Modulating Charge Mobility in Microwave Synthesized Ti-doped ZnS Nanoparticles for Potential Photoanode Applications
Abstract
1. Introduction
2. Experimental Details
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussions
3.1. X-ray Diffraction
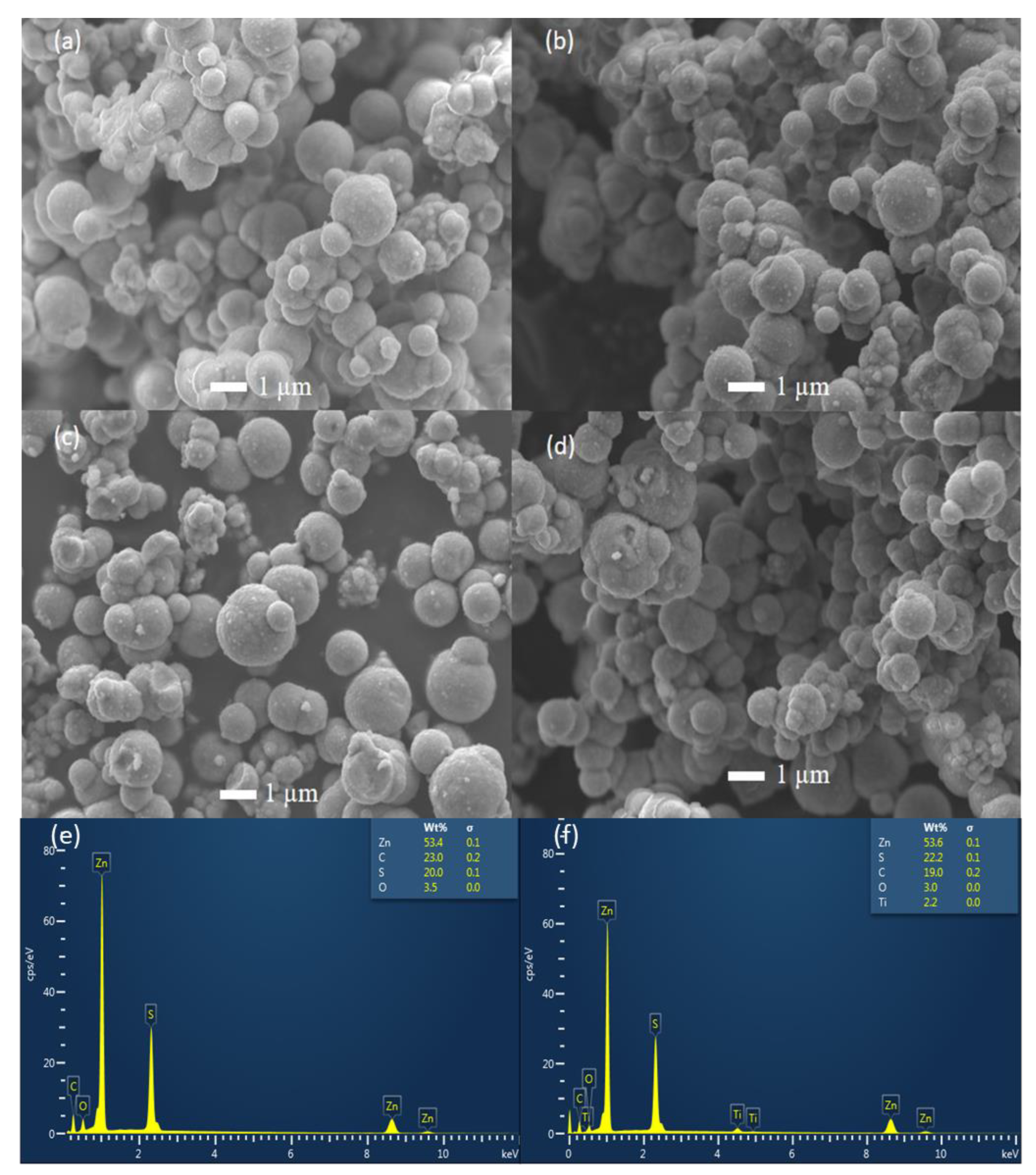
3.2. Scanning Electron Microscopy (SEM) and EDS
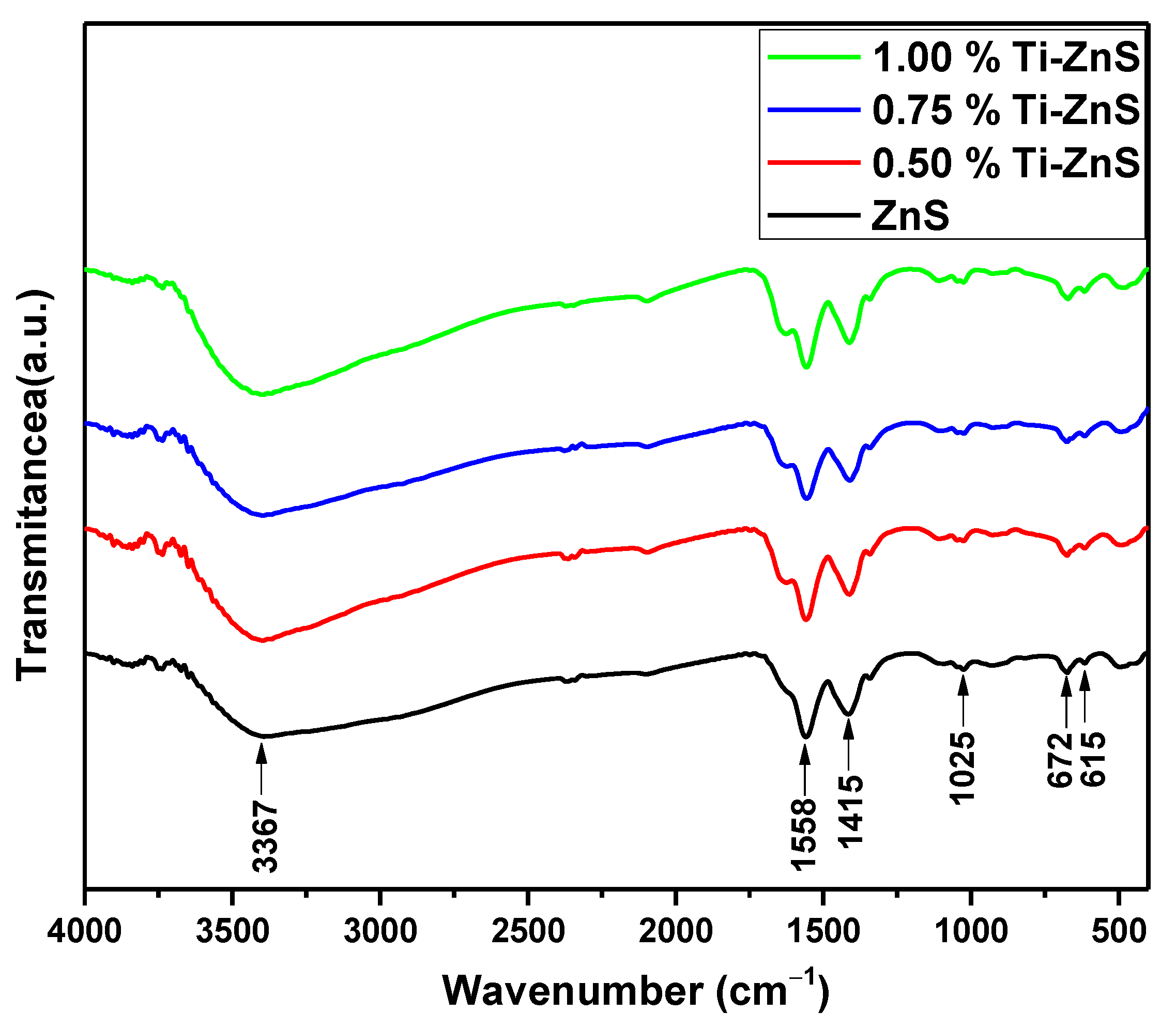
3.3. FTIR Spectroscopy
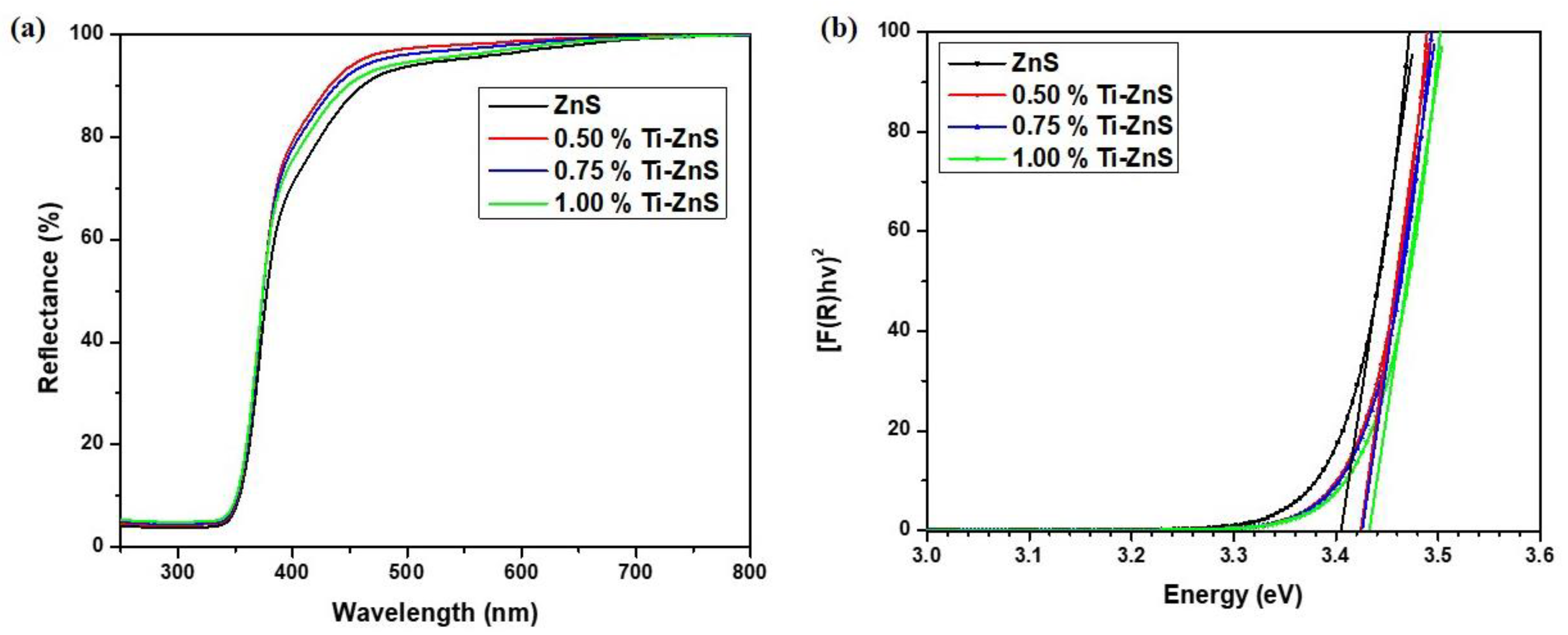
3.4. UV-Vis Spectroscopy
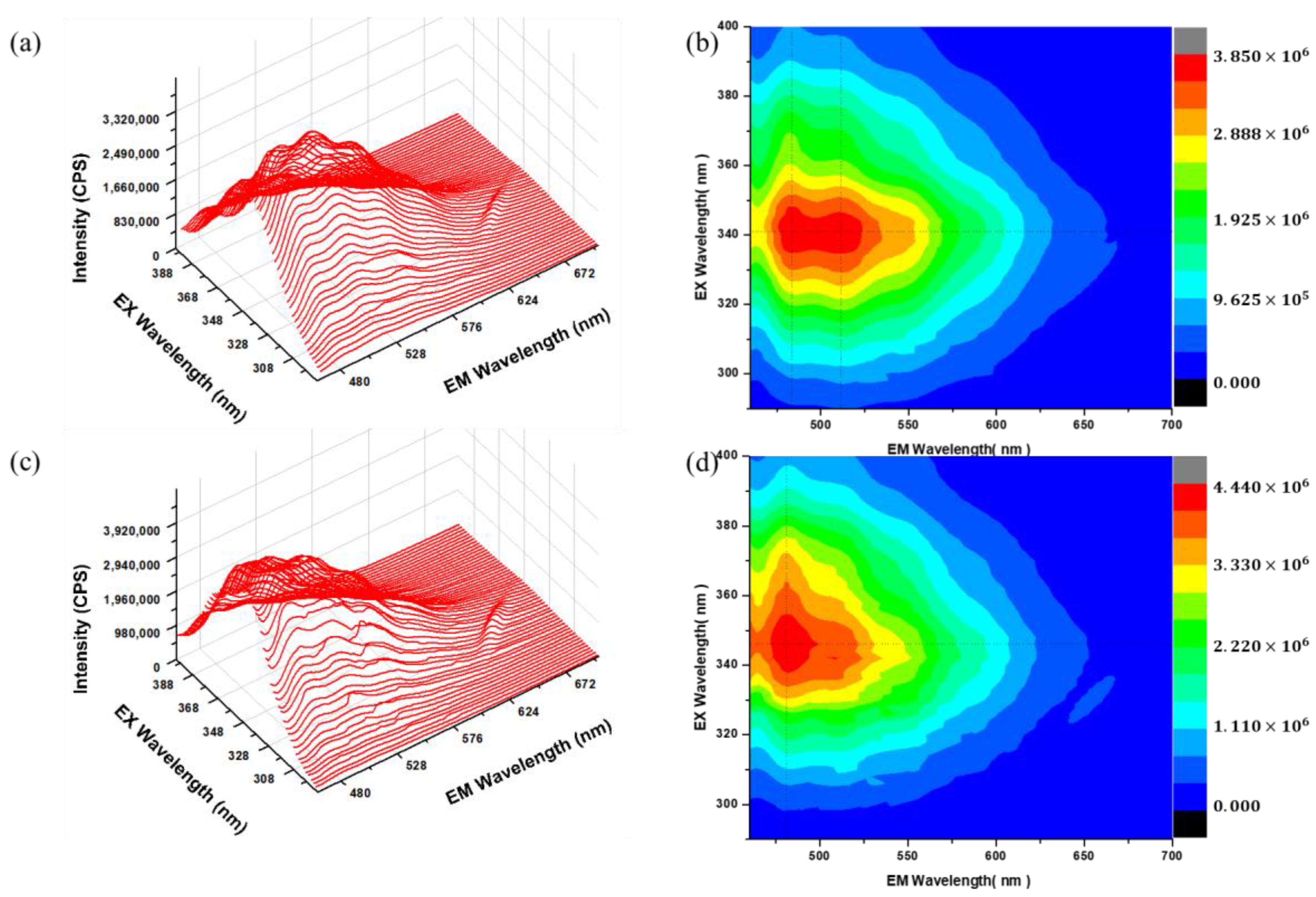
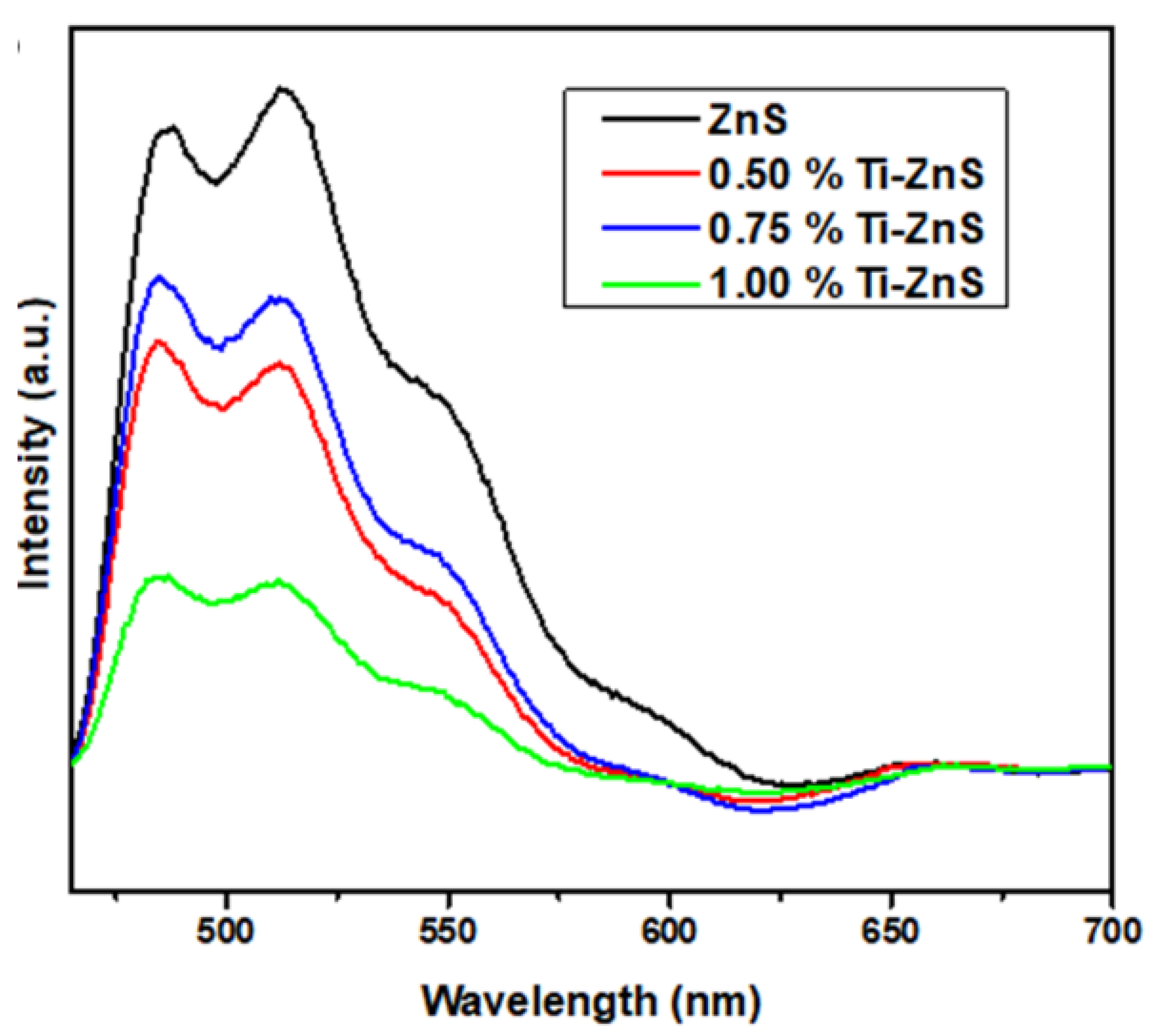
3.5. PL Spectroscopy
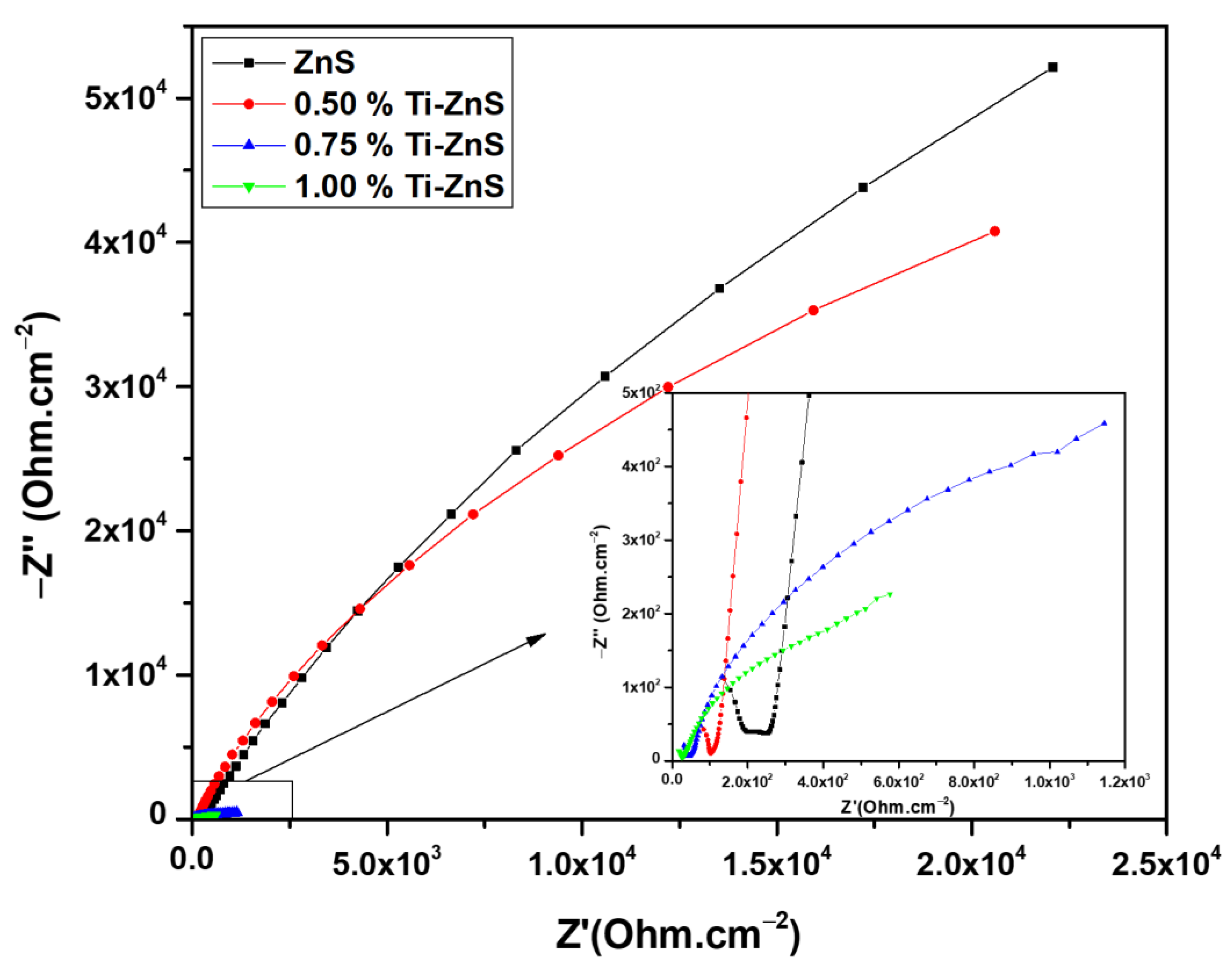
3.6. Electrochemical Impedance spectroscopy
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shamsudin, N.H.; Shafie, S.; Abidin Ab Kadir, M.Z.; Ahmad, F.; Sulaiman, Y.; Chachuli, S.A.M.; Razali, M.C. Flexible Back-Illuminated Dye Sensitised Solar Cells (DSSCs) with Titanium Dioxide/Silver Nanoparticles Composite Photoanode for Improvement of Power Conversion Efficiency. Optik 2022, 272, 170237. [Google Scholar] [CrossRef]
- Mohammadian-Sarcheshmeh, H.; Arazi, R.; Mazloum-Ardakani, M. Application of Bifunctional Photoanode Materials in DSSCs: A Review. Renew. Sustain. Energy Rev. 2020, 134, 110249. [Google Scholar] [CrossRef]
