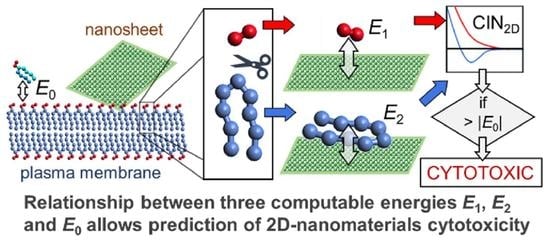
Computational Indicator Approach for Assessment of Nanotoxicity of Two-Dimensional Nanomaterials
Abstract
1. Introduction
2. Method and Models
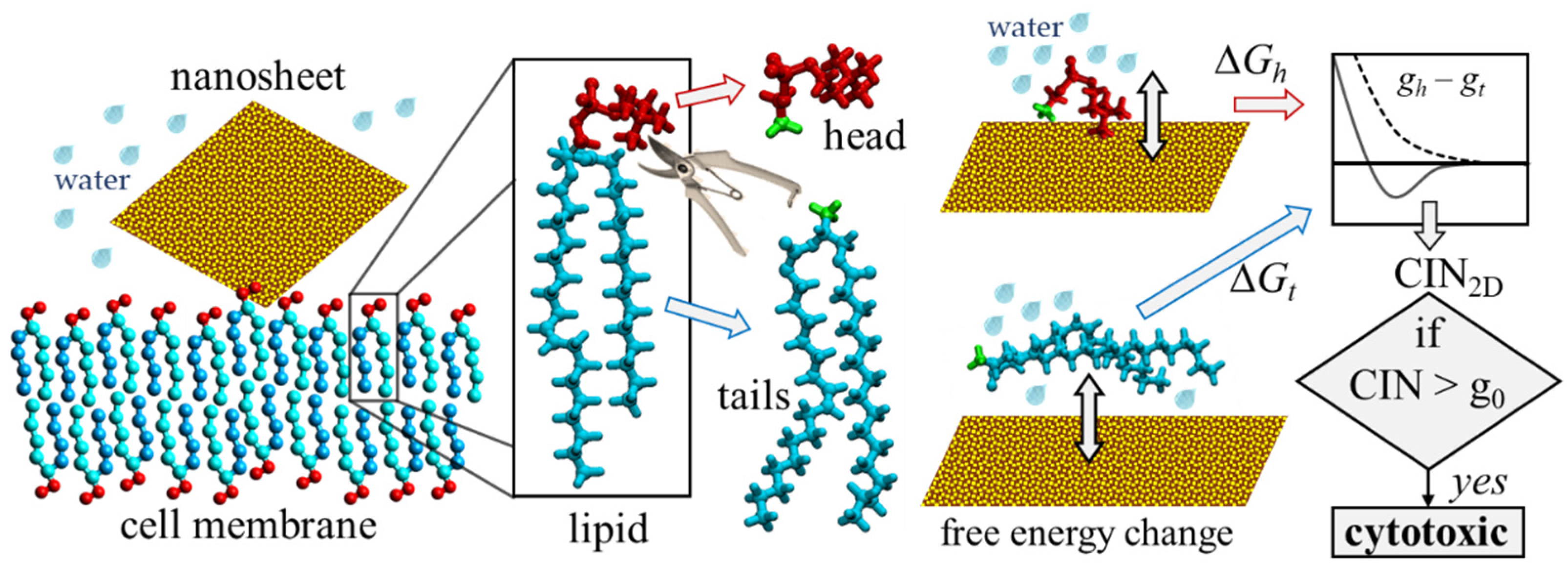
2.1. Computational Indicator of Nanotoxicity of Two-Dimensional Nanomaterials (CIN2D)
2.2. Numerical Procedure for Rapid Assessment of CIN2D
2.3. Graphene and Graphene Oxide Models
2.4. Layered Double Hydroxide and Aloohene Models
2.5. Boron Nitride Nanosheet Models
2.6. Lipid and Water Models
2.7. Simulation Details
3. Results and Discussion
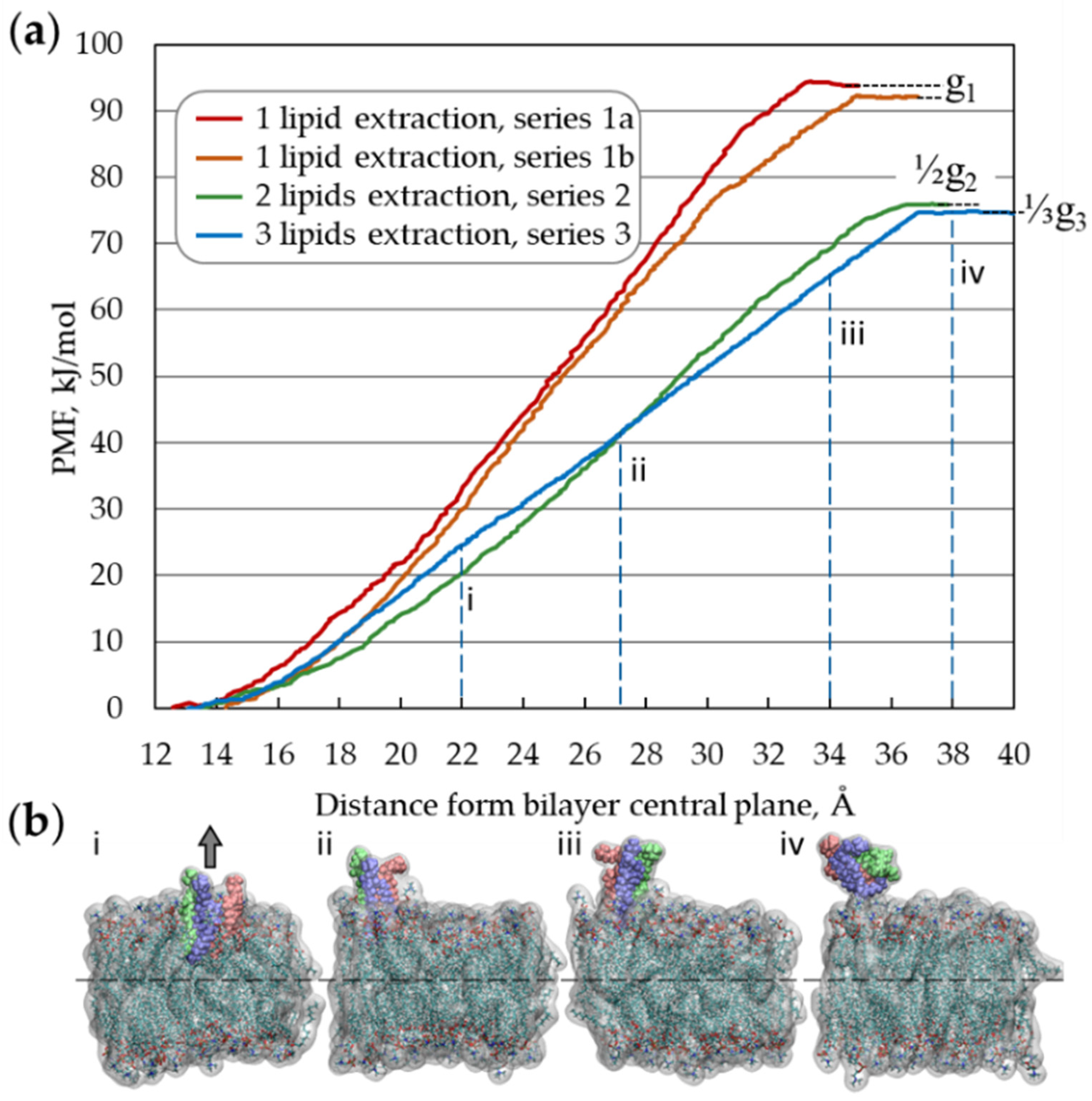
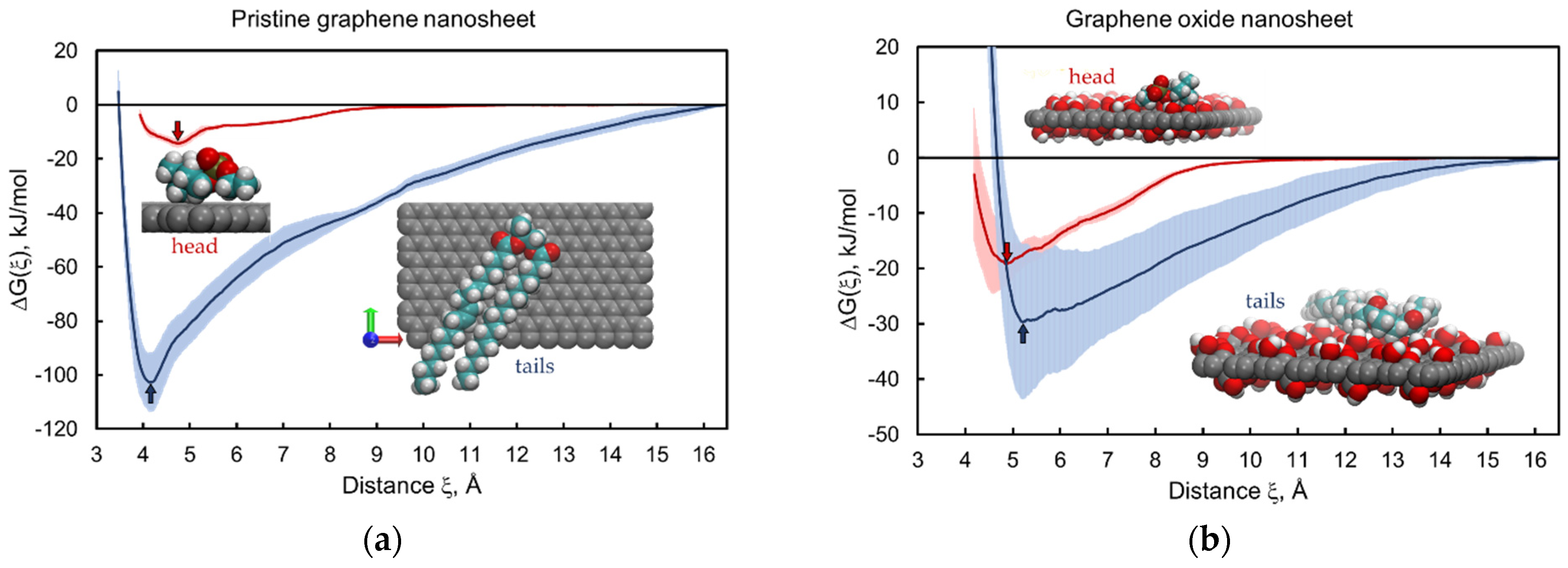
3.1. Graphene and Graphene Oxide Nanosheets
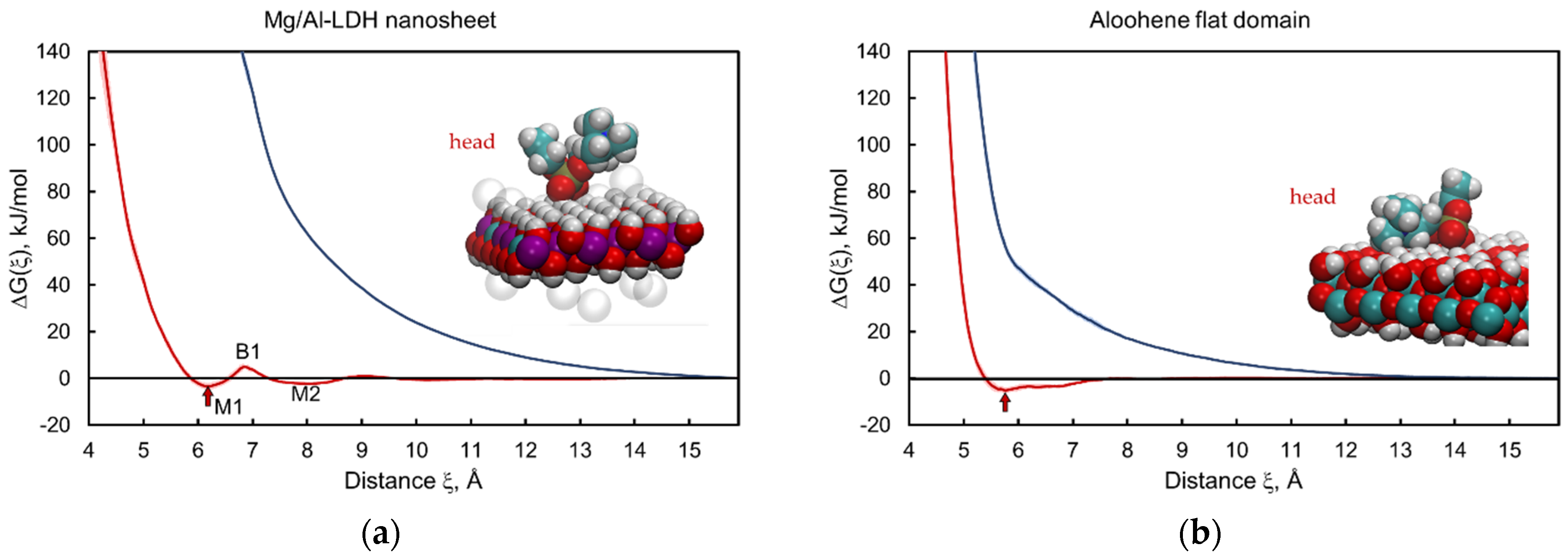
3.2. Layered Double Hydroxide Nanosheet and Aloohene Flat Domain
3.3. Boron Nitride Nanosheets
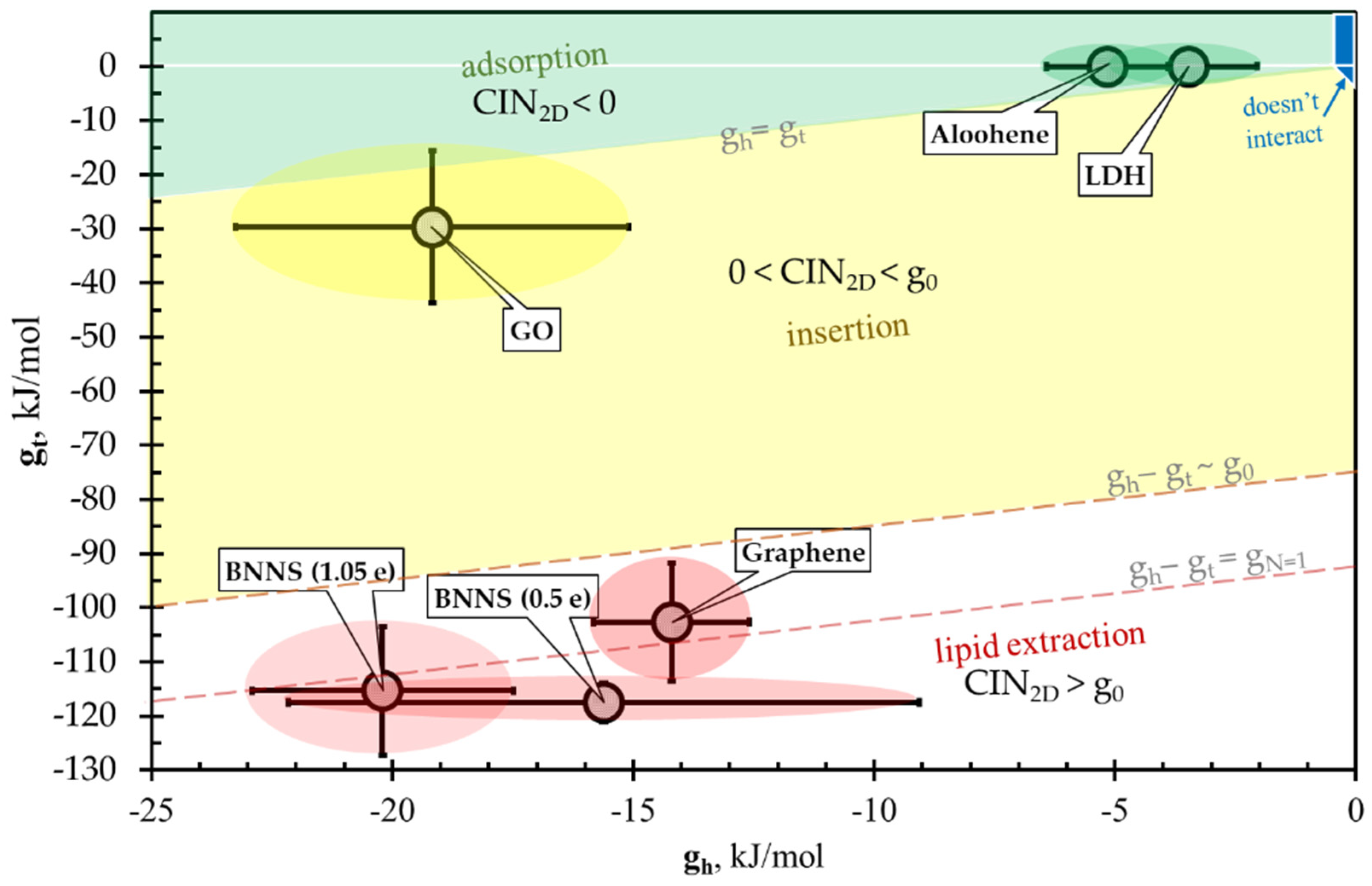
3.4. CIN2D Diagram
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.; Song, C.; Cho, H.R.; Ghaffari, R.; Choi, T.K.; Kim, K.H.; Lee, Y.B.; Ling, D.; Lee, H.; et al. An endoscope with integrated transparent bioelectronics and theranostic nanoparticles for colon cancer treatment. Nat. Commun. 2015, 6, 10059. [Google Scholar] [CrossRef]
- Mikhaylov, G.; Mikac, U.; Magaeva, A.A.; Itin, V.I.; Naiden, E.P.; Psakhye, I.; Babes, L.; Reinheckel, T.; Peters, C.; Zeiser, R.; et al. Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nat. Nanotechnol. 2011, 6, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, Y.T.; Yu, M.; Guo, J.; Chaudhary, D.; Wang, C.C. Cancer therapy and fluorescence imaging using the active release of doxorubicin from MSPs/Ni-LDH folate targeting nanoparticles. Biomaterials 2013, 34, 7913–7922. [Google Scholar] [CrossRef]
- Liang, L.; Shen, J.W.; Wang, Q. Molecular dynamics study on DNA nanotubes as drug delivery vehicle for anticancer drugs. Colloids Surf. B Biointerfaces 2017, 153, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Lerner, M.I.; Mikhaylov, G.; Tsukanov, A.A.; Lozhkomoev, A.S.; Gutmanas, E.; Gotman, I.; Bratovs, A.; Turk, V.; Turk, B.; Psakhye, S.G.; et al. Crumpled aluminum hydroxide nanostructures as a microenvironment dysregulation agent for cancer treatment. Nano Lett. 2018, 18, 5401–5410. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Landis, R.F.; Li, C.H.; Schnurr, M.; Das, R.; Lee, Y.W.; Yazdani, M.; Liu, Y.; Kozlova, A.; Rotello, V.M. Engineered polymer nanoparticles with unprecedented antimicrobial efficacy and therapeutic indices against multidrug-resistant bacteria and biofilms. J. Am. Chem. Soc. 2018, 140, 12137–12143. [Google Scholar] [CrossRef]
- Zhou, W.; Pan, T.; Cui, H.; Zhao, Z.; Chu, P.K.; Yu, X.F. Black phosphorus: Bioactive nanomaterials with inherent and selective chemotherapeutic effects. Angew. Chem. 2019, 131, 779–784. [Google Scholar] [CrossRef]
