Recent Advances in Colloidal Quantum Dots or Perovskite Quantum Dots as a Luminescent Downshifting Layer Embedded on Solar Cells
Abstract
1. Introduction
2. Physical and Optical Characteristics of QDs
3. Synthesis Method of QDs
4. Strategies of Stability Improvement for PQDs
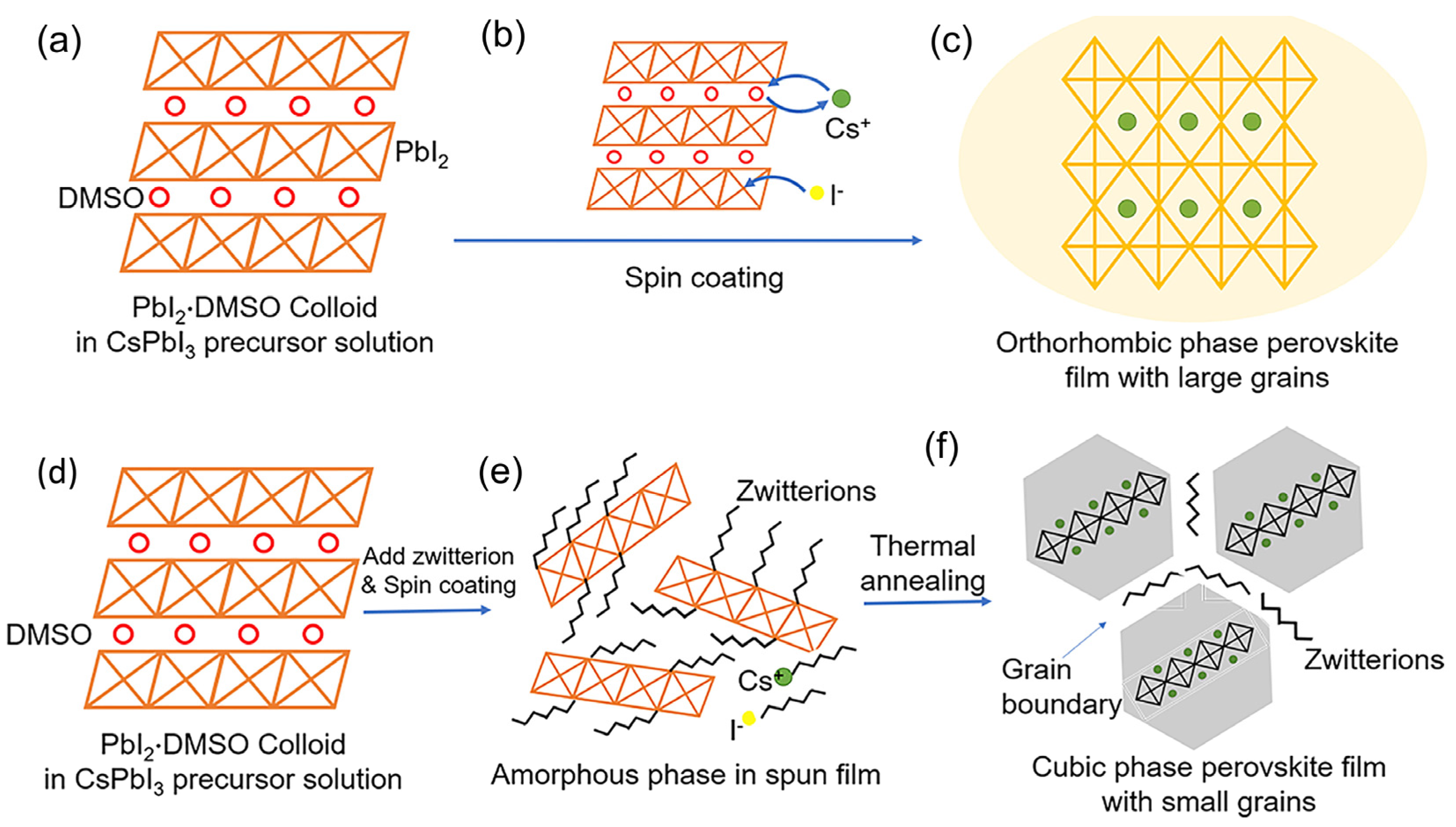
4.1. 2D Nanocrystal Engineering
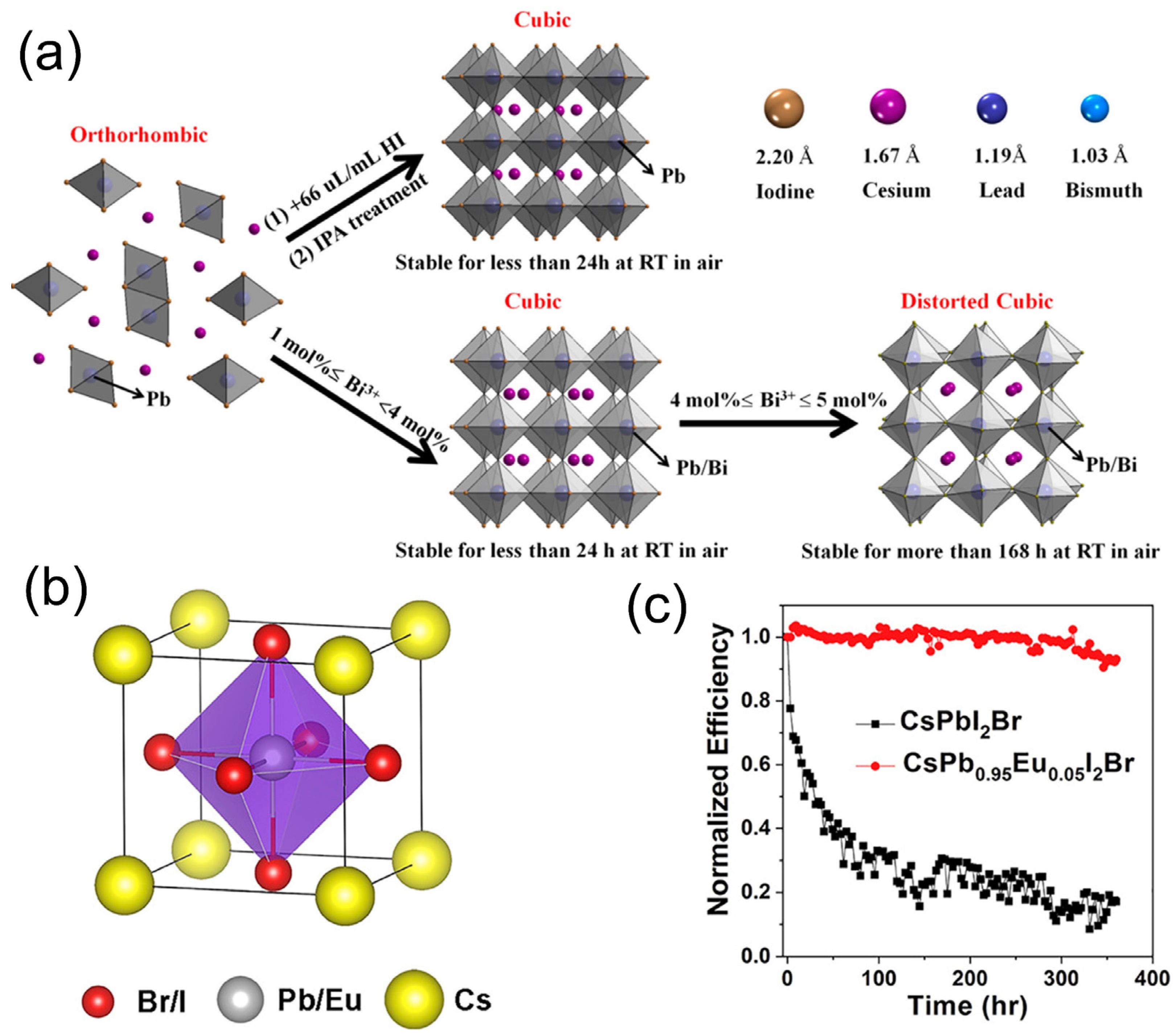
4.2. Doping with Alloying Compound
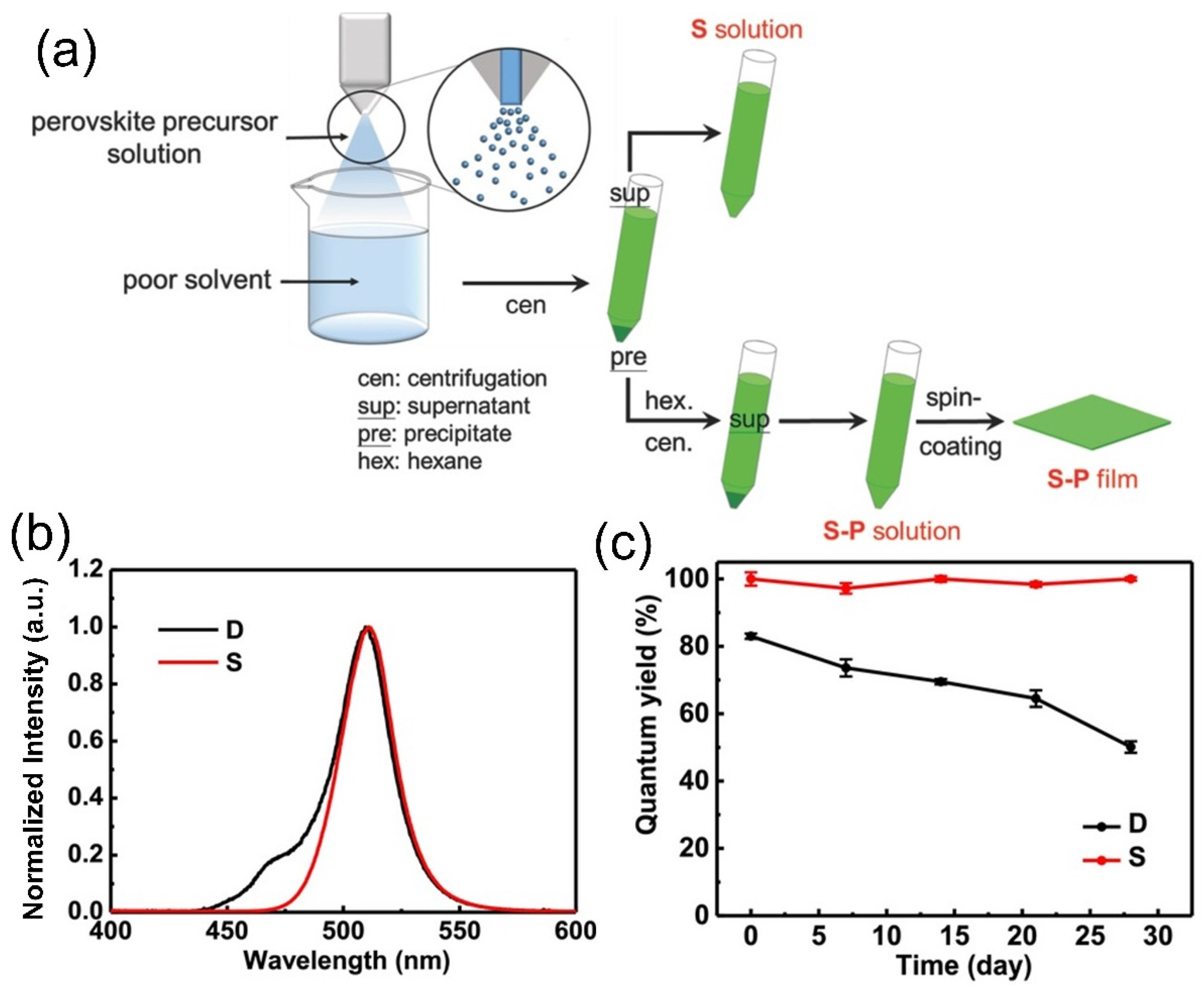
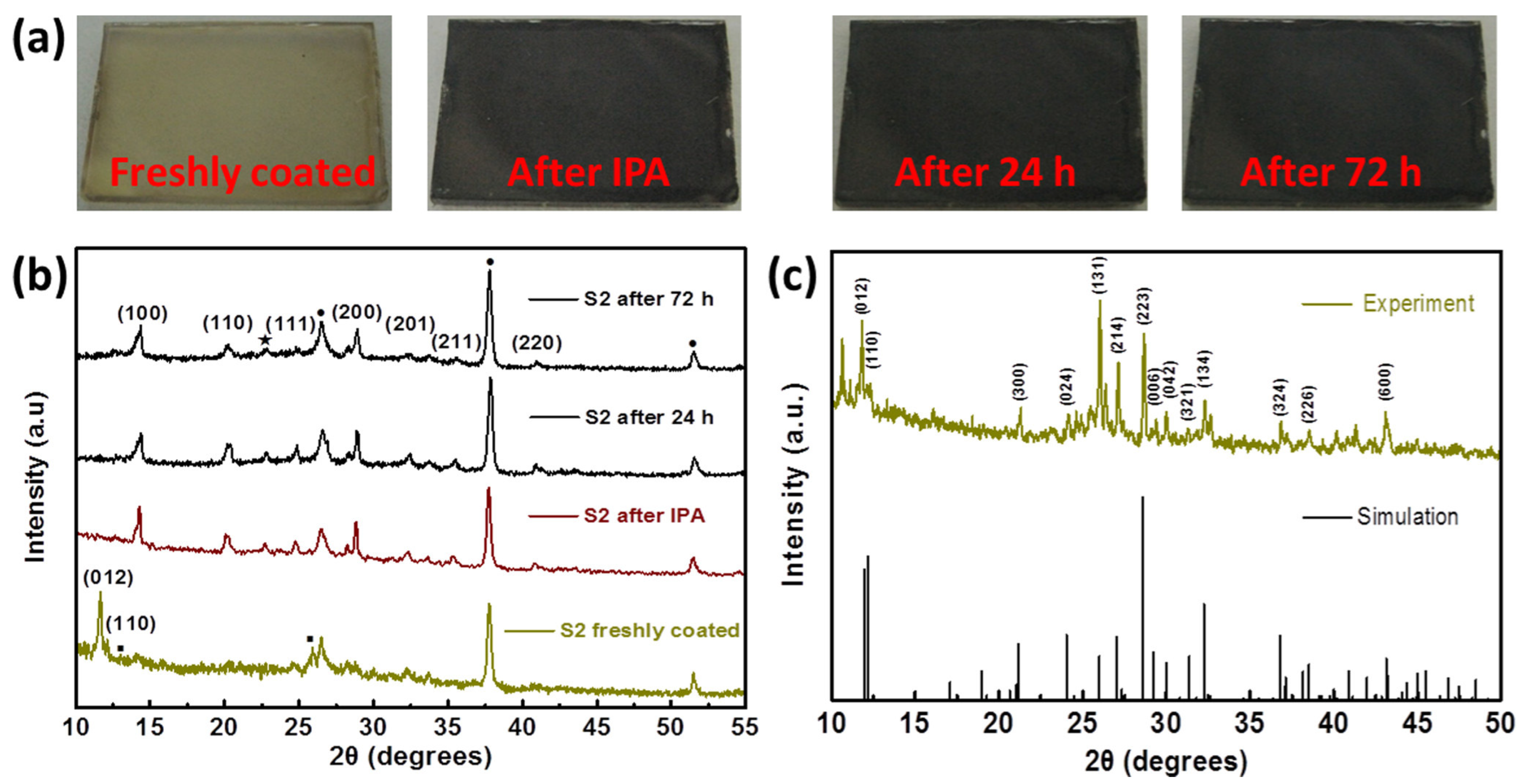
4.3. Solvent-Additive Technique
5. QDs with Various Solar Cells
5.1. Si-Based Solar Cells
5.2. GaAs-Based Solar Cells
5.3. CdTe-Based Solar Cells
5.4. CIGS-Based Solar Cells
5.5. Organic and Perovskite-Based Solar Cells
6. Challenges of QD-Based LDS Layers in Solar Cells
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- BP Statistical Review of World Energy June 2017. Available online: https://www.connaissancedesenergies.org/sites/default/files/pdf-actualites/bp-statistical-review-of-world-energy-2017-full-report.pdf (accessed on 30 June 2017).