- Wali, Q.; Jose, R. SnO2 Dye-Sensitized Solar Cells. In Nanomaterials for Solar Cell Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 205–285. ISBN 9780128133378. [Google Scholar]
- Upadhyaya, H.M.; Senthilarasu, S.; Hsu, M.H.; Kumar, D.K. Recent Progress and the Status of Dye-Sensitised Solar Cell (DSSC) Technology with State-of-the-Art Conversion Efficiencies. Sol. Energy Mater. Sol. Cells 2013, 119, 291–295. [Google Scholar] [CrossRef]
- Infahsaeng, Y.; Ummartyotin, S. Optical Properties of Composited TiO2-Aluminium-Doped ZnS Photoanode. Mater. Today Proc. 2019, 17, 1693–1701. [Google Scholar] [CrossRef]
- Lun, Y.; Lin, Y.; Meng, Y.; Wang, Y. CdS Quantum Dots Sensitized ZnO Spheres via ZnS Overlayer to Improve Efficiency for Quantum Dots Sensitized Solar Cells. Ceram. Int. 2014, 40, 8157–8163. [Google Scholar] [CrossRef]
- Lalitha Devi, B.; Mohan Rao, K.; Ramananda, D. Effect of Microwave Irradiation Time on Structural and Optical Properties of ZnS Nanoparticles. Inorg. Chem. Commun. 2022, 140, 109460. [Google Scholar] [CrossRef]
- Zakerian, F.; Kafashan, H. Investigation the Effect of Annealing Parameters on the Physical Properties of Electrodeposited ZnS Thin Films. Superlattices Microstruct. 2018, 124, 92–106. [Google Scholar] [CrossRef]
