Identification of Molecular Fragments in Equilibrium with Polysiloxane Ultrasmall Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
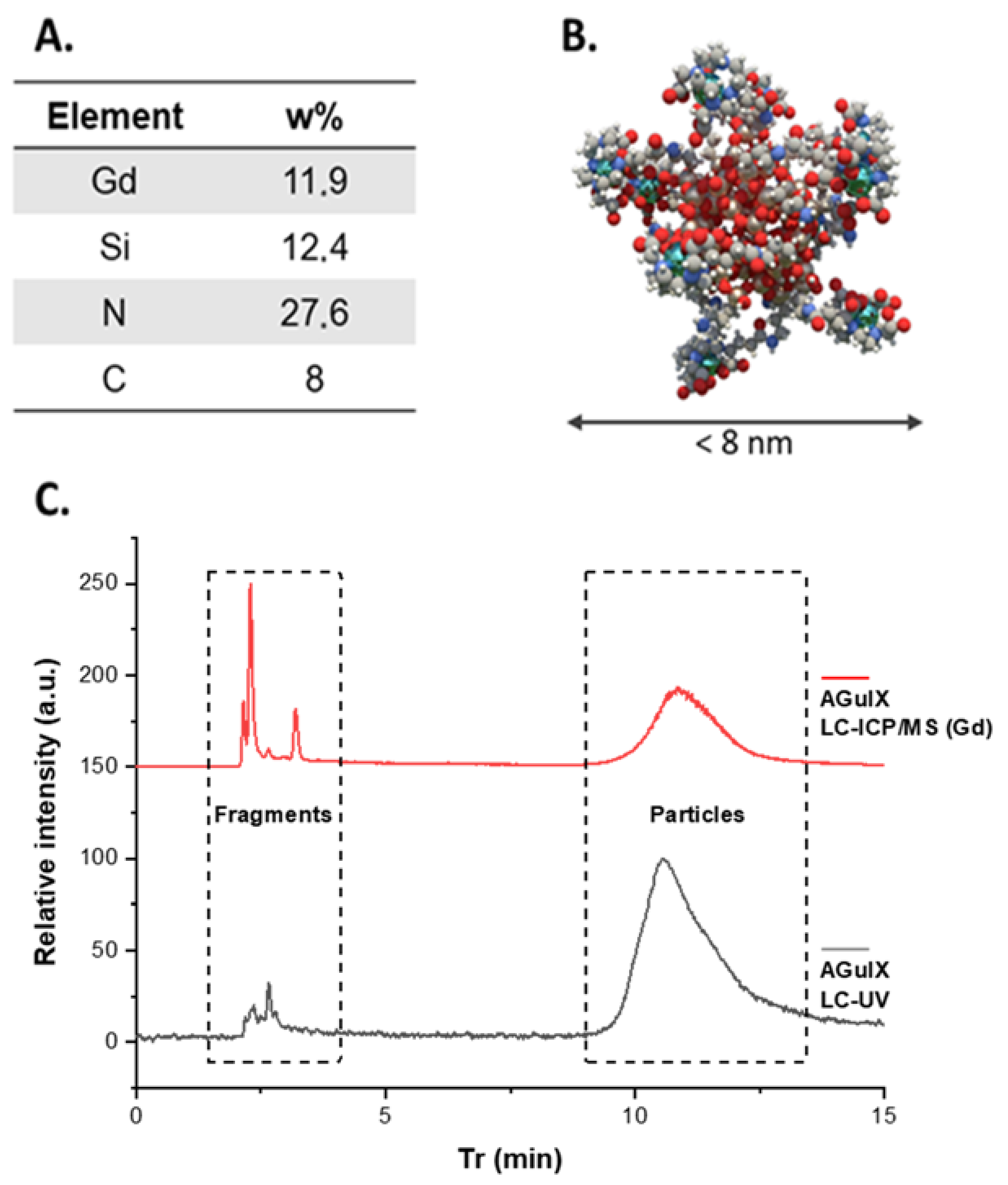
2.3. Elemental Analysis
2.4. Separation of Both Nanoparticles and Fragments in a Single Run
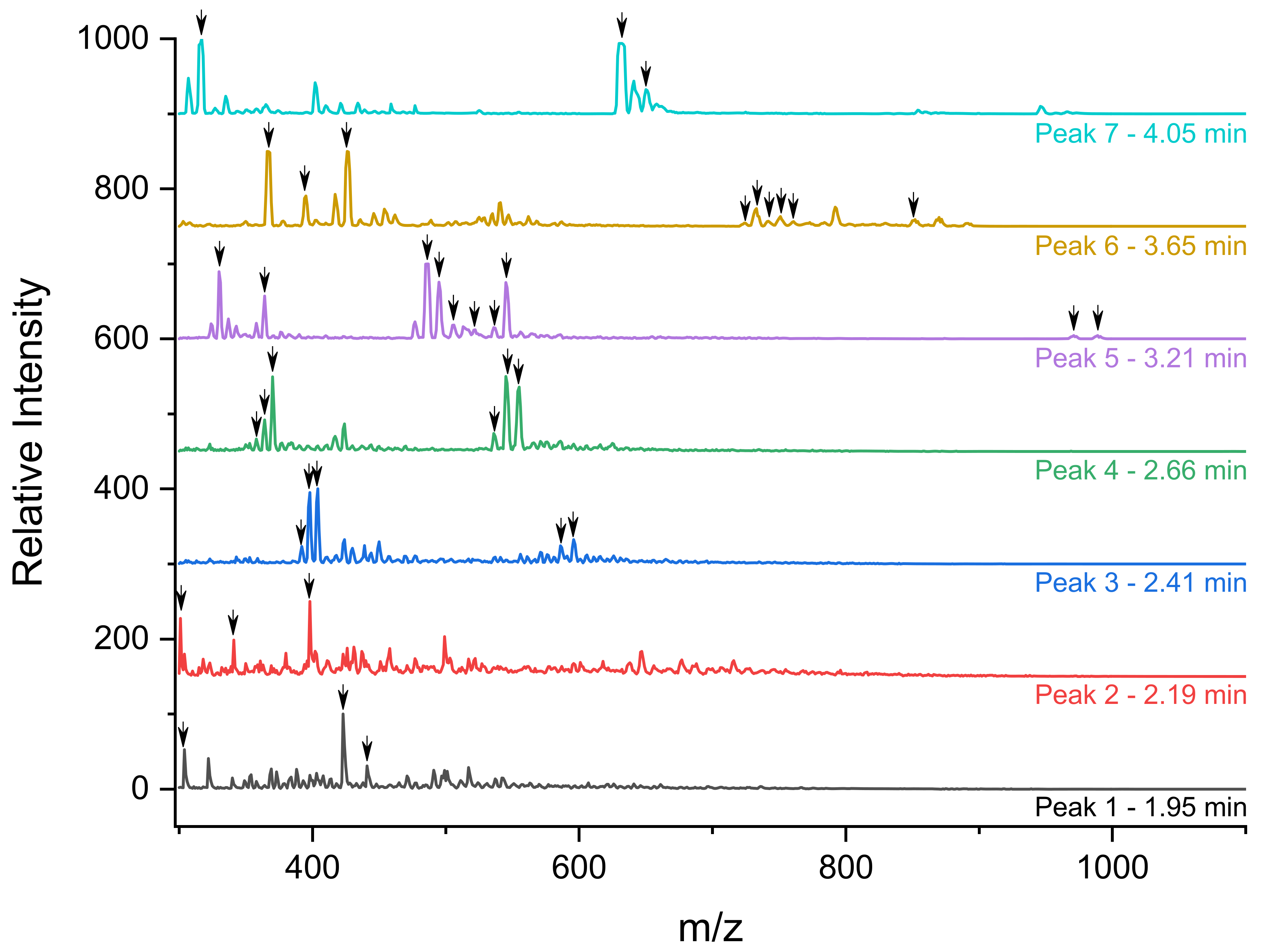
2.5. Fragment Separations and Identification by HPLC Coupled to Different Detectors
3. Results and Discussion
3.1. Synthesis and Purification of AGuIX Nanoparticles
3.2. Separating Nanoparticles from Fragments by HPLC Coupled to Different Detection Methods
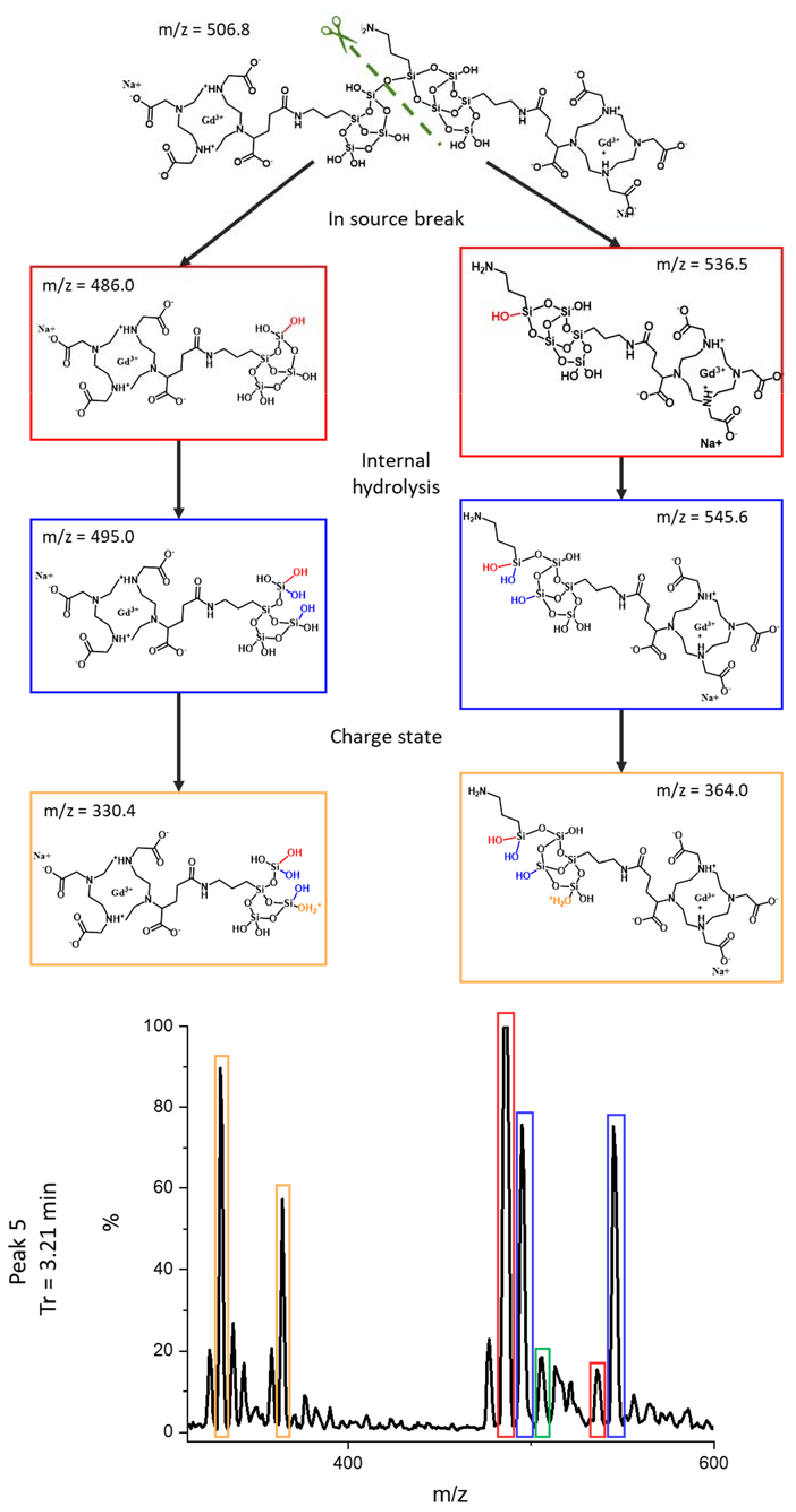
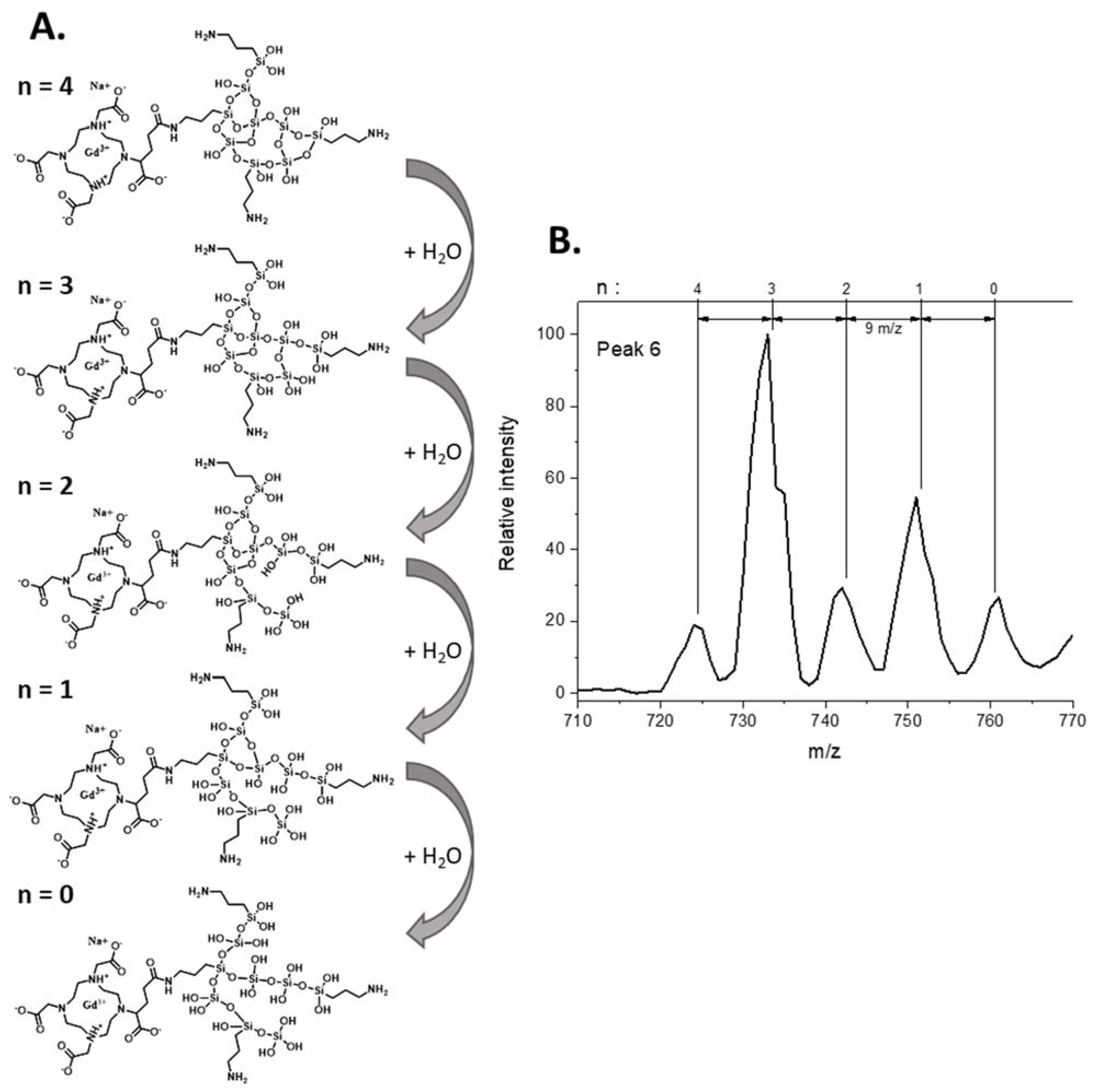
3.3. Identification of the Different Species by ESI/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lim, E.K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.M.; Lee, K. Nanomaterials for theranostics: Recent advances and future challenges. Chem. Rev. 2014, 115, 327. [Google Scholar]
- Johnson, K.K.; Koshy, P.; Yang, J.-L.; Sorrell, C. Preclinical Cancer Theranostics—From Nanomaterials to Clinic: The Missing Link. Adv. Func. Mat. 2021, 31, 2104199. [Google Scholar] [CrossRef]
- Bort, G.; Lux, F.; Dufort, S.; Crémillieux, Y.; Verry, C.; Tillement, O. EPR-mediated tumor targeting using ultrasmall-hybrid nanoparticles: From animal to human with theranostic AGuIX nanoparticles. Theranostics 2020, 10, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xing, Y.; Wang, R.; Yu, F.; Yu, F. Self-Assembled Nanomaterials for Enhanced Phototherapy of Cancer. ACS Appl. Bio Mater. 