Nanomaterial Probes for Nuclear Imaging
Abstract
1. Introduction
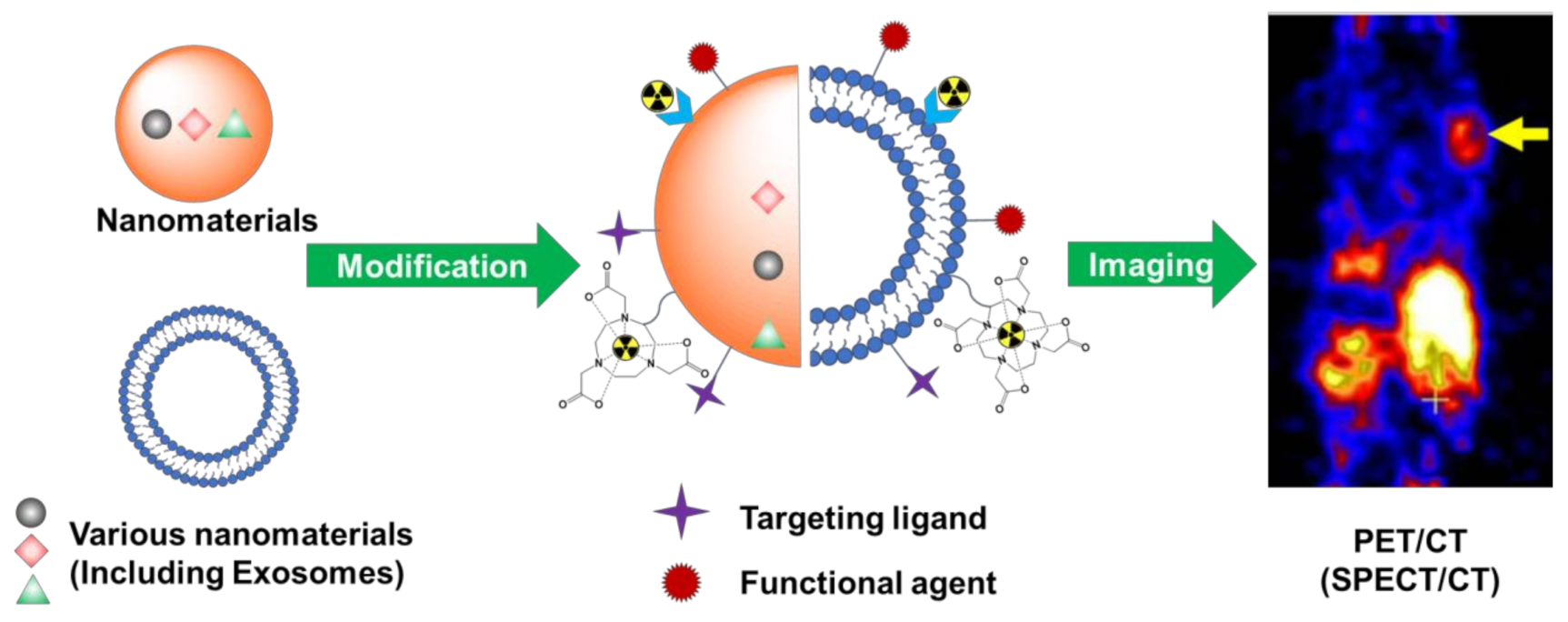
2. Challenges of Nuclear Imaging and the Role of Nanomaterials
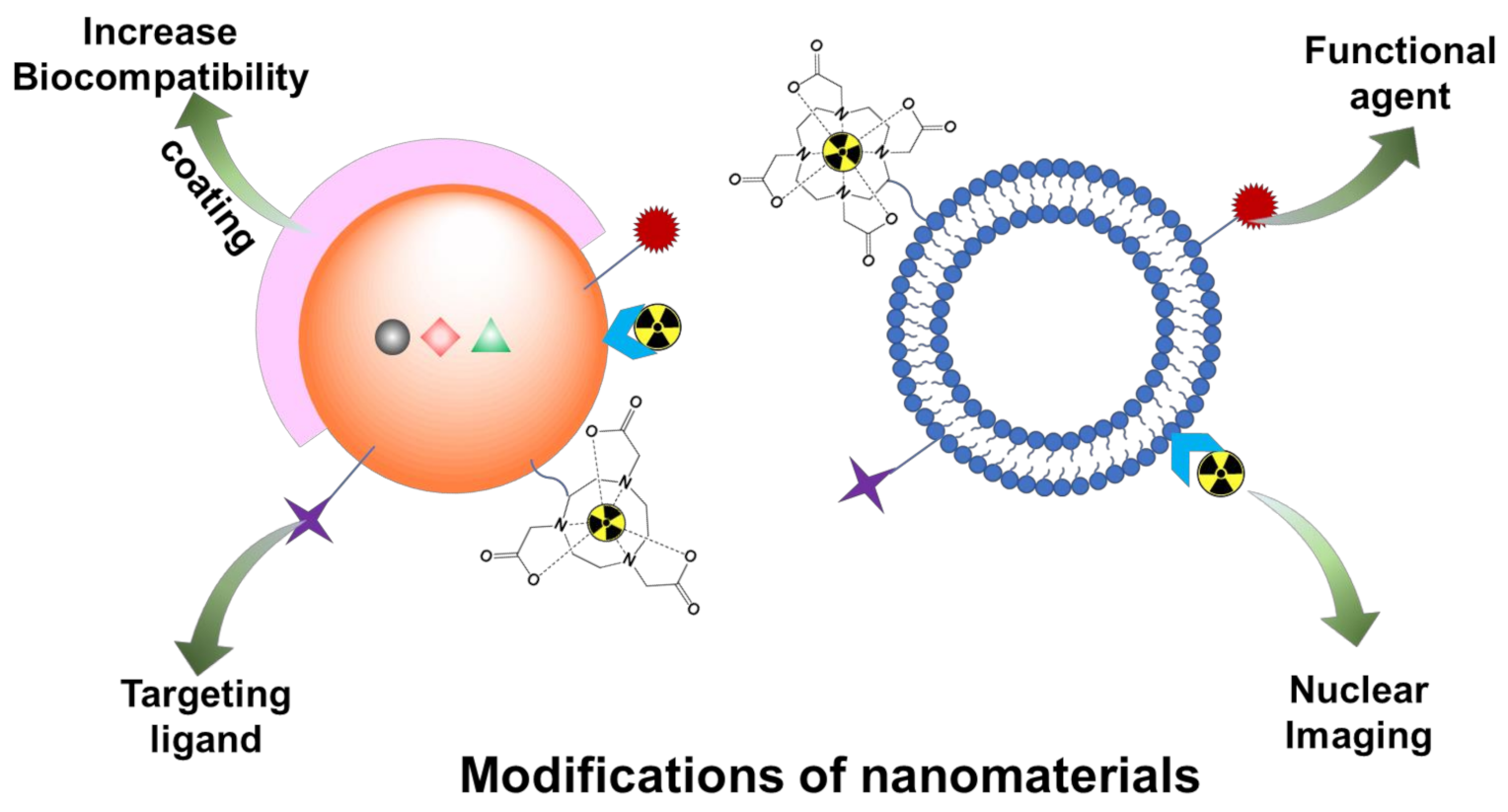
3. Modifications of Nanomaterials for Nuclear Imaging
3.1. Coating
3.2. Active Targeting Moieties for Disease-Specific Receptors
4. Nanomaterials for Theranostic Nuclear Imaging Probes
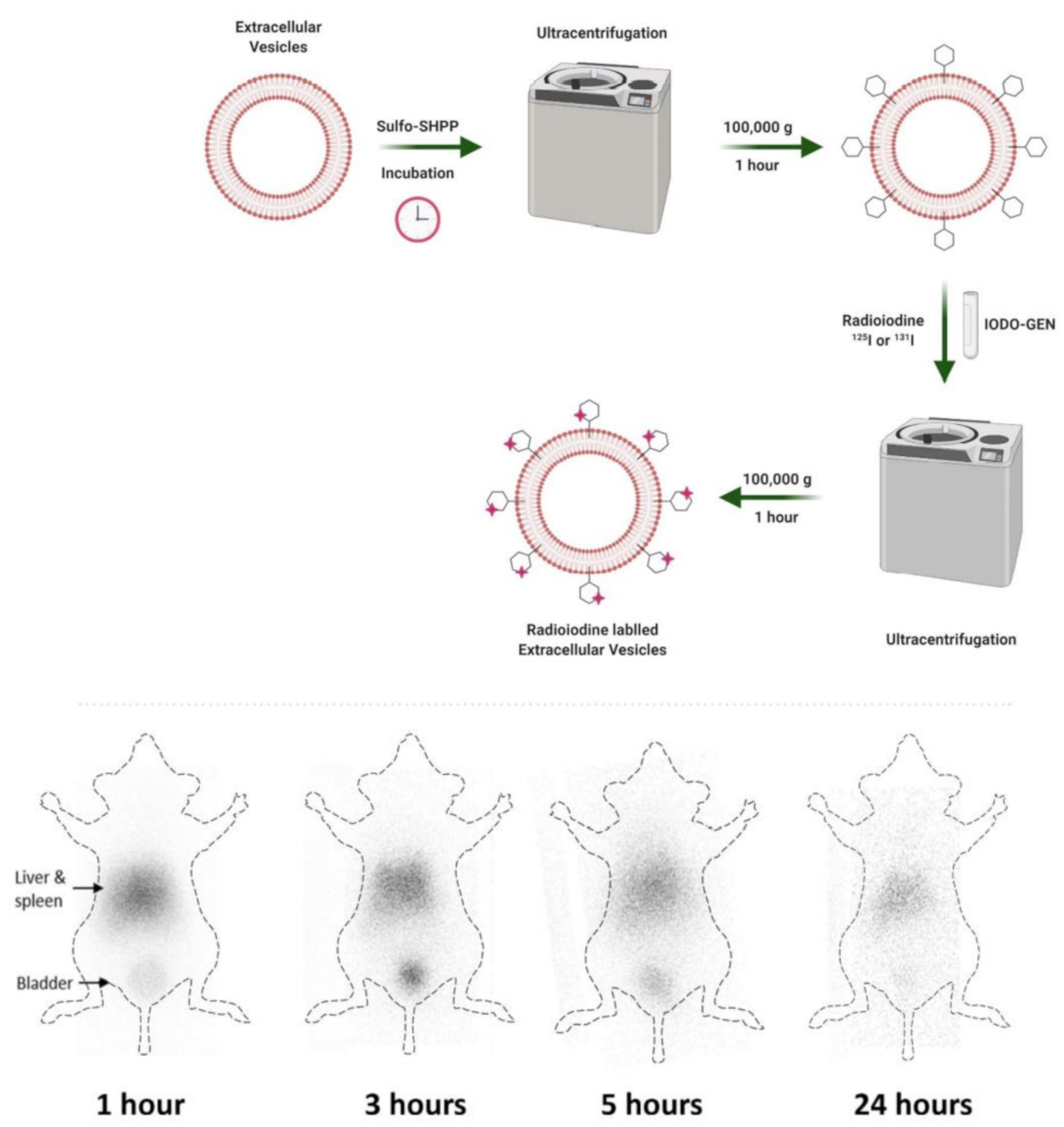
5. Radiolabeled Exosomes for Nuclear Imaging
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Chen, K.; Chen, X. Design and development of molecular imaging probes. Curr. Top. Med. Chem. 2010, 10, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Nguyen, Q.T. Molecular imaging for cancer diagnosis and surgery. Adv. Drug Deliv. Rev. 2014, 66, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Truijman, M.T.; Kwee, R.M.; van Hoof, R.H.; Hermeling, E.; van Oostenbrugge, R.J.; Mess, W.H.; Backes, W.H.; Daemen, M.J.; Bucerius, J.; Wildberger, J.E.; et al. Combined 18 F-FDG PET-CT and DCE-MRI to Assess Inflammation and Microvascularization in Atherosclerotic Plaques. Stroke 2013, 44, 3568–3570. [Google Scholar] [CrossRef]
- Chou, C.-P.; Lewin, J.M.; Chiang, C.-L.; Hung, B.-H.; Yang, T.-L.; Huang, J.-S.; Liao, J.-B.; Pan, H.-B. Clinical evaluation of contrast-enhanced digital mammography and contrast enhanced tomosynthesis—Comparison to contrast-enhanced breast MRI. Eur. J. Radiol. 2015, 84, 2501–2508. [Google Scholar] [CrossRef]
- Wells, R.G. Instrumentation in molecular imaging. J. Nucl. Cardiol. 2016, 23, 1343–1347. [Google Scholar] [CrossRef]
- Erdi, Y.E. Limits of Tumor Detectability in Nuclear Medicine and PET. Mol. Imaging Radionucl. Ther. 2012, 21, 23–28. [Google Scholar] [CrossRef]
- Shen, K.; Liu, B.; Zhou, X.; Ji, Y.; Chen, L.; Wang, Q.; Xue, W. The Evolving Role of 18F-FDG PET/CT in Diagnosis and Prognosis Prediction in Progressive Prostate Cancer. Front. Oncol. 2021, 11, 683793. [Google Scholar] [CrossRef]
- Pellico, J.; Gawne, P.J.; de Rosales, R.T.M. Radiolabelling of nanomaterials for medical imaging and therapy. Chem. Soc. Rev. 2021, 50, 3355–3423. [Google Scholar] [CrossRef]
