Abstract
Electrochemical supercapacitors as an energy storage device have become trademark in current electronic, medical and industrial applications, as they are sources of impressive power output. Supercapacitors supply fast power output, suitable to cover the energy demand of future electronic devices. Electrode material design is a subject of intense research in the area of energy development and advancement, due to its essential role in the electrochemical process of charge storage and the cost of capacitors. The nano-dimensions allow for more electroactive sites, different pore size distributions, and a large specific surface area, making nanostructured electrode materials more promising. Electrode materials based on metal oxides, metal nitrides, and metal carbides are considered ideal for highly efficient electrochemical supercapacitors. Recently, much effort has been devoted to metal nitride-based electrodes and their diverse compositions as they possess higher electrical conductivity and better corrosion resistance, electrochemical stability, and chemical reactivity. Among these, titanium nitride (TiN), possesses high electrochemical stability, outstanding electrical conductivity, and a unique electronic structure. Nanocomposites based on titanium nitrides are known to deliver higher electrochemical performance than pristine nanostructured TiNs due to potential synergetic effects from both the materials. In this paper, recent advancements made in the field of nanostructural TiN electrode materials for SCs are reviewed along with their challenges and future opportunities. Additionally, some of the major techniques involved in the synthesis process are discussed, along with some basic concepts.
1. Introduction
Uncontrolled use of fossil fuels not only leads to their rapid depletion but also raises substantial concerns about global warming and energy shortages. The progress made possible by the use of such energy sources is not said to be long-term and sustainable [1,2,3]. Henceforth an urgent need has arisen for the clean, green and economic generation and utilization of energy. Renewable energy sources are the preferred solution to such concerns. Electric energy production through solar, wind and hydro energy is said to be the future of energy [4,5]. However, the non-continuous supply of such energy sources is a major limitation. Therefore, electric energy storage is equally important as its generation [6]. Among several energy storage systems, electrochemical energy storage (EES) is the most popular and efficient method for storing renewable energy, such as solar and wind energy [7,8]. Batteries and supercapacitors (SCs) are the two most popular EES technologies, both of which are known for their low cost and high performance [9]. Batteries can deliver high energy densities in the range of 10–265 Wh kg−1 and are used in a variety of applications including portable devices, electric vehicles, and powering homes. However, because of their short life cycle and low power density, they are unsuitable for a variety of applications that require a large amount of energy in a short period [10,11]. A supercapacitor (SC) is a well-renowned technology equipped with the novel features of high-power density (>10 kW kg−1), elongated cycle life (>105), rapid charge–discharge processes and low cost. The device is promising in a wide range of applications ranging from hybrid electric vehicles, space crafts, memory back-up devices and portable electronic devices [12,13]. The charge storage mechanism of SCs relies on two principles, known as electrostatic storage of charges and the faradaic process of charge storage. The resulting capacitances from electrostatic charge storage are known as electric double-layer capacitance, in which charges are stored at the interface of electrode and electrolyte through physical attractive forces between the opposite polarities. This is a very fast charge storage process and the SCs possessing such capacitances are known to deliver the highest power densities. Electric double-layer capacitors (EDLCs) consist of electrodes primarily made of carbonaceous materials, which hold the key to high conductivity, large specific surface area, and long lifespan [14,15]. On the other hand, pseudocapacitance arises from the faradaic charge storage process in which the actual transfer of charges takes place at the interface of electrode and electrolyte. The capacitors which own such a charge storage mechanism are called pseudocapacitors (PCs) and possess higher energy densities than EDLCs [16]. Conducting polymers and metal oxides are the most preferred choice of electrode materials for PCs [17,18]. However, EDLCs have poor energy density delivery, whereas PCs have lower power densities. To combine the benefits of both the EDLCs and PCs into a single capacitor, a third category of hybrid capacitors came into existence which are known to possess both high energy and power densities [19]. Electrode materials are considered a prominent component, one that plays a major role in the EC performance of a SC device. Carbonaceous materials and pseudocapacitive materials (metal oxides and conducting polymers) are the two most common forms of electrode materials for SCs [20,21]. The low cost, good electrochemical stability, and large surface area of carbon-based electrode materials are well-known novel features. The availability of carbonaceous materials in a variety of forms has led to the commercialization of EDLCs with high power capabilities. Activated carbon, carbon nanotubes, graphene, and carbon aerogel are used as electrode materials in EDLCs [22]. These are enriched by various dimensional structures, porosity, large surface areas, and conductivity. Liu et al. for example, investigated a wide range of carbon-based electrode materials for SCs [23]. However, in addition to their distinct characteristics, these materials have poor specific capacitance capability and a low energy density. The pseudocapacitive electrode materials are known for good specific capacitance storage capability, high redox activity, and high energy density. Several metal oxides that have been explored for SCs are ruthenium oxides, iron oxides, manganese oxides, zinc oxides, nickel oxides, and cobalt oxides [24,25,26]. Although the presence of a large number of oxidation states enriches their working performance, the inferior electrochemical stability on continuous charge–discharge cycles and poor power performance are the major drawbacks. Conducting polymers are also considered pseudocapacitive materials due to their high specific capacitance capability, low cost, eco-friendly nature, and high capacitive nature. These include polyaniline, polypyrrole, polythiophenes, polyvinyl alcohol, and polyindole [27,28]. However, on continuous intercalation and de-intercalation of electrolyte ions to their surface, a significant change in the volume is observed, which results in inferior electrochemical stability. In this domain, transition metal nitrides (TMNs) have attracted much research attention as SC electrode materials [29,30,31]. The TMNs possess metallic structures, with their interstitial sites occupied by nitrogen atoms. The bonding between metals and nitrogen greatly affects the physical and chemical nature of the complete structure. Such configuration provides an enriched electrical conductivity comparable to metals, higher redox activity than their metal oxide counterparts, and excellent electrochemical stability. Several groups have explored the potential application of TMNs in energy storage [32,33,34]. In this series, the explored TMNs are vanadium nitrides, molybdenum nitrides, titanium nitrides, and niobium nitrides [35]. Although all of these metal nitrides possess unique behavior for energy storage, Titanium nitride has been a pressing topic of research for SC electrodes [36,37]. This is promising due to the presence of several characteristic features, such as high electrical conductivity comparable to that of metals (4 × 103–5 × 10 4 S cm−1), good electrochemical stability, extreme mechanical strength, and low production cost [38]. Interestingly, it has been shown that the electrochemical performance of TiN is boosted with the introduction of oxygen vacancies at its surface due to the growth of TiOx by air exposure. This signifies that the charge storing behavior of TiN greatly depends upon its surface chemistry [39]. The surface-dependent properties of TiN open a new window through which to explore its electrochemical performance by varying different parameters such as surface area, porosity, morphology, the density of materials, and crystal structures. The charge storing characteristics of TiN involves the combined effect of electrostatic charge storage as well as the reversible faradaic charge-storage process [40]. Along with the choice of suitable electrode materials such as TiN, the SC device performance greatly depends on the configuration and design of the electrodes. In recent years, it has been observed that the nanostructuring of electrodes provides a large surface area, porosity, abundance of redox-active sites, conductivity, and electrochemical stability.
Nanostructuring of electrode materials provides short ionic diffusion pathways and helps in increasing the specific capacitance [41,42,43]. Therefore, the electrode should be designed in such a way that electrolyte ions can quickly travel through the conducting channels. The different techniques are used to realize the 0D, 1D, 2D, 3D, and hierarchical nanostructures for electrode materials [44]. TiN has been synthesized in a range of unique nanostructures, as shown in Figure 1 [45,46,47,48]. The above-mentioned TiN nanomorphologies are rich in electroactive sites, and their stable nano geometries provide short paths for ions to move rapidly, efficiently, and reversibly to the surface of active materials. Although sufficient work has been carried out on the synthesis of different nanostructures of TiN, no review has summarized the potential application of nanostructured titanium nitride for SCs. In this critical and comprehensive review, the progress achieved in the fabrication of nanostructured TiN-based SCs is explored in depth. Additionally, the fabrication of electrode materials requires a synthesis approach and therefore the understanding of each synthesis technique is of equal importance. Herein, we have provided a brief introduction of different synthesis techniques used in the fabrication of SC electrodes with suitable examples. Following that, the application of various forms of TiN, such as pure TiN, TiN–carbonaceous composites, TiN–metal oxides, and TiN–polymers composites, is reviewed along with their major challenges, to provide an in-depth overview of the prospective use of nanostructured TiNs for SCs.
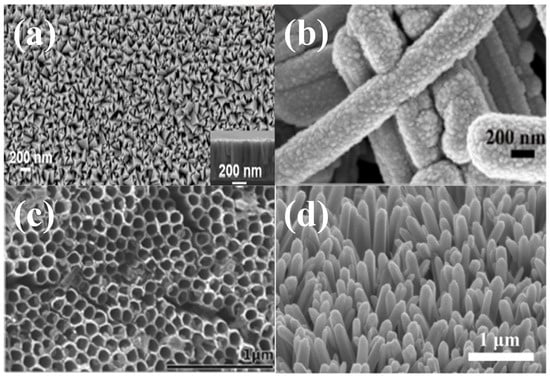
Figure 1.
(a) FE-SEM image of nanopyramid shaped TiN thin film synthesized by sputtering process [45], reproduced with permission, copyright 2018, Elsevier. (b) Magnified SEM image of rod-like Si60 NWs–TiN electrode [46], reproduced with permission, copyright 2021, Elsevier. (c) Mesoporous SEM images of the top surface view of TiN nanotube arrays [47], reproduced with permission, copyright 2014, Royal Society of Chemistry. (d) SEM image of the TiN nanowire arrays on the carbon nanotube fibers [48], reproduced with permission, copyright 2018, American Chemical Society.
2. Synthesis Techniques for Nanostructured Titanium Nitride
The development of innovative and stable potential electrode materials relies on the synthesis techniques used. Different synthesis techniques are employed in the fabrication of nanostructured TiNs for SC electrodes, a few of which are briefly explained here.