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 153, 1513–1524. [Google Scholar] [CrossRef]
- Kannan, N.; Vakeesan, D. Solar energy for future world—A review. Renew. Sustain. Energy Rev. 2016, 62, 1092–1105. [Google Scholar] [CrossRef]
- Tanabe, K. A review of ultrahigh efficiency III-V semiconductor compound solar cells: Multijunction tandem, lower dimensional, photonic up/down conversion and plasmonic nanometallic structures. Energies 2009, 2, 504–530. [Google Scholar] [CrossRef]
- Baur, C.; Bett, A.W.; Dimroth, F.; Siefer, G.; Meusel, M.; Bensch, W.; Köstler, W.; Strobl, G. Triple-junction III–V based concentrator solar cells: Perspectives and challenges. ASME J. Sol. Energy Eng. 2007, 129, 258–265. [Google Scholar] [CrossRef]
- Takamoto, T.; Ikeda, E.; Kurita, H.; Ohmori, M. Over 30% efficient InGaP/GaAs tandem solar cells. Appl. Phys. Lett. 1997, 70, 381–383. [Google Scholar] [CrossRef]
- Kongkanand, A.; Tvrdy, K.; Takechi, K.; Kuno, M.; Kamat, P.V. Quantum dot solar cells. Tuning photoresponse through size and shape control of CdSe−TiO2 architecture. J. Am. Chem. Soc. 2008, 130, 4007–4015. [Google Scholar] [CrossRef]
- Standridge, S.D.; Schatz, G.C.; Hupp, J.T. Distance dependence of plasmon-enhanced photocurrent in dye-sensitized solar cells. J. Am. Chem. Soc. 2009, 131, 8407–8409. [Google Scholar] [CrossRef]
- Zhang, Q.; Chou, T.P.; Russo, B.; Jenekhe, S.A.; Cao, G. Polydisperse aggregates of ZnO nanocrystallites: A method for energy-conversion-efficiency enhancement in dye-sensitized solar cells. Adv. Funct. Mater. 2008, 18, 1654–1660. [Google Scholar] [CrossRef]
- Siebentritt, S. Wide gap chalcopyrites: Material properties and solar cells. Thin Solid Films 2002, 403, 1–8. [Google Scholar] [CrossRef]
- Klenk, R.; Klaer, J.; Scheer, R.; Lux-Steiner, M.C.; Luck, I.; Meyer, N.; Rühle, U. Solar cells based on CuInS2—An overview. Thin Solid Films 2005, 480, 509–514. [Google Scholar] [CrossRef]
- Green, M.A. Thin-film solar cells: Review of materials, technologies and commercial status. J. Mater. Sci. Mater. Electron. 2007, 18, 15–19. [Google Scholar] [CrossRef]
- Masuko, K.; Shigematsu, M.; Hashiguchi, T.; Fujishima, D.; Kai, M.; Yoshimura, N.; Yamaguchi, T.; Ichihashi, Y.; Mishima, T.; Matsubara, N.; et al. Achievement of more than 25% conversion efficiency with crystalline silicon heterojunction solar cell. IEEE J. Photovolt. 2014, 4, 1433–1435. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Kawasaki, H.; Yoshida, W.; Irie, T.; Konishi, K.; Nakano, K.; Uto, T.; Adachi, D.; Kanematsu, M.; Uzu, H.; et al. Silicon heterojunction solar cell with interdigitated back contacts for a photoconversion efficiency over 26%. Nat. Energy 2017, 2, 17032. [Google Scholar] [CrossRef]
- Green, M.A. Corrigendum to ‘Solar cell efficiency tables (version 49). Prog. Photovolt. Res. Appl. 2017, 25, 333–334. [Google Scholar] [CrossRef]
- NREL, Best Research-Cell Efficiency Chart. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 26 January 2022).
- Meng, L.; Wu, X.G.; Ma, S.; Shi, L.; Zhang, M.; Wang, L.; Chen, Y.; Chen, Q.; Zhong, H. Improving the efficiency of silicon solar cells using in situ fabricated perovskite quantum dots as luminescence downshifting materials. Nanophotonics 2020, 9, 93–100. [Google Scholar] [CrossRef]
- Ahn, Y.; Kim, J.; Shin, S.; Ganorkar, S.; Kim, Y.H.; Kim, Y.T.; Kim, S.I. Efficiency enhancement of GaAs solar cell using luminescent down-shifting layer consisting of (CdSe) ZnS quantum dots with calculation and experiment. ASME J. Sol. Energy Eng. 2015, 137, 021011. [Google Scholar] [CrossRef]
- Jeong, W.L.; Jang, J.; Kim, J.; Joo, S.K.; Park, M.D.; Kwak, H.M.; Baik, J.; Kim, H.J.; Kim, J.H.; Lee, D.S. Improving Ultraviolet Responses in Cu2ZnSn(S,Se)4 Thin-Film Solar Cells Using Quantum Dot-Based Luminescent Down-Shifting Layer. Nanomaterials 2021, 11, 1166. [Google Scholar] [CrossRef]
- Hodgson, S.D.; Brooks, W.S.; Clayton, A.J.; Kartopu, G.; Barrioz, V.; Irvine, S.J. Enhancing blue photoresponse in CdTe photovoltaics by luminescent down-shifting using semiconductor quantum dot/PMMA films. Nano Energy 2013, 2, 21–27. [Google Scholar] [CrossRef]
- Lee, Y.J.; Ruby, D.S.; Peters, D.W.; McKenzie, B.B.; Hsu, J.W. ZnO nanostructures as efficient antireflection layers in solar cells. Nano Lett. 2008, 8, 1501–1505. [Google Scholar] [CrossRef]
- Yu, P.; Chang, C.H.; Chiu, C.H.; Yang, C.S.; Yu, J.C.; Kuo, H.C.; Hsu, S.H.; Chang, Y.C. Efficiency enhancement of GaAs photovoltaics employing antireflective indium tin oxide nanocolumns. Adv. Mater. 2009, 21, 1618–1621. [Google Scholar] [CrossRef]
- Tsai, M.A.; Tseng, P.C.; Chen, H.C.; Kuo, H.C.; Yu, P. Enhanced conversion efficiency of a crystalline silicon solar cell with frustum nanorod arrays. Opt. Express 2011, 19, A28–A34. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N. Plasma-chemical vapor-deposited silicon oxide/silicon oxynitride double-layer antireflective coating for solar cells. Jpn. J. Appl. Phys. 1991, 30, 997. [Google Scholar] [CrossRef]
- Aiken, D.J. High performance anti-reflection coatings for broadband multi-junction solar cells. Sol. Energy Mater. Sol. Cells 2000, 64, 393–404. [Google Scholar] [CrossRef]
- Richards, B.S. Luminescent layers for enhanced silicon solar cell performance: Down-conversion. Sol. Energy Mater. Sol. Cells 2006, 90, 1189–1207. [Google Scholar] [CrossRef]
- Klampaftis, E.; Ross, D.; McIntosh, K.R.; Richards, B.S. Enhancing the performance of solar cells via luminescent down-shifting of the incident spectrum: A review. Sol. Energy Mater. Sol. Cells 2009, 93, 1182–1194. [Google Scholar] [CrossRef]
- Richards, B.S.; McIntosh, K.R. Overcoming the poor short wavelength spectral response of CdS/CdTe photovoltaic modules via luminescence down-shifting: Ray-tracing simulations. Prog. Photovolt. Res. Appl. 2007, 15, 27–34. [Google Scholar] [CrossRef]
- Lesyuk, R.; Marinov, V.; Hobbie, E.K.; Elbaradei, A.; Tarnavchyk, I.; Bobitski, Y. Toward cadmium-free spectral down-shifting converters for photovoltaic applications. Sol. Energy Mater. Sol. Cells 2016, 151, 52–59. [Google Scholar] [CrossRef]
- McKenna, B.; Evans, R.C. Towards efficient spectral converters through materials design for luminescent solar devices. Adv. Mater. 2017, 29, 1606491. [Google Scholar] [CrossRef]
- Hovel, H.J.; Hodgson, R.T.; Woodall, J.M. The effect of fluorescent wavelength shifting on solar cell spectral response. Sol. Energy Mater. 1979, 2, 19–29. [Google Scholar] [CrossRef]
- Chen, H.C.; Lin, C.C.; Han, H.W.; Tsai, Y.L.; Chang, C.H.; Wang, H.W.; Tsai, M.A.; Kuo, H.C.; Yu, P. Enhanced efficiency for c-Si solar cell with nanopillar array via quantum dots layers. Opt. Express 2011, 19, A1141–A1147. [Google Scholar] [CrossRef]
- Han, H.V.; Lin, C.C.; Tsai, Y.L.; Chen, H.C.; Chen, K.J.; Yeh, Y.L.; Lin, W.Y.; Kuo, H.C.; Yu, P. A highly efficient hybrid GaAs solar cell based on colloidal-quantum-dot-sensitization. Sci. Rep. 2014, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.M.; dos Santos Torres, E.T.; de Jesus, J.P.A.; Lourenço, S.A.; Fernandes, R.V.; Laureto, E.; da Silva, M.A.T. Application of heterostructured CdS/ZnS quantum dots as luminescence down-shifting layer in P3HT: PCBM solar cells. J. Lumin. 2021, 237, 118178. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Zeng, J.; Chen, J.; Chen, H. Enhance short-wavelength response of CIGS solar cell by CdSe quantum disks as luminescent down-shifting material. Sol. Energy 2019, 193, 303–308. [Google Scholar] [CrossRef]
