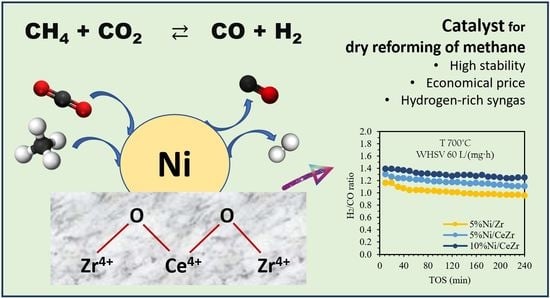
Coke-Resistant Ni/CeZrO2 Catalysts for Dry Reforming of Methane to Produce Hydrogen-Rich Syngas
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Catalysts
2.2.1. Catalyst Based on the ZrO2 Support
2.2.2. Catalyst Based on the Ce0.1Zr0.9O2 Support
2.3. Characterization of Catalysts
2.4. Catalytic Performance Evaluation
3. Results and Discussion
3.1. Characterization of Catalysts
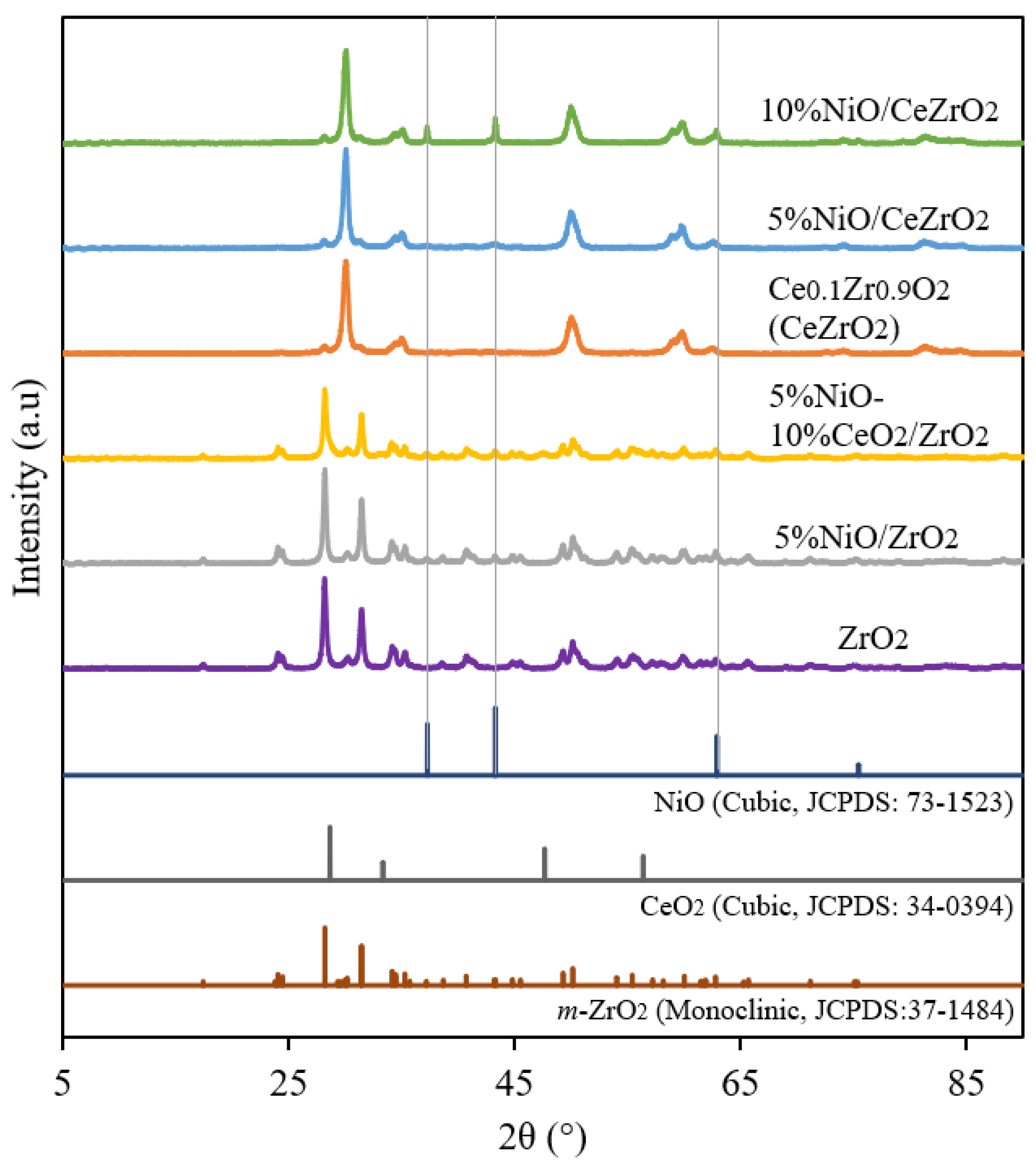
3.1.1. Crystalline Phase and Catalyst Crystallinity
3.1.2. Surface Area and Pore Size Distribution of the Catalysts
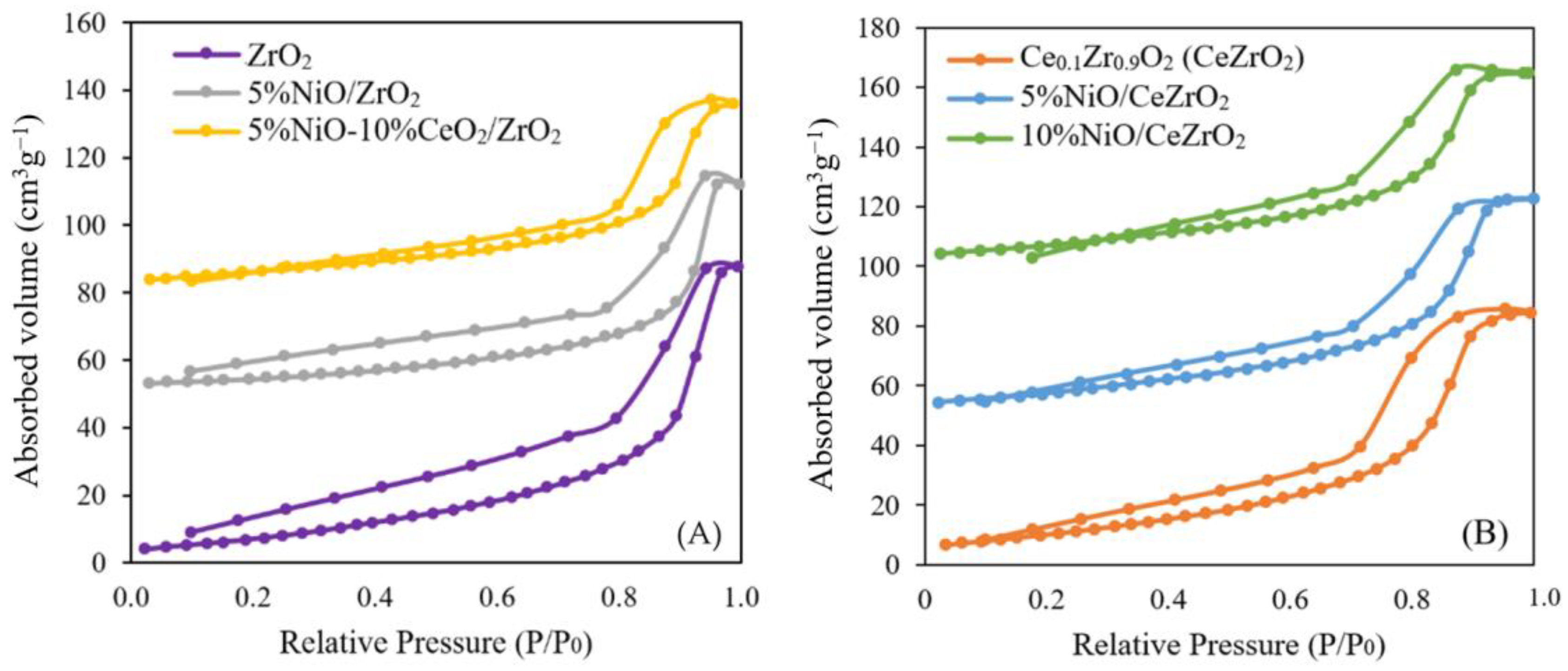
- Catalyst Surface Area (via the BET Method)
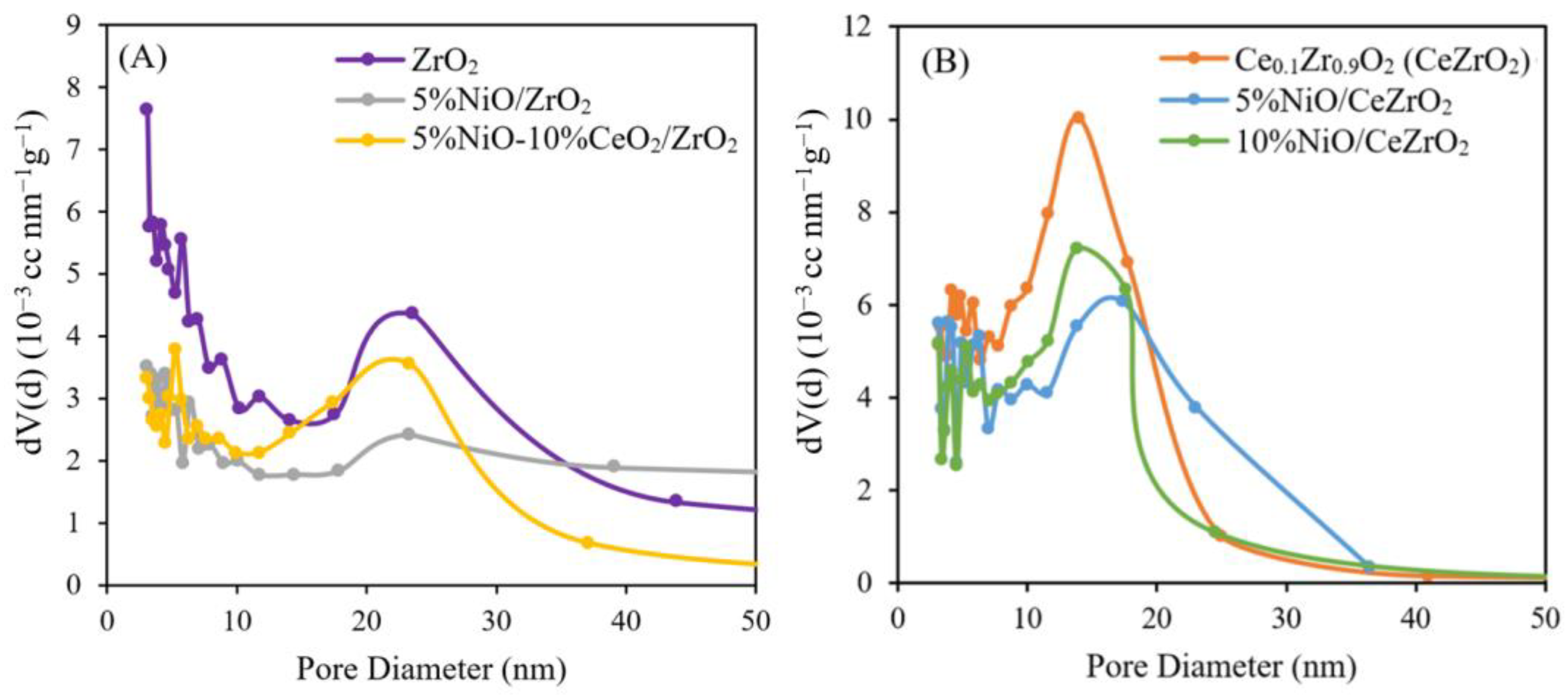
- Catalyst Pore Size Distribution (via the BJH Method)
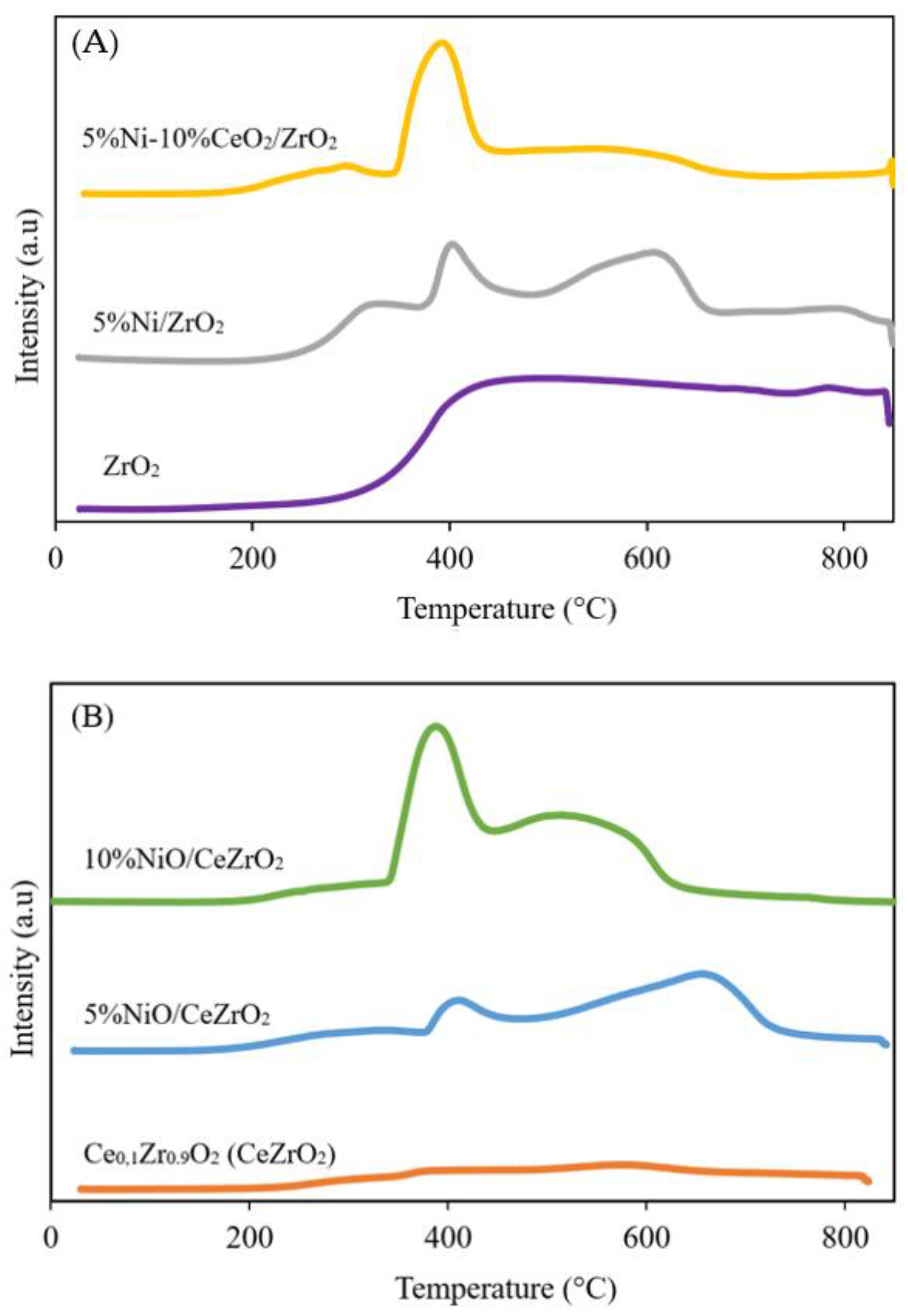
3.1.3. Catalyst Reducibility
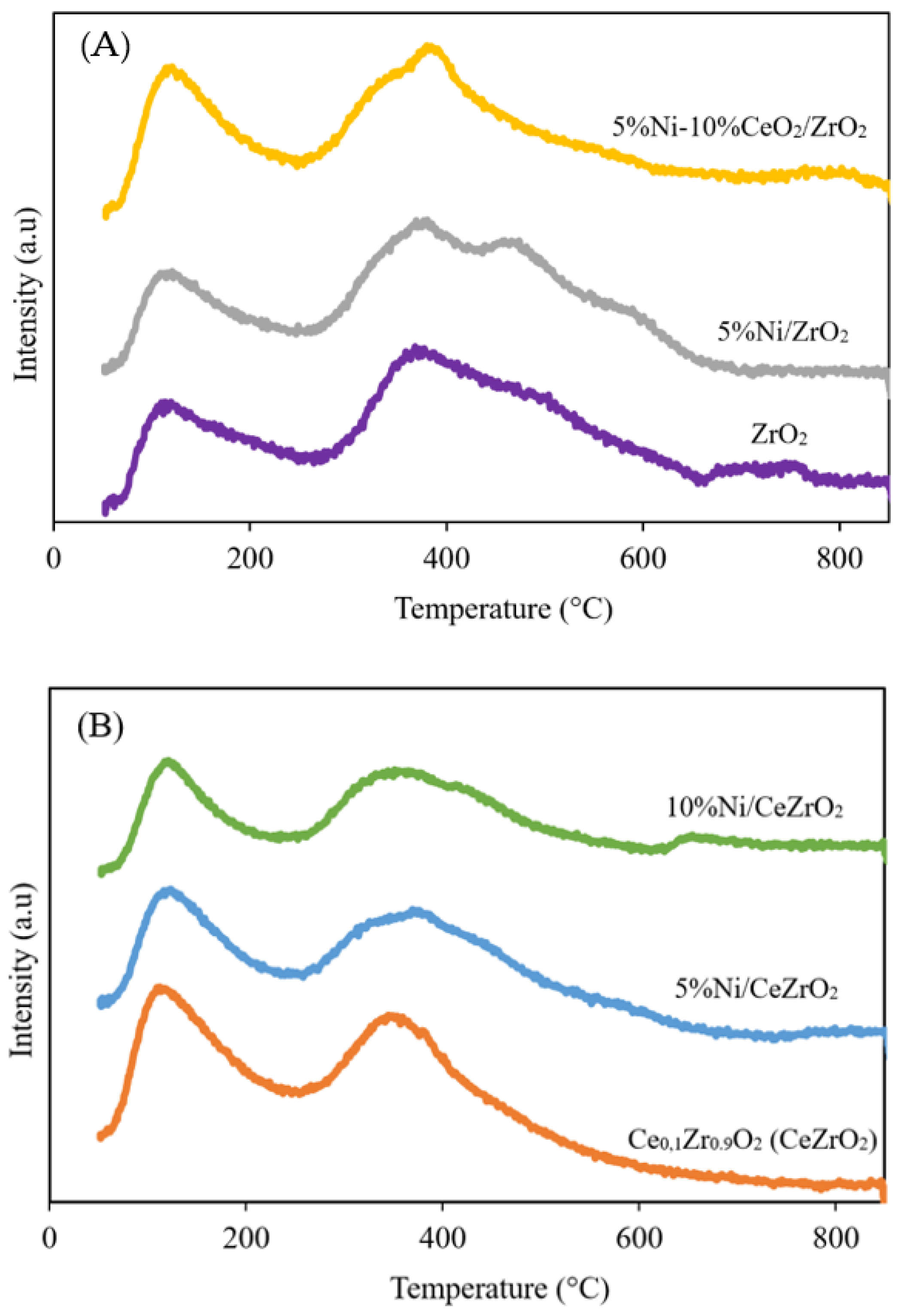
3.1.4. Basicity of the Catalysts
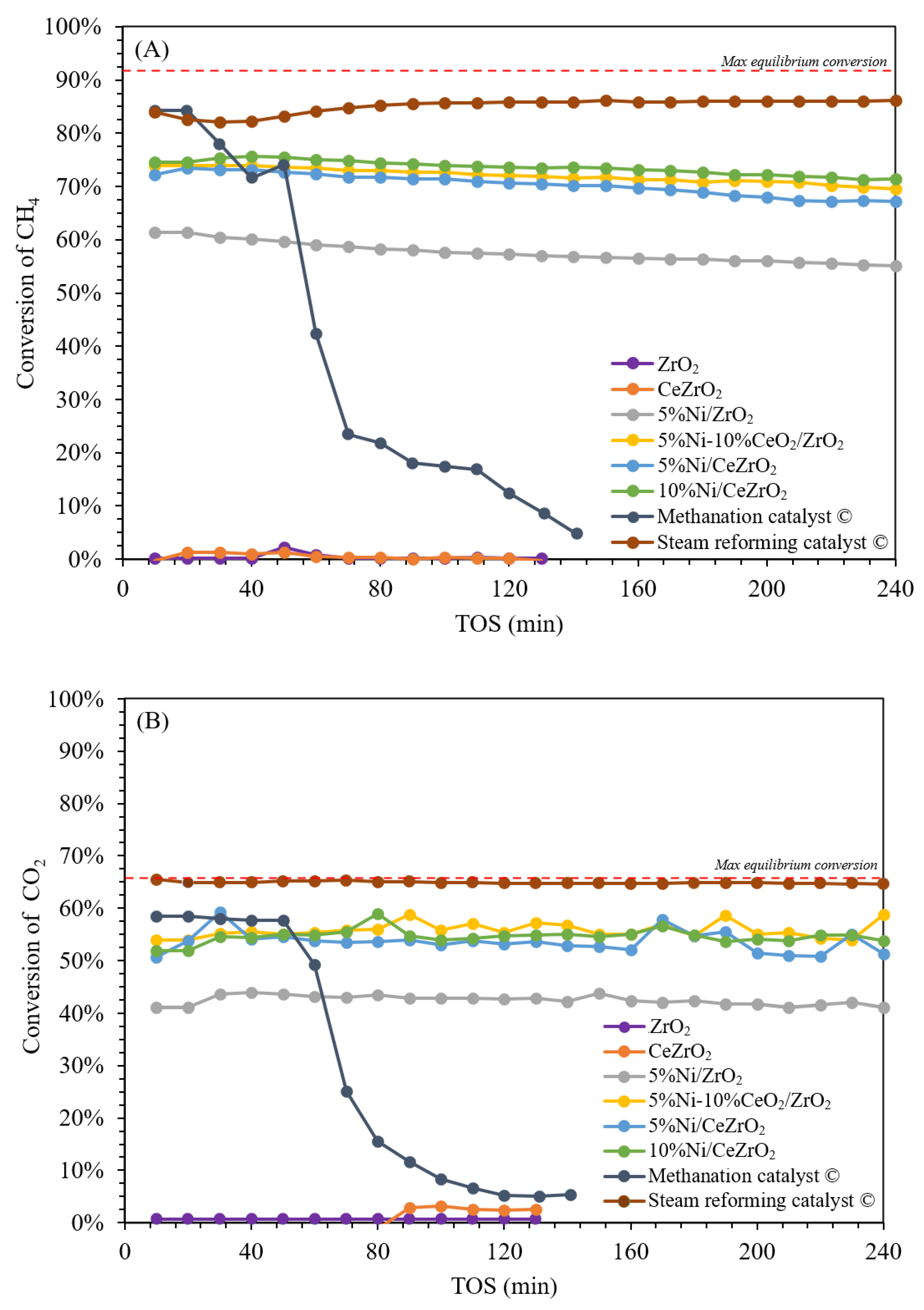
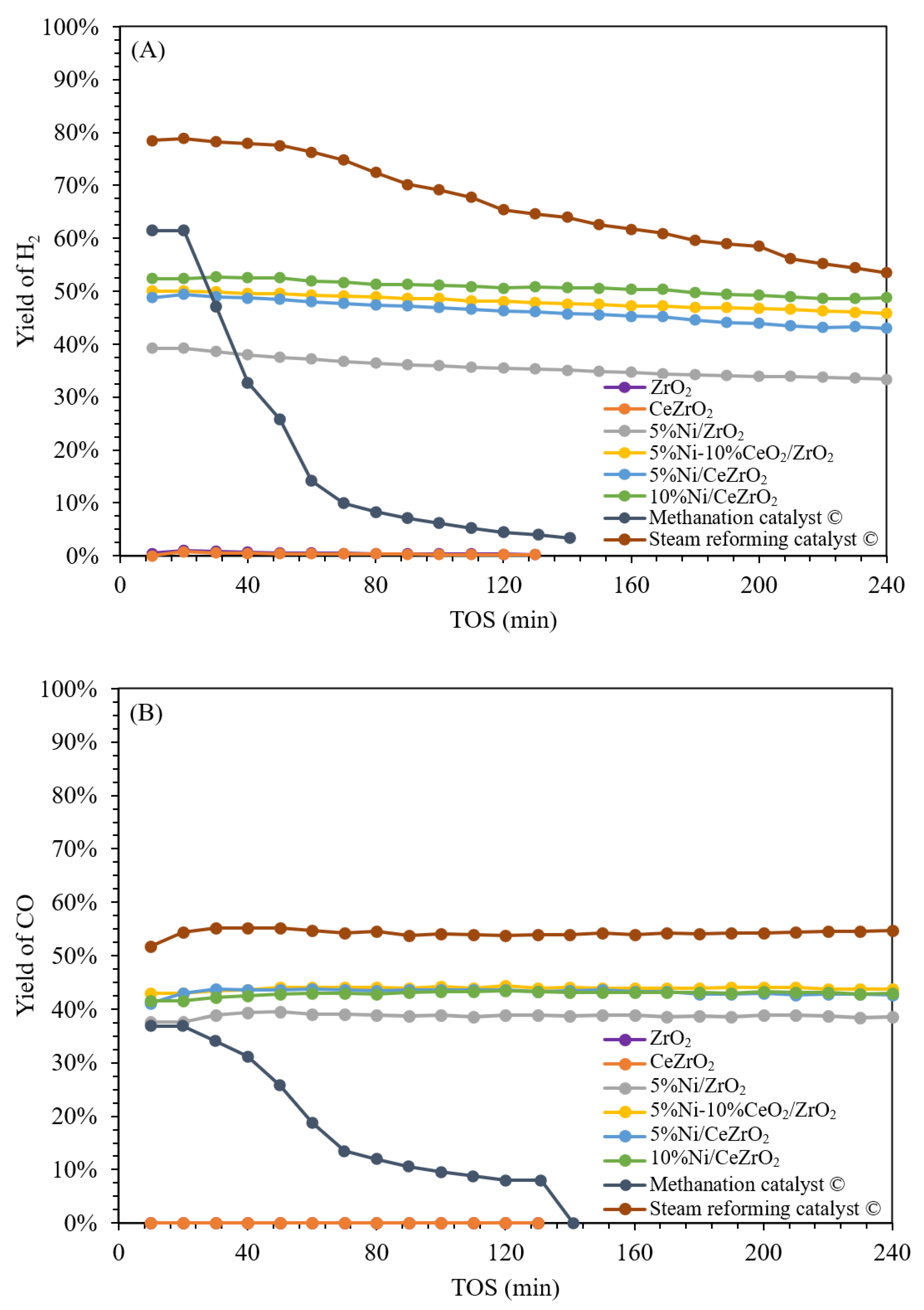
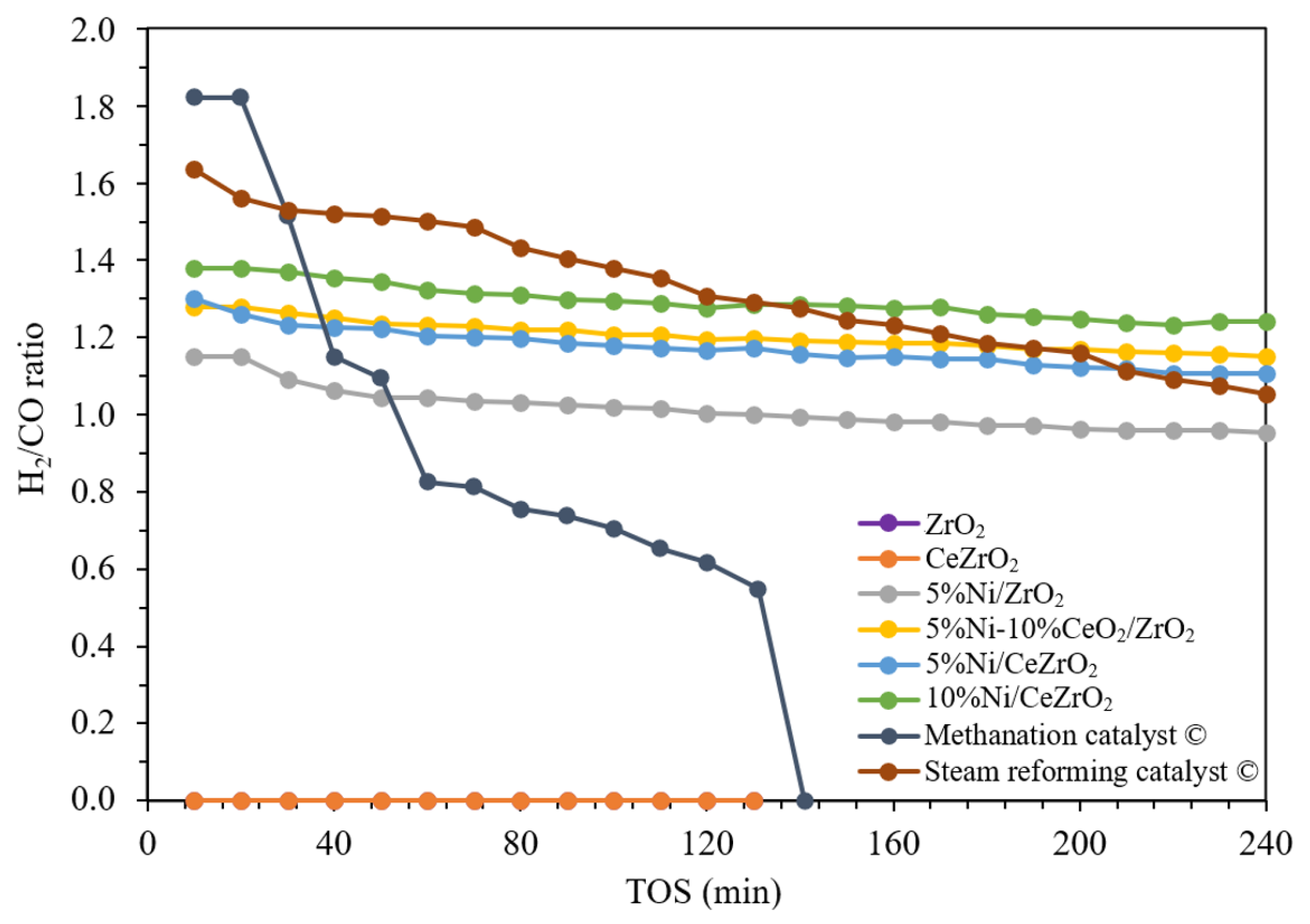
3.2. Activity and Stability of the Catalysts
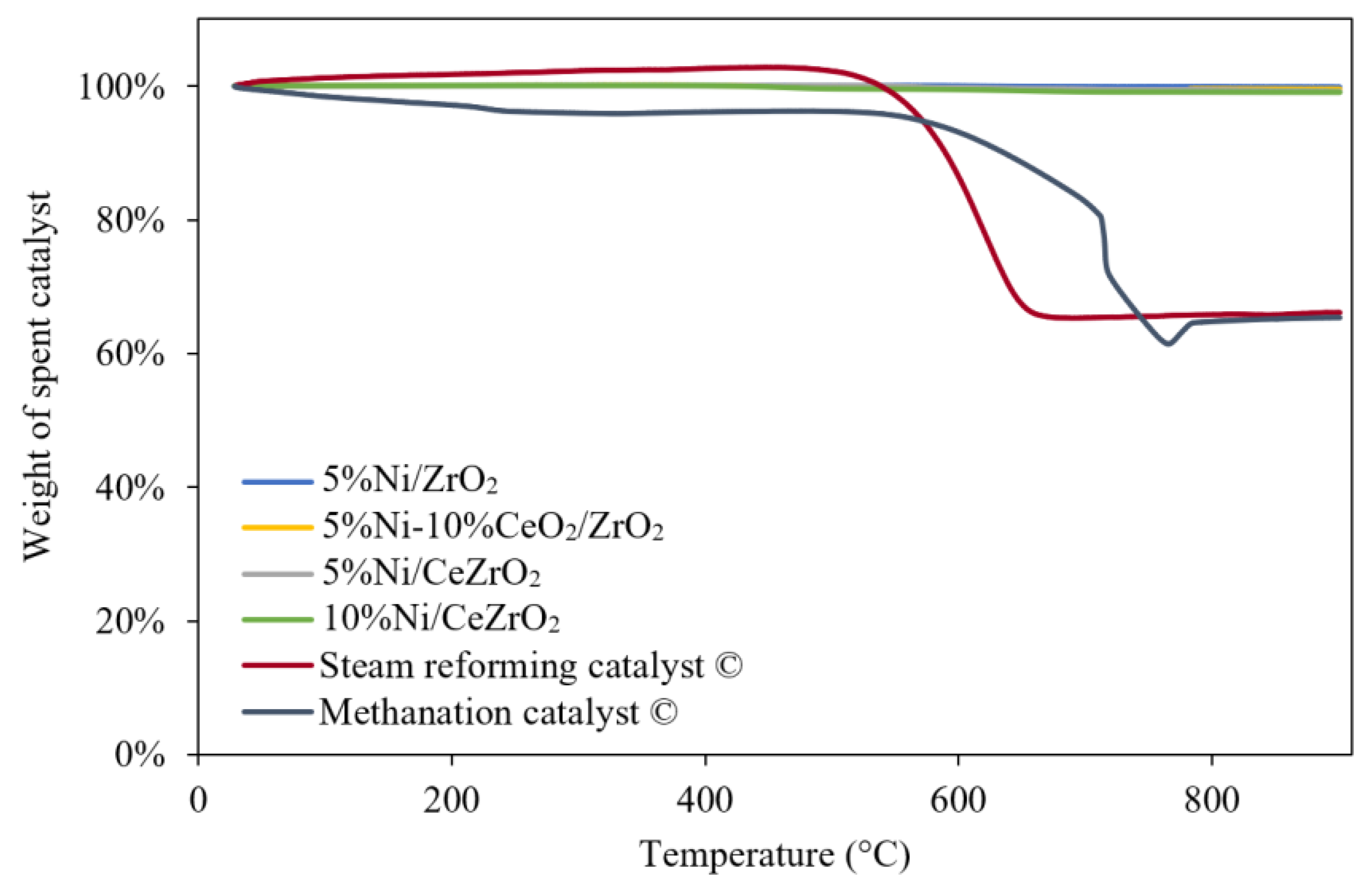
3.3. Carbon Formation in the Spent Catalyst
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Budhi, Y.W.; Effendy, M.; Bindar, Y. Subagjo Dynamic Behavior of Reverse Flow Reactor for Lean Methane Combustion. J. Eng. Technol. Sci. 2014, 46, 299–317. [Google Scholar] [CrossRef]
- Riemer, P. Greenhouse Gas Mitigation Technologies, an Overview of the C02 Capture, Storage and Future Activities of the IEA Greenhouse Gas R&D Programme. Energy Convers. Manag. 1996, 37, 665–670. [Google Scholar]
- Lavoie, J.M. Review on Dry Reforming of Methane, a Potentially More Environmentally-Friendly Approach to the Increasing Natural Gas Exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Xu, Y.; Sun, Y. A Review of CH4–CO2 Reforming to Synthesis Gas over Ni-Based Catalysts in Recent Years (2010–2017). Int. J. Hydrogen Energy 2018, 43, 15030–15054. [Google Scholar] [CrossRef]
- Usman, M.; Wan Daud, W.M.A.; Abbas, H.F. Dry Reforming of Methane: Influence of Process Parameters–A Review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef]
