Photoelectrochemical Response Enhancement for Metallofullerene-[12]Cycloparaphenylene Supramolecular Complexes
Abstract
:1. Introduction
2. Materials and Methods
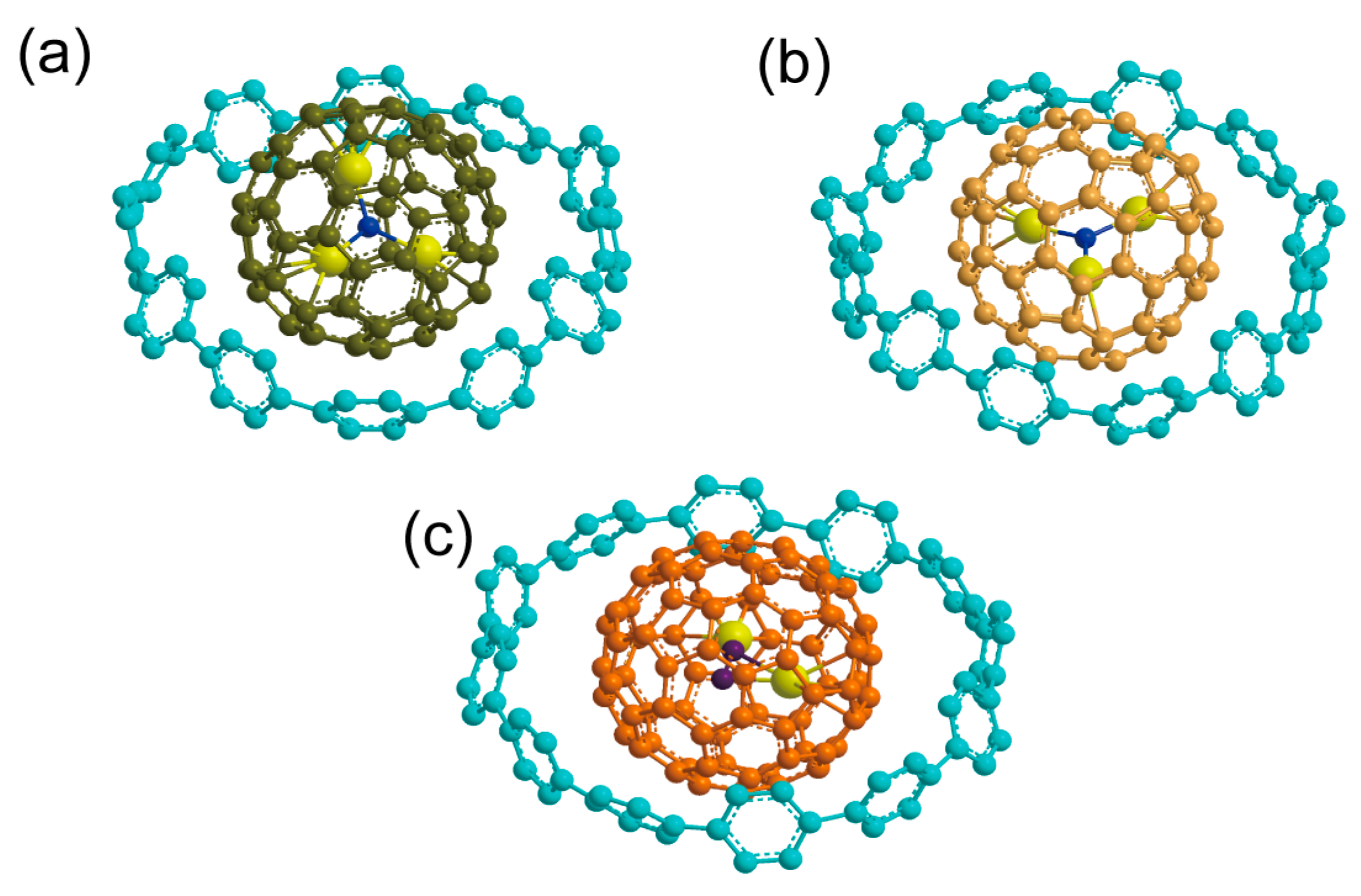
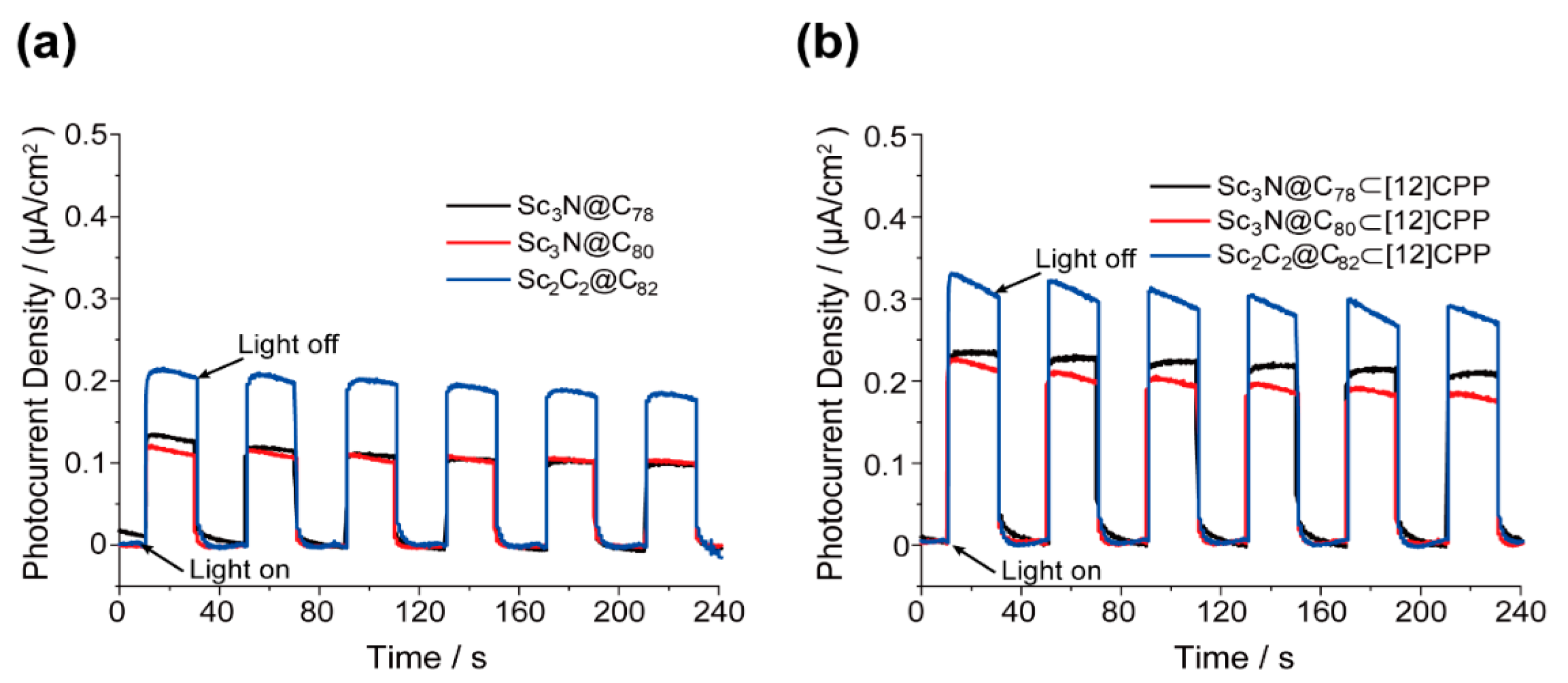
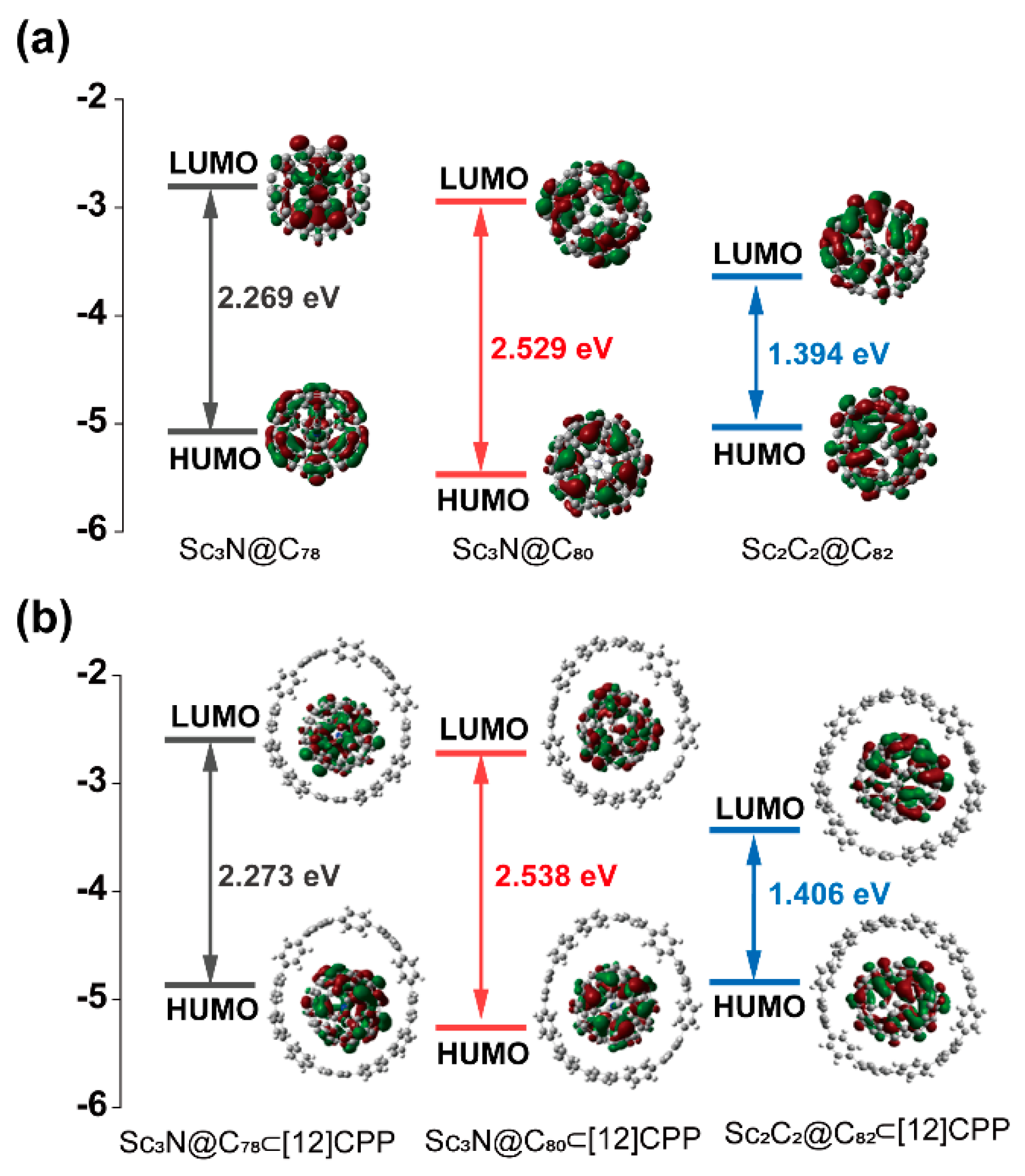
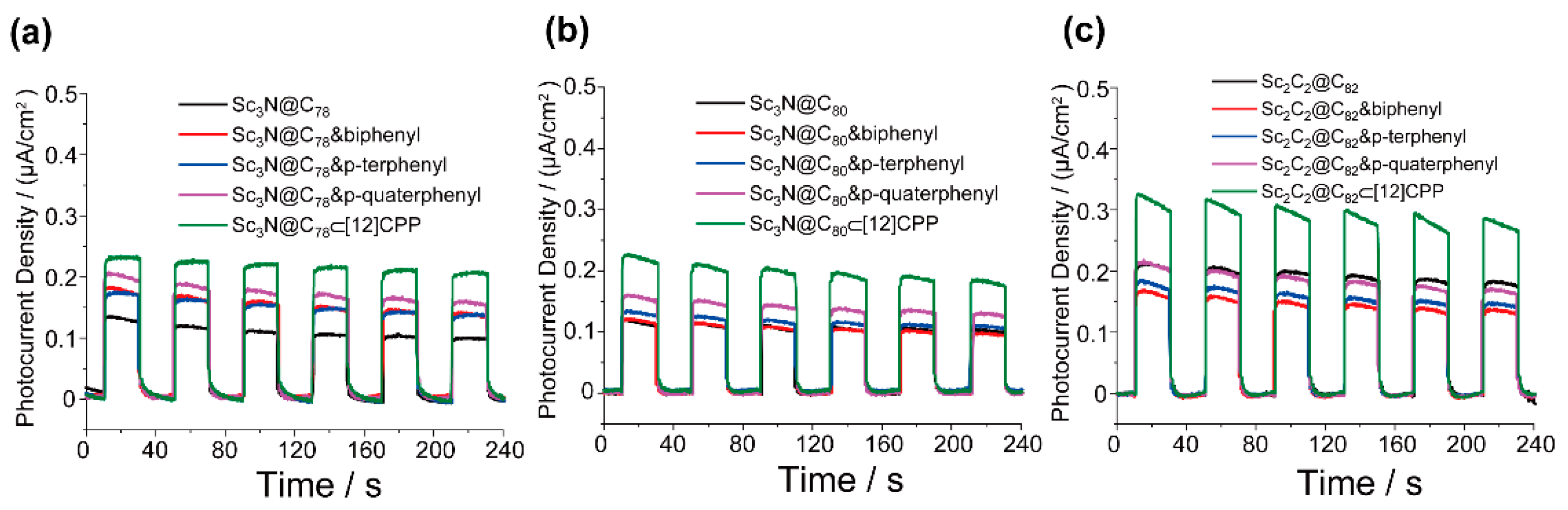
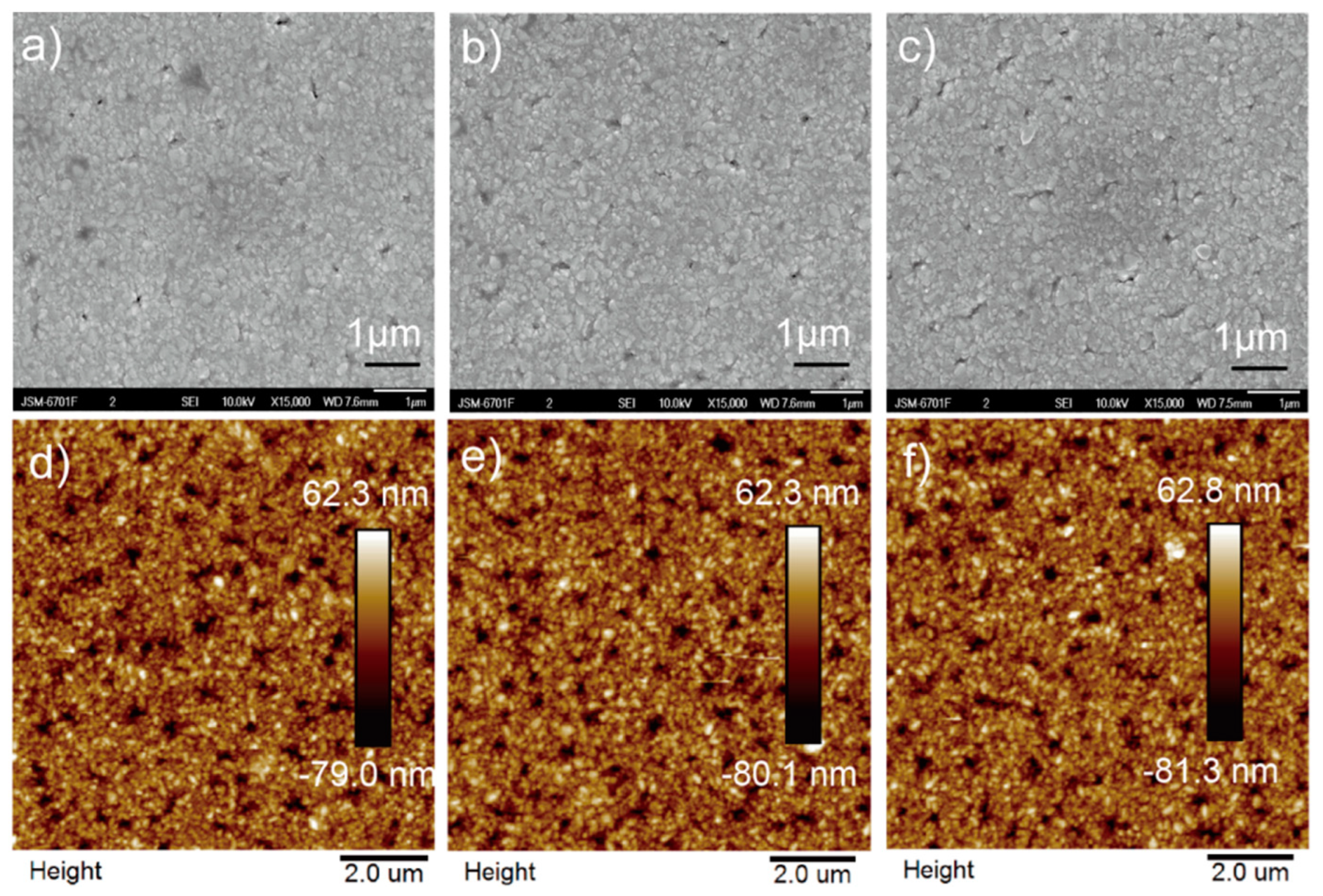
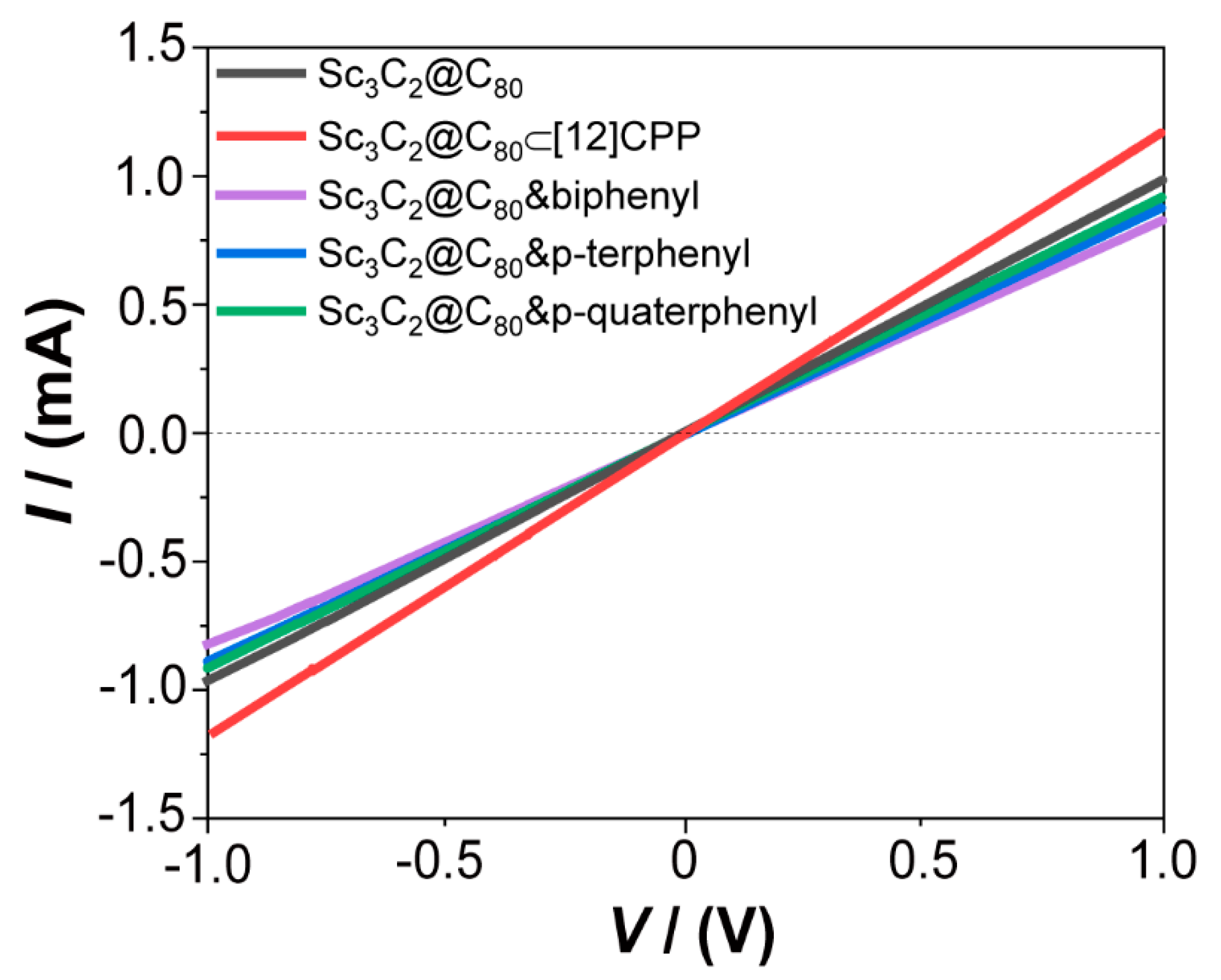
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shinohara, H. Endohedral metallofullerenes. Rep. Prog. Phys. 2000, 6, 843. [Google Scholar] [CrossRef]
- Lu, X.; Feng, L.; Akasaka, T.; Nagase, S. Current status and future developments of endohedral metallofullerenes. Chem. Soc. Rev. 2012, 23, 7723–7760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Yang, S. Photoelectrochemistry of Langmuir−Blodgett Films of the Endohedral Metallofullerene Dy@C82 on ITO Electrodes. J. Phys. Chem. B 2001, 39, 9406–9412. [Google Scholar] [CrossRef]