- Dart, A. Chemotherapy: Less is more. Nat. Rev. Cancer 2017, 17, 3. [Google Scholar] [CrossRef]
- Seton-Rogers, S. Chemotherapy: Preventing competitive release. Nat. Rev. Cancer 2016, 16, 199. [Google Scholar] [CrossRef]
- Fiorito, S.; Serafino, A.; Andreola, F.; Bernier, P. Effects of fullerenes and single-wall carbon nanotubes on murine and human macrophages. Carbon 2006, 44, 1100–1105. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Altman, S.A.; Randers, L.; Rao, G. Comparison of trypan blue dye exclusion and fluorometric assays for mammalian cell viability determinations. Biotechnol. Prog. 1993, 9, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.M.; Fortner, J.D.; Guo, W.; Lyon, D.; Boyd, A.M.; Ausman, K.D.; Colvin, V.L. The differential cytotoxicity of water-soluble fullerenes. Nano Lett. 2004, 4, 1881–1887. [Google Scholar] [CrossRef]
- Sambale, F.; Wagner, S.; Stahl, F.; Khaydarov, R.R.; Scheper, T.; Bahnemann, D. Investigations of the toxic effect of silver nanoparticles on mammalian cell lines. J. Nanomater. 2015, 16, 6. [Google Scholar] [CrossRef]
- Chng, E.; Chua, C.; Pumera, M. Graphene oxide nanoribbons exhibit significantly greater toxicity than graphene oxide nanoplatelets. Nanoscale 2014, 6, 10792–10797. [Google Scholar] [CrossRef]
- Kargar, H.; Ghasemi, F.; Darroudi, M. Bioorganic polymer-based synthesis of cerium oxide nanoparticles and their cell viability assays. Ceram. Int. 2015, 41, 1589–1594. [Google Scholar] [CrossRef]
- Malich, G.; Markovic, B.; Winder, C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997, 124, 179–192. [Google Scholar] [CrossRef]
- Mannerström, M.; Zou, J.; Toimela, T.; Pyykkö, I.; Heinonen, T. The applicability of conventional cytotoxicity assays to predict safety/toxicity of mesoporous silica nanoparticles, silver and gold nanoparticles and multi-walled carbon nanotubes. Toxicol. Vitr. 2016, 37, 113–120. [Google Scholar] [CrossRef]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L.; Hume, C.W. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959; Volume 238. [Google Scholar]
- Passini, E.; Britton, O.J.; Lu, H.R.; Rohrbacher, J.; Hermans, A.N.; Gallacher, D.J.; Greig, R.J.H.; Bueno-Orovio, A.; Rodriguez, B. Human in silico drug trials demonstrate higher accuracy than animal models in predicting clinical pro-arrhythmic cardiotoxicity. Front. Physiol. 2017, 8, 668. [Google Scholar] [CrossRef]
- Gleeson, M.P.; Modi, S.; Bender, A.; Marchese Robinson, R.L.; Kirchmair, J.; Promkatkaew, M.; Hannongbua, S.; Glen, R. The challenges involved in modeling toxicity data in silico: A review. Curr. Pharm. Des. 2012, 18, 1266–1291. [Google Scholar] [CrossRef] [PubMed]