2020, 3, 86–106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, H.; Xing, Y.; Wang, R.; Cheng, Z.; Lv, C.; Lv, Z.; Yu, F. Tumor microenvironment-specific functional nanomaterials for biomedical applications. J. Biomed. Nanotechnol. 2020, 16, 1325–1358. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Feng, W.; Chen, Y.; Shi, J. Inorganic nanoparticles in clinical trials and translations. Nano Today 2020, 35, 100972. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update post COVID-19 vaccines. Bioeng. Transl. Med. 2021, 13, e10246. [Google Scholar] [CrossRef]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef]
- Agrahari, V.; Hiremath, P. Challenges associated and approaches for successful translation of nanomedicines into commercial products. Nanomedicine 2017, 12, 819–823. [Google Scholar] [CrossRef] [Green Version]
- Sayes, C.M.; Aquino, G.V.; Hickey, A.J. Nanomaterial drug products: Manufacturing and analytical perspectives. AAPS J. 2017, 19, 18–25. [Google Scholar] [CrossRef]
- Cassano, D.; Pocovi-Martinez, S.; Voliani, V. Ultrasmall-in-nano approach: Enabling the translation of metal nanomaterials to clinics. Bioconj. Chem. 2018, 29, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Zarschler, K.; Rocks, L.; Licciardello, N.; Boselli, L.; Polo, E.; Garcia, K.P.; de Cola, L.; Stephan, H.; Dawson, K.A. Ultrasmall inorganic nanoparticles: State-of-the-art and perspectives for biomedical applications. Nanomedicine 2016, 12, 1663–1701. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ma, K.; Benezra, M.; Zhang, L.; Cheal, S.M.; Phillips, E.; Yoo, B.; Pauliah, M.; Overholtzer, M.; Zanzonico, P.; et al. Cancer-targeting ultrasmall silica nanoparticles for clinical translation: Physicochemical structure and biological property correlations. Chem. Mater. 2017, 29, 8766–8779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lux, F.; Tran, V.-L.; Thomas, E.; Dufort, S.; Rossetti, F.; Martini, M.; Truillet, C.; Doussineau, T.; Bort, G.; Denat, F.; et al. AGuIX® from bench to bedside—Transfer of an ultrasmall theranostic gadolinium-based nanoparticle to clinical medicine. Br. J. Radiol. 2018, 91, 20180365. [Google Scholar] [CrossRef] [PubMed]
- Verry, C.; Dufort, S.; Lemasson, B.; Grand, S.; Pietras, J.; Troprès, I.; Crémillieux, Y.; Lux, F.; Mériaux, S.; Larrat, B.; et al. Targeting brain metastases with ultrasmall theranostic nanoparticles, a first-in-human trial from an MRI perspective. Sci. Adv. 2020, 6, eaay5279. [Google Scholar] [CrossRef]