- Huang, X.; Han, S.; Huang, W.; Liu, X. Enhancing solar cell efficiency: The search for luminescent materials as spectral converters. Chem. Soc. Rev. 2013, 42, 173–201. [Google Scholar] [CrossRef]
- Marchionna, S.; Meinardi, F.; Acciarri, M.; Binetti, S.; Papagni, A.; Pizzini, S.; Malatesta, V.; Tubino, R. Photovoltaic quantum efficiency enhancement by light harvesting of organo-lanthanide complexes. J. Lumin. 2006, 118, 325–329. [Google Scholar] [CrossRef]
- LeDonne, A.; Acciarri, M.; Binetti, S.; Marchionna, S.; Narducci, D.; Rotta, D. Enhancement of Solar Energy Conversion Efficiency by Light Harvesting of Organolanthanide Complexes. In Proceedings of the 23rd European Photovoltaic Solar Energy Conference and Exhibition EU PVSEC, Valencia, Spain, 1–4 September 2008. [Google Scholar]
- Slooff, L.H.; Kinderman, R.; Burgers, A.R.; Bakker, N.J.; Van Roosmalen, J.A.M.; Büchtemann, A.; Danz, R.; Schleusener, M. Efficiency enhancement of solar cells by application of a polymer coating containing a luminescent dye. ASME J. Sol. Energy Eng. 2007, 129, 272–276. [Google Scholar] [CrossRef]
- Chen, H.C.; Lin, C.C.; Han, H.V.; Chen, K.J.; Tsai, Y.L.; Chang, Y.A.; Shih, M.H.; Kuo, H.C.; Yu, P. Enhancement of power conversion efficiency in GaAs solar cells with dual-layer quantum dots using flexible PDMS film. Sol. Energy Mater. Sol. Cells 2012, 104, 92–96. [Google Scholar] [CrossRef]
- Cho, Y.Y.; Huang, Y.M.; Hsu, S.C.; Kao, S.F.; Shih, H.Y.; Lee, T.Y.; Liu, Y.H.; Chen, T.M.; Kuo, H.C.; Lin, C.C. The Luminescent Down Shifting Effect of Single-Junction GaAs Solar Cell with Perovskite Quantum Dots. In Proceedings of the 46th Photovoltaic Specialists Conference (PVSC), Chicago, IL, USA, 16–21 June 2019. [Google Scholar]
- Kawano, K.; Hashimoto, N.; Nakata, R. Effects on solar cell efficiency of fluorescence of rare-earth ions. Mater. Sci. Forum. 1997, 239, 311–314. [Google Scholar] [CrossRef]
- Cheng, Z.; Su, F.; Pan, L.; Cao, M.; Sun, Z. CdS quantum dot-embedded silica film as luminescent down-shifting layer for crystalline Si solar cells. J. Alloys Compd. 2010, 494, L7–L10. [Google Scholar] [CrossRef]
- Kawano, K.; Arai, K.; Yamada, H.; Hashimoto, N.; Nakata, R. Application of rare-earth complexes for photovoltaic precursors. Sol. Energy Mater. Sol. Cells 1997, 48, 35–41. [Google Scholar] [CrossRef]
- Maruyama, T.; Bandai, J. Solar cell module coated with fluorescent coloring agent. J. Electrochem. Soc. 1999, 146, 4406. [Google Scholar] [CrossRef]
- Maruyama, T.; Shinyashiki, Y. Solar cells coated with fluorescent coloring agent. J. Electrochem. Soc. 1998, 145, 2955. [Google Scholar] [CrossRef]
- Maruyama, T.; Shinyashiki, Y.; Osako, S. Energy conversion efficiency of solar cells coated with fluorescent coloring agent. Sol. Energy Mater. Sol. Cells 1998, 56, 1–6. [Google Scholar] [CrossRef]
- Švrček, V.; Slaoui, A.; Muller, J.C. Silicon nanocrystals as light converter for solar cells. Thin Solid Films 2004, 451, 384–388. [Google Scholar] [CrossRef]
- Muffler, H.J.; Bär, M.; Lauermann, I.; Rahne, K.; Schröder, M.; Lux-Steiner, M.C.; Fischer, C.H.; Niesen, T.P.; Karg, F. Colloid attachment by ILGAR-layers: Creating fluorescing layers to increase quantum efficiency of solar cells. Sol. Energy Mater. Sol. Cells 2006, 90, 3143–3150. [Google Scholar] [CrossRef]
- Van Sark, W.G.J.H.M.; Meijerink, A.; Schropp, R.E.I.; Van Roosmalen, J.A.M.; Lysen, E.H. Modeling improvement of spectral response of solar cells by deployment of spectral converters containing semiconductor nanocrystals. Semiconductors 2004, 38, 962–969. [Google Scholar] [CrossRef]
- Van Sark, W.G.J.H.M. Enhancement of solar cell performance by employing planar spectral converters. Appl. Phys. Lett. 2005, 87, 151117. [Google Scholar] [CrossRef]
- Van Sark, W.G.J.H.M.; Meijerink, A.; Schropp, R.E.I.; Van Roosmalen, J.A.M.; Lysen, E.H. Enhancing solar cell efficiency by using spectral converters. Sol. Energy Mater. Sol. Cells 2005, 87, 395–409. [Google Scholar] [CrossRef]
- Valizadeh, A.; Mikaeili, H.; Samiei, M.; Farkhani, S.M.; Zarghami, N.; Akbarzadeh, A.; Davaran, S. Quantum dots: Synthesis, bioapplications, and toxicity. Nanoscale Res. Lett. 2012, 7, 480. [Google Scholar] [CrossRef]
- Alonso-Álvarez, D.; Ross, D.; Klampaftis, E.; McIntosh, K.R.; Jia, S.; Storiz, P.; Stolz, T.; Richards, B.S. Luminescent down-shifting experiment and modelling with multiple photovoltaic technologies. Prog. Photovolt. Res. Appl. 2015, 23, 479–497. [Google Scholar] [CrossRef]
- Rowan, B.C.; Wilson, L.R.; Richards, B.S. Advanced material concepts for luminescent solar concentrators. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 1312–1322. [Google Scholar] [CrossRef]
- Seybold, G.; Wagenblast, G. New perylene and violanthrone dyestuffs for fluorescent collectors. Dyes Pigm. 1989, 11, 303–317. [Google Scholar] [CrossRef]
- Sah, R.E.; Baur, G.; Kelker, H. Influence of the solvent matrix on the overlapping of the absorption and emission bands of solute fluorescent dyes. Appl. Phys. 1980, 23, 369–372. [Google Scholar] [CrossRef]
- Viehmann, W. Thin-film scintillators for extended ultraviolet (UV) response silicon detectors. Meas. Opt. Radiat. 1979, 196, 90–95. [Google Scholar]
- Hsu, S.C.; Huang, Y.M.; Kao, Y.C.; Kuo, H.C.; Horng, R.H.; Lin, C.C. The Analysis of Dual-Junction tandem solar cells enhanced by surface dispensed quantum dots. IEEE Photonics J. 2018, 10, 1–11. [Google Scholar] [CrossRef]
- Fu, Q.; Tang, X.; Huang, B.; Hu, T.; Tan, L.; Chen, L.; Chen, Y. Recent progress on the long-term stability of perovskite solar cells. Adv. Sci. 2018, 5, 1700387. [Google Scholar] [CrossRef]
- Niu, G.; Guo, X.; Wang, L. Review of recent progress in chemical stability of perovskite solar cells. J. Mater. Chem. A 2015, 3, 8970–8980. [Google Scholar] [CrossRef]
- Girma, W.M.; Fahmi, M.Z.; Permadi, A.; Abate, M.A.; Chang, J.Y. Synthetic strategies and biomedical applications of I–III–VI ternary quantum dots. J. Mater. Chem. B 2017, 5, 6193–6216. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Pons, T.; Medintz, I.L.; Mattoussi, H. Biosensing with luminescent semiconductor quantum dots. Sensors 2006, 6, 925–953. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Zhang, W.; Schneider, J.; Halpert, J.E.; Rogach, A.L. Luminescent Down-Conversion Semiconductor Quantum Dots and Aligned Quantum Rods for Liquid Crystal Displays. Adv. Sci. 2019, 6, 1901345. [Google Scholar] [CrossRef]
- Ashoori, R.C. Electrons in artificial atoms. Nature 1996, 379, 413–419. [Google Scholar] [CrossRef]
- Kastner, M.A. The single electron transistor and artificial atoms. Ann. Phys. 2000, 9, 885–894. [Google Scholar] [CrossRef]
- Bawendi, M.G.; Steigerwald, M.L.; Brus, L.E. The quantum mechanics of larger semiconductor clusters (“quantum dots”). Annu. Rev. Phys. Chem. 1990, 41, 477–496. [Google Scholar] [CrossRef]
- Reimann, S.M.; Manninen, M. Electronic structure of quantum dots. Rev. Mod. Phys. 2002, 74, 1283. [Google Scholar] [CrossRef]
- Hetsch, F.; Zhao, N.; Kershaw, S.V.; Rogach, A.L. Quantum dot field effect transistors. Mater. Today 2013, 16, 312–325. [Google Scholar] [CrossRef]
- Deng, Z.; Jeong, K.S.; Guyot-Sionnest, P. Colloidal quantum dots intraband photodetectors. ACS Nano 2014, 8, 11707–11714. [Google Scholar] [CrossRef]
- Hu, Z.; Yin, Y.; Ali, M.U.; Peng, W.; Zhang, S.; Li, D.; Zou, T.; Li, Y.; Jiao, S.; Chen, S.J.; et al. Inkjet printed uniform quantum dots as color conversion layers for full-color OLED displays. Nanoscale 2020, 12, 2103–2110. [Google Scholar] [CrossRef]
- Nikolenko, L.M.; Razumov, V.F. Colloidal quantum dots in solar cells. Russ. Chem. Rev. 2013, 82, 429. [Google Scholar] [CrossRef]