- Ellison, C.M.; Enoch, S.J.; Cronin, M.T. A review of the use of in silico methods to predict the chemistry of molecular initiating events related to drug toxicity. Expert Opin. Drug Metab. Toxicol. 2011, 7, 1481–1495. [Google Scholar] [CrossRef] [PubMed]
- Fourches, D.; Pu, D.; Li, L.; Zhou, H.; Mu, Q.; Su, G.; Yan, B.; Tropsha, A. Computer-aided design of carbon nanotubes with the desired bioactivity and safety profiles. Nanotoxicology 2016, 10, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Kleandrova, V.V.; Gonzalez-Diaz, H.; Ruso, J.M.; Melo, A.; Speck-Planche, A.; Cordeiro, M.N.D. Computer-aided nanotoxicology: Assessing cytotoxicity of nanoparticles under diverse experimental conditions by using a novel QSTR-perturbation approach. Nanoscale 2014, 6, 10623–10630. [Google Scholar] [CrossRef]
- Puzyn, T.; Rasulev, B.; Gajewicz, A.; Hu, X.; Dasari, T.P.; Michalkova, A.; Hwang, H.-M.; Toropov, A.; Leszczynska, D.; Leszczynski, J. Using nano-QSAR to predict the cytotoxicity of metal oxide nanoparticles. Nat. Nanotechnol. 2011, 6, 175–178. [Google Scholar] [CrossRef]
- Sosnin, S.; Misin, M.; Palmer, D.; Fedorov, M. 3D matters! 3D-RISM and 3D convolutional neural network for accurate bioaccumulation prediction. J. Phys. Condens. Matter 2018, 30, 32LT03. [Google Scholar] [CrossRef]
- Ma, C.; Kang, H.; Liu, Q.; Zhu, R.; Cao, Z. Insight into potential toxicity mechanisms of melamine: An in silico study. Toxicology 2011, 283, 96–100. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Ung, C.Y. Prediction of potential toxicity and side effect protein targets of a small molecule by a ligand–protein inverse docking approach. J. Mol. Graph. Model. 2001, 20, 199–218. [Google Scholar] [CrossRef]
- Kraszewski, S.; Tarek, M.; Ramseyer, C. Uptake and translocation mechanisms of cationic amino derivatives functionalized on pristine C60 by lipid membranes: A molecular dynamics simulation study. ACS Nano 2011, 5, 8571–8578. [Google Scholar] [CrossRef]
- Tsukanov, A.; Psakhie, S.G. Molecular level in silico studies for oncology. Direct models review. AIP Conf. Proc. 2017, 1882, 020058. [Google Scholar]
- Shi, X.; von Dem Bussche, A.; Hurt, R.H.; Kane, A.B.; Gao, H. Cell entry of one-dimensional nanomaterials occurs by tip recognition and rotation. Nat. Nanotechnol. 2011, 6, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Guo, R.; Yan, L.T. Simulation and analysis of cellular internalization pathways and membrane perturbation for graphene nanosheets. Biomaterials 2014, 35, 6069–6077. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Jin, X.; Guan, Z.; Lin, J.; Cai, C.; Wang, L.; Gao, L. Membrane Nanopores Induced by Nanotoroids via an Insertion and Pore-Forming Pathway. Nano Lett. 2021, 21, 8545–8553. [Google Scholar] [CrossRef]