2.1. Hydrothermal–Solvothermal Technique
One of the most often used methods for fabricating nanostructured SC electrodes is the solution-based hydrothermal synthesis method, which is simple and environmentally benign [49]. This method allows for the development of a wide variety of materials with nano morphologies, ranging from zero to three-dimensional architectures. The closed system approach, which uses high temperatures and pressures, produces materials with excellent purity, homogeneity, crystallinity, and controlled morphology [50]. The synthesized materials’ physical and chemical natures are dependent on their solvent properties, with the supercritical phase allowing particle formation due to the fast reaction kinetics. When water is replaced by another solvent, the same process becomes solvothermal [51]. Although pristine TiN offers excellent electrical conductivity and rapid charge–discharge rates, the poor cyclability and inferior capacitance are the major drawbacks [32]. To resolve such problems, Qin et al. employed a facile hydrothermal method to synthesize nanopillars of hierarchical TiN nanoparticles. The binder-free electrode is fabricated via the nitration of TiO2 nanotubes and results in H-TiN nanopillars with a surface area of 23.1 m2g−1 and diameter under the range of 100–150 nm, with a length of 6 µm, as shown in Figure 2a,b. As an electrode, the H-TiN NPs showed a capacitance of 69 Fcm−3 at a current density of 0.83 Acm−3. Both the large specific surface area and increased electroactive sites are attributed to the remarkable EC performance [52]. To provide extra stability to TiN nanostructures, coating with other potential materials is highly encouraged. A hydrothermal technique effectively provides the formation of such a configuration. For example, Lu et al. emphasized that the continuous intercalation–deinteracalation of electrolyte ions during the cycling process results in the structural degradation of TiN electrodes. The group used hydrothermal technique to grow a thin layer of amorphous carbon onto the TiN nanowires grown onto the carbon cloth. The resulting TiN@C electrode delivered an improved specific capacitance of 124.5 Fg−1 at a current density of 5 Ag−1, improving on the 107 Fg−1 for a pure TiN electrode. Additionally, for a long cyclic process (15,000 charge-discharge cycles), the TiN@C electrode retained 91.7% of its initial capacitance [53]. Liu et al. explored the mechanism behind the capacitance loss of hydrothermally grown TiN nanowires on carbon cloth. An XPS data analysis revealed that the irreversible oxidation of TiN during the continuous electrochemical cycling process is the reason the capacitance fades. Interestingly, the use of poly vinyl alcohol (PVA–KOH) as a polymer electrolyte provided a high stability to the TiN solid-state SC over 15,000 cycles with a volumetric energy density of 0.05 mWh cm−3. The morphology and the synthesis process of TiN nanowires is shown in Figure 2c [54]. Sun and co-workers reported a fiber SC based on a core–shell TiN@C nanotube-shaped electrode. After performing a complete nitrification of the TiN core, a hydrothermal process was used to coat a shell of carbon on the core of the TiN. Such a hybrid configuration resulted in a capacitance of 2.4 mF cm−1 at a 10 mV s−1 of scan rate. Additionally, high capacitance retention of 80% was achieved after running 10,000 charge–discharge cycles. The carbon coating is attributed to the enhanced performance of PVA–KOH gel electrolyte-based flexible SC [55]. The electrochemical performance of TiN nanostructures can also be enhanced by combining the TiN with other metal nitrides. For example, vanadium nitride is promising for energy storage due to its high theoretical capacitance value and good conductivity. Therefore, its incorporation with TiN can further boost the electrochemical performance. Recently, Wei et al. reported a bimetallic nitride TiVN for SC application synthesized through solvothermal process. The mesoporous hollow spheres morphology of the TiVN composite contributes to the improved electronic conductivity and specific capacitance with a value of 729 Fg−1 at 2 Ag−1 of current density. The synergetic contribution from both the counterparts is attributed to the remarkable performance [56]. The above reviewed literature signifies the potential use of the hydrothermal technique in the fabrication of nanostructured TiN-based SC electrodes. Although the cyclic stability over a large number of cycles is greatly improved by following different strategies, the limited specific capacitance is still a challenge.
2.2. Magnetron Sputtering Technique
Magnetron sputtering has evolved to become an advanced technique for the deposition of highly uniform and pure thin films of different metal oxides, nitrides and sulfides [57]. The technique is utilized in several application areas such as memory devices, optical devices, semiconducting devices, decoratives and electrical devices. Magnetron sputtering consists of RF and DC sputtering with the difference in the working mechanism. In a normal sputtering process, a target whose film is to be deposited on the substrate is bombarded by highly accelerated and energetic ions. This results in the emission of atoms from the target which get deposited as thin films onto the surface of the substrate placed at a height, just opposite to the target. A high control over the operating conditions, such as pressure (10−3 mbar–10−2 mbar), temperature and voltage, results in the formation of defect-free thin films [58,59,60]. The synthesis of TiN nanostructures using other techniques such as the hydrothermal process and thermal decomposition involves the participation of other gases such as oxygen, ammonia, and the requirement of extremely high temperatures (~800 °C). Depositing TiN nanostructures directly onto the substrates helps in improving the electrochemical performance due to the absence of any binder and conducting agents [61]. Magnetron sputtering facilitates the fabrication of highly conformal and pure binder-free thin films of TiN directly onto the substrates. For example, Arif et al. used the DC sputtering at 80 W of power supply to obtain the pyramid-shaped thin films of nanostructured TiN with an atomic percentage ratio of Ti:N as 54.24:15.26%. A symmetric SC device based on TiN thin films showed a capacitance of 112 Fg−1 at 1 Ag−1 with awesome capacitance retention of 92.6% for a long number of cycles of 30,000 with a remarkable energy density of 26.28 Wh kg−1 and power density of 12.9 kW kg−1. The nanopyramid configuration is attributed to the enhanced performance [45]. Recently, the demand for micro supercapacitors has risen due to their reliability and their ability to power other on-chip devices. However, good cyclic stability and improved energy density are the major requirements for enhanced performance. In this direction, the deposition of TiN thin films through magnetron sputtering adds value to the fabrication of micro SCs. For example, Achour et al. used a sputtering-based micro-fabrication process for the fabrication of TiN thin films onto flat silicon substrates. The films with controlled porosity delivered a volumetric specific capacitance of 146.4 F cm−3 with negligible decay of capacitance over 20,000 cycles. The performance is attributed to the doping of nitrogen into TiO2 layers, porosity and thickness of the deposited film [38]. In another study, a micro-SC based on binary metal nitride composite TiVN thin films was fabricated by using DC magnetron sputtering technique. A relation between specific capacitance and Ti–V atomic ratio has been explored and it was found that at an optimized 1:1 Ti–V ratio, a maximum areal capacitance of 15 mF cm−2 is delivered in the presence of 1M aqueous KOH electrolyte. Moreover, the TiVN thin film-based electrode showed negligible capacitance decay over 10,000 cycles. A synergetic contribution from both the counterparts attribute to such good capacitance retention [62]. The concept of flexible and wearable electronic devices has attracted much research attention in recent years [63]. In this sequence, thin films with nanostructures and porosities provide short ionic diffusion pathways and facilitate the motion of electrolyte ions. Recently, Sial et al. fabricated a flexible solid-state SC device based on TiN–Ni thin film deposited by DC magnetron sputtering at a power of 150 W under 10 m Torr of pressure for 30 min. The porous TiN thin films grown possess a thickness of 103 nm with high uniformity, shown in Figure 2d. The presence of a thin layer of Ni enhanced the conductivity and electrochemical stability of the TiN–Ni electrode, with the assembled device showing a specific capacitance of 10.21 mF g−1 at 5 mV s−1 of scan rate with good energy and power densities [64].
2.3. Electrochemical Deposition
This deposition technique is well known for rapid synthesis, low cost, non-toxic by-products, and high purity for the synthesis of a variety of materials varying from metal oxides to conducting polymers. The electrodeposition process is carried out using several techniques, such as cyclic voltammetry, double-pulse deposition and potential step, with the help of reference, counter and working electrodes [65,66,67]. It results in the formation of a variety of nanostructures from 0D to 3D with porosity varying from micropores to mesopores. The technique also holds potential for depositing nanostructured TiN thin films on different substrates which are used as binder-free electrodes for SCs [68]. It has been demonstrated that the cyclic performance of TiN-based thin films improves with the growth of an oxide layer on its surface. Therefore, it is interesting to explore the relation between capacitance and surface activation by the formation of an oxide layer onto the surface of TiN thin films. For example, Gray et al. tried to understand the effect of surface oxide layers on the EC performance of TiN thin films. Following the electrodeposition process, the titanium foils were converted into TiN thin films by the anodization process in ammonia. Their CV analysis predicted that, with the increase in oxidative treatment, the initial cycles would not show an increase in capacitance values. However, the subsequent cycles showed greater capacitance with 39 Fcm−2 at a high scan rate of 100 mV s−1 [69]. The EC performance is highly influenced by the morphology of electrodes. For instance, 1D nanostructural morphology provides an efficient and rapid transport of ions through the conducting channels [70]. A study based on the fabrication of co-axial 1D MnO2–TiN nanotube arrays through the facile electrodeposition process has been reported by Dang et al. The TiN nanotubes were synthesized using anodization of Ti foil using ammonia. The 1D MnO2–TiN nanocomposite showed an improved specific capacitance of 681 Fg−1 at a high current density of 2 Ag−1 with only 3% capacitance decay after 1000 continuous cycles. The high conductivity of TiN nanotubes and redox active behavior of MnO2 contributed significantly to the EC performance [71]. The EC performance of TiN nanostructures can be further enhanced by merging the TiN with other metal nitrides. For instance, molybdenum nitride has been explored as a potential active electrode material due to its layered structure (γ-Mo2N), high electric conductivity (0.2 S cm−1), enriched electrochemical stability and high charge storing ability. Xie et al. tried to incorporate MoNx with TiN as MoNx–TiN NTA with the help of electrodeposition process followed by nitration process in ammonia. The average diameter of the nanotubes was found to be 110–130 nm with a length of around 4 µm (Figure 3a–c). The synergetic contribution of both MoNx and TiN contributed to the improved capacitance of 121.50 mF cm−2 with a maximum rate capability of 93.8% over 1000 continuous charge–discharge cycles [72]. TiN–Li4Ti5O12 NTAs were reported by the group of Xie et al. Applying the lithiation process of TiO2, the Li4Ti5O12 NTAs were synthesized with a further coating of TiO2 sol and annealing in an ammonia atmosphere. The TiN-Li4T5O12 delivered an enhanced specific capacitance of 143.83 Fg−1 at a current density of 0.5 Ag−1 with good capacitance retention of 82.41% over 1000 cycles. A solid-state SC showed a capacitance of 40.45 Fg−1 at 0.5 Ag−1 of current density [73]. The above reviewed literature signifies the potential use of electrochemical deposition for fabrication of SC electrodes.
