Green Nanotechnology: Plant-Mediated Nanoparticle Synthesis and Application
Abstract
:1. Introduction
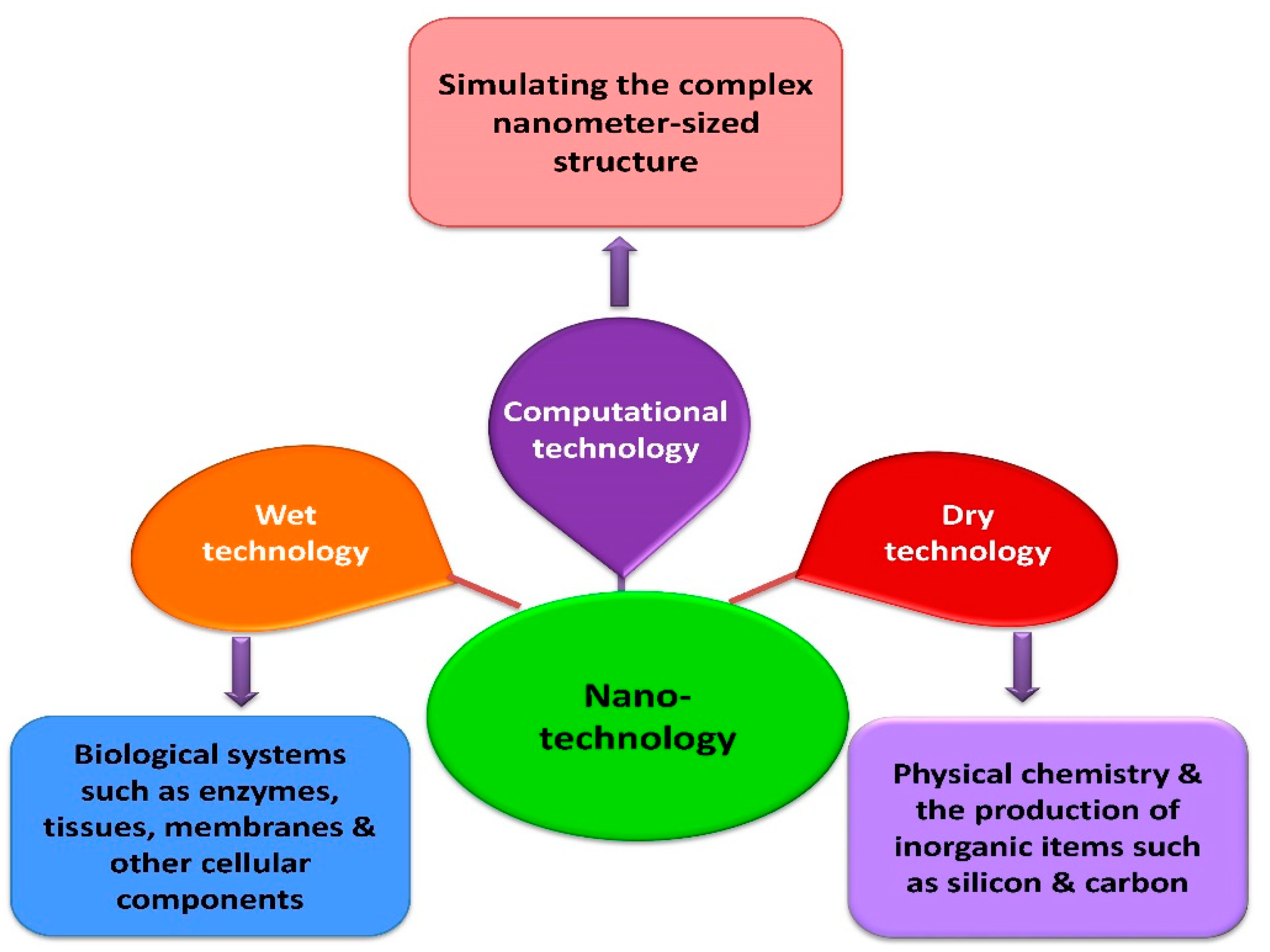
2. Different Types of Nanotechnologies
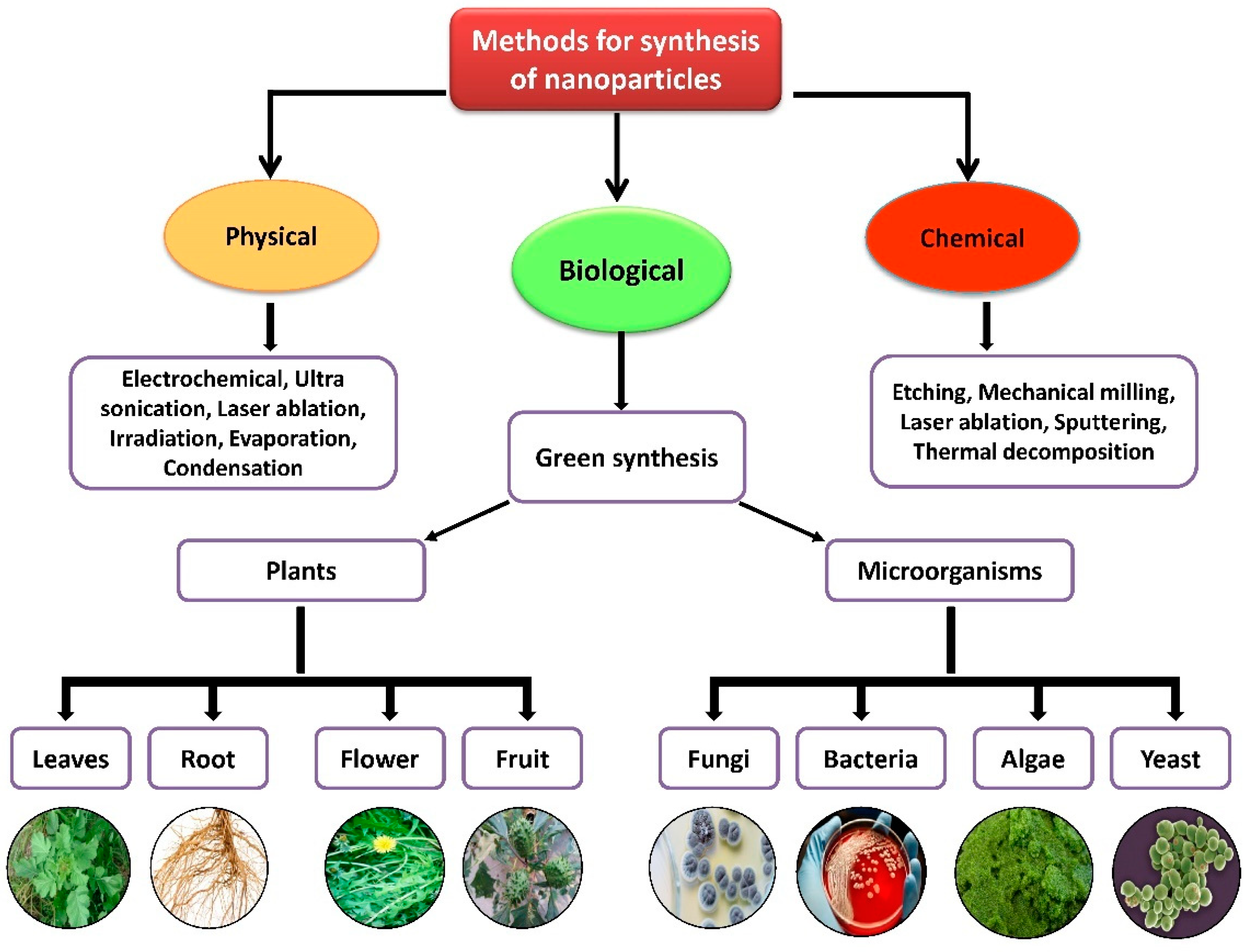
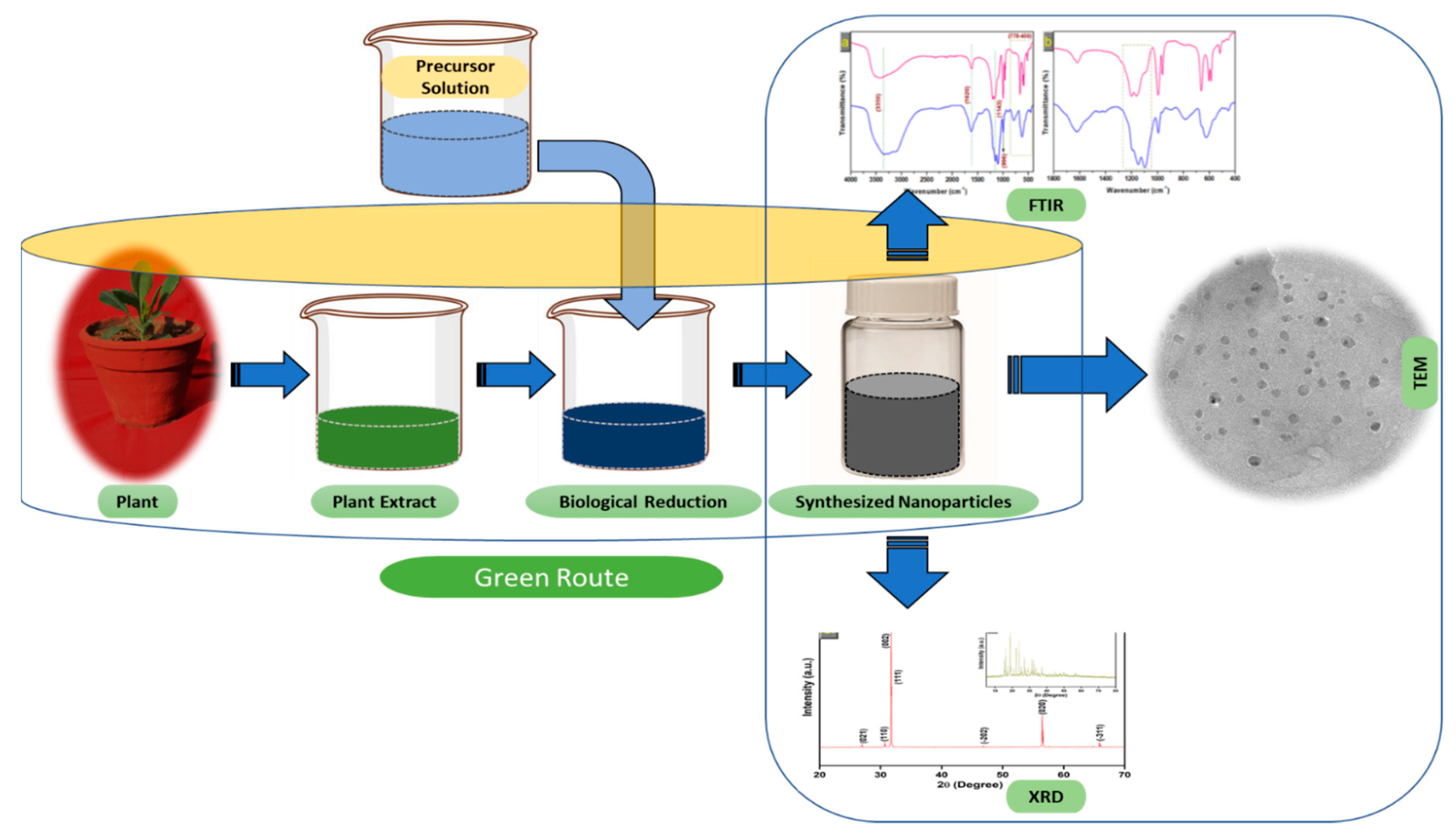
3. Biosynthesis of Novel Metal Nanoparticles Using Plant Extracts
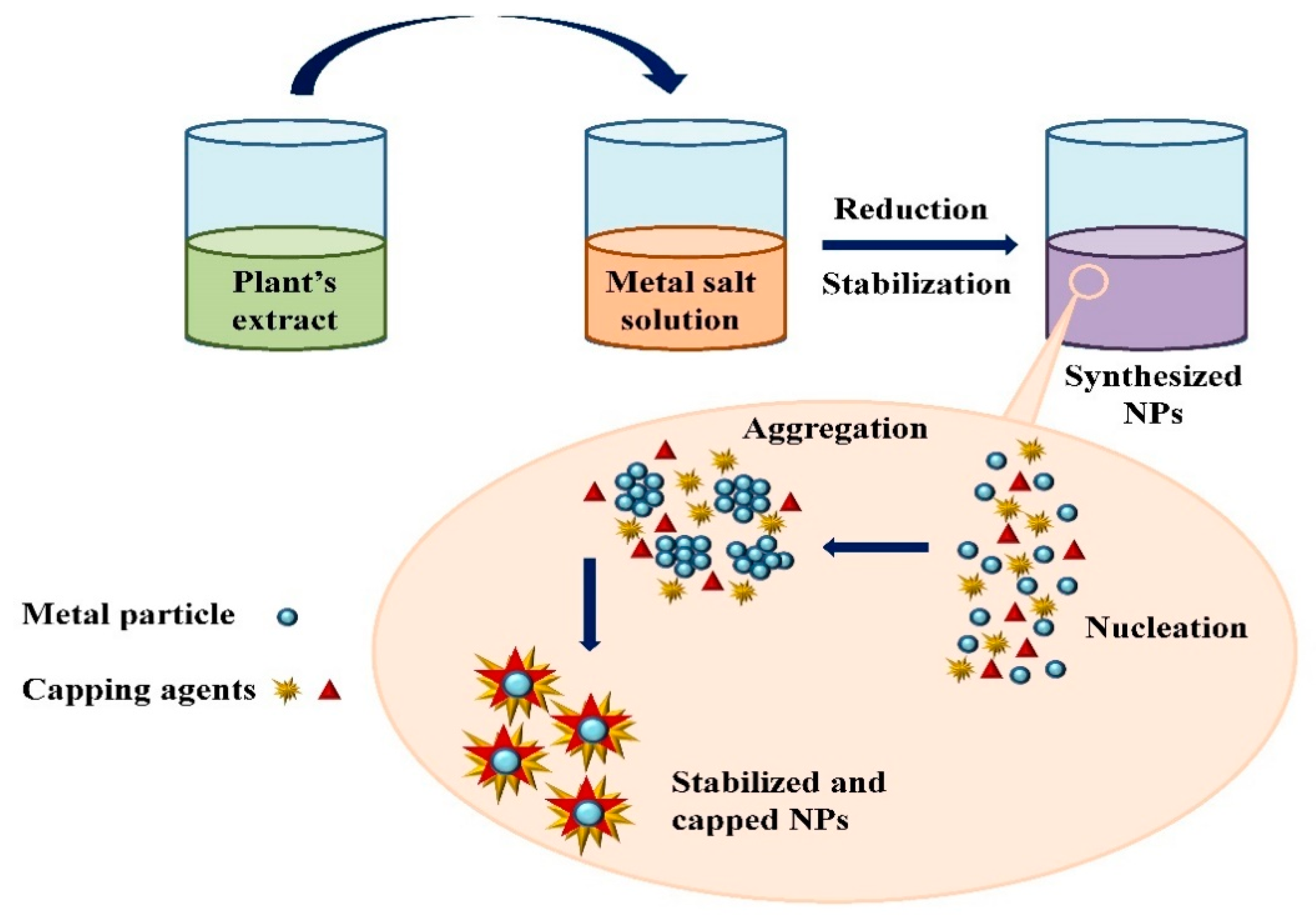
3.1. Mechanism of Nanoparticle Synthesis
3.2. Silver Nanoparticles
3.3. Gold Nanoparticles
3.4. Zinc Nanoparticles
3.5. Titanium Nanoparticles
3.6. Palladium Nanoparticles
4. Factors Affecting Plant-Assisted Synthesis of Nanoparticles
4.1. Effect of pH
4.2. Temperature Role in Plant-Assisted Synthesis
4.3. Contact or Incubation Role in Plant-Assisted Synthesis
5. Application of Nanoparticles
6. Conclusions and Future Roles
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sergeev, G.B.; Shabatina, T.I. Cryochemistry of nanometals. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Korbekandi, H.; Iravani, S.; Abbasi, S. Production of nanoparticles using organisms Production of nanoparticles using organisms. Crit. Rev. Biotechnol. 2009, 29, 279–306. [Google Scholar] [CrossRef]
- Luangpipat, T.; Beattie, I.R.; Chisti, Y.; Haverkamp, R.G. Gold nanoparticles produced in a microalga. J. Nanopart. Res. 2011, 13, 6439–6445. [Google Scholar] [CrossRef]
- Arumugam, A.; Karthikeyan, C.; Haja Hameed, A.S.; Gopinath, K.; Gowri, S.; Karthika, V. Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties. Mater. Sci. Eng. C 2015, 49, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Mehata, M.S. Enhancement of Charge Transfer and Quenching of Photoluminescence of Capped CdS Quantum Dots. Sci. Rep. 2015, 5, 12056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. Nanostructured titanium oxide hybrids-based electrochemical biosensors for healthcare applications. Colloids Surf. B Biointerfaces 2019, 178, 385–394. [Google Scholar] [CrossRef]
- Zhang, M.; Yuan, P.; Zhou, N.; Su, Y.; Shao, M.; Chi, C. pH-Sensitive N-doped carbon dots–heparin and doxorubicin drug delivery system: Preparation and anticancer research. RSC Adv. 2017, 7, 9347–9356. [Google Scholar] [CrossRef] [Green Version]
- Niculescu, A.G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Nanomaterials Synthesis through Microfluidic Methods: An Updated Overview. Nanomaterials 2021, 11, 864. [Google Scholar] [CrossRef]
- Chung, I.M.; Park, I.; Seung-Hyun, K.; Thiruvengadam, M.; Rajakumar, G. Plant-Mediated Synthesis of Silver Nanoparticles: Their Characteristic Properties and Therapeutic Applications. Nanoscale Res. Lett. 2016, 11, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Khatib, A.M.; Badawi, M.S.; Ghatass, Z.F.; Mohamed, M.M.; Elkhatib, M. Synthesize of Silver Nanoparticles by Arc Discharge Method Using Two Different Rotational Electrode Shapes. J. Clust. Sci. 2018, 29, 1169–1175. [Google Scholar] [CrossRef]
- Kumar, P.S.M.; Francis, A.P.; Devasena, T. Biosynthesized and Chemically Synthesized Titania Nanoparticles: Comparative Analysis of Antibacterial Activity. J. Environ. Nanotechnol. 2014, 3, 73–81. [Google Scholar]
- Kumar, P.V.; Kala, S.M.J.; Prakash, K.S. Green synthesis of gold nanoparticles using Croton Caudatus Geisel leaf extract and their biological studies. Mater. Lett. 2019, 236, 19–22. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yeh, C.S. Laser ablation method: Use of surfactants to form the dispersed Ag nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2002, 197, 133–139. [Google Scholar] [CrossRef]
- Sen, P.; Ghosh, J.; Abdullah, A.; Kumar, P. Preparation of Cu, Ag, Fe and Al nanoparticles by the exploding wire technique. J. Chem. Sci. 2003, 115, 499–508. [Google Scholar] [CrossRef]
- Sangar, S.; Sharma, S.; Vats, V.K.; Mehta, S.K.; Singh, K. Biosynthesis of silver nanocrystals, their kinetic profile from nucleation to growth and optical sensing of mercuric ions. J. Clean. Prod. 2019, 228, 294–302. [Google Scholar] [CrossRef]
- Melchert, W.R.; Reis, B.F.; Rocha, F.R.P. Green chemistry and the evolution of flow analysis. A review. Anal. Chim. Acta 2012, 714, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Shankar, R.; Malik, V.; Sharma, N.; Mukherjee, T.K. Green Chemistry Based Benign Routes for Nanoparticle Synthesis. J. Nanopart. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sofi, H.S.; Ashraf, R.; Khan, A.H.; Beigh, M.A.; Majeed, S.; Sheikh, F.A. Reconstructing nanofibers from natural polymers using surface functionalization approaches for applications in tissue engineering, drug delivery and biosensing devices. Mater. Sci. Eng. C 2019, 94, 1102–1124. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef] [Green Version]
- Căprărescu, S.; Modrogan, C.; Purcar, V.; Dăncilă, A.M.; Orbuleț, O.D. Study of Polyvinyl Alcohol-SiO2 Nanoparticles Polymeric Membrane in Wastewater Treatment Containing Zinc Ions. Polymers 2021, 13, 1875. [Google Scholar] [CrossRef]
- Sinha, S.; Pan, I.; Chanda, P.; Sen, S.K. Nanoparticles fabrication using ambient biological resources. J. Appl. Biosci. 2009, 19, 1113–1130. [Google Scholar]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotech. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Gowramma, B.; Keerthi, U.; Rafi, M.; Muralidhara Rao, D. Biogenic silver nanoparticles production and characterization from native stain of Corynebacterium species and its antimicrobial activity. 3 Biotech 2015, 5, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Wang, L.; Yao, J.; Zhang, X.; Zhang, Y.; Xu, C.; Lee, R.; Yu, G.; Yu, B.; Teng, L. Delivery of paclitaxel using nanoparticles composed of poly (ethylene oxide)-b-poly (butylene oxide) (PEO-PBO). Colloids Surf. B Biointerfaces 2018, 161, 464–470. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. Polymer-Based Nanosystems—A Versatile Delivery Approach. Materials 2021, 14, 6812. [Google Scholar] [CrossRef]
- Ahirwar, D.; Bano, M.; Khan, I.; Gound, S.S.; Ud Din Sheik, M.; Mondal, R.; Khan, F. Facile synthesis of macroporous Ag and CuO monoliths as an efficient nonenzymatic electrochemical sensor and antimicrobial agent. J. Solid State Chem. 2019, 273, 233–242. [Google Scholar] [CrossRef]
- Zare, M.; Namratha, K.; Alghamdi, S.; Mohammad, Y.H.E.; Hezam, A.; Zare, M.; Drmosh, Q.A.; Byrappa, K.; Nanjegowda, B.; Chandrashekar, S.R.; et al. Novel green biomimetic approach for synthesis of ZnO-Ag nanocomposite; antimicrobial activity against food-borne pathogen, biocompatibility and solar. Sci. Rep. 2019, 9, 8303. [Google Scholar] [CrossRef] [Green Version]
- Nadagouda, M.N.; Varma, R.S. Green synthesis of silver and palladium nanoparticles at room temperature using coffee and tea extract. Green Chem. 2008, 10, 859–886. [Google Scholar] [CrossRef]