- Shityakov, S.; Roewer, N.; Broscheit, J.A.; Förster, C. In silico models for nanotoxicity evaluation and prediction at the blood-brain barrier level: A mini-review. Comput. Toxicol. 2017, 2, 20–27. [Google Scholar] [CrossRef]
- Tsukanov, A.; Psakhie, S.G. Review of Computer Simulation Studies of Cell Membrane Interaction with Neutral and Charged Nano-Objects. Quasi-Zero-Dimensional Nanoparticles, Drugs and Fullerenes. Adv. Biomater. Devices Med. 2015, 2, 44–53. [Google Scholar]
- Jimenez-Cruz, C.A.; Kang, S.G.; Zhou, R. Large scale molecular simulations of nanotoxicity. Wiley Interdiscip. Rev. Syst. Biol. Med. 2014, 6, 329–343. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef]
- Gonzalez, L.; Lison, D.; Kirsch-Volders, M. Genotoxicity of engineered nanomaterials: A critical review. Nanotoxicology 2008, 2, 252–273. [Google Scholar] [CrossRef]
- Gennis, R.B. Biomembranes: Molecular Structure and Function; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Mitaku, S. The role of hydrophobic interaction in phase transition and structure formation of lipid membranes and proteins. Phase Transit. A Multinatl. J. 1993, 45, 137–155. [Google Scholar] [CrossRef]
- Jo, B.C.; Yoon, H.J.; Ok, M.R.; Wu, S. Molecular dynamics simulation of cytotoxicity of graphene nanosheets to blood-coagulation protein. Biointerphases 2017, 12, 01A403. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Kanno, S.; Furuyama, A. Multi-walled carbon nanotubes injure the plasma membrane of macrophages. Toxicol. Appl. Pharmacol. 2008, 232, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Doshi, N.; Mitragotri, S. Needle-shaped polymeric particles induce transient disruption of cell membranes. J. R. Soc. Interface 2010, 7, S403–S410. [Google Scholar] [CrossRef] [PubMed]
- Izrailev, S.; Stepaniants, S.; Isralewitz, B.; Kosztin, D.; Lu, H.; Molnar, F.; Wriggers, W.; Schulten, K. Steered Molecular Dynamics. Computational Molecular Dynamics: Challenges, Methods, Ideas; Springer: Berlin, Germany, 1997; pp. 39–65. [Google Scholar]
- Isralewitz, B.; Gao, M.; Schulten, K. Steered molecular dynamics and mechanical functions of proteins. Curr. Opin. Struct. Biol. 2001, 11, 224–230. [Google Scholar] [CrossRef]
- Titov, A.V.; Král, P.; Pearson, R. Sandwiched graphene−membrane superstructures. ACS Nano 2009, 4, 229–234. [Google Scholar] [CrossRef]
- Tsukanov, A.; Psakhie, S.G. From the soft matter-hard matter interface to bio-self-organization and hybrid systems. Phys. Mesomech. 2017, 20, 43–54. [Google Scholar] [CrossRef]
- Tsukanov, A.; Psakhie, S.G. The role of defects in the formation of hierarchical AlOOH-based nanomaterials for biomedical applications. AIP Conf. Proc. 2018, 2053, 040099. [Google Scholar]
