large pore size of leaf stomata facilitates entry of Ag NPs [199]. The uptake of Ag NPs through leaves in Salvia officinalis suggests that it can pass the cell wall and plasma membrane and enter the cell through endocytosis. It is localized in the cytoplasm and intercellular space of S. officinalis leaves [200]. After entering the body, NPs are distantly transported along with the sugar flow of the phloem sieve tube. Transportation of NPs by phloem allows bidirectional movement and accumulation in different parts of the plant. It is widely agreed that the apoplastic route favors the passage of water nutrients and nonessential metal complexes [201]. Translocation factors (TF) are the ratio of NP levels in the shoot and root. TF vary with particle properties and plant species. The small-size nano copper nanoparticles (nCu NPs) tended to transfer upward more feasibly than Cu NPs in Cucumis sativus that was exposed to 100 mg/kg Cu for 65 days [202]. Toxicity and bioaccumulation of NPs are influenced by total surface area and size; likewise, in Lolium multiflorum, Ag NPs (6 nm) were more toxic than (25 nm) Ag NPs because of their higher accumulation [203]. Foliar uptake of an NP is influenced by application methods and the shape, size, concentration and surface properties of the NPs. Leaf morphology (leaf area, size of stomata and cuticle thickness) also affects the trapping of NPs on the surface of the leaf [199,200]. The accumulation rate of NPs depends on the route of exposure. A study performed on Glycine max seedlings revealed more accumulation of silver in foliar exposure than in root application in leaves [204]. Recently, another study concluded that different forms of silver nanoparticles (20 nm Ag2S NPs, 3–8 nm Ag NPs, 50 nm Ag NPs and AgNO3) had differing uptake and bioaccumulation in the life cycle of Brassica rapa. They showed that pristine Ag NPs accumulated 14 times more Ag than other sulfide silver forms [205]. Therefore, a major factor determining the difference in uptake and NP accumulation is the stability of the NPs.
5. Nanomaterial Interaction with Plants
The nanotechnology market has expanded significantly in agricultural sectors. Various nano-enabled agrochemical products have been launched in the market that include fertilizers for crop production, pesticides for disease resistance and nanosensors for monitoring plant health and soil quality. The emergence of nanotechnological applications in consumer products has also raised ethical and social concerns, including those about environmental safety issues. Plants, being the producers, are the most essential components of the terrestrial ecosystem. In the past few years, a large number of studies on the phytotoxic effect of NMs have been reported, but the mechanistic action of toxicity is still not well understood. Small-size NPs can easily be taken up by the plant and induce cytotoxic effects (Figure 3). The accumulation of NPs in different plant parts has been confirmed in Oriza sativa [206], Lycopersicon esculentum [207], Cucumis sativus, Triticum aestivum [194], Sinapis alba and Lepidium sativum [208]. The accumulation of NPs causes dysfunction of photosystem II (PSII) and PSI and ultimately affects photosynthetic rate [209]. For example, wheat chlorophyll content, photosynthetic rate and efficiency of PSII were suppressed by P25 (a commercial formulation of TiO2 NPs) [210]. The toxicity of cerium oxide NPs (25 nm) was also observed by measuring photosynthetic activity in Glycine max. CeO2 NPs-exposure-induced changes in the thylakoid membrane, which subsequently reduced chlorophyll content and inhibited the quantum yield of PSII [204]. Systemic biological approaches have been implemented to resolve the behavior of genes, proteins and metabolites in response to NMs interaction. In plants, gene expression analysis is the most studied predictive marker of NPs phytotoxicity through which NPs-induced phenotypic changes can be correlated. Likewise, a dose-dependent phytotoxic study of Cu NPs was performed in Lactuca sativa. Transcriptomic analysis suggested that the ATP-binding cassette (ABC) transporter or other metal-binding protein transporters are actively involved in Cu NPs accumulation, and an increase in the expression of antioxidants (POD, MDHAR, APX and FSDs) was also observed under NPs stress [211]. Recent analysis on Zn ONP- and MWCNT-exposed Arabidopsis thaliana seedlings suggested that inorganic NPs induce stronger inhibitory effects than MWCNT via analyzing the expression pattern of genes involved in ROS homeostasis [212]. NPs exposure in plants influences the expression of genes catalase (CAT), ascorbate (APX) [213], superoxide dismutase (SOD) [214], auxin signaling F-box protein and DNA mismatch repair protein (MSH5) [215]. In response to NPs stress, multiple metabolomic pathways are differentially regulated in order to protect the plant from oxidative damage. In this manner, the phenolics and amino acid synthesis are controlled in stress conditions via the phenylpropanoid pathway, glutathione metabolism, GABA shunt, shikimate pathway and flavonoid pathway [216,217]. Researchers are also trying to evaluate the behavior of NPs with other soil contaminants. A study reported the comparative toxic behavior of mixtures of NPs. The study showed that combined NPs binary systems have a lower toxic impact on seed germination and root growth than individual ones [218]. Toxicological-based studies showed the effect of various factors such as the shape, size, coating, composition and physiochemistry of the NPs on their phytotoxic behavior. In S. lycopersicon, CuO2 NPs accumulation was higher compared to in Al2O3 NPs and consequently caused mitochondrial membrane defects and changes in growth dynamics [143]. NPs also impugn the rhizospheric soil microbial community and functions. Ag NPs treatment negatively influenced the bacterial and fungal microbiota of the Populus nigra plant [219]. Direct application of CuO2 NPs as fertilizer inhibits important soil nitrification kinetics and diminishes the activity of soil nitrifiers [220]. Copper NPs are used as a component of fertilizers, but Cu NPs drastically affect microbial nitrogen cycle processes (nitrification and denitrification). They also decreased the activity of heterotrophic microbe populations in Triticum aestivum rhizospheres [221]. The rhizospheric microbial community plays a crucial role in maintaining plant growth and soil health. The non-target effect of NPs application should be monitored to sustain the biogeochemical process.
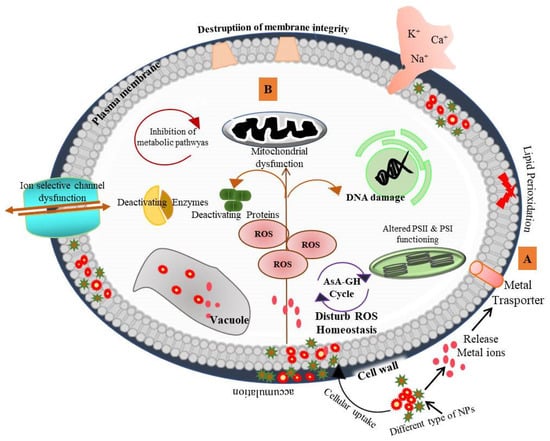
Figure 3.
Cellular toxicity mechanism of various nanoparticles in plants. [A]—Direct attachment of NPs to the cell surface releases metal ions which enter through ion channels and accumulate in cell wall intracellular space and vacuoles. Uptake and accumulation of NPs varies with the characteristics of NPs (shape, size, surface and charge). [B]—NP stress causes reactive oxygen species (ROS) generation and induces damaging responses [217] available at CC BY 4.0.
6. Soil Health and Biodiversity: Engineered Nanomaterials (ENMs) in Soil
It is not possible to gauge the concentration of NMs pollution in the environment due to a lack of proper detection tools and analytical approaches, so modeling predicted environmental concentrations are used. Based on the NMs production volume, it is estimated by a prediction model that the highest concentrations are expected for carbon-based NMs, followed by titanium oxide NPs and copper NPs in the aquatic systems; however, in soil, the highest concentrations are assumed for CeO2 and TiO2, followed by other NMs [222]. This information infers that NMs pollution reaches the soil by the end of the shelf life of nano-enabled products. ENMs enter the soil either by direct application or indirectly via atmospheric deposition, sludge application or agricultural irrigation [223]. The burden of NM pollution is much higher in soil than in water and air due to their low mobility [224]. Therefore, soil becomes the final sink for NMs released into the environment. Once incorporated in the soil matrix, NMs threaten the stability and function of the soil ecosystem. NMs interact with the soil’s organic and inorganic components and undergo a series of environmental transformations. Furthermore, NMs alter the porosity of soil, influencing the water dynamics and soil aggregation properties. Uncontrolled release of NMs into the soil matrix can have an adverse effect as they may potentially aggregate and not undergo degradation in the soil. Studies by Cao et al. and Kolesnikov et al. reported the impact of NMs on soil microscopic properties where ENMs at high concentrations negatively affected the dehydrogenase enzyme activity [225,226]. The effect of nanoparticles on soil enzymatic activity is varied by the nature of the nanoparticles; for example, the degree of influence on enzymatic activity in a soil sample is not the same when exposed to different NPs (Cu-, Ni- and Zn NPs). Catalase activity is greatly affected by the presence of Zn NPs in comparison to Ni- and Cu NPs. The overall enzyme activity of soil is sensitive to metal NPs in an order of Cu = Zn > Ni [226]. The duration of the NPs exposure also has a significant impact on enzymatic activity. As observed in silver-treated soil, the activities of -glucosaminidase, glucosidase, phosphatase and arylsulfatase decreased after 1 h and 1 week of treatment [227]. Another of the most studied issues of NM application in soil is their negative effect on soil microbial communities and the soil nutrient cycle. A study by Chen et al. showed the effect of multiwalled carbon nanotubes (MCNT) on soil enzyme activity and the diversity of rhizospheric microbial communities. They demonstrated the increased urease, phosphatase and sucrose activities under MCNT; however, the availability of available nitrogen and potassium was negatively affected by MCNT. The soil microbial taxonomic compositions were changed by the influence of MCNT [228]. Some researchers attribute the significant shift in the composition of the soil microbial community to metal NPs, especially highly dissolved heavy metal NPs. By exploring the relationship between bacteria and nitrogen functional genes, it was found that CuO NPs positively influence N fixation in the bacterial community by significantly increasing the expression of nifH and amoA genes (involved in nitrogen fixation) and negatively influencing the genes norB and nosZ (involved in denitrification) in bacteria [229]. Table 4 shows that soil microbial functional diversity and abundance significantly change when exposed to engineered nanomaterial. The homeostasis of microbes in soil is critical for maintaining plant and soil health. In the soil matrix, NMs can also affect the behavior of other soil pollutants. Recently, many researchers have studied the interaction of NMs with other pollutants. According to available literature, elevated CO2 (eCO2) can mitigate the toxicity of nano-Cr2O3 with respect to microbial biomass, soil enzymatic activities and bacterial alpha diversity in loamy soil [230].

Table 4.
