Silica Nanoparticles Enhance the Disease Resistance of Ginger to Rhizome Rot during Postharvest Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Fungal Pathogen
2.3. Effect of SiNPs on F. solani In Vitro
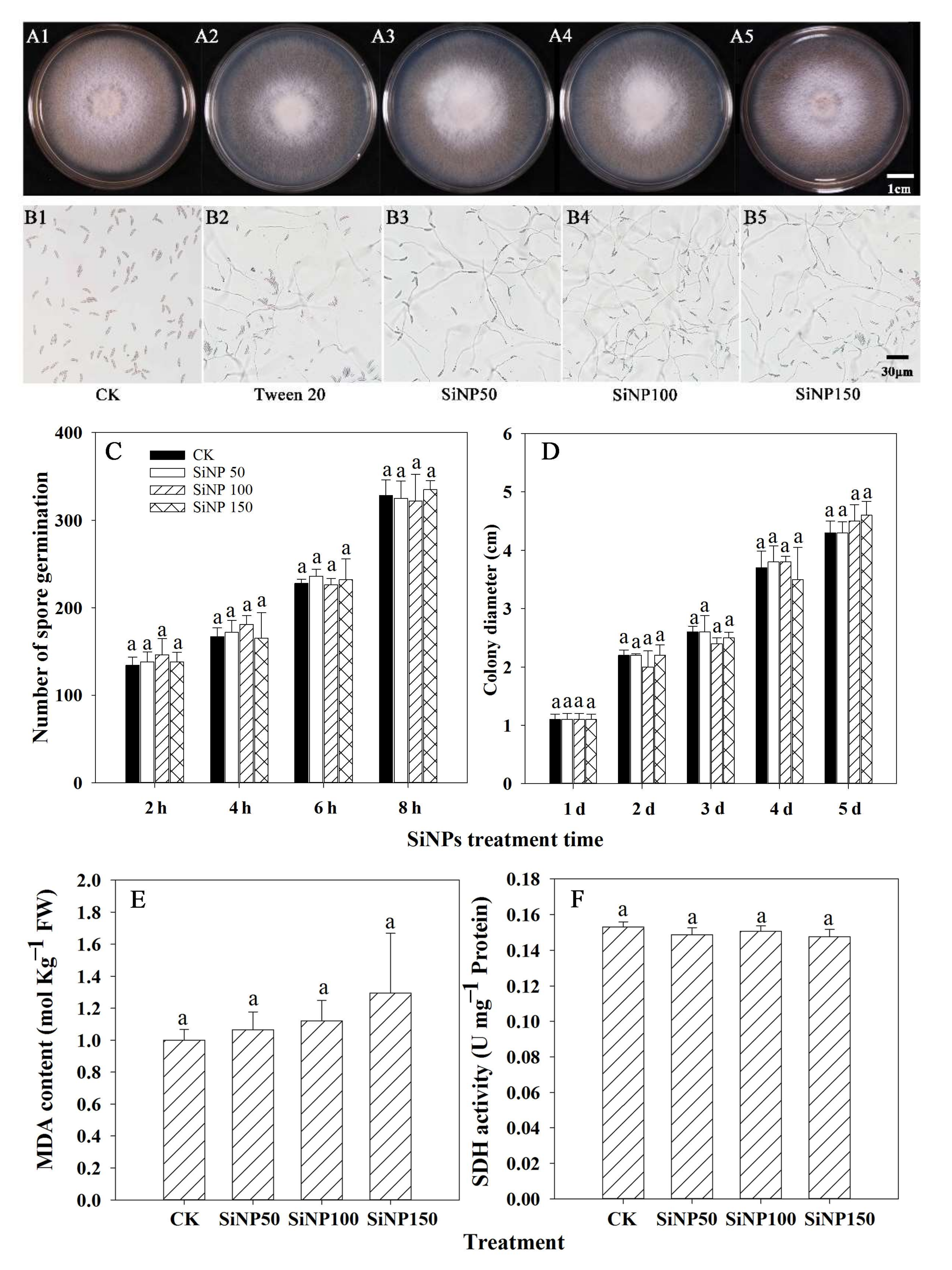
2.3.1. Determination of Mycelial Growth
2.3.2. Determination of Spore Germination
2.3.3. Determination of Malondialdehyde (MDA) Content and Succinate Dehydrogenase (SDH) of F. solani
2.4. SiNPs Dioxide Treatment of Postharvest Ginger Rhizomes
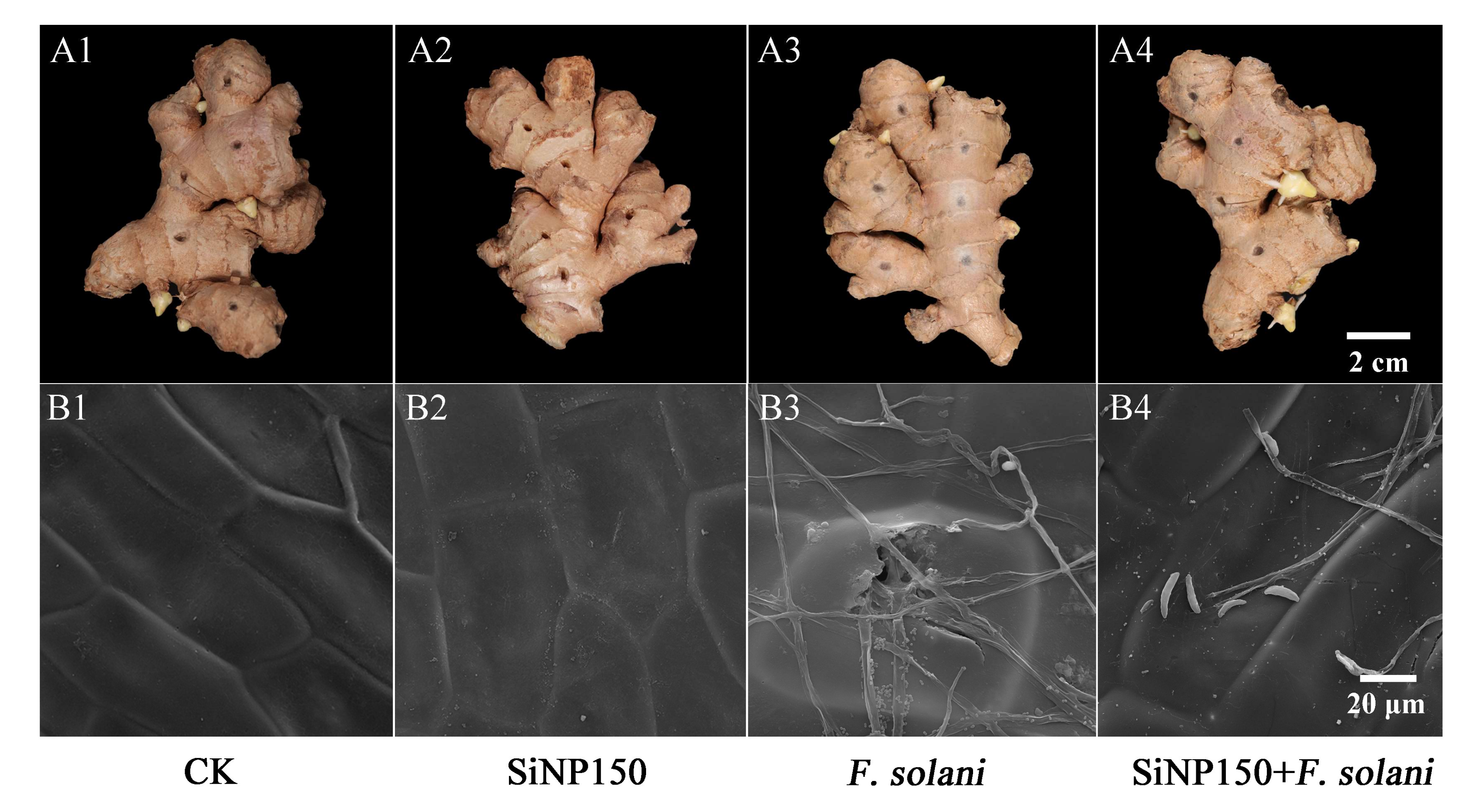
2.5. Scanning Electron Microscope (SEM) Analysis
2.6. Determination of H2O2, O2− and MDA
2.6.1. DAB and NBT Histochemical Staining and Determination of H2O2 and O2− Content
2.6.2. Determination of Malondialdehyde (MDA) Content
2.7. Enzyme Assays
2.8. Determination of Total Phenolics, Total Flavonoid and Lignin Contents
2.9. Quantitative Real-Time PCR (qRT-PCR) Assays
2.10. Statistical Analysis
3. Results
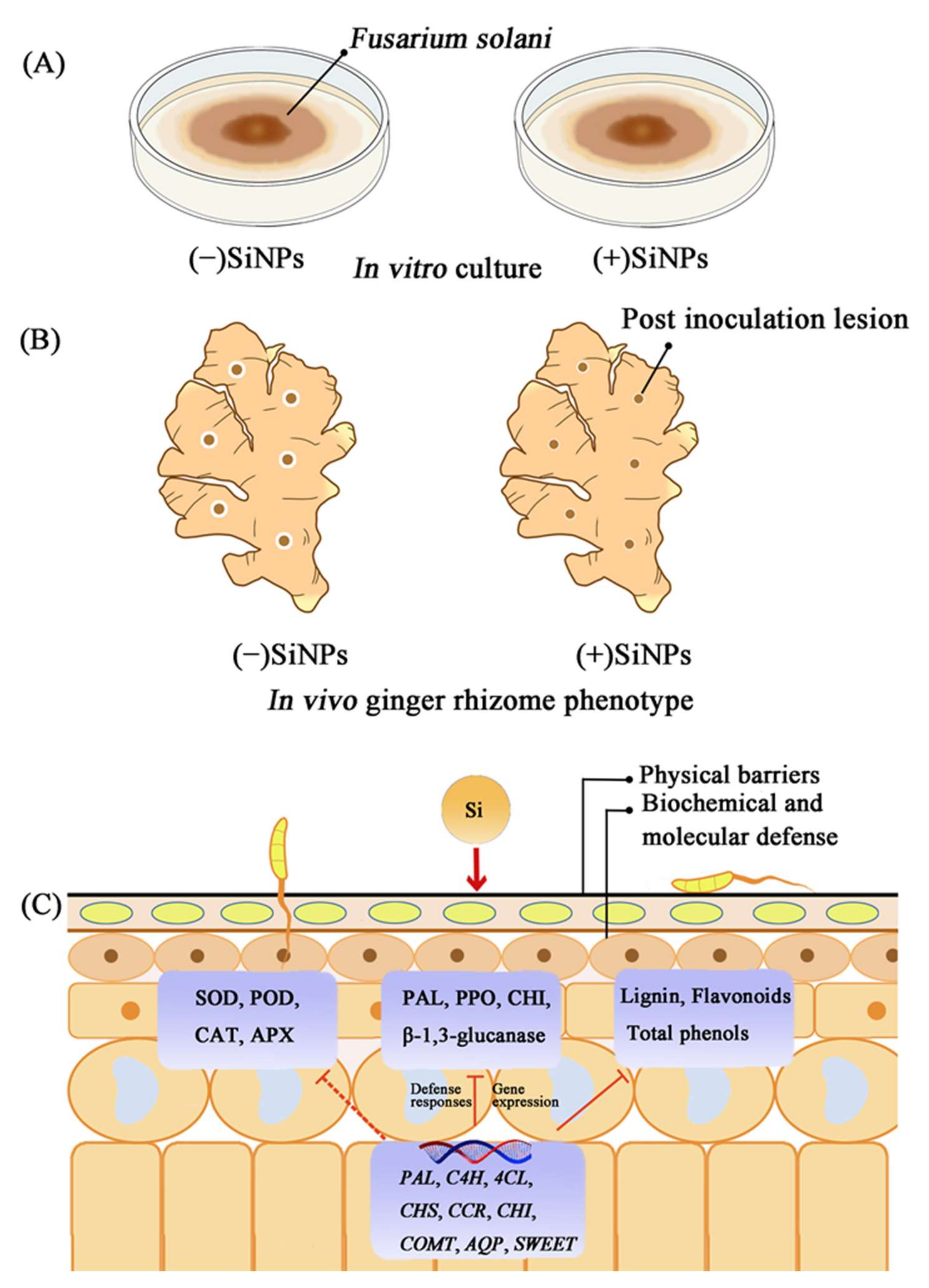
3.1. Effect of SiNPs Treatment on F. solani In Vitro
3.2. Effect of SiNPs Treatment on Rhizome of Ginger Inoculated with F. solani
3.3. Effect of SiNP150 on In Vivo Visualization and Content of Reactive Oxygen Species (ROS)
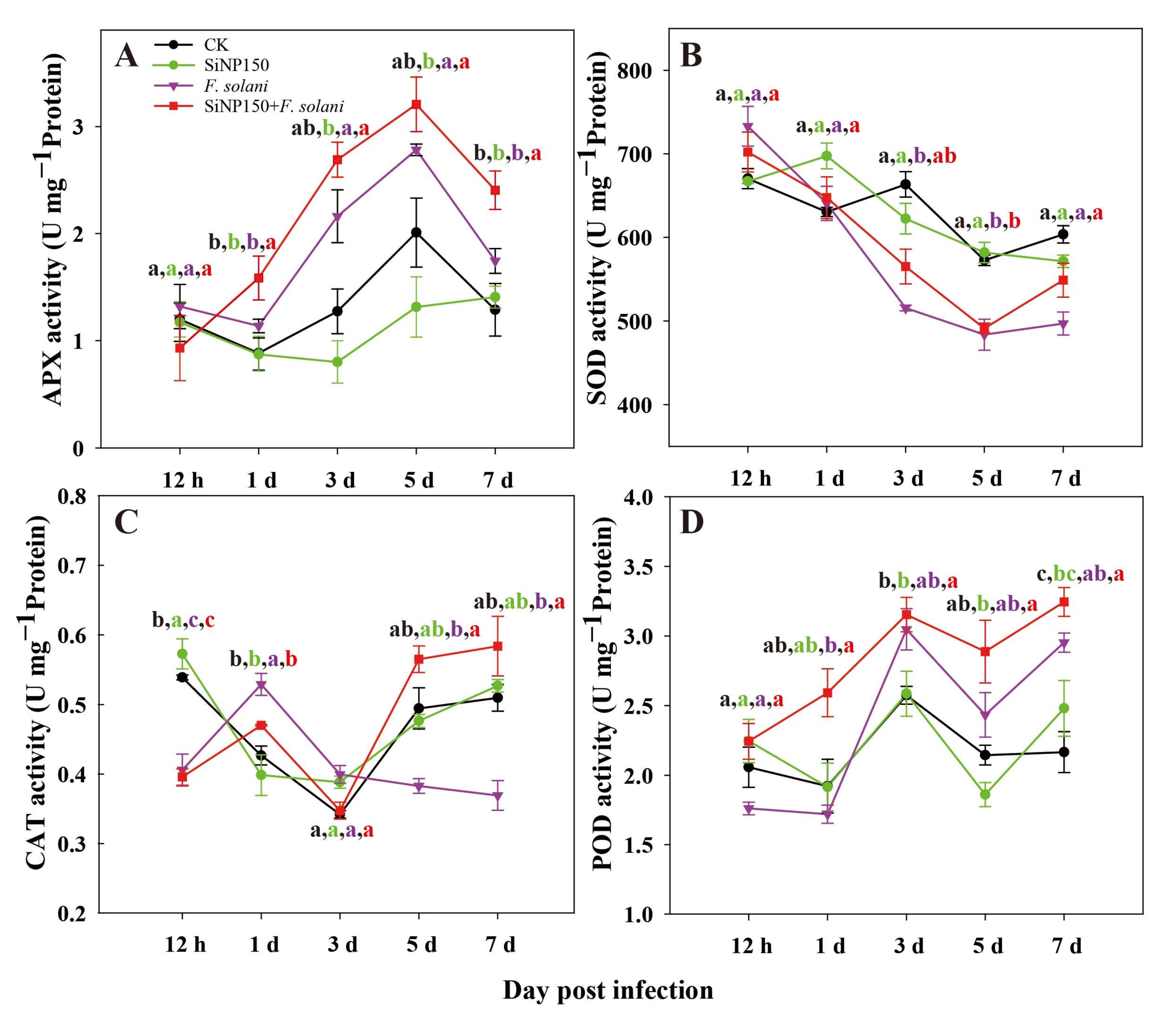
3.4. Effects of the SiNPs Treatment on Antioxidant Enzyme Activities in Ginger Rhizome
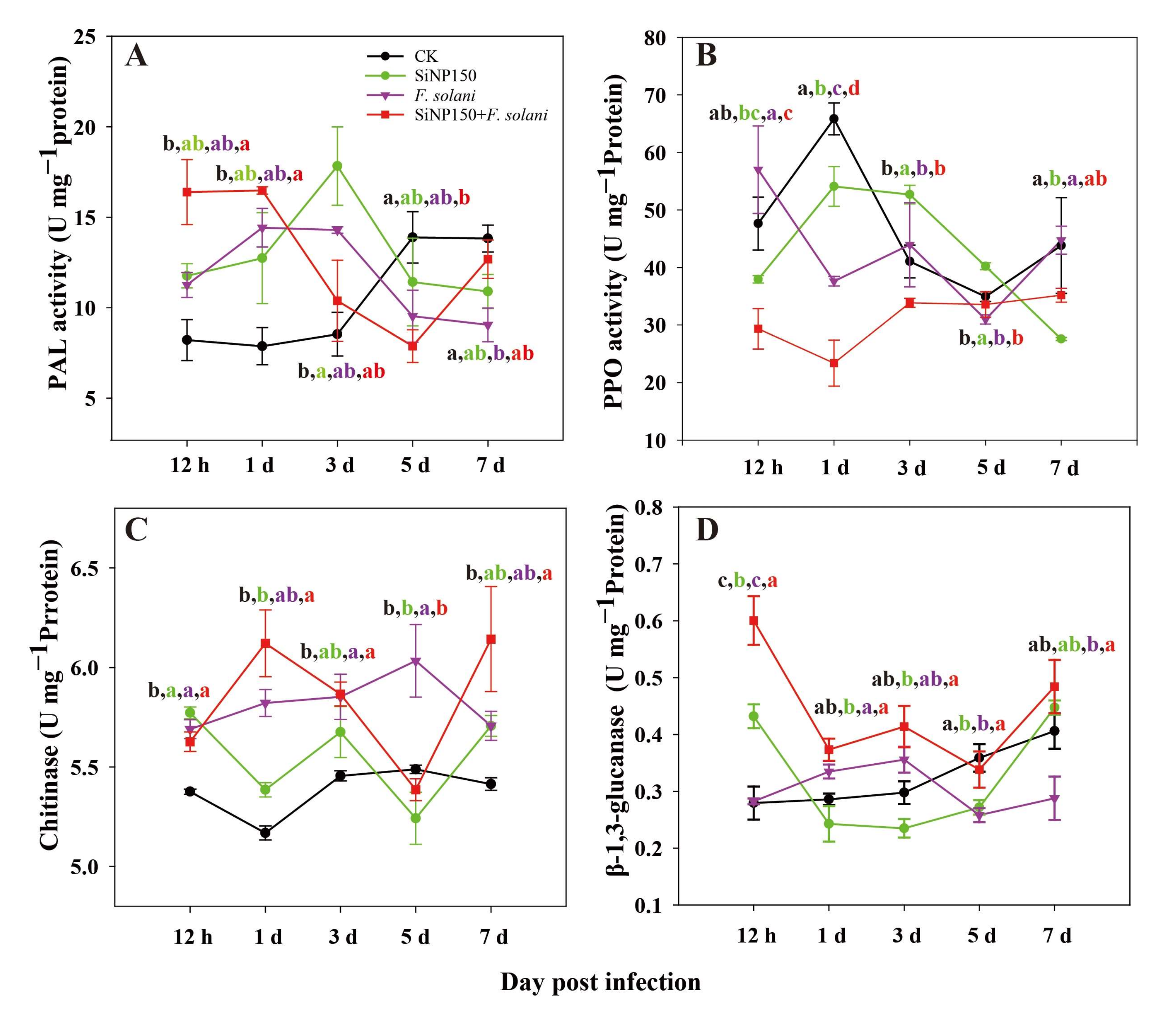
3.5. Effects of the SiNPs Treatment on Disease Resistance-Related Enzyme Activities in Ginger Rhizome