- Sharath, D.; Gaikwad, A.P.; Choudhury, S.; Gupta, N.; Sasikala, R.; Betty, C.A. Effect of Indium Doping on the Photoelectrochemical and Photocatalytic Properties of Zinc Sulphide. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2017, 226, 57–63. [Google Scholar] [CrossRef]
- Rodríguez, S.; Zandalazini, C.; Navarro, J.; Vadiraj, K.T.; Albanesi, E.A. First Principles Calculations and Experimental Study of the Optical Properties of Ni-Doped ZnS. Mater. Res. Express 2019, 7, 016303. [Google Scholar] [CrossRef]
- Tiwary, K.P.; Choubey, S.K.; Sharma, K. Structural and Optical Properties of ZnS Nanoparticles Synthesized by Microwave Irradiation Method. Chalcogenide Lett. 2013, 10, 319–323. [Google Scholar]
- Devi, B.L.; Rao, K.M.; Kekuda, D.; Ramananda, D. Evolution of Defects and Their Effect on Photoluminescence and Conducting Properties of Green-Synthesized ZnS Nanoparticles. Appl. Phys. A Mater. Sci. Process. 2018, 124, 767. [Google Scholar] [CrossRef]
- Messalti, A.S.; El-Ghozzi, M.; Zambon, D.; Mahiou, R.; Setifi, Z. Investigating Photoluminescence Properties of Ca-Doped ZnS Nanoparticles Prepared via Hydrothermal Method. J. Lumin. 2021, 238, 118227. [Google Scholar] [CrossRef]
- Kumar, K.C.; Rao, N.M.; Kaleemulla, S.; Rao, G.V. Structural, Optical and Magnetic Properties of Sn Doped ZnS Nano Powders Prepared by Solid State Reaction. Phys. B Phys. Condens. Matter 2017, 522, 75–80. [Google Scholar] [CrossRef]
