A Micro Electrochemical Sensor for Multi-Analyte Detection Based on Oxygenated Graphene Modified Screen-Printed Electrode
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Solutions
2.2. Apparatus
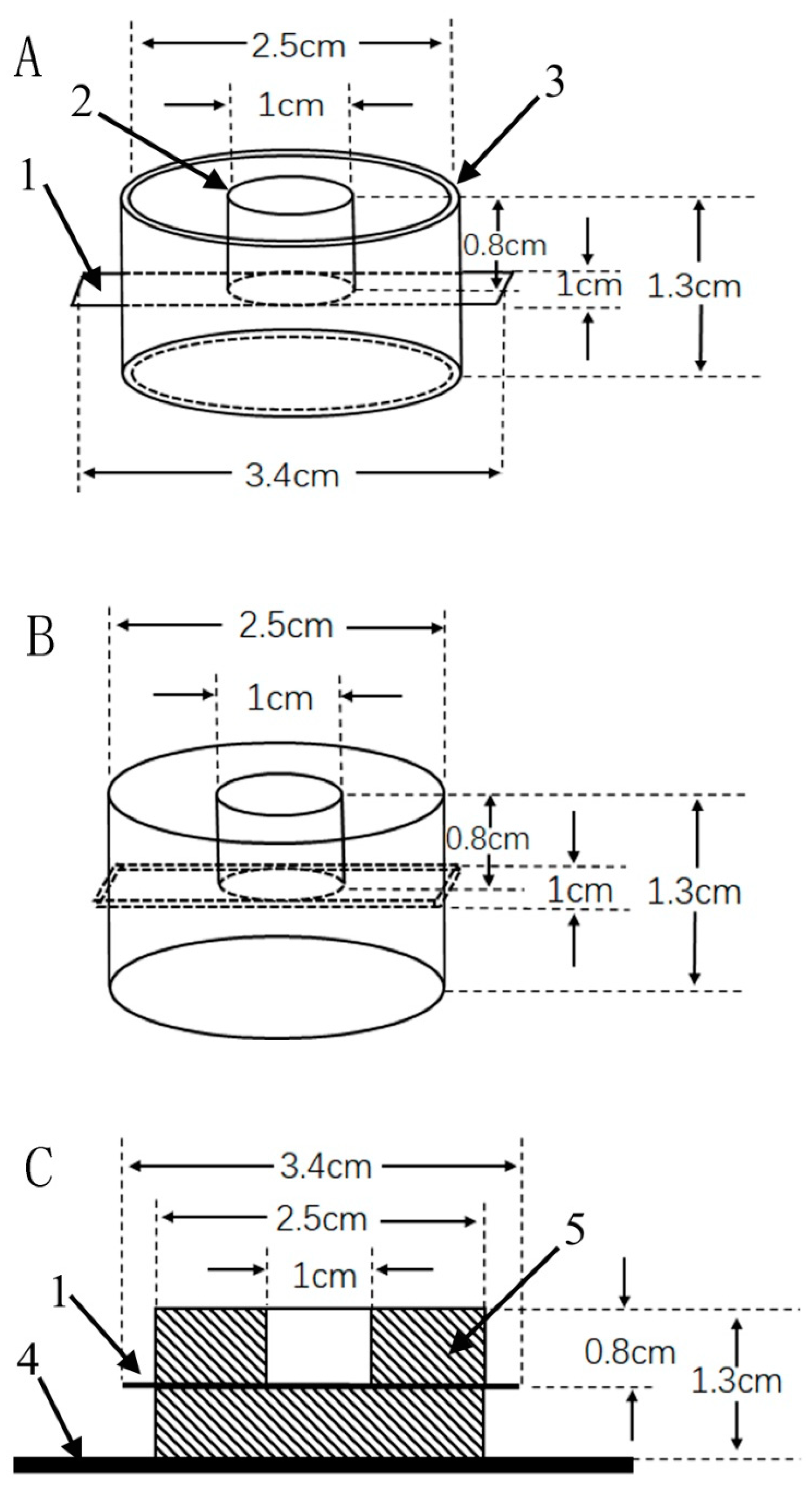
2.3. Electrode Preparation and Modification
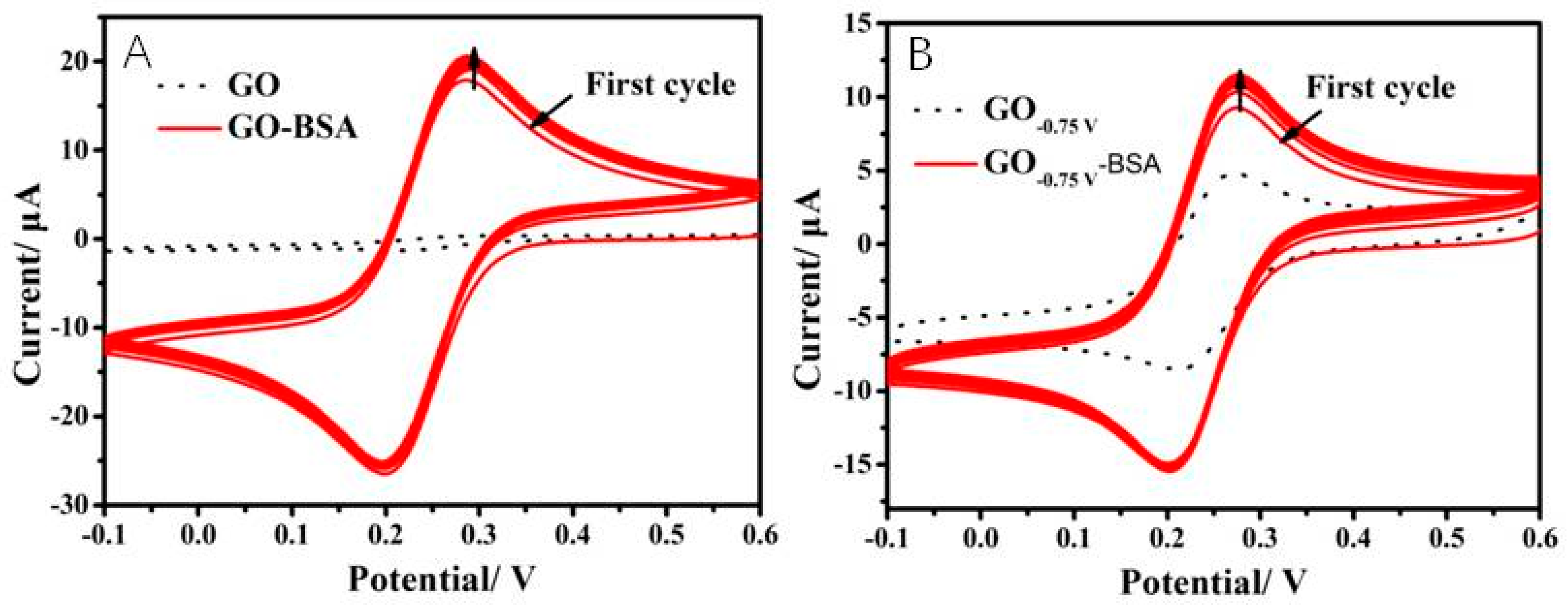
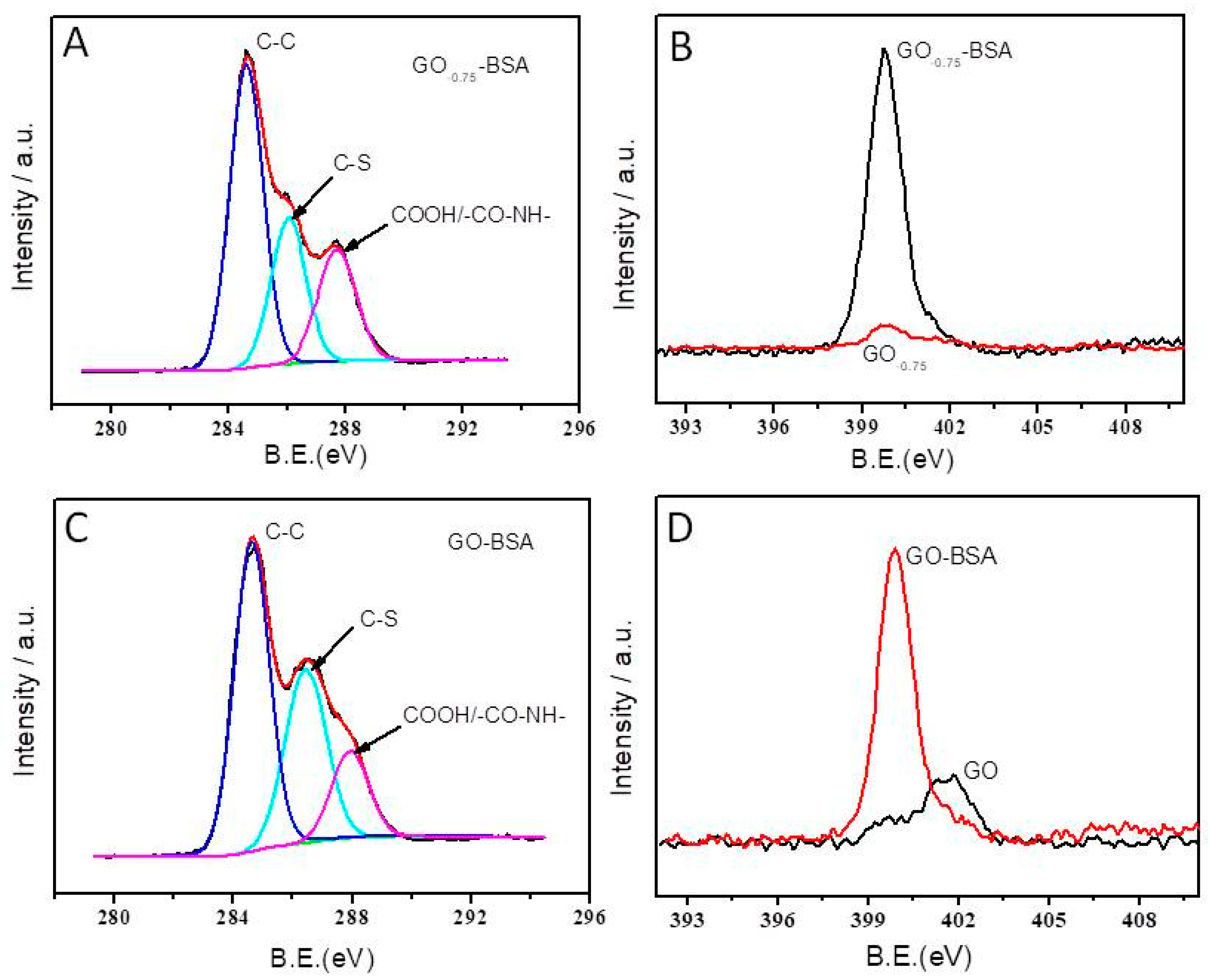
2.4. Interaction between Electrode and Proteins
2.5. Serum Sample Analysis for UA
3. Results and Discussion
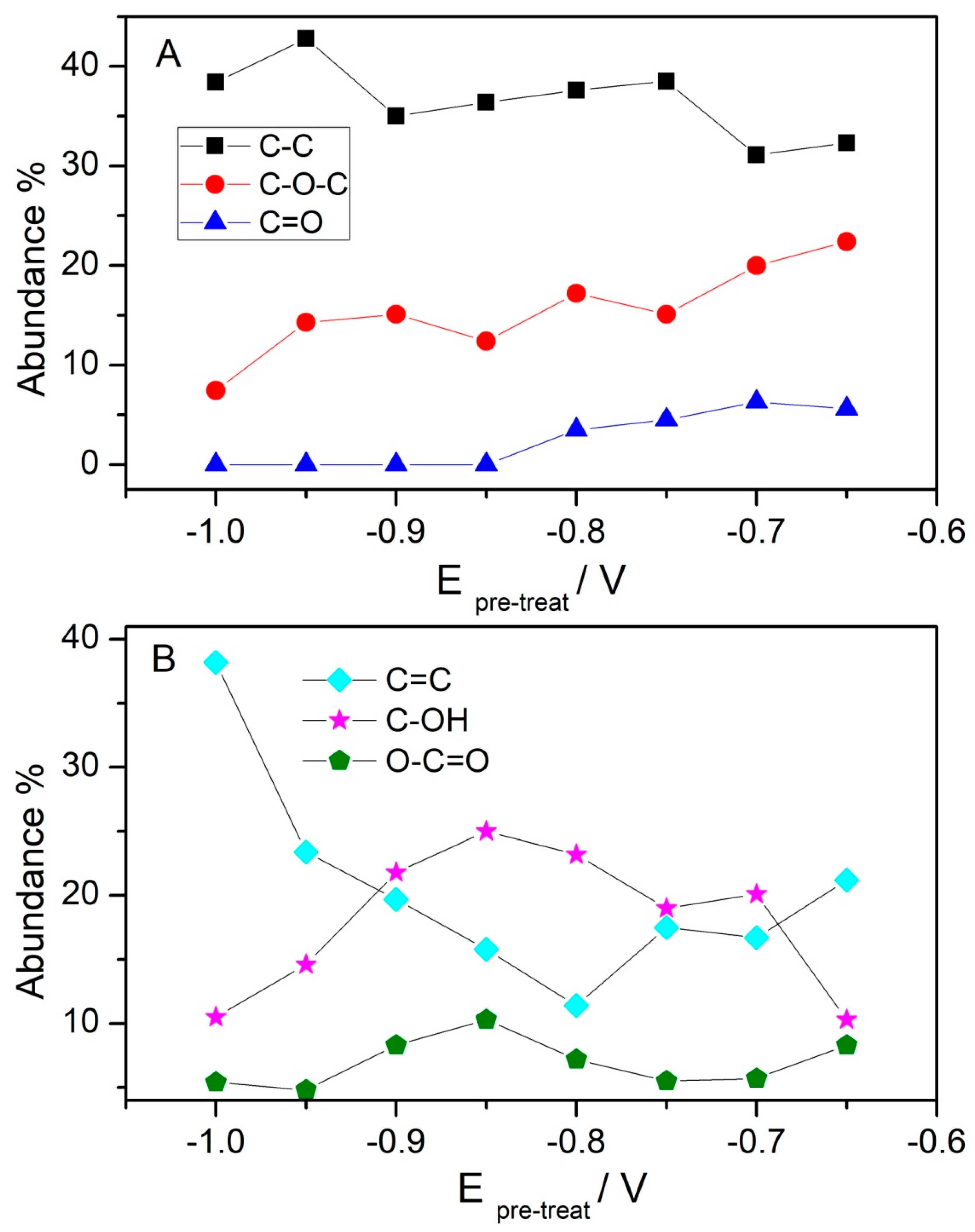
3.1. XPS Characterization of the Oxo Functionalities Present on GOpotential
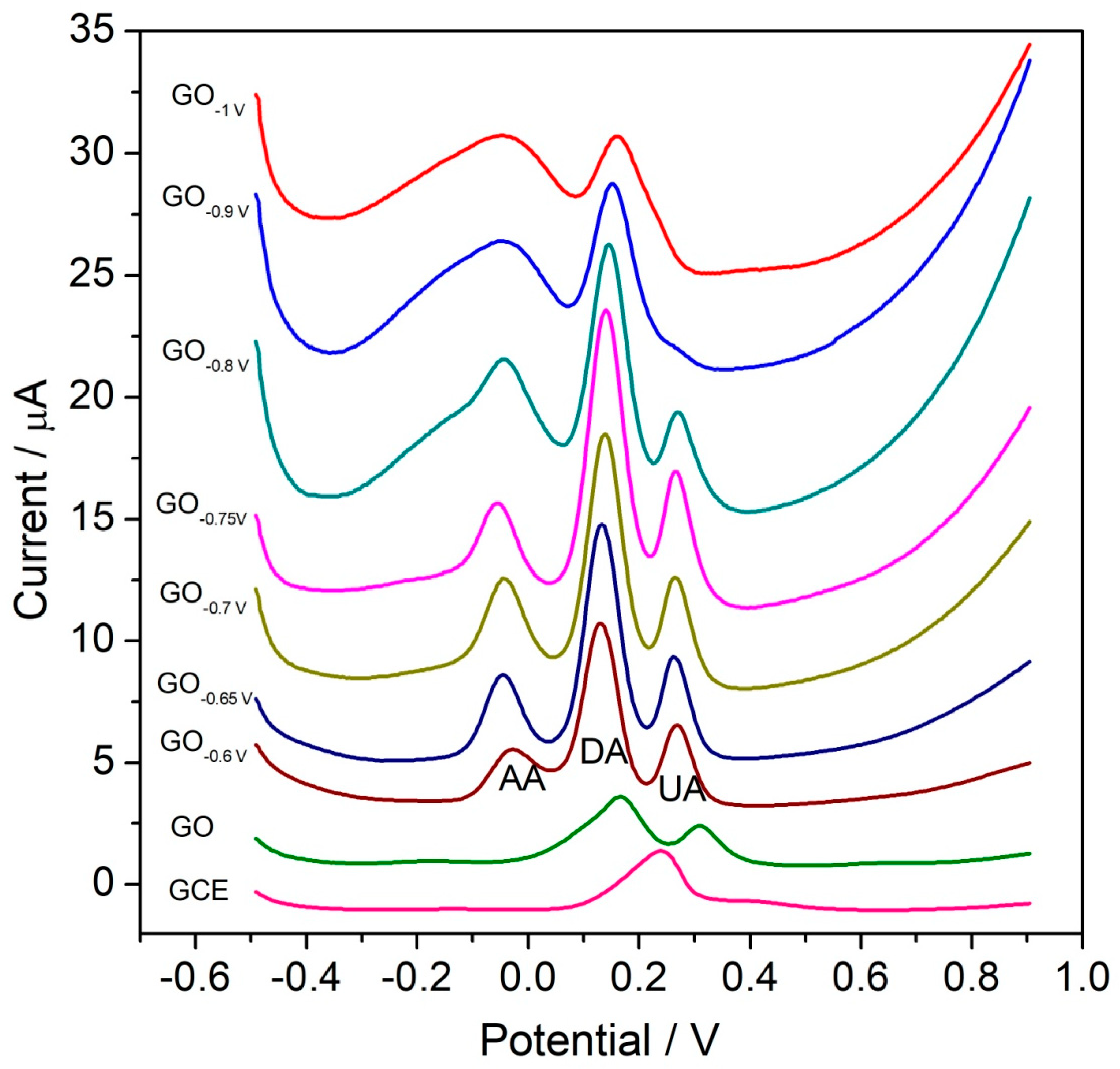
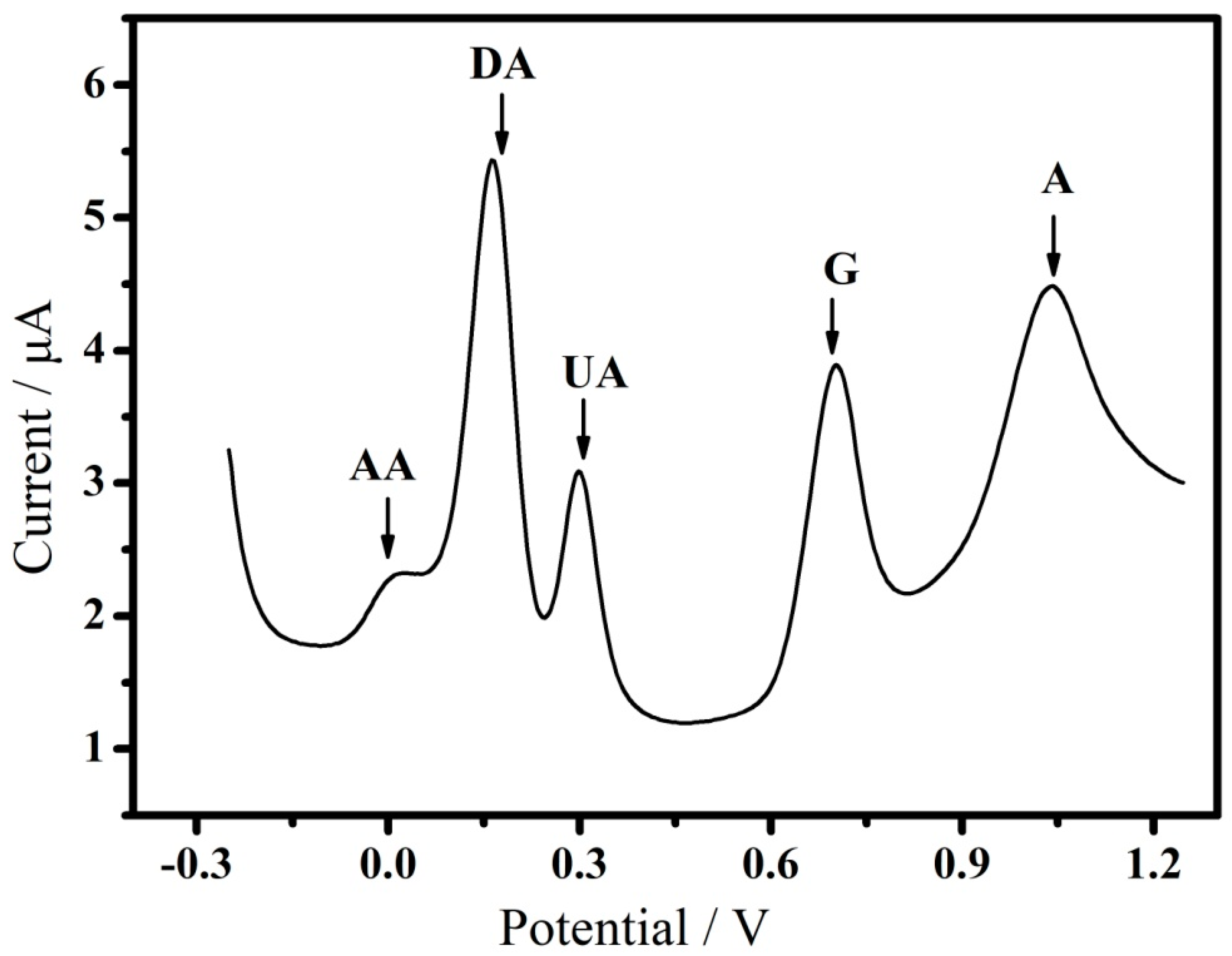
3.2. Electrochemical Sensing of Multiple-Analyte
3.3. Antibiofouling Property
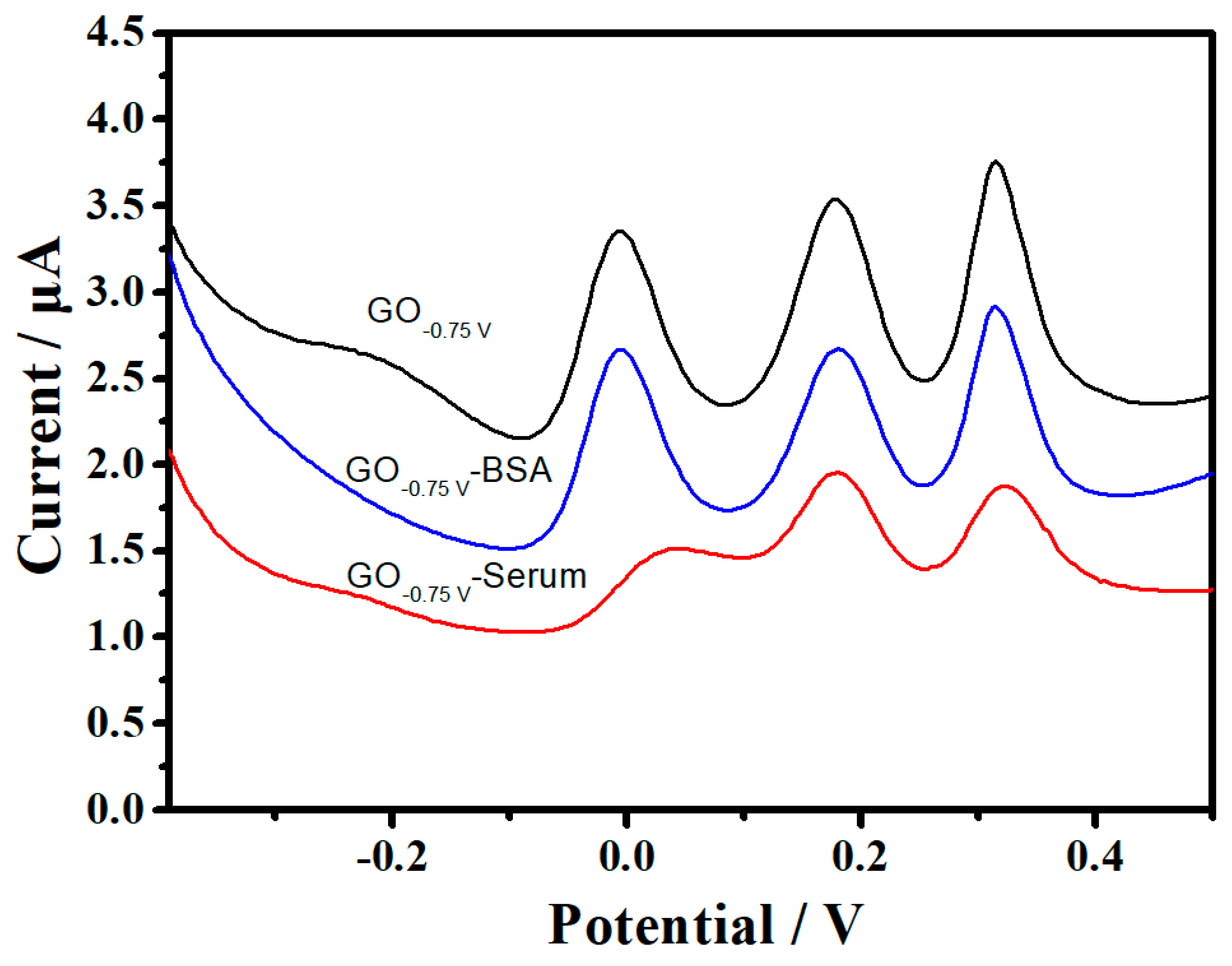
3.4. Analysis of UA in Serum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PB | Prussian blue |
| TiN-rGO | titanium nitride-reduced graphene oxide |
| N-rGO | nitrogen-doped reduced graphene oxide |
| PVP | polyvinylpyrrolidone |
| GR | graphene |
| h-BN | hexagonal boron nitride |
| PrGO/MnO2 | poly(3,4-ethylenedioxythiophene)/reduced graphene oxide/manganese dioxide |
| AuNPs | gold nanoparticles |
| MNP | magnetic nanoparticles |
| ERGO-pEBT | electrochemically reduced graphene oxide-poly(eriochrome black T) |
| HCONS | hexagonal cobalt oxide nanosheets |
| Ni@CHS/rGO | Ni@carbon hollow nanosphere/reduced graphene oxide |
| PGE | pencil graphite electrode |
| PPyox | polypyrrole |
| p-GLY/GO | poly(glycine)/graphene oxide composite based sensor |
| CQD/PAPox | carbon quantum dots (CQDs) and overoxidized poly(2-aminopyridine) (PAPox) |
| βCD/IRGO | β-cyclodextrin functionalized reduced graphite oxide with the aid of ionic liquid |
| CTME | carbon-thread microelectrode |
References
- Del Río, J.S.; Henry, O.Y.; Jolly, P.; Ingber, D.E. An antifouling coating that enables affinity-based electrochemical biosensing in complex biological fluids. Nat. Nanotechnol. 2019, 14, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, G.; Hein, R.; Liu, N.; Luo, X.; Davis, J.J. Antifouling strategies for selective in vitro and in vivo sensing. Chem. Rev. 2020, 120, 3852–3889. [Google Scholar] [CrossRef] [PubMed]
- Barfidokht, A.; Gooding, J.J. Approaches toward allowing electroanalytical devices to be used in biological fluids. Electroanalysis 2014, 26, 1182–1196. [Google Scholar] [CrossRef]