3.6. Effects of the SiNPs Treatment on Lignin, Total Flavonoid and Phenolics Contents
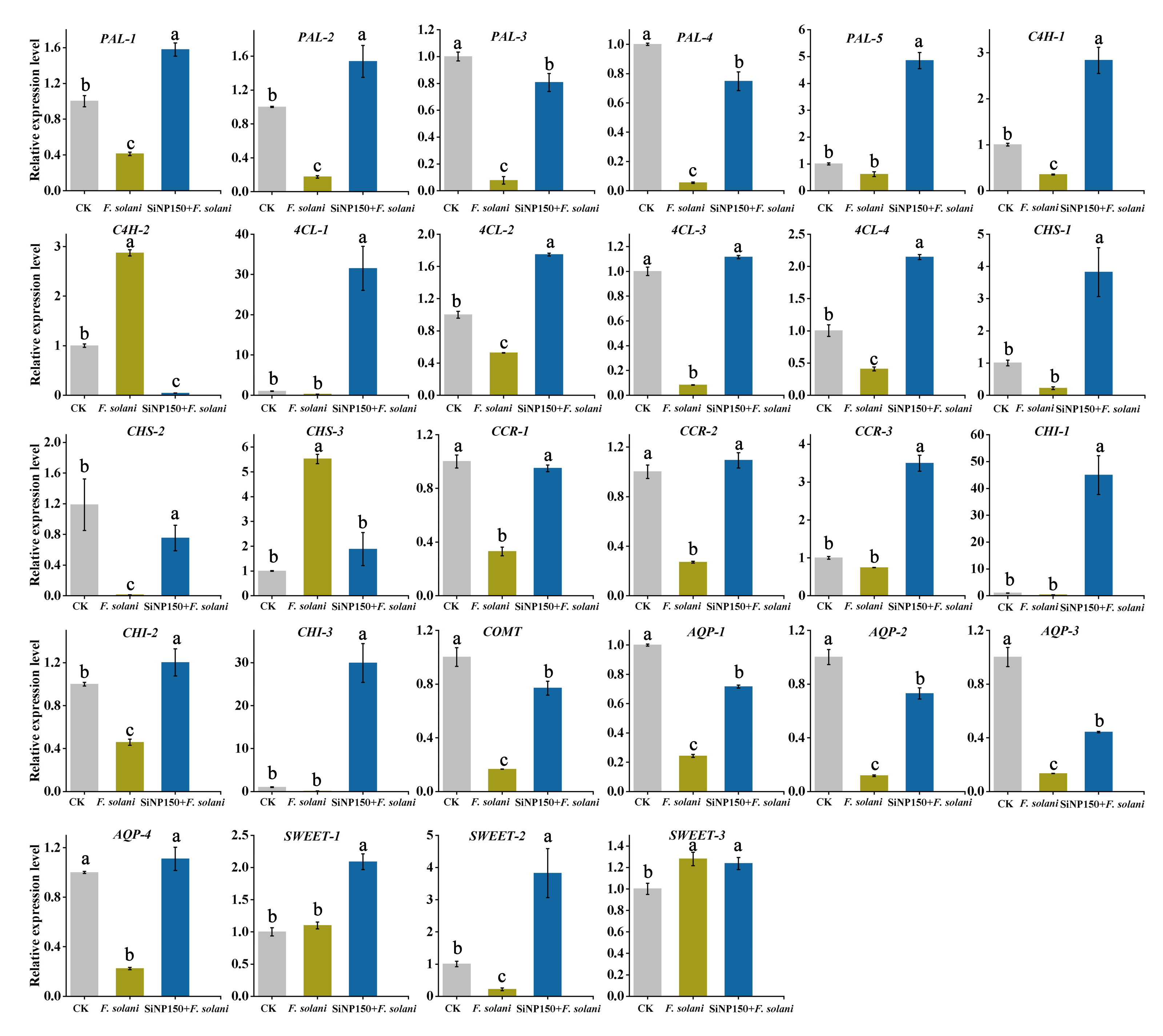
3.7. Effects of the SiNP150 Treatment on the Expression of Key Phenylpropanoid Pathway Genes Related to Lignin and Flavonoid
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rai, M.; Ingle, A.P.; Paralikar, P.; Anasane, N.; Gade, R.; Ingle, P. Effective management of soft rot of ginger caused by Pythium spp. and Fusarium spp.: Emerging role of nanotechnology. Appl. Microbiol. Biotechnol. 2018, 102, 6827–6839. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, L.; Tang, N.; Liu, R.; Jin, Z.; Liu, Y.; Li, Z. Analysis of transcriptome and phytohormone profiles reveal novel insight into ginger (Zingiber officinale Rose) in response to postharvest dehydration stress. Postharvest Biol. Technol. 2020, 161, 111087. [Google Scholar] [CrossRef]
- Kaushal, M.; Gupta, A.; Vaidya, D.; Gupta, M. Postharvest management and value addition of Ginger (Zingiber Officinale Roscoe): A Review. Int. J. Environ. Agric. Biotechnol. 2017, 2, 397–412. [Google Scholar] [CrossRef]
- Liu, Y.; Wisniewski, M.; Kennedy, J.F.; Jiang, Y.; Tang, J.; Liu, J. Chitosan and oligochitosan enhance ginger (Zingiber officinale Roscoe) resistance to rhizome rot caused by Fusarium oxysporum in storage. Carbohydr. Polym. 2016, 151, 474–479. [Google Scholar] [CrossRef]