- Selopal, G.S.; Zhao, H.; Tong, X.; Benetti, D.; Navarro-Pardo, F.; Zhou, Y.; Barba, D.; Vidal, F.; Wang, Z.M.; Rosei, F. Highly stable colloidal “giant” quantum dots sensitized solar cells. Adv. Funct. Mater. 2017, 27, 1701468. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, C.H.; Hyun, B.R.; Sher, C.W.; Lv, Z.; Luo, B.; Jiang, F.; Wu, T.; Ho, C.H.; Kuo, H.C.; et al. Micro-light-emitting diodes with quantum dots in display technology. Light Sci. Appl. 2020, 9, 83. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Y.A.; Li, L.S.; Wang, D.; Zhu, T.; Xu, J.; Yang, C.; Li, Y. Bright, multicoloured light-emitting diodes based on quantum dots. Nat. Photonics 2007, 1, 717–722. [Google Scholar] [CrossRef]
- Klostranec, J.M.; Chan, W.C. Quantum dots in biological and biomedical research: Recent progress and present challenges. Adv. Mater. 2006, 18, 1953–1964. [Google Scholar] [CrossRef]
- Rosenthal, S.J.; Chang, J.C.; Kovtun, O.; McBride, J.R.; Tomlinson, I.D. Biocompatible quantum dots for biological applications. Chem. Biol. 2011, 18, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.J.; Fan, X.; Sadhu, A.S.; Lin, C.H.; Liou, F.J.; Wu, T.; Lu, Y.J.; He, J.H.; Chen, Z.; Wu, T.; et al. CsPbBr3 perovskite quantum-dot paper exhibiting a highest 3 dB bandwidth and realizing a flexible white-light system for visible-light communication. Photonics Res. 2021, 9, 2341–2350. [Google Scholar] [CrossRef]
- Oh, E.; Liu, R.; Nel, A.; Gemill, K.B.; Bilal, M.; Cohen, Y.; Medintz, I.L. Meta-analysis of cellular toxicity for cadmium-containing quantum dots. Nat. Nanotechnol. 2016, 11, 479–486. [Google Scholar] [CrossRef]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Milstein, T.J.; Kroupa, D.M.; Gamelin, D.R. Picosecond quantum cutting generates photoluminescence quantum yields over 100% in ytterbium-doped CsPbCl3 nanocrystals. Nano Lett. 2018, 18, 3792–3799. [Google Scholar] [CrossRef]
- Huang, H.; Polavarapu, L.; Sichert, J.A.; Susha, A.S.; Urban, A.S.; Rogach, A.L. Colloidal lead halide perovskite nanocrystals: Synthesis, optical properties and applications. NPG Asia Mater. 2016, 8, e328. [Google Scholar] [CrossRef]
- Li, X.; Cao, F.; Yu, D.; Chen, J.; Sun, Z.; Shen, Y.; Zhu, Y.; Wang, L.; Wei, Y.; Wu, Y.; et al. All inorganic halide perovskites nanosystem: Synthesis, structural features, optical properties and optoelectronic applications. Small 2017, 13, 1603996. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, K. Organic–inorganic hybrid lead halide perovskites for optoelectronic and electronic applications. Chem. Soc. Rev. 2016, 45, 655–689. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Y.; Wang, S.; Guo, J.; Yu, W.W.; Rogach, A.L. Metal halide perovskite light-emitting devices: Promising technology for next-generation displays. Adv. Funct. Mater. 2019, 29, 1902008. [Google Scholar] [CrossRef]
- Wu, H.; Yao, L.; Cao, W.; Yang, Y.; Cui, Y.; Yang, D.; Qian, G. Stable and wide-wavelength tunable luminescence of CsPbX3 nanocrystals encapsulated in metal-organic frameworks. J. Mater. Chem. C, 2022; advance article. [Google Scholar] [CrossRef]
- Møller, C.K. Crystal structure and photoconductivity of caesium plumbohalides. Nature 1958, 182, 1436. [Google Scholar] [CrossRef]
- Chen, Y.; Jing, H.; Ling, F.; Kang, W.; Zhou, T.; Liu, X.; Zeng, W.; Zhang, Y.; Qi, L.; Fang, L.; et al. Tuning the electronic structures of all-inorganic lead halide perovskite CsPbI3 via heterovalent doping: A first-principles investigation. Chem. Phys. Lett. 2019, 722, 90–95. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, Y.; Shao, Y.; Yan, Y. Understanding the physical properties of hybrid perovskites for photovoltaic applications. Nat. Rev. Mater. 2017, 2, 1–19. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, S.; Chen, W.; Zhu, H.; Xiong, Z.; Yang, Z.; Chen, C.; Chen, R.; Han, L.; Chen, W. Solvent engineering for efficient inverted perovskite solar cells based on inorganic CsPbI2Br light absorber. Mater. Today Energy 2018, 8, 125–133. [Google Scholar] [CrossRef]
- Huang, G.; Huang, Y.; Xu, W.; Yao, Q.; Liu, X.; Ding, C.; Chen, X. Cesium lead halide perovskite nanocrystals for ultraviolet and blue light blocking. Chin. Chem. Lett. 2019, 30, 1021–1023. [Google Scholar] [CrossRef]
- Sutton, R.J.; Eperon, G.E.; Miranda, L.; Parrott, E.S.; Kamino, B.A.; Patel, J.B.; Hörantner, M.T.; Johnston, M.B.; Haghighirad, A.A.; Moore, D.T.; et al. Bandgap-tunable cesium lead halide perovskites with high thermal stability for efficient solar cells. Adv. Energy Mater. 2016, 6, 1502458. [Google Scholar] [CrossRef]
- Bhalla, A.S.; Guo, R.; Roy, R. The perovskite structure—A review of its role in ceramic science and technology. Mater. Res. Innov. 2000, 4, 3–26. [Google Scholar] [CrossRef]
- Wang, H.C.; Bao, Z.; Tsai, H.Y.; Tang, A.C.; Liu, R.S. Perovskite quantum dots and their application in light-emitting diodes. Small 2018, 14, 1702433. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, H.Y.; Zhang, Z.Y.; Zhang, Y.; Sun, C.; Yue, Y.Y.; Wang, L.; Chen, Q.D.; Sun, H.B. Photoluminescence quenching of inorganic cesium lead halides perovskite quantum dots (CsPbX3) by electron/hole acceptor. Phys. Chem. Chem. Phys. 2017, 19, 1920–1926. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Chen, D.; Chang, S.; Huang, H.; Tong, K.; Xiao, C.; Chou, S.; Zhong, H.; Pei, Q. Highly flexible organometal halide perovskite quantum dot based light-emitting diodes on a silver nanowire–polymer composite electrode. J. Mater. Chem. C 2017, 5, 531–538. [Google Scholar] [CrossRef]
- Lin, H.; Xie, P.; Liu, Y.; Zhou, X.; Li, B. Tuning luminescence and reducing reabsorption of CdSe quantum disks for luminescent solar concentrators. Nanotechnology 2015, 26, 335401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, H.H.Y.; Evans, C.M.; Swartz, B.D.; Neukirch, A.J.; Young, J.; Prezhdo, O.V.; Krauss, T.D. Colloidal semiconductor quantum dots with tunable surface composition. Nano Lett. 2012, 12, 4465–4471. [Google Scholar] [CrossRef]
- Bansal, A.K.; Antolini, F.; Zhang, S.; Stroea, L.; Ortolani, L.; Lanzi, M.; Serra, E.; Allard, S.; Scherf, U.; Samuel, I.D.W. Highly luminescent colloidal CdS quantum dots with efficient near-infrared electroluminescence in light-emitting diodes. J. Phys. Chem. C 2016, 120, 1871–1880. [Google Scholar] [CrossRef]
- Anderson, N.C.; Hendricks, M.P.; Choi, J.J.; Owen, J.S. Ligand exchange and the stoichiometry of metal chalcogenide nanocrystals: Spectroscopic observation of facile metal-carboxylate displacement and binding. J. Am. Chem. Soc. 2013, 135, 18536–18548. [Google Scholar] [CrossRef]
- Lee, T.; Noguchi, K.; Nishimura, H.; Kim, D. Absorption and photoluminescence properties of CdSe quantum dots prepared by hydrothermal method. J. Phys. Conf. Ser. 2019, 1220, 012028. [Google Scholar] [CrossRef]
- Schmidt, L.C.; Pertegás, A.; González-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Minguez Espallargas, G.; Bolink, H.J.; Galian, R.E.; Pérez-Prieto, J. Nontemplate synthesis of CH3NH3PbBr3 perovskite nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef]
- Gonzalez-Carrero, S.; Galian, R.E.; Pérez-Prieto, J. Maximizing the emissive properties of CH3NH3PbBr3 perovskite nanoparticles. J. Mater. Chem. A 2015, 3, 9187–9193. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Protesescu, L.; Bodnarchuk, M.I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals. Science 2017, 358, 745–750. [Google Scholar] [CrossRef]