- Jang, W.J.; Shim, J.O.; Kim, H.M.; Yoo, S.Y.; Roh, H.S. A Review on Dry Reforming of Methane in Aspect of Catalytic Properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Q.; Shen, W.; Zhu, Z.; Fang, Y. (Ni/MgAl2O4)@SiO2 Core–Shell Catalyst with High Coke-Resistance for the Dry Reforming of Methane. React. Kinet. Mech. Catal. 2018, 125, 127–139. [Google Scholar] [CrossRef]
- Das, S.; Pérez-Ramírez, J.; Gong, J.; Dewangan, N.; Hidajat, K.; Gates, B.C.; Kawi, S. Core-Shell Structured Catalysts for Thermocatalytic, Photocatalytic, and Electrocatalytic Conversion of CO2. Chem. Soc. Rev. 2020, 49, 2937–3004. [Google Scholar] [CrossRef]
- Hoseinzade, L.; Adams, T.A. Dynamic Modeling of Integrated Mixed Reforming and Carbonless Heat Systems. Ind. Eng. Chem. Res. 2018, 57, 6013–6023. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Bajaj, I.; Hasan, M.M.F. Simulation and Optimization of Reforming Reactors for Carbon Dioxide Utilization Using Both Rigorous and Reduced Models. J. CO2 Util. 2018, 23, 80–104. [Google Scholar] [CrossRef]
- Mahboob, S.; Haghighi, M.; Rahmani, F. Sonochemically Preparation and Characterization of Bimetallic Ni-Co/Al2O3-ZrO2 Nanocatalyst: Effects of Ultrasound Irradiation Time and Power on Catalytic Properties and Activity in Dry Reforming of CH4. Ultrason. Sonochem. 2017, 38, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yao, L.; Wang, Y.; Wang, S.; Zhao, Q.; Mao, D.; Hu, C. Low-Temperature Catalytic CO2 Dry Reforming of Methane on Ni-Si/ZrO2 Catalyst. ACS Catal. 2018, 8, 6495–6506. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Alonso-Fariñas, B.; Vilches Arenas, L.F.; Navarrete, B. Carbon Capture and Utilization Technologies: A Literature Review and Recent Advances. Energy Sour. Part A Recovery Util. Environ. Eff. 2019, 41, 1403–1433. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Oemar, U.; Saw, E.T.; Li, Z.; Kawi, S. Kinetic and Mechanistic Aspects for CO2 Reforming of Methane over Ni Based Catalysts. Chem. Eng. J. 2015, 278, 62–78. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A Review of Dry (CO2) Reforming of Methane over Noble Metal Catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Khani, Y.; Shariatinia, Z.; Bahadoran, F. High Catalytic Activity and Stability of ZnLaAlO4 Supported Ni, Pt and Ru Nanocatalysts Applied in the Dry, Steam and Combined Dry-Steam Reforming of Methane. Chem. Eng. J. 2016, 299, 353–366. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Carvalho, D.C.; de Souza, H.S.A.; Filho, J.M.; Oliveira, A.C.; Campos, A.; Milet, É.R.C.; de Sousa, F.F.; Padron-Hernandez, E. A Study on the Modification of Mesoporous Mixed Oxides Supports for Dry Reforming of Methane by Pt or Ru. Appl. Catal. A Gen. 2014, 473, 132–145. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Shi, W.; Zhang, J.; Wu, K.; Zhao, Y.; Li, H.; Li, H.X.; Xu, G.Q.; Chen, W. Thermally Stable Ir/Ce0.9La0.1O2 Catalyst for High Temperature Methane Dry Reforming Reaction. Nano Res. 2017, 10, 364–380. [Google Scholar] [CrossRef]
- Li, L.; Zhou, L.; Ould-Chikh, S.; Anjum, D.H.; Kanoun, M.B.; Scaranto, J.; Hedhili, M.N.; Khalid, S.; Laveille, P.V.; D’Souza, L.; et al. Controlled Surface Segregation Leads to Efficient Coke-Resistant Nickel/Platinum Bimetallic Catalysts for the Dry Reforming of Methane. ChemCatChem 2015, 7, 819–829. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Panagiotopoulou, P.; Katsoni, A.; Diamadopoulos, E.; Mantzavinos, D.; Delimitis, A. Dry Reforming of Methane: Catalytic Performance and Stability of Ir Catalysts Supported on γ-Al2O3, Zr0.92Y0.08O2-δ (YSZ) or Ce0.9Gd0.1O2-δ (GDC) Supports. Top. Catal. 2015, 58, 1228–1241. [Google Scholar] [CrossRef]
- Singh, S.A.; Madras, G. Sonochemical Synthesis of Pt, Ru Doped TiO2 for Methane Reforming. Appl. Catal. A Gen. 2016, 518, 102–114. [Google Scholar] [CrossRef]
- Pakhare, D.; Shaw, C.; Haynes, D.; Shekhawat, D.; Spivey, J. Effect of Reaction Temperature on Activity of Pt- and Ru-Substituted Lanthanum Zirconate Pyrochlores (La2Zr2O7) for Dry (CO2) Reforming of Methane (DRM). J. CO2 Util. 2013, 1, 37–42. [Google Scholar] [CrossRef]
- Jabbour, K.; el Hassan, N.; Casale, S.; Estephane, J.; el Zakhem, H. Promotional Effect of Ru on the Activity and Stability of Co/SBA-15 Catalysts in Dry Reforming of Methane. Int. J. Hydrogen Energy 2014, 39, 7780–7787. [Google Scholar] [CrossRef]