- Wei, Y.; Tang, T.; Pang, H.-B. Cellular internalization of bystander nanomaterial induced by TAT-nanoparticles and regulated by extracellular cysteine. Nat. Commun. 2019, 10, 3646. [Google Scholar] [CrossRef]
- Yang, C.-T.; Hattiholi, A.; Selvan, S.T.; Yan, S.X.; Fang, W.-W.; Chandrasekharan, P.; Koteswaraiah, P.; Herold, C.J.; Gulyás, B.; Aw, S.E.; et al. Gadolinium-based bimodal probes to enhance T1-Weighted magnetic resonance/optical imaging. Acta Biomater. 2020, 110, 15–36. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Xia, Y.; Matham, M.V.; Su, H.; Padmanabhan, P.; Gulyás, B. Nanoparticulate Contrast Agents for Multimodality Molecular Imaging. J. Biomed. Nanotechnol. 2016, 12, 1553–1584. [Google Scholar] [CrossRef]
- Kim, J.; Lee, N.; Hyeon, T. Recent development of nanoparticles for molecular imaging. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20170022. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jeong, J.M.; Yoo, B.C.; Kim, K.; Kim, Y.; Yang, B.Y.; Lee, Y.-S.; Lee, D.S.; Chung, J.-K.; Lee, M.C. Development of 68Ga-labeled mannosylated human serum albumin (MSA) as a lymph node imaging agent for positron emission tomography. Nucl. Med. Biol. 2011, 38, 371–379. [Google Scholar] [CrossRef]
- Aranda-Laraa, L.; Morales-Avilab, E.; Luna-Gutiérrezc, M.A.; Olivé-Alvarezd, E.; Isaac-Olivéa, K. Radiolabeled liposomes and lipoproteins as lipidic nanoparticles for imaging and therapy. Chem. Phys. Lipids 2020, 230, 104934. [Google Scholar] [CrossRef]
- Pérez-Medina, C.; Tang, J.; Abdel-Atti, D.; Hogstad, B.; Merad, M.; Fisher, E.A.; Fayad, Z.A.; Lewis, J.S.; Mulder, W.J.M.; Reiner, T. PET imaging of tumor-associated macrophages with 89Zr-labeled high-density lipoprotein nanoparticles. J. Nucl. Med. 2015, 56, 1272–1277. [Google Scholar] [CrossRef]
- Vera, D.B.; Fontaine, S.D.; VanBrocklin, H.F.; Hearn, B.R.; Reid, R.; Ashley, G.W.; Santi, D.V. PET Imaging of the EPR Effect in Tumor Xenografts Using Small 15 nm Diameter Polyethylene Glycols Labeled with Zirconium-89. Mol. Cancer Ther. 2020, 19, 673–679. [Google Scholar] [CrossRef]