Effect of different ENMs on soil microbial activity.
7. Environmental Concern of NMs (Toxicological Aspects)
The use of NMs in agroscience is expanding as a result of the population’s ever-growing demand for agricultural yields and more efficient methods to compensate for agricultural practices that are negatively impacting the environment [227]. In reality, the growth of precision farming and sustainable agriculture may be significantly impacted by nanotechnology. This strategy seeks to balance lowering inputs (such as fertilizers, pesticides and herbicides) with increasing agriculture output (i.e., crop yields). It monitors environmental variables and takes targeted action to achieve desired results [167,168]. Researchers are contemplating the potential negative impacts on human and environmental health due to emerging applications of nanotechnology in agriculture and other sectors influencing the global economy [237]. In fact, the intentional introduction of NMs into agro processes may have unforeseen health effects [238]. According to this scenario, bioaccumulation in the environment and food chain is one exposure route that could lead to increased uptake of nanomaterial residues by humans and other environmental entities.
The NPs, also known as nanostructured materials, frequently enter the soil, water and air in the environment. Lead and tin NPs, among others, have been shown to be extremely stable, stiff and non-degradable. Furthermore, these NPs have toxicological effects when they infiltrate the tissues and organs of plants, people and animals [239]. Furthermore, the usage of silver nanoparticles (NPs) in consumer goods is widespread and harms the environment of the aquatic system by altering fish, algae, bacteria and other aquatic animals [240].
The hazardous significance of nano-agrochemicals is determined by their configuration, the mass and structure of the NPs. Nanocomposites with crystal structures are more dangerous than those with amorphous structures because size and adverse effects of NPs are related and diminish the size of NPs. It has also been discovered that large doses of nanofertilizers can change molecules in a variety of ways and disrupt plant feeding. Moreover, IAA and ABA levels in plant cells can be lowered by an overdose of Cu NPs. Parallel to this, the application of Fe3O4 NPs to second-generation maize crops resulted in severe physiological damage due to a greater buildup of Fe, despite the fact that the same dose (100 ppm) of NFs was observed to be beneficial for first-generation maize [241]. The knowledge of the “dose effect” on the organism’s level should not be the sole basis for the ecotoxicological risk assessment of nano-agrochemicals. It should include a study of the lethal process starting at the level of the cell and cell organelles [211].
Although NPs have been extensively utilized in sustainability applications, it is still unclear how long-term exposure to them can have negative effects on both human health and the environment. As a result, an integrated risk analysis based on the life cycle of NMs is required, as well as an assessment of exposure and hazards using a predetermined method for testing and monitoring. Moreover, fewer toxic NMs are carbon based (e.g., fullerene, CNTs and graphene). NPs can be employed as an alternative to reduce the toxicity of NPs [212].
8. Future Perspectives
Nano-enabled technology and nano-based carrier system applications in agricultural sectors can undoubtedly tackle the challenges of the food security issues of growing populations and climate change. Nanotechnological application has developed in every sector by leaps and bounds, but our understanding of nanomaterial-associated environmental challenges is at its nascent stage. The development of suitable analytical technology for the detection of transformed NMs in environmental matrices is needed. This might be possible to a certain extent by modifying and customizing the currently available advanced techniques. Additionally, there is an urgent need to gauge the bioavailable portion of NMs to assess their toxicity in terrestrial ecosystems. As the nanomaterial market is expanding every year, strict guidelines, testing and legislation must be enforced to regulate the production, handling and disposal of NMs.
Sustainable development is the key to balancing the challenges of growth with its opportunities. Thus, ecofriendly processes should be used in the synthesis, administration and dissemination of NMs. Nanotechnological interventions such as nanopesticides, nanoherbicides and nanofertilizers have increased the output of agriculture. The research on how nano-enabled strategies or products produce the intended outcome is still lacking. The impacts of NMs’ interactions with living systems are defined by their size, shape, charge and hydrophobicity. The sensitivity of each species to the NMs is quite different. The ecotoxicological behaviors of any ENM are governed by their characteristics (i.e., type, size, surface charge, coating and crystal chemistry) and exposure conditions (i.e., concentrations, duration and soil physicochemical properties). A thorough characterization of the NMs’ epitope is therefore requisite for concluding the toxicological effects. Furthermore, MNMs’ influence on soil physicochemical properties should be evaluated completely. The transformed forms of different nanofabricated products in soil and their degradation should be fully investigated in environmental matrices.
In the past few years, NMs with a wide range of physical and chemical properties, such as type, size, surface charge, coating and crystal chemistry, have been made, dumped into the environment and deposited there. Future nanotechnology design requires a functional understanding of NMs’ interactions with biological systems. Complete knowledge of each NM’s potential toxicological aspects is an essentially limitless task. According to the evidence that is currently available, various NMs frequently demonstrate diverse toxicity tendencies in many complex biological systems, as well as in the environment. To address the gaps in the issues of nanotechnology and environmental safety in the future, a clear mechanistic mechanism of NMs toxicity in complex systems should be clarified.
Author Contributions
S.T., S.S. (Shivesh Sharma) and S.S. (Shivendra Sahi) made a substantial contribution to the concept and design of the article. S.T. and S.M. wrote the manuscript, and V.J. and K.T. assisted in the preparation and editing of figures and tables. S.R. and D.K.T. critically reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No data, models or code were generated or used during the study.
Acknowledgments
The authors are thankful to MNNIT Allahabad for providing the necessary facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Surendranath, A.; Mohanan, P.V. Impact of Nanoparticles in Balancing the Ecosystem. Biointerface Res. Appl. Chem 2020, 11, 10461–10481. [Google Scholar]
- Pramanik, P.; Krishnan, P.; Maity, A.; Mridha, N.; Mukherjee, A.; Rai, V. Application of nanotechnology in agriculture. Environ. Nanotechnol. 2020, 4, 317348. [Google Scholar]
- Mazayen, Z.M.; Ghoneim, A.M.; Elbatanony, R.S.; Basalious, E.B.; Bendas, E.R. Pharmaceutical nanotechnology: From the bench to the market. Future J. Pharm. Sci. 2022, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, P.; Giaouris, E.; Gardikis, K. Applications and Prospects of Nanotechnology in Food and Cosmetics Preservation. Nanomaterials 2022, 12, 1196. [Google Scholar] [CrossRef]
- Sudha, P.N.; Sangeetha, K.; Vijayalakshmi, K.; Barhoum, A. Nanomaterials history, classification, unique properties, production and market. In Emerging Applications of Nanoparticles and Architecture Nanostructures; Elsevier: Amsterdam, The Netherlands, 2018; pp. 341–384. [Google Scholar]
- Iravani, S. Bacteria in Nanoparticle Synthesis: Current Status and Future Prospects. Int. Sch. Res. Not. 2014, 2014, 59361. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Mughal, S.S.; Hassan, S.M. Comparative study of AgO nanoparticles synthesize via biological, chemical and physical methods: A review. AJMSP 2022, 15, 28. [Google Scholar]
- Sajid, M. Nanomaterials: Types, Properties, Recent Advances, and Toxicity Concerns. Curr. Opin. Environ. Sci. Health 2022, 25, 100319. [Google Scholar] [CrossRef]
- Sahu, P.K.; Brahmaprakash, G.P. Microbial Inoculants in Sustainable Agricultural Productivity Formulations of biofertilizers–approaches and advances. Funct. Appl. 2016, 2, 179–198. [Google Scholar]
- Bhatia, M. Encapsulation of Active Molecules and Their Delivery System A Review on Application of Encapsulation in Agricultural Processes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 131–140. [Google Scholar]
- Kour, D.; Rana, K.L.; Yadav, A.N.; Yadav, N.; Kumar, M.; Kumar, V.; Saxena, A.K. Microbial biofertilizers: Bioresources and eco-friendly technologies for agricultural and environmental sustainability. ISBAB 2020, 23, 101487. [Google Scholar] [CrossRef]