- Kamieniak, J.; Bernalte, E.; Doyle, A.M.; Kelly, P.J.; Banks, C.E. Can Ultrasound or PH Influence Pd Distribution on the Surface of HAP to Improve Its Catalytic Properties in the Dry Reforming of Methane? Catal. Lett. 2017, 147, 2200–2208. [Google Scholar] [CrossRef][Green Version]
- Singha, R.K.; Yadav, A.; Shukla, A.; Kumar, M.; Bal, R. Low Temperature Dry Reforming of Methane over Pd-CeO2 Nanocatalyst. Catal. Commun. 2017, 92, 19–22. [Google Scholar] [CrossRef]
- Pizzolitto, C.; Pupulin, E.; Menegazzo, F.; Ghedini, E.; di Michele, A.; Mattarelli, M.; Cruciani, G.; Signoretto, M. Nickel Based Catalysts for Methane Dry Reforming: Effect of Supports on Catalytic Activity and Stability. Int. J. Hydrogen Energy 2019, 44, 28065–28076. [Google Scholar] [CrossRef]
- Dekkar, S.; Tezkratt, S.; Sellam, D.; Ikkour, K.; Parkhomenko, K.; Martinez-Martin, A.; Roger, A.C. Dry Reforming of Methane over Ni–Al2O3 and Ni–SiO2 Catalysts: Role of Preparation Methods. Catal. Lett. 2020, 150, 2180–2199. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, G.; Xue, Q.; Chen, L.; Lu, Y. High Carbon-Resistance Ni/CeAlO3-Al2O3 Catalyst for CH4/CO2 Reforming. Appl. Catal. B Environ. 2013, 136–137, 260–268. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, L.; Zhang, X.; Wang, Y.; Pan, H.; Su, H. Modification Effect of Natural Mixed Rare Earths on Co/γ-Al2O3 Catalysts for CH4/CO2 Reforming to Synthesis Gas. Int. J. Hydrogen Energy 2012, 37, 9994–10001. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Shi, W. Syngas Production from CO2 Reforming with Methane over Core-Shell Ni@SiO2 Catalysts. J. CO2 Util. 2016, 16, 318–327. [Google Scholar] [CrossRef]
- Baktash, E.; Littlewood, P.; Schomäcker, R.; Thomas, A.; Stair, P.C. Alumina Coated Nickel Nanoparticles as a Highly Active Catalyst for Dry Reforming of Methane. Appl. Catal. B Environ. 2015, 179, 122–127. [Google Scholar] [CrossRef]