- Dirin, D.N.; Protesescu, L.; Trummer, D.; Kochetygov, I.V.; Yakunin, S.; Krumeich, F.; Stadie, N.P.; Kovalenko, M.V. Harnessing defect-tolerance at the nanoscale: Highly luminescent lead halide perovskite nanocrystals in mesoporous silica matrixes. Nano Lett. 2016, 16, 5866–5874. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, Y.; Ding, C.; Kobayashi, S.; Izuishi, T.; Nakazawa, N.; Toyoda, T.; Ohta, T.; Hayase, S.; Minemoto, T.; et al. Highly luminescent phase-stable CsPbI3 perovskite quantum dots achieving near 100% absolute photoluminescence quantum yield. ACS Nano 2017, 11, 10373–10383. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Reifsnyder, D.; Xie, R.; Aldana, J.; Peng, X. Surface ligand dynamics in growth of nanocrystals. J. Am. Chem. Soc. 2007, 129, 9500–9509. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Alivisatos, A.P. Colloidal nanocrystal synthesis and the organic–inorganic interface. Nature 2005, 437, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhong, H.; Chen, C.; Wu, X.G.; Hu, X.; Huang, H.; Han, J.; Zou, B.; Dong, Y. Brightly luminescent and color-tunable colloidal CH3NH3PbX3 (X = Br, I, Cl) quantum dots: Potential alternatives for display technology. ACS Nano 2015, 9, 4533–4542. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 quantum dots for lighting and displays: Room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Xu, L.; Li, J.; Zhang, F.; Han, B.; Shan, Q.; Zeng, H. Room-temperature triple-ligand surface engineering synergistically boosts ink stability, recombination dynamics, and charge injection toward EQE-11.6% perovskite QLEDs. Adv. Mater. 2018, 30, 1800764. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; D’Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the optical properties of cesium lead halide perovskite nanocrystals by anion exchange reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef]
- Kim, Y.; Yassitepe, E.; Voznyy, O.; Comin, R.; Walters, G.; Gong, X.; Kanjanaboos, P.; Nogueira, A.F.; Sargent, E.H. Efficient luminescence from perovskite quantum dot solids. ACS Appl. Mater. Interfaces 2015, 7, 25007–25013. [Google Scholar] [CrossRef]
- Zhang, F.; Huang, S.; Wang, P.; Chen, X.; Zhao, S.; Dong, Y.; Zhong, H. Colloidal synthesis of air-stable CH3NH3PbI3 quantum dots by gaining chemical insight into the solvent effects. Chem. Mater. 2017, 29, 3793–3799. [Google Scholar] [CrossRef]
- Dai, S.W.; Hsu, B.W.; Chen, C.Y.; Lee, C.A.; Liu, H.Y.; Wang, H.F.; Huang, Y.C.; Wu, T.L.; Manikandan, A.; Ho, R.M.; et al. Perovskite quantum dots with near unity solution and neat-film photoluminescent quantum yield by novel spray synthesis. Adv. Mater. 2018, 30, 1705532. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, X.; Matras-Postolek, K.; Chen, H.S.; Yang, P. An anion-driven Sn2+ exchange reaction in CsPbBr3 nanocrystals towards tunable and high photoluminescence. J. Mater. Chem. C 2018, 6, 5506–5513. [Google Scholar] [CrossRef]
- Huang, X.; Hu, J.; Bi, C.; Yuan, J.; Lu, Y.; Sui, M.; Tian, J. B-site doping of CsPbI3 quantum dot to stabilize the cubic structure for high-efficiency solar cells. Chem. Eng. J. 2021, 421, 127822. [Google Scholar] [CrossRef]
- Singh, R.K.; Sharma, P.; Kumar, R.; Som, S.; Dutta, S.; Jain, N.; Chaurasiya, R.; Meena, M.L.; Ho, J.S.; Dai, S.W.; et al. CH3NH3Pb1–xCoxBr3–2xCl2x Perovskite Quantum Dots for Wide-Color Backlighting. ACS Appl. Nano Mater. 2021, 4, 717–728. [Google Scholar] [CrossRef]
- Li, T.Y.; Xu, X.; Lin, C.H.; Guan, X.; Hsu, W.H.; Tsai, M.L.; Fang, X.; Wu, T.; He, J.H. Highly UV Resistant Inch-Scale Hybrid Perovskite Quantum Dot Papers. Adv. Sci. 2020, 7, 1902439. [Google Scholar] [CrossRef]
- Grabolle, M.; Ziegler, J.; Merkulov, A.; Nann, T.; Resch-Genger, U. Stability and fluorescence quantum yield of CdSe–ZnS quantum dots—influence of the thickness of the ZnS shell. Ann. N. Y. Acad. Sci. 2008, 1130, 235–241. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Ruan, C.; Yin, C.; Wang, X.; Wang, Y.; Yu, W.W. Efficient and stable white LEDs with silica-coated inorganic perovskite quantum dots. Adv. Mater. 2016, 28, 10088–10094. [Google Scholar] [CrossRef]
- Kulbak, M.; Cahen, D.; Hodes, G. How important is the organic part of lead halide perovskite photovoltaic cells? Efficient CsPbBr3 cells. J. Phys. Chem. Lett. 2015, 6, 2452–2456. [Google Scholar] [CrossRef]
- Dualeh, A.; Gao, P.; Seok, S.I.; Nazeeruddin, M.K.; Grätzel, M. Thermal behavior of methylammonium lead-trihalide perovskite photovoltaic light harvesters. Chem. Mater. 2014, 26, 6160–6164. [Google Scholar] [CrossRef]
- Baikie, T.; Fang, Y.; Kadro, J.M.; Schreyer, M.; Wei, F.; Mhaisalkar, S.G.; Graetzel, M.; White, T.J. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications. J. Mater. Chem. A 2013, 1, 5628–5641. [Google Scholar] [CrossRef]
- Pan, J.; Quan, L.N.; Zhao, Y.; Peng, W.; Murali, B.; Sarmah, S.P.; Yuan, M.; Sinatra, L.; Alyami, N.M.; Liu, J.; et al. Highly efficient perovskite-quantum-dot light-emitting diodes by surface engineering. Adv. Mater. 2016, 28, 8718–8725. [Google Scholar] [CrossRef] [PubMed]
- Eperon, G.E.; Paternò, G.M.; Sutton, R.J.; Zampetti, A.; Haghighirad, A.A.; Cacialli, F.; Snaith, H.J. Inorganic caesium lead iodide perovskite solar cells. J. Mater. Chem. A 2015, 3, 19688–19695. [Google Scholar] [CrossRef]
- Luo, P.; Xia, W.; Zhou, S.; Sun, L.; Cheng, J.; Xu, C.; Lu, Y. Solvent engineering for ambient-air-processed, phase-stable CsPbI3 in perovskite solar cells. J. Phys. Chem. Lett. 2016, 7, 3603–3608. [Google Scholar] [CrossRef]
- Hu, Y.; Bai, F.; Liu, X.; Ji, Q.; Miao, X.; Qiu, T.; Zhang, S. Bismuth incorporation stabilized α-CsPbI3 for fully inorganic perovskite solar cells. ACS Energy Lett. 2017, 2, 2219–2227. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Kanatzidis, M.G. The renaissance of halide perovskites and their evolution as emerging semiconductors. Acc. Chem. Res. 2015, 48, 2791–2802. [Google Scholar] [CrossRef]
- Sharma, S.; Weiden, N.; Weiss, A. Phase-Diagrams of Quasi-Binary Systems of the Type-ABX3-A’BX3 ABX3-AB’X3, and ABX3-ABX’3 X= Halogen. Z. Phys. Chem. 1992, 175, 63–80. [Google Scholar] [CrossRef]
- Hoffman, J.B.; Schleper, A.L.; Kamat, P.V. Transformation of sintered CsPbBr3 nanocrystals to cubic CsPbI3 and gradient CsPbBrxI3–x through halide exchange. J. Am. Chem. Soc. 2016, 138, 8603–8611. [Google Scholar] [CrossRef]
- Yunakova, O.N.; Miloslavskii, V.K.; Kovalenko, E.N. Exciton absorption spectrum of thin CsPbI3 and Cs4PbI6 films. Opt. Spectrosc. 2012, 112, 91–96. [Google Scholar] [CrossRef]
- Ripolles, T.S.; Nishinaka, K.; Ogomi, Y.; Miyata, Y.; Hayase, S. Efficiency enhancement by changing perovskite crystal phase and adding a charge extraction interlayer in organic amine free-perovskite solar cells based on cesium. Sol. Energy Mater. Sol. Cells 2016, 144, 532–536. [Google Scholar] [CrossRef]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot–induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.J.; Filip, M.R.; Haghighirad, A.A.; Sakai, N.; Wenger, B.; Giustino, F.; Snaith, H.J. Cubic or orthorhombic? Revealing the crystal structure of metastable black-phase CsPbI3 by theory and experiment. ACS Energy Lett. 2018, 3, 1787–1794. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Zhou, S.; Li, Z.; Zhang, B.; Xia, X.; Liu, X.; Dai, S.; Yao, J. Preparation of Tortuous 3D γ-CsPbI3 Films at Low Temperature by CaI2 as Dopant for Highly Efficient Perovskite Solar Cells. Adv. Funct. Mater. 2019, 29, 1808986. [Google Scholar] [CrossRef]