- Palou, L. Postharvest treatments with GRAS salts to control fresh fruit decay. Horticulturae 2018, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Ruffo Roberto, S.; Youssef, K.; Hashim, A.F.; Ippolito, A. Nanomaterials as alternative control means against postharvest diseases in fruit crops. Nanomaterials 2019, 9, 1752. [Google Scholar] [CrossRef]
- Lyu, L.; Bi, Y.; Li, S.; Xue, H.; Li, Y.; Prusky, D.B. Sodium silicate prime defense responses in harvested muskmelon by regulating mitochondrial energy metabolism and reactive oxygen species production. Food Chem. 2019, 289, 369–376. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Alsaif, M.A.; Al-Warthan, A.A.; Ali, A. Presence of nanosilica (E551) in commercial food products: TNF-mediated oxidative stress and altered cell cycle progression in human lung fibroblast cells. Cell Biol. Toxicol. 2014, 30, 89–100. [Google Scholar] [CrossRef]
- El-Shetehy, M.; Moradi, A.; Maceroni, M.; Reinhardt, D.; Petri-Fink, A.; Rothen-Rutishauser, B.; Mauch, F.; Schwab, F. Silica nanoparticles enhance disease resistance in Arabidopsis plants. Nat. Nanotechnol. 2021, 16, 344–353. [Google Scholar] [CrossRef]
- Yao, H.J.; Tian, S.P. Effects of a biocontrol agent and methyl jasmonate on postharvest diseases of peach fruit and the possible mechanisms involved. J. Appl. Microbiol. 2005, 98, 941–950. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Peng, H.M.; Hu, H.J.; Xi, K.Y.; Zhu, X.M.; Zhou, J.; Yin, J.L.; Guo, F.L.; Liu, Y.Q.; Zhu, Y.X. Silicon Nanoparticles Enhance Ginger Rhizomes Tolerance to Postharvest Deterioration and Resistance to Fusarium solani. Front. Plant Sci. 2022, 13, 816143. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jiang, X.; Zhang, J.; He, Y.; Zhu, X.; Zhou, X.; Gong, H.; Yin, J.; Liu, Y. Silicon confers cucumber resistance to salinity stress through regulation of proline and cytokinins. Plant Physiol. Biochem. 2020, 156, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Yusuf, M.; Singh, P.; Sardar, M.; Sarin, N. Histochemical detection of superoxide and H2O2 accumulation in Brassica juncea Seedlings. Bio-Protocol 2014, 4, e1108. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wang, W.B.; Kim, Y.H.; Lee, H.S.; Kim, K.Y.; Deng, X.P.; Kwak, S.S. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 2009, 47, 570–577. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Bioch. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Fikret, Y.; Manar, T.; Şebnem, E.; Sebnem, K.; Ozlem, U. SOD, CAT, GR and APX enzyme activities in callus tissues of susceptible and tolerant eggplant varieties under salt stress. Res. J. Biotech. 2013, 8, 45–51. [Google Scholar]
- Wei, Y.; Zhou, D.; Peng, J.; Pan, L.; Tu, K. Hot air treatment induces disease resistance through activating the phenylpropanoid metabolism in Cherry tomato fruit. J. Agric. Food Chem. 2017, 65, 8003–8010. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Tian, S.; Qin, G.; Li, B. Reactive oxygen species involved in regulating fruit senescence and fungal pathogenicity. Plant Mol. Biol. 2013, 82, 593–602. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, L.; Zhao, J.; Bi, Y. Use of silicon dioxide and sodium silicate for controlling Trichothecium roseum postharvest rot in Chinese cantaloupe (Cucumis melo L.). Int. J. Food Sci. Tech. 2007, 42, 1012–1018. [Google Scholar] [CrossRef]
- Dorneles, K.R.; Dallagnol, L.J.; Pazdiora, P.C.; Rodrigues, F.A.; Deuner, S. Silicon potentiates biochemical defense responses of wheat against tan spot. Physiol. Mol. Plant Pathol. 2017, 97, 69–78. [Google Scholar] [CrossRef]
- Hawerroth, C.; Araujo, L.; Bermúdez-Cardona, M.B.; Silveira, P.R.; Wordell Filho, J.A.; Rodrigues, F.A. Silicon-mediated maize resistance to macrospora leaf spot. Tropical. Plant Pathol. 2018, 44, 192–196. [Google Scholar] [CrossRef]
- Shetty, N.P.; Mehrabi, R.; Lutken, H.; Haldrup, A.; Kema, G.H.J.; Collinge, D.B.; Jorgensen, H.J.L. Role of hydrogen peroxide during the interaction between the hemibiotrophic fungal pathogen Septoria tritici and wheat. New Phytol. 2007, 174, 637–647. [Google Scholar] [CrossRef]
- Khan, M.R.; Siddiqui, Z.A. Use of silicon dioxide nanoparticles for the management of Meloidogyne incognita, Pectobacterium betavasculorum and Rhizoctonia solani disease complex of beetroot (Beta vulgaris L.). Sci. Hortic. 2020, 265, 109211. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R. Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol. Environ. Saf. 2018, 147, 881–896. [Google Scholar] [CrossRef]