- Rai, P.; Singh, V.P.; Sharma, S.; Tripathi, D.K.; Sharma, S. Iron oxide nanoparticles impart cross tolerance to arsenate stress in rice roots through involvement of nitric oxide. Environ. Pollut. 2022, 307, 119320. [Google Scholar] [CrossRef]
- Bansal, K.; Hooda, V.; Verma, N.; Kharewal, T.; Tehri, N.; Dhull, V.; Gahlaut, A. Stress alleviation and crop improvement using silicon nanoparticles in agriculture: A review. Silicon 2022, 14, 10173–10186. [Google Scholar] [CrossRef]
- Keerthana, P.; Vijayakumar, S.; Vidhya, E.; Punitha, V.N.; Nilavukkarasi, M.; Praseetha, P.K. Biogenesis of ZnO nanoparticles for revolutionizing agriculture: A step towards anti-infection and growth promotion in plants. Ind. Crops Prod. 2021, 170, 113762. [Google Scholar]
- Chakraborty, R.; Mukhopadhyay, A.; Paul, S.; Sarkar, S.; Mukhopadhyay, R. Nanocomposite-based smart fertilizers: A boon to agricultural and environmental sustainability. Sci. Total Environ. 2023, 863, 160859. [Google Scholar] [CrossRef]
- Wirunchit, S.; Gansa, P.; Koetniyom, W. Synthesis of ZnO nanoparticles by Ball-milling process for biological applications. Mater. Today Proc. 2021, 47, 3554–3559. [Google Scholar] [CrossRef]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of copper nanoparticles: An overview of the various methods. Korean J. Chem. Eng. 2014, 31, 1105–1109. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Xu, K.; Chen, J. High-resolution scanning probe lithography technology: A review. Appl. Nanosci. 2019, 10, 1013–1022. [Google Scholar] [CrossRef]
- Garg, V.; Mote, R.G.; Fu, J. Facile fabrication of functional 3D micro-nano architectures with focused ion beam implantation and selective chemical etching. Appl. Surf. Sci. 2020, 526, 146644. [Google Scholar] [CrossRef]
- Suja, S.K.; Mathiya, S. Tribology of Nanofiber-and Nanofibril-Reinforced Polymeric Composites; Elsevier: Amsterdam, The Netherlands, 2023; pp. 297–333. [Google Scholar]
- Wang, X.-D.; Vinodgopal, K.; Dai, G.-P. Chapter 2: Synthesis of carbon nanotubes by catalytic chemical vapor deposition. In Perspective of Carbon Nanotubes; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol–gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Kurian, J.; Mathew, M.J. Structural, optical and magnetic studies of CuFe2O4, MgFe2O4 and ZnFe2O4 nanoparticles prepared by hydrothermal/solvothermal method. J. Magn. Magn. 2018, 451, 121–130. [Google Scholar] [CrossRef]
- Lone, I.H.; Radwan, N.R.; Aslam, J.; Akhter, A. Concept of reverse micelle method for the synthesis of nano-structured materials. Curr. Nanosci. 2019, 15, 129–136. [Google Scholar] [CrossRef]
- Zikalala, N.; Matshetshe, K.; Parani, S.; Oluwafemi, O.S. Biosynthesis protocols for colloidal metal oxide nanoparticles. Nano-Struct. Nano-Objects 2018, 16, 288–299. [Google Scholar] [CrossRef]
- Devatha, C.P.; Thalla, A.K. Green Synthesis of Nanomaterials. In Synthesis of Inorganic Nanomaterials; Woodhead Publishing: Sawston, UK, 2018; pp. 169–184. [Google Scholar]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green synthesis of nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N.; Han, S.S. From chemistry to biology: Applications and advantages of green, biosynthesized/biofabricated metal-and carbon-based nanoparticles. Fibers Polym. 2021, 22, 877–897. [Google Scholar] [CrossRef]
- Sarkar, J.; Mridha, D.; Davoodbasha, M.A.; Banerjee, J.; Chanda, S.; Ray, K.; Roychowdhury, T.; Acharya, K.; Sarkar, J. A State-of-the-Art Systemic Review on Selenium Nanoparticles: Mechanisms and Factors Influencing Biogenesis and Its Potential Applications. Biol. Trace Elem. Res. 2023. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Fouda, A.; Abdel-Rahman, M.A.; Hassan, S.E.-D.; El-Gamal, M.S.; Salem, S.S.; Shaheen, T.I. Fungal strain impacts the shape, bioactivity and multifunctional properties of green synthesized zinc oxide nanoparticles. Biocatal. Agric. Biotechnol. 2019, 19, 101103. [Google Scholar] [CrossRef]
- Tsekhmistrenko, S.I.; Bityutskyy, V.S.; Tsekhmistrenko, O.S.; Horalskyi, L.P.; Tymoshok, N.O.; Spivak, M.Y. Bacterial synthesis of nanoparticles: A green approach. Biosyst. Divers. 2020, 28, 9–17. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Ashtaputre, N.; Varadarajan, P.; Nachane, R.; Paralikar, K.; Balasubramanya, R. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater. Lett. 2007, 61, 1413–1418. [Google Scholar] [CrossRef]
- Balakumaran, M.; Ramachandran, R.; Kalaichelvan, P. Exploitation of endophytic fungus, Guignardia mangiferae for extracellular synthesis of silver nanoparticles and their in vitro biological activities. Microbiol. Res. 2015, 178, 9–17. [Google Scholar] [CrossRef]
- Elgorban, A.M.; Aref, S.M.; Seham, S.M.; Elhindi, K.M.; Bahkali, A.H.; Sayed, S.R.; Manal, M.A. Extracellular synthesis of silver nanoparticles using Aspergillus versicolor and evaluation of their activity on plant pathogenic fungi. Mycosphere 2016, 7, 844–852. [Google Scholar] [CrossRef]
- Hulikere, M.M.; Joshi, C.G. Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus—Cladosporium cladosporioides. Process Biochem. 2019, 82, 199–204. [Google Scholar] [CrossRef]
- Tyagi, S.; Tyagi, P.K.; Gola, D.; Chauhan, N.; Bharti, R.K. Extracellular synthesis of silver nanoparticles using entomopathogenic fungus: Characterization and antibacterial potential. SN Appl. Sci. 2019, 1, 1545. [Google Scholar] [CrossRef]
- Feroze, N.; Arshad, B.; Younas, M.; Afridi, M.I.; Saqib, S.; Ayaz, A. Fungal mediated synthesis of silver nanoparticles and evaluation of antibacterial activity. Microsc. Res. Tech. 2020, 83, 72–80. [Google Scholar] [CrossRef]
- Osorio-Echavarría, J.; Osorio-Echavarría, J.; Ossa-Orozco, C.P.; Gómez-Vanegas, N.A. Synthesis of silver nanoparticles using white-rot fungus Anamorphous Bjerkandera sp. R1: Influence of silver nitrate concentration and fungus growth time. Sci. Rep. 2021, 11, 3842. [Google Scholar] [CrossRef]
- Boldt, A.; Walter, J.; Hofbauer, F.; Stetter, K.; Aubel, I.; Bertau, M.; Jäger, C.M.; Walther, T. Cell-free synthesis of silver nanoparticles in spent media of different Aspergillus species. Eng. Life Sci. 2023, 23, e202200052. [Google Scholar] [CrossRef]
- Gupta, T.; Saxena, J. Biogenic synthesis of silver nanoparticles from Aspergillus oryzae mtcc 3107 against plant pathogenic fungi Sclerotinia sclerotiorum mtcc 8785. J. Microbiol. Biotechnol. Food Sci. 2023, 12, e9387. [Google Scholar] [CrossRef]
- Gurunathan, B.; Bathrinarayanan, P.V.; Muthukumarasamy, V.K.; Thangavelu, D. Characterization of Intracellular Gold Nanoparticles Synthesized by Biomass of Aspergillus terreus. Acta Met. Sin. Engl. Lett. 2014, 27, 569–572. [Google Scholar] [CrossRef]
- Molnár, Z.; Bódai, V.; Szakacs, G.; Erdélyi, B.; Fogarassy, Z.; Sáfrán, G.; Varga, T.; Kónya, Z.; Tóth-Szeles, E.; Szűcs, R.; et al. Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci. Rep. 2018, 8, 3943. [Google Scholar] [CrossRef]
- Vetchinkina, E.; Loshchinina, E.; Kupryashina, M.; Burov, A.; Pylaev, T.; Nikitina, V. Green synthesis of nanoparticles with extracellular and intracellular extracts of basidiomycetes. PeerJ 2018, 6, e5237. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Sivapriya, D. Fungus-mediated nanoparticles: Characterization and biomedical advances. In Nanoparticles in Medicine; Springer: Singapore, 2020; pp. 185–199. [Google Scholar] [CrossRef]
- Hietzschold, S.; Walter, A.; Davis, C.; Taylor, A.A. Sepunaru, LDoes nitrate reductase play a role in silver nanoparticle synthesis Evidence for NADPH as the sole reducing agent. ACS Sustain. Chem. Eng. 2019, 7, 8070–8076. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahmad, R.; Zeyaullah, M.; Khare, S.K. Microbial Nano-Factories: Synthesis and Biomedical Applications. Front. Chem. 2021, 9, 626834. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.H.; Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; Jin, C.-G.; Yang, D.C. Pseudomonas deceptionensis DC5-mediated synthesis of extracellular silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1576–1581. [Google Scholar] [CrossRef]
- Huq, M.A. Green Synthesis of Silver Nanoparticles Using Pseudoduganella eburnea MAHUQ-39 and Their Antimicrobial Mechanisms Investigation against Drug Resistant Human Pathogens. Int. J. Mol. Sci. 2020, 21, 1510. [Google Scholar] [CrossRef]
- Alsamhary, K.I. Eco-friendly synthesis of silver nanoparticles by Bacillus subtilis and their antibacterial activity. Saudi J. Biol. Sci. 2020, 27, 2185–2191. [Google Scholar] [CrossRef]
- Ameen, F.; AlYahya, S.; Govarthanan, M.; ALjahdali, N.; Al-Enazi, N.; Alsamhary, K.; Alharbi, S.A. Soil bacteria Cupriavidus sp. mediates the extracellular synthesis of antibacterial silver nanoparticles. J. Mol. Struct. 2020, 1202, 127233. [Google Scholar] [CrossRef]
- Harir, M.; Bendif, H.; Bellahcene, M.; Fortas, Z.; Pogni, R. Chapter 6: Streptomyces secondary metabolites. In Basic Biology and Applications of Actinobacteria; IntechOpen: London, UK, 2018; pp. 99–122. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.; Abdo, A.M.; El-Gamal, M.S. Antimicrobial, Antioxidant and Larvicidal Activities of Spherical Silver Nanoparticles Synthesized by Endophytic Streptomyces spp. Biol. Trace Elem. Res. 2020, 195, 707–724. [Google Scholar] [CrossRef]