- Zhao, B.; Jin, S.F.; Huang, S.; Liu, N.; Ma, J.Y.; Xue, D.J.; Han, Q.; Ding, J.; Ge, Q.Q.; Feng, Y.; et al. Thermodynamically stable orthorhombic γ-CsPbI3 thin films for high-performance photovoltaics. J. Am. Chem. Soc. 2018, 140, 11716–11725. [Google Scholar] [CrossRef]
- Mir, W.J.; Swarnkar, A.; Nag, A. Postsynthesis Mn-doping in CsPbI3 nanocrystals to stabilize the black perovskite phase. Nanoscale 2019, 11, 4278–4286. [Google Scholar] [CrossRef]
- Lai, M.; Kong, Q.; Bischak, C.G.; Yu, Y.; Dou, L.; Eaton, S.W.; Ginsberg, N.S.; Yang, P. Structural, optical, and electrical properties of phase-controlled cesium lead iodide nanowires. Nano Res. 2017, 10, 1107–1114. [Google Scholar] [CrossRef]
- Frolova, L.A.; Anokhin, D.V.; Piryazev, A.A.; Luchkin, S.Y.; Dremova, N.N.; Stevenson, K.J.; Troshin, P.A. Highly efficient all-inorganic planar heterojunction perovskite solar cells produced by thermal coevaporation of CsI and PbI2. J. Phys. Chem. Lett. 2017, 8, 67–72. [Google Scholar] [CrossRef]
- Li, H.; Tong, G.; Chen, T.; Zhu, H.; Li, G.; Chang, Y.; Wang, L.; Jiang, Y. Interface engineering using a perovskite derivative phase for efficient and stable CsPbBr3 solar cells. J. Mater. Chem. A 2018, 6, 14255–14261. [Google Scholar] [CrossRef]
- Liu, X.; Tan, X.; Liu, Z.; Ye, H.; Sun, B.; Shi, T.; Tang, Z.; Liao, G. Boosting the efficiency of carbon-based planar CsPbBr3 perovskite solar cells by a modified multistep spin-coating technique and interface engineering. Nano Energy 2019, 56, 184–195. [Google Scholar] [CrossRef]
- Dutta, A.; Dutta, S.K.; Das Adhikari, S.; Pradhan, N. Phase-Stable CsPbI3 Nanocrystals: The Reaction Temperature Matters. Angew. Chem. Int. Ed. Engl. 2018, 57, 9083–9087. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, A.; Mir, W.J.; Nag, A. Can B-site doping or alloying improve thermal-and phase-stability of all-inorganic CsPbX3 (X = Cl, Br, I) perovskites? ACS Energy Lett. 2018, 3, 286–289. [Google Scholar] [CrossRef]
- Huang, L.Y.; Lambrecht, W.R. Electronic band structure trends of perovskite halides: Beyond Pb and Sn to Ge and Si. Phys. Rev. B 2016, 93, 195211. [Google Scholar] [CrossRef]
- Bremner, S.P.; Levy, M.Y.; Honsberg, C.B. Analysis of tandem solar cell efficiencies under AM1. 5G spectrum using a rapid flux calculation method. Prog. Photovolt. Res. Appl. 2008, 16, 225–233. [Google Scholar] [CrossRef]
- Xiang, W.; Wang, Z.; Kubicki, D.J.; Tress, W.; Luo, J.; Prochowicz, D.; Akin, S.; Emsley, L.; Zhou, J.; Dietler, G.; et al. Europium-doped CsPbI2Br for stable and highly efficient inorganic perovskite solar cells. Joule 2019, 3, 205–214. [Google Scholar] [CrossRef]
- Akkerman, Q.A.; Meggiolaro, D.; Dang, Z.; De Angelis, F.; Manna, L. Fluorescent alloy CsPbxMn1–xI3 perovskite nanocrystals with high structural and optical stability. ACS Energy Lett. 2017, 2, 2183–2186. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, P.; Wang, C.; Wang, Y.; Hu, Y.; Zhu, G.; Ma, L.; Liu, J.; Jin, Z. CsPb0.9Sn0.1IBr2 based all-inorganic perovskite solar cells with exceptional efficiency and stability. J. Am. Chem. Soc. 2017, 139, 14009–14012. [Google Scholar] [CrossRef]
- Li, F.; Pei, Y.; Xiao, F.; Zeng, T.; Yang, Z.; Xu, J.; Sun, J.; Peng, B.; Liu, M. Tailored dimensionality to regulate the phase stability of inorganic cesium lead iodide perovskites. Nanoscale 2018, 10, 6318–6322. [Google Scholar] [CrossRef]
- Zhang, T.; Dar, M.I.; Li, G.; Xu, F.; Guo, N.; Grätzel, M.; Zhao, Y. Bication lead iodide 2D perovskite component to stabilize inorganic α-CsPbI3 perovskite phase for high-efficiency solar cells. Sci. Adv. 2017, 3, e1700841. [Google Scholar] [CrossRef]
- Liao, J.F.; Rao, H.S.; Chen, B.X.; Kuang, D.B.; Su, C.Y. Dimension engineering on cesium lead iodide for efficient and stable perovskite solar cells. J. Mater. Chem. A 2017, 5, 2066–2072. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, X.; Deng, Y.; Zhao, J.; Chen, Z.; Huang, J. Stabilizing the α-phase of CsPbI3 perovskite by sulfobetaine zwitterions in one-step spin-coating films. Joule 2017, 1, 371–382. [Google Scholar] [CrossRef]
- Hanrahan, P.; Krueger, W. Reflection from layered surfaces due to subsurface scattering. In Proceedings of the 20th Annual Conference on Computer Graphics and Interactive Techniques, Anaheim, CA, USA, 2–6 August 1993. [Google Scholar]
- McIntosh, K.R.; Richards, B.S. Increased mc-Si module efficiency using fluorescent organic dyes: A ray-tracing study. In Proceedings of the 4th World Conference on Photovoltaic Energy Conference, Waikoloa, HI, USA, 7–12 May 2006. [Google Scholar]
- Kainarbay, A.Z.; Nurakhmetov, T.N.; Daurenbekov, D.K.; Eliseev, A.A.; Sachkova, T.Y.; Salikhodzha, Z.M.; Zhunusbekov, A.M. Luminescent down shifting CdTe colloidal quantum dots for enhancing polycrystalline silicon solar cells. Optik 2018, 169, 41–47. [Google Scholar] [CrossRef]
- Liu, W.; Lin, Q.; Li, H.; Wu, K.; Robel, I.; Pietryga, J.M.; Klimov, V.I. Mn2+-doped lead halide perovskite nanocrystals with dual-color emission controlled by halide content. J. Am. Chem. Soc. 2016, 138, 14954–14961. [Google Scholar] [CrossRef] [PubMed]
- Mir, W.J.; Jagadeeswararao, M.; Das, S.; Nag, A. Colloidal Mn-doped cesium lead halide perovskite nanoplatelets. ACS Energy Lett. 2017, 2, 537–543. [Google Scholar] [CrossRef]
- Van der Stam, W.; Geuchies, J.J.; Altantzis, T.; Van Den Bos, K.H.; Meeldijk, J.D.; Van Aert, S.; Bals, S.; Vanmaekelbergh, D.; de Mello Donega, C. Highly emissive divalent-ion-doped colloidal CsPb1–xMxBr3 perovskite nanocrystals through cation exchange. J. Am. Chem. Soc. 2017, 139, 4087–4097. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, M.; Ji, S.; Yang, L.; Zhao, J.; Liu, H.; Sui, Y.; Sun, Y.; Yang, J.; Zhang, X. Boosting spectral response of multi-crystalline Si solar cells with Mn2+ doped CsPbCl3 quantum dots downconverter. J. Power Sources 2018, 395, 85–91. [Google Scholar] [CrossRef]
- Beaulac, R.; Archer, P.I.; Ochsenbein, S.T.; Gamelin, D.R. Mn2+-doped CdSe quantum dots: New inorganic materials for spin-electronics and spin-photonics. Adv. Funct. Mater. 2008, 18, 3873–3891. [Google Scholar] [CrossRef]
- Jung, J.Y.; Zhou, K.; Bang, J.H.; Lee, J.H. Improved photovoltaic performance of Si nanowire solar cells integrated with ZnSe quantum dots. J. Phys. Chem. C 2012, 116, 12409–12414. [Google Scholar] [CrossRef]
- Baek, S.W.; Shim, J.H.; Seung, H.M.; Lee, G.S.; Hong, J.P.; Lee, K.S.; Park, J.G. Effect of core quantum-dot size on power-conversion-efficiency for silicon solar-cells implementing energy-down-shift using CdSe/ZnS core/shell quantum dots. Nanoscale 2014, 6, 12524–12531. [Google Scholar] [CrossRef]
- Sadeghimakki, B.; GaO, Z.; Sivoththaman, S. Proof of down-conversion by CdSe/ZnS quantum dots on silicon solar cells. In Proceedings of the 40th Photovoltaic Specialist Conference (PVSC), Denver, CO, USA, 8–13 June 2014. [Google Scholar]
- Ko, Y.H.; Baek, S.W.; Park, J.G. Effect of Cd0.5Zn0.5S/ZnS Core/Shell Quantum Dots on Power-Conversion-Efficiency Enhancement for Silicon Solar Cells. ECS Meet. Abstr. 2014, MA2014-02, 2065. [Google Scholar] [CrossRef]
- Gardelis, S.; Nassiopoulou, A.G. Evidence of significant down-conversion in a Si-based solar cell using CuInS2/ZnS core shell quantum dots. Appl. Phys. Lett. 2014, 104, 183902. [Google Scholar] [CrossRef]
- Xuan, T.T.; Liu, J.Q.; Li, H.L.; Sun, H.C.; Pan, L.K.; Chen, X.H.; Sun, Z. Microwave synthesis of high luminescent aqueous CdSe/CdS/ZnS quantum dots for crystalline silicon solar cells with enhanced photovoltaic performance. RSC Adv. 2015, 5, 7673–7678. [Google Scholar] [CrossRef]