- Li, T.; Hu, X.; Fan, Q.; Chen, Z.; Zheng, Z.; Zhang, R. The Novel DPP-BDT Nanoparticles as Efficient Photoacoustic Imaging and Positron Emission Tomography Agents in Living Mice. Int. J. Nanomed. 2020, 15, 5017–5026. [Google Scholar] [CrossRef]
- Nagachinta, S.; Becker, G.; Dammicco, S.; Serrano, M.E.; Leroi, N.; Bahri, M.A.; Plenevaux, A.; Lemaire, C.; Lopez, R.; Luxen, A.; et al. Radiolabelling of lipid-based nanocarriers with fluorine-18 for in vivo tracking by PET. Colloids Surf. B Biointerfaces 2020, 188, 110793. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, Q.; Cheng, Y.; Xiang, L.; Shen, G.; Wu, X.; Cai, H.; Li, D.; Zhu, H.; Zhang, R.; et al. 64Cu-labeled melanin nanoparticles for PET/CT and radionuclide therapy of tumor. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102248. [Google Scholar] [CrossRef] [PubMed]
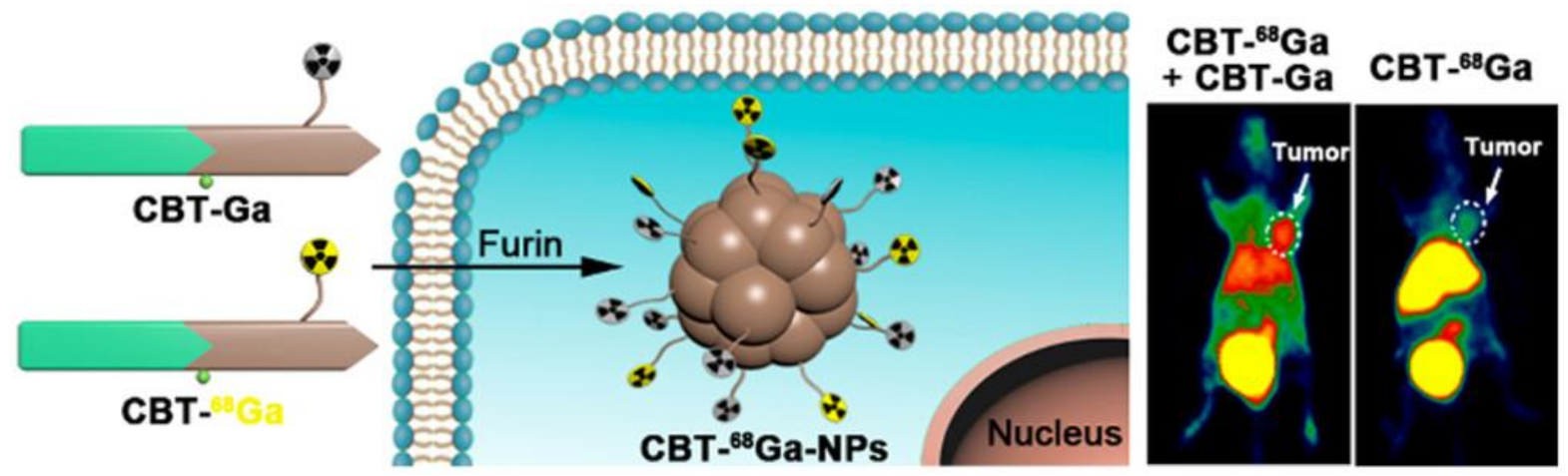
- Wang, H.; Chen, P.; Wu, H.; Zou, P.; Wu, J.; Liu, Y.; Liang, G. Furin-Guided Intracellular 68Ga Nanoparticle Formation Enhancing Tumor MicroPET Imaging. Anal. Chem. 2019, 91, 14842–14845. [Google Scholar] [CrossRef] [PubMed]
- De Sá, A.; Prata, M.I.M.; Geraldes, C.F.; André, J.P.G.C. Triaza-based amphiphilic chelators: Synthetic route, in vitro characterization and in vivo studies of their Ga(III) and Al(III) chelates. J. Inorg. Biochem. 2010, 104, 1051–1062. [Google Scholar] [CrossRef]
- Fontes, A.; Prata, M.I.M.; Geraldes, C.; André, J.P. Ga(III) chelates of amphiphilic DOTA-based ligands: Synthetic route and in vitro and in vivo studies. Nucl. Med. Biol. 2011, 38, 363–370. [Google Scholar] [CrossRef]
- Hong, H.; Zhang, Y.; Engle, J.W.; Nayak, T.R.; Theuer, C.P.; Nickles, R.J.; Barnhart, T.E.; Cai, W. In vivo targeting and positron emission tomography imaging of tumor vasculature with 66Ga-labeled nano-graphene. Biomaterials 2012, 33, 4147–4156. [Google Scholar] [CrossRef]
- Hong, H.; Yang, K.; Zhang, Y.; Engle, J.W.; Feng, L.; Yang, Y.; Nayak, T.R.; Goel, S.; Bean, J.; Theuer, C.P.; et al. In Vivo Targeting and Imaging of Tumor Vasculature with Radiolabeled, Antibody-Conjugated Nanographene. ACS Nano 2012, 6, 2361–2370. [Google Scholar] [CrossRef]