2.4. Atomic Layer Deposition Technique
This vapor deposition technique is well known for developing thin films of various nanostructures, development of large surface area current collectors, hard resistive coatings and fabrication of semiconducting and electrical microchip devices [74,75]. The process can be applied to a variety of materials including carbonaceous materials, metal oxides, metal sulfides and metal nitrides. This technique offers a precise control over the porosity, thickness, and purity of the thin film grown onto different substrates, which helps in improving the electrochemical performance of the device [76]. The one step conformal surface coatings provided by the ALD also allows the fabrication of composite electrodes. Therefore, to improve the cyclic performance, electrical conductivity, electrochemical stability, energy and power densities of SCs, the ALD technique for the fabrication of thin-film-based electrode materials is highly encouraged [77]. The major limitations for other deposition techniques in the synthesis of TiN nanostructures are the difficulty in achieving a precise control over the geometry, high temperature requirement and long processing time. The ALD process also supports the synthesis of TiN–carbon composite for SC electrodes. A large surface area with porosity is the foremost requirement for the high performance of SCs. Carbon nanotubes (CNTs) are considered to have the most potential as an EDLC’s electrode material [78]. Their use is promising in the development of SCs possessing high voltage, high energy and power density. Composites based on the incorporation of a tubular backbone such as TiN, with the outer surface coated by a highly conducting pseudocapacitive material, leads to the formation of a new generation of SCs. The three main factors that can illustrate this are: (1) the good settlement and bonding of metal nitride counterparts with carbonaceous materials, (2) the large surface area with porous network that provides a smooth conducting channel for electrolyte ions to diffuse rapidly, and (3) the way that the composite gains extra strength when less volumetric changes occur in the material on continuous charge–discharge cycles. A good example of fabricating a TiN–CNT nanocomposite through highly conformal ALD technique has been reported by Kao et al. On performing a 400-cycle run of ALD, a thick TiN layer of around 20 nm is well grown on each CNT. This results in a 500% increment of the EC capacitance of an ALD-grown TiN-CNT-based device—81 mF cm−2 as opposed to 14 mF cm−2—over CNT-based electrodes (shown in Figure 3d,e). The use of CNTs increases the porosity and surface area while TiN thin film grown over CNTs enhances the electronic conductivity and electrochemical stability of the composite [79]. On-chip SCs have recently gained a lot of attention from both the research and the industry points of view. To power portable electronic devices with printed boards a smooth and controlled flow of electricity is highly important. However, it becomes quite difficult to assemble the powering devices at microscale level. The ALD technique has been found to be quite important in the fabrication of on-chip micro-SC devices. For example, Grigoras et al. successfully grew an ultrathin TiN layer by utilizing an ALD technique. The nanostructured TiN thin film (thickness 15 nm), grown onto porous silicon substrate (thickness 1.5 µm) improved the specific capacitance due to an increase in the surface-to-volume ratio (Figure 4). The assembled on-chip SC delivered a remarkable specific capacitance of 15 F cm−3, with negligible loss in capacitance after 23,000 continuous charge–discharge cycles [80].
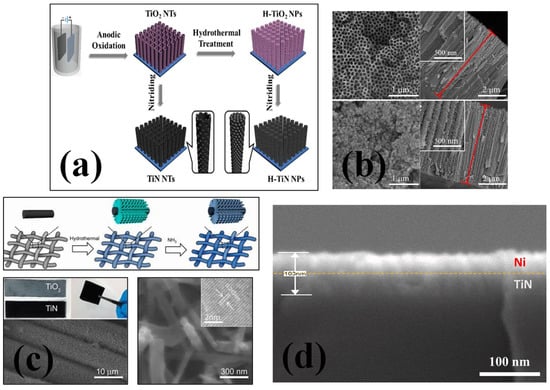
Figure 2.
(a) The synthesis mechanism and (b) SEM images from different surfaces for TiN nanotubes and H-TiN nanopillars [52], reproduced with permission, copyright 2013, Royal Society of Chemistry. (c) Schematic diagram illustrating the synthesis process for TiN nanowires grown on carbon cloth substrate and magnified SEM and TEM images of TiN nanowires [54], reproduced with permission, copyright 2012, American Chemical Society. (d) The SEM cross sectional image of TiN–Ni nanocomposite [64], reproduced with permission, copyright 2021, Elsevier.
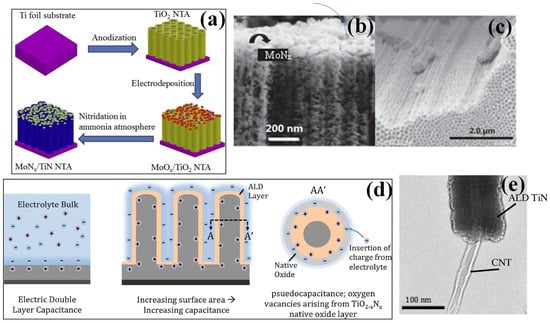
Figure 3.
(a) The experimental procedure and synthesis mechanism of MoNx−TiN NTAs using electrochemical deposition process, (b) SEM image of side view for TiN nanotubes, and (c) SEM images of the side view of MoNx−TiN nanotube arrays respectively [72], reproduced with permission, copyright 2016, Elsevier. (d) Conceptual representation showing the effect of increased surface area on the capacitance and a rise in pseudo capacitive effect. (e) TEM image of ALD-grown TiN coated with the carbon nanotube showing a uniform coating with a layer of around 20 nm respectively [79], reproduced with permission, copyright 2016, Elsevier.
2.5. Molten Salt Technique
The molten salt technique is a co-effective and very simple method for the synthesis of the various ceramic materials and oxide powders. In this synthesis procedure molten salt enhances the fluidity of the reaction components in the liquid phase, which is responsible for improving the rate of the diffusion and stopping the aggregation of the particles during the synthesis procedure. This results in a shortened reaction time and lower temperatures required for synthesis. Recently, molten salt techniques have been used for the synthesis of various materials [81,82,83,84].
Metal nitrides such as vanadium nitrides, titanium nitrides, iron nitrides etc. have been synthesized through a molten salt-based synthesis method. In the past, Ding et al. have reported TiN whiskers can be synthesized through the molten salt media on a graphitic substrate. In this synthesis method, titanium metal (Ti), NaF and NaCl are utilized as raw materials. The authors used molar ratios of the Ti and graphite of 1:1, 1:2, 1:3 and 1:4, whereas the NaCl and NaF weight ratio was 10:1. Firstly NaF and NaCl were added equally to the Ti and graphite and were then mixed in an alumina crucible used to place the powdered mixture and maintain the temperature at 1100 to 1400 °C for 3 hours in an argon atmosphere [85]. The formatted TiN whisker with a diameter of 500–600 nm also modified the surface area of the graphite. Recently Guan et al. have reported porous three-dimensional high surface area metal nitrides such as VN, MoN, WN and TiN using the mild molten salt route. They used enviro-friendly ZnCl2 as the molten salt phase and maintained a temperature of 290 C at 1 atm [86].
3. Different Forms of Nanostructured TiNs for Supercapacitor Electrodes
3.1. Pure Nanostructured TiN for SC Electrodes
Nanostructured titanium nitride is well known for its electronic conductivity comparable to that of metals (3.70 × 106 S m−1), cubic crystal structure, high melting point of 3050 °C and density ~5.40 g cm−3 [87]. The material is reported to synthesize in a variety of nanostructural morphologies with the help of different techniques. To enhance the energy density of TiN-based electrodes several approaches are applied. For example, the concept of a binder-free electrode is helpful in the rapid transport of electrolyte ions through the conducting networks. Fabrication of TiN as a binder-free electrode avoids the use of conducting agents and binders and consequently enhances the EC performance [88]. This strategy can be used for the development of thin and foldable metallic foils as flexible electrodes for SCs and are of keen importance in wearable electronic devices. Hou et al. reported a flexible solid-state SC based on chrysanthemum-like TiN as an electrode synthesized by the facile hydrothermal method. The unique morphology of CL-TiN possesses a diameter of 2 µm with a nanorod width of 200 nm and provides an efficient ion diffusion channel (Figure 4a,b). The assembled SC device delivered a volumetric capacitance of 7.48 F cm−3 with an energy density of 0.34 mWh cm−3 at a current density of 0.05 A cm−3. Moreover, remarkable capacitance retention of 136% was achieved after running 20,000 charge–discharge cycles. The 3D flower-like morphology, high conductivity and good electrochemical stability are attributed as reasons for the remarkable EC performance [89]. In another study, Achour et al. provided evidence of the influence of nitrogen doping on the surface of TiN thin films with charge storing ability. The grown films using DC reactive magnetron sputtering resulted in pyramid-shaped nanostructures with a film thickness of 760 nm. An increase in β-N dopant yielded a threefold increase in areal capacitance of 8.2 mF cm−2 with negligible capacitance loss after 10,000 cycles [90]. The large surface area of the electrode materials is quite prominent for high performance SC electrodes since accumulation of more charges at the electrode surface result in higher capacitance value. Additionally, if TiN can be synthesized with a large specific surface area, then it becomes quite useful. Following a similar approach, Choi et al. successfully synthesized nanocrystalline TiN with a large surface area of 128 m2g−1 using a two-step halide approach. The different nanostructures were obtained by varying the heat treatment temperatures and were evaluated for their EC performances [91]. Although nanostructured TiN is explored as a promising SC electrode due to its high electric conductivity and mechanical strength, the limited specific surface area and lesser number of electroactive sites for charge storage are some limitations that need to be resolved.