- Hong, M.; Xuan, T.; Liu, J.; Jiang, Z.; Chen, Y.; Chen, X.; Li, H. Air-exposing microwave-assisted synthesis of CuInS2/ZnS quantum dots for silicon solar cells with enhanced photovoltaic performance. RSC Adv. 2015, 5, 102682–102688. [Google Scholar] [CrossRef]
- Mirershadi, S.; Ahmadi-Kandjani, S. Efficient thin luminescent solar concentrator based on organometal halide perovskite. Dyes Pigm. 2015, 120, 15–21. [Google Scholar] [CrossRef]
- Ahmed, H.; Doran, J.; McCormack, S.J. Increased short-circuit current density and external quantum efficiency of silicon and dye sensitised solar cells through plasmonic luminescent down-shifting layers. Sol. Energy 2016, 126, 146–155. [Google Scholar] [CrossRef]
- Liu, N.; Xue, H.; Ji, Y.; Wang, J. ZnSe/ZnS core-shell quantum dots incorporated with Ag nanoparticles as luminescent down-shifting layers to enhance the efficiency of Si solar cells. J. Alloys Compd. 2018, 747, 696–702. [Google Scholar] [CrossRef]
- Krishnan, C.; Mercier, T.; Rahman, T.; Piana, G.; Brossard, M.; Yagafarov, T.; To, A.; Pollard, M.E.; Shaw, P.; Bagnall, D.M.; et al. Efficient light harvesting in hybrid quantum dot–interdigitated back contact solar cells via resonant energy transfer and luminescent downshifting. Nanoscale 2019, 11, 18837–18844. [Google Scholar] [CrossRef]
- Meng, L.; Shi, L.; Ge, Y.; Tang, J.; Chen, Y.; Zhong, H. Photon management of combining nanostructural antireflection and perovskite down-shifting composite films for improving the efficiency of silicon solar cells. Sol. Energy Mater. Sol. Cells 2021, 220, 110856. [Google Scholar] [CrossRef]
- Nakamura, Y.; Iso, Y.; Isobe, T. Bandgap-Tuned CuInS2/ZnS Core/Shell Quantum Dots for a Luminescent Downshifting Layer in a Crystalline Silicon Solar Module. ACS Appl. Nano Mater. 2020, 3, 3417–3426. [Google Scholar] [CrossRef]
- Lin, C.C.; Chen, H.C.; Tsai, Y.L.; Han, H.V.; Shih, H.S.; Chang, Y.A.; Kuo, H.C.; Yu, P. Highly efficient CdS-quantum-dot-sensitized GaAs solar cells. Opt. Express 2012, 20, A319–A326. [Google Scholar] [CrossRef]
- Chen, C.H.; Yu, C.P.; Huang, Y.M.; Chan, Y.L.; Huang, C.P.; Chang, S.H.; Hsu, S.C.; Liou, J.J.; Chen, T.M.; Lin, C.C. Evaluation of Luminescent Downshifting Effect of Perovskite Quantum Dots on Semiconductor Solar Cells. In Proceedings of the 2018 IEEE 7th World Conference on Photovoltaic Energy Conversion (WCPEC) (A Joint Conference of 45th IEEE PVSC, 28th PVSEC & 34th EU PVSEC), Waikoloa Village, HI, USA, 10–15 June 2018. [Google Scholar]
- Safie, N.E.; Azam, M.A.; Aziz, M.F.; Ismail, M. Recent progress of graphene-based materials for efficient charge transfer and device performance stability in perovskite solar cells. Int. J. Energy Res. 2021, 45, 1347–1374. [Google Scholar] [CrossRef]
- Huang, Y.M.; Huang, C.P.; Huang, S.K.; Lee, T.Y.; Liu, Y.H.; Chen, T.M.; Kuo, H.C.; Lin, C.C. The Reliability and Prolonged time in Single-Junction GaAs Solar Cell with Perovskite Quantum Dots. In Proceedings of the 47th IEEE Photovoltaic Specialists Conference (PVSC), Calgary, ON, Canada, 15 June–21 August 2020. [Google Scholar]
- Rwaimi, M.; Bailey, C.G.; Shaw, P.J.; Mercier, T.M.; Krishnan, C.; Rahman, T.; Lagoudakis, P.G.; Horng, R.H.; Boden, S.A.; Charlton, M.D. FAPbBr3 perovskite quantum dots as a multifunctional luminescent-downshifting passivation layer for GaAs solar cells. Sol. Energy Mater. Sol. Cells 2022, 234, 111406. [Google Scholar] [CrossRef]
- Lee, Y.J.; Yao, Y.C.; Tsai, M.T.; Liu, A.F.; Yang, M.D.; Lai, J.T. Current matching using CdSe quantum dots to enhance the power conversion efficiency of InGaP/GaAs/Ge tandem solar cells. Opt. Express 2013, 21, A953–A963. [Google Scholar] [CrossRef] [PubMed]
- Ghymn, Y.H.; Jung, K.; Shin, M.; Ko, H. A luminescent down-shifting and moth-eyed anti-reflective film for highly efficient photovoltaic devices. Nanoscale 2015, 7, 18642–18650. [Google Scholar] [CrossRef]
- Hsu, S.C.; Kao, Y.C.; Han, H.V.; Kuo, H.C.; Horng, R.H.; Lin, C.C. Colloidal quantum dot enhanced dual-junction tandem solar cells. In Proceedings of the IEEE 43rd Photovoltaic Specialists Conference (PVSC), Portland, OR, USA, 5–10 June 2016. [Google Scholar]
- Shu, C.J.; Huang, Y.M.; Hsu, S.C.; Shu, J.K.; Tsai, J.L.; Yu, P.C.; Hung, Y.J.; Lin, C.C. Performance Enhancement of A GaAs Solar Cell with Colloidal Quantum Dots Embedded in Trenches. In Proceedings of the IEEE 44th Photovoltaic Specialist Conference (PVSC), Washington, DC, USA, 25–30 June 2017. [Google Scholar]
- Moutinho, H.R.; Hasoon, F.S.; Kazmerski, L.L. Studies of the micro-and nanostructure of polycrystalline CdTe and CuInSe2 using atomic force and scanning tunneling microscopy. Prog. Photovolt. Res. Appl. 1995, 3, 39–46. [Google Scholar] [CrossRef]
- Jones, E.W.; Barrioz, V.; Irvine, S.J.C.; Lamb, D. Towards ultra-thin CdTe solar cells using MOCVD. Thin Solid Films 2009, 517, 2226–2230. [Google Scholar] [CrossRef]
- Hodgson, S.D.; Brooks, W.S.M.; Clayton, A.J.; Kartopu, G.; Barrioz, V.; Irvine, S.J.C. The impact of quantum dot concentration on the optical properties of QD/PMMA luminescent down-shifting films applied to CdTe photovoltaic devices. Nano Energy 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Hodgson, S.D.; Brooks, W.S.; Clayton, A.J.; Kartopu, G.; Lamb, D.A.; Barrioz, V.; Irvine, S.J. Increased conversion efficiency in cadmium telluride photovoltaics by luminescent downshifting with quantum dot/poly (methyl methacrylate) films. Prog. Photovolt. Res. Appl. 2015, 23, 150–159. [Google Scholar] [CrossRef]
- Pudov, A.O.; Sites, J.R.; Contreras, M.A.; Nakada, T.; Schock, H.W. CIGS J–V distortion in the absence of blue photons. Thin Solid Films 2005, 480, 273–278. [Google Scholar] [CrossRef]
- Liao, Y.K.; Brossard, M.; Hsieh, D.H.; Lin, T.N.; Charlton, M.D.; Cheng, S.J.; Chen, C.H.; Shen, J.L.; Cheng, L.T.; Hsieh, T.P.; et al. Highly efficient flexible hybrid nanocrystal-Cu(In,Ga)Se2 (CIGS) solar cells. Adv. Energy Mater. 2015, 5, 1401280. [Google Scholar] [CrossRef]
- Jeong, H.J.; Kim, Y.C.; Lee, S.K.; Jeong, Y.; Song, J.W.; Yun, J.H.; Jang, J.H. Ultrawide spectral response of CIGS solar cells integrated with luminescent down-shifting quantum dots. ACS Appl. Mater. Interfaces 2017, 9, 25404–25411. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Jeong, H.J.; Kim, S.T.; Song, Y.H.; Kim, B.Y.; Kim, J.P.; Kang, B.K.; Yun, J.H.; Jang, J.H. Luminescent down-shifting CsPbBr3 perovskite nanocrystals for flexible Cu(In,Ga)Se2 solar cells. Nanoscale 2020, 12, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Wehrenfennig, C.; Eperon, G.E.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. High charge carrier mobilities and lifetimes in organolead trihalide perovskites. Adv. Mater. 2014, 26, 1584–1589. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Wang, B.; Li, B.; Shen, T.; Li, M.; Tian, J. ZnSe quantum dots downshifting layer for perovskite solar cells. J. Energy Chem. 2018, 27, 736–741. [Google Scholar] [CrossRef]
- Khafajeh, R.; Molaei, M.; Karimipour, M. Synthesis of ZnSe and ZnSe: Cu quantum dots by a room temperature photochemical (UV-assisted) approach using Na2SeO3 as Se source and investigating optical properties. Luminescence 2017, 32, 581–587. [Google Scholar] [CrossRef]
- Chen, C.; Zhuang, X.; Bi, W.; Wu, Y.; Gao, Y.; Pan, G.; Liu, D.; Dai, Q.; Song, H. Chemical inhibition of reversible decomposition for efficient and super-stable perovskite solar cells. Nano Energy 2020, 68, 104315. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Liu, L.; Gao, Y.; Chen, X.; Bi, W.; Chen, X.; Liu, D.; Dai, Q.; Song, H. Interfacial engineering and photon downshifting of CsPbBr3 nanocrystals for efficient, stable, and colorful vapor phase perovskite solar cells. Adv. Sci. 2019, 6, 1802046. [Google Scholar] [CrossRef]