- Uzun Çam, Ş.; Seri̇n, T.; Yazıcı, A.N. Effect of Sn Doping Concentration on Structural, Optical and Electrical Properties of ZnS/p-Si (111) Diodes Fabricated by Sol-Gel Dip-Coating Method. Mater. Sci. Semicond. Process. 2021, 127, 105693. [Google Scholar] [CrossRef]
- Jothibas, M.; Manoharan, C.; Johnson Jeyakumar, S.; Praveen, P.; Kartharinal Punithavathy, I.; Prince Richard, J. Synthesis and Enhanced Photocatalytic Property of Ni Doped ZnS Nanoparticles. Sol. Energy 2018, 159, 434–443. [Google Scholar] [CrossRef]
- Othman, A.A.A.; Osman, M.A.A.; Ali, M.A.; Ibrahim, E.M.M.M.M. Influence of Doping with Sb3+, In3+, and Bi3+ Ions on the Structural, Optical and Dielectric Properties of ZnS Nanoparticles Synthesized by Ultrasonication Process. Phys. B Condens. Matter 2021, 614, 413041. [Google Scholar] [CrossRef]
- Daneu, N.; Bernik, S.; Renik, A. Inversion Boundary Induced Grain Growth in ZnO Ceramics: From Atomic-Scale Investigations to Microstructural Engineering. J. Phys. Conf. Ser. 2011, 326, 012003. [Google Scholar] [CrossRef]
- Suzuki, H.; Bradt, R.C. Grain Growth of ZnO in ZnO-Bi2O3 Ceramics with TiO2 Additions. J. Am. Ceram. Soc. 1995, 78, 1354–1360. [Google Scholar] [CrossRef]