- Yang, S.; Fan, L.; Yang, S. Significantly enhanced photocurrent efficiency of a poly (3-hexylthiophene) photoelectrochemical device by doping with the endohedral metallofullerene Dy@C82. Chem. Phys. Lett. 2004, 4–6, 253–258. [Google Scholar] [CrossRef]
- Bikram, C.K.C.; Subbaiyan, N.K.; D’Souza, F. Supramolecular Donor–Acceptor Assembly Derived from Tetracarbazole–Zinc Phthalocyanine Coordinated to Fullerene: Design, Synthesis, Photochemical, and Photoelectrochemical Studies. J. Phys. Chem. C 2012, 22, 11964–11972. [Google Scholar] [CrossRef]
- Sandanayaka, A.S.; Murakami, T.; Hasobe, T. Preparation and photophysical and photoelectrochemical properties of supramolecular porphyrin nanorods structurally controlled by encapsulated fullerene derivatives. J. Phys. Chem. C 2009, 42, 18369–18378. [Google Scholar] [CrossRef]
- Chen, M.; Guan, R.; Yang, S. Hybrids of fullerenes and 2d nanomaterials. Adv. Sci. 2019, 1, 1800941. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.J.; Girotto, E.M.; Nogueira, A.F. Photoelectrochemical properties of poly (terthiophene) films modified with a fullerene derivative. Thin Solid Film. 2006, 4, 2644–2649. [Google Scholar] [CrossRef]
