Investigating the Morphology and Mechanics of Biogenic Hierarchical Materials at and below Micrometer Scale
Abstract
1. Introduction
2. Materials and Methods
2.1. Diatom Culture
2.2. Sample Preparation for SEM Imaging and Mechanical Testing
2.3. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS)
2.4. Mechanical Manipulation and In Situ Deformation Tests
2.5. Scanning Transmission Electron Microscopy (STEM) Tomography and Segmentation
2.6. FEM Simulations
3. Results and Discussion
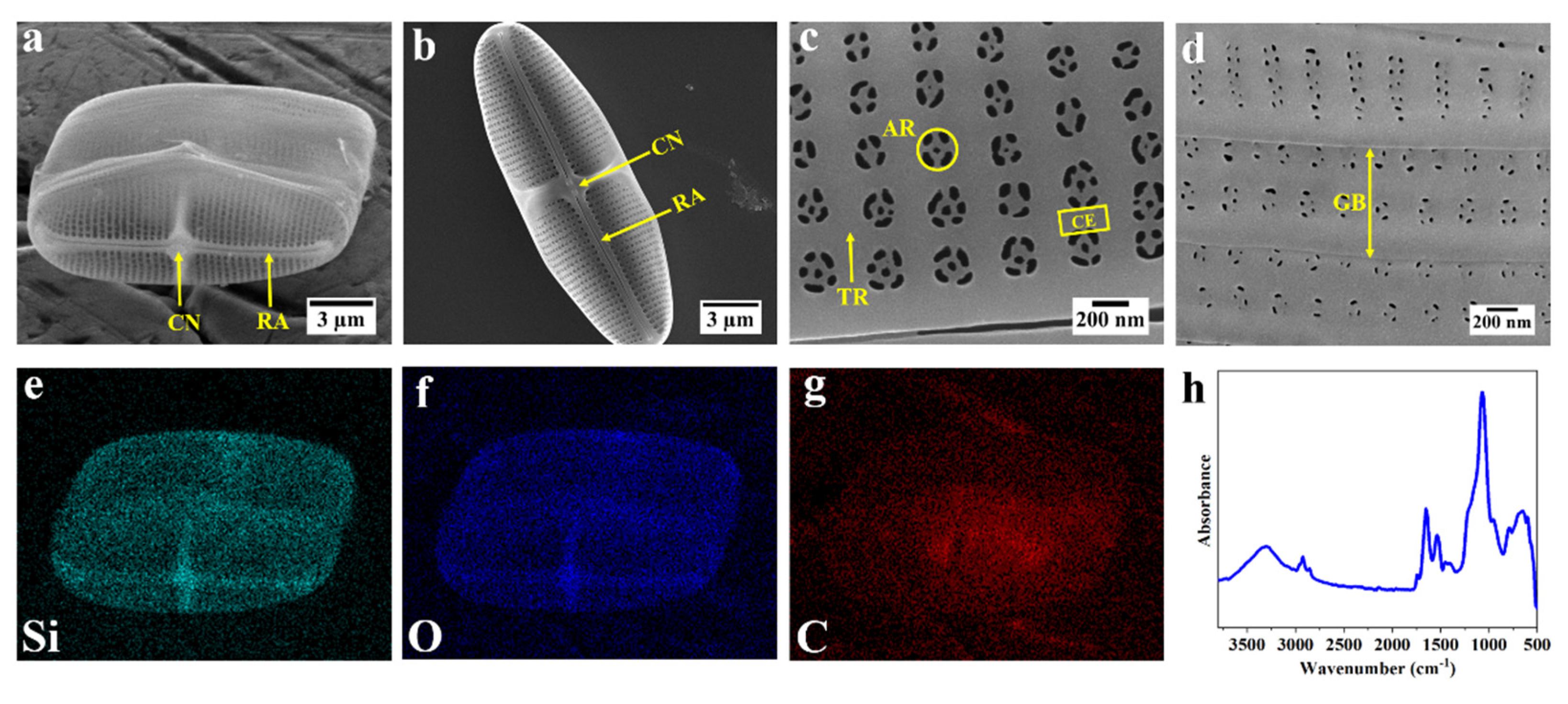
3.1. Morphology and Elemental Composition of the Frustule of Diatom C.sp.
3.2. Positioning of the Frustule for In Situ Mechanical Testing
3.3. In Situ Deformation Experiment, STEM Electron Tomography, and FEM Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arakaki, A.; Shimizu, K.; Oda, M.; Sakamoto, T.; Nishimura, T.; Kato, T. Biomineralization-inspired synthesis of functional organic/inorganic hybrid materials: Organic molecular control of self-organization of hybrids. Org. Biomol. Chem. 2015, 13, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Luz, G.M.; Mano, J.F. Mineralized structures in nature: Examples and inspirations for the design of new composite materials and biomaterials. Compos. Sci. Technol. 2010, 70, 1777–1788. [Google Scholar] [CrossRef]
- Libonati, F.; Buehler, M.J. Advanced structural materials by bioinspiration. Adv. Eng. Mater. 2017, 19, 1600787. [Google Scholar] [CrossRef]
- Espinosa, H.D.; Rim, J.E.; Barthelat, F.; Buehler, M.J. Merger of structure and material in nacre and bone–Perspectives on de novo biomimetic materials. Prog. Mater. Sci. 2009, 54, 1059–1100. [Google Scholar] [CrossRef]
- Thula, T.T.; Rodriguez, D.E.; Lee, M.H.; Pendi, L.; Podschun, J.; Gower, L.B. In vitro mineralization of dense collagen substrates: A biomimetic approach toward the development of bone-graft materials. Acta Biomater. 2011, 7, 3158–3169. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Gao, H. Mechanical properties of nanostructure of biological materials. J. Mech. Phys. Solids 2004, 52, 1963–1990. [Google Scholar] [CrossRef]
- Ji, B.; Gao, H. Mechanical principles of biological nanocomposites. Annu. Rev. Mater. Res. 2010, 40, 77–100. [Google Scholar] [CrossRef]
- Grossman, M.; Pivovarov, D.; Bouville, F.; Dransfeld, C.; Masania, K.; Studart, A.R. Hierarchical toughening of nacre-like composites. Adv. Funct. Mater. 2019, 29, 1806800. [Google Scholar] [CrossRef]
- Rim, J.E.; Zavattieri, P.; Juster, A.; Espinosa, H.D. Dimensional analysis and parametric studies for designing artificial nacre. J. Mech. Behav. Biomed. Mater. 2011, 4, 190–211. [Google Scholar] [CrossRef]
- Hildebrand, M. Diatoms, biomineralization processes, and genomics. Chem. Rev. 2008, 108, 4855–4874. [Google Scholar] [CrossRef]