- Park, Y.H.; Chandra Mishra, R.; Yoon, S.; Kim, H.; Park, C.; Seo, S.T.; Bae, H. Endophytic Trichoderma citrinoviride isolated from mountain-cultivated ginseng (Panax ginseng) has great potential as a biocontrol agent against ginseng pathogens. J. Ginseng Res. 2019, 43, 408–420. [Google Scholar] [CrossRef]
- Souri, Z.; Khanna, K.; Karimi, N.; Ahmad, P. Silicon and plants: Current knowledge and future prospects. J. Plant Growth Regul. 2020, 40, 906–925. [Google Scholar] [CrossRef]
- Fortunato, A.A.; da Silva, W.L.; Rodrigues, F.A. Phenylpropanoid pathway is potentiated by silicon in the roots of banana plants during the infection process of Fusarium oxysporum f. sp. cubense. Phytopathology 2014, 104, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerriero, G.; Hausman, J.F.; Legay, S. Silicon and the plant extracellular matrix. Front Plant Sci. 2016, 7, 463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, D.; Li, F.; Cui, X.; Zhou, J.; Li, J.; Ai, W.; Shu, P.; Zhang, X.; Li, X.; Meng, D.; et al. SlMYC2 are required for methyl jasmonate-induced tomato fruit resistance to Botrytis cinerea. Food Chem. 2020, 310, 125901. [Google Scholar] [CrossRef]
- Minaeva, O.M.; Akimova, E.E.; Tereshchenko, N.N.; Zyubanova, T.I.; Apenysheva, M.V.; Kravets, A.V. Effect of oseudomonas bacteria on peroxidase activity in wheat plants when infected with bipolaris sorokiniana. Russ. J. Plant Physiol. 2018, 65, 717–725. [Google Scholar] [CrossRef]