- Sato, S.; Seki, S.; Luo, G.; Suzuki, M.; Lu, J.; Nagase, S.; Akasaka, T. Tunable charge-transport properties of Ih-C80 endohedral metallofullerenes: Investigation of La2@C80, Sc3N@C80, and Sc3C2@C80. J. Am. Chem. Soc. 2012, 28, 11681–11686. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.-I.; Mori, S.; Iida, S.; Ando, H.; Takenobu, T.; Taguchi, Y.; Fujiwara, A.; Taninaka, A.; Shinohara, H.; Iwasa, Y. Conductivity and field effect transistor of La2@C80 metallofullerene. J. Am. Chem. Soc. 2013, 27, 8116–8117. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Zhang, L.; Zheng, S.; Xie, Y.; Lu, X. Lu2@C82 Nanorods with Enhanced Photoluminescence and Photoelectrochemical Properties. ACS Appl. Mater. Interfaces 2017, 34, 28838–28843. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.-I.; Akiyama, T.; Yamada, S. Step-by-Step Fabrication of Porphyrin−Fullerene Supramolecular Assemblies and Their Photoelectrochemical Properties. J. Phys. Chem. C 2008, 17, 7015–7020. [Google Scholar] [CrossRef]
- Ogoshi, T.; Ueshima, N.; Sakakibara, F.; Yamagishi, T.A.; Haino, T. Conversion from pillar[5]arene to pillar[6-15]arenes by ring expansion and encapsulation of C60 by pillar[n]arenes with nanosize cavities. Org. Lett. 2014, 11, 2896–2899. [Google Scholar] [CrossRef]
- Iwamoto, T.; Slanina, Z.; Mizorogi, N.; Guo, J.; Akasaka, T.; Nagase, S.; Takaya, H.; Yasuda, N.; Kato, T.; Yamago, S. Partial charge transfer in the shortest possible metallofullerene peapod, La@C82⊂[11] cycloparaphenylene. Chem. Eur. J. 2014, 44, 14403–14409. [Google Scholar] [CrossRef]
- Zhao, C.; Meng, H.; Nie, M.; Wang, X.; Cai, Z.; Chen, T.; Wang, D.; Wang, C.; Wang, T. Supramolecular Complexes of C80-Based Metallofullerenes with [12]Cycloparaphenylene Nanoring and Altered Property in a Confined Space. J. Phys. Chem. C 2019, 19, 12514–12520. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, B.; Kaur, R.; Minameyer, M.B.; Bothe, M.; Drewello, T.; Guldi, D.M.; von Delius, M. A Supramolecular [10]CPP Junction Enables Efficient Electron Transfer in Modular Porphyrin-[10]CPP supersetFullerene Complexes. Angew. Chem. Int. Ed. 2018, 36, 11549–11553. [Google Scholar] [CrossRef] [PubMed]