- Elavazhagan, T.; Arunachalam, K.D. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int. J. Nanomed. 2011, 6, 1265–1278. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.W.; Kim, M.; Kim, Z.H.; Han, S.W. One-step synthesis of Au@Pd core-shell nanooctahedron. J. Am. Chem. Soc. 2009, 131, 17036–17037. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Z.; Xiao, Z.; Huang, J.; Li, Q.; Wang, Y.; Sun, D. Ni2P-graphite nanoplatelets supported Au-Pd core-shell nanoparticles with superior electrochemical properties. J. Phys. Chem. C 2015, 119, 10469–10477. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Sastry, M. Geranium Leaf Assisted Biosynthesis of Silver Nanoparticles. Biotechnol. Prog. 2003, 19, 1627–1631. [Google Scholar] [CrossRef]
- Ankamwar, B.; Chaudhary, M.; Sastry, M. Gold Nanotriangles Biologically Synthesized using Tamarind Leaf Extract and Potential Application in Vapor Sensing. Synth. React. Inorg. Met. Nano-Metal Chem. 2005, 35, 19–26. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Gomez, E.; Peralta-Videa, J.R.; Parsons, J.G.; Troiani, H.; Jose-Yacaman, M. Alfalfa sprouts: A natural source for the synthesis of silver nanoparticles. Langmuir 2003, 19, 1357–1361. [Google Scholar] [CrossRef]
- Xin Lee, K.; Shameli, K.; Miyake, M.; Kuwano, N.; Bt Ahmad Khairudin, N.B.; Bt Mohamad, S.E.; Yew, Y.P. Green Synthesis of Gold Nanoparticles Using Aqueous Extract of Garcinia mangostana Fruit Peels. J. Nanomater. 2016, 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Davids, J.S.; Ackah, M.; Okoampah, E.; Fometu, S.S.; Guohua, W.; Jianping, Z. Biocontrol of Bacteria Associated with Pine Wilt Nematode, Bursaphelenchus xylophilus by using Plant mediated Gold Nanoparticles. Int. J. Agric. Biol. 2021, 26, 517–526. [Google Scholar]
- Jiang, H.; Manolache, S.; Wong, A.C.L.; Denes, F.S. Plasma-enhanced deposition of silver nanoparticles onto polymer and metal surfaces for the generation of antimicrobial characteristics. J. Appl. Polym. Sci. 2004, 93, 1411–1422. [Google Scholar] [CrossRef]
- Thirunavoukkarasu, M.; Balaji, U.; Behera, S.; Panda, P.K.; Mishra, B.K. Biosynthesis of silver nanoparticle from leaf extract of Desmodium gangeticum (L.) DC. and its biomedical potential. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 116, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Parlinska-Wojtan, M.; Kus-Liskiewicz, M.; Depciuch, J.; Sadik, O. Green synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using camomile terpenoids as a combined reducing and capping agent. Bioprocess Biosyst. Eng. 2016, 39, 1213–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-León, E.; Iñiguez-Palomares, R.; Navarro, R.E.; Herrera-Urbina, R.; Tánori, J.; Iñiguez-Palomares, C.; Maldonado, A. Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts). Nanoscale Res. Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopinath, V.; MubarakAli, D.; Priyadarshini, S.; Priyadharsshini, N.M.; Thajuddin, N.; Velusamy, P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: A novel biological approach. Colloids Surf. B Biointerfaces 2012, 96, 69–74. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pohl, P.; Epifano, F.; Álvarez-Suarez, J.M. Green Synthesis of Silver Nanoparticles Using Astragalus tribuloides Delile. Root Extract: Characterization, Antioxidant, Antibacterial, and Anti-Inflammatory Activities. Nanomaterials 2020, 10, 2383. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K. Green Synthesis of Silver Nanoparticles Using Cycas Leaf. Int. J. Green Nanotechnol. Phys. Chem. 2010, 1, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Sathishkumar, M.; Sneha, K.; Yun, Y.S. Immobilization of silver nanoparticles synthesized using Curcuma longa tuber powder and extract on cotton cloth for bactericidal activity. Bioresour. Technol. 2010, 101, 7958–7965. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Synthesis of stable, polyshaped silver, and gold nanoparticles using leaf extract of Lonicera japonica L. Int. J. Green Nanotechnol. Biomed. 2011, 3, 281–291. [Google Scholar] [CrossRef]
- Banerjee, J.; Narendhirakannan, R.T. Biosynthesis of silver nanoparticles from Syzygium cumini (L.) seed extract and evaluation of their in vitro antioxidant activities. Dig. J. Nanomater. Biostruct. 2011, 6, 961–968. [Google Scholar]
- Patil, C.D.; Patil, S.V.; Borase, H.P.; Salunke, B.K.; Salunkhe, R.B. Larvicidal activity of silver nanoparticles synthesized using Plumeria rubra plant latex against Aedes aegypti and Anopheles stephensi. Parasitol. Res. 2012, 110, 1815–1822. [Google Scholar] [CrossRef]
- Ponarulselvam, S.; Panneerselvam, C.; Murugan, K.; Aarthi, N.; Kalimuthu, K.; Thangamani, S. Synthesis of silver nanoparticles using leaves of Catharanthus roseus Linn. G. Don and their antiplasmodial activities. Asian Pac. J. Trop. Biomed. 2012, 2, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Sathishkumar, M.; Sneha, K.; Won, S.W.; Cho, C.W.; Kim, S.; Yun, Y.S. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf. B Biointerfaces 2009, 73, 332–338. [Google Scholar] [CrossRef]
- Harshiny, M.; Matheswaran, M.; Arthanareeswaran, G.; Kumaran, S.; Rajasree, S. Enhancement of antibacterial properties of silver nanoparticles-ceftriaxone conjugate through Mukia maderaspatana leaf extract mediated synthesis. Ecotoxicol. Environ. Saf. 2015, 121, 135–141. [Google Scholar] [CrossRef]
- Anandalakshmi, K.; Venugobal, J.; Ramasamy, V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl. Nanosci. 2016, 6, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Raju, D.; Paneliya, N.; Mehta, U.J. Extracellular synthesis of silver nanoparticles using living peanut seedling. Appl. Nanosci. 2014, 4, 875–879. [Google Scholar] [CrossRef] [Green Version]
- Morales-Lozoya, V.; Espinoza-Gómez, H.; Flores-López, L.Z.; Sotelo-Barrera, E.L.; Núñez-Rivera, A.; Cadena-Nava, R.D.; Alonso-Nuñez, G.; Rivero, I.A. Study of the effect of the different parts of Morinda citrifolia L. (noni) on the green synthesis of silver nanoparticles and their antibacterial activity. Appl. Surf. Sci. 2021, 537, 147855. [Google Scholar] [CrossRef]
- Gudimalla, A.; Jose, J.; Varghese, R.J.; Thomas, S. Green Synthesis of Silver Nanoparticles Using Nymphae odorata Extract Incorporated Films and Antimicrobial Activity. J. Polym. Environ. 2021, 29, 1412–1423. [Google Scholar] [CrossRef]
- Nilavukkarasi, M.; Vijayakumar, S.; Prathip Kumar, S. Biological synthesis and characterization of silver nanoparticles with Capparis zeylanica L. leaf extract for potent antimicrobial and anti proliferation efficiency. Mater. Sci. Energy Technol. 2020, 3, 371–376. [Google Scholar] [CrossRef]
- Moteriya, P.; Chanda, S. Green Synthesis of Silver Nanoparticles from Caesalpinia pulcherrima Leaf Extract and Evaluation of Their Antimicrobial, Cytotoxic and Genotoxic Potential (3-in-1 System). J. Inorg. Organomet. Polym. Mater. 2020, 30, 3920–3932. [Google Scholar] [CrossRef]
- Dalir, S.J.B.; Djahaniani, H.; Nabati, F.; Hekmati, M. Characterization and the evaluation of antimicrobial activities of silver nanoparticles biosynthesized from Carya illinoinensis leaf extract. Heliyon 2020, 6, e03624. [Google Scholar] [CrossRef]