3.2. TiN–Carbon Based Nanocomposite for SC Electrodes
High power density and long cycle life are the key properties of SCs while the inferior energy density is the major limitation that needs to be overcome. To overcome this, the concept of nanocompositing an electrode material has been highly recommended [53,55]. A composite based on TiN–C holds excellent EC properties with a large surface area, various porosities and high conductivity. A long cyclability test of 10,000 cycles for TiN–rGO has been performed by Haldorai et al. following a two-step process; the nanoparticles of TiN were uniformly dispersed into an rGO matrix and delivered a capacitance of 415 Fg−1 at 0.5 Ag−1 of current density. Good interaction and dispersion of the TiN nanoparticles with rGO sheets were attributed as reasons for the enhanced EC performance [92]. Wang et al. utilized a green chemical synthesis route to fabricate a TiN–C composite-based SC electrode. Incorporating carbon with TiN, the surface area was increased to 148 m2g−1 with the composite delivering a specific capacitance of 159 Fg−1 at a low current density of 0.5 Ag−1. The EC performance was attributed to the well dispersed TiN nanoparticles at an annealing temperature of 700 °C in the carbon matrix, which had a large electroactive surface area [93]. The inferior electrochemical stability and poor cyclability are the major problems associated with metal nitrides such as TiNs. The application of large surface area carbon materials with high cyclic performance and stability have been proven to improve the EC performance. Among various carbonaceous materials, graphene is the most researched electrode material with the highest potential due to its versatile properties such as high electrical conductivity, large specific surface area and good mechanical strength [94,95]. Incorporating graphene with TiN can boost the EC performance due to the synergetic contribution from both the materials. Considering a similar concept, Lee et al. successfully reported a nanocomposite of TiN–graphene with a large portion of Ti in the composite. Synthesized through the two different routes, the TiN–G composite delivered an improved capacitance over the TiN–G-TE nanocomposite. The presence of a large number of oxygen vacancies in the TiN–G composite was attributed to the higher capacitance it had over TiN–G-TE [96]. Synthesis routes can affect the EC performance of an electrode and a good example of this concept is given by Tian et al. For their study, TiN nanotube arrays grown onto nickel foam and bare nickel foam were considered as substrate to coat graphene onto the surfaces (shown in Figure 4c,d) which were then referred to as G–TiN NTAs and G–NF. The difference in reducing GO into graphene through chemical reduction and thermal reduction resulted in a major difference in the capacitance—333.7 Fg−1 for G–TiN and 198.7 Fg−1 for G–NF. A solid-state symmetric SC device based on G–NF NTA showed an energy density of 34.2 Wh kg−1 at a higher power density of 11.3 KW kg−1. The high electronic conductivity of TiN and the large surface area of graphene were attributed as reasons for the enhanced EC performance [97]. An interconnected porous structure with high electrochemical stability and purity is the most demanding characteristic property of high performing nanostructures of TiN-based electrodes. The thermal and chemical synthesis routes are most utilized but the use of templates, hazardous chemicals and extreme high temperature conditions over long periods are the major drawbacks of these synthesis routes. In contrast, the plasma-related synthesis methods under high vacuum conditions are quite effective due to their short reaction times, impurity free environments and uniform depositions. The application of such growth techniques have been emphasized by the work reported by Qi et al. Utilizing the transferred arc method, the TiN nanoparticles with cubic crystal structure and a size of 5–20 nm were successfully grown onto vertical graphene to fabricate a TiN–VG hybrid electrode. With the high voltage window of 1.8 V, the composite delivered a remarkable cyclic stability of 89.5% over 10,000 continuous charge–discharge cycles (Figure 4e). The good crystallinity, high metallic content, and porous morphology were attributed as reasons for the good cyclic performance [98]. Although the TiN–C composite holds promising application for SC electrodes, reports based on this material are scarcely reported. Thus, the material needs to be further explored.
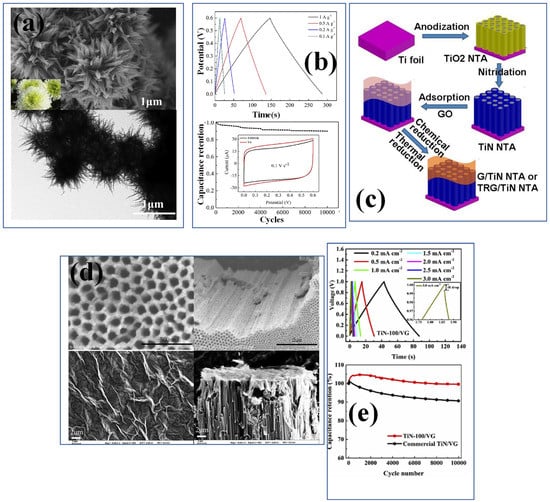
Figure 4.
(a) The SEM–TEM image of chrysanthemum-like CL−TiN and GCD, (b) capacitance retention curve of a CL–TiN nanostructure [89], reproduced with permission, copyright 2018, Elsevier. (c) The synthesis mechanism of G–TiN nanotube arrays and (d) SEM images of top surface and cross-section view of TiN nanotubes and graphene coated TiN nanostructures [97], reproduced with permission, copyright 2014, Royal Society of Chemistry. (e) The GCD and capacitance retention curve for TiN-100 vertically grown graphene and commercial TiN-VG [98], reproduced with permission, copyright 2019, Elsevier.
3.3. TiN–Conducting Polymer-based Composite for SC Electrodes
Recently the organic–inorganic composite for SC electrodes have been widely explored [99,100]. The organic part consists of conducting polymers (CPs), showing the charge storage from pseudocapacitance while the inorganic part consists of metal oxides, metal sulfides or metal nitrides [101]. CPs are promising due to their high energy storage capability, environmentally benign nature, large operating potential window, low cost and ease of fabrication. The different polymers that hold potential uses in SCs are polypyrrole, polypropylene, polyvinyl alcohol, polyacryl amide etc. [102]. These are suitable for organic–inorganic composite form due to their easy doping mechanism and excellent electric conductivity. Among various metal nitrides, TiN is highly suited for such combination because of its excellent electronic conductivity, high mechanical strength, and good electrochemical stability. Such a composite includes the noble features of both the types of materials and therefore help in increasing the overall performance of the device. Electrochemical deposition is the preferred choice of fabrication technique for a CPs–TiNs composite-based electrode [103]. In this sequence, conducting polypyrrole (PPy) is the most promising CP due to the high electrical conductivity, effective interface area, porous nature and nanoscale dimension architecture. These can be synthesized in a range of nanostructures such as nanofibers, thin films, nanosheets etc. [104]. The incorporation of nanostructured TiNs in the composite also acts as a substrate material with a large surface area, high conductivity and good electrochemical stability. They facilitate a smooth and rapid diffusion of electrolyte ions to the surface of active electrode materials and serve as a backbone for the composite [98]. Following the same approach, Du et al. successfully synthesized a PPy–TiN nanocomposite using the electrodeposition approach and compared the EC performance with a PPy–TiO2 composite. The TiO2 nanotube arrays were converted into TiN nanotubes at an annealing temperature of 800 °C in the presence of NH3. The resulting TiN nanotubes possessed a diameter of 100–110 nm with wall-coated PPy thin layers on the surface (shown in Figure 5a). The PPy–TiN composite delivered a highly specific capacitance of 1265 Fg−1 compared to 382 Fg−1 for PPy–TiO2 at a small current density of 0.6 Ag−1. This represents the high conducting nature of TiN NTAs and the same is ascribed for the increase in the EC performance [47]. Incorporation of a p-type polymer such as PPy and an n-type polymer such as PANI with a highly conducting TMN such as TiN greatly helps in improving the charge-storing ability of SCs. This configuration provides effective and short pathways for the rapid diffusion of electrolyte ions. For instance, Xie et al. fabricated a solid-state flexible SC by incorporating PPy–TiN–PANI nanotube array as a composite electrode. The presence of two different types of polymers induced more electroactive sites for the storage of electric charges with the TiN nanotube arrays providing a good electric conducting channel. The PPy–TiN–PANI composite synthesized through the pulse voltammetry and cyclic voltammetry outperformed with a capacitance of 1471.9 Fg−1 in presence of 1M H2SO4 electrolyte at a current density of 0.5 Ag−1. Even at a high current density of 10 Ag−1, the hybrid material delivered a high capacitance of 1077.4 Fg−1. All the counterparts of the hybrid composite contributed significantly to the remarkable EC performance [105]. Low mass transfer resistance and high conductivity of the electrode is key to obtaining good efficiency in EC energy storage. In this perspective, the concept of self-standing current collectors with directly grown active materials on their surfaces has gained considerable attention. This provides fast electronic and ionic transport, large specific surface area, good wettability of electrode surface by electrolyte ions and high electrochemical stability. The overall synergetic contribution leads to high energy and power density of the SC device. A unique combination based on phosphomolybdic acid–polyaniline–titanium nitride core–shell as composite electrode has been successfully fabricated by Lu et al. (shown in Figure 5b). The good dispersion of both the polymers with each other and the high conducting backbone of TiN in the form of binder-free electrodes fabricated by an electrodeposition process resulted in a specific capacitance delivery of 469 Fg−1 at a current density of 1 Ag−1 with the device showing an energy density of 216 Wh kg−1 at an operating voltage window of 1.5 V [106]. A ternary nanocomposite based on PANI–MnO2–TiN as electrode material synthesized by a combination of hydrothermal and electrochemical deposition is reported by Xia et al. A three-layered structure as TiN NWA of diameter 10–30 nm uniformly covered with a layer of MnO2 and then a PANI layer result in an enhanced capacitance of 674 Fg−1 at a 1 Ag−1 of current density. The introduction of MnO2 increased the pseudocapacitance effect and was attributed as reason for the increase in capacitive performance [107]. The application of CPs provides flexibility to the SC electrodes and is quite useful in wearable electronics devices. Among various CPs, polyaniline (PANI) is the most versatile due to its low cost, high specific energy, good theoretical capacitance and environmentally benign nature. However, the major problem associated with such polymers is the degradation in their structure due to swelling and shrinking after a long cycling process [28]. This limits their application for the long-term use of flexible SCs. Several research directions have been suggested to overcome this limitation and one of these is to incorporate them with other carbon, metal oxide or metal nitride species. Such an incorporation could provide mechanical strength to the CPs. Along with this, a novel nanoarchitecture such as a core–shell structure is of great importance. For instance, Xie et al. developed a ternary nanocomposite PANI–C–TiN, incorporating the combine properties of a polymer, a carbonaceous material and titanium nitride. The multicomponent material is synthesized in a shell–shell–core-shaped nanostructure with TiN NWA of diameter 40–60 nm. The fabricated novel electrode delivered a remarkable specific capacitance of 1092 Fg−1 at a current density of 1 Ag−1 with high capacitance retention of 98% over the 2000 continuous cycles. The presence of a middle carbon layer is attributed as reason for the extra strength provided to PANI along with the positive contribution from all individual components of the hybrid composite [70]. Peng et al. reported a hybrid co-axial PANI–TiN–PANI NTA as SC electrode through electrochemical deposition of PANI on the outer, upper and inner surface of TiN NTAs. The electrode showed a specific capacitance of 242 mF cm−2 with a good capacitance retention of 83% over 3000 charge–discharge cycles. The 3D nanoporous structure with large surface area was responsible for the improved electrochemical performance [108]. Nanostructured TiN supported CPs have been found to be promising electrode material for SCs. However, there is still a large challenge in synthesizing such hybrid nanostructures with various dimensions, porosities and morphologies. The fabrication techniques are only limited to electrochemical deposition. Thus, to further explore the potential use of TiN–CPs hybrids for SCs, other low-cost and scalable synthesis methods should be researched and utilized. Polymers in SCs are not only applied in composite form with TiN but also work as templates in the synthesis. TiN synthesized through this process possesses higher capacitance then by other methods. This is due to the fact that template-grown TiN possesses a large specific surface area, various pore size distributions and conducting channels with short pathways. For example, Kin et al. synthesized mesoporous TiN thin films by using poly(vinyl chloride)-graft-poly(oxyethylene methacrylate) (PVC-g-POEM) polymers as a template. The flexible SC device that was fabricated did not lose the capacitance even on bending and delivered a capacitance of 266.8 Fg−1 with high cycling stability [109].