- Kayahara, E.; Sun, L.; Onishi, H.; Suzuki, K.; Fukushima, T.; Sawada, A.; Kaji, H.; Yamago, S. Gram-Scale Syntheses and Conductivities of [10]Cycloparaphenylene and Its Tetraalkoxy Derivatives. J. Am. Chem. Soc. 2017, 51, 18480–18483. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhuang, G.; Jia, H.; Qian, M.; Cui, S.; Yang, S.; Du, P. Photoconductive Curved-Nanographene/Fullerene Supramolecular Heterojunctions. Angew. Chem. Int. Ed. 2019, 19, 6244–6249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, F.; Feng, L.; Nie, M.; Lu, Y.; Zhang, J.; Wang, C.; Wang, T. Construction of a double-walled carbon nanoring. Nanoscale 2021, 9, 4880–4886. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, X.; Hu, W.; Zhang, M.; Ding, L.; Wang, M.; Qiao, Q.; Yang, S. Beyond Metal Oxides: Introducing Low-Temperature Solution-Processed Ultrathin Layered Double Hydroxide Nanosheets into Polymer Solar Cells Toward Improved Electron Transport. Sol. RRL 2019, 2, 1970025. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, L.; Xia, J.; Lu, X. Controllable Synthesis of Sc3N@C78 Microspindles with Excellent Electrophotonic Properties. ACS Appl. Energy Mater. 2019, 2, 1489–1493. [Google Scholar] [CrossRef]