- Marathe, K.; Naik, J.; Maheshwari, V. Biogenic synthesis of silver nanoparticles using Streptomyces spp. and their antifungal activity against Fusarium verticillioides. J. Clust. Sci. 2021, 32, 1299–1309. [Google Scholar] [CrossRef]
- Manivasagan, P.; Oh, J. Production of a Novel Fucoidanase for the Green Synthesis of Gold Nanoparticles by Streptomyces sp. and Its Cytotoxic Effect on HeLa Cells. Mar. Drugs 2015, 13, 6818–6837. [Google Scholar] [CrossRef]
- Balraj, B.; Senthilkumar, N.; Siva, C.; Krithikadevi, R.; Julie, A.; Potheher, I.V.; Arulmozhi, M. Synthesis and characterization of zinc oxide nanoparticles using marine Streptomyces sp. with its investigations on anticancer and antibacterial activity. Res. Chem. Intermed. 2017, 43, 2367–2376. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lim, J.S.; Harris, M.T. Synthesis and application of virus-based hybrid nanomaterials. Biotechnol. Bioeng. 2012, 109, 16–30. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Han, S.S. Helical plant viral nanoparticles—Bioinspired synthesis of nanomaterials and nanostructures. Bioinspir. Biomim. 2017, 12, 031001. [Google Scholar] [CrossRef]
- Love, A.J.; Makarov, V.; Yaminsky, I.; Kalinina, N.O.; Taliansky, M.E. The use of tobacco mosaic virus and cowpea mosaic virus for the production of novel metal nanomaterials. Virology 2014, 449, 133–139. [Google Scholar] [CrossRef]
- Alemzadeh, E.; Dehshahri, A.; Izadpanah, K.; Ahmadi, F. Plant virus nanoparticles: Novel and robust nanocarriers for drug delivery and imaging. Colloids Surf. B Biointerfaces 2018, 167, 20–27. [Google Scholar] [CrossRef]
- Ahiwale, S.S.; Bankar, A.V.; Tagunde, S.; Kapadnis, B.P. A Bacteriophage Mediated Gold Nanoparticles Synthesis and Their Anti-biofilm Activity. Indian J. Microbiol. 2017, 57, 188–194. [Google Scholar] [CrossRef]
- Thangavelu, R.M.; Ganapathy, R.; Ramasamy, P.; Krishnan, K. Fabrication of virus metal hybrid nanomaterials: An ideal reference for bio semiconductor. Arab. J. Chem. 2020, 13, 2750–2765. [Google Scholar] [CrossRef]
- Górzny, M.Ł.; Walton, A.S.; Evans, S.D. Synthesis of high-surface-area platinum nanotubes using a viral template. Adv. Funct. Mater. 2010, 20, 1295–1300. [Google Scholar] [CrossRef]
- Patel, J.M.; Kim, M.C.; Vartabedian, V.F.; Lee, Y.N.; He, S.; Song, J.M.; Choi, H.J.; Yamanaka, S.; Amaram, N.; Lukacher, A.; et al. Protein transfer-mediated surface engineering to adjuvantate virus-like nanoparticles for enhanced anti-viral immune responses. Nanomedicine 2015, 11, 1097–1107. [Google Scholar] [CrossRef]
- Kale, A.; Bao, Y.; Zhou, Z.; Prevelige, P.E.; Gupta, A. Directed self-assembly of CdS quantum dots on bacteriophage P22 coat protein templates. Nanotechnology 2013, 24, 045603. [Google Scholar] [CrossRef]
- Khan, A.A.; Fox, E.K.; Gorzny, M.Ł.; Nikulina, E.; Brougham, D.F.; Wege, C.; Bittner, A.M. pH Control of the Electrostatic Binding of Gold and Iron Oxide Nanoparticles to Tobacco Mosaic Virus. Langmuir 2013, 29, 2094–2098. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Zhang, S.; Zhang, Y.; He, S.; Tian, Y. Biosynthesis, characterization and antimicrobial activity of silver nanoparticles by a halotolerant Bacillus endophyticus SCU-L. Prep. Biochem. Biotechnol. 2018, 48, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Manikprabhu, D.; Cheng, J.; Chen, W.; Sunkara, A.K.; Mane, S.B.; Kumar, R.; Hozzein, W.N.; Duan, Y.Q.; Li, W.J. Sunlight mediated synthesis of silver nanoparticles by a novel actinobacterium (Sinomonas mesophila MPKL 26) and its antimicrobial activity against multi drug resistant Staphylococcus aureus. J. Photochem. Photobiol. B Biol. 2016, 158, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Monowar, T.; Rahman, M.S.; Bhore, S.J.; Raju, G.; Sathasivam, K.V. Silver Nanoparticles Synthesized by Using the Endophytic Bacterium Pantoea ananatis are Promising Antimicrobial Agents against Multidrug Resistant Bacteria. Molecules 2018, 23, 3220. [Google Scholar] [CrossRef]
- John, M.S.; Nagoth, J.A.; Ramasamy, K.P.; Mancini, A.; Giuli, G.; Natalello, A.; Ballarini, P.; Miceli, C.; Pucciarelli, S. Synthesis of Bioactive Silver Nanoparticles by a Pseudomonas Strain Associated with the Antarctic Psychrophilic Protozoon Euplotes focardii. Mar. Drugs 2020, 18, 38. [Google Scholar] [CrossRef]
- Singh, P.S.; Vidyasagar, G.M. Biosynthesis of antibacterial silver nano-particles from Aspergillus terreus. WNOFNS 2018, 16, 109–116. [Google Scholar]
- Ma, L.; Su, W.; Liu, J.X.; Zeng, X.X.; Huang, Z.; Li, W.; Liu, Z.C.; Tang, J.X. Optimization for extracellular biosynthesis of silver nanoparticles by Penicillium aculeatum Su1 and their antimicrobial activity and cytotoxic effect compared with silver ions. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 963–971. [Google Scholar] [CrossRef]
- Husseiny, S.M.; Salah, T.A.; Anter, H.A. Biosynthesis of size-controlled silver nanoparticles by Fusarium oxysporum, their antibacterial and antitumor activities. BJBAS 2015, 4, 225–231. [Google Scholar] [CrossRef]
- Amerasan, D.; Nataraj, T.; Murugan, K.; Panneerselvam, C.; Madhiyazhagan, P.; Nicoletti, M.; Benelli, G. Myco-synthesis of silver nanoparticles using Metarhizium anisopliae against the rural malaria vector Anopheles culicifacies Giles (Diptera: Culicidae). J. Pest Sci. 2015, 89, 249–256. [Google Scholar] [CrossRef]
- Fatima, R.; Priya, M.; Indurthi, L.; Radhakrishnan, V.; Sudhakaran, R. Biosynthesis of silver nanoparticles using red algae Portieria hornemannii and its antibacterial activity against fish pathogens. Microb. Pathog. 2020, 138, 103780. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.A.; Sundararaju, S.; Ramaraj, R.; Maniam, G.P.; Govindan, N. Synthesis of silver nanoparticles using marine macroalgae Padina sp. and its antibacterial activity towards pathogenic bacteria. BJBAS 2020, 9, 3. [Google Scholar] [CrossRef]
- Malhotra, A.; Dolma, K.; Kaur, N.; Rathore, Y.S.; Mayilraj, S.; Choudhury, A.R. Biosynthesis of gold and silver nanoparticles using a novel marine strain of Stenotrophomonas. Bioresour. Technol. 2013, 142, 727–731. [Google Scholar] [CrossRef]
- Srinath, B.S.; Namratha, K.; Byrappa, K. Eco-friendly synthesis of gold nanoparticles by Bacillus subtilis and their environmental applications. Adv. Sci. Lett. 2018, 24, 5942–5946. [Google Scholar] [CrossRef]
- Camas, M.; Sazak Camas, A.; Kyeremeh, K. Extracellular Synthesis and Characterization of Gold Nanoparticles Using Mycobacterium sp. BRS2A-AR2 Isolated from the Aerial Roots of the Ghanaian Mangrove Plant, Rhizophora racemosa. Indian J. Microbiol. 2018, 58, 214–221. [Google Scholar] [CrossRef]
- Joshi, C.G.; Danagoudar, A.; Poyya, J.; Kudva, A.K.; Dhananjaya, B.L. Biogenic synthesis of gold nanoparticles by marine endophytic fungus-Cladosporium cladosporioides isolated from seaweed and evaluation of their antioxidant and antimicrobial properties. Process Biochem. 2017, 63, 137–144. [Google Scholar]
- Shen, W.; Qu, Y.; Pei, X.; Li, S.; You, S.; Wang, J.; Zhang, Z.; Zhou, J. Catalytic reduction of 4-nitrophenol using gold nanoparticles biosynthesized by cell-free extracts of Aspergillus sp. WL-Au. J. Hazard. Mater. 2017, 321, 299–306. [Google Scholar] [CrossRef]
- Kitching, M.; Choudhary, P.; Inguva, S.; Guo, Y.; Ramani, M.; Das, S.K.; Marsili, E. Fungal surface protein mediated one-pot synthesis of stable and hemocompatible gold nanoparticles. Enzym. Microb. Technol. 2016, 95, 76–84. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Surendran, L.; Sudhagar, B.; Ranjith Santhosh Kumar, D.S. Facile green synthesis of gold nanoparticles from marine algae Gelidiella acerosa and evaluation of its biological Potential. SN Appl. Sci. 2019, 1, 284. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Prado-López, S.; Rodríguez-González, J.B.; Lastra, M.; Rodríguez-Argüelles, M.C. Green synthesis of gold nanoparticles using brown algae Cystoseira baccata: Its activity in colon cancer cells. Colloids Surf. B Biointerfaces 2017, 153, 190–198. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Z. Biosynthesis of gold nanoparticles using green alga Pithophora oedogonia with their electrochemical performance for determining carbendazim in soil. Int. J. Electrochem. Sci 2016, 11, 4550–4559. [Google Scholar] [CrossRef]
- Lv, Q.; Zhang, B.; Xing, X.; Zhao, Y.; Cai, R.; Wang, W.; Gu, Q. Biosynthesis of copper nanoparticles using Shewanella loihica PV-4 with antibacterial activity: Novel approach and mechanisms investigation. J. Hazard. Mater. 2018, 347, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Kimber, R.L.; Lewis, E.A.; Parmeggiani, F.; Smith, K.; Bagshaw, H.; Starborg, T.; Joshi, N.; Figueroa, A.I.; van der Laan, G.; Cibin, G.; et al. Biosynthesis and Characterization of Copper Nanoparticles Using Shewanella oneidensis: Application for Click Chemistry. Small 2018, 14, 1703145. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Dong, Y.; Wu, M.; Zhao, Y.; Liu, L.; Zhou, H. Characterization of selenite reduction by Lysinibacillus sp. ZYM-1 and photocatalytic performance of biogenic selenium nanospheres. ACS Sustain. Chem. Eng. 2017, 5, 2535–2543. [Google Scholar] [CrossRef]