- Russo, M.J.; Han, M.; Desroches, P.E.; Manasa, C.S.; Dennaoui, J.; Quigley, A.F.; Kapsa, R.M.I.; Moulton, S.E.; Guijt, R.M.; Greene, G.W.; et al. Antifouling strategies for electrochemical biosensing: Mechanisms and performance toward point of care based diagnostic applications. ACS Sens. 2021, 6, 1482–1507. [Google Scholar] [CrossRef]
- Silva, T.A.; Khan, M.R.K.; Fatibello-Filho, O.; Collinson, M.M. Simultaneous electrochemical sensing of ascorbic acid and uric acid under biofouling conditions using nanoporous gold electrodes. J. Electroanal. Chem. 2019, 846, 113160. [Google Scholar] [CrossRef]
- Brisebois, P.P.; Siaj, M. Harvesting graphene oxide–years 1859 to 2019: A review of its structure, synthesis, properties and exfoliation. J. Mater. Chem. C 2020, 8, 1517–1547. [Google Scholar] [CrossRef]
- Kim, J.; Cote, L.J.; Kim, F.; Yuan, W.; Shull, K.R.; Huang, J. Graphene oxide sheets at interfaces. J. Am. Chem. Soc. 2010, 132, 8180–8186. [Google Scholar] [CrossRef]
- Joshi, D.J.; Koduru, J.R.; Malek, N.I.; Hussain, C.M.; Kailasa, S.K. Surface modifications and analytical applications of graphene oxide: A review. Trac-Trend. Anal. Chem. 2021, 144, 116448. [Google Scholar] [CrossRef]
- Agarwal, V.; Zetterlund, P.B. Strategies for reduction of graphene oxide–A comprehensive review. Chem. Eng. J. 2021, 405, 127018. [Google Scholar] [CrossRef]
- Lavin-Lopez, M.D.P.; Paton-Carrero, A.; Sanchez-Silva, L.; Valverde, J.L.; Romero, A. Influence of the reduction strategy in the synthesis of reduced graphene oxide. Adv. Powder Technol. 2017, 28, 3195–3203. [Google Scholar] [CrossRef] [Green Version]
- Renteria, J.A.Q.; Ruiz-Garcia, C.; Sauvage, T.; Chazaro-Ruiz, L.F.; Rangel-Mendez, J.R.; Ania, C.O. Photochemical and electrochemical reduction of graphene oxide thin films: Tuning the nature of surface defects. Phys. Chem. Chem. Phys. 2020, 22, 20732–20743. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Ross, M.B.; Kornienko, N.; Zhang, L.; Guo, J.; Yang, P.; McCloskey, B.D. Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts. Nat. Catal. 2018, 1, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Tahini, H.A.; Smith, S.C. Understanding the high activity of mildly reduced graphene oxide electrocatalysts in oxygen reduction to hydrogen peroxide. Mater. Horiz. 2019, 6, 1409–1415. [Google Scholar] [CrossRef]
- Kim, H.W.; Park, H.; Roh, J.S.; Shin, J.E.; Lee, T.H.; Zhang, L.; McCloskey, B.D. Carbon defect characterization of nitrogen-doped reduced graphene oxide electrocatalysts for the two-electron oxygen reduction reaction. Chem. Mater. 2019, 31, 3967–3973. [Google Scholar] [CrossRef]