- Verry, C.; Dufort, S.; Villa, J.; Gavard, M.; Iriart, C.; Grand, S.; Charles, J.; Chovelon, B.; Cracowski, J.L.; Quesada, J.L.; et al. Theranostic AGuIX nanoparticles as radiosensitizer: A phase I, dose-escalation study in patients with multiple brain metastases (NANO-RAD trial). J. Balosso Radiother. Oncol. 2021, 160, 159–165. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Kashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef]
- Truillet, C.; Lux, F.; Tillement, O.; Dugourd, P.; Antoine, R. Coupling of HPLC with electrospray ionization mass spectrometry for studying the aging of ultrasmall multifunctional gadolinium-based silica nanoparticles. Anal. Chem. 2013, 85, 10440–10447. [Google Scholar] [CrossRef]
- Labied, L.; Rocchi, P.; Doussineau, T.; Randon, J.; Tillement, O.; Cottet, H.; Lux, F.; Hagège, A. Biodegradation of metal-based ultra-small nanoparticles: A combined approach using TDA-ICP-MS and CE-ICP-MS. Anal. Chim. Acta 2021, 1185, 339081. [Google Scholar] [CrossRef]
- Le Duc, G.; Roux, S.; Paruta-Tuarez, A.; Dufort, S.; Brauer, E.; Marais, A.; Truillet, C.; Sancey, L.; Perriat, P.; Lux, F.; et al. Advantages of gadolinium based ultrasmall nanoparticles vs molecular gadolinium chelates for radiotherapy guided by MRI for glioma treatment. Cancer Nanotechnol. 2014, 5, 4. [Google Scholar] [CrossRef]