- Wang, T.; Yang, L.; Zhang, B.; Liu, J. Extracellular biosynthesis and transformation of selenium nanoparticles and application in H2O2 biosensor. Colloids Surf. B Biointerfaces 2010, 80, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.Y.; Mao, C.; Gao, X.; Burt, J.L.; Belcher, A.M.; Georgiou, G.; Iverson, B.L. Bacterial biosynthesis of cadmium sulfide nanocrystals. Chem. Biol. 2004, 11, 1553–1559. [Google Scholar] [CrossRef]
- Raj, R.; Dalei, K.; Chakraborty, J.; Das, S. Extracellular polymeric substances of a marine bacterium mediated synthesis of CdS nanoparticles for removal of cadmium from aqueous solution. J. Colloid Interface Sci. 2016, 462, 166–175. [Google Scholar] [CrossRef]
- Ordenes-Aenishanslins, N.A.; Saona, L.A.; Durán-Toro, V.M.; Monrás, J.P.; Bravo, D.M.; Pérez-Donoso, J.M. Use of titanium dioxide nanoparticles biosynthesized by Bacillus mycoides in quantum dot sensitized solar cells. Microb. Cell Factories 2014, 13, 90. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Roopan, S.M.; Kirthi, A.V.; Venkatesan, J.; Kim, S.K.; Iyappan, M.; Siva, C. Biological approach to synthesize TiO2 nanoparticles using Aeromonas hydrophila and its antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 107, 82–89. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef]
- Jain, D.; Bhojiya, A.A.; Singh, H.; Daima, H.K.; Singh, M.; Mohanty, S.R.; Singh, A. Microbial fabrication of zinc oxide nanoparticles and evaluation of their antimicrobial and photocatalytic properties. Front. Chem. 2020, 8, 778. [Google Scholar] [CrossRef]
- Mashrai, A.; Khanam, H.; Aljawfi, R.N. Biological synthesis of ZnO nanoparticles using C. albicans and studying their catalytic performance in the synthesis of steroidal pyrazolines. Arab. J. Chem. 2017, 10, S1530–S1536. [Google Scholar]
- Fouda, A.; El-Din Hassan, S.; Salem, S.S.; Shaheen, T.I. In-Vitro cytotoxicity, antibacterial, and UV protection properties of the biosynthesized Zinc oxide nanoparticles for medical textile applications. Microb. Pathog. 2018, 125, 252–261. [Google Scholar] [CrossRef]
- Khalafi, T.; Buazar, F.; Ghanemi, K. Phycosynthesis and Enhanced Photocatalytic Activity of Zinc Oxide Nanoparticles Toward Organosulfur Pollutants. Sci. Rep. 2019, 9, 6866. [Google Scholar] [CrossRef]
- Sanaeimehr, Z.; Javadi, I.; Namvar, F. Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using Sargassum muticum algae extraction. Cancer Nanotechnol. 2018, 9, 3. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ashokkumar, T. Plant-mediated biosynthesis of metallic nanoparticles: A review of literature, factors affecting synthesis, characterization techniques and applications. J. Environ. Chem. Eng. 2017, 5, 4866–4883. [Google Scholar] [CrossRef]
- Oza, G.; Reyes-Calderón, A.; Mewada, A.; Arriaga, L.G.; Cabrera, G.B.; Luna, D.E.; Sharma, A. Plant-based metal and metal alloy nanoparticle synthesis: A comprehensive mechanistic approach. J. Mater. Sci. 2020, 55, 1309–1330. [Google Scholar] [CrossRef]
- Ettadili, F.E.; Aghris, S.; Laghrib, F.; Farahi, A.; Saqrane, S.; Bakasse, M.; El Mhammedi, M.A. Recent advances in the nanoparticle’s synthesis using plant extract: Applications and future recommendations. J. Mol. Struct. 2022, 1248, 131538. [Google Scholar] [CrossRef]
- Paulkumar, K.; Gnanajobitha, G.; Vanaja, M.; Rajeshkumar, S.; Malarkodi, C.; Pandian, K.; Annadurai, G. Piper nigrum leaf and stem assisted green synthesis of silver nanoparticles and evaluation of its antibacterial activity against agricultural plant pathogens. Sci. World J. 2014, 2014, 829894. [Google Scholar] [CrossRef]
- Hosny, M.; Fawzy, M.; El-Borady, O.M.; Mahmoud, A.E.D. Comparative study between Phragmites australis root and rhizome extracts for mediating gold nanoparticles synthesis and their medical and environmental applications. Adv. Powder Technol. 2021, 32, 2268–2279. [Google Scholar] [CrossRef]
- Rafique, M.; Tahir, R.; Gillani, S.S.A.; Tahir, M.B.; Shakil, M.; Iqbal, T.; Abdellahi, M.O. Plant-mediated green synthesis of zinc oxide nanoparticles from Syzygium cumini for seed germination and wastewater purification. J. Environ. Anal. Chem. 2022, 102, 23–38. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Javed, M.N.; Alam, M.S.; Rishishwar, P.; Rishishwar, S.; Ali, S.; Nayak, A.K.; Beg, S. Purple heart plant leaves extract-mediated silver nanoparticle synthesis: Optimization by Box-Behnken design. Mater. Sci. Eng. C 2019, 99, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Abdallah, Y.; Ali, M.A.; Masum, M.M.I.; Li, B.; Sun, G.; Meng, Y.; Wang, Y.; An, Q. Lemon-Fruit-Based Green Synthesis of Zinc Oxide Nanoparticles and Titanium Dioxide Nanoparticles against Soft Rot Bacterial Pathogen Dickeya dadantii. Biomolecules 2019, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Burlacu, E.; Tanase, C.; Coman, N.A.; Berta, L. A Review of Bark-Extract-Mediated Green Synthesis of Metallic Nanoparticles and Their Applications. Molecules 2019, 24, 4354. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Bhardwaj, K.; Kuča, K.; Kalia, A.; Nepovimova, E.; Verma, R.; Kumar, D. Flower-Based Green Synthesis of Metallic Nanoparticles: Applications beyond Fragrance. Nanomaterials 2020, 10, 766. [Google Scholar] [CrossRef]
- Heidari, Z.; Salehzadeh, A.; Sadat Shandiz, S.A.; Tajdoost, S. Anti-cancer and anti-oxidant properties of ethanolic leaf extract of Thymus vulgaris and its bio-functionalized silver nanoparticles. 3 Biotech 2018, 8, 177. [Google Scholar] [CrossRef]
- Shaik, M.R.; Khan, M.; Kuniyil, M.; Al-Warthan, A.; Alkhathlan, H.Z.; Siddiqui, M.R.H.; Adil, S.F. Plant-extract-assisted green synthesis of silver nanoparticles using Origanum vulgare L. extract and their microbicidal activities. Sustainability 2018, 10, 913. [Google Scholar] [CrossRef]
- Razavi, R.; Molaei, R.; Moradi, M.; Tajik, H.; Ezati, P.; Shafipour Yordshahi, A. Biosynthesis of metallic nanoparticles using mulberry fruit (Morus alba L.) extract for the preparation of antimicrobial nanocellulose film. Appl. Nanosci. 2020, 10, 465–476. [Google Scholar] [CrossRef]
- Chahardoli, A.; Karimi, N.; Sadeghi, F.; Fattahi, A. Green approach for synthesis of gold nanoparticles from Nigella arvensis leaf extract and evaluation of their antibacterial, antioxidant, cytotoxicity and catalytic activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 579–588. [Google Scholar] [CrossRef]
- Gopinath, V.; Priyadarshini, S.; MubarakAli, D.; Loke, M.F.; Thajuddin, N.; Alharbi, N.S.; Vadivelu, J. Anti-Helicobacter pylori, cytotoxicity and catalytic activity of biosynthesized gold nanoparticles: Multifaceted application. Arab. J. Chem. 2019, 12, 33–40. [Google Scholar] [CrossRef]
- Sharma, G.; Sharma, A.R.; Bhavesh, R.; Park, J.; Ganbold, B.; Nam, J.S.; Lee, S.S. Biomolecule-mediated synthesis of selenium nanoparticles using dried Vitis vinifera (raisin) extract. Molecules 2014, 19, 2761–2770. [Google Scholar] [CrossRef]
- Liang, T.; Qiu, X.; Ye, X.; Liu, Y.; Li, Z.; Tian, B.; Yan, D. Biosynthesis of selenium nanoparticles and their effect on changes in urinary nanocrystallites in calcium oxalate stone formation. 3 Biotech 2020, 10, 23. [Google Scholar] [CrossRef]
- Thakur, B.K.; Kumar, A.; Kumar, D. Green synthesis of titanium dioxide nanoparticles using Azadirachta indica leaf extract and evaluation of their antibacterial activity. S. Afr. J. Bot. 2019, 124, 223–227. [Google Scholar] [CrossRef]
- Rueda, D.; Arias, V.; Zhang, Y.; Cabot, A.; Agudelo, A.C.; Cadavid, D. Low-cost tangerine peel waste mediated production of titanium dioxide nanocrystals: Synthesis and characterization. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100285. [Google Scholar] [CrossRef]
- Santhoshkumar, J.; Kumar, S.V.; Rajeshkumar, S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resour.-Effic. Technol. 2017, 3, 459–465. [Google Scholar] [CrossRef]
- Iqbal, M.; Raja, N.I.; Mashwani, Z.-U.-R.; Hussain, M.; Ejaz, M.; Yasmeen, F. Effect of Silver Nanoparticles on Growth of Wheat Under Heat Stress. Iran. J. Sci. Technol. Trans. A Sci. 2019, 43, 387–395. [Google Scholar] [CrossRef]
- Venkatachalam, P.; Priyanka, N.; Manikandan, K.; Ganeshbabu, I.; Indiraarulselvi, P.; Geetha, N.; Muralikrishna, K.; Bhattacharya, R.; Tiwari, M.; Sharma, N.; et al. Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2017, 110, 118–127. [Google Scholar] [CrossRef]
- Azimi, R.; Feizi, H.; Hosseini, M.K. Can Bulk and Nanosized Titanium Dioxide Particles Improve Seed Germination Features of Wheatgrass (Agropyron desertorum). Not. Sci. Biol. 2013, 5, 325–331. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, X.; Yang, W.; Xie, H.; Ashraf, U.; Mo, Z.; Liu, J.; Li, G.; Li, W. Seed Priming with Multiwall Carbon Nanotubes (MWCNTs) Modulates Seed Germination and Early Growth of Maize Under Cadmium (Cd) Toxicity. J. Soil Sci. Plant Nutr. 2021, 21, 1793–1805. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Q. Graphene ameliorates saline-alkaline stress-induced damage and improves growth and tolerance in alfalfa (Medicago sativa L.). Plant Physiol. Biochem. 2021, 163, 128–138. [Google Scholar] [CrossRef]