- Khatoon, A.; Khan, F.; Ahmad, N.; Shaikh, S.; Rizvi, S.M.D.; Shakil, S.; Al-Qahtani, M.H.; Abuzenadah, A.M.; Tabrez, S.; Ahmed, A.B.F.; et al. Silver nanoparticles from leaf extract of Mentha piperita: Eco-friendly synthesis and effect on acetylcholinesterase activity. Life Sci. 2018, 209, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; De, S.P.; Misra, A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 134–139. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef]
- Philip, D. Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Phys. E Low-Dimens. Syst. Nanostruct. 2010, 42, 1417–1424. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M.; Abdeen, A.O. Green synthesis of silver nanoparticles using carob leaf extract and its antibacterial activity. Int. J. Ind. Chem. 2013, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Satyavani, K.; Gurudeeban, S.; Ramanathan, T.; Balasubramanian, T. Toxicity study of silver nanoparticles synthesized from Suaeda monoica on Hep-2 cell line. Avicenna J. Med. Biotechnol. 2012, 4, 35–39. [Google Scholar] [PubMed]
- Philip, D.; Unni, C. Extracellular biosynthesis of gold and silver nanoparticles using Krishna tulsi (Ocimum sanctum) leaf. Phys. E Low-Dimens. Syst. Nanostruct. 2011, 43, 1318–1322. [Google Scholar] [CrossRef]
- Patil, R.S.; Kokate, M.R.; Kolekar, S.S. Bioinspired synthesis of highly stabilized silver nanoparticles using Ocimum tenuiflorum leaf extract and their antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 91, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Kim, B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng. 2009, 32, 79–84. [Google Scholar] [CrossRef]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010, 45, 1065–1071. [Google Scholar] [CrossRef]
- Singh, A.; Jain, D.; Upadhyay, M.K.; Khandelwal, N.; Verma, H.N. Green synthesis of silver nanoparticles using Argemone mexicana leaf extract and evaluation of their antimicrobial activities. Dig. J. Nanomater. Biostruct. 2010, 5, 483–489. [Google Scholar]
- Nabikhan, A.; Kandasamy, K.; Raj, A.; Alikunhi, N.M. Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L. Colloids Surf. B Biointerfaces 2010, 79, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Yadav, S.C.; Yadav, S.K. Syzygium cumini leaf and seed extract mediated biosynthesis of silver nanoparticles and their characterization. J. Chem. Technol. Biotechnol. 2010, 85, 1301–1309. [Google Scholar] [CrossRef]
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N.; et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 2007, 18, 105104. [Google Scholar] [CrossRef]
- Sukirtha, R.; Priyanka, K.M.; Antony, J.J.; Kamalakkannan, S.; Thangam, R.; Gunasekaran, P.; Krishnan, M.; Achiraman, S. Cytotoxic effect of Green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process Biochem. 2012, 47, 273–279. [Google Scholar] [CrossRef]
- Mittal, A.K.; Kaler, A.; Banerjee, U.C. Free radical scavenging and antioxidant activity of silver nanoparticles synthesized from flower extract of Rhododendron dauricum. Nano Biomed. Eng. 2012, 4, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Cruz, D.; Falé, P.L.; Mourato, A.; Vaz, P.D.; Luisa Serralheiro, M.; Lino, A.R.L. Preparation and physicochemical characterization of Ag nanoparticles biosynthesized by Lippia citriodora (Lemon Verbena). Colloids Surf. B Biointerfaces 2010, 81, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Satyavani, K.; Gurudeeban, S.; Ramanathan, T.; Balasubramanian, T. Biomedical potential of silver nanoparticles synthesized from calli cells of Citrullus colocynthis (L.) Schrad. J. Nanobiotechnol. 2011, 9, 1–8. [Google Scholar]
- Aromal, S.A.; Philip, D. Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1–5. [Google Scholar] [CrossRef]
- Singh, A.K.; Talat, M.; Singh, D.P.; Srivastava, O.N. Biosynthesis of gold and silver nanoparticles by natural precursor clove and their functionalization with amine group. J. Nanopart. Res. 2010, 12, 1667–1675. [Google Scholar] [CrossRef]
- Sharma, R.K.; Gulati, S.; Mehta, S. Preparation of gold nanoparticles using tea: A green chemistry experiment. J. Chem. Educ. 2012, 89, 1316–1318. [Google Scholar] [CrossRef]
- Suman, T.Y.; Radhika Rajasree, S.R.; Ramkumar, R.; Rajthilak, C.; Perumal, P. The Green synthesis of gold nanoparticles using an aqueous root extract of Morinda citrifolia L. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Gogoi, N.; Bora, U. Green synthesis of gold nanoparticles using Nyctanthes arbortristis flower extract. Bioprocess Biosyst. Eng. 2011, 34, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Prasad, K. Biosynthesis of gold nanoparticles using bael (Aegle marmelos) leaf: Mythology meets technology. Int. J. Green Nanotechnol. Biomed. 2011, 3, 92–97. [Google Scholar] [CrossRef]
- Rodríguez-León, E.; Rodríguez-Vázquez, B.E.; Martínez-Higuera, A.; Rodríguez-Beas, C.; Larios-Rodríguez, E.; Navarro, R.E.; López-Esparza, R.; Iñiguez-Palomares, R.A. Synthesis of Gold Nanoparticles Using Mimosa tenuiflora Extract, Assessments of Cytotoxicity, Cellular Uptake, and Catalysis. Nanoscale Res. Lett. 2019, 14, 334. [Google Scholar] [CrossRef] [Green Version]
- Anuradha, J.; Tasneem, A.; Abbasi, S.A. “Green” Synthesis of Gold Nanoparticles with Aqueous Extracts of Neem (Azadirachta indica). Res. J. Biotechnol. 2010, 5, 75–79. [Google Scholar]
- Kasthuri, J.; Kathiravan, K.; Rajendiran, N. Phyllanthin-assisted biosynthesis of silver and gold nanoparticles: A novel biological approach. J. Nanopart. Res. 2009, 11, 1075–1085. [Google Scholar] [CrossRef]
- Aromal, S.A.; Philip, D. Benincasa hispida seed mediated green synthesis of gold nanoparticles and its optical nonlinearity. Phys. E Low-Dimens. Syst. Nanostruct. 2012, 44, 1329–1334. [Google Scholar] [CrossRef]
- Muniyappan, N.; Pandeeswaran, M.; Amalraj, A. Green synthesis of gold nanoparticles using Curcuma pseudomontana isolated curcumin: Its characterization, antimicrobial, antioxidant and anti-inflammatory activities. Environ. Chem. Ecotoxicol. 2021, 3, 117–124. [Google Scholar] [CrossRef]
- Kumari, P.; Meena, A. Green synthesis of gold nanoparticles from Lawsoniainermis and its catalytic activities following the Langmuir-Hinshelwood mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125447. [Google Scholar] [CrossRef]
- ElMitwalli, O.S.; Barakat, O.A.; Daoud, R.M.; Akhtar, S.; Henari, F.Z. Green synthesis of gold nanoparticles using cinnamon bark extract, characterization, and fluorescence activity in Au/eosin Y assemblies. J. Nanopart. Res. 2020, 22, 1–9. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Prasad, K. Biosynthesis of gold nanoparticles using common aromatic plants. Int. J. Green Nanotechnol. Biomed. 2012, 4, 219–224. [Google Scholar] [CrossRef]
- Shukla, D.; Vankar, P.S. Synthesis of plant parts mediated gold nanoparticles. Int. J. Green Nanotechnol. Biomed. 2012, 4, 277–288. [Google Scholar] [CrossRef]
- Philip, D.; Unni, C.; Aromal, S.A.; Vidhu, V.K. Murraya Koenigii leaf-assisted rapid green synthesis of silver and gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 899–904. [Google Scholar] [CrossRef]