- Gao, X.; Tan, Z.; Hidajat, K.; Kawi, S. Highly Reactive Ni-Co/SiO2 Bimetallic Catalyst via Complexation with Oleylamine/Oleic Acid Organic Pair for Dry Reforming of Methane. Catal. Today 2017, 281, 250–258. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, Q.; Zhang, J.; Sun, Y.; Zhu, Y. NiO-MgO Nanoparticles Confined inside SiO2 Frameworks to Achieve Highly Catalytic Performance for CO2 Reforming of Methane. Mol. Catal. 2017, 432, 31–36. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Chen, C.; Zou, X.; Ding, W.; Lu, X. Dry Reforming of Methane to Syngas over Lanthanum-Modified Mesoporous Nickel Aluminate/Γ-Alumina Nanocomposites by One-Pot Synthesis. Int. J. Hydrogen Energy 2017, 42, 11333–11345. [Google Scholar] [CrossRef]
- Ghods, B.; Meshkani, F.; Rezaei, M. Effects of Alkaline Earth Promoters on the Catalytic Performance of the Nickel Catalysts Supported on High Surface Area Mesoporous Magnesium Silicate in Dry Reforming Reaction. Int. J. Hydrogen Energy 2016, 41, 22913–22921. [Google Scholar] [CrossRef]
- Titus, J.; Roussière, T.; Wasserschaff, G.; Schunk, S.; Milanov, A.; Schwab, E.; Wagner, G.; Oeckler, O.; Gläser, R. Dry Reforming of Methane with Carbon Dioxide over NiO-MgO-ZrO2. Catal. Today 2016, 270, 68–75. [Google Scholar] [CrossRef]
- Budiman, A.W.; Song, S.H.; Chang, T.S.; Shin, C.H.; Choi, M.J. Dry Reforming of Methane Over Cobalt Catalysts: A Literature Review of Catalyst Development. Catal. Surv. Asia 2012, 16, 183–197. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effect of Ni Loadings on the Activity and Coke Formation of MgO-Modified Ni/Al2O3 Nanocatalyst in Dry Reforming of Methane. J. Energy Chem. 2014, 23, 633–638. [Google Scholar] [CrossRef]
- Faria, E.C.; Neto, R.C.R.; Colman, R.C.; Noronha, F.B. Hydrogen Production through CO2 Reforming of Methane over Ni/CeZrO2/Al2O3 Catalysts. Catal. Today 2014, 228, 138–144. [Google Scholar] [CrossRef]
- Teh, L.P.; Setiabudi, H.D.; Timmiati, S.N.; Aziz, M.A.A.; Annuar, N.H.R.; Ruslan, N.N. Recent Progress in Ceria-Based Catalysts for the Dry Reforming of Methane: A Review. Chem. Eng. Sci. 2021, 242, 116606. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Huerta, M.V.M.; Fierro, J.L.G. The Effect of CeO2 on the Surface and Catalytic Properties of Pt/CeO2-ZrO2 Catalysts for Methane Dry Reforming. Appl. Catal. B Environ. 2009, 89, 149–159. [Google Scholar] [CrossRef]