- Ndiege, N.; Raidoo, R.; Schultz, M.K.; Larsen, S. Preparation of a Versatile Bifunctional Zeolite for Targeted Imaging Applications. Langmuir 2011, 27, 2904–2909. [Google Scholar] [CrossRef]
- Chen, K.; Li, Z.-B.; Wang, H.; Cai, W.; Chen, X. Dual-modality optical and positron emission tomography imaging of vascular endothelial growth factor receptor on tumor vasculature using quantum dots. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 2235–2244. [Google Scholar] [CrossRef]
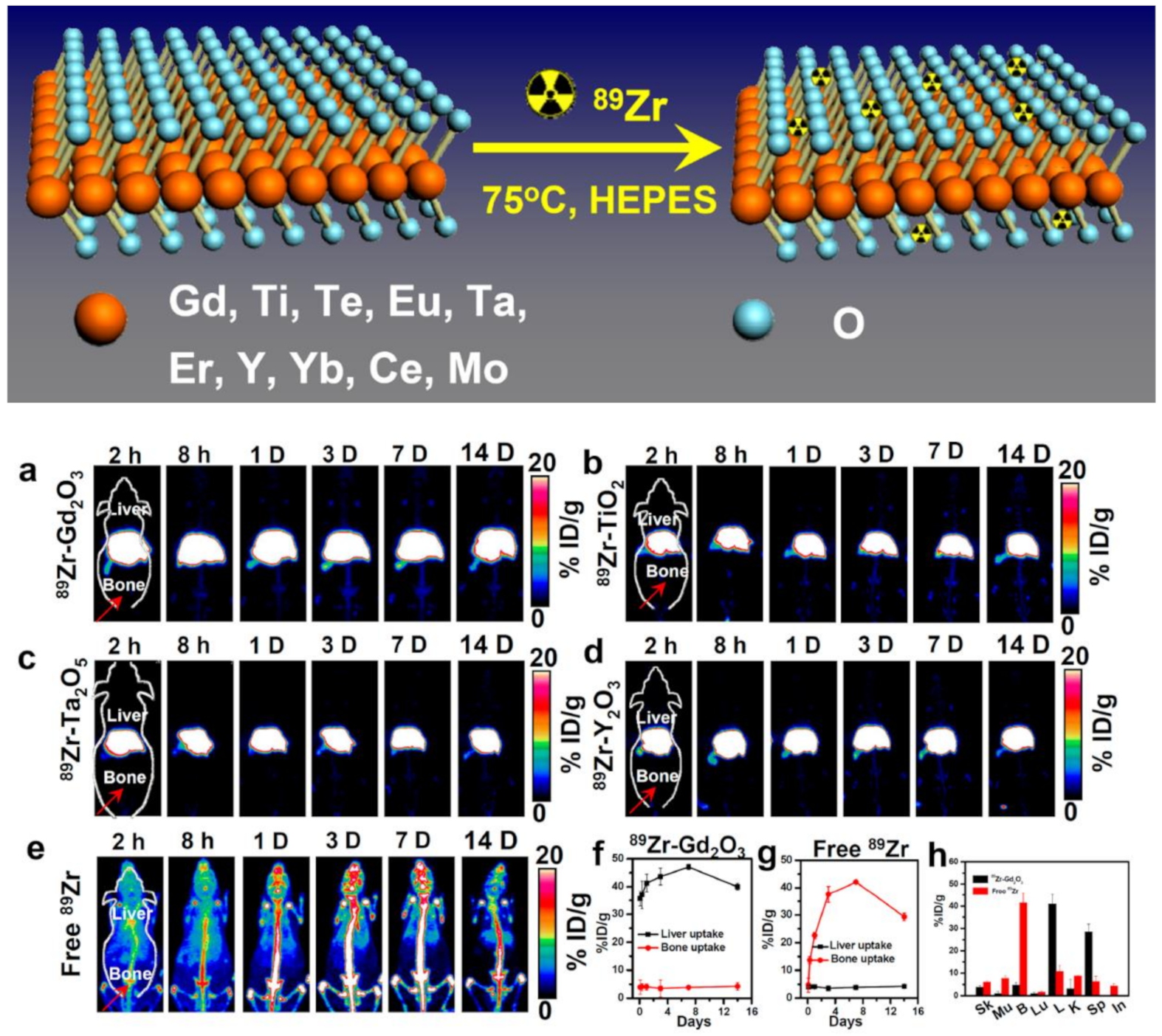
- Cheng, L.; Shen, S.; Jiang, D.; Jin, Q.; Ellison, P.A.; Ehlerding, E.B.; Goel, S.; Song, G.; Huang, P.; Barnhart, T.E.; et al. Chelator-Free Labeling of Metal Oxide Nanostructures with Zirconium-89 for Positron Emission Tomography Imaging. ACS Nano 2017, 11, 12193–12201. [Google Scholar] [CrossRef]
- Kumar, A.; Zhang, S.R.; Hao, G.Y.; Hassan, G.; Ramezani, S.; Sagiyama, K.; Lo, S.-T.; Takahashi, M.; Sherry, A.D.; Öz, O.K.; et al. Molecular Platform for Design and Synthesis of Targeted Dual-Modality Imaging Probes. Bioconjug. Chem. 2015, 26, 549–558. [Google Scholar] [CrossRef]
- Tu, C.; Ng, T.C.; Jacobs, R.; Louie, A. Multimodality PET/MRI agents targeted to activated macrophages. JBIC J. Biol. Inorg. Chem. 2014, 19, 247–258. [Google Scholar] [CrossRef]
- Starmans, L.W.; Hummelink, M.A.; Rossin, R.; Kneepkens, E.; Lamerichs, R.; Donato, K.; Nicolay, K.; Grüll, H. 89Zr- and Fe-Labeled Polymeric Micelles for Dual Modality PET and T1-Weighted MR Imaging. Adv. Health Mater. 2015, 4, 2137–2145. [Google Scholar] [CrossRef]
- Xie, J.; Chen, K.; Huang, J.; Lee, S.; Wang, J.; Gao, J.; Li, X.; Chen, X. PET/NIRF/MRI triple functional iron oxide nanoparticles. Biomaterials 2010, 31, 3016–3022. [Google Scholar] [CrossRef]
- Park, J.C.; Yu, M.K.; An, G.I.; Park, S.-I.; Oh, J.; Kim, H.J.; Kim, J.-H.; Wang, E.K.; Hong, I.-H.; Ha, Y.S.; et al. Facile Preparation of a Hybrid Nanoprobe for Triple-Modality Optical/PET/MR Imaging. Small 2010, 6, 2863–2868. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, J.; Zhao, L.; Zhao, P.; Yang, J.; Zhao, J.; Miao, W. 131I-Labeled Multifunctional Polyethylenimine/Doxorubicin Complexes with pH-Controlled Cellular Uptake Property for Enhanced SPECT Imaging and Chemo/Radiotherapy of Tumors. Int. J. Nanomed. 2021, 16, 5167–5183. [Google Scholar] [CrossRef]