- Noruzi, M.; Zare, D.; Khoshnevisan, K.; Davoodi, D. Rapid green synthesis of gold nanoparticles using Rosa hybrida petal extract at room temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.M.; Mandal, B.K.; Sinha, M.; Krishnakumar, V. Terminalia chebula mediated green and rapid synthesis of gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 86, 490–494. [Google Scholar] [CrossRef]
- Pandey, S.; Oza, G.; Mewada, A.; Sharon, M. Green Synthesis of Highly Stable Gold Nanoparticles using Momordica charantia as Nano fabricator. Arch. Appl. Sci. Res. 2012, 4, 1135–1141. [Google Scholar]
- Annamalai, A.; Babu, S.T.; Jose, N.A.; Sudha, D.; Lyza, C.V. Biosynthesis and characterization of silver and gold nanoparticles using aqueous leaf extraction of Phyllanthus amarus Schum. & Thonn. World Appl. Sci. J. 2011, 13, 1833–1840. [Google Scholar]
- Philip, D. Rapid green synthesis of spherical gold nanoparticles using Mangifera indica leaf. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 807–810. [Google Scholar] [CrossRef]
- Mishra, A.N.; Bhadauria, S.; Gaur, M.S.; Pasricha, R.; Kushwah, B.S. Synthesis of Gold Nanoparticles by Leaves of Zero-Calorie Sweetener Herb ( Stevia rebaudiana ) and Their Nanoscopic Characterization by Spectroscopy and Microscopy. Int. J. Green Nanotechnol. Phys. Chem. 2010, 1, 118–124. [Google Scholar] [CrossRef]
- Sneha, K.; Sathishkumar, M.; Lee, S.Y.; Bae, M.A.; Yun, Y.S. Biosynthesis of Au nanoparticles using cumin seed powder extract. J. Nanosci. Nanotechnol. 2011, 11, 1811–1814. [Google Scholar] [CrossRef]
- Dubey, S.P.; Lahtinen, M.; Särkkä, H.; Sillanpää, M. Bioprospective of Sorbus aucuparia leaf extract in development of silver and gold nanocolloids. Colloids Surf. B Biointerfaces 2010, 80, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kajbafvala, A.; Ghorbani, H.; Paravar, A.; Samberg, J.P.; Kajbafvala, E.; Sadrnezhaad, S.K. Effects of morphology on photocatalytic performance of Zinc oxide nanostructures synthesized by rapid microwave irradiation methods. Superlattices Microstruct. 2012, 51, 512–522. [Google Scholar] [CrossRef]
- Kumar, S.S.; Venkateswarlu, P.; Rao, V.R.; Rao, G.N. Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. Nano Lett. 2013, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lingaraju, K.; Raja Naika, H.; Manjunath, K.; Basavaraj, R.B.; Nagabhushana, H.; Nagaraju, G.; Suresh, D. Biogenic synthesis of zinc oxide nanoparticles using Ruta graveolens (L.) and their antibacterial and antioxidant activities. Appl. Nanosci. 2016, 6, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, S.K.; Malodia, L. Biosynthesis of zinc oxide nanoparticles using leaf extract of calotropis gigantea: Characterization and its evaluation on tree seedling growth in nursery stage. Appl. Nanosci. 2017, 7, 501–512. [Google Scholar] [CrossRef]
- Ahmad, H.; Venugopal, K.; Rajagopal, K.; De Britto, S.; Nandini, B.; Pushpalatha, H.G.; Konappa, N.; Udayashankar, A.C.; Geetha, N.; Jogaiah, S. Green synthesis and characterization of zinc oxide nanoparticles using eucalyptus globules and their fungicidal ability against pathogenic fungi of apple orchards. Biomolecules 2020, 10, 425. [Google Scholar] [CrossRef] [Green Version]
- Gomathi, R.; Suhana, H. Green synthesis, characterization and antimicrobial activity of zinc oxide nanoparticles using Artemisia pallens plant extract. Inorg. Nano-Metal Chem. 2021, 51, 1663–1672. [Google Scholar] [CrossRef]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Ambika, S.; Sundrarajan, M. Antibacterial behaviour of Vitex negundo extract assisted ZnO nanoparticles against pathogenic bacteria. J. Photochem. Photobiol. B Biol. 2015, 146, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Anbuvannan, M.; Ramesh, M.; Viruthagiri, G.; Shanmugam, N.; Kannadasan, N. Anisochilus carnosus leaf extract mediated synthesis of zinc oxide nanoparticles for antibacterial and photocatalytic activities. Mater. Sci. Semicond. Process. 2015, 39, 621–628. [Google Scholar] [CrossRef]
- Singh, R.P.; Shukla, V.K.; Yadav, R.S.; Sharma, P.K.; Singh, P.K.; Pandey, A.C. Biological approach of zinc oxide nanoparticles formation and its characterization. Adv. Mater. Lett. 2011, 2, 313–317. [Google Scholar] [CrossRef]
- Ramesh, P.; Rajendran, A.; Sundaram, M. Green Synthesis of Zinc Oxide Nanoparticles Using Flower Extract Cassia Auriculata. J. Nanosci. Nanotechnol. 2014, 2, 41–45. [Google Scholar]
- Jayachandran, A.; Aswathy, T.R.; Nair, A.S. Green synthesis and characterization of zinc oxide nanoparticles using Cayratia pedata leaf extract. Biochem. Biophys. Rep. 2021, 26, 100995. [Google Scholar] [CrossRef]
- Ahmad, W.; Kalra, D. Green synthesis, characterization and anti-microbial activities of ZnO nanoparticles using Euphorbia hirta leaf extract. J. King Saud Univ.-Sci. 2020, 32, 2358–2364. [Google Scholar] [CrossRef]
- Sharmila, G.; Thirumarimurugan, M.; Muthukumaran, C. Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: Characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem. J. 2019, 145, 578–587. [Google Scholar] [CrossRef]
- Raj, L.F.A.; Jayalakshmy, E. Biosynthesis and characterization of zinc oxide nanoparticles using root extract of Zingiber officinale. Orient. J. Chem. 2015, 31, 51–56. [Google Scholar] [CrossRef]
- Noorjahan, C.M.; Shahina, S.K.J.; Deepika, T.; Rafiq, S. Green Synthesis and Characterization of Zinc Oxide Nanoparticles from Neem (Azadirachta indicia). Int. J. Sci. Eng. Technol. Res. 2015, 4, 5751–5753. [Google Scholar]
- Savithramma, N.; Bhumi, G. Biological Synthesis of Zinc oxide Nanoparticles from C atharanthus roseus (l.) G. Don. Leaf extract and validation for antibacterial activity. Int. J. Drug Dev. Res. 2014, 6, 208–214. [Google Scholar]
- Ramesh, M.; Anbuvannan, M.; Viruthagiri, G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Awwad, A.M.; Albiss, B.; Ahmad, A.L. Green synthesis, characterization and optical properties of zinc oxide nanosheets using Olea europea leaf extract. Adv. Mater. Lett. 2014, 5, 520–524. [Google Scholar] [CrossRef]
- Oudhia, A.; Kulkarni, P.; Sharma, S. Green Synthesisof ZnO nanotubes for Bioapplications. J. Adv. Eng. Res. Stud. 2015, 280–281. [Google Scholar]
- Jamdagni, P.; Khatri, P.; Rana, J.S. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Univ.-Sci. 2018, 30, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Devi, R.; Gayathri, R. Green Synthesis of Zinc Oxide Nanoparticles by using Hibiscus rosa-sinensis. Int. J. Curr. Eng. Technol. 2014, 44, 2444–2446. [Google Scholar]
- Varghese, E.; George, M. Green synthesis of zinc oxide nanoparticles. Int. J. Adv. Res. Sci. Eng. 2015, 4, 307–314. [Google Scholar]
- Raut, S.; Thorat, P.V.; Thakre, R. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Ocimum Tenuiflorum Leaves. Int. J. Sci. Res. 2013, 4, 2319–7064. [Google Scholar]
- Azizi, S.; Ahmad, M.B.; Namvar, F.; Mohamad, R. Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Mater. Lett. 2014, 116, 275–277. [Google Scholar] [CrossRef]