- Soleimani, M.; Rutten, L.; Maddala, S.P.; Wu, H.; Eren, E.D.; Mezari, B.; Schreur-Piet, I.; Friedrich, H.; van Benthem, R.A.T.M. Modifying the thickness, pore size, and composition of diatom frustule in Craspedostauros sp. with Al3+ ions. Sci. Rep. 2020, 10, 19498. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, M.; De Stefano, L. Nanostructures in diatom frustules: Functional morphology of valvocopulae in Cocconeidacean monoraphid taxa. J. Nanosci. Nanotechnol. 2005, 5, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, J.; Jiang, Y.; Jiang, X.; Zhang, D. Preparation of biosilica structures from frustules of diatoms and their applications: Current state and perspectives. Appl. Microbiol. Biotechnol. 2013, 97, 453–460. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, L.; Lamberti, A.; Rotiroti, L.; De Stefano, M. Interfacing the nanostructured biosilica microshells of the marine diatom Coscinodiscus wailesii with biological matter. Acta Biomater. 2008, 4, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Kröger, N.; Poulsen, N. Diatoms—from cell wall biogenesis to nanotechnology. Annu. Rev. Genet. 2008, 42, 83–107. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.A.; Pack, S.P. Biomimetic and bioinspired silicifications: Recent advances for biomaterial design and applications. Acta Biomater. 2021, 120, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, S.; Prieto-Simón, B.; Campàs, M. Past, present and future of diatoms in biosensing. Trends. Anal. Chem. 2016, 79, 276–285. [Google Scholar] [CrossRef]
- Losic, D.; Mitchell, J.G.; Voelcker, N.H. Complex gold nanostructures derived by templating from diatom frustules. ChemComm 2005, 39, 4905–4907. [Google Scholar] [CrossRef]
- Losic, D.; Rosengarten, G.; Mitchell, J.G.; Voelcker, N.H. Pore architecture of diatom frustules: Potential nanostructured membranes for molecular and particle separations. J. Nanosci. Nanotechnol. 2006, 6, 982–989. [Google Scholar] [CrossRef]
- Aw, M.S.; Simovic, S.; Yu, Y.; Addai-Mensah, J.; Losic, D. Porous silica microshells from diatoms as biocarrier for drug delivery applications. Powder Technol. 2012, 223, 52–58. [Google Scholar] [CrossRef]
- Lang, Y.; Monte, F.d.; Rodriguez, B.J.; Dockery, P.; Finn, D.P.; Pandit, A. Integration of TiO2 into the diatom Thalassiosira weissflogii during frustule synthesis. Sci. Rep. 2013, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jeffryes, C.; Solanki, R.; Rangineni, Y.; Wang, W.; Chang, C.H.; Rorrer, G.L. Electroluminescence and photoluminescence from nanostructured diatom frustules containing metabolically inserted germanium. Adv. Mater. 2008, 20, 2633–2637. [Google Scholar] [CrossRef]
- Köhler, L.; Machill, S.; Werner, A.; Selzer, C.; Kaskel, S.; Brunner, E. Are diatoms “green” aluminosilicate synthesis microreactors for future catalyst production? Molecules 2017, 22, 2232. [Google Scholar] [CrossRef] [PubMed]
- Hamm, C.E.; Merkel, R.; Springer, O.; Jurkojc, P.; Maier, C.; Prechtel, K.; Smetacek, V. Architecture and material properties of diatom shells provide effective mechanical protection. Nature 2003, 421, 841–843. [Google Scholar] [CrossRef]
- Pančić, M.; Torres, R.R.; Almeda, R.; Kiørboe, T. Silicified cell walls as a defensive trait in diatoms. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190184. [Google Scholar] [CrossRef]
- Losic, D.; Short, K.; Mitchell, J.G.; Lal, R.; Voelcker, N.H. AFM nanoindentations of diatom biosilica surfaces. Langmuir 2007, 23, 5014–5021. [Google Scholar] [CrossRef]