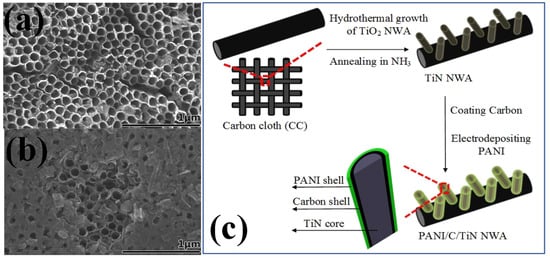
Figure 5.
(a) The SEM images of the top view of TiN nanotubes and (b) PPy coated TiN nanotubes [47], reproduced with permission, copyright 2014, Royal Society of Chemistry. (c) The synthesis mechanism of PANI–C–TiN NWA grown onto CC substrate [70], reproduced with permission, copyright 2015, Elsevier.
3.4. TiN–Other Materials-Based Composite for SC Electrodes
Due to the excellent electrical conductivity and electrochemical stability, nanostructured TiNs provide a conducting framework to hold various transition metal oxides (TMOs) such as MoOx, MnO2, NiCo2O4 etc. [36,42]. The presence of TiN offers mechanical strength and rapid transfer of electrolyte ions which helps to enhance the capacitance nature of the hybrid electrode material. Another unique property of TiNs are their easy tunability with other metal oxides, resulting in extra stable nanostructures. The metal oxides have been widely explored as pseudocapacitive electrode materials. They possess higher energy density and capacitance than EDLC electrodes (>2000 Fg−1) but suffer from poor electronic conductivity and low specific surface area. One of the strategies to improve the electric conductivity of metal oxides is to incorporate them with conducting TiNs as a conducting substrate or as a composite material [46]. Peng et al. synthesized a double-layer coated MoOx NTAs over highly conducting TiN cores to assemble MoOx–TiN–MoOx as a composite electrode, one that comprises the high conductivity and pseudocapacitance of a TiN core with the pseudocapacitance arising from a TiN shell, as well as the electroactive sites. This in turn improved the capacitance and cycling performance, with the specific capacitance delivery of 97 mF cm−2 at a scan rate of 1 mA cm−2. The electrochemical stability of MoOx contributed significantly to the symmetrical SC device, showing a volumetric capacitance of 24 Fcm−3 and the device exhibited the full capacitance retention over 10,000 cycles. The dual-layered MoOx NTA over the thin TiN provided high mass loading with a large surface area and was attributed as the reason for the increase in the device’s overall performance [110]. Fibers possessing mesoporous nanostructural materials are the most suitable for SCs, in which flexible electrodes with desired porosities are an innovative research avenue for the development of high-performance SC electrodes. This configuration provides a large surface-to-volume ratio with more electroactive regions and short diffusion ionic pathways. Among several metal nitrides, Vanadium nitride is also promising due to its improved capacitance storage. However, it possesses good electronic conductivity [111]. Incorporating the two different metal nitrides is a good way to enhance the rapid transportation of electrons and ions with enlarged charge storing ability. In this perspective, a composite based on core–shell TiN–VN fibers as flexible SC electrodes has been explored by Zhou et al. Using the co-axial electrospinning method, highly porous and large surface area core–shell fibers with an outer diameter of 600 nm were synthesized. Both the materials contributed significantly and delivered a specific capacitance of 247.5 Fg−1 at a scan rate of 2 mV s−1 due to their fibrous structure that provided a large area of access to the electrolyte ions. The electrode maintained a capacitance retention of 88% over 500 cycles. The improved specific capacitance delivery and good rate capability were ascribed to the presence of TiN and VN in the composite [112]. The poor energy density of SCs is the major limitation that hampers their wide-scale application. The device energy density is proportional to the voltage window and capacitance. Therefore, optimization of these two parameters through the use of suitable electrode materials and device configuration is the forefront need. The fabrication of an asymmetric SC device is the most emphasized strategy to obtain a large energy density. Along with this, the choice of electrode materials such as nanostructured TiN is the perfect combination. Recently, Wei et al. fabricated an asymmetric SC device based on a bimetallic nitride TiNbN as cathode and VN as anode with 0.5 M H2SO4 as electrolyte. The magnetron sputtered grown bimetallic nitride TiNbN as binder-free electrode and delivered a capacitance of 59.3 mF cm−2 at a scan rate of 1 mA cm−2 with outstanding cyclability after 20,000 cycles. The asymmetric configuration led to an extended voltage window of 1.6 V with enhanced energy and power densities as 74.9 mWh cm−3 and 8.8 W cm−3 respectively. The synergetic contribution from Ti and Nb were ascribed as reason for the boost in EC performance [35]. Although the TiN–other materials-based electrodes discussed above have excellent electrochemical characteristics, the majority of the nanostructured TiN reported work as a current collector or a scaffold to load other materials. This leads to an unnecessarily high electrode weight, as well as the inability to properly utilize nanostructured TiNs, resulting in inferior capacitive performance.
3.5. TiN-based Electrode Material Used in Flexible–Wearable SC
Flexible energy storage devices are fundamental to the development of next generation wearable, compact and portable electronics for medical, military and civil applications such as health tracking devices, computers, television, or flexible displays on phones. Flexible supercapacitor devices are highly attractive in comparison with batteries as they combine the inherent high power density (≥10 kW–kg), fast charging–discharging capability, longer life cycles and good mechanical flexibility. In conventional supercapacitors they consist of the outer case, the current collectors in the form of the metal foils, and cathode and anode electrodes in the electrolyte separated by the ion transport layer. In the case of flexible supercapacitors, the highly conducting and flexible carbon network serves as both the electrodes and current collector. Therefore, the architecture of the flexible supercapacitors is highly conducting and lightweight and is further simplified for portable electronic devices. Thus, self-supported and flexible electrodes have been paid intensive attention. Deposition of the active material on the flexible textile is one of the most effective and widely reported methods to fabricate flexible free-standing electrodes. Recently, highly conductive transition metal nitrides such as TiN, VN, MoN, FeN have been used in flexible SCs due to their high capacitance (100–1340 Fg−1). Among these, TiNs have attracted more attention due to their higher conductivity, which is closer to pure metal. For example, Lu et al. have reported free-standing TiN nanowires grown on a carbon cloth using a two-step synthesis method. Firstly, they uniformly grew 100 to 200 nm TiO2 nanowires on carbon cloth via a seed-assisted hydrothermal synthesis method. After that, these nanowires were thermally annealed in a NH3 atmosphere and with a constant temperature in the range of 700–1000 °C to obtain the TiN. The fabricated flexible free-standing TiN electrode shows an excellent electrochemical performance. In this work the authors claim that the flexible solid-state TiN SC retained 83% retention after 15,000 cycles. The electrochemical performance of the flexible solid-state TiN SC could open up new opportunities for a flexible solid-state SC based on TiN and its composites [55]. Recently, Bin et al. fabricated porous tin nitride paper as an effective electrode for ultrafast charging–discharging SC. The fabricated TiN paper achieved excellent conductivity. In this work the authors fabricated a porous and highly conductive TiN paper as a flexible electrode which enabled the rapid electron transport and ion diffusion that are required for ultrafast charging [113]. Table 1. summarizes the synthesis methodology, obtained morphology and the electrochemical parameters of few metal nitrides.

Table 1.
Synthesis methodology, morphology and electrochemical parameters of a few metal nitrides.
4. Conclusions
To increase the performance of supercapacitors (SCs) enough to power advanced electronics and other technologies, the development of innovative and promising electrodes is the most pressing need. Recent research carried out in the development of SCs has emphasized the nanostructuring of electrode materials. These changes are vital due to the following facts: (1) a smaller particle size with sub-nanoscale dimensions provides a large specific surface area for the generation of electrons and the accumulation of ions on the electrode surface; (2) nano-dimensional architecture facilitates short paths for the diffusion of electrolyte ions, resulting in higher wettability of electrode surface; (3) nanostructuring of electrode materials strengthens the whole electrode, which helps in providing electrochemical stability; (4) fabrication of SC electrodes with a wide range of pore-size distributions with various dimensions are only possible by nanostructuring, and is therefore very important for accessing the maximum surface area of electrodes by electrolytes; and (5) nanostructuring provides high surface-to-volume ratio to electrodes, which increases their capacitance. Along with the strategy of nanostructuring electrodes, choice and optimization of an electrode material is equally important. Among several types of electrode materials, transition metal nitrides are of huge importance due to their high conductivity, structural stability and remarkable electrochemical performance. Titanium nitride (TiN) has recently been explored for potential use in SC electrodes. The material is well known for its excellent electronic conductivity, comparable to that of metals, and its good mechanical strength. The nanostructures of TiNs add value to their novel characteristics in terms of higher specific energy, specific power, cyclic stability and large operating potential window. Considering the promising features of nanostructural TiNs, it is important to summarize the latest advancements made in nanostructural TiNs, with the help of different synthesis techniques, incorporating their importance, advantages and complications. In this review, a comprehensive overview of SC performance based on nanostructural TiNs was provided, including a brief introduction of some techniques involved in the fabrication of electrodes. The EC performance of pristine nanostructured TiNs can be enhanced by incorporating these with other materials such as carbonaceous materials, metal oxides, metal sulfides and conducting polymers. The composite hybrid form of nanostructural TiNs incorporates the synergetic effect of all the counterparts, resulting in extraordinary conductivity, electrochemical stability and mechanical flexibility. The concept of free-standing and binder-free nanostructural TiNs or composites avoids the usage of any binder or conducting agent and provides ultra-long cycling performance to the electrodes. However, in comparison with other SC electrode materials, TiN has received little attention, and, as a result, research on this material is limited. The lack of understanding of the original and fundamental mechanism of charge store behavior in the crystal lattice is the cause of this scarcity. The process of their capacitance decay is similarly poorly understood. As a result, there are numerous research gaps in existing nanostructured TiNs that need be investigated in order to increase the EC performance of SCs. Apart from the obvious advantages of TiNs, there are also some intrinsic drawbacks limiting their practical applications. There is a challenge in large-scale controllable synthesis. Fabrication of TiNs with controlled/specific structures and crystal planes usually requires multistep processes and high temperature treatment, which make the process costly, complicated, or even uneconomical.
Author Contributions
Conceptualization, M.O.A. and S.A.A.; formal analysis, N.P., M.O.A., and P.K.; writing—original draft preparation, N.P., M.O.A., S.A.A. and P.K.; writing—review and editing, N.P., M.O.A., S.A.A. and P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Institutional Funds Projects under grant no. (IFPRP: 604-903-1442). Therefore, authors gratefully acknowledge technical and financial support from the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Data Availability Statement
Not applicable.