- Yuan, B.; Zeng, X.; Xu, C.; Liu, L.; Ma, Y.; Zhang, D.; Fan, Y. Electrochemical modification of graphene oxide bearing different types of oxygen functional species for the electro-catalytic oxidation of reduced glutathione. Sens. Actuators B-Chem. 2013, 184, 15–20. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, C.; Li, S.; Zhang, R.; Yan, H.; Miao, H.; Yong, F.; Yuan, B. Electrochemically controlling oxygen functional groups in graphene oxide for the optimization in the electro-catalytic oxidation of dihydroxybenzene isomers and L-methionine. J. Electroanal. Chem. 2014, 717, 219–224. [Google Scholar] [CrossRef]
- Uhm, S.; Tuyen, N.H.; Lee, J. Controlling oxygen functional species of graphene oxide for an electro-oxidation of L-ascorbic acid. Electrochem. Commun. 2011, 13, 677–680. [Google Scholar] [CrossRef]
- Hui, K.H.; Ambrosi, A.; Pumera, M.; Bonanni, A. Improving the analytical performance of graphene oxide towards the assessment of polyphenols. Chem.-Eur. J. 2016, 22, 3830–3834. [Google Scholar] [CrossRef]
- Maccaferri, G.; Zanardi, C.; Xia, Z.Y.; Kovtun, A.; Liscio, A.; Teizi, F.; Palermo, V.; Seeber, R. Systematic study of the correlation between surface chemistry, conductivity and electrocatalytic properties of graphene oxide nanosheets. Carbon 2017, 120, 165–175. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, S.; Lin, X.; Nie, L.; Rui, L. Selective determination of uric acid by using a β-cyclodextrin modified electrode. Electroanalysis 2001, 13, 1351–1354. [Google Scholar] [CrossRef]
- Zen, J.M.; Chen, P.J. A selective voltammetric method for uric acid and dopamine detection using clay-modified electrodes. Anal. Chem. 1997, 69, 5087–5093. [Google Scholar] [CrossRef]
- Dos Santos, P.L.; Katic, V.; Toledo, K.C.F.; Bonacin, J.A. Photochemical one-pot synthesis of reduced graphene oxide/Prussian blue nanocomposite for simultaneous electrochemical detection of ascorbic acid, dopamine, and uric acid. Sens. Actuators B-Chem. 2018, 255, 2437–2447. [Google Scholar] [CrossRef]
- Feng, J.; Li, Q.; Cai, J.P.; Yang, T.; Chen, J.H.; Hou, X.M. Electrochemical detection mechanism of dopamine and uric acid on titanium nitride-reduced graphene oxide composite with and without ascorbic acid. Sens. Actuators B-Chem. 2019, 298, 126872. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Feng, J.; Xiao, J.; Li, J.; Liu, J.; Li, G.; He, Q. Simultaneous and sensitive determination of ascorbic acid, dopamine and uric acid via an electrochemical sensor based on PVP-graphene composite. J. Nanobiotechnol. 2020, 18, 112. [Google Scholar] [CrossRef]