- Singha, R.K.; Das, S.; Pandey, M.; Kumar, S.; Bal, R.; Bordoloi, A. Ni Nanocluster on Modified CeO2-ZrO2 Nanoporous Composite for Tri-Reforming of Methane. Catal. Sci. Technol. 2016, 6, 7122–7136. [Google Scholar] [CrossRef]
- Wolfbeisser, A.; Sophiphun, O.; Bernardi, J.; Wittayakun, J.; Föttinger, K.; Rupprechter, G. Methane Dry Reforming over Ceria-Zirconia Supported Ni Catalysts. Catal. Today 2016, 277, 234–245. [Google Scholar] [CrossRef]
- Muñoz, M.A.; Calvino, J.J.; Rodríguez-Izquierdo, J.M.; Blanco, G.; Arias, D.C.; Pérez-Omil, J.A.; Hernández-Garrido, J.C.; González-Leal, J.M.; Cauqui, M.A.; Yeste, M.P. Highly Stable Ceria-Zirconia-Yttria Supported Ni Catalysts for Syngas Production by CO2 Reforming of Methane. Appl. Surf. Sci. 2017, 426, 864–873. [Google Scholar] [CrossRef]
- Mesrar, F.; Kacimi, M.; Liotta, L.F.; Puleo, F.; Ziyad, M. Syngas Production from Dry Reforming of Methane over Ni/Perlite Catalysts: Effect of Zirconia and Ceria Impregnation. Int. J. Hydrogen Energy 2018, 43, 17142–17155. [Google Scholar] [CrossRef]
- Paladino Lino, A.V.; Rodella, C.B.; Assaf, E.M.; Assaf, J.M. Methane Tri-Reforming for Synthesis Gas Production Using Ni/CeZrO2/MgAl2O4 Catalysts: Effect of Zr/Ce Molar Ratio. Int. J. Hydrogen Energy 2020, 45, 8418–8432. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.; Chen, X.; Rui, N.; Betancourt, L.E.; Lin, L.; Xu, W.; Sun, C.J.; Abeykoon, A.M.M.; Rodriguez, J.A.; et al. Effects of Zr Doping into Ceria for the Dry Reforming of Methane over Ni/CeZrO2 Catalysts: In Situ Studies with XRD, XAFS, and AP-XPS. ACS Catal. 2020, 10, 3274–3284. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 Catalysts for the Dry Reforming of Methane. Appl. Catal. A Gen. 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Kumar, P.; Sun, Y.; Idem, R.O. Nickel-Based Ceria, Zirconia, and Ceria-Zirconia Catalytic Systems for Low-Temperature Carbon Dioxide Reforming of Methane. Energy Fuels 2007, 21, 3113–3123. [Google Scholar] [CrossRef]
- Basahel, S.N.; Ali, T.T.; Mokhtar, M.; Narasimharao, K. Influence of Crystal Structure of Nanosized ZrO2 on Photocatalytic Degradation of Methyl Orange. Nanoscale Res. Lett. 2015, 10, 73. [Google Scholar] [CrossRef]