- Kumar, M.A.P.; Suresh, D.; Nagabhushana, H.; Sharma, S.C. Beta vulgaris aided green synthesis of ZnO nanoparticles and their luminescence, photocatalytic and antioxidant properties. Eur. Phys. J. Plus 2015, 130, 1–7. [Google Scholar]
- Fatimah, I.; Yudha, S.P.; Mutiara, N.A.L. Green synthesis of ZnO nanoparticles via complex formation by using Curcuma longa extract. Proceedings of 6th Nanoscience and Nanotechnology Symposium (NNS2015), Sukarta, Indonesia, 4–5 November 2015; Purwano, A., Nur, A., Rahmawati, F., Dyartanti, E.R., Jumari, A., Eds.; AIP Publishing LLC: Melville, NY, USA, 2015. [Google Scholar]
- Yuvakkumar, R.; Suresh, J.; Hong, S.I. Green synthesis of zinc oxide nanoparticles. Adv. Mater. Res. 2014, 952, 137–140. [Google Scholar] [CrossRef]
- Suresh, D.; Shobharani, R.M.; Nethravathi, P.C.; Pavan Kumar, M.A.; Nagabhushana, H.; Sharma, S.C. Artocarpus gomezianus aided green synthesis of ZnO nanoparticles: Luminescence, photocatalytic and antioxidant properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 141, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Sindhura, K.S.; Tnvkv, P.; Selvam, P.P.; Hussain, O.M. Green Synthesis of Zinc Nanoparticles from Senna Auriculata and Influence on Peanut Pot-Culture. Int. J. Res. Agric. Sci. 2015, 2, 61–69. [Google Scholar]
- Raj, A.; Lawrence, R.S.; Jalees, M.; Lawrence, K. Anti-Bacterial Activity of Zinc Oxide Nanoparticles Prepared from Brassica Oleraceae Leaves Extract. Int. J. Adv. Res. 2015, 3, 322–328. [Google Scholar]
- Gnanasangeetha, D.; Thambavani, D. Biological and Physical Sciences Biogenic Production of Zinc Oxide Nanoparticles Using Acalypha Indica. J. Chem. Biol. Phys. Sci. 2014, 4, 238–246. [Google Scholar]
- Vijayakumar, S.; Vinoj, G.; Malaikozhundan, B.; Shanthi, S.; Vaseeharan, B. Plectranthus amboinicus leaf extract mediated synthesis of zinc oxide nanoparticles and its control of methicillin resistant Staphylococcus aureus biofilm and blood sucking mosquito larvae. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 886–891. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Sreekanth, T.V.M.; Tettey, C.O.; Jun, Y.I.; Mook, S.H. Characterization, antibacterial, antioxidant, and cytotoxic activities of ZnO nanoparticles using Coptidis Rhizoma. Bioorganic Med. Chem. Lett. 2014, 24, 4298–4303. [Google Scholar] [CrossRef] [PubMed]
- Janaki, A.C.; Sailatha, E.; Gunasekaran, S. Synthesis, characteristics and antimicrobial activity of ZnO nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 144, 17–22. [Google Scholar] [CrossRef]
- Lai, Y.; Wang, L.; Liu, D.; Chen, Z.; Lin, C. TiO2-Based Nanomaterials: Design, Synthesis, and Applications. J. Nanomater. 2015, 2015, 1–3. [Google Scholar] [CrossRef]
- Altikatoglu Yapaoz, M.; Attar, A. Salvia officinalis-derived rutile TiO2NPs: Production, characterization, antibacterial evaluation and its effect on decolorization. Mater. Res. Express 2019, 6, 55039. [Google Scholar] [CrossRef]
- Mahmoud, W.M.M.; Rastogi, T.; Kümmerer, K. Application of titanium dioxide nanoparticles as a photocatalyst for the removal of micropollutants such as pharmaceuticals from water. Curr. Opin. Green Sustain. Chem. 2017, 6, 1–10. [Google Scholar] [CrossRef]
- Abel, S.; Jule, L.T.; Belay, F.; Shanmugam, R.; Dwarampudi, L.P.; Nagaprasad, N.; Krishnaraj, R. Application of Titanium Dioxide Nanoparticles Synthesized by Sol-Gel Methods in Wastewater Treatment. J. Nanomater. 2021, 2021, 3039761. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A.; Priyamvada, B.; Khanna, V.G.; Kumar, D.K.; Sujin, P.J. Eclipta prostrata leaf aqueous extract mediated synthesis of titanium dioxide nanoparticles. Mater. Lett. 2012, 68, 115–117. [Google Scholar] [CrossRef]
- Subhashini, D.; Nachiyar, C.V. Albizia saman: A Green Route for the Reduction of Bulk TiO2. Int. J. Chem Tech. Res. 2014, 6, 5137–5141. [Google Scholar]
- Jalill, R.D.A.; Nuaman, R.S.; Abd, A.N. Biological synthesis of Titanium Dioxide nanoparticles by Curcuma longa plant extract and study its biological properties. World Sci. News 2016, 49, 204–222. [Google Scholar]
- Dobrucka, R. Synthesis of titanium dioxide nanoparticles using Echinacea purpurea herba. Iran. J. Pharm. Res. 2017, 16, 753–759. [Google Scholar]
- Aswini, R.; Murugesan, S.; Kannan, K. Bio-engineered TiO2 nanoparticles using Ledebouria revoluta extract: Larvicidal, histopathological, antibacterial and anticancer activity. Int. J. Environ. Anal. Chem. 2021, 101, 2926–2936. [Google Scholar] [CrossRef]
- Narayanan, M.; Devi, P.G.; Natarajan, D.; Kandasamy, S.; Devarayan, K.; Alsehli, M.; Elfasakhany, A.; Pugazhendhi, A. Green synthesis and characterization of titanium dioxide nanoparticles using leaf extract of Pouteria campechiana and larvicidal and pupicidal activity on Aedes aegypti. Environ. Res. 2021, 200, 111333. [Google Scholar] [CrossRef]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process. Synth. 2020, 9, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, W.; Jaiswal, K.K.; Soni, S. Green synthesis of titanium dioxide (TiO2) nanoparticles by using Mentha arvensis leaves extract and its antimicrobial properties. Inorg. Nano-Metal Chem. 2020, 50, 1032–1038. [Google Scholar] [CrossRef]
- Thakur, B.K.; Kumar, A.; Kumar, D. Green synthesis of titanium dioxide nanoparticles using Azadirachta indica leaf extract and evaluation of their antibacterial activity. S. Afr. J. Bot. 2019, 124, 223–227. [Google Scholar] [CrossRef]
- Santhoshkumar, T.; Rahuman, A.A.; Jayaseelan, C.; Rajakumar, G.; Marimuthu, S.; Kirthi, A.V.; Velayutham, K.; Thomas, J.; Venkatesan, J.; Kim, S.K. Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac. J. Trop. Med. 2014, 7, 968–976. [Google Scholar] [CrossRef] [Green Version]
- Sundrarajan, M.; Gowri, S. Green synthesis of titanium dioxide nanoparticles by nyctanthes arbor-tristis leaves extract. Chalcogenide Lett. 2011, 8, 447–451. [Google Scholar]
- Marimuthu, S.; Rahuman, A.A.; Jayaseelan, C.; Kirthi, A.V.; Santhoshkumar, T.; Velayutham, K.; Bagavan, A.; Kamaraj, C.; Elango, G.; Iyappan, M.; et al. Acaricidal activity of synthesized titanium dioxide nanoparticles using Calotropis gigantea against Rhipicephalus microplus and Haemaphysalis bispinosa. Asian Pac. J. Trop. Med. 2013, 6, 682–688. [Google Scholar] [CrossRef] [Green Version]
- Rajakumar, G.; Rahuman, A.A.; Jayaseelan, C.; Santhoshkumar, T.; Marimuthu, S.; Kamaraj, C.; Bagavan, A.; Zahir, A.A.; Kirthi, A.V.; Elango, G.; et al. Solanum trilobatum extract-mediated synthesis of titanium dioxide nanoparticles to control Pediculus humanus capitis, Hyalomma anatolicum anatolicum and Anopheles subpictus. Parasitol. Res. 2014, 113, 469–479. [Google Scholar] [CrossRef]
- Sankar, R.; Rizwana, K.; Shivashangari, K.S.; Ravikumar, V. Ultra-rapid photocatalytic activity of Azadirachta indica engineered colloidal titanium dioxide nanoparticles. Appl. Nanosci. 2015, 5, 731–736. [Google Scholar] [CrossRef] [Green Version]