- Fayez, H.; El-Motaleb, M.A.; Selim, A.A. Synergistic Cytotoxicity of Shikonin-Silver Nanoparticles As An Opportunity For Lung Cancer. J. Label. Compd. Radiopharm. 2020, 63, 25–32. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Lin, J.-J.; Chang, W.-Y.; Hsieh, C.-Y.; Wu, C.-C.; Chen, H.-S.; Hsu, H.-J.; Yang, A.-S.; Hsu, M.-H.; Kuo, W.-Y. Development of theranostic active-targeting boron-containing gold nanoparticles for boron neutron capture therapy (BNCT). Colloids Surf. B Biointerfaces 2019, 183, 110387. [Google Scholar] [CrossRef]
- Yi, X.; Zhou, X.; Zhang, Z.; Xiong, S.; Yang, K. X-rays-optimized delivery of radiolabeled albumin for cancer theranostics. Biomaterials 2020, 233, 119764. [Google Scholar] [CrossRef]
- Royo, F.; Cossío, U.; de Angulo, A.R.; Llop, J.; Falcon-Perez, J.M. Modification of the glycosylation of extracellular vesicles alters their biodistribution in mice. Nanoscale 2019, 11, 1531–1537. [Google Scholar] [CrossRef]
- Hong, C.M.; Gangadaran, P.; Oh, J.M.; Rajendran, R.L.; Gopal, A.; Zhu, L.; Ahn, B.-C. Radioiodine labeling and in vivo trafficking of extracellular vesicles. Sci. Rep. 2021, 11, 5041. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.O.; Kim, Y.-H.; Chung, S.-J.; Lee, C.-H.; Rhee, S.; Pratx, G.; Chung, J.-K.; Youn, H. Identification of Lymphatic and Hematogenous Routes of Rapidly Labeled Radioactive and Fluorescent Exosomes through Highly Sensitive Multimodal Imaging. Int. J. Mol. Sci. 2020, 21, 7850. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Man, F.; Faruqu, F.; Kim, J.; Al-Salemee, F.; Volpe, A.; Fruhwirth, G.O.; Al-Jamal, K.T.; de Rosale, R.T.M. [89Zr]Zr(oxinate)4 allows direct radiolabelling and PET imaging of small extracellular vesicles. ChemRxiv 2021. Available online: https://doi.org/10.26434/chemrxiv.12730463.v2 (accessed on 10 February 2021).