- Almqvist, N.; Delamo, Y.; Smith, B.; Thomson, N.; Bartholdson, Å.; Lal, R.; Brzezinski, M.; Hansma, P. Micromechanical and structural properties of a pennate diatom investigated by atomic force microscopy. J. Microsc. 2001, 202, 518–532. [Google Scholar] [CrossRef]
- Moreno, M.D.; Ma, K.; Schoenung, J.; Dávila, L.P. An integrated approach for probing the structure and mechanical properties of diatoms: Toward engineered nanotemplates. Acta Biomater. 2015, 25, 313–324. [Google Scholar] [CrossRef]
- Aitken, Z.H.; Luo, S.; Reynolds, S.N.; Thaulow, C.; Greer, J.R. Microstructure provides insights into evolutionary design and resilience of Coscinodiscus sp. frustule. Proc. Natl. Acad. Sci. USA 2016, 113, 2017–2022. [Google Scholar] [CrossRef]
- Görlich, S.; Pawolski, D.; Zlotnikov, I.; Kröger, N. Control of biosilica morphology and mechanical performance by the conserved diatom gene Silicanin-1. Commun. Biol. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Lu, J.; Sun, C.; Wang, Q.J. Mechanical simulation of a diatom frustule structure. J. Bionic Eng. 2015, 12, 98–108. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Gordon, R.; Dávila, L.P. Deformation modes and structural response of diatom frustules. J. Mater. Sci. Eng. Adv. Technol. 2017, 15, 105–134. [Google Scholar]
- Gutiérrez, A.; Guney, M.G.; Fedder, G.K.; Dávila, L.P. The role of hierarchical design and morphology in the mechanical response of diatom-inspired structures via simulation. J. Biomater. Sci. 2018, 6, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Möbus, G.; Inkson, B.J. Nanoscale tomography in materials science. Mater. Today 2007, 10, 18–25. [Google Scholar] [CrossRef]
- Saghi, Z.; Midgley, P.A. Electron tomography in the (S) TEM: From nanoscale morphological analysis to 3D atomic imaging. Annu. Rev. Mater. Res. 2012, 42, 59–79. [Google Scholar] [CrossRef]
- Fijneman, A.J.; Goudzwaard, M.; Keizer, A.D.; Bomans, P.H.; Gebäck, T.; Palmlöf, M.; Persson, M.; Högblom, J.; de With, G.; Friedrich, H. Local quantification of mesoporous silica microspheres using multiscale electron tomography and lattice Boltzmann simulations. Microporous Mesoporous Mater. 2020, 302, 110243. [Google Scholar] [CrossRef]
- Lu, X.; Bertei, A.; Finegan, D.P.; Tan, C.; Daemi, S.R.; Weaving, J.S.; O’Regan, K.B.; Heenan, T.M.; Hinds, G.; Kendrick, E. 3D microstructure design of lithium-ion battery electrodes assisted by X-ray nano-computed tomography and modelling. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Zgłobicka, I.; Li, Q.; Gluch, J.; Płocińska, M.; Noga, T.; Dobosz, R.; Szoszkiewicz, R.; Witkowski, A.; Zschech, E.; Kurzydłowski, K.J. Visualization of the internal structure of Didymosphenia geminata frustules using nano X-ray tomography. Sci. Rep. 2017, 7, 9086. [Google Scholar]
- Zglobicka, I.; Chmielewska, A.; Topal, E.; Kutukova, K.; Gluch, J.; Krüger, P.; Kilroy, C.; Swieszkowski, W.; Kurzydlowski, K.J.; Zschech, E. 3D diatom–designed and selective laser melting (SLM) manufactured metallic structures. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Topal, E.; Rajendran, H.; Zgłobicka, I.; Gluch, J.; Liao, Z.; Clausner, A.; Kurzydłowski, K.J.; Zschech, E. Numerical and experimental study of the mechanical response of diatom frustules. Nanomaterials 2020, 10, 959. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y.; Cai, J.; Pan, J.; Jiang, X.; Jiang, Y. Bio-manufacturing technology based on diatom micro-and nanostructure. Chin. Sci. Bull. 2012, 57, 3836–3849. [Google Scholar] [CrossRef]