- Sophiana, I.C.; Topandi, A.; Iskandar, F.; Devianto, H.; Nishiyama, N.; Budhi, Y.W. Catalytic Oxidation of Benzene at Low Temperature over Novel Combination of Metal Oxide Based Catalysts: CuO, MnO2, NiO with Ce0.75Zr0.25O2 as Support. Mater. Today Chem. 2020, 17, 100305. [Google Scholar] [CrossRef]
- Deng, Q.F.; Ren, T.Z.; Agula, B.; Liu, Y.; Yuan, Z.Y. Mesoporous CexZr1-XO2 Solid Solutions Supported CuO Nanocatalysts for Toluene Total Oxidation. J. Ind. Eng. Chem. 2014, 20, 3303–3312. [Google Scholar] [CrossRef]
- Pham, P.T.M.; le Minh, T.; Nguyen, T.T.; van Driessche, I. Ceo2 Based Catalysts for the Treatment of Propylene in Motorcycle’s Exhaust Gases. Materials 2014, 7, 7379–7397. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Vagia, E.C.; Lemonidou, A.A. Hydrogen Production via Steam Reforming of Bio-Oil Components over Calcium Aluminate Supported Nickel and Noble Metal Catalysts. Appl. Catal. A Gen. 2008, 351, 111–121. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Al-Fatesh, A.A.; Atia, H.; Fakeeha, A.H.; Kasim, S.O.; Abasaeed, A.E. Influence of Promoted 5%Ni/MCM-41 Catalysts on Hydrogen Yield in CO2 Reforming of CH4. Int. J. Energy Res. 2018, 42, 4120–4130. [Google Scholar] [CrossRef]
- Tan, M.; Wang, X.; Hu, Y.; Shang, X.; Zhang, L.; Zou, X.; Ding, W.; Lu, X. Influence of Nickel Content on Structural and Surface Properties, Reducibility and Catalytic Behavior of Mesoporous γ-Alumina-Supported Ni-Mg Oxides for Pre-Reforming of Liquefied Petroleum Gas. Catal. Sci. Technol. 2016, 6, 3049–3063. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, S.; Mao, D.; Hu, C. Low-Temperature Catalytic CO2 Dry Reforming of Methane on Ni-Based Catalysts: A Review. Fuel Process. Technol. 2018, 169, 199–206. [Google Scholar] [CrossRef]
- Nguyen-Huy, C.; Shin, E.W. Oxidative Cracking of Vacuum Residue with Steam over NiK/CeZr–Al Catalysts. Fuel 2017, 192, 149–157. [Google Scholar] [CrossRef]