- Zhao, C.; Meng, H.; Nie, M.; Huang, Q.; Du, P.; Wang, C.; Wang, T. Construction of a short metallofullerene-peapod with a spin probe. Chem. Commun. 2019, 77, 11511–11514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, C.; Meng, H.; Nie, M.; Li, Q.; Xiang, J.; Zhang, Z.; Wang, C.; Wang, T. Size-selective encapsulation of metallofullerenes by [12]Cycloparaphenylene and dissociation using metal-organic framework. Carbon 2020, 161, 694–701. [Google Scholar] [CrossRef]
- Fujii, A.; Umeda, T.; Shirakawa, T.; Akasaka, T.; Yoshino, K. Photocurrent Enhancement in Conducting Polymer Device by Doping with Endohedral Metallofullerene La@C82. Jpn J. Appl. Phys. 2002, 41, 2254–2255. [Google Scholar] [CrossRef]
- Manzetti, S.; Lu, T. Alternant conjugated oligomers with tunable and narrow HOMO–LUMO gaps as sustainable nanowires. RSC Adv. 2013, 48, 25881–25890. [Google Scholar] [CrossRef]
- Tan, S.; Shi, H.; Fu, L.; Ma, J.; Du, X.; Song, J.; Liu, Y.; Zeng, Q.; Xu, H.; Wan, J. Superlubricity of Fullerene Derivatives Induced by Host–Guest Assembly. ACS Appl. Mater. 2020, 16, 18924–18933. [Google Scholar] [CrossRef]
- Barendt, T.A.; Ball, M.L.; Xu, Q.; Zhang, B.; Fowler, B.; Schattman, A.; Ritter, V.C.; Steigerwald, M.L.; Nuckolls, C. Supramolecular Assemblies for Electronic Materials. Chem. Eur. J. 2020, 26, 3744–3748. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Wang, F.; Tang, L.; Xiang, J.; Teng, K.S.; Lau, S.P. High-Performance Deep Ultraviolet Photodetector Based on NiO/β-Ga2O3 Heterojunction. Nanoscale Res. Lett. 2020, 1, 47. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Chen, P.; Yuan, S.; Huang, T.; Zhang, S.; Wuu, D. Improved Performance of Deep Ultraviolet Photodetector From Sputtered Ga2O3 Films Using Post-Thermal Treatments. IEEE Photon. J. 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Qian, L.X.; Wang, Y.; Wu, Z.H.; Sheng, T.; Liu, X.Z. β-Ga2O3 solar-blind deep-ultraviolet photodetector based on annealed sapphire substrate. Vacuum 2017, 140, 106–110. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Qiu, L.; Liu, L.; Liu, Y.; Cui, P.; Wang, F.; Zhang, Z. Photoelectrochemical Response Enhancement for Metallofullerene-[12]Cycloparaphenylene Supramolecular Complexes. Nanomaterials 2022, 12, 1408. https://doi.org/10.3390/nano12091408
Zhang J, Qiu L, Liu L, Liu Y, Cui P, Wang F, Zhang Z. Photoelectrochemical Response Enhancement for Metallofullerene-[12]Cycloparaphenylene Supramolecular Complexes. Nanomaterials. 2022; 12(9):1408. https://doi.org/10.3390/nano12091408
Chicago/Turabian StyleZhang, Jie, Ling Qiu, Linshan Liu, Yang Liu, Peng Cui, Fang Wang, and Zhuxia Zhang. 2022. "Photoelectrochemical Response Enhancement for Metallofullerene-[12]Cycloparaphenylene Supramolecular Complexes" Nanomaterials 12, no. 9: 1408. https://doi.org/10.3390/nano12091408
APA StyleZhang, J., Qiu, L., Liu, L., Liu, Y., Cui, P., Wang, F., & Zhang, Z. (2022). Photoelectrochemical Response Enhancement for Metallofullerene-[12]Cycloparaphenylene Supramolecular Complexes. Nanomaterials, 12(9), 1408. https://doi.org/10.3390/nano12091408