- Mignot, A.; Truillet, C.; Lux, F.; Sancey, L.; Louis, C.; Denat, F.; Boschetti, F.; Bocher, L.; Gloter, A.; Stephan, O.; et al. A Top-Down synthesis route to ultrasmall multifunctional Gd-Based silica nanoparticles for theranostic applications. Chem. Eur. J. 2013, 19, 6122–6136. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Meng, X.; Choi, M.M.F.; Gong, X.; Chan, W. Elucidating the structure of carbon nanoparticles by ultra-performance liquid chromatography coupled with electrospray ionisation quadrupole time-of-flight tandem mass spectrometry. Anal. Chim. Acta 2016, 911, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Sancey, L.; Kotb, S.; Truillet, C.; Appaix, F.; Marais, A.; Thomas, E.; van der Sanden, B.; Klein, J.-P.; Laurent, B.; Cottier, M.; et al. Long-Term in Vivo Clearance of Gadolinium-Based AGuIX Nanoparticles and Their Biocompatibility after Systemic Injection. ACS Nano 2015, 9, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Bolsa-Ferruz, M.; Marichal, L.; Porcel, E.; Salado-Leza, D.; Lux, F.; Tillement, O.; Renault, J.P.; Pin, S.; Wien, F.; et al. Human serum albumin in the presence of AGuIX nanoagents: Structure stabilisation without direct interaction. Int. J. Mol. Sci. 2020, 21, 4673. [Google Scholar] [CrossRef]

| Time (min) | m/z | Species | Peak |
|---|---|---|---|
| 1.95 | 303.0 | C6H22N2O5Si22+ + CHO2− | 1 |
| 423.0 | C6H22N2O9Si42+ + CHO2− | ||
| 441.1 | C6H24N2O10Si42+ + CHO2− | ||