- Xia, Z.; Guo, S. Strain Engineering of Metal-Based Nanomaterials for Energy Electrocatalysis. Chem. Soc. Rev. 2019, 48, 3265–3278. [Google Scholar] [CrossRef]
- Basnet, P.; Samanta, D.; Chanu, T.I.; Chatterjee, S. Visible Light Facilitated Degradation of Alternate Dye Solutions by Highly Reusable Mn-ZnO Nano-Photocatalyst. J. Alloys Compd. 2021, 867, 158870. [Google Scholar] [CrossRef]
- Aadnan, I.; Zegaoui, O.; El Mragui, A.; Daou, I.; Moussout, H.; Esteves da Silva, J.C.G. Structural, Optical and Photocatalytic Properties of Mn Doped ZnO Nanoparticles Used as Photocatalysts for Azo-Dye Degradation under Visible Light. Catalysts 2022, 12, 1382. [Google Scholar] [CrossRef]
- Jafari-Rad, A.; Kafashan, H. Preparation and Characterization of Electrochemically Deposited Nanostructured Ti-Doped ZnS Thin Films. Ceram. Int. 2019, 45, 21413–21422. [Google Scholar] [CrossRef]
- Alwany, A.B.; Youssef, G.M.; Saleh, E.E.; Samir, O.M.; Algradee, M.A.; Alnehia, A. Structural, Optical and Radiation Shielding Properties of ZnS Nanoparticles QDs. Optik 2022, 260, 169124. [Google Scholar] [CrossRef]
- Kole, A.K.; Kumbhakar, P. Cubic-to-Hexagonal Phase Transition and Optical Properties of Chemically Synthesized ZnS Nanocrystals. Results Phys. 2012, 2, 150–155. [Google Scholar] [CrossRef]