- Kraeber-Bodéré, F.; Barbet, J. Challenges in Nuclear Medicine: Innovative Theranostic Tools for Personalized Medicine. Front. Med. 2014, 1, 16. [Google Scholar] [CrossRef]
- Chen, Z.; Haider, A.; Chen, J.; Xiao, Z.; Gobbi, L.; Honer, M.; Grether, U.; Arnold, S.E.; Josephson, L.; Liang, S.H. The Repertoire of Small-Molecule PET Probes for Neuroinflammation Imaging: Challenges and Opportunities beyond TSPO. J. Med. Chem. 2021, 64, 17656–17689. [Google Scholar] [CrossRef]
- Farzin, L.; Sheibani, S.; Moassesi, M.E.; Shamsipur, M. An overview of nanoscale radionuclides and radiolabeled nanomaterials commonly used for nuclear molecular imaging and therapeutic functions. J. Biomed. Mater. Res. A 2019, 107, 251–285. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood–brain barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef]
- Silva, G.A. Nanotechnology approaches for drug and small molecule delivery across the blood brain barrier. Surg. Neurol. 2007, 67, 113–116. [Google Scholar] [CrossRef]
- Singh, K.R.B.; Nayak, V.; Singh, R.P. Introduction to Bionanomaterials: An Overview; IOP Publishing: Bristol, UK, 2021; pp. 1–21. [Google Scholar] [CrossRef]
- Prashant, C.; Dipak, M.; Yang, C.-T.; Chuang, K.-H.; Jun, D.; Feng, S.-S. Superparamagnetic iron oxide—Loaded poly (lactic acid)-d-α-tocopherol polyethylene glycol 1000 succinate copolymer nanoparticles as MRI contrast agent. Biomaterials 2010, 31, 5588–5597. [Google Scholar] [CrossRef]
- Dipak, M.; Prashant, C.; Yang, C.-T.; Chuang, K.-H.; Feng, S.-S.; Ding, J. Facile synthesis of water-stable fine magnetite nanoparticles for MRI and magnetic hyperthermia applications. Nanomedicine 2010, 5, 1571–1584. [Google Scholar]
- Fang, R.H.; Hu, C.-M.J.; Zhang, L. Nanoparticles disguised as red blood cells to evade the immune system. Expert Opin. Biol. Ther. 2012, 12, 385–389. [Google Scholar] [CrossRef]
- Vlashi, E.; Kelderhouse, L.; Sturgis, J.E.; Low, P.S. Effect of Folate-Targeted Nanoparticle Size on Their Rates of Penetration into Solid Tumors. ACS Nano 2013, 7, 8573–8582. [Google Scholar] [CrossRef]
- Gu, F.; Zhang, L.; Teply, B.A.; Mann, N.; Wang, A.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc. Natl. Acad. Sci. USA 2008, 105, 2586–2591. [Google Scholar] [CrossRef]
- Shmeeda, H.; Tzemach, D.; Mak, L.; Gabizon, A. Her2-targeted pegylated liposomal doxorubicin: Retention of target-specific binding and cytotoxicity after in vivo passage. J. Control. Release 2009, 136, 155–160. [Google Scholar] [CrossRef]
- Dos Santos, N.; Allen, C.; Doppen, A.-M.; Anantha, M.K.; Cox, A.K.; Gallagher, R.C.; Karlsson, G.; Edwards, K.; Kenner, G.; Samuels, L.; et al. Influence of poly(ethylene glycol) grafting density and polymer length on liposomes: Relating plasma circulation lifetimes to protein binding. Biochim. Biophys. Acta Biomembr. 2007, 1768, 1367–1377. [Google Scholar] [CrossRef]
- Gbadamosi, J.K.; Hunter, A.C.; Moghimi, S.M. PEGylation of microspheres generates a heterogeneous population of particles with differential surface characteristics and biological performance. FEBS Lett. 2002, 532, 338–344. [Google Scholar] [CrossRef]
- Fang, J.; Chandrasekharan, P.; Liu, X.-L.; Yang, Y.; Lv, Y.-B.; Yang, C.-T.; Ding, J. Manipulating the surface coating of ultra-small Gd2O3 nanoparticles for improved T1-weighted MR imaging. Biomaterials 2014, 35, 1636–1642. [Google Scholar] [CrossRef]
- Lao, L.L.; Ramanujan, R.V. Magnetic and hydrogel composite materials for hyperthermia applications. J. Mater. Sci. Mater. Med. 2004, 15, 1061–1064. [Google Scholar] [CrossRef]
- Kratz, H.; Taupitz, M.; de Schellenberger, A.A.; Kosch, O.; Eberbeck, D.; Wagner, S.; Trahms, L.; Hamm, B.; Schnorr, J. Novel magnetic multicore nanoparticles designed for MPI and other biomedical applications: From synthesis to first in vivo studies. PLoS ONE 2018, 13, e0190214. [Google Scholar] [CrossRef]
- Chen, B.-W.; Hatamie, S.; Garu, P.; Heravi, P.; Chen, J.-Y.; Liu, B.-T.; Wei, Z.-H.; Yao, D.-J. Synthesis of iron-oxide magnetic nanoparticles coated with dextran of varied molecular mass using a facile ball-milling method. Micro Nano Lett. 2020, 15, 645–650. [Google Scholar] [CrossRef]
- Li, M.; Gu, H.; Zhang, C. Highly sensitive magnetite nano clusters for MR cell imaging. Nanoscale Res. Lett. 2012, 7, 204. [Google Scholar] [CrossRef]
- Ferrauto, G.; Castelli, D.D.; Di Gregorio, E.; Terreno, E.; Aime, S. LipoCEST and cellCEST imaging agents: Opportunities and challenges. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 602–618. [Google Scholar] [CrossRef]