- Haugen, A.; May, S. The influence of zwitterionic lipids on the electrostatic adsorption of macroions onto mixed lipid membranes. J. Chem. Phys. 2007, 127, 215104. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Tang, H.; Liu, D.; Zhao, Y.; Yang, X.; Lu, J.; Cui, F. A Molecular dynamics study of the aggregation process of graphene oxide in water. J. Phys. Chem. C 2015, 119, 26712–26718. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Yakovenchuk, V.N.; Zhitova, E.S.; Zolotarev, A.A.; Pakhomovsky, Y.A.; Ivanyuk, G.Y. Crystal chemistry of natural layered double hydroxides. I. Quintinite-2H-3c from the Kovdor alkaline massif, Kola peninsula, Russia. Mineral. Mag. 2010, 74, 821–832. [Google Scholar] [CrossRef]
- Noel, Y.; Demichelis, R.; Pascale, F.; Ugliengo, P.; Orlando, R.; Dovesi, R. Ab initio quantum mechanical study of γ-AlOOH boehmite: Structure and vibrational spectrum. Phys. Chem. Miner. 2009, 36, 47–59. [Google Scholar] [CrossRef]
- Lozhkomoev, A.S.; Lerner, M.I.; Tsukanov, A.A.; Kazantsev, S.O.; Bakina, O.V.; Psakhie, S.G. On the possibility of soft matter nanostructure formation based on mesoporous aluminum hydroxide. Prospects for biomedical applications. Phys. Mesomech. 2017, 20, 134–141. [Google Scholar] [CrossRef]
- Cygan, R.T.; Liang, J.-J.; Kalinichev, A.G. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field. J. Phys. Chem. B 2004, 108, 1255–1266. [Google Scholar] [CrossRef]
- MacKerell, A.D., Jr.; Bashford, D.; Bellott, M.L.D.R.; Dunbrack, R.L., Jr.; Evanseck, J.D.; Field, M.J.; Fischer, S.; Gao, J.; Guo, H.; Ha, S.J. All-atom Empirical Potential for Molecular Modeling and Dynamics Studies of Proteins. Phys. Chem. B 1998, 102, 3586–3616. [Google Scholar] [CrossRef]
- Tsukanov, A.; Psakhie, S.G. Energy and structure of bonds in the interaction of organic anions with layered double hydroxide nanosheets: A molecular dynamics study. Sci. Rep. 2016, 6, 19986. [Google Scholar] [CrossRef]
- Azamat, J.; Sardroodi, J.J. The permeation of potassium and chloride ions through nanotubes: A molecular simulation study. Mon. Für Chem.-Chem. Mon. 2014, 145, 881–890. [Google Scholar] [CrossRef]
- Tsukanov, A.; Psakhie, S.G. Potential of mean force analysis of short boron nitride and carbon nanotubes insertion into cell membranes. Adv. Biomater. Devices Med. 2016, 3, 1–9. [Google Scholar]
- Won, C.; Aluru, N.J. Structure and dynamics of water confined in a boron nitride nanotube. Phys. Chem. C 2008, 112, 1812–1818. [Google Scholar] [CrossRef]