- Ho, C.R.; Tsai, M.L.; Jhuo, H.J.; Lien, D.H.; Lin, C.A.; Tsai, S.H.; Wei, T.C.; Huang, K.P.; Chen, S.A.; He, J.H. An energy-harvesting scheme employing CuGaSe2 quantum dot-modified ZnO buffer layers for drastic conversion efficiency enhancement in inorganic–organic hybrid solar cells. Nanoscale 2013, 5, 6350–6355. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Jin, Z.; Zhang, J.; Gao, Z.; Li, Y.; Liu, S.F. Energy-down-shift CsPbCl3:Mn quantum dots for boosting the efficiency and stability of perovskite solar cells. ACS Energy Lett. 2017, 2, 1479–1486. [Google Scholar] [CrossRef]
- Lintangpradipto, M.N.; Tsevtkov, N.; Moon, B.C.; Kang, J.K. Size-controlled CdSe quantum dots to boost light harvesting capability and stability of perovskite photovoltaic cells. Nanoscale 2017, 9, 10075–10083. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Z.; Zhang, J.; Chen, W.; Yang, J.; Wen, X.; Wang, B.; Kobamoto, N.; Yuan, L.; Stride, J.A.; et al. Significant improvement in the performance of PbSe quantum dot solar cell by introducing a CsPbBr3 perovskite colloidal nanocrystal back layer. Adv. Energy Mater. 2017, 7, 1601773. [Google Scholar] [CrossRef]
- Gao, F.; Dai, H.; Pan, H.; Chen, Y.; Wang, J.; Chen, Z. Performance enhancement of perovskite solar cells by employing TiO2 nanorod arrays decorated with CuInS2 quantum dots. J. Colloid Interface Sci. 2018, 513, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Xiong, H.; Wang, G.; Xie, H.; Jia, W.; Zhang, Q.; Li, Y.; Wang, H. High-performance solar cells with induced crystallization of perovskite by an evenly distributed CdSe quantum dots seed-mediated underlayer. J. Power Sources 2018, 376, 46–54. [Google Scholar] [CrossRef]
- Rothemund, R. Optical modelling of the external quantum efficiency of solar cells with luminescent down-shifting layers. Sol. Energy Mater. Sol. Cells 2014, 120, 616–621. [Google Scholar] [CrossRef]
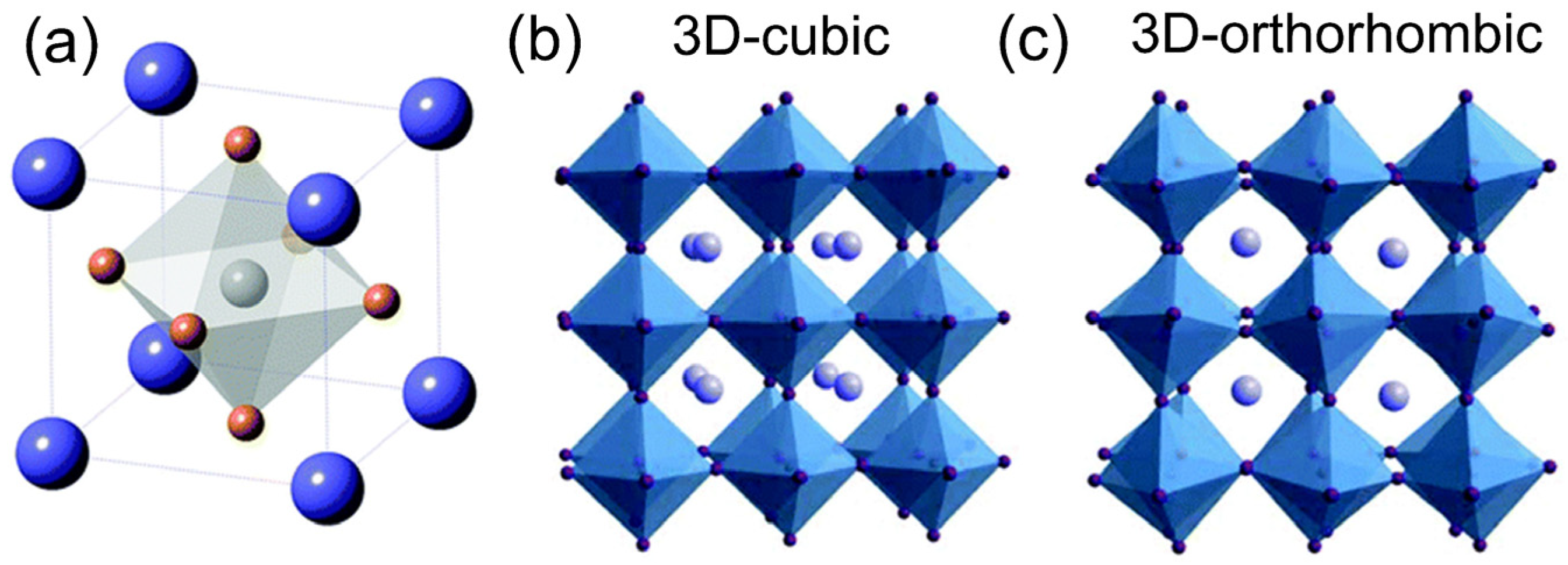
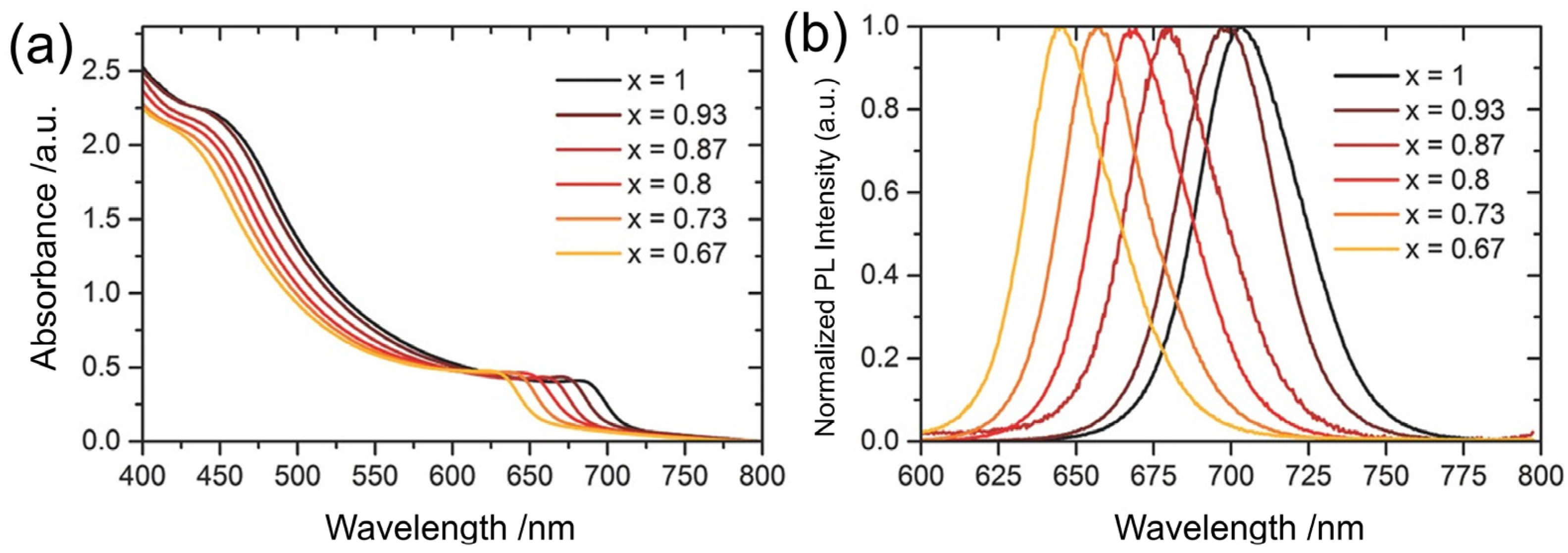

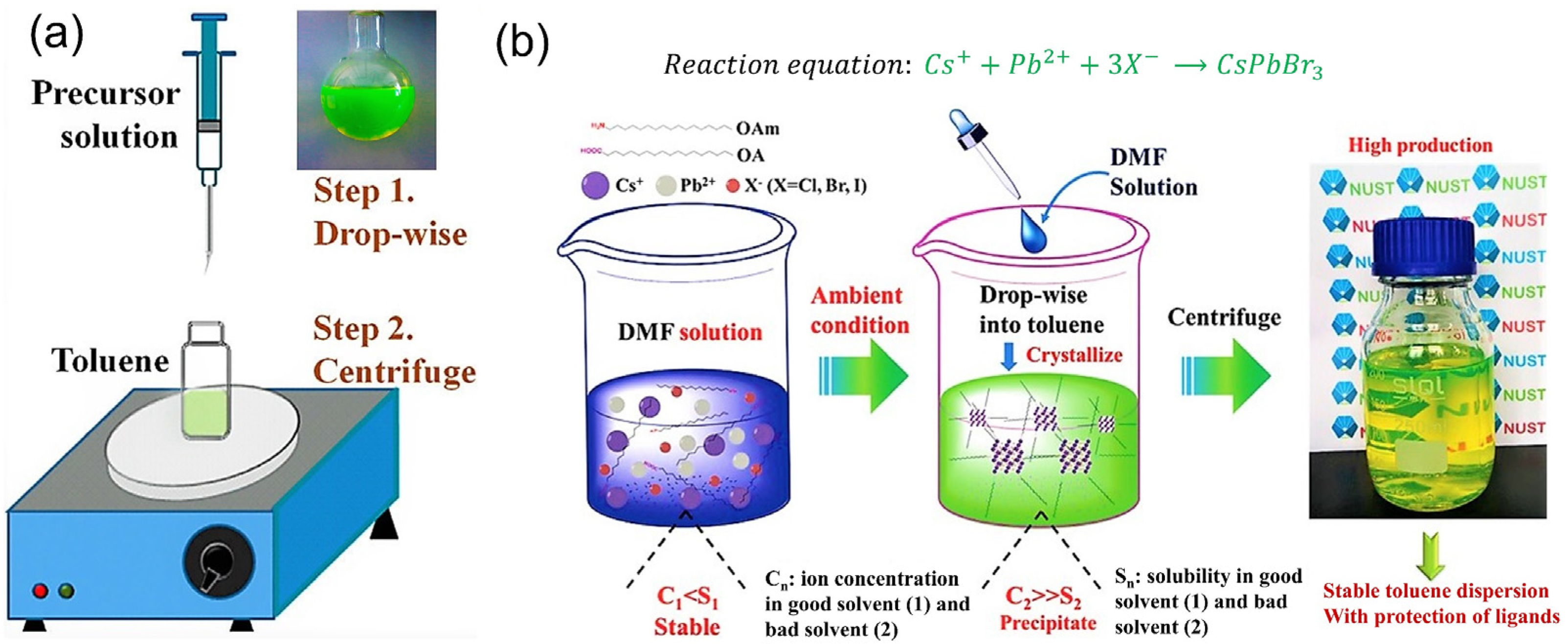
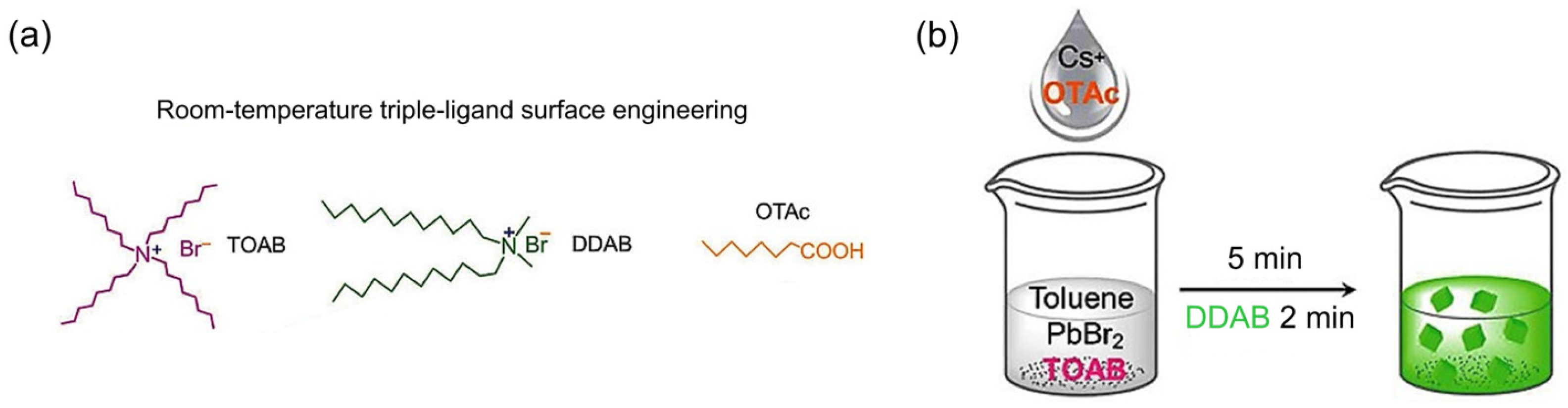


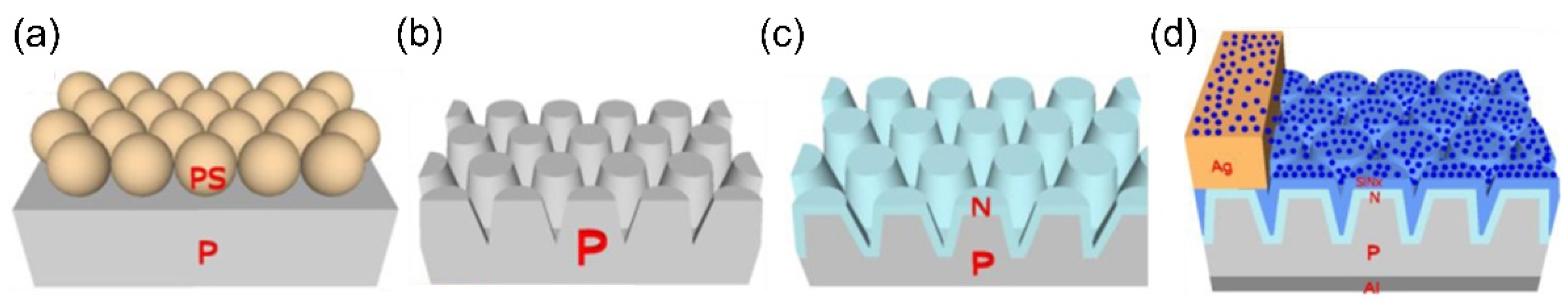
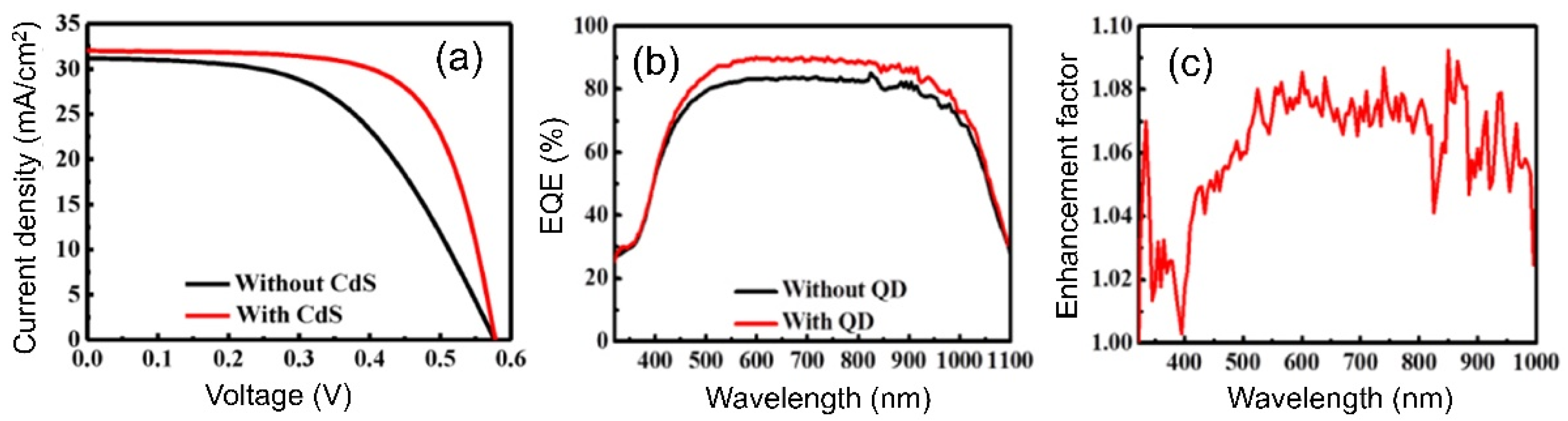
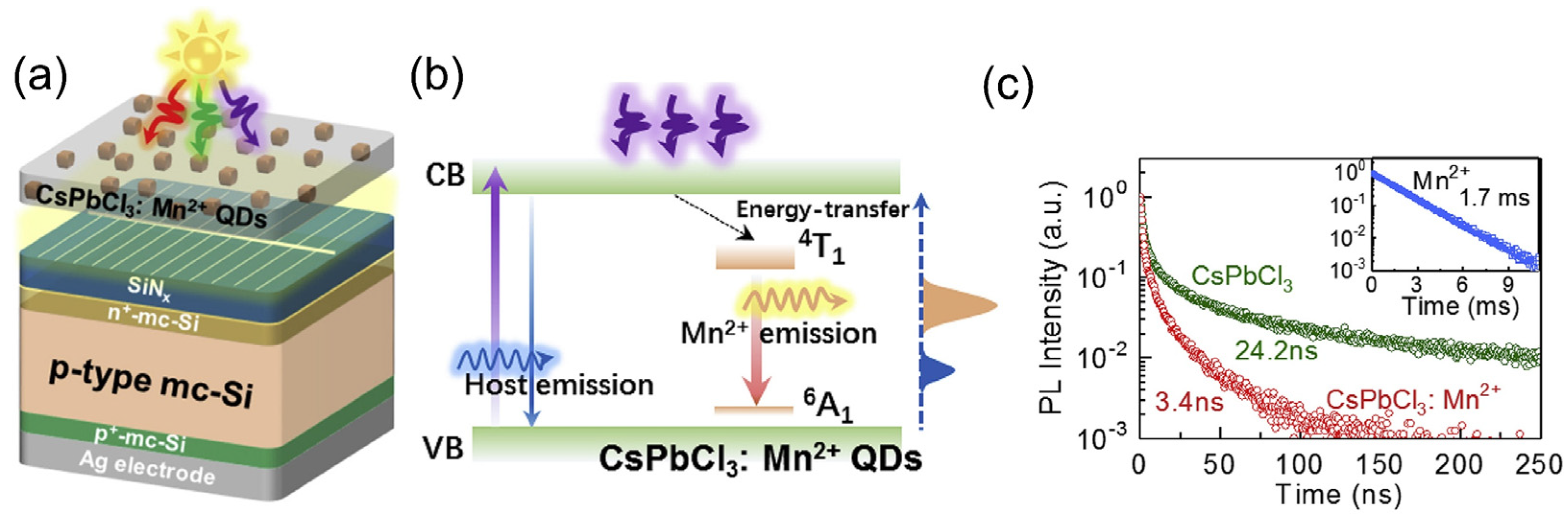
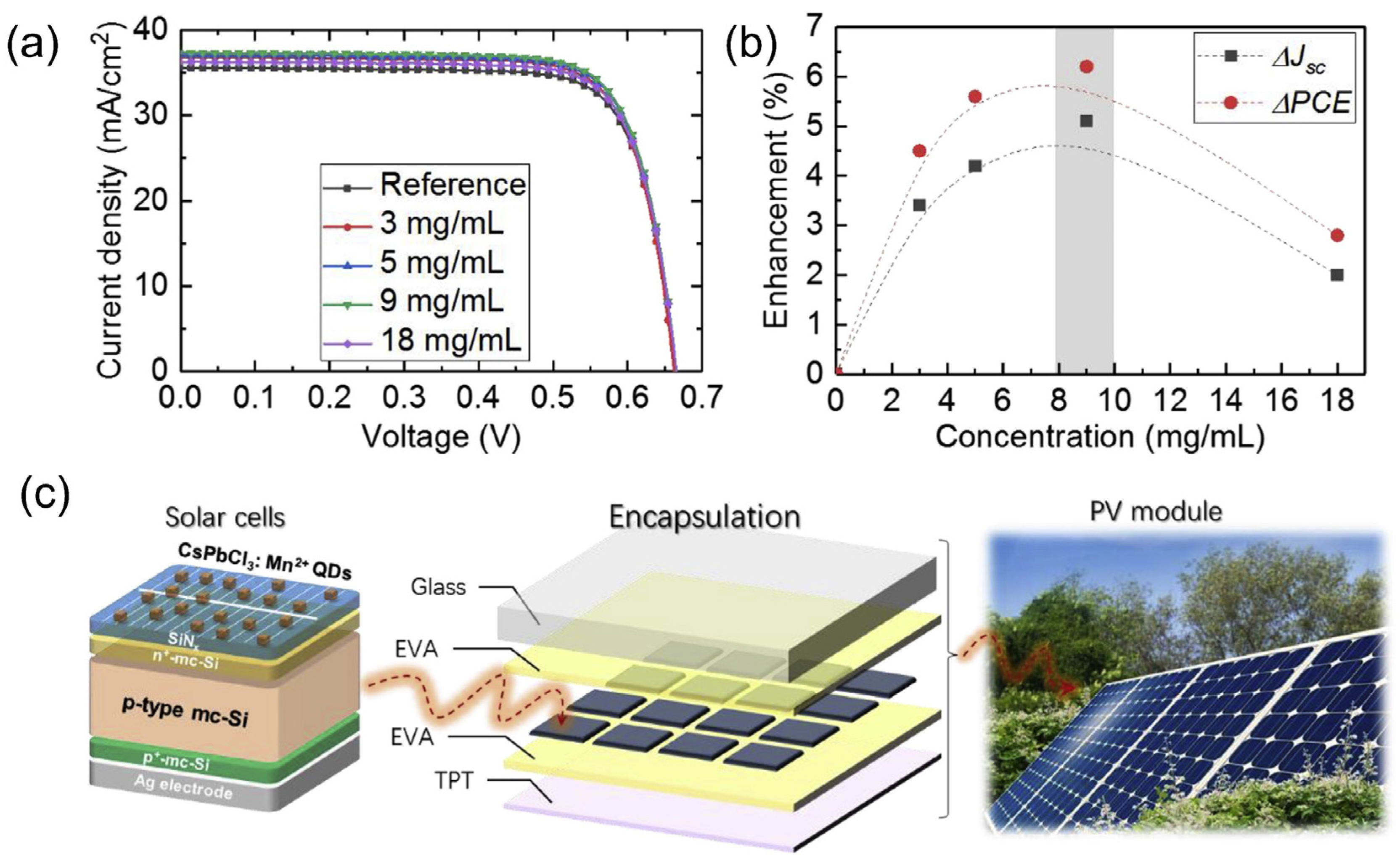

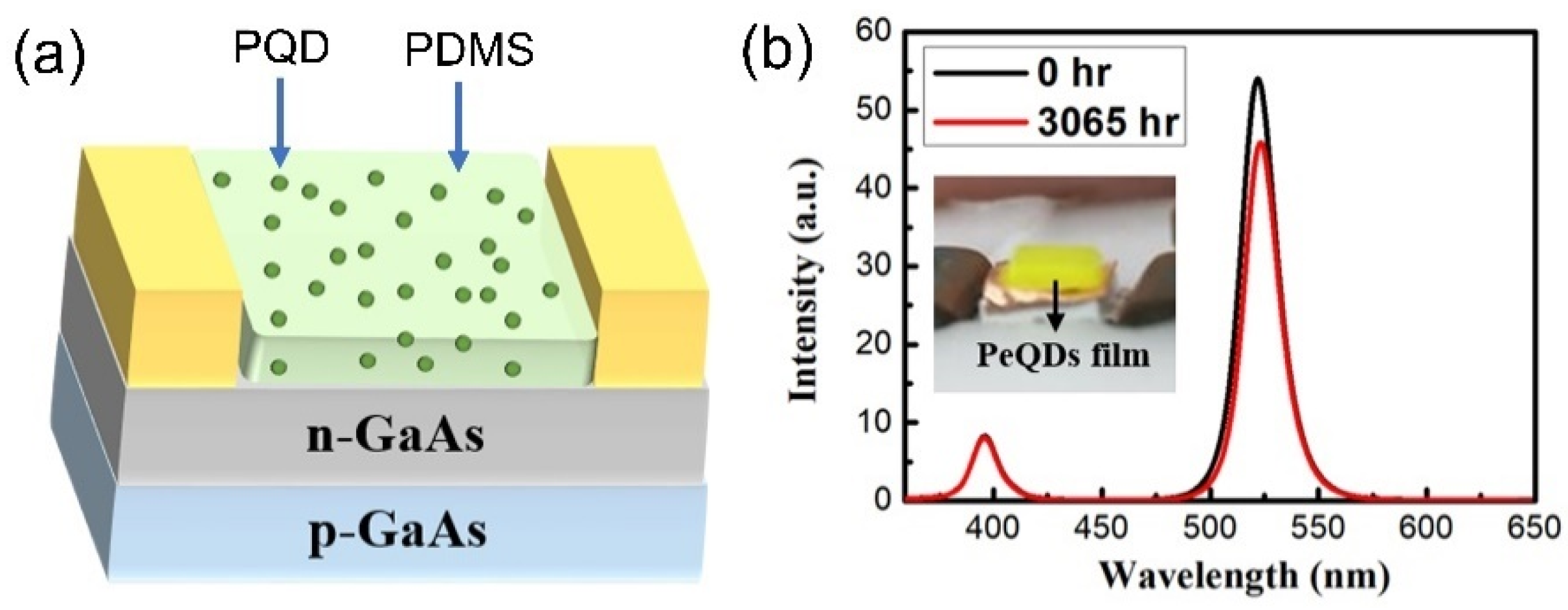
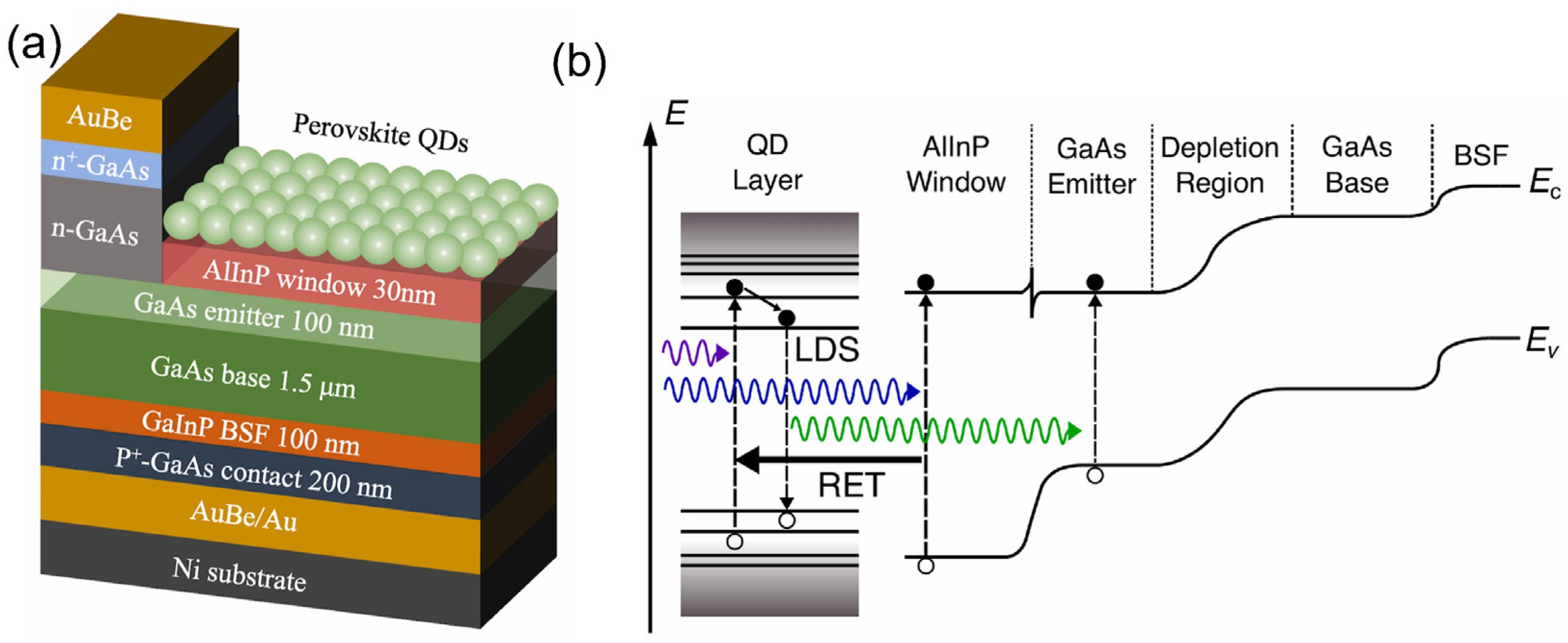


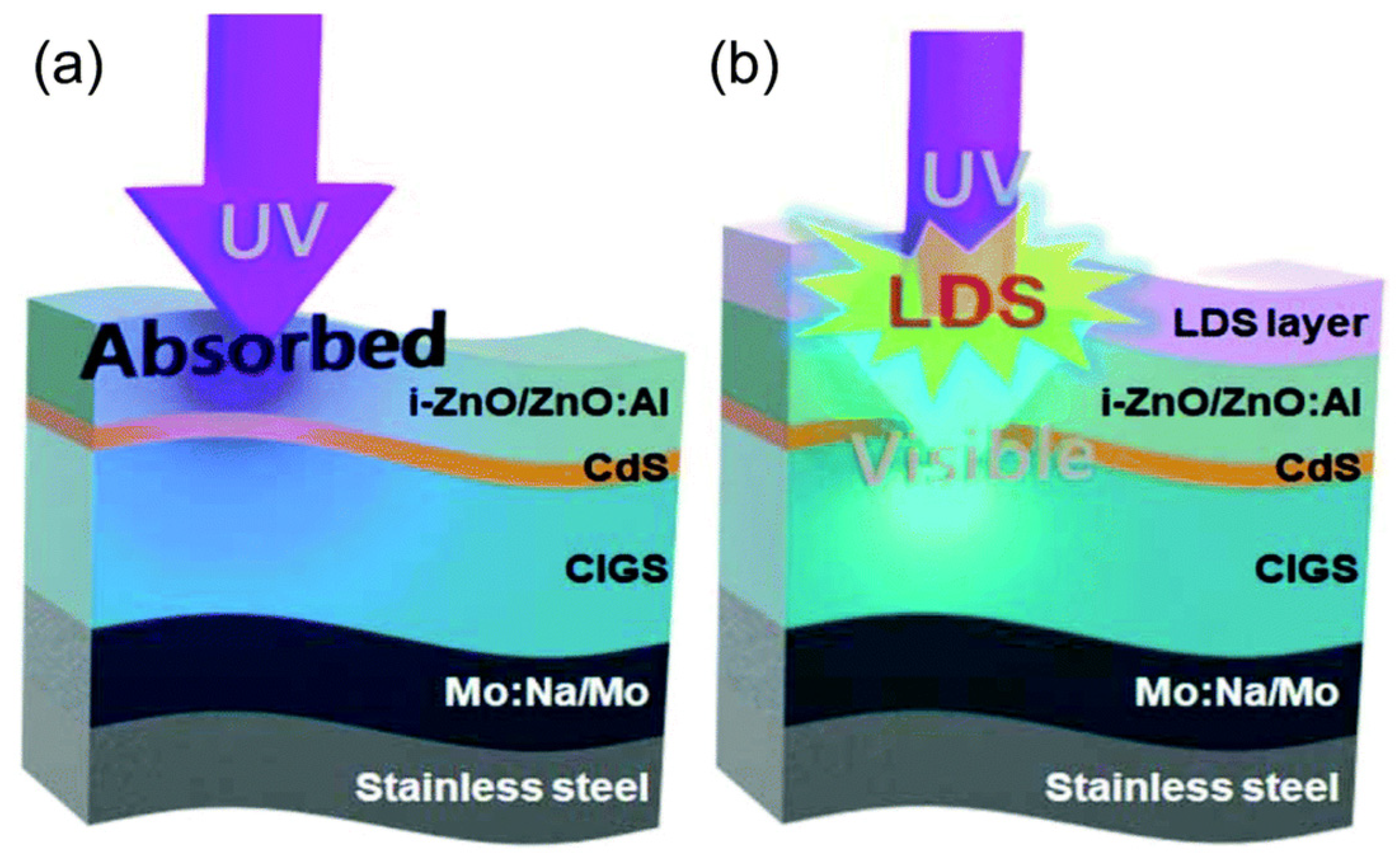

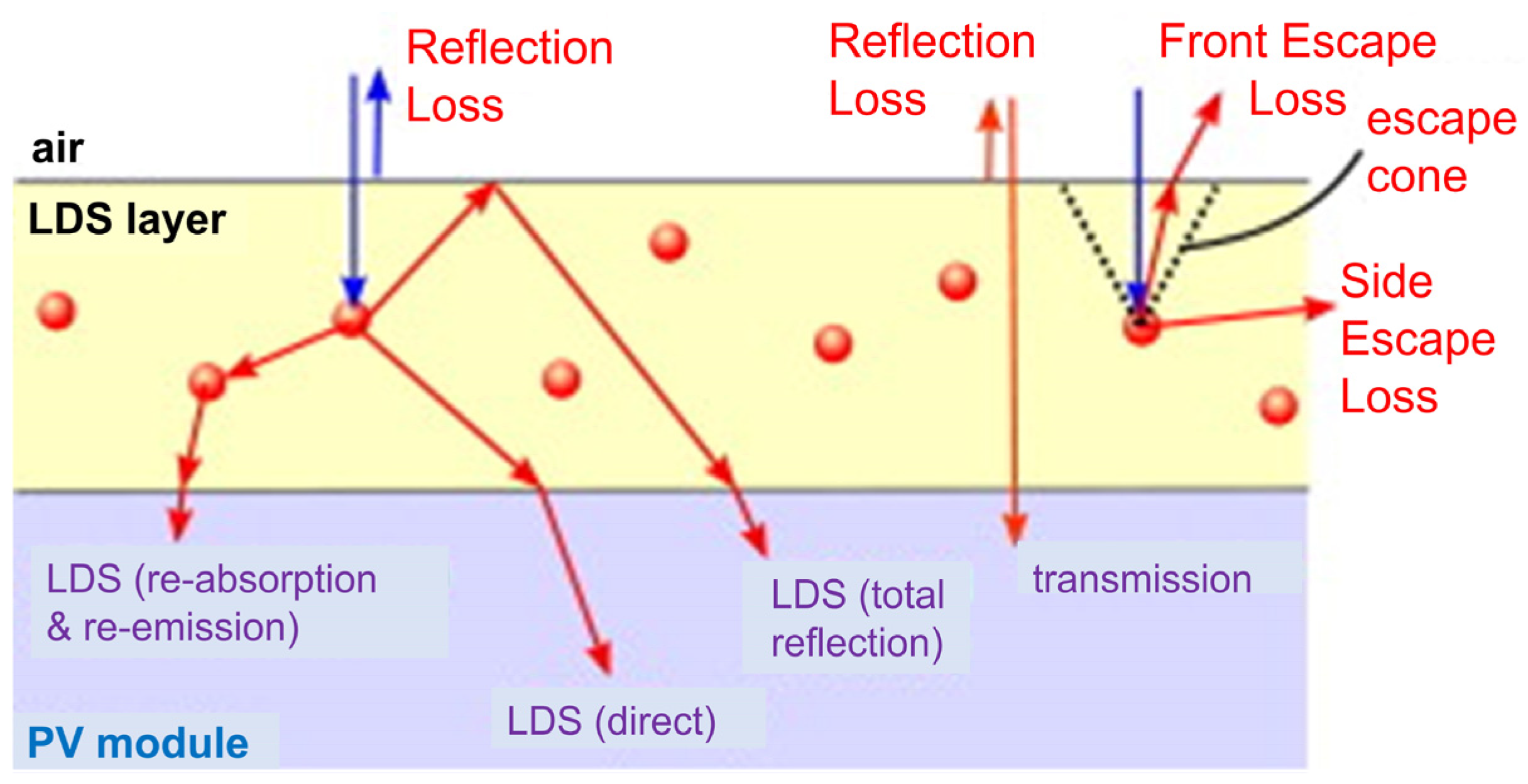
| QD Type | Synthesis Method | PLQY (%) | Ref./Year |
|---|---|---|---|
| CdSe–ZnS | Microwave | 50 | [121]/2008 |
| CH3NH3PbBr3 | Ligand-assisted reprecipitation (LARP) | 70 | [110]/2015 |
| CsPbBr3 | Hot injection and RT-SR | 90 | [111]/2016 |
| Ligand-capped CdS | Solvothermal | 69 | [99]/2016 |
| CH3NH3PbBr3 | Spray synthesis | 100 | [116]/2018 |
| CsPbBr3 | Room-temperature triple-ligand surface engineering | 93 | [112]/2018 |
| CdSe/ZnS core/shell | Hydrothermal | 47 | [106]/2019 |
| MAPbBr3/PQDP | Oleic acid/oleylamine (traditional surface ligands)-free | 80 | [120]/2020 |
| CH3NH3Pb1−xCoxBr3−2xCl2x | Cation and anion exchange | 95 | [119]/2021 |
| Si Type | QD Type | Matrix | Enhancement in Jsc (%) | Enhancement in PCE (%) | Ref./Year |
|---|---|---|---|---|---|
| c-Si | CdS | Silica | 4 | N/A | [43]/2010 |
| c-Si | CdS | N/A | 2.76 | 33 | [32]/2011 |
| N/A | ZnSe | SiO2 | 23.75 | 12.88 | [164]/2012 |
| N/A | CdSe/ZnS | N/A | 6.21 | 5.5 | [165]/2014 |
| c-Si | CdSe/ZnS | N/A | 6.55 | 3.58 | [166]/2014 |
| N/A | CdZnS/ZnS | N/A | 6.45 | 6.4 | [167]/2014 |
| N/A | CuInS2/ZnS | N/A | 28 | 37.5 | [168]/2014 |
| c-Si | CdSe/CdS/ZnS | SiO2 | 2.61 | 5.2 | [169]/2015 |
| N/A | CuInS2/ZnS | PMMA | 2.78 | 3.95 | [170]/2015 |
| N/A | CH3NH3PbBr3 | PVA & | 12.96 (Isc) | 45 | [171]/2015 |
| c-Si | CdSe/ZnS/Ag NPs | PMMA | 7.84 | 5.95 | [172]/2016 |
| mc-Si | CsPbCl3:Mn2+ | N/A | 5.1 | 6.2 | [162]/2018 |
| N/A | ZnSe/ZnS/Ag NPs | N/A | 12.5 | N/A | [173]/2018 |
| p-Si | CdTe | PMMA | 17.39 (Isc) | 48.47 | [158]/2018 |
| c-si | CH3NH3PbBr3 | PAN $ | 4.03 | 6.69 | [17]/2019 |
| mc-Si | CH3NH3PbBr3 | PAN $ | 2.07 | 8.43 | [17]/2019 |
| IBC * | CdSe1−xSx/ZnS | N/A | 39.5 | 40 | [174]/2019 |
| a-Si | CdxZn1−xSeyS1−y/ZnS | PDMS | 9.7 (Isc) | 11.73 | [175]/2020 |
| c-Si | CuInS2/ZnS/ZnS | EVA # | 1.8 (Isc) | 2.1 | [176]/2020 |
| QD Type | Matrix | Enhancement in Jsc (%) | Enhancement in PCE (%) | Ref./Year |
|---|---|---|---|---|
| CdS | PDMS | 18.37 | 21.52 | [40]/2012 |
| CdS | N/A | 20.98 | 18.91 | [177]/2012 |
| CdSe | N/A | 9.78 | 10.4 | [182]/2013 |
| CdSe/ZnS | N/A | 21.9 | 24.65 | [33]/2014 |
| CdZnS/ZnS | PDMS | 3.36 | 3.24 | [183]/2015 |
| CdSe/ZnS | InvisiSil | N/A | 2.87 | [18]/2015 |
| CdS/ZnS | N/A | 4.43 | 3.77 | [184]/2016 |
| CdS/ZnS | N/A | 9.44 | 5.78 | [184]/2016 |
| CdS/ZnS | N/A | 12.24 | 17.64 | [185]/2017 |
| CdSe/ZnS | N/A | 44.6 | 5.47 | [59]/2018 |
| CsPbBr3 | N/A | 10.9 | 14.06 | [178]/2018 |
| CsPbBr3 | PDMS/SiO2 | 5.22 | 7.12 | [41]/2018 |
| CsPbBr3 | PDMS/SiO2 | 3.60 | 10.56 | [180]/2020 |
| FAPbBr3 | N/A | 18 | N/A | [181]/2021 |
| QD Type | Matrix | Enhancement in Jsc (%) | Enhancement in PCE (%) | Ref./Year |
|---|---|---|---|---|
| CdS | PMMA | 30.3 | 1.7 | [188]/2013 |
| Commercial | PMMA | 4 | N/A | [189]/2013 |
| QD Type | Matrix | Enhancement in Jsc (%) | Enhancement in PCE (%) | Ref./Year |
|---|---|---|---|---|
| CdS/ZnS | N/A | 12.2 | 10.9 | [191]/2015 |
| CdSe/CdZnS | N/A | 4.35 | 3.85 | [192]/2017 |
| CdSe | PMMA | 9.54 | N/A | [35]/2019 |
| CdSe/CdZnS | N/A | 7.33 | 7.40 | [19]/2019 |
| CsPbBr3 | N/A | 4.5 | 4.5 | [193]/2020 |
| Cell Type | QD Type | Position of LDS Layer | Enhancement in Jsc (%) | Enhancement in PCE (%) | Ref./Year |
|---|---|---|---|---|---|
| Organic | CuGaSe2 | Inside | 9.43 | 23.8 | [200]/2012 |
| PSCs | ZnSe | Outside | 0.5 | 4.21 | [196]/2017 |
| PSCs | CsPbCl3:Mn | Outside | 3.77 | 3.34 | [201]/2017 |
| PSCs | CdSe | Inside | 8.06 | 14.67 | [202]/2017 |
| QDSCs | CsPbBr3 | Inside | −4.84 (Voc enhancement: 23.40%) | 50.41 | [203]/2017 |
| PSCs | CuInS2 | Inside | 18.12 | 31.46 | [204]/2018 |
| PSCs | CdSe | Inside | 12.03 | 35.5 | [205]/2018 |
| PSCs | Cs3+-CsPbI3 | Outside | 2.9 | 3.06 | [198]/2019 |
| PSCs | CsPbBr3 | Inside | 3.64 | 11.6 | [199]/2019 |
| PSCs | CsPbBr3@SiO2 | Outside | 4.53 | 5.6 | [199]/2019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadhu, A.S.; Huang, Y.-M.; Chen, L.-Y.; Kuo, H.-C.; Lin, C.-C. Recent Advances in Colloidal Quantum Dots or Perovskite Quantum Dots as a Luminescent Downshifting Layer Embedded on Solar Cells. Nanomaterials 2022, 12, 985. https://doi.org/10.3390/nano12060985
Sadhu AS, Huang Y-M, Chen L-Y, Kuo H-C, Lin C-C. Recent Advances in Colloidal Quantum Dots or Perovskite Quantum Dots as a Luminescent Downshifting Layer Embedded on Solar Cells. Nanomaterials. 2022; 12(6):985. https://doi.org/10.3390/nano12060985
Chicago/Turabian StyleSadhu, Annada Sankar, Yu-Ming Huang, Li-Yin Chen, Hao-Chung Kuo, and Chien-Chung Lin. 2022. "Recent Advances in Colloidal Quantum Dots or Perovskite Quantum Dots as a Luminescent Downshifting Layer Embedded on Solar Cells" Nanomaterials 12, no. 6: 985. https://doi.org/10.3390/nano12060985
APA StyleSadhu, A. S., Huang, Y.-M., Chen, L.-Y., Kuo, H.-C., & Lin, C.-C. (2022). Recent Advances in Colloidal Quantum Dots or Perovskite Quantum Dots as a Luminescent Downshifting Layer Embedded on Solar Cells. Nanomaterials, 12(6), 985. https://doi.org/10.3390/nano12060985