Acknowledgments
This research was funded by Institutional Funds Projects under grant no. (IFPRP: 604-903-1442). Therefore, authors gratefully acknowledge technical and financial support from the Ministry of Education and King Abdulaziz University, DSR, Jeddah, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Steckel, J.C.; Brecha, R.J.; Jakob, M.; Strefler, J.; Luderer, G. Development without energy? Assessing future scenarios of energy consumption in developing countries. Ecol. Econ. 2013, 90, 53–67. [Google Scholar] [CrossRef]
- Wang, J.; Feng, L.; Tang, X.; Bentley, Y.; Höök, M. The implications of fossil fuel supply constraints on climate change projections: A supply-side analysis. Futures 2017, 86, 58–72. [Google Scholar] [CrossRef]
- Kreps, B.H. The Rising Costs of Fossil-Fuel Extraction: An Energy Crisis That Will Not Go Away. Am. J. Econ. Sociol. 2020, 79, 695–717. [Google Scholar] [CrossRef]
- Kalair, A.; Abas, N.; Saleem, M.S.; Kalair, A.R.; Khan, N. Role of energy storage systems in energy transition from fossil fuels to renewables. Energy Storage 2021, 3, e135. [Google Scholar] [CrossRef]
- Zheng, X.; Miao, L.; Song, Z.; Du, W.; Zhu, D.; Lv, Y.; Li, L.; Gan, L.; Liu, M. In situ nanoarchitecturing of conjugated polyamide network-derived carbon cathodes toward high energy-power Zn-ion capacitors. J. Mater. Chem. A 2022, 10, 611–621. [Google Scholar] [CrossRef]
- Weitzel, T.; Glock, C.H. Energy management for stationary electric energy storage systems: A systematic literature review. Eur. J. Oper. Res. 2018, 264, 582–606. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.; Wang, Z.; Ruan, J.; Ma, C.; Song, Z.; Dorrell, D.G.; Pecht, M.G. Hybrid electrochemical energy storage systems: An overview for smart grid and electrified vehicle applications. Renew. Sustain. Energy Rev. 2021, 139, 110581. [Google Scholar] [CrossRef]
- Maddukuri, S.; Malka, D.; Chae, M.S.; Elias, Y.; Luski, S.; Aurbach, D. On the challenge of large energy storage by electrochemical devices. Lectrochimica Acta 2020, 354, 136771. [Google Scholar] [CrossRef]
- Zou, C.; Zhang, L.; Hu, X.; Wang, Z.; Wik, T.; Pecht, M. A review of fractional-order techniques applied to lithium-ion batteries, lead-acid batteries, and supercapacitors. J. Power Sources 2018, 390, 286–296. [Google Scholar] [CrossRef]
- Fang, S.; Bresser, D.; Passerini, S. Transition metal oxide anodes for electrochemical energy storage in lithium-and sodium-ion batteries. Adv. Energy Mater. 2020, 10, 1902485. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, B.; Shen, Y.; Wu, T.; Zang, X.; Zhao, Y.; Zhong, C.; Ma, F.; Hu, W. Comparative study of intrinsically safe zinc-nickel batteries and lead-acid batteries for energy storage. J. Power Sources 2021, 510, 230393. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Wang, H. Review on reliability of supercapacitors in energy storage applications. Appl. Energy 2020, 278, 115436. [Google Scholar] [CrossRef]
- Saikia, B.K.; Benoy, S.M.; Bora, M.; Tamuly, J.; Pandey, M.; Bhattacharya, D. A brief review on supercapacitor energy storage devices and utilization of natural carbon resources as their electrode materials. Fuel 2020, 282, 118796. [Google Scholar] [CrossRef]
- Zhao, J.; Burke, A.F. Electrochemical capacitors: Materials, technologies and performance. Energy Storage Mater. 2021, 36, 31–55. [Google Scholar] [CrossRef]
- Ping, G.; Miao, L.; Awati, A.; Qian, X.; Shi, T.; Lv, Y.; Liu, Y.; Gan, L.; Liu, M.; Zhu, D. Porous carbon globules with moss-like surfaces from semi-biomass interpenetrating polymer network for efficient charge storage. Chin. Chem. Lett. 2021, 32, 3811–3816. [Google Scholar] [CrossRef]
- Ghadimi, L.S.; Arsalani, N.; Ahadzadeh, I.; Hajalilou, A.; Abouzari-Lotf, E. Effect of synthesis route on the electrochemical performance of CoMnFeO4 nanoparticles as a novel supercapacitor electrode material. Appl. Surf. Sci. 2019, 494, 440–451. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest advances in supercapacitors: From new electrode materials to novel device designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Xie, P.; Yuan, W.; Liu, X.; Peng, Y.; Yin, Y.; Li, Y.; Wu, Z. Advanced carbon nanomaterials for state-of-the-art flexible supercapacitors. Energy Storage Mater. 2020, 36, 56–76. [Google Scholar] [CrossRef]
- Qin, Y.; Miao, L.; Mansuer, M.; Hu, C.; Lv, Y.; Gan, L.; Liu, M. Spatial Confinement Strategy for Micelle-Size-Mediated Modulation of Mesopores in Hierarchical Porous Carbon Nanosheets with an Efficient Capacitive Response. ACS Appl. Mater. Interfaces 2022, 14, 33328–33339. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Guruviah, V. Review of carbon-based electrode materials for supercapacitor energy storage. Ionics 2019, 25, 1419–1445. [Google Scholar] [CrossRef]
- Liu, C.-F.; Liu, Y.-C.; Yi, T.-Y.; Hu, C.-C. Carbon materials for high-voltage supercapacitors. Carbon 2019, 145, 529–548. [Google Scholar] [CrossRef]
- An, C.; Zhang, Y.; Guo, H.; Wang, Y. Metal oxide-based supercapacitors: Progress and prospectives. Nanoscale Adv. 2019, 1, 4644–4658. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Devi, A. Recent advancements of metal oxides/Nitrogen-doped graphene nanocomposites for supercapacitor electrode materials. J. Energy Storage 2020, 30, 101486. [Google Scholar] [CrossRef]
- Delbari, S.A.; Ghadimi, L.S.; Hadi, R.; Farhoudian, S.; Nedaei, M.; Babapoor, A.; Namini, A.S.; Van Le, Q.; Shokouhimehr, M.; Asl, M.S.; et al. Transition metal oxide-based electrode materials for flexible supercapacitors: A review. J. Alloys Compd. 2021, 857, 158281. [Google Scholar] [CrossRef]
- Naskar, P.; Maiti, A.; Chakraborty, P.; Kundu, D.; Biswas, B.; Banerjee, A. Chemical supercapacitors: A review focusing on metallic compounds and conducting polymers. J. Mater. Chem. A 2021, 9, 1970–2017. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, Y.; Guo, X.; Yu, G. Conductive polymers for stretchable supercapacitors. Nano Res. 2019, 12, 1978–1987. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, X.; Pi, C.; Song, H.; Gao, B.; Chu, P.K.; Huo, K. Recent advances of two-dimensional transition metal nitrides for energy storage and conversion applications. FlatChem 2020, 19, 100149. [Google Scholar] [CrossRef]
- Gao, B.; Li, X.; Ding, K.; Huang, C.; Li, Q.; Chu, P.K.; Huo, K. Recent progress in nanostructured transition metal nitrides for advanced electrochemical energy storage. J. Mater. Chem. A 2019, 7, 14–37. [Google Scholar] [CrossRef]
- Yuan, S.; Pang, S.-Y.; Hao, J. 2D transition metal dichalcogenides, carbides, nitrides, and their applications in supercapacitors and electrocatalytic hydrogen evolution reaction. Appl. Phys. Rev. 2020, 7, 021304. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Li, K.; Lin, Y.; Chen, J.; Gao, L.; Nicolosi, V.; Xiao, X.; Lee, J.-M. Transition metal nitrides for electrochemical energy applications. Chem. Soc. Rev. 2021, 50, 1354–1390. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Guo, W.; Li, T. A review on transition metal nitrides as electrode materials for supercapacitors. Ceram. Int. 2019, 45, 21062–21076. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Huang, Y.; Qiu, W.; Yang, H.; Ji, H.; Tong, Y. Updates on the development of nanostructured transition metal nitrides for electrochemical energy storage and water splitting. Mater. Today 2017, 20, 425–451. [Google Scholar] [CrossRef]
- Wei, B.; Ming, F.; Liang, H.; Qi, Z.; Hu, W.; Wang, Z. All nitride asymmetric supercapacitors of niobium titanium nitride-vanadium nitride. J. Power Sources 2021, 481, 228842. [Google Scholar] [CrossRef]
- Yang, P.; Chao, D.; Zhu, C.; Xia, X.; Zhang, Y.; Wang, X.; Sun, P.; Tay, B.K.; Shen, Z.X.; Mai, W.; et al. Ultrafast-charging supercapacitors based on corn-like titanium nitride nanostructures. Adv. Sci. 2016, 3, 1500299. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, N.A.; Hasan, Z.; Shaikh, A.A.; Ferdousi, F.K.; Barai, H.R.; Lopa, N.S.; Rahman, M. Electrochemical synthesis of titanium nitride nanoparticles onto titanium foil for electrochemical supercapacitors with ultrafast charge/discharge. Sustain. Energy Fuels 2020, 4, 2480–2490. [Google Scholar] [CrossRef]
- Achour, A.; Porto, R.L.; Soussou, M.-A.; Islam, M.; Boujtita, M.; Aissa, K.A.; Le Brizoual, L.; Djouadi, A.; Brousse, T. Titanium nitride films for micro-supercapacitors: Effect of surface chemistry and film morphology on the capacitance. J. Power Sources 2015, 300, 525–532. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, Y.; Du, H. Electrochemical capacitance performance of titanium nitride nanoarray. Mater. Sci. Eng. B 2013, 178, 1443–1451. [Google Scholar] [CrossRef]
- Tang, S.; Cheng, Q.; Zhao, J.; Liang, J.; Liu, C.; Lan, Q.; Cao, Y.-C.; Liu, J. Preparation of titanium nitride nanomaterials for electrode and application in energy storage. Results Phys. 2017, 7, 1198–1201. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Q.; Wang, R.; Shen, G. Ternary oxide nanostructured materials for supercapacitors: A review. J. Mater. Chem. A 2015, 3, 10158–10173. [Google Scholar] [CrossRef]