- Asgari, F.; Majd, A.; Jonoubi, P.; Najafi, F. Effects of silicon nanoparticles on molecular, chemical, structural and ultrastructural characteristics of oat (Avena sativa L.). Plant Physiol. Bioch. 2018, 127, 152–160. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Yang, Y. Mechanisms of silicon-induced fungal disease resistance in plants. Plant Physiol. Bioch. 2021, 165, 200–206. [Google Scholar] [CrossRef]
- Ratnayake, R.M.R.N.K.; Daundasekera, W.A.M.; Ariyarathne, H.M.; Ganehenege, M.Y.U. Some biochemical defense responses enhanced by soluble silicon in bitter gourd-powdery mildew pathosystem. Australas Plant Path. 2016, 45, 425–433. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.J. Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol. Plant 2015, 8, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.X.; Yang, L.; Liu, N.; Yang, J.; Zhou, X.K.; Xia, Y.C.; He, Y.; He, Y.Q.; Gong, H.J.; Ma, D.F.; et al. Genome-wide identification, structure characterization, and expression pattern profiling of aquaporin gene family in cucumber. BMC Plant Biol. 2019, 19, 345. [Google Scholar] [CrossRef] [Green Version]
- Jeena, G.S.; Kumar, S.; Shukla, R.K. Structure, evolution and diverse physiological roles of SWEET sugar transporters in plants. Plant Mol. Biol. 2019, 100, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, T.; Zhang, Z.; Li, B.; Tian, S. Roles of aquaporins in plant-pathogen interaction. Plants 2020, 9, 1134. [Google Scholar] [CrossRef] [PubMed]