| 2.19 | 301.5 | C9H32N3O13Si63+ + CHO2− | 2 |
| 340.5 | C9H34N3O16Si73+ + CHO2− | ||
| 397.5 | C9H40N3O21Si83+ + CHO2− | ||
| 2.41 | 391.7 | C28H56GdN7O21Si62+ + Na+ | 3 |
| 397.7 | C28H58GdN7O22Si62+ + Na+ | ||
| 403.7 | C28H60GdN7O23Si62+ + Na+ | ||
| 587.1 | C28H55GdN7O21Si6+ + Na+ | ||
| 596.1 | C28H57GdN7O22Si6+ + Na+ | ||
| 2.66 | 358.0 | C25H49GdN6O20Si52+ + Na+ | 4 |
| 364.0 | C25H51GdN6O21Si52+ + Na+ | ||
| 370.0 | C25H53GdN6O22Si52+ + Na+ | ||
| 536.5 | C25H48GdN6O20Si5+ + Na+ | ||
| 545.6 | C25H50GdN6O21Si5+ + Na+ | ||
| 554.6 | C25H52GdN6O22Si5+ + Na+ | ||
| 3.21 | 330.4 | C22H44GdN5O20Si42+ + Na+ | 5 |
| 364.0 | C25H51GdN6O21Si52+ + Na+ | ||
| 486.0 | C22H41GdN5O19Si4+ + Na+ | ||
| 495.0 | C22H43GdN5O20Si4+ + Na+ | ||
| 506.8 | C47H87Gd2N11O38Si92+ +2 Na+ | ||
| 515.5 | C47H91Gd2N11O40Si92+ + 2 Na+ | ||
| 536.5 | C25H48GdN6O20Si5+ + Na+ | ||
| 545.6 | C25H50GdN6O21Si5+ + Na+ | ||
| 971.1 | C22H40GdN5O19Si4 + Na+ | ||
| 989.1 | C22H42GdN5O20Si4 + Na+ | ||
| 3.65 | 367.3 | C31H72GdN8O30Si93+ + Na+ | 6 |
| 394.2 | C65H157Gd2N17O66Si206+ + 2 Na+ | ||
| 425.5 | C34H87GdN9O37Si113+ + Na+ | ||
| 724.6 | C31H68GdN8O29Si9+ + Na+ | ||
| 733.6 | C31H70GdN8O30Si9+ + Na+ | ||
| 742.6 | C31H72GdN8O31Si9+ + Na+ | ||
| 751.6 | C31H74GdN8O32Si9+ + Na+ | ||
| 760.6 | C31H76GdN8O33Si9+ + Na+ | ||
| 850.1 | C34H85GdN9O37Si11+ + Na+ | ||