- Zhi, M.; Xiang, C.; Li, J.; Li, M.; Wu, N. Nanostructured carbon–metal oxide composite electrodes for supercapacitors: A review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, L.; Sun, X.; Zhang, J.; Hou, L.; Li, L.; Yang, S.; Yuan, C. One-dimensional nanostructured pseudocapacitive materials: Design, synthesis and applications in supercapacitors. Batter. Supercaps 2019, 2, 820–841. [Google Scholar] [CrossRef]
- Yu, Z.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730. [Google Scholar] [CrossRef]
- Arif, M.; Sanger, A.; Singh, A. One-step sputtered titanium nitride nano-pyramid thin electrodes for symmetric super-capacitor device. Mater. Lett. 2019, 245, 142–146. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, B.; Liu, Z.; Li, C.; Yan, F.; Liu, X.; Li, H.; Yang, C.; Dong, D.; Hao, J. Sputtered titanium nitride films on nanowires Si substrate as pseudocapacitive electrode for supercapacitors. Ceram. Int. 2021, 47, 26758–26767. [Google Scholar] [CrossRef]
- Du, H.; Xie, Y.; Xia, C.; Wang, W.; Tian, F. Electrochemical capacitance of polypyrrole–titanium nitride and polypyrrole–titania nanotube hybrids. New J. Chem. 2014, 38, 1284–1293. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Q.; Li, Q.; Sun, J.; Li, C.; He, B.; Zhou, Z.; Xie, L.; Li, M.; Yao, Y. Rational design of hierarchical titanium nitride@ vanadium pentoxide core–shell heterostructure fibrous electrodes for high-performance 1.6 V nonpolarity wearable supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 29705–29711. [Google Scholar] [CrossRef]
- Chen, H.; Du, X.; Wu, R.; Wang, Y.; Sun, J.; Zhang, Y.; Xu, C. Facile hydrothermal synthesis of porous MgCo2O4 nanoflakes as an electrode material for high-performance asymmetric supercapacitors. Nanoscale Adv. 2020, 2, 3263–3275. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, A. Importance and challenges of hydrothermal technique for synthesis of transition metal oxides and composites as supercapacitor electrode materials. J. Energy Storage 2021, 44, 103295. [Google Scholar] [CrossRef]
- Wu, S.; Cui, T.; Hu, Q.; Yin, F.; Feng, Q.; Zhou, S.; Su, Q.; Wu, L.; Yang, Q. Mixing solvothermal synthesis nickel selenide on the surface of graphene for high-efficiency asymmetric supercapacitors. Synth. Met. 2020, 268, 116490. [Google Scholar] [CrossRef]
- Qin, P.; Li, X.; Gao, B.; Fu, J.; Xia, L.; Zhang, X.; Huo, K.; Shen, W.; Chu, P.K. Hierarchical TiN nanoparticles-assembled nanopillars for flexible supercapacitors with high volumetric capacitance. Nanoscale 2018, 10, 8728–8734. [Google Scholar] [CrossRef]
- Lu, X.; Liu, T.; Zhai, T.; Wang, G.; Yu, M.; Xie, S.; Ling, Y.; Liang, C.; Tong, Y.; Li, Y. “Improving the cycling stability of metal–nitride supercapacitor electrodes with a thin carbon shell. Adv. Energy Mater. 2014, 4, 1300994. [Google Scholar] [CrossRef]
- Lu, X.; Wang, G.; Zhai, T.; Yu, M.; Xie, S.; Ling, Y.; Liang, C.; Tong, Y.; Li, Y. Stabilized TiN nanowire arrays for high-performance and flexible supercapacitors. Nano Lett. 2012, 12, 5376–5381. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Lin, R.; Wang, Z.; Qiu, M.; Chai, Z.; Zhang, B.; Meng, H.; Tan, S.; Zhao, C.; Mai, W. Rational design of carbon shell endows TiN@ C nanotube based fiber supercapacitors with significantly enhanced mechanical stability and electrochemical performance. Nano Energy 2017, 31, 432–440. [Google Scholar] [CrossRef]
- Wei, B.; Shang, C.; Shui, L.; Wang, X.; Zhou, G. TiVN composite hollow mesospheres for high-performance supercapacitors. Mater. Res. Express 2018, 6, 025801. [Google Scholar] [CrossRef]
- Kelly, P.J.; Arnell, R.D. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Shen, H.; Wei, B.; Zhang, D.; Qi, Z.; Wang, Z. Magnetron sputtered NbN thin film electrodes for supercapacitors. Mater. Lett. 2018, 229, 17–20. [Google Scholar] [CrossRef]
- Qi, Z.; Wei, B.; Wang, J.; Yang, Y.; Wang, Z. Nanostructured porous CrN thin films by oblique angle magnetron sputtering for symmetric supercapacitors. J. Alloys Compd. 2019, 806, 953–959. [Google Scholar] [CrossRef]
- Wei, B.; Liang, H.; Zhang, D.; Wu, Z.; Qi, Z.; Wang, Z. CrN thin films prepared by reactive DC magnetron sputtering for symmetric supercapacitors. J. Mater. Chem. A 2017, 5, 2844–2851. [Google Scholar] [CrossRef]
- Wei, B.; Liang, H.; Zhang, D.; Qi, Z.; Shen, H.; Wang, Z. Magnetron sputtered TiN thin films toward enhanced performance supercapacitor electrodes. Mater. Renew. Sustain. Energy 2018, 7, 11. [Google Scholar] [CrossRef]
- Achour, A.; Lucio-Porto, R.; Chaker, M.; Arman, A.; Ahmadpourian, A.; Soussou, M.A.; Boujtita, M.; Le Brizoual, L.; Djouadi, M.A.; Brousse, T. Titanium vanadium nitride electrode for micro-supercapacitors. Electrochem. Commun. 2017, 77, 40–43. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, C.; Liang, J.; Wu, W. Electrode materials and device architecture strategies for flexible supercapacitors in wearable energy storage. J. Mater. Chem. A 2021, 9, 8099–8128. [Google Scholar] [CrossRef]
- Sial, Q.A.; Duy, L.T.; Singh, R.; Iqbal, S.; Yeasmin, R.; Lee, Y.-J.; Kalanur, S.S.; Seo, H. A multifunctional TiN/Ni electrode for wearable supercapacitor and sensor with an insight into charge storage mechanism. Appl. Surf. Sci. 2021, 555, 149718. [Google Scholar] [CrossRef]
- Islam, S.; Mia, M.; Shah, S.S.; Naher, S.; Shaikh, M.N.; Aziz, A.; Ahammad, A.J.S. Recent Advancements in Electrochemical Deposition of Metal-Based Electrode Materials for Electrochemical Supercapacitors. Chem. Rec. 2022, 22, e202200013. [Google Scholar] [CrossRef]
- Parnell, C.M.; Chhetri, B.P.; Mitchell, T.B.; Watanabe, F.; Kannarpady, G.; RanguMagar, A.B.; Zhou, H.; Alghazali, K.M.; Biris, A.S.; Ghosh, A. Simultaneous electrochemical deposition of cobalt complex and poly (pyrrole) thin films for supercapacitor electrodes. Sci. Rep. 2019, 9, 5650. [Google Scholar] [CrossRef]
- Dey, M.K.; Sahoo, P.K.; Satpati, A.K. Electrochemically deposited layered MnO2 films for improved supercapacitor. J. Electroanal. Chem. 2017, 788, 175–183. [Google Scholar] [CrossRef]
- Xie, Y.; Xia, C.; Du, H.; Wang, W. Enhanced electrochemical performance of polyaniline/carbon/titanium nitride nanowire array for flexible supercapacitor. J. Power Sources 2015, 286, 561–570. [Google Scholar] [CrossRef]
- Gray, B.M.; Hector, A.L.; Jura, M.; Owen, J.R.; Whittam, J. Effect of oxidative surface treatments on charge storage at titanium nitride surfaces for supercapacitor applications. J. Mater. Chem. A 2017, 5, 4550–4559. [Google Scholar] [CrossRef]
- Li, X.; Wang, J. One-dimensional and two-dimensional synergized nanostructures for high-performing energy storage and conversion. InfoMat 2020, 2, 3–32. [Google Scholar] [CrossRef]
- Dong, S.; Chen, X.; Gu, L.; Zhou, X.; Li, L.; Liu, Z.; Han, P.; Xu, H.; Yao, J.; Wang, H.; et al. One dimensional MnO2/titanium nitride nanotube coaxial arrays for high performance electrochemical capacitive energy storage. Energy Environ. Sci. 2011, 4, 3502–3508. [Google Scholar] [CrossRef]
- Xie, Y.; Tian, F. Capacitive performance of molybdenum nitride/titanium nitride nanotube array for supercapacitor. Mater. Sci. Eng. B 2017, 215, 64–70. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, R. Electrochemical capacitance of titanium nitride modified lithium titanate nanotube array. J. Alloys Compd. 2017, 725, 1–13. [Google Scholar] [CrossRef]
- Wang, X.; Yushin, G. Chemical vapor deposition and atomic layer deposition for advanced lithium ion batteries and supercapacitors. Energy Environ. Sci. 2015, 8, 1889–1904. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Selvaraj, S.; Ji, S.H.; Kwon, Y.; Kim, D.H. Interface-Engineered Nickel Cobaltite Nanowires through NiO Atomic Layer Deposition and Nitrogen Plasma for High-Energy, Long-Cycle-Life Foldable All-Solid-State Supercapacitors. Small 2019, 15, 1803716. [Google Scholar] [CrossRef]
- Naeem, F.; Naeem, S.; Zhao, Z.; Shu, G.-Q.; Zhang, J.; Mei, Y.; Huang, G. Atomic layer deposition synthesized ZnO nanomembranes: A facile route towards stable supercapacitor electrode for high capacitance. J. Power Sources 2020, 451, 227740. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Parveen, N.; Nandi, D.K.; Ramesh, R.; Ansari, S.A.; Cheon, T.; Kim, S.-H. Enhanced activity of highly conformal and layered tin sulfide (SnSx) prepared by atomic layer deposition (ALD) on 3D metal scaffold towards high performance supercapacitor electrode. Sci. Rep. 2019, 9, 10225. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube-and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Kao, E.; Yang, C.; Warren, R.; Kozinda, A.; Lin, L. ALD titanium nitride on vertically aligned carbon nanotube forests for electrochemical supercapacitors. Sens. Actuators A Phys. 2016, 240, 160–166. [Google Scholar] [CrossRef]
- Grigoras, K.; Keskinen, J.; Grönberg, L.; Yli-Rantala, E.; Laakso, S.; Välimäki, H.; Kauranen, P.; Ahopelto, J.; Prunnila, M. Conformal titanium nitride in a porous silicon matrix: A nanomaterial for in-chip supercapacitors. Nano Energy 2016, 26, 340–345. [Google Scholar] [CrossRef]
- Ding, J.; Deng, C.; Yuan, W.; Zhu, H.; Zhang, X. Novel synthesis and characterization of silicon carbide nanowires on graphite flakes. Ceram. Int. 2014, 40, 4001–4007. [Google Scholar] [CrossRef]