- Kampschulte, M.; Langheinirch, A.C.; Sender, J.; Litzlbauer, H.D.; Althöhn, U.; Schwab, J.D.; Alejandre-Lafont, E.; Martels, G.; Krombach, G.A. Nano-Computed Tomography: Technique and Applications. RöFo 2016, 188, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, H.; De Jongh, P.E.; Verkleij, A.J.; De Jong, K.P. Electron tomography for heterogeneous catalysts and related nanostructured materials. Chem. Rev. 2009, 109, 1613–1629. [Google Scholar] [CrossRef] [PubMed]
- Neumüller, J. Electron tomography—A tool for ultrastructural 3D visualization in cell biology and histology. Wien. Med. Wochenschr. Suppl. 2018, 168, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.E.; Friedrich, H. Advanced tomography techniques for inorganic, organic, and biological materials. MRS Bull. 2016, 41, 516–521. [Google Scholar] [CrossRef]
- Levitan, O.; Chen, M.; Kuang, X.; Cheong, K.Y.; Jiang, J.; Banal, M.; Nambiar, N.; Gorbunov, M.Y.; Ludtke, S.J.; Falkowski, P.G. Structural and functional analyses of photosystem II in the marine diatom Phaeodactylum tricornutum. Proc. Natl. Acad. Sci. USA 2019, 116, 17316–17322. [Google Scholar] [CrossRef]
- Arshad, R.; Calvaruso, C.; Boekema, E.J.; Büchel, C.; Kouřil, R. Revealing the architecture of the photosynthetic apparatus in the diatom Thalassiosira pseudonana. J. Plant Physiol. 2021, 186, 2124–2136. [Google Scholar] [CrossRef]
- Mayzel, B.; Aram, L.; Varsano, N.; Wolf, S.G.; Gal, A. Structural evidence for extracellular silica formation by diatoms. Nat. Commun. 2021, 12, 4639. [Google Scholar] [CrossRef]
- Soleimani, M.; van Breemen, L.C.; Maddala, S.P.; Joosten, R.R.; Wu, H.; Schreur-Piet, I.; van Benthem, R.A.; Friedrich, H. In Situ Manipulation and Micromechanical Characterization of Diatom Frustule Constituents Using Focused Ion Beam Scanning Electron Microscopy. Small Methods 2021, 5, 2100638. [Google Scholar] [CrossRef]
- Komissarenko, F.; Zograf, G.; Makarov, S.; Petrov, M.; Mukhin, I. Manipulation Technique for Precise Transfer of Single Perovskite Nanoparticles. Nanomaterials 2020, 10, 1306. [Google Scholar] [CrossRef]
- Wang, C.; Peng, Q.; Wu, J.; He, X.; Tong, L.; Luo, Q.; Li, J.; Moody, S.; Liu, H.; Wang, R. Mechanical characteristics of individual multi-layer graphene-oxide sheets under direct tensile loading. Carbon 2014, 80, 279–289. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Wang, N.; Li, Y.; Feng, X.; Huang, Y.; Zhao, C.; Liu, Z.; Fang, M.; Ou, G. Ultralight, scalable, and high-temperature–resilient ceramic nanofiber sponges. Sci. Adv. 2017, 3, e1603170. [Google Scholar] [CrossRef] [PubMed]
- Romeis, S.; Paul, J.; Herre, P.; Hanisch, M.; Taylor, R.N.K.; Schmidt, J.; Peukert, W. In situ deformation and breakage of silica particles inside a SEM. Procedia Eng. 2015, 102, 201–210. [Google Scholar] [CrossRef]