| 4.05 | 316.6 | C19H31GdN4O102+ | 7 |
| 632.1 | C19H30GdN4O10+ | ||
| 650.1 | C19H30GdN4O10+ + H2O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocchi, P.; Labied, L.; Doussineau, T.; Julien, M.; Giroud, B.; Vulliet, E.; Randon, J.; Tillement, O.; Hagège, A.; Lux, F. Identification of Molecular Fragments in Equilibrium with Polysiloxane Ultrasmall Nanoparticles. Nanomaterials 2022, 12, 738. https://doi.org/10.3390/nano12050738
Rocchi P, Labied L, Doussineau T, Julien M, Giroud B, Vulliet E, Randon J, Tillement O, Hagège A, Lux F. Identification of Molecular Fragments in Equilibrium with Polysiloxane Ultrasmall Nanoparticles. Nanomaterials. 2022; 12(5):738. https://doi.org/10.3390/nano12050738
Chicago/Turabian StyleRocchi, Paul, Lucie Labied, Tristan Doussineau, Michel Julien, Barbara Giroud, Emmanuelle Vulliet, Jérôme Randon, Olivier Tillement, Agnès Hagège, and François Lux. 2022. "Identification of Molecular Fragments in Equilibrium with Polysiloxane Ultrasmall Nanoparticles" Nanomaterials 12, no. 5: 738. https://doi.org/10.3390/nano12050738
APA StyleRocchi, P., Labied, L., Doussineau, T., Julien, M., Giroud, B., Vulliet, E., Randon, J., Tillement, O., Hagège, A., & Lux, F. (2022). Identification of Molecular Fragments in Equilibrium with Polysiloxane Ultrasmall Nanoparticles. Nanomaterials, 12(5), 738. https://doi.org/10.3390/nano12050738