- Li, Q.; Huo, C.R.; Yi, K.; Zhou, L.L.; Su, L.; Hou, X.M. Preparation of flake hexagonal BN and its application in electrochemical detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B-Chem. 2018, 260, 346–356. [Google Scholar] [CrossRef]
- Tukimin, N.; Abdullah, J.; Sulaiman, Y. Electrodeposition of poly(3,4-ethylenedioxythiophene)/reduced graphene oxide/manganese dioxide for simultaneous detection of uric acid, dopamine and ascorbic acid. J. Electroanal. Chem. 2018, 820, 74–81. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Zang, Y.; Ruan, S.J.; Zhang, Y.Y.; Gao, P.P. A high efficiency N, P doped porous carbon nanoparticles derived from lotus leaves for simultaneous electrochemical determination of ascorbic acid, dopamine, and uric acid. Microchem. J. 2021, 165, 106152. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Liu, S. Electrochemical sensors based on nitrogen-doped reduced graphene oxide for the simultaneous detection of ascorbic acid, dopamine and uric acid. J. Alloys Compd. 2020, 842, 155873. [Google Scholar] [CrossRef]
- Lee, C.S.; Yu, S.H.; Kim, T.H. One-step electrochemical fabrication of reduced graphene oxide/gold nanoparticles nanocomposite-modified electrode for simultaneous detection of dopamine, ascorbic acid, and uric acid. Nanomaterials 2017, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Rosli, A.R.M.; Yusoff, F.; Loh, S.H.; Yusoff, H.M.; Jamil, M.M.A.; Shamsudin, S.H. Simultaneous electrochemical detection of ascorbic acid, dopamine, and uric acid at magnetic nanoparticles/reduced graphene oxide modified electrode. J. Teknol. 2021, 83, 85–92. [Google Scholar] [CrossRef]
- Mohammed, N.; Abdullah, J.; Kamaruzaman, S.; Saiman, M.; Sulaiman, Y. Electrochemical reduced graphene oxide-poly(eriochrome black T)/gold nanoparticles modified glassy carbon electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. Arab. J. Chem. 2018, 11, 1301–1312. [Google Scholar] [CrossRef]
- Minta, D.; Gonzalez, Z.; Wiench, P.; Gryglewicz, S.; Gryglewicz, G. N-Doped reduced graphene oxide/gold nanoparticles composite as an improved sensing platform for simultaneous detection of dopamine, ascorbic acid, and uric acid. Sensors 2020, 20, 4427. [Google Scholar] [CrossRef] [PubMed]