- Kremer, J.R.; Mastronarde, D.N.; McIntosh, J.R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 1996, 116, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Machill, S.; Köhler, L.; Ueberlein, S.; Hedrich, R.; Kunaschk, M.; Paasch, S.; Schulze, R.; Brunner, E. Analytical studies on the incorporation of aluminium in the cell walls of the marine diatom Stephanopyxis turris. BioMetals 2013, 26, 141–150. [Google Scholar] [CrossRef]
- Viji, S.; Anbazhagi, M.; Ponpandian, N.; Mangalaraj, D.; Jeyanthi, S.; Santhanam, P.; Devi, A.S.; Viswanathan, C. Diatom-based label-free optical biosensor for biomolecules. Appl. Biochem. Biotechnol. 2014, 174, 1166–1173. [Google Scholar] [CrossRef]
- Heredia, A.; Figueira, E.; Rodrigues, C.T.; Rodríguez-Galván, A.; Basiuk, V.A.; Vrieling, E.G.; Almeida, S.F. Cd2+ affects the growth, hierarchical structure and peptide composition of the biosilica of the freshwater diatom Nitzschia palea (Kützing) W. Smith. Psychol. Res. 2012, 60, 229–240. [Google Scholar] [CrossRef]
- Schweizer, P.; Dolle, C.; Dasler, D.; Abellán, G.; Hauke, F.; Hirsch, A.; Spiecker, E. Mechanical cleaning of graphene using in situ electron microscopy. Nat. Commun. 2020, 11, 1743. [Google Scholar] [CrossRef]
- Shi, C.; Luu, D.K.; Yang, Q.; Liu, J.; Chen, J.; Ru, C.; Xie, S.; Luo, J.; Ge, J.; Sun, Y. Recent advances in nanorobotic manipulation inside scanning electron microscopes. Microsyst. Nanoeng. 2016, 2, 1–16. [Google Scholar] [CrossRef]
- Subhash, G.; Yao, S.; Bellinger, B.; Gretz, M. Investigation of mechanical properties of diatom frustules using nanoindentation. J. Nanosci. Nanotechnol. 2005, 5, 50–56. [Google Scholar] [CrossRef]
- Dahmani, F.; Lambropoulos, J.; Schmid, A.; Burns, S.; Pratt, C. Nanoindentation technique for measuring residual stress field around a laser-induced crack in fused silica. J. Mater. Sci. 1998, 33, 4677–4685. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soleimani, M.; van den Broek, S.J.J.; Joosten, R.R.M.; van Hazendonk, L.S.; Maddala, S.P.; van Breemen, L.C.A.; van Benthem, R.A.T.M.; Friedrich, H. Investigating the Morphology and Mechanics of Biogenic Hierarchical Materials at and below Micrometer Scale. Nanomaterials 2022, 12, 1549. https://doi.org/10.3390/nano12091549
Soleimani M, van den Broek SJJ, Joosten RRM, van Hazendonk LS, Maddala SP, van Breemen LCA, van Benthem RATM, Friedrich H. Investigating the Morphology and Mechanics of Biogenic Hierarchical Materials at and below Micrometer Scale. Nanomaterials. 2022; 12(9):1549. https://doi.org/10.3390/nano12091549
Chicago/Turabian StyleSoleimani, Mohammad, Sten J. J. van den Broek, Rick R. M. Joosten, Laura S. van Hazendonk, Sai P. Maddala, Lambert C. A. van Breemen, Rolf A. T. M. van Benthem, and Heiner Friedrich. 2022. "Investigating the Morphology and Mechanics of Biogenic Hierarchical Materials at and below Micrometer Scale" Nanomaterials 12, no. 9: 1549. https://doi.org/10.3390/nano12091549
APA StyleSoleimani, M., van den Broek, S. J. J., Joosten, R. R. M., van Hazendonk, L. S., Maddala, S. P., van Breemen, L. C. A., van Benthem, R. A. T. M., & Friedrich, H. (2022). Investigating the Morphology and Mechanics of Biogenic Hierarchical Materials at and below Micrometer Scale. Nanomaterials, 12(9), 1549. https://doi.org/10.3390/nano12091549