- Zhu, J.; Zou, Z.; Shen, Y.; Li, J.; Shi, S.; Han, S.; Zhan, X. Increased ZnO nanoparticle toxicity to wheat upon co-exposure to phenanthrene. Environ. Pollut. 2019, 247, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, U.; Luo, X.; Wang, Q.; Shu, K. Are There Unidentified Factors Involved in the Germination of Nanoprimed Seeds. Front. Plant Sci. 2020, 11, 832. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Gui, X.; Deng, Y.; Rui, Y.; Gao, B.; Luo, W.; Chen, S.; Van Nhan, L.; Li, X.; Liu, S.; Han, Y.; et al. Response difference of transgenic and conventional rice (Oryza sativa) to nanoparticles (γFe2O3). Environ. Sci. Pollut. Res. 2015, 22, 17716–17723. [Google Scholar] [CrossRef] [PubMed]
- Faraji, J.; Sepehri, A. Efeitos benéficos de nanopartículas de TiO2e nitroprussiato de sódio na germinação de sementes e no crescimento de plântulas de trigo sob estresse hídrico estimulado por PEGJ. Seed Sci. 2019, 41, 309–317. [Google Scholar] [CrossRef]
- Rhaman, M.S.; Tania, S.S.; Imran, S.; Rauf, F.; Kibria, M.G.; Ye, W.; Hasanuzzaman, M.; Murata, Y. Seed Priming with Nanoparticles: An Emerging Technique for Improving Plant Growth, Development, and Abiotic Stress Tolerance. J. Soil Sci. Plant Nutr. 2022, 22, 4047–4062. [Google Scholar] [CrossRef]
- Shang, Y.; Hasan, M.K.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of Nanotechnology in Plant Growth and Crop Protection: A Review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef]
- Ahmed, B.; Khan, M.S.; Musarrat, J. Toxicity assessment of metal oxide nano-pollutants on tomato (Solanum lycopersicon): A study on growth dynamics and plant cell death. Environ. Pollut. 2018, 240, 802–816. [Google Scholar] [CrossRef]
- Hassanisaadi, M.; Barani, M.; Rahdar, A.; Heidary, M.; Thysiadou, A.; Kyzas, G.Z. Role of agrochemical-based nanomaterials in plants: Biotic and abiotic stress with germination improvement of seeds. Plant Growth Regul. 2022, 97, 375–418. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Mundekkad, D.; Ramalingam, C.; Shanker, R.; Kumar, A. Nanotechnology in agro-food: From field to plate. Int. Food Res. J. 2015, 69, 381–400. [Google Scholar] [CrossRef]
- de Oliveira, J.L.; Campos, E.V.; Gonçalves da Silva, C.M.; Pasquoto, T.; Lima, R.; Fraceto, L.F. Solid lipid nanoparticles co-loaded with simazine and atrazine: Preparation, characterization, and evaluation of herbicidal activity. J. Agric. Food Chem. 2015, 63, 422–432. [Google Scholar] [CrossRef]
- Chidambaram, R. Application of rice husk nanosorbents containing 2,4-dichlorophenoxyacetic acid herbicide to control weeds and reduce leaching from soil. J. Taiwan Inst. Chem. Eng. 2016, 63, 318–326. [Google Scholar]
- Silva, M.d.S.; Cocenza, D.S.; Grillo, R.; de Melo, N.F.; Tonello, P.S.; de Oliveira, L.C.; Cassimiro, D.L.; Rosa, A.H.; Fraceto, L.F. Paraquat-loaded alginate/chitosan nanoparticles: Preparation, characterization and soil sorption studies. J. Hazard. Mater. 2011, 190, 366–374. [Google Scholar] [CrossRef]
- Sharma, A.; Sood, K.; Kaur, J.; Khatri, M. Agrochemical loaded biocompatible chitosan nanoparticles for insect pest management. ISBAB 2019, 18, 101079. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Zinc encapsulated chitosan nanoparticle to promote maize crop yield. Int. J. Biol. Macromol. 2019, 127, 126–135. [Google Scholar] [CrossRef]
- Ghareeb, R.Y.; Shams El-Din, N.G.E.D.; Maghraby, D.M.E.; Ibrahim, D.S.; Abdel-Megeed, A.; Abdelsalam, N.R. Nematicidal activity of seaweed-synthesized silver nanoparticles and extracts against Meloidogyne incognita on tomato plants. Sci. Rep. 2022, 12, 3841. [Google Scholar] [CrossRef]
- Mózner, Z.; Tabi, A.; Csutora, M. Modifying the yield factor based on more efficient use of fertilizer—The environmental impacts of intensive and extensive agricultural practices. Ecol. Indic. 2012, 16, 58–66. [Google Scholar] [CrossRef]
- Solanki, P.; Bhargava, A.; Chhipa, H.; Jain, N.; Panwar, J. Nano-fertilizers and Their Smart Delivery System. In Nanotechnologies in Food and Agriculture; Springer: Cham, Switzerland, 2015; pp. 81–101. [Google Scholar]
- Huang, S.; Wang, L.; Liu, L.; Hou, Y.; Li, L. Nanotechnology in agriculture, livestock, and aquaculture in China: A review. ASD 2015, 35, 369–400. [Google Scholar] [CrossRef]
- Sharma, S.K.; Sharma, P.K.; Mandeewal, R.L.; Sharma, V.; Chaudhary, R.; Pandey, R.; Gupta, S. Effect of Foliar Application of Nano-Urea Under Different Nitrogen Levels on Growth and Nutrient Content of Pearl millet (Pennisetum glaucum L.). Int. J. Plant Soil Sci. 2022, 34, 149–155. [Google Scholar] [CrossRef]
- Rady, M.M.; Mossa, A.-T.H.; Youssof, A.M.; Osman, A.S.; Ahmed, S.M.; Mohamed, I.A. Exploring the reinforcing effect of nano-potassium on the antioxidant defense system reflecting the increased yield and quality of salt-stressed squash plants. Sci. Hortic. 2023, 308, 111609. [Google Scholar] [CrossRef]
- El-Desouky, H.S.; Islam, K.R.; Bergefurd, B.; Gao, G.; Harker, T.; Abd-El-Dayem, H.; Ismail, F.; Mady, M.; Zewail, R.M.Y. Nano iron fertilization significantly increases tomato yield by increasing plants’ vegetable growth and photosynthetic efficiency. J. Plant Nutr. 2021, 44, 1649–1663. [Google Scholar] [CrossRef]
- Gatahi, D.M.; Wanyika, H.; Kihurani, A.W.; Ateka, E.; Kavoo, A. Use of Bio-Nanocomposites in Enhancing Bacterial Wilt Plant Resistance, Tomato Production and Water Conservation in Greenhouse Farming. In Proceedings of the 2015 JKUAT Scientific Conference: Agricultural Sciences, Technologies and Global Networking, Juja, Kenya, 12–13 November 2015. [Google Scholar]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Singh, D.P. Nano-biofertilizer: An emerging eco-friendly approach for sustainable agriculture. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2020, 90, 733–741. [Google Scholar] [CrossRef]
- Rajak, J.; Bawaskar, M.; Rathod, D.; Agarkar, G.; Nagaonkar, D.; Gade, A.; Rai, M. Interaction of copper nanoparticles and an endophytic growth promoter Piriformospora indica with Cajanus cajan. J. Sci. Food Agric. 2017, 97, 4562–4570. [Google Scholar] [CrossRef]
- Mathur, S.; Pareek, S.; Shrivastava, D. Nanofertilizers for Development of Sustainable Agriculture. Commun. Soil Sci. Plant Anal. 2022, 53, 1999–2016. [Google Scholar] [CrossRef]
- Hojjat, S.S.; Kamyab, M. The effect of silver nanoparticle on Fenugreek seed germination under salinity levels. Russ. Agric. Sci. 2017, 43, 61–65. [Google Scholar] [CrossRef]
- Prakash, V.; Rai, P.; Sharma, N.C.; Singh, V.P.; Tripathi, D.K.; Sharma, S.; Sahi, S. Application of zinc oxide nanoparticles as fertilizer boosts growth in rice plant and alleviates chromium stress by regulating genes involved in oxidative stress. Chemosphere 2022, 303, 134554. [Google Scholar] [CrossRef]
- Singh, J.; Lee, B.K. Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): A possible mechanism for the removal of Cd from the contaminated soil. J. Environ. Manag. 2016, 170, 88–96. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Yue, L.; Wang, C.; Tao, M.; Wang, Z.; Xing, B. Foliar-applied cerium oxide nanomaterials improve maize yield under salinity stress: Reactive oxygen species homeostasis and rhizobacteria regulation. Environ. Pollut. 2022, 299, 118900. [Google Scholar] [CrossRef]
- Hajizadeh, H.S.; Asadi, M.; Zahedi, S.M.; Hamzehpour, N.; Rasouli, F.; Helvacı, M.; Alas, T. Silicon dioxide-nanoparticle nutrition mitigates salinity in gerbera by modulating ion accumulation and antioxidants. Folia Hortic. 2021, 33, 91–105. [Google Scholar] [CrossRef]
- Rezvani, N.; Sorooshzadeh, A.; Farhadi, N. Effect of nano-silver on growth of saffron in flooding stress. Int. J. Agric. Biol. Eng. 2012, 6, 11–16. [Google Scholar]
- Mustafa, G.; Komatsu, S. Insights into the Response of Soybean Mitochondrial Proteins to Various Sizes of Aluminum Oxide Nanoparticles under Flooding Stress. J. Proteome Res. 2016, 15, 4464–4475. [Google Scholar] [CrossRef]
- Hatami, M.; Ghorbanpour, M. Effect of nanosilver on physiological performance of pelargonium plants exposed to dark storage. Hortic. Res. 2013, 21, 15–20. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Tang, Y.; Naz, I.; Alam, S.S.; Wang, W.; Ahmad, M.; Najeeb, S.; Rao, C.; Li, Y.; Xie, B.; et al. Management of Ralstonia solanacearum in Tomato Using ZnO Nanoparticles Synthesized Through Matricaria chamomilla. Plant Dis. 2021, 105, 3224–3230. [Google Scholar] [CrossRef]
- Boxi, S.S.; Mukherjee, K.; Paria, S. Ag doped hollow TiO2 nanoparticles as an effective green fungicide against Fusarium solani and Venturia inaequalis phytopathogens. Nanotechnology 2016, 27, 085103. [Google Scholar] [CrossRef]
- Norman, D.J.; Chen, J. Effect of foliar application of titanium dioxide on bacterial blight of geranium and Xanthomonas leaf spot of poinsettia. HortScience 2011, 46, 426–428. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, P.; Gu, W.; Jiang, J. Application of anatasa TiO2 sol derived from peroxotitannic acid in crop diseases control and growth regulation. NSTI-Nanotech 2009, 2, 286–289. [Google Scholar]
- Sampathkumar, K.; Tan, K.X.; Loo, S.C.J. Developing Nano-Delivery Systems for Agriculture and Food Applications with Nature-Derived Polymers. iScience 2020, 23, 101055. [Google Scholar] [CrossRef]
- Mishra, A.; Saini, R.K.; Bajpai, A.K. Polymer Formulations for Pesticide Release. In Controlled Release of Pesticides for Sustainable Agriculture; Springer: Cham, Switzerland, 2020; pp. 185–206. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 321–334. [Google Scholar] [CrossRef]