- Zahed, M.A.; Barman, S.C.; Toyabur, R.M.; Sharifuzzaman, M.; Xuan, X.; Nah, J.; Park, J.Y. Ex situ hybridized hexagonal cobalt oxide nanosheets and RGO@MWCNT based nanocomposite for ultra-selective electrochemical detection of ascorbic acid, dopamine, and uric Acid. J. Electrochem. Soc. 2019, 166, B304–B311. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J. 3D hierarchical bayberry-like Ni@carbon hollow nanosphere/rGO hybrid as a new interesting electrode material for simultaneous detection of small biomolecules. Talanta 2018, 178, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, N.; Badhulika, S. Single step grown MoS2 on pencil graphite as an electrochemical sensor for guanine and adenine: A novel and low cost electrode for DNA studies. Biosens. Bioelectron. 2018, 124, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.T.; Bao, J.; Huang, T.; Wang, X.J.; Hou, C.J.; Hou, J.Z.; Samalo, M.; Yang, M.; Huo, D.Q. Electrochemical Sensor for the Simultaneous Detection of Guanine and Adenine Based on a PPyox/MWNTs-MoS2 Modified Electrode. J. Electrochem. Soc. 2019, 166, B498–B504. [Google Scholar] [CrossRef]
- Wang, D.; Huang, B.; Liu, J.; Guo, X.; Abudukeyoumu, G.; Zhang, Y.; Ye, B.C.; Li, Y. A novel electrochemical sensor based on Cu@Ni/MWCNTs nanocomposite for simultaneous determination of guanine and adenine. Biosens. Bioelectron. 2018, 102, 389–395. [Google Scholar] [CrossRef]
- Arvand, M.; Sanayeei, M.; Hemmati, S. Label-free electrochemical DNA biosensor for guanine and adenine by ds-DNA/poly(L-cysteine)/Fe3O4 nanoparticles-graphene oxide nanocomposite modified electrode. Biosens. Bioelectron. 2018, 102, 70–79. [Google Scholar] [CrossRef]
- He, S.; He, P.; Zhang, X.; Zhang, X.; Liu, K.; Jia, L.; Dong, F. Poly(glycine)/graphene oxide modified glassy carbon electrode: Preparation, characterization and simultaneous electrochemical determination of dopamine, uric acid, guanine and adenine. Anal. Chim. Acta 2018, 1031, 75–82. [Google Scholar] [CrossRef]
- Zhang, S.; Zhuang, X.; Chen, D.; Luan, F.; He, T.; Tian, C.; Chen, L. Simultaneous voltammetric determination of guanine and adenine using MnO2 nanosheets and ionic liquid-functionalized graphene combined with a permeation-selective polydopamine membrane. Microchim. Acta 2019, 186, 1–10. [Google Scholar] [CrossRef]