- Kavi Rasu, K.; Sakthivel, P.; Prasanna Venkatesan, G.K.D. Effect of Pd2+ Co-Doping on the Structural and Optical Properties of Mn2+:ZnS Nanoparticles. Opt. Laser Technol. 2020, 130, 106365. [Google Scholar] [CrossRef]
- Zafar, S.; Zubair, M.; Shah, S.M.; Khan, M.I.; Khan, A.A.; Iqbal, M.F.; Hassan, A.; Din, M.F.U. Effect of Fe Doping on the Structural and Optical Properties of ZnS Macro-Spheres. Optik 2022, 262, 169342. [Google Scholar] [CrossRef]
- Gesesse, G.D.; Gomis-Berenguer, A.; Barthe, M.F.; Ania, C.O. On the Analysis of Diffuse Reflectance Measurements to Estimate the Optical Properties of Amorphous Porous Carbons and Semiconductor/Carbon Catalysts. J. Photochem. Photobiol. A Chem. 2020, 398, 112622. [Google Scholar] [CrossRef]
- Kalu, O.; Duarte Moller, J.A.; Reyes Rojas, A. Structural and Optical Properties of Cadmium Magnesium Zinc Oxide (CdMgZnO) Nanoparticles Synthesized by Sol–Gel Method. Phys. Lett. Sect. A Gen. At. Solid State Phys. 2019, 383, 1037–1046. [Google Scholar] [CrossRef]
- Kaur, J.; Kahagalla, K.G.P.; Rani, S. Investigation Of La Doped Zns For Visible Emission. Eur. J. Mol. Clin. Med. 2020, 7, 3640–3645. [Google Scholar]
- Onwudiwe, D.C.; Ajibade, P.A. ZnS, CdS and HgS Nanoparticles via Alkyl-Phenyl Dithiocarbamate Complexes as Single Source Precursors. Int. J. Mol. Sci. 2011, 12, 5538–5551. [Google Scholar] [CrossRef]