- Chanratana, M.; Han, G.H.; Joe, M.M.; Choudhury, A.R.; Sundaram, S.; Halim, A.; Sa, T. Evaluation of chitosan and alginate immobilized Methylobacterium oryzae CBMB20 on tomato plant growth. Arch. Agron. Soil Sci. 2018, 64, 1489–1502. [Google Scholar] [CrossRef]
- Fernández, M.; Pagnussat, L.A.; Borrajo, M.P.; Perez Bravo, J.J.; Francois, N.J.; Creus, C.M. Chitosan/starch beads as bioinoculants carrier: Long-term survival of bacteria and plant growth promotion. Appl. Microbiol. Biotechnol. 2022, 106, 7963–7972. [Google Scholar] [CrossRef] [PubMed]
- Sinha, T.; Bhagwatwar, P.; Krishnamoorthy, C.; Chidambaram, R. Polymer based micro- and nanoencapsulation of agrochemicals. In Polymers for Agri-Food Applications; Springer: Cham, Switzerland, 2019; pp. 5–28. [Google Scholar] [CrossRef]
- Tesfay, S.Z.; Magwaza, L.S.; Mbili, N.; Mditshwa, A. Carboxyl methylcellulose (CMC) containing moringa plant extracts as new postharvest organic edible coating for Avocado (Persea americana Mill.) fruit. Sci. Hortic. 2017, 226, 201–207. [Google Scholar] [CrossRef]
- Saekow, M.; Naradisorn, M.; Tongdeesoontorn, W.; Hamauzu, Y. Effect of carboxymethyl cellulose coating containing ZnO-nanoparticles for prolonging shelf life of persimmon and tomato fruit. JFAT 2019, 5, 41–48. [Google Scholar]
- Aouada, F.A.; De Moura, M.R. Nanotechnology applied in agriculture: Controlled release of agrochemicals. In Nanotechnologies in Food and Agriculture; Springer: Cham, Switzerland, 2015; pp. 103–118. [Google Scholar] [CrossRef]
- Kumar, S.; Bhanjana, G.; Sharma, A.; Sidhu, M.C.; Dilbaghi, N. Synthesis, characterization and on field evaluation of pesticide loaded sodium alginate nanoparticles. Carbohydr. Polym. 2014, 101, 1061–1067. [Google Scholar] [CrossRef]
- Xie, Y.L.; Jiang, W.; Li, F.; Zhang, Y.; Liang, X.Y.; Wang, M.; Zhou, X.; Wu, S.Y.; Zhang, C.H. Controlled Release of Spirotetramat Using Starch-Chitosan-Alginate-Encapsulation. Bull. Environ. Contam. Toxicol. 2020, 104, 149–155. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Ebrahimi-Zarandi, M.; Tamanadar, E.; Moradi Pour, M.; Thakur, V.K. Salinity stress: Toward sustainable plant strategies and using plant growth-promoting rhizobacteria encapsulation for reducing it. Sustainability 2021, 13, 12758. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; van Hullebusch, E.D.; Rtimi, S. Preparation and applications of chitosan and cellulose composite materials. J. Environ. Manage 2022, 301, 113850. [Google Scholar] [CrossRef]
- Chen, S.; Han, Y.; Jian, L.; Liao, W.; Zhang, Y.; Gao, Y. Fabrication, characterization, physicochemical stability of zein-chitosan nanocomplex for co-encapsulating curcumin and resveratrol. Carbohydr. Polym. 2020, 236, 116090. [Google Scholar] [CrossRef]
- Hossain, F.; Follett, P.; Salmieri, S.; Vu, K.D.; Fraschini, C.; Lacroix, M. Antifungal activities of combined treatments of irradiation and essential oils (EOs) encapsulated chitosan nanocomposite films in in vitro and in situ conditions. Int. J. Food Microbiol. 2019, 295, 33–40. [Google Scholar] [CrossRef]
- Abd El-Mohdy, H.L.; Abd El-Rehim, H.A. Radiation synthesis of kappa-carrageenan/acrylamide graft copolymers as superabsorbents and their possible applications. J. Polym. Res. 2009, 16, 63–72. [Google Scholar] [CrossRef]
- Alves, S.F.; Borges, L.L.; dos Santos, T.O.; de Paula, J.R.; Conceição, E.C.; Bara, M.T. Microencapsulation of essential oil from fruits of Pterodon emarginatus using gum Arabic and maltodextrin as wall materials: Composition and stability. Dry. Technol. 2014, 32, 96–105. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Campos, E.V.R.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: Prospects and promises. Biotechnol. Adv. 2014, 32, 1550–1561. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, G.; Dong, H.; Geng, Q.; Niu, J.; Tang, J.; Yang, J.; Huo, H.; Cao, Y. Targeted release mechanism of λ-cyhalothrin nanocapsules using dopamine-conjugated silica as carrier materials. Colloids Surf. B Biointerfaces 2019, 178, 153–162. [Google Scholar] [CrossRef]
- Pachapur, V.L.; Dalila Larios, A.; Cledón, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y. Behavior and characterization of titanium dioxide and silver nanoparticles in soils. Sci. Total Environ. 2016, 563–564, 933–943. [Google Scholar] [CrossRef]
- Ogunkunle, C.O.; Oyedeji, S.; Okoro, H.K.; Adimula, V. Interaction of Nanoparticles with Soil. In Nanomaterials for Soil Remediation; Elsevier: Amsterdam, The Netherlands, 2021; pp. 101–132. [Google Scholar]
- Tripathi, D.K.; Tripathi, A.; Shweta; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K.; et al. Uptake, Accumulation and Toxicity of Silver Nanoparticle in Autotrophic Plants, and Heterotrophic Microbes: A Concentric Review. Front Microbiol. 2017, 8, 7. [Google Scholar] [CrossRef]
- Stegemeier, J.P.; Colman, B.P.; Schwab, F.; Wiesner, M.R.; Lowry, G.V. Uptake and Distribution of Silver in the Aquatic Plant Landoltia punctata (Duckweed) Exposed to Silver and Silver Sulfide Nanoparticles. Environ. Sci. Technol. 2017, 51, 4936–4943. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, W.; Liu, G.; Song, M.; Tan, Z.; Mao, Y.; Jiang, G. Transformation and uptake of silver nanoparticles and silver ions in rice plant (Oryza sativa L.): The effect of iron plaque and dissolved iron. Environ. Sci. Nano 2020, 7, 599–609. [Google Scholar] [CrossRef]
- Yusefi-Tanha, E.; Fallah, S.; Rostamnejadi, A.; Pokhrel, L.R. Particle size and concentration dependent toxicity of copper oxide nanoparticles (CuONPs) on seed yield and antioxidant defense system in soil grown soybean (Glycinemax cv. Kowsar). Sci. Total Environ. 2020, 715, 136994. [Google Scholar] [CrossRef]
- Pagano, L.; Rossi, R.; White, J.C.; Marmiroli, N.; Marmiroli, M. Nanomaterials biotransformation: In planta mechanisms of action. Environ. Pollut. 2023, 318, 120834. [Google Scholar] [CrossRef]
- Adams, J.; Wright, M.; Wagner, H.; Valiente, J.; Britt, D.; Anderson, A. Cu from dissolution of CuO nanoparticles signals changes in root morphology. Plant Physiol. Biochem. 2017, 110, 108–117. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Sun, S.; Scheckel, K.G.; Malysheva, A.; McKenna, B.A.; Kopittke, P.M. Characterizing the uptake, accumulation and toxicity of silver sulfide nanoparticles in plants. Environ. Sci. Nano 2017, 4, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Barrios, A.C.; Rico, C.M.; Trujillo-Reyes, J.; Medina-Velo, I.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of uncoated and citric acid coated cerium oxide nanoparticles, bulk cerium oxide, cerium acetate, and citric acid on tomato plants. Sci. Total Environ. 2016, 563, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Chen, F.; Yu, K.; Xiao, Z.; Yu, X.; Wang, Z.; Xing, B. Early development of apoplastic barriers and molecular mechanisms in juvenile maize roots in response to La2O3 nanoparticles. Sci. Total Environ. 2019, 653, 675–683. [Google Scholar] [CrossRef]
- Liu, Y.; Persson, D.P.; Li, J.; Liang, Y.; Li, T. Exposure of cerium oxide nanoparticles to the hyperaccumulator Sedum alfredii decreases the uptake of cadmium via the apoplastic pathway. J. Hazard. Mater. 2021, 417, 125955. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Qi, L.; Li, Y.; Wang, J.; Shen, C.; Ge, Y. Negative effects of rare earth oxide nanoparticles of La2O3, Nd2O3, and Gd2O3 on the ammonia-oxidizing microorganisms. JSS 2020, 20, 3114–3123. [Google Scholar] [CrossRef]
- Larue, C.; Castillo-Michel, H.; Sobanska, S.; Cecillon, L.; Bureau, S.; Barthès, V.; Sarret, G. Foliar exposure of the crop Lactuca sativa to silver nanoparticles: Evidence for internalization and changes in Ag speciation. J. Hazard. Mater. 2014, 264, 98–106. [Google Scholar] [CrossRef]
- Moazzami Farida, S.H.; Karamian, R.; Albrectsen, B.R. Silver nanoparticle pollutants activate oxidative stress responses and rosmarinic acid accumulation in sage. Physiol. Plant. 2020, 170, 415–432. [Google Scholar] [CrossRef]
- Banijamali, S.M.; Feizian, M.; Alinejadian Bidabadi, A.; Mehdipour, E. Evaluation Uptake and Translocation of Iron Oxide Nanoparticles and Its Effect on Photosynthetic Pigmentation of Chrysanthemum (Chrysanthemum morifolium) ‘Salvador’. J. Ornam. Plants 2019, 9, 245–258. [Google Scholar]
- Zong, X.; Wu, D.; Zhang, J.; Tong, X.; Yin, Y.; Sun, Y.; Guo, H. Size-dependent biological effect of copper oxide nanoparticles exposure on cucumber (Cucumis sativus). Environ. Sci. Pollut. Res. Int. 2022, 29, 69517–69526. [Google Scholar] [CrossRef]
- Yin, L.; Cheng, Y.; Espinasse, B.; Colman, B.P.; Auffan, M.; Wiesner, M.; Bernhardt, E.S. More than the ions: The effects of silver nanoparticles on Lolium multiflorum. Environ. Sci. Technol. 2011, 45, 2360–2367. [Google Scholar] [CrossRef]
- Li, J.; Mu, Q.; Du, Y.; Luo, J.; Liu, Y.; Li, T. Growth and Photosynthetic Inhibition of Cerium Oxide Nanoparticles on Soybean (Glycine max). Bull. Environ. Contam. Toxicol. 2020, 105, 119–126. [Google Scholar] [CrossRef]