- Siddegowda, K.; Mahesh, N.B.; Kumara, S. Fabrication of copper oxide nanoparticles modified carbon paste electrode and its application in simultaneous electroanalysis of guanine, adenine and thymine. Sens. Actuators A-Phys. 2018, 280, 277–286. [Google Scholar]
- Zhao, X.; Guo, H.; Xue, R.; Wang, M.Y.; Guan, Q.X.; Fan, T.; Yang, W.H.; Yang, W. Electrochemical sensing and simultaneous determination of guanine and adenine based on covalent organic frameworks/NH2-rG/MoS2 modified glassy carbon electrode. Microchem. J. 2021, 160, 105759. [Google Scholar] [CrossRef]
- Gao, S.H.; Li, H.J.; Li, M.J.; Li, C.P.; Qian, L.R.; Yang, B.H. A gold-nanoparticle/horizontal-graphene electrode for the simultaneous detection of ascorbic acid, dopamine, uric acid, guanine, and adenine. J.Solid State Electrochem. 2018, 22, 3245–3254. [Google Scholar] [CrossRef]
- He, S.; He, P.; Zhang, X.; Zhang, X.; Dong, F.; Jia, L.; Du, L.; Lei, H. Simultaneous voltammetric determination of guanine and adenine by using a glassy carbon electrode modified with a composite consisting of carbon quantum dots and overoxidized poly(2-aminopyridine). Microchim. Acta 2018, 185, 107. [Google Scholar] [CrossRef]
- Aktürk, M.; Karabiberoğlu, S.U.; Dursun, Z. Fabrication of Cu−CeO2 coated multiwall carbon nanotube composite electrode for simultaneous determination of guanine and adenine. Electroanalysis 2018, 30, 238–249. [Google Scholar] [CrossRef]
- Mahanthappa, M.; Kottam, N.; Yellappa, S. Electrocatalytic performance of a zinc sulphide nanoparticles-modified carbon paste electrode for the simultaneous determination of acetaminophen, guanine and adenine. Anal. Methods 2018, 10, 1362–1371. [Google Scholar] [CrossRef]
- Wang, M.L.; Cui, M.Z.; Liu, W.F.; Liu, X.G.; Xu, B.S. Facile Synthesis of Cyclodextrin Functionalized Reduced Graphite Oxide with the Aid of Ionic Liquid for Simultaneous Determination of Guanine and Adenine. Electroanalysis 2018, 30, 842–851. [Google Scholar] [CrossRef]
- Zhang, N.; Zhong, W.L.; Zhang, Z.Q.; Fan, Y.W. Electrochemical behaviours of guanine and adenine and their simultaneous determination using a three-dimensional porous poly(dopamine)/Reduced Graphene Oxide-Modified Electrode. Int. J. Electrochem. Sci. 2020, 15, 5287–5295. [Google Scholar] [CrossRef]
- Kim, I.; Im Kim, Y.; Lee, S.W.; Jung, H.G.; Lee, G.; Yoon, D.S. Highly permselective uric acid detection using kidney cell membrane–functionalized enzymatic biosensors. Biosens. Bioelectron. 2021, 190, 113411. [Google Scholar] [CrossRef]