- Sade, D.; Brotman, Y.; Eybishtz, A.; Cuadros-Inostroza, A.; Fernie, A.R.; Willmitzer, L.; Czosnek, H. Involvement of the hexose transporter gene LeHT1 and of sugars in resistance of tomato to tomato yellow leaf curl virus. Mol. Plant. 2013, 6, 1707–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Liu, X.; Sun, C.; Li, G.; Yang, P.; Jia, Q.; Cai, X.; Zhu, Y.; Yin, J.; Liu, Y. Silica Nanoparticles Enhance the Disease Resistance of Ginger to Rhizome Rot during Postharvest Storage. Nanomaterials 2022, 12, 1418. https://doi.org/10.3390/nano12091418
Zhou J, Liu X, Sun C, Li G, Yang P, Jia Q, Cai X, Zhu Y, Yin J, Liu Y. Silica Nanoparticles Enhance the Disease Resistance of Ginger to Rhizome Rot during Postharvest Storage. Nanomaterials. 2022; 12(9):1418. https://doi.org/10.3390/nano12091418
Chicago/Turabian StyleZhou, Jie, Xuli Liu, Chong Sun, Gang Li, Peihua Yang, Qie Jia, Xiaodong Cai, Yongxing Zhu, Junliang Yin, and Yiqing Liu. 2022. "Silica Nanoparticles Enhance the Disease Resistance of Ginger to Rhizome Rot during Postharvest Storage" Nanomaterials 12, no. 9: 1418. https://doi.org/10.3390/nano12091418
APA StyleZhou, J., Liu, X., Sun, C., Li, G., Yang, P., Jia, Q., Cai, X., Zhu, Y., Yin, J., & Liu, Y. (2022). Silica Nanoparticles Enhance the Disease Resistance of Ginger to Rhizome Rot during Postharvest Storage. Nanomaterials, 12(9), 1418. https://doi.org/10.3390/nano12091418