- Khodaparast, Z.; van Gestel, C.A.; Verweij, R.A.; Papadiamantis, A.G.; Gonçalves, S.F.; Lynch, I.; Loureiro, S. Effects of sulfidation of silver nanoparticles on the Ag uptake kinetics in Brassica rapa plants. JHM Lett. 2022, 435, 128880. [Google Scholar] [CrossRef]
- Tan, J.; Chen, Y.; Mo, Z.; Tan, C.; Wen, R.; Chen, Z.; Tian, H. Zinc oxide nanoparticles and polyethylene microplastics affect the growth, physiological and biochemical attributes, and Zn accumulation of rice seedlings. Environ. Sci. Pollut. Res. 2022, 29, 61534–61546. [Google Scholar] [CrossRef]
- Noori, A.; Ngo, A.; Gutierrez, P.; Theberge, S.; White, J.C. Silver nanoparticle detection and accumulation in tomato (Lycopersicon esculentum). J. Nanopart. Res. 2020, 22, 1–16. [Google Scholar] [CrossRef]
- Asztemborska, M.; Steborowski, R.; Kowalska, J.; Bystrzejewska-Piotrowska, G. Accumulation of Platinum Nanoparticles by Sinapis alba and Lepidium sativum Plants. Water Air Soil Pollut. 2015, 226, 126. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Mishra, R.K.; Singh, S.; Singh, S.; Vishwakarma, K.; Sharma, S.; Singh, V.P.; Singh, P.K.; Prasad, S.M.; Dubey, N.K.; et al. Nitric Oxide Ameliorates Zinc Oxide Nanoparticles Phytotoxicity in Wheat Seedlings: Implication of the Ascorbate-Glutathione Cycle. Front. Plant Sci. 2017, 8, 1. [Google Scholar] [CrossRef]
- Dias, M.C.; Santos, C.; Pinto, G.; Silva, A.M.S.; Silva, S. Titanium dioxide nanoparticles impaired both photochemical and non-photochemical phases of photosynthesis in wheat. Protoplasma 2019, 256, 69–78. [Google Scholar] [CrossRef]
- Xiong, T.; Zhang, S.; Kang, Z.; Zhang, T.; Li, S. Dose-dependent physiological and transcriptomic responses of lettuce (Lactuca sativa L.) to copper oxide nanoparticles—Insights into the phytotoxicity mechanisms. Int. J. Mol. Sci. 2021, 22, 3688. [Google Scholar] [CrossRef]
- Yang, S.; Yin, R.; Wang, C.; Yang, Y.; Wang, J. Phytotoxicity of zinc oxide nanoparticles and multi-walled carbon nanotubes, alone or in combination, on Arabidopsis thaliana and their mutual effects on oxidative homeostasis. PLoS ONE 2023, 18, e0281756. [Google Scholar] [CrossRef]
- Gupta, S.D.; Agarwal, A.; Pradhan, S. Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: An insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicol. Environ. Saf. 2018, 161, 624–633. [Google Scholar] [CrossRef]
- Mosa, K.A.; El-Naggar, M.; Ramamoorthy, K.; Alawadhi, H.; Elnaggar, A.; Wartanian, S.; Hani, H. Copper nanoparticles induced genotoxicty, oxidative stress, and changes in superoxide dismutase (SOD) gene expression in cucumber (Cucumis sativus) plants. Front. Plant Sci. 2018, 9, 872. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, L.; Zhao, J.; Wang, X.; White, J.C.; Xing, B. CuO nanoparticle interaction with Arabidopsis thaliana: Toxicity, parent-progeny transfer, and gene expression. Environ. Sci. Technol. 2016, 50, 6008–6016. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Pagano, L.; Wohlschlegel, J.A.; Villani, M.; Zappettini, A.; White, J.C.; Keller, A.A. Proteomic, gene and metabolite characterization reveal the uptake and toxicity mechanisms of cadmium sulfide quantum dots in soybean plants. Environ. Sci. Nano 2019, 6, 3010–3026. [Google Scholar] [CrossRef]
- Hossain, Z.; Mustafa, G.; Komatsu, S. Plant Responses to Nanoparticle Stress. Int. J. Mol. Sci. 2015, 16, 26644–26653. [Google Scholar] [CrossRef] [PubMed]
- Jośko, I.; Oleszczuk, P.; Skwarek, E. Toxicity of combined mixtures of nanoparticles to plants. J. Hazard. Mater. 2017, 331, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Vitali, F.; Raio, A.; Sebastiani, F.; Cherubini, P.; Cavalieri, D.; Cocozza, C. Environmental pollution effects on plant microbiota: The case study of poplar bacterial-fungal response to silver nanoparticles. Appl. Microbiol. Biotechnol. 2019, 103, 8215–8227. [Google Scholar] [CrossRef]
- VandeVoort, A.R.; Arai, Y. Macroscopic Observation of Soil Nitrification Kinetics Impacted by Copper Nanoparticles: Implications for Micronutrient Nanofertilizer. Nanomaterials 2018, 8, 927. [Google Scholar] [CrossRef]
- Simonin, M.; Cantarel, A.A.M.; Crouzet, A.; Gervaix, J.; Martins, J.M.F.; Richaume, A. Negative Effects of Copper Oxide Nanoparticles on Carbon and Nitrogen Cycle Microbial Activities in Contrasting Agricultural Soils and in Presence of Plants. Front. Microbiol. 2018, 9, 3102. [Google Scholar] [CrossRef]
- Gottschalk, F.; Lassen, C.; Kjoelholt, J.; Christensen, F.; Nowack, B. Modeling flows and concentrations of nine engineered nanomaterials in the Danish environment. Int. J. Environ. Res. Public Health 2015, 12, 5581–5602. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Qian, S.; Zhou, X.; Fu, Y.; Song, B.; Yan, H.; Chen, Z.; Sun, Q.; Ye, H.; Qin, L.; Lai, C. Biochar-compost as a new option for soil improvement: Application in various problem soils. Sci. Total Environ. 2023, 870, 162024. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Huang, J.; Cai, W.S.; Yan, C.N.; Liu, J.L.; Jiang, Y.D. Effects of silver nanoparticles on soil enzyme activity of different wetland plant soil systems. Soil Sediment Contam. Int. J. 2017, 26, 558–567. [Google Scholar] [CrossRef]
- Kolesnikov, S.; Timoshenko, A.; Minnikova, T.; Tsepina, N.; Kazeev, K.; Akimenko, Y.; Zhadobin, A.; Shuvaeva, V.; Rajput, V.D.; Mandzhieva, S.; et al. Impact of Metal-Based Nanoparticles on Cambisol Microbial Functionality, Enzyme Activity, and Plant Growth. Plants 2021, 10, 2080. [Google Scholar] [CrossRef] [PubMed]
- Eivazi, F.; Afrasiabi, Z.; Elizabeth, J.O.S.E. Effects of silver nanoparticles on the activities of soil enzymes involved in carbon and nutrient cycling. Pedosphere 2018, 28, 209–214. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; You, Y.; Wang, R.; Chu, S.; Chi, Y.; Hayat, K.; Hui, N.; Liu, X.; Zhang, D.; et al. When nanoparticle and microbes meet: The effect of multi-walled carbon nanotubes on microbial community and nutrient cycling in hyperaccumulator system. J. Hazard. Mater. 2022, 423, 126947. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Gao, X.; Avellan, A.; Spielman-Sun, E.; Xu, J.; Laughton, S.; Yun, J.; Zhang, Y.; Bland, G.D.; Zhang, Y.; et al. CuO Nanoparticles Alter the Rhizospheric Bacterial Community and Local Nitrogen Cycling for Wheat Grown in a Calcareous Soil. Environ. Sci. Technol. 2020, 54, 8699–8709. [Google Scholar] [CrossRef]
- Luo, J.; Guo, X.; Liang, J.; Song, Y.; Liu, Y.; Li, J.; Du, Y.; Mu, Q.; Jiang, Y.; Zhao, H.; et al. The influence of elevated CO2 on bacterial community structure and its co-occurrence network in soils polluted with Cr2O3 nanoparticles. Sci. Total Environ. 2021, 779, 146430. [Google Scholar] [CrossRef]
- Chai, H.; Yao, J.; Sun, J.; Zhang, C.; Liu, W.; Zhu, M.; Ceccanti, B. The effect of metal oxide nanoparticles on functional bacteria and metabolic profiles in agricultural soil. Bull. Environ. Contam. Toxicol. 2015, 94, 490–495. [Google Scholar] [CrossRef]
- Bhattacharjya, S.; Adhikari, T.; Sahu, A.; Patra, A.K. Ecotoxicological effect of TiO2 nano particles on different soil enzymes and microbial community. Ecotoxicology 2021, 30, 719–732. [Google Scholar] [CrossRef]
- Huang, J.; Qian, X.; Li, X.; Hu, Q.; Cao, C.; Yan, C. A Comparative Study on Responses of Soil under Three Typical Nanoparticles Exposure: Enzyme Activities and Microbial Community Structure. Water Air Soil Pollut. 2023, 234, 229. [Google Scholar] [CrossRef]
- Yan, C.; Huang, J.; Cao, C.; Li, R.; Ma, Y.; Wang, Y. Effects of PVP-coated silver nanoparticles on enzyme activity, bacterial and archaeal community structure and function in a yellow-brown loam soil. Environ. Sci. Pollut. Res. 2020, 27, 8058–8070. [Google Scholar] [CrossRef]
- Samarajeewa, A.D.; Velicogna, J.R.; Schwertfeger, D.M.; Princz, J.I.; Subasinghe, R.M.; Scroggins, R.P.; Beaudette, L.A. Ecotoxicological effects of copper oxide nanoparticles (nCuO) on the soil microbial community in a biosolids-amended soil. Sci. Total Environ. 2021, 763, 143037. [Google Scholar] [CrossRef]
- Fahimmunisha, B.A.; Ishwarya, R.; AlSalhi, M.S.; Devanesan, S.; Govindarajan, M.; Vaseeharan, B. Green fabrication, characterization and antibacterial potential of zinc oxide nanoparticles using Aloe socotrina leaf extract: A novel drug delivery approach. J. Drug Deliv. Sci. Technol. 2020, 55, 101465. [Google Scholar] [CrossRef]
- Scott, N.; Chen, H. Nanoscale science and engineering for agriculture and food systems. Ind. Biotechnol. 2012, 8, 340–343. [Google Scholar] [CrossRef]
- Singh, J.; Vishwakarma, K.; Ramawat, N.; Rai, P.; Singh, V.K.; Mishra, R.K.; Kumar, V.; Tripathi, D.K.; Sharma, S. Nanomaterials and microbes’ interactions: A contemporary overview. 3 Biotech 2019, 9, 68. [Google Scholar] [CrossRef]
- Singh, K.; Sharma, S. Quantum Dots: Fabrication, Functionalization, and Applications. In Functionalized Nanomaterials II; CRC Press: Boca Raton, FL, USA, 2021; pp. 129–164. [Google Scholar]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef]
- Van, N.L.; Ma, C.; Shang, J.; Rui, Y.; Liu, S.; Xing, B. Effects of CuO nanoparticles on insecticidal activity and phytotoxicity in conventional and transgenic cotton. Chemosphere 2016, 144, 661–670. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).