- Velayutham, K.; Rahuman, A.A.; Rajakumar, G.; Santhoshkumar, T.; Marimuthu, S.; Jayaseelan, C.; Bagavan, A.; Kirthi, A.V.; Kamaraj, C.; Zahir, A.A.; et al. Evaluation of Catharanthus roseus leaf extract-mediated biosynthesis of titanium dioxide nanoparticles against Hippobosca maculata and Bovicola ovis. Parasitol. Res. 2012, 111, 2329–2337. [Google Scholar] [CrossRef]
- Nwanya, A.C.; Ugwuoke, P.E.; Ejikeme, P.M.; Oparaku, O.U.; Ezema, F.I. Jathropha curcas and citrus aurantium leaves dye extract for use in dye sensitized solar cell with TiO2 films. Int. J. Electrochem. Sci. 2012, 7, 11219–11235. [Google Scholar]
- Hudlikar, M.; Joglekar, S.; Dhaygude, M.; Kodam, K. Green synthesis of TiO2 nanoparticles by using aqueous extract of Jatropha curcas L. latex. Mater. Lett. 2012, 75, 196–199. [Google Scholar] [CrossRef]
- Zahir, A.A.; Chauhan, I.S.; Bagavan, A.; Kamaraj, C.; Elango, G.; Shankar, J.; Arjaria, N.; Roopan, S.M.; Rahuman, A.A.; Singh, N. Green synthesis of silver and titanium dioxide nanoparticles using Euphorbia prostrata extract shows shift from apoptosis to G0/G1 arrest followed by necrotic cell death in Leishmania donovani. Antimicrob. Agents Chemother. 2015, 59, 4782–4799. [Google Scholar] [CrossRef] [Green Version]
- Rao, K.G.; Ashok, C.; Rao, K.V.; Chakra, C.S.; Rajendar, V. Synthesis of TiO2 nanoparticles from orange fruit waste. Int. J. Multidiscip. Adv. Res. Trends 2015, 2, 82–90. [Google Scholar]
- Valli, D.G.; Geetha, S. A green method for the synthesis of titanium dioxide nanoparticles using Cassia auriculata leaves extract. Eur. J. Biomed. Pharm. Sci. 2015, 2, 490–497. [Google Scholar]
- Salam, H.A.; Sivaraj, R. Ocimum basilicum L. var. purpurascens Benth.-Lamiaceae Mediated Green Synthesis and Characterization of Titanium Dioxide Nanoparticles. Adv. Biores. 2014, 5, 10–16. [Google Scholar]
- Valli, G.; Jayalakshmi, A. Erythrina variegate leaves extract assisted synthesis of titanium dioxide nanoparticles in an eco-friendly approach. Eur. J. Biomed. Pharm. Sci. 2015, 2, 1228–1236. [Google Scholar]
- Siddiqi, K.S.; Husen, A. Green Synthesis, Characterization and Uses of Palladium/Platinum Nanoparticles. Nanoscale Res. Lett. 2016, 11, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurunathan, S.; Kim, E.S.; Han, J.W.; Park, J.H.; Kim, J.-H.H.; Grumezescu, A.M. Green chemistry approach for synthesis of effective anticancer palladium nanoparticles. Molecules 2015, 20, 22476–22498. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Maham, M. Green synthesis of palladium nanoparticles using Hippophae rhamnoides Linn leaf extract and their catalytic activity for the Suzuki-Miyaura coupling in water. J. Mol. Catal. A Chem. 2015, 396, 297–303. [Google Scholar] [CrossRef]
- Khan, M.; Albalawi, G.H.; Shaik, M.R.; Khan, M.; Adil, S.F.; Kuniyil, M.; Alkhathlan, H.Z.; Al-Warthan, A.; Siddiqui, M.R.H. Miswak mediated green synthesized palladium nanoparticles as effective catalysts for the Suzuki coupling reactions in aqueous media. J. Saudi Chem. Soc. 2017, 21, 450–457. [Google Scholar] [CrossRef] [Green Version]
- Sathishkumar, M.; Sneha, K.; Kwak, I.S.; Mao, J.; Tripathy, S.J.; Yun, Y.S. Phyto-crystallization of palladium through reduction process using Cinnamom zeylanicum bark extract. J. Hazard. Mater. 2009, 171, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Khan, M.M.; Kuniyil, M.; Adil, S.F.; Al-Warthan, A.; Alkhathlan, H.Z.; Tremel, W.; Tahir, M.N.; Siddiqui, M.R.H. Biogenic synthesis of palladium nanoparticles using Pulicaria glutinosa extract and their catalytic activity towards the Suzuki coupling reaction. Dalt. Trans. 2014, 43, 9026–9031. [Google Scholar] [CrossRef]
- Kumar Petla, R.; Vivekanandhan, S.; Misra, M.; Kumar Mohanty, A.; Satyanarayana, N. Soybean (Glycine Max) Leaf Extract Based Green Synthesis of Palladium Nanoparticles. J. Biomater. Nanobiotechnol. 2012, 3, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Jia, L.; Zhang, Q.; Li, Q.; Song, H. The biosynthesis of palladium nanoparticles by antioxidants in Gardenia jasminoides Ellis: Long lifetime nanocatalysts for p-nitrotoluene hydrogenation. Nanotechnology 2009, 20, 385601–385611. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Fawzy, I.M.; Saleh, B.M.; Issa, M.Y.; Bakowsky, U.; Azzazy, H.M.E.S. Green synthesis of platinum and palladium nanoparticles using Peganum harmala L. Seed alkaloids: Biological and computational studies. Nanomaterials 2021, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Bathula, C.; Subalakshmi, K.; Kumar, A.; Yadav, H.; Ramesh, S.; Shinde, S.; Shrestha, N.K.; Mallikarjuna, K.; Kim, H. Ultrasonically driven green synthesis of palladium nanoparticles by Coleus amboinicus for catalytic reduction and Suzuki-Miyaura reaction. Colloids Surf. B Biointerfaces 2020, 192, 111026. [Google Scholar] [CrossRef]
- Kora, A.J.; Rastogi, L. Green synthesis of palladium nanoparticles using gum ghatti (Anogeissus latifolia) and its application as an antioxidant and catalyst. Arab. J. Chem. 2018, 11, 1097–1106. [Google Scholar] [CrossRef] [Green Version]
- Sharmila, G.; Farzana Fathima, M.; Haries, S.; Geetha, S.; Manoj Kumar, N.; Muthukumaran, C. Green synthesis, characterization and antibacterial efficacy of palladium nanoparticles synthesized using Filicium decipiens leaf extract. J. Mol. Struct. 2017, 1138, 35–40. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Wang, H.; Huang, J.; Lin, L.; Wang, W.; Sun, D.; Su, Y.; Opiyo, J.B.; Hong, L.; et al. Green synthesis of palladium nanoparticles using broth of Cinnamomum camphora leaf. J. Nanopart. Res. 2010, 12, 1589–1598. [Google Scholar] [CrossRef] [Green Version]
- Bankar, A.; Joshi, B.; Kumar, A.R.; Zinjarde, S. Banana peel extract mediated novel route for the synthesis of palladium nanoparticles. Mater. Lett. 2010, 64, 1951–1953. [Google Scholar] [CrossRef]
- Kalaiselvi, A.; Roopan, S.M.; Madhumitha, G.; Ramalingam, C.; Elango, G. Synthesis and characterization of palladium nanoparticles using Catharanthus roseus leaf extract and its application in the photo-catalytic degradation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, M.; Sneha, K.; Yun, Y. Palladium Nanocrystal Synthesis Using Curcuma longa Tuber Extract. Int. J. Mater. Sci. 2009, 4, 11–17. [Google Scholar]
- Muthu, K.; Priya, S. Green synthesis, characterization and catalytic activity of silver nanoparticles using Cassia auriculata flower extract separated fraction. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 179, 66–72. [Google Scholar] [CrossRef]
- Ramesh, A.V.; Devi, D.R.; Battu, G.R.; Basavaiah, K. A Facile plant mediated synthesis of silver nanoparticles using an aqueous leaf extract of Ficus hispida Linn. f. for catalytic, antioxidant and antibacterial applications. S. Afr. J. Chem. Eng. 2018, 26, 25–34. [Google Scholar] [CrossRef]