- Lee, S.J.; Oh, B.K.; Sung, K.C. Uric acid and cardiometabolic diseases. Clin. Hypertens. 2020, 26, 1–7. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, K.; Wu, Z.; Dong, H.; Sun, H.; Cheng, X.; Zhang, H.; Zhou, H.; Jia, C.; Jin, Q.; et al. Investigation of controllable nanoscale heat-denatured bovine serum albumin films on graphene. Langmuir 2016, 32, 12623–12631. [Google Scholar] [CrossRef] [PubMed]
- Casero, E.; Parra-Alfambra, A.M.; Petit-Domínguez, M.D.; Pariente, F.; Lorenzo, E.; Alonso, C. Differentiation between graphene oxide and reduced graphene by electrochemical impedance spectroscopy (EIS). Electrochem. Commun. 2012, 20, 63–66. [Google Scholar] [CrossRef]
- Makarska-Bialokoz, M.; Lipke, A. Study of the binding interactions between uric acid and bovine serum albumin using multiple spectroscopic techniques. J. Mol. Liq. 2019, 276, 595–604. [Google Scholar] [CrossRef]


| Electrocatalysts | Linear Range | Detection Limit | pH | Samples | Ref. |
|---|---|---|---|---|---|
| PrGO/PB | AA: 34.7 μM DA: 26.2 μM UA: 8.0 μM | 7.4 | [22] | ||
| TiN-rGO | AA: 50–100 μM DA: 5–175 μM UA: 30–215 μM | AA: DA: 0.159 μM UA: 0.350 μM | 7.0 | spiked in urine | [23] |
| PVP-GR | AA: 4.0–1000 μM DA: 0.02–100 μM UA: 0.04–100 μM | AA: 0.8 μM DA: 0.002 μM UA: 0.02 μM | 6.0 | urine | [24] |
| h-BN | AA: 30–1000 μM DA: 0.5–150 μM UA: 1–300 μM | AA: 3.77 μM DA: 0.02 μM UA: 0.15 μM | 6.0 | spiked in urine | [25] |
| PrGO/MnO2 | AA: 1–800 μM DA: 0.03–45 μM UA: 0.3–80 μM | AA: 1.00 μM DA: 0.02 μM UA: 0.05 μM | 6.0 | spiked in urine | [26] |
| CNP | AA: 20–250 μM DA: 10–480 μM UA: 25–2500 μM | AA: 4.25 μM DA: 0.86 μM UA: 0.20 μM | 7.0 | spiked in serum | [27] |
| N-rGO | AA: 0.1–4 μM DA: 1–60 μM UA: 1–30 μM | AA: 9.6 μM DA: 0.1 μM UA: 0.2 μM | 7.0 | [28] | |
| RGO/AuNPs | AA: 9.5 μM DA: 0.14 μM UA: 25 μM | 7.4 | [29] | ||
| MNP/rGO/GCE | AA: 10–100 μM DA: 15 nm–100 μM UA: 10–100 μM | AA: 0.22 μM DA: 0.19 nM UA: 45 nM | [30] | ||
| ERGO-pEBT | AA: 10–900 μM DA: 0.5–20 μM UA: 2–70 μM | AA: 0.53 μM DA: 0.009 μM UA: 0.046 μM | 6.0 | urine | [31] |
| N-rGO | AA: 550–1500 µM DA: 3–100 µM UA: 20–1000 µM | AA:58 μM DA: 2.4 μM UA: 8.7 μM | 7.4 | [32] | |
| HCONS | AA: 12.5–1382 μM DA: 1.6–23.6 μM UA: 46.5–806.5 μM | AA: 12.5 μM DA: 0.012 μM UA: 1.5 μM | 7.4 | serum | [33] |
| Ni@CHS/rGO | AA: 2–4000 μM DA: 0.25–126 μM UA: 0.25–126 μM | AA: 0.37 μM DA: 0.05 μM UA: 0.05 μM | 7.0 | spiked in urine | [34] |
| MoS2-PGE | G: 15–120 μM A: 15–120 μM | G:0.76 μM A:2.38 μM | 7.4 | [35] | |