- Ding, J.; Deng, C.; Zhu, H.; Zhang, X.; Guo, D. Novel preparation of a porous composite insulating scaffold from forsterite and sodium carbonate media. Int. J. Mater. Res. 2014, 105, 1140–1144. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, H.; Deng, C.; Li, G.; Wang, K.; Liu, J. Preparation and characterisation of porous biomorphic sic/c ceramic from molten salt. Ceram. Int. 2015, 41, 11539–11545. [Google Scholar] [CrossRef]
- Ding, J.; Zhu, H.; Li, G.; Deng, C.; Chai, Z. Catalyst-assisted synthesis of α-Si3N4 in molten salt. Ceram. Int. 2016, 42, 2892–2898. [Google Scholar] [CrossRef]
- Ding, J.; Deng, C.; Yuan, W.; Zhu, H.; Li, J. The synthesis of titanium nitride whiskers on the surface of graphite by molten salt media. Ceram. Int. 2013, 39, 2995–3000. [Google Scholar] [CrossRef]
- Guan, H.; Li, W.; Han, J.; Yi, W.; Bai, H.; Kong, Q.; Xi, G. General molten-salt route to three-dimensional porous transition metal nitrides as sensitive and stable Raman substrates. Nat. Commun. 2021, 12, 1376. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, B.; Li, C.; Yan, F.; Wang, D.; Yang, C.; Wan, J. Review of transition metal nitrides and transition metal nitrides/carbon nanocomposites for supercapacitor electrodes. Mater. Chem. Phys. 2020, 245, 122533. [Google Scholar] [CrossRef]
- Mariappan, V.K.; Krishnamoorthy, K.; Pazhamalai, P.; Sahoo, S.; Nardekar, S.S.; Kim, S.-J. Nanostructured ternary metal chalcogenide-based binder-free electrodes for high energy density asymmetric supercapacitors. Nano Energy 2019, 57, 307–316. [Google Scholar] [CrossRef]
- Hou, X.; Li, Q.; Zhang, L.; Yang, T.; Chen, J.; Su, L. Tunable preparation of chrysanthemum-like titanium nitride as flexible electrode materials for ultrafast-charging/discharging and excellent stable supercapacitors. J. Power Sources 2018, 396, 319–326. [Google Scholar] [CrossRef]
- Achour, A.; Chaker, M.; Achour, H.; Arman, A.; Islam, M.; Mardani, M.; Boujtita, M.; Le Brizoual, L.; Djouadi, M.A.; Brousse, T. Role of nitrogen doping at the surface of titanium nitride thin films towards capacitive charge storage enhancement. J. Power Sources 2017, 359, 349–354. [Google Scholar] [CrossRef]
- Choi, D.; Kumta, P.N. Nanocrystalline TiN derived by a two-step halide approach for electrochemical capacitors. J. Electrochem. Soc. 2006, 153, A2298–A2303. [Google Scholar] [CrossRef]
- Haldorai, Y.; Arreaga-Salas, D.; Rak, C.S.; Huh, Y.S.; Han, Y.-K.; Voit, W. Platinized titanium nitride/graphene ternary hybrids for direct methanol fuel cells and titanium nitride/graphene composites for high performance supercapacitors. Electrochim. Acta 2016, 220, 465–474. [Google Scholar] [CrossRef]
- Wang, T.; Li, K.; An, S.; Song, C.; Guo, X. Facile and green synthesis of TiN/C as electrode materials for supercapacitors. Appl. Surf. Sci. 2018, 470, 241–249. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, J.; Chen, Y. An overview of the applications of graphene-based materials in supercapacitors. Small 2012, 8, 1805–1813. [Google Scholar] [CrossRef]
- Kannappan, S.; Yang, H.; Kaliyappan, K.; Manian, R.K.; Pandian, A.S.; Lee, Y.S.; Jang, J.-H.; Lu, W. Thiolated-graphene-based supercapacitors with high energy density and stable cycling performance. Carbon 2018, 134, 326–333. [Google Scholar] [CrossRef]
- Lee, J.; Sohn, Y.; Park, J.H.; Lee, S.; Kim, B.-S.; Park, I.-S.; Kim, P. Preparation and electrochemical performance of titanium nitride-graphene nanocomposite with high Ti contents and tailored morphology. Curr. Appl. Phys. 2019, 19, 961–967. [Google Scholar] [CrossRef]
- Tian, F.; Xie, Y.; Du, H.; Zhou, Y.; Xia, C.; Wang, W. Preparation and electrochemical capacitance of graphene/titanium nitride nanotube array. RSC Adv. 2014, 4, 41856–41863. [Google Scholar] [CrossRef]
- Qi, H.; Yick, S.; Francis, O.; Murdock, A.; van der Laan, T.; Ostrikov, K.; Bo, Z.; Han, Z.; Bendavid, A. Nanohybrid TiN/Vertical graphene for high-performance supercapacitor applications. Energy Storage Mater. 2019, 26, 138–146. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Z.; Yin, H.; Hou, S.; Lin, H.; Zhou, J.; Zhuo, S. Investigation on the role of different conductive polymers in supercapacitors based on a zinc sulfide/reduced graphene oxide/conductive polymer ternary composite electrode. RSC Adv. 2020, 10, 3122–3129. [Google Scholar] [CrossRef]
- Fu, L.; Qu, Q.; Holze, R.; Kondratiev, V.V.; Wu, Y. Composites of metal oxides and intrinsically conducting polymers as supercapacitor electrode materials: The best of both worlds? J. Mater. Chem. A 2019, 7, 14937–14970. [Google Scholar] [CrossRef]
- Chen, M.; Fan, H.; Zhang, Y.; Liang, X.; Chen, Q.; Xia, X. Coupling PEDOT on Mesoporous Vanadium Nitride Arrays for Advanced Flexible All-Solid-State Supercapacitors. Small 2020, 16, 2003434. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Li, H.; Huang, Y.; Zhi, C. Polymers for supercapacitors: Boosting the development of the flexible and wearable energy storage. Mater. Sci. Eng. R Rep. 2019, 139, 100520. [Google Scholar] [CrossRef]
- Rajesh, M.; Manikandan, R.; Kim, B.C.; Becuwe, M.; Yu, K.H.; Raj, C.J. Electrochemical polymerization of chloride doped PEDOT hierarchical porous nanostructure on graphite as a potential electrode for high performance supercapacitor. Electrochim. Acta 2020, 354, 136669. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, N.; Chen, W.; Wang, X.; Sun, C.; Su, Z. Supercapacitor with high cycling stability through electrochemical deposition of metal–organic frameworks/polypyrrole positive electrode. Dalton Trans. 2018, 47, 13472–13478. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, D. Supercapacitance performance of polypyrrole/titanium nitride/polyaniline coaxial nanotube hybrid. J. Alloys Compd. 2016, 665, 323–332. [Google Scholar] [CrossRef]
- Lu, L.; Xie, Y. Fabrication and supercapacitor behavior of phosphomolybdic acid/polyaniline/titanium nitride core–shell nanowire array. New J. Chem. 2016, 41, 335–346. [Google Scholar] [CrossRef]
- Xia, C.; Xie, Y.; Du, H.; Wang, W. Ternary nanocomposite of polyaniline/manganese dioxide/titanium nitride nanowire array for supercapacitor electrode. J. Nanoparticle Res. 2015, 17, 30. [Google Scholar] [CrossRef]
- Peng, X.; Huo, K.; Fu, J.; Zhang, X.; Gao, B.; Chu, P.K. Coaxial PANI/TiN/PANI nanotube arrays for high-performance supercapacitor electrodes. Chem. Commun. 2013, 49, 10172–10174. [Google Scholar] [CrossRef]
- Kim, D.J.; Kim, J.K.; Lee, J.H.; Cho, H.H.; Bae, Y.-S.; Kim, J.H. Scalable and bendable organized mesoporous TiN films templated by using a dual-functional amphiphilic graft copolymer for solid supercapacitors. J. Mater. Chem. A 2016, 4, 12497–12503. [Google Scholar] [CrossRef]
- Peng, X.; Huo, K.; Fu, J.; Gao, B.; Wang, L.; Hu, L.; Zhang, X.; Chu, P.K. Porous Dual-Layered MoOx Nanotube Arrays with Highly Conductive TiN Cores for Supercapacitors. ChemElectroChem 2015, 2, 512–517. [Google Scholar] [CrossRef]
- Cui, G.; Gu, L.; Thomas, A.; Fu, L.; van Aken, P.A.; Antonietti, M.; Maier, J. A carbon/titanium vanadium nitride composite for lithium storage. ChemPhysChem 2010, 11, 3219–3223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shang, C.; Gu, L.; Dong, S.; Chen, X.; Han, P.; Li, L.; Yao, J.; Liu, Z.; Xu, H.; et al. Mesoporous coaxial titanium nitride-vanadium nitride fibers of core–shell structures for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2011, 3, 3058–3063. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Li, M.; Zhang, J.; Zhang, L.; Song, Y.; Xiao, W.; Cruz, A.; Tong, Y.; Li, Y. TiN Paper for Ultrafast-Charging Supercapacitors. Nano-Micro Lett. 2019, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Barsoum, Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Shi, C.; Sun, J.; Chen, W.; Pang, Y.; Liu, B.T. Mesoporous vanadium nitride as anion storage electrode for reverse dual-ion hybrid supercapacitor. Iscience 2022, 25, 104141. [Google Scholar] [CrossRef]
- Bi, W.; Hu, Z.; Li, X.; Wu, C.; Wu, J.; Wu, Y.; Xie, Y. Metallic mesocrystal nanosheets of vanadium nitride for high-performance all-solid-state pseudocapacitors. Nano Res. 2015, 8, 193–200. [Google Scholar] [CrossRef]
- Ghimbeu, C.M.; Raymundo-Piñero, E.; Fioux, P.; Béguin, F.; Vix-Guterl, C. Vanadium nitride/carbon nanotube nanocomposites as electrodes for supercapacitors. J. Mater. Chem. 2011, 21, 13268. [Google Scholar] [CrossRef]
- Shang, C.; Dong, S.; Wang, S.; Xiao, D.; Han, P.; Wang, X.; Gu, L.; Cui, G. Coaxial NixCo2x(OH)6x/TiN Nanotube Arrays as Supercapacitor Electrodes. ACS Nano 2013, 7, 5430. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, P.; Chao, D.; Wang, X.; Zhang, X.; Chen, S.; Tay, B.K.; Huang, H.; Zhang, H.; Mai, W.; et al. All Metal Nitrides Solid-State Asymmetric Supercapacitors. Adv. Mater. 2015, 27, 4566. [Google Scholar] [CrossRef]
- Yu, M.; Han, Y.; Cheng, X.; Hu, L.; Zeng, Y.; Chen, M.; Cheng, F.; Lu, X.; Tong, Y. Holey tungsten oxynitride nanowires: Novel anodes efficiently integrate microbial chemical energy conversion and electrochemical energy storage. Adv. Mater. 2015, 27, 3085. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).