- Hao, X.; Wang, Y.; Zhou, J.; Cui, Z.; Wang, Y.; Zou, Z. Zinc Vacancy-Promoted Photocatalytic Activity and Photostability of ZnS for Efficient Visible-Light-Driven Hydrogen Evolution. Appl. Catal. B Environ. 2018, 221, 302–311. [Google Scholar] [CrossRef]
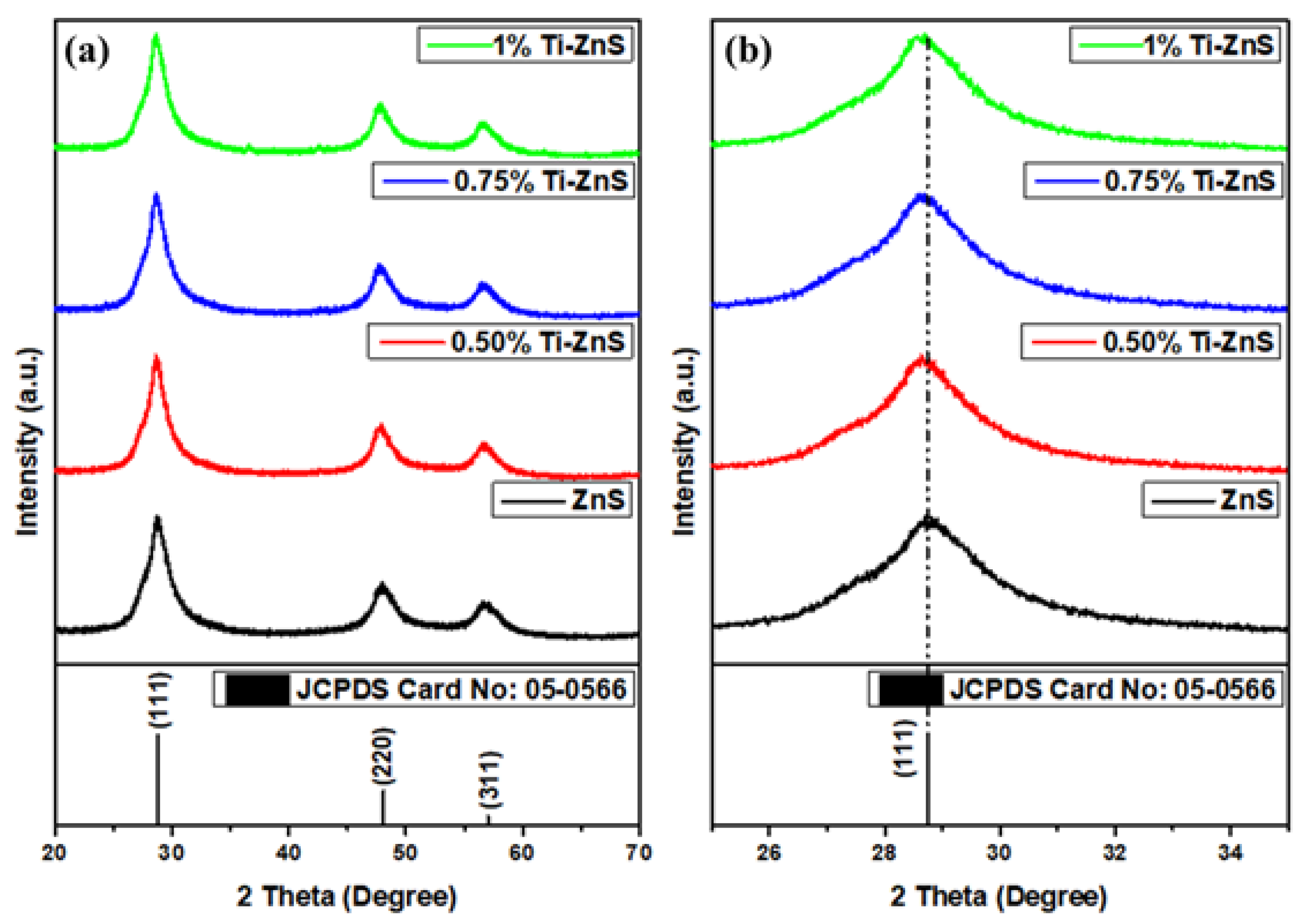
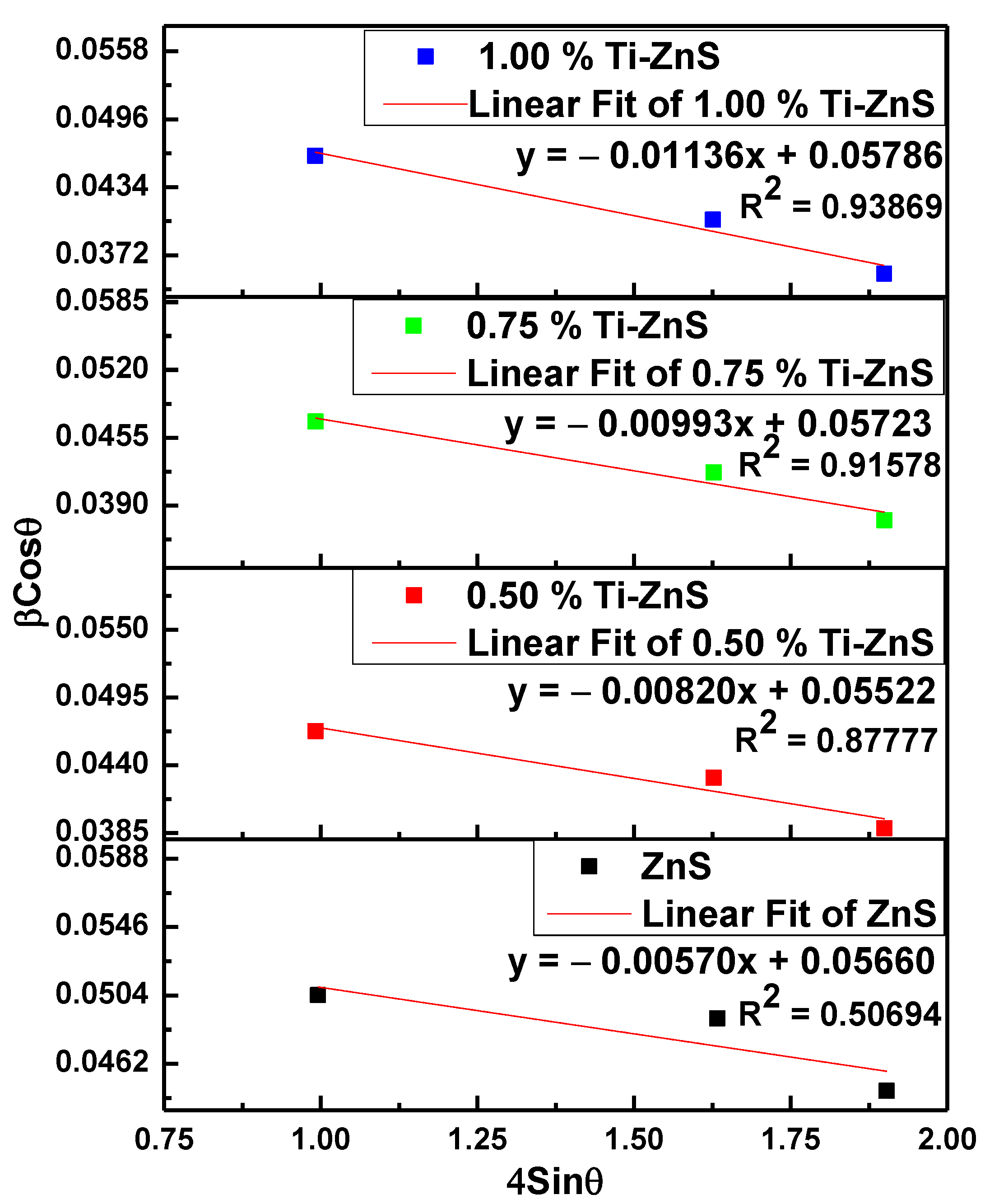

| Sample Name | (Deg.) | d (Å) | a (Å) | Ave. of a (Å) | Ave. of V (Å)3 | D (nm) | ε (10−3) | δ (1017 lines/m2) | SF |
|---|---|---|---|---|---|---|---|---|---|
| ZnS | 28.833 | 3.094 | 5.359 | 5.355 | 153.564 | 2.558 | −5.700 | 1.528 | 0.022 |
| 48.188 | 1.887 | 5.337 | |||||||
| 56.827 | 1.619 | 5.369 | |||||||
| 0.5% Ti-ZnS | 28.714 | 3.106 | 5.381 | 5.372 | 155.004 | 2.622 | −8.200 | 1.454 | 0.020 |
| 48.010 | 1.893 | 5.356 | |||||||
| 56.713 | 1.622 | 5.379 | |||||||
| 0.75% Ti-ZnS | 28.713 | 3.107 | 5.381 | 5.372 | 155.026 | 2.530 | −9.930 | 1.562 | 0.019 |
| 48.006 | 1.894 | 5.356 | |||||||
| 56.711 | 1.622 | 5.379 | |||||||
| 1.00% Ti-ZnS | 28.697 | 3.108 | 5.383 | 5.375 | 155.242 | 2.503 | −11.360 | 1.596 | 0.018 |
| 47.976 | 1.895 | 5.360 | |||||||
| 56.405 | 1.622 | 5.380 |
| Sample | Eg (eV) |
|---|---|
| ZnS | 3.4052 |
| 0.5% Ti-ZnS | 3.4244 |
| 0.75% Ti-ZnS | 3.4260 |
| 1.00% Ti-ZnS | 3.4335 |
| Sample | Rs (Ω) | R1 (kΩ) | CPE (µF) | n | C (µF) |