- Hilder, T.; Gaston, N. Interaction of Boron Nitride Nanosheets with Model Cell Membranes. Chem. Phys. Chem. 2016, 17, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Enciso, M.; Hilder, T.A. Insertion mechanism and stability of boron nitride nanotubes in lipid bilayers. J. Phys. Chem. B 2015, 119, 4929–4936. [Google Scholar] [CrossRef] [PubMed]
- Klauda, J.B.; Venable, R.M.; Freites, J.A.; O’Connor, J.W.; Tobias, D.J.; Mondragon-Ramirez, C.; Vorobyov, I.; MacKerell, A.D., Jr.; Pastor, R.W. Update of the CHARMM all-atom additive force field for lipids: Validation on six lipid types. J. Phys. Chem. B 2010, 114, 7830–7843. [Google Scholar] [CrossRef] [PubMed]
- Neria, E.; Fischer, S.; Karplus, M. Simulation of activation free energies in molecular systems. J. Chem. Phys. 1996, 105, 1902–1921. [Google Scholar] [CrossRef]
- Hockney, R.W.; Goel, S.P.; Eastwood, J.W. A 10000 particle molecular dynamics model with long range forces. Chem. Phys. Lett. 1973, 21, 589–591. [Google Scholar] [CrossRef]
- Hockney, R.W.; Eastwood, J.W. Computer Simulation Using Particles; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Moradi, M.; Tajkhorshid, E. Driven metadynamics: Reconstructing equilibrium free energies from driven adaptive-bias simulations. J. Phys. Chem. Lett. 2013, 4, 1882–1887. [Google Scholar] [CrossRef]
- Tsukanov, A.; Psakhie, S.G. Adhesion effects within the hard matter–soft matter interface: Molecular dynamics. Facta Univ. Ser. Mech. Eng. 2016, 14, 269–280. [Google Scholar] [CrossRef][Green Version]
- Jarzynski, C. Nonequilibrium equality for free energy differences. Phys. Rev. Lett. 1997, 78, 2690. [Google Scholar] [CrossRef]
- Frenkel, D.; Smit, B. Understanding Molecular Simulation: From Algorithms to Applications; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Voevodin, V.; Antonov, A.; Nikitenko, D.; Shvets, P.; Sobolev, S.; Sidorov, I.; Stefanov, K.; Voevodin, V.; Zhumatiy, S. Supercomputer Lomonosov-2: Large scale, deep monitoring and fine analytics for the user community. Supercomput. Front. Innov. 2019, 6, 4–11. [Google Scholar]
- Adinets, A.; Bryzgalov, P.; Voevodin, V.; Zhumatii, S.; Nikitenko, D.; Stefanov, K. Job digest: An approach to dynamic analysis of job characteristics on supercomputers. Numer. Methods Program. Adv. Comput. 2012, 13, 160–166. [Google Scholar]
- Zacharov, I.; Arslanov, R.; Gunin, M.; Stefonishin, D.; Pavlov, S.; Panarin, O.; Maliutin, A.; Rykovanov, S.; Fedorov, M. “Zhores”—Petaflops supercomputer for data-driven modeling, machine learning and artificial intelligence installed in Skolkovo Institute of Science and Technology. Open Eng. 2019, 9, 512–520. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Plimpton, S.; Crozier, P.; Thompson, A. LAMMPS-Large-Scale Atomic/Molecular Massively Parallel Simulator; Sandia National Laboratories: Albuquerque, NM, USA, 2007; Volume 18, p. 43.