| PPyox/MWNTs-MoS2/GCE | G: 5–30 μM A: 30–120 μM | G: 1.6 μM A: 1.7 μM | 7.0 | salmon sperm DNA | [36] |
| Cu@Ni/MWCNTs | G: 5–180 μM A: 8–150 μM | G: 0.35 μM A: 0.56 μM | 4.0 | dsDNA from brain tissue of mice | [37] |
| ds-DNA/p(L-Cys)/Fe3O4 NPs-GO/CPE | G: 0.01–25 μM A: 0.01–30 μM | G: 1.59 μM A: 3.48 μM | 6.5 | blood DNA | [38] |
| p-GLY/GO | G: 0.15–48 μM A: 0.09–103 μM | G: 0.026 μM A: 0.03 μM | 3.7 | urine and fish sperm DNA | [39] |
| PDA/MnO2/IL-G | G: 10–300 μM A: 10–300 μM | G: 0.25 μM A: 0.15 μM | 4.0 | mouse whole blood | [40] |
| CuO NPs | G: 1–80 μM A: 1–80 μM | G: 0.687 μM A: 0.472 μM | 7.0 | [41] | |
| COFS/NH2-rG/MoS2 | G: 0.5–150 μM A: 1–280 μM | G: 0.51 μM A: 0.44 μM | 2.0 | herring sperm DNA | [42] |
| Au/HG | G: 6–500 μM A: 0.6–40 μM | G: 2.5 μM A: 0.42 μM | 7.0 | [43] | |
| CQD/PAPox | G: 1–65 μM A: 2–70 μM | G: 0.51 μM A: 0.39 μM | 3.7 | fish sperm DNA | [44] |
| Cu-CeO2/MWCNT/GCE | G: 0.2–6 μM A: 0.1–8 μM | G: 0.128 μM A: 0.062 μM | 7.0 | a calf thymus DNA | [45] |
| ZnS NPs/CPE | G: 1–15 μM A: 15–120 μM | G: 0.036 μM A: 0.031 μM | 7.4 | [46] | |
| βCD/IRGO | G: 0.03–10 μM A: 0.02–7 μM | G: 0.01 μM A: 0.01 μM | 7.0 | [47] | |
| poly(dopamine)/GR | G: 1–58 μM A: 1–58 μM | G: 0.34 μM A: 0.31 μM | 7.0 | a calf thymus DNA | [48] |
| GO−0.75V | DA: 1–100 μM UA: 0.2–20 μM G: 0.5–100 μM A: 2–100 μM | DA: 0.5 μM UA: 0.12 μM G: 0.2 μM A: 0.3 μM | 7.2 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, B.; Gan, L.; Li, G.; Xu, C.; Liu, G. A Micro Electrochemical Sensor for Multi-Analyte Detection Based on Oxygenated Graphene Modified Screen-Printed Electrode. Nanomaterials 2022, 12, 711. https://doi.org/10.3390/nano12040711
Yuan B, Gan L, Li G, Xu C, Liu G. A Micro Electrochemical Sensor for Multi-Analyte Detection Based on Oxygenated Graphene Modified Screen-Printed Electrode. Nanomaterials. 2022; 12(4):711. https://doi.org/10.3390/nano12040711
Chicago/Turabian StyleYuan, Baiqing, Liju Gan, Gang Li, Chunying Xu, and Gang Liu. 2022. "A Micro Electrochemical Sensor for Multi-Analyte Detection Based on Oxygenated Graphene Modified Screen-Printed Electrode" Nanomaterials 12, no. 4: 711. https://doi.org/10.3390/nano12040711
APA StyleYuan, B., Gan, L., Li, G., Xu, C., & Liu, G. (2022). A Micro Electrochemical Sensor for Multi-Analyte Detection Based on Oxygenated Graphene Modified Screen-Printed Electrode. Nanomaterials, 12(4), 711. https://doi.org/10.3390/nano12040711