- Armendariz, V.; Herrera, I.; Peralta-Videa, J.R.; Jose-Yacaman, M.; Troiani, H.; Santiago, P.; Gardea-Torresdey, J.L. Size controlled gold nanoparticle formation by Avena sativa biomass: Use of plants in nanobiotechnology. J. Nanopart. Res. 2004, 6, 377–382. [Google Scholar] [CrossRef]
- Zulfiqar, H.; Zafar, A.; Rasheed, M.N.; Ali, Z.; Mehmood, K.; Mazher, A.; Hasan, M.; Mahmood, N. Synthesis of silver nanoparticles using Fagonia cretica and their antimicrobial activities. Nanoscale Adv. 2019, 1, 1707–1713. [Google Scholar] [CrossRef] [Green Version]
- Pasca, R.D.; Mocanu, A.; Cobzac, S.C.; Petean, I.; Horovitz, O.; Tomoaia-Cotisel, M. Biogenic Syntheses of Gold Nanoparticles Using Plant Extracts. Part. Sci. Technol. 2014, 32, 131–137. [Google Scholar] [CrossRef]
- Dhamecha, D.; Jalalpure, S.; Jadhav, K. Nepenthes khasiana mediated synthesis of stabilized gold nanoparticles: Characterization and biocompatibility studies. J. Photochem. Photobiol. B Biol. 2016, 154, 108–117. [Google Scholar] [CrossRef]
- Lee, K.X.; Shameli, K.; Mohamad, S.E.; Yew, Y.P.; Isa, E.D.M.; Yap, H.Y.; Lim, W.L.; Teow, S.Y. Bio-Mediated Synthesis and Characterisation of Silver Nanocarrier, and Its Potent Anticancer Action. Nanomaterials 2019, 9, 1423. [Google Scholar] [CrossRef] [Green Version]
- Silva-De-Hoyos, L.E.; Sánchez-Mendieta, V.; Rico-Moctezuma, A.; Vilchis-Nestor, A.R.; Camacho-López, M.A.; Avalos-Borja, M. Silver nanoparticles biosynthesized using Opuntia ficus aqueous extract. Superf. Vacio 2012, 25, 31–35. [Google Scholar]
- Fayaz, A.M.; Balaji, K.; Kalaichelvan, P.T.; Venkatesan, R. Fungal based synthesis of silver nanoparticles-An effect of temperature on the size of particles. Colloids Surf. B Biointerfaces 2009, 74, 123–126. [Google Scholar] [CrossRef]
- Khalil, M.M.H.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Song, J.Y.; Jang, H.K.; Kim, B.S. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 2009, 44, 1133–1138. [Google Scholar] [CrossRef]
- Philip, D. Mangifera Indica leaf-assisted biosynthesis of well-dispersed silver nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Ghoreishi, S.M.; Behpour, M.; Khayatkashani, M. Green synthesis of silver and gold nanoparticles using Rosa damascena and its primary application in electrochemistry. Phys. E Low-Dimens. Syst. Nanostruct. 2011, 44, 97–104. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Gopal, K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf. A Physicochem. Eng. Asp. 2010, 369, 27–33. [Google Scholar] [CrossRef]
- Masum, M.I.; Siddiqa, M.M.; Ali, K.A.; Zhang, Y.; Abdallah, Y.; Ibrahim, E.; Qiu, W.; Yan, C.; Li, B. Biogenic synthesis of silver nanoparticles using phyllanthus emblicafruit extract and its inhibitory action against the pathogen acidovorax oryzaestrain RS-2 of rice bacterial brown stripe. Front. Microbiol. 2019, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Elahi, N.; Kamali, M.; Baghersad, M. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Viet Long, N.; Yang, Y.; Teranishi, T.; Minh Thi Ho Chi, C.; Minh Thi, C.; Cao, Y.; Nogami, M. Biomedical applications of advanced multifunctional magnetic nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 10091–10107. [Google Scholar] [CrossRef]
- Rubilar, O.; Rai, M.; Tortella, G.; Diez, M.C.; Seabra, A.B.; Durán, N. Biogenic nanoparticles: Copper, copper oxides, copper sulphides, complex copper nanostructures and their applications. Biotechnol. Lett. 2013, 35, 1365–1375. [Google Scholar] [CrossRef]
- Wang, J.; Liu, R.; Liu, B. Cadmium-containing quantum dots: Current perspectives on their application as nanomedicine and toxicity concerns. Mini Rev. Med. Chem. 2016, 16, 905–916. [Google Scholar] [CrossRef]
- Khan, S.T.; Malik, A. Engineered nanomaterials for water decontamination and purification: From lab to products. J. Hazard. Mater. 2019, 363, 295–308. [Google Scholar] [CrossRef]
- Wilson, M.P.; Schwarzman, M.R. Toward a new U.S. chemicals policy: Rebuilding the foundation to advance new science, green chemistry, and environmental health. Environ. Health Perspect. 2009, 117, 1202–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamin, S.R.; de Lima, F.; Florean, E.O.P.T.; Guedes, M.I.F. Current trends in nanotechnology for bioremediation. Int. J. Environ. Pollut. 2019, 66, 19–40. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, A.; Chen, A.; Gao, Y.; He, C.; Kai, X. A functionalized gold nanoparticles and Rhodamine 6G based fluorescent sensor for high sensitive and selective detection of mercury (II) in environmental water. Anal. Chim. Acta 2007, 599, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xiao, X.; Zhang, S.; Ren, F.; Jiang, C. Facile method to synthesize magnetic iron oxides/TiO 2 hybrid nanoparticles and their photodegradation application of methylene blue. Nanoscale Res. Lett. 2011, 6, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, P.; Rumjit, N.P.; George, P.J.; Lai, C.W.; Tyagi, P.; Johan, M.R.B.; Saravanakumar, M.P. Remediation of heavy metal Ions using nanomaterials sourced from wastewaters. In Nanotechnology for Food, Agriculture and Environment; Springer Science and Business Media B.V.: Berlin/Heidelberg, Germany, 2020; pp. 255–296. [Google Scholar]
- Bashir, A.; Razanamahandry, L.; Nwanya, A.; Kaviyarasu, K.; Saban, W.; Mohamed, H.; Ntwampe, S.; Ezema, F.; Maaza, M. Biosynthesis of NiO nanoparticles for photodegradation of free cyanide solutions under ultraviolet light. J. Phys. Chem. Solids 2019, 134, 133–140. [Google Scholar] [CrossRef]
- Varadavenkatesan, T.; Lyubchik, E.; Pai, S.; Pugazhendhi, A.; Vinayagam, R.; Selvaraj, R. Photocatalytic degradation of Rhodamine B by zinc oxide nanoparticles synthesized using the leaf extract of Cyanometra ramiflora. J. Photochem. Photobiol. B Biol. 2019, 199, 111621. [Google Scholar] [CrossRef] [PubMed]
- Rogozea, E.; Petcu, A.; Olteanu, N.; Lazar, C.; Cadar, D.; Mihaly, M. Tandem adsorption-photodegradation activity induced by light on NiO-ZnO p–n couple modified silica nanomaterials. Mater. Sci. Semicond. Process. 2017, 57, 1–11. [Google Scholar] [CrossRef]


| Plant Name | Parts Used | Size (nm) | Shapes | Reference |
|---|---|---|---|---|
| Morinda citrifolia L. | Leaves, fruit pulp, seeds | 3–11 | Spherical | [56] |
| Nymphae odorata | Leaves | 15 ± 5 | Spherical | [57] |
| Capparis zeylanica | Leaves | 23 | Spherical | [58] |
| Caesalpinia pulcherrima | Leaves | 9 | Spherical | [59] |
| Carya illinoinensis | Leaves | 12–30 | Spherical | [60] |
| Mentha piperita | Leaves extract | 35 | Spherical | [61] |
| Jatropha curcas | Latex | 10–20 | Face-centered cubic | [62] |
| Acalypha indica | Leaves extract | 20–30 | Spherical | [63] |
| Hibiscus rosa sinensis | Leaves | 14 | Spherical/prism | [64] |
| Cycas | Leaves | 2–6 | Spherical | [46] |
| Ceratonia siliqua | Leaves extract | 5–40 | Spherical | [65] |
| Suaeda monoica | Leaves | 31 | Spherical | [66] |
| Catharanthtus roseus | Leaves | 35–55 | Cubical | [51] |
| Ocimum sanctum | Leaves extract | 10–20 | Spherical | [67] |
| Ocimum tenuiflorum | Leaves | 25–40 | Spherical | [68] |
| Ginkgo biloba | Leaves | 15–500 | Cubic | [69] |