- Park, S.-H.; Yoon, Y.I.; Moon, H.; Lee, G.-H.; Lee, B.; Yoon, T.; Lee, H. Development of a novel microbubble-liposome complex conjugated with peptide ligands targeting IL4R on brain tumor cells. Oncol. Rep. 2016, 36, 131–136. [Google Scholar] [CrossRef][Green Version]
- Yeh, C.; Hsiao, J.-K.; Wang, Y.-P.; Lan, C.-H.; Wu, H.-C. Peptide-conjugated nanoparticles for targeted imaging and therapy of prostate cancer. Biomaterials 2016, 99, 1–15. [Google Scholar] [CrossRef]
- Jackson, A.W.; Chandrasekharan, P.; Shi, J.; Rannard, S.P.; Liu, Q.; Yang, C.-T.; He, T. Synthesis and in vivo magnetic resonance imaging evaluation of biocompatible branched copolymer nanocontrast agents. Int. J. Nanomed. 2015, 10, 5895–5907. [Google Scholar] [CrossRef][Green Version]
- Dundas, C.; Demonte, D.; Park, S. Streptavidin–biotin technology: Improvements and innovations in chemical and biological applications. Appl. Microbiol. Biotechnol. 2013, 97, 9343–9353. [Google Scholar] [CrossRef]
- Frampas, E.; Rousseau, C.; Bodet-Milin, C.; Barbet, J.; Chatal, J.-F.; Kraeber-Bodéré, F. Improvement of Radioimmunotherapy Using Pretargeting. Front. Oncol. 2013, 3, 159. [Google Scholar] [CrossRef]
- Wu, L.Y.; Liu, T.; Hopkins, M.R.; Davis, W.C.; Berkman, C.E. Chemoaffinity capture of pre-targeted prostate cancer cells with magnetic beads. Prostate 2012, 72, 1532–1541. [Google Scholar] [CrossRef]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 2011, 153, 198–205. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 206–212. [Google Scholar] [CrossRef]
- Yu, M.K.; Park, J.; Jon, S. Targeting Strategies for Multifunctional Nanoparticles in Cancer Imaging and Therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, G.; Baeza, A. Targeting strategies for improving the efficacy of nanomedicine in oncology. Beilstein J. Nanotechnol. 2019, 10, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhou, L.; Xing Wu, F. Tracking Neural Stem Cells in Patients with Brain Trauma. N. Engl. J. Med. 2006, 355, 2376–2378. [Google Scholar] [CrossRef] [PubMed]
- Berry, N.C.; Sosnovik, D.E. Cardiomyocyte Death: Insights from Molecular and Microstructural Magnetic Resonance Imaging. Pediatr. Cardiol. 2011, 32, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Yoo, J.-W.; Kolhar, P.; Wakankar, A.; Gokarn, Y.R.; Mitragotri, S. Particle shape enhances specificity of antibody-displaying nanoparticles. Proc. Natl. Acad. Sci. USA 2013, 110, 3270–3275. [Google Scholar] [CrossRef] [PubMed]
- Danila, D.; Partha, R.; Elrod, D.B.; Lackey, M.; Casscells, S.W.; Conyers, J.L. Antibody-labeled liposomes for CT imaging of atherosclerotic plaques: In vitro investigation of an anti-ICAM antibody-labeled liposome containing iohexol for molecular imaging of atherosclerotic plaques via computed tomography. Tex. Heart Inst. J. 2009, 36, 393–403. [Google Scholar]
- Serres, S.; Mardiguian, S.; Campbell, S.J.; McAteer, M.A.; Akhtar, A.; Krapitchev, A.; Choudhury, R.P.; Anthony, D.C.; Sibson, N.R. VCAM-1-targeted magnetic resonance imaging reveals subclinical disease in a mouse model of multiple sclerosis. FASEB J. 2011, 25, 4415–4422. [Google Scholar] [CrossRef]
- Böhmová, E.; Machová, D.; Pechar, M.; Pola, R.; Venclíková, K.; Janoušková, O.; Etrych, T. Cell-Penetrating Peptides: A Useful Tool for the Delivery of Various Cargoes Into Cells. Physiol. Res. 2018, 67 (Suppl. 2), S267–S279. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Deyev, S.M.; Lebedenko, E.N. Multivalency: The hallmark of antibodies used for optimization of tumor targeting by design. BioEssays 2008, 30, 904–918. [Google Scholar] [CrossRef]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef]
- Hwang, D.W.; Ko, H.Y.; Lee, J.H.; Kang, H.; Ryu, S.H.; Song, I.-C.; Lee, D.S.; Kim, S. A Nucleolin-Targeted Multimodal Nanoparticle Imaging Probe for Tracking Cancer Cells Using an Aptamer. J. Nucl. Med. 2009, 51, 98–105. [Google Scholar] [CrossRef]
- Cho, E.J.; Lee, J.-W.; Ellington, A.D. Applications of Aptamers as Sensors. Annu. Rev. Anal. Chem. 2010, 2, 241–264. [Google Scholar] [CrossRef]
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262. [Google Scholar] [CrossRef]
- Yang, R.; Kolb, E.A.; Qin, J.; Chou, A.; Sowers, R.; Hoang, B.; Healey, J.H.; Huvos, A.G.; Meyers, P.A.; Gorlick, R. The Folate Receptor α Is Frequently Overexpressed in Osteosarcoma Samples and Plays a Role in the Uptake of the Physiologic Substrate 5-Methyltetrahydrofolate. Clin. Cancer Res. 2007, 13, 2557–2567. [Google Scholar] [CrossRef]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; De Jong, J.S.; Arts, H.J.G.; Van Der Zee, A.G.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315–1319. [Google Scholar] [CrossRef]