- Humphrey, W.; Dalke, A.; Schulten, K. VMD - visual molecular dynamics. J. Molec. Graphics 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Catania, F.; Marras, E.; Giorcelli, M.; Jagdale, P.; Lavagna, L.; Tagliaferro, A.; Bartoli, M. A Review on Recent Advancements of Graphene and Graphene-Related Materials in Biological Applications. Appl. Sci. 2021, 11, 614. [Google Scholar] [CrossRef]
- Shim, G.; Ko, S.; Park, J.Y.; Suh, J.H.; Le, Q.V.; Kim, D.; Oh, Y.K. Tannic acid-functionalized boron nitride nanosheets for theranostics. J. Control. Release 2020, 327, 616–626. [Google Scholar] [CrossRef]
- Qian, Y.; Xu, Y.; Yan, Z.; Jin, Y.; Chen, X.; Yuan, W.E.; Fan, C. Boron nitride nanosheets functionalized channel scaffold favors microenvironment rebalance cocktail therapy for piezocatalytic neuronal repair. Nano Energy 2021, 83, 105779. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Bu, W. Bioactive nanomaterials for ion-interference therapy. View 2020, 1, e18. [Google Scholar] [CrossRef]
- Lozhkomoev, A.S.; Mikhaylov, G.; Turk, V.; Turk, B.; Vasiljeva, O. Application of Crumpled Aluminum Hydroxide Nanostructures for Cancer Treatment. In Multiscale Biomechanics and Tribology of Inorganic and Organic Systems; Springer: Cham, Switzerland, 2021; pp. 211–223. [Google Scholar]
- Tsukanov, A.A.; Vasiljeva, O. Nanomaterials Interaction with Cell Membranes: Computer Simulation Studies. In Multiscale Biomechanics and Tribology of Inorganic and Organic Systems; Springer: Cham, Switzerland, 2021; pp. 189–210. [Google Scholar]
- Cao, Z.; Li, B.; Sun, L.; Li, L.; Xu, Z.P.; Gu, Z. 2D layered double hydroxide nanoparticles: Recent progress toward preclinical/clinical nanomedicine. Small Methods 2020, 4, 1900343. [Google Scholar] [CrossRef]
- Zhang, L.X.; Hu, J.; Jia, Y.B.; Liu, R.T.; Cai, T.; Xu, Z.P. Two-dimensional layered double hydroxide nanoadjuvant: Recent progress and future direction. Nanoscale 2021, 13, 7533–7549. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.H.; Lin, Y.S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yang, S.-T.; Liu, J.-H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, A.; Panchakarla, L.S.; Chandran, P.; Menon, D.; Nair, S.; Rao, C.N.R.; Koyakutty, M. Differential nano-bio interactions and toxicity effects of pristine versus functionalized graphene. Nanoscale 2011, 3, 2461–2464. [Google Scholar] [CrossRef]
- Bahrami, A.H.; Raatz, M.; Agudo-Canalejo, J.; Michel, R.; Curtis, E.M.; Hall, C.K.; Gradzielski, M.; Lipowsky, R.; Weikl, T.R. Wrapping of nanoparticles by membranes. Adv. Colloid Interface Sci. 2014, 208, 214–224. [Google Scholar] [CrossRef]
- Ladewig, K.; Niebert, M.; Xu, Z.P.; Gray, P.P.; Lu, G.Q. Efficient siRNA delivery to mammalian cells using layered double hydroxide nanoparticles. Biomaterials 2010, 31, 1821–1829. [Google Scholar] [CrossRef]
- Choi, S.J.; Oh, J.M.; Choy, J.H. Safety aspect of inorganic layered nanoparticles: Size-dependency in vitro and in vivo. J. Nanosci. Nanotechnol. 2008, 8, 5297–5301. [Google Scholar] [CrossRef]
- Kura, A.U.; Hussein, M.Z.; Fakurazi, S.; Arulselvan, P. Layered double hydroxide nanocomposite for drug delivery systems; bio-distribution, toxicity and drug activity enhancement. Chem. Cent. J. 2014, 8, 1–8. [Google Scholar] [CrossRef]
- Cunha, V.R.R.; de Souza, R.B. Accessing the biocompatibility of layered double hydroxide by intramuscular implantation: Histological and microcirculation evaluation. Sci. Rep. 2016, 6, 30547. [Google Scholar] [CrossRef]
- Fomenko, A.N.; Korovin, M.S. Comparative analysis of the effect of low-dimensional alumina structures on cell lines L929 and Neuro-2a. AIP Conf. Proc. 2016, 1760, 020015. [Google Scholar]
- Ciofani, G.; Raffa, V.; Menciassi, A.; Cuschieri, A. Boron nitride nanotubes: An innovative tool for nanomedicine. Nano Today 2009, 4, 8–10. [Google Scholar] [CrossRef]
- Sukhorukova, I.V.; Zhitnyak, I.Y.; Kovalskii, A.M.; Matveev, A.T.; Lebedev, O.I.; Li, X.; Gloushankova, N.A.; Golberg, D.; Shtansky, D.V. Boron nitride nanoparticles with a petal-like surface as anticancer drug-delivery systems. ACS Appl. Mater. Interfaces 2015, 7, 17217–17225. [Google Scholar] [CrossRef] [PubMed]
- Weng, Q.; Wang, B.; Wang, X.; Hanagata, N.; Li, X.; Liu, D.; Wang, X.; Jiang, X.; Bando, Y.; Golberg, D. Highly water-soluble, porous, and biocompatible boron nitrides for anticancer drug delivery. ACS Nano 2014, 8, 6123–6130. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Danti, S.; Genchi, G.G.; Mazzolai, B.; Mattoli, V. Boron nitride nanotubes: Biocompatibility and potential spill-over in nanomedicine. Small 2013, 9, 1672–1685. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, C.; Li, Z.; Ma, J.; Meng, Q.; Zhi, C.; Fan, J. Nanotoxicity of Boron Nitride Nanosheet to Bacterial Membranes. Langmuir 2019, 35, 6179–6187. [Google Scholar] [CrossRef]