| Tanacetum vulgare | Fruit | 16 | Spherical | [70] |
| Argemone mexicana | Leaves extract | 30 | Spherical, hexagonal | [71] |
| Sesuvium portulacastrum | Callus extract | 5–20 | Spherical | [72] |
| Syzygium cumini | Leaves and seed | 29–92 | Spherical | [49,73] |
| Cinnamomum camphora | Sun dried leaves | 3.2–20 | Cubic hexagonal crystalline | [74] |
| Melia azedarach | Leaves | 78 | Spherical | [75] |
| Rhododedendron dauricam | Flower extract | 25–40 | Spherical | [76] |
| Lippia citriodora | Leaves extract | 15–30 | Crystalline | [77] |
| Tribulus terrestris | Fruit | 16–28 | Spherical | [44] |
| Citrullusm colocynthis | Leaves | 31 | Spherical | [78] |
| Plant Name | Parts Used | Size (nm) | Shapes | Reference |
|---|---|---|---|---|
| Parkia biglobosa | Leaves | 1–35 | Truncated, pentagonal, spherical, triangular | [39] |
| Curcuma pseudomontana | Rhizome | 20 | Spherical | [89] |
| Lawsonia inermis | Leaves | 20 | Spherical | [90] |
| Cinnamon | Bark | 35 | Spherical | [91] |
| Croton Caudatus Geisel | Leaves | 20 | Spherical | [13] |
| Tamarind | Leaves | 20–40 | Triangle | [36] |
| Aloe vera | Plant extract | 50/350 | Crystalline | [92] |
| Mentha, Ocimum, Eucalyptus | Leaves | 3–16 | Spherical | [93] |
| Canna indica, Quisqualis indica | Leaves and flower | 30–130 | Polymorphic/stable | [94] |
| Murraya koenigii | Leaves | 20 | Spherical | [95] |
| Aegle marmelos | Leaves | 4–10 | Spherical | [84] |
| Rosa hybrid | Rose petals | 10 | Cubic | [96] |
| Terminalia chebula | Plant extract | 6–60 | Anisotropic | [97] |
| Momordica charantia | Fruit | 30–40 | Cubical | [98] |
| Phyllanthus amarus | Leaves | 65–99 | Cubic | [99] |
| Mangifera indica | Leaves | 17–20 | Spherical | [100] |
| Stevia rebaudiana | Leaves | 8–20 | Octahedral | [101] |
| Nyctanthes arbortristis | Flower extract | 19.8 | Spherical, hexagonal | [83] |
| Trigonella foneum-graecum | Leaves | 15–25 | Spherical | [79] |
| Tanacetum vulgare | Fruit | 11 | Triangular | [70] |
| Cuminum cyminum | Seeds | 1–10 | Spherical | [102] |
| Sorbus aucuparia | Leaf extract | 16–18 | Spherical, triangular, hexagonal | [103] |
| Plant Name | Parts Used | Size (nm) | Shapes | Reference |
|---|---|---|---|---|
| Artemisia pallens | Leaves along with stem | 50–100 | Hexagonal | [109] |
| Cayratia pedata | Leaves | 52.24 | Spherical | [115] |
| Euphorbia hirta | Leaves | 20–50 | Spherical | [116] |
| Eucalyptus globules | Leaves | 52–70 | Spherical, elongated | [108] |
| Tecoma castanifolia | Leaves | 70–75 | Spherical | [117] |
| Zingiber officinale | Root | 30–50 | Spherical | [118] |
| Azadirachta indica | Leaves | 50 | Spindle shaped | [119] |
| Catharanthus roseus | Leaves | 23–57 | Spherical | [120] |
| Solanum nigrum | Leaves | 20–30 | Hexagonal | [121] |
| Olea europea | Leaves | 18–30 | Crystalline | [122] |
| Azadirachta indica | Leaves | 25 | Crystalline | [123] |
| Nyctanthes arbor-tristis | Flowers | 12–32 | Crystalline | [124] |
| Hibiscus rosa-sinensis | Leaves | 30–35 | Crystal, spongy | [125] |
| Ruta graveolens | Stem | 28 | Spherical | [106] |
| Aloe vera | Leaves | 22.18 | Hexagonal | [126] |
| Ocimum tenuiflorum | Leaves | 11–25 | Hexagonal | [127] |
| Sargassum muticum | Leaves | 30–57 | Hexagonal | [128] |
| Calotropis gigantea | Leaves | 1.5–8.5 | Spherical | [107] |
| Beta vulgaris | Root | 52–76 | Hexagonal | [129] |
| Curcuma longa | Root | 20–80 | Hexagonal | [130] |
| Nephelium lappaceum | Peel | 20 | Spherical | [131] |
| Artocarpus gomezianus | Fruit | 50 | Spherical | [132] |
| Senna auriculata | Leaves | 2 | Spherical | [133] |
| Brassica oleraceae | Leaves | 1–100 | Spherical and sheet shaped | [134] |
| Acalypha Indica | Leaves | 100–200 | Cube | [135] |
| Plectranthus amboinicus | Leaves | 20–50 | Crystalline | [136] |
| Coptidis rhizome | Rhizome | 2.9–25.2 | Spherical and rod shaped | [137] |
| Ginger | Rhizome | 23–26 | Crystalline | [138] |
| Plant Name | Parts Used | Size (nm) | Shapes | Reference |
|---|---|---|---|---|
| Ledebouria revoluta | Bulb | 47 | Tetragonal | [147] |
| Pouteria campechiana | Leaves | 73–140 | Spherical | [148] |
| Syzygium cumini | Leaves | 22 | Spherical round | [149] |
| Mentha arvensis | Leaves | 20–70 | Spherical | [150] |
| Azadirachta indica | Leaves | 15–50 | Spherical | [151] |
| Psidium guajava | Leaves | 32.58 | Spherical | [152] |
| Nyctanthes arbor-tristis | Leaves | 100–150, 100–200 | Cubic, crystalline, Spherical | [153] |
| Calotropis gigantea | Flower | 10–52 | Crystalline, Spherical oval | [154] |
| Salvia officinalis | Leaves | 15–20 | Spherical | [140] |
| Solanum trilobatum | Leaves | 70 | Spherical, oval | [155] |
| Azadirachta indica | Leaves | 124 | Spherical | [156] |
| Annona squamosal | Leaves | 40–60 | Spherical | [157] |
| Jatropha curcas, citrus aurantium | Leaves | 25–50 | Spherical | [158] |
| Jatropha curcas | Latex | 25–50 | Spherical, uneven | [159] |
| Euphorbia prostrata | Leaves | 81–84 | Spherical | [160] |
| Citrus sinensis | Fruit peel | 19 | Tetragonal | [161] |
| Cassia auriculata | Leaves | 38 | Spherical | [162] |
| Ocimum basilicum | Leaves | 50 | Hexagonal | [163] |
| Hibiscus-rosa-sinensis | Petals | 7–24 | Spherical | [12] |
| Erythrina variegates | Leaves | 39 | Crystalline, spherical | [164] |
| Plant Name | Parts Used | Size (nm) | Shapes | Reference |
|---|---|---|---|---|
| Peganum harmala | Seed | 22.5 ± 5.7 | Spherical | [173] |
| Coleus amboinicus | Leaves | 40–50 | Spherical | [174] |
| Anogeissus latifolia | Gum ghatti | 4.8 ± 1.6 | Spherical | [175] |
| Filicium decipiens | Leaves | 2–22 | Spherical | [176] |
| Cinnamomum camphora | Leaves | 3.2–6 | Multiple | [177] |
| Pulicariaglutinosa | Leaves | 3–5 | Spherical | [170] |
| Musa paradisica | Peeled banana | 50 | Crystalline | [178] |
| Cinnamom zeylanicum | Bark | 15–20 | Crystalline | [169] |
| Catharanthus roseus | Leaves | 38 | Spherical | [179] |
| Curcuma longa | Tuber | 10–15 | Spherical | [180] |
| Glycine max | Leaves | 15 | Spherical | [171] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, F.; Shariq, M.; Asif, M.; Siddiqui, M.A.; Malan, P.; Ahmad, F. Green Nanotechnology: Plant-Mediated Nanoparticle Synthesis and Application. Nanomaterials 2022, 12, 673. https://doi.org/10.3390/nano12040673
Khan F, Shariq M, Asif M, Siddiqui MA, Malan P, Ahmad F. Green Nanotechnology: Plant-Mediated Nanoparticle Synthesis and Application. Nanomaterials. 2022; 12(4):673. https://doi.org/10.3390/nano12040673
Chicago/Turabian StyleKhan, Faryad, Mohammad Shariq, Mohd Asif, Mansoor Ahmad Siddiqui, Pieter Malan, and Faheem Ahmad. 2022. "Green Nanotechnology: Plant-Mediated Nanoparticle Synthesis and Application" Nanomaterials 12, no. 4: 673. https://doi.org/10.3390/nano12040673
APA StyleKhan, F., Shariq, M., Asif, M., Siddiqui, M. A., Malan, P., & Ahmad, F. (2022). Green Nanotechnology: Plant-Mediated Nanoparticle Synthesis and Application. Nanomaterials, 12(4), 673. https://doi.org/10.3390/nano12040673