- Velikyan, I. Prospective of 68Ga-Radiopharmaceutical Development. Theranostics 2014, 4, 47–80. [Google Scholar] [CrossRef]
- Augustine, R.; Al Mamun, A.; Hasan, A.; Salam, S.A.; Chandrasekaran, R.; Ahmed, R.; Thakor, A.S. Imaging cancer cells with nanostructures: Prospects of nanotechnology driven non-invasive cancer diagnosis. Adv. Colloid Interface Sci. 2021, 294, 102457. [Google Scholar] [CrossRef]
- Stoffels, I.; Herrmann, K.; Rekowski, J.; Jansen, P.; Schadendorf, D.; Stang, A.; Klode, J. Sentinel lymph node excision with or without preoperative hybrid single-photon emission computed tomography/computed tomography (SPECT/CT) in melanoma: Study protocol for a multicentric randomized controlled trial. Trials 2019, 20, 99. [Google Scholar] [CrossRef]
- Maus, S.; Buchholz, H.-G.; Ament, S.; Brochhausen, C.; Bausbacher, N.; Schreckenberger, M. Labelling of commercially available human serum albumin kits with Ga-68-as surrogates for 99mTc-MAA microspheres. Appl. Radiat. Isot. 2011, 69, 171–175. [Google Scholar] [CrossRef]
- Cutler, C.S.; Chanda, N.; Shukla, R.; Sisay, N.; Cantorias, M.; Zambre, A.; McLaughlin, M.; Kelsey, J.; Upenandran, A.; Robertson, D.; et al. Nanoparticles and Phage Display Selected Peptides for Imaging and Therapy of Cancer. Recent Results Cancer Res. 2013, 194, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Zhang, K.; Aruva, M.R.; Cardi, C.A.; Opitz, A.W.; Wagner, N.J.; Thakur, M.L.; Wickstrom, E. Radiohybridization PET imaging of KRAS G12D mRNA expression in with [64Cu]DO3A-peptide nucleic acid-peptide nanoparticles. Cancer Biol. Ther. 2007, 6, 948–956. [Google Scholar] [CrossRef] [PubMed]
- De Barros, A.L.B.; Tsourkas, A.; Saboury, B.; Cardoso, V.N.; Alavi, A. Emerging role of radiolabeled nanoparticles as an effective diagnostic technique. EJNMMI Res. 2012, 2, 39. [Google Scholar] [CrossRef] [PubMed]
- Man, F.; Gawne, P.J.; de Rosales, R.T.M. Nuclear imaging of liposomal drug delivery systems: A critical review of radiolabelling methods and applications in nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 134–160. [Google Scholar] [CrossRef]
- Helbok, A.; Decristoforo, C.; Dobrozemsky, G.; Rangger, C.; Diederen, E.; Stark, B.; Prassl, R.; Von Guggenberg, E. Radiolabeling of lipid-based nanoparticles for diagnostics and therapeutic applications: A comparison using different radiometals. J. Liposome Res. 2010, 20, 219–227. [Google Scholar] [CrossRef]
- Andreozzi, E.; Seo, J.W.; Ferrara, K.; Louie, A. Novel Method to Label Solid Lipid Nanoparticles with 64Cu for Positron Emission Tomography Imaging. Bioconjug. Chem. 2011, 22, 808–818. [Google Scholar] [CrossRef]
- Hood, E.D.; Greineder, C.F.; Shuvaeva, T.; Walsh, L.; Villa, C.H.; Muzykantov, V.R. Vascular Targeting of Radiolabeled Liposomes with Bio-Orthogonally Conjugated Ligands: Single Chain Fragments Provide Higher Specificity than Antibodies. Bioconjug. Chem. 2018, 29, 3626–3637. [Google Scholar] [CrossRef]
- Wang, X.; Sheng, J.; Yang, M. Melanin-based nanoparticles in biomedical applications: From molecular imaging to treatment of diseases. Chin. Chem. Lett. 2019, 30, 533–540. [Google Scholar] [CrossRef]
- Liu, Q.; Fang, H.; Lan, X. pH-triggered assembly of natural melanin nanoparticle for enhanced PET imaging. J. Nucl. Med. 2020, 61, 1088. [Google Scholar] [CrossRef]
- Ni, D.; Jiang, D.; Ehlerding, E.B.; Huang, P.; Cai, W. Radiolabeling Silica-Based Nanoparticles via Coordination Chemistry: Basic Principles, Strategies, and Applications. Acc. Chem. Res. 2018, 51, 778–788. [Google Scholar] [CrossRef]
- Hall, M.A.; Kwon, S.; Robinson, H.; Lachance, P.-A.; Azhdarinia, A.; Ranganathan, R.; Price, R.E.; Chan, W.; Sevick-Muraca, E.M. Imaging prostate cancer lymph node metastases with a multimodality contrast agent. Prostate 2012, 72, 129–146. [Google Scholar] [CrossRef]
- Lee, Y.K.; Jeong, J.M.; Hoigebazar, L.; Yang, B.Y.; Lee, Y.-S.; Lee, B.C.; Youn, H.; Lee, D.S.; Chung, J.-K.; Lee, M.C. Nanoparticles Modified by Encapsulation of Ligands with a Long Alkyl Chain to Affect Multispecific and Multimodal Imaging. J. Nucl. Med. 2012, 53, 1462–1470. [Google Scholar] [CrossRef]
- Pretze, M.; van der Meulen, N.P.; Wangler, C.; Schibli, R.; Wangler, B. Targeted 64