|---|---|---|---|---|---|
| ZnS | 208.23 | 527.68 | 26.24 | 0.84 | 43.29 |
| 0.50% Ti-ZnS | 103.76 | 174.79 | 29.66 | 0.88 | 37.12 |
| 0.75% Ti-ZnS | 38.38 | 1.65 | 387.91 | 0.56 | 273.19 |
| 1.00% Ti-ZnS | 23.34 | 0.60 | 500.00 | 0.58 | 209.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maswanganye, M.W.; Kabongo, G.L.; Dhlamini, M.S. Modulating Charge Mobility in Microwave Synthesized Ti-doped ZnS Nanoparticles for Potential Photoanode Applications. Nanomaterials 2023, 13, 77. https://doi.org/10.3390/nano13010077
Maswanganye MW, Kabongo GL, Dhlamini MS. Modulating Charge Mobility in Microwave Synthesized Ti-doped ZnS Nanoparticles for Potential Photoanode Applications. Nanomaterials. 2023; 13(1):77. https://doi.org/10.3390/nano13010077
Chicago/Turabian StyleMaswanganye, Mpho W., Guy L. Kabongo, and Mokhotjwa S. Dhlamini. 2023. "Modulating Charge Mobility in Microwave Synthesized Ti-doped ZnS Nanoparticles for Potential Photoanode Applications" Nanomaterials 13, no. 1: 77. https://doi.org/10.3390/nano13010077
APA StyleMaswanganye, M. W., Kabongo, G. L., & Dhlamini, M. S. (2023). Modulating Charge Mobility in Microwave Synthesized Ti-doped ZnS Nanoparticles for Potential Photoanode Applications. Nanomaterials, 13(1), 77. https://doi.org/10.3390/nano13010077