- Jabbour, K.; Massiani, P.; Davidson, A.; Casale, S.; el Hassan, N. Ordered Mesoporous “One-Pot” Synthesized Ni-Mg(Ca)-Al2O3 as Effective and Remarkably Stable Catalysts for Combined Steam and Dry Reforming of Methane (CSDRM). Appl. Catal. B Environ. 2017, 201, 527–542. [Google Scholar] [CrossRef]
- Rahbar Shamskar, F.; Rezaei, M.; Meshkani, F. The Influence of Ni Loading on the Activity and Coke Formation of Ultrasound-Assisted Co-Precipitated Ni–Al2O3 Nanocatalyst in Dry Reforming of Methane. Int. J. Hydrogen Energy 2017, 42, 4155–4164. [Google Scholar] [CrossRef]
- Makri, M.M.; Vasiliades, M.A.; Petallidou, K.C.; Efstathiou, A.M. Effect of Support Composition on the Origin and Reactivity of Carbon Formed during Dry Reforming of Methane over 5 wt% Ni/Ce1-XMxO2-δ (M = Zr4+, Pr3+) Catalysts. Catal. Today 2016, 259, 150–164. [Google Scholar] [CrossRef]

| Catalyst | Surface Area (m2 g−1) | Pore Diameter (nm) | Total Pore Volume (cm3 g−1) |
|---|---|---|---|
| ZrO2 | 29.19 | 18.54 | 0.135 |
| 5%NiO/ZrO2 | 17.23 | 22.16 | 0.095 |
| 5%NiO-10%CeO2/ZrO2 | 24.62 | 7.73 | 0.047 |
| Ce0.1Zr0.9O2 (CeZrO2) | 39.83 | 13.14 | 0.131 |
| 5%NiO/CeZrO2 | 30.40 | 14.78 | 0.112 |
| 10%NiO/CeZrO2 | 28.75 | 16.10 | 0.116 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sophiana, I.C.; Iskandar, F.; Devianto, H.; Nishiyama, N.; Budhi, Y.W. Coke-Resistant Ni/CeZrO2 Catalysts for Dry Reforming of Methane to Produce Hydrogen-Rich Syngas. Nanomaterials 2022, 12, 1556. https://doi.org/10.3390/nano12091556
Sophiana IC, Iskandar F, Devianto H, Nishiyama N, Budhi YW. Coke-Resistant Ni/CeZrO2 Catalysts for Dry Reforming of Methane to Produce Hydrogen-Rich Syngas. Nanomaterials. 2022; 12(9):1556. https://doi.org/10.3390/nano12091556
Chicago/Turabian StyleSophiana, Intan Clarissa, Ferry Iskandar, Hary Devianto, Norikazu Nishiyama, and Yogi Wibisono Budhi. 2022. "Coke-Resistant Ni/CeZrO2 Catalysts for Dry Reforming of Methane to Produce Hydrogen-Rich Syngas" Nanomaterials 12, no. 9: 1556. https://doi.org/10.3390/nano12091556
APA StyleSophiana, I. C., Iskandar, F., Devianto, H., Nishiyama, N., & Budhi, Y. W. (2022). Coke-Resistant Ni/CeZrO2 Catalysts for Dry Reforming of Methane to Produce Hydrogen-Rich Syngas. Nanomaterials, 12(9), 1556. https://doi.org/10.3390/nano12091556