- Xie, X.; Hou, Z.; Duan, G.; Zhang, S.; Zhou, H.; Yang, Z.; Zhou, R. Boron nitride nanosheets elicit significant hemolytic activity via destruction of red blood cell membranes. Colloids Surf. B Biointerfaces 2021, 203, 111765. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Danti, S.; D’Alessandro, D.; Moscato, S.; Menciassi, A. Assessing cytotoxicity of boron nitride nanotubes: Interference with the MTT assay. Biochem. Biophys. Res. Commun. 2010, 394, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Ciofani, G.; Danti, S.; Genchi, G.G.; D’Alessandro, D.; Pellequer, J.-L.; Odorico, M.; Mattoli, V.; Giorgi, M. Pilot in vivo toxicological investigation of boron nitride nanotubes. Int. J. Nanomed. 2012, 7, 19–24. [Google Scholar] [CrossRef]
- Chen, X.; Wu, P.; Rousseas, M.; Okawa, D.; Gartner, Z.; Zettl, A.; Bertozzi, C. Boron nitride nanotubes are noncytotoxic and can be functionalized for interaction with proteins and cells. J. Am. Chem. Soc. 2009, 131, 890–891. [Google Scholar] [CrossRef]
- Ciofani, G.; Danti, S.; Nitti, S.; Mazzolai, B.; Mattoli, V.; Giorgi, M. Biocompatibility of boron nitride nanotubes: An up-date of in vivo toxicological investigation. Int. J. Pharm. 2013, 444, 85–88. [Google Scholar] [CrossRef]
- Jo, S.; Lim, J.B.; Klauda, J.B.; Im, W. CHARMM-GUI Membrane Builder for mixed bilayers and its application to yeast membranes. Biophys. J. 2009, 97, 50–58. [Google Scholar]
- Nosé, S. A molecular dynamics method for simulations in the canonical ensemble. Mol. Phys. 1984, 52, 255–268. [Google Scholar]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar]
- Molecular Dynamics Models and Related Data Will Be Available at GitHub Repository via Link. Available online: https://github.com/AATsukanov/CIN2D (accessed on 11 February 2022).
- Available online: https://tsukanov-lab.moy.su (accessed on 11 February 2022).

| Relationship | Description | Behavior | |
|---|---|---|---|
| 1° | < 0 | Interaction with the head group is energetically more favorable than with the tails, leading to nanosheet adsorption by the membrane surface | 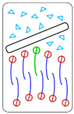 Adsorption of nanomaterial on membrane surface |
| 2° | Nanosheet insertion into the bilayer is energetically favorable. Size-dependent mechanical disruption of membrane is also possible | 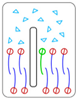 Insertion of nanomaterial into bilayer | |
| 3° | < | Nanosheet insertion into the bilayer with lipid extraction and membrane disruption | 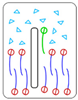 Disruptive lipid extraction |
| 4° | Nanosheet does not, or weakly, interacts with the cell membrane—non-critical impact | 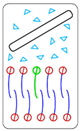 No interaction |
| Nanomaterial | gh, kJ/mol | gt, kJ/mol | CIN2D, kJ/mol | Relationship | Prediction |
|---|---|---|---|---|---|
| GN | −14 ± 2 | −103 ± 11 | 88 ± 13 | lipid extraction | |
| GON | −19 ± 4 | −30 ± 14 | 10 ± 18 * | insertion into bilayer | |
| Mg/Al-LDH | −3.5 ± 1.4 | 0.00 ± 0.12 | −3.5 ± 1.5 | CIN < 0 | adsorption by bilayer |
| Aloohene | −5.2 ± 1.3 | −0.03 ± 0.05 | −5.1 ± 1.3 | CIN < 0 | adsorption by bilayer |
| BNN (PAC ± 1.05 e) | −20 ± 3 | −115 ± 12 | 95 ± 15 | lipid extraction | |
| BNN (PAC ± 0.5 e) | −16 ± 7 | −118 ± 4 | 102 ± 10 | lipid extraction |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsukanov, A.A.; Turk, B.; Vasiljeva, O.; Psakhie, S.G. Computational Indicator Approach for Assessment of Nanotoxicity of Two-Dimensional Nanomaterials. Nanomaterials 2022, 12, 650. https://doi.org/10.3390/nano12040650
Tsukanov AA, Turk B, Vasiljeva O, Psakhie SG. Computational Indicator Approach for Assessment of Nanotoxicity of Two-Dimensional Nanomaterials. Nanomaterials. 2022; 12(4):650. https://doi.org/10.3390/nano12040650
Chicago/Turabian StyleTsukanov, Alexey A., Boris Turk, Olga Vasiljeva, and Sergey G. Psakhie. 2022. "Computational Indicator Approach for Assessment of Nanotoxicity of Two-Dimensional Nanomaterials" Nanomaterials 12, no. 4: 650. https://doi.org/10.3390/nano12040650
APA StyleTsukanov, A. A., Turk, B., Vasiljeva, O., & Psakhie, S. G. (2022). Computational Indicator Approach for Assessment of Nanotoxicity of Two-Dimensional Nanomaterials. Nanomaterials, 12(4), 650. https://doi.org/10.3390/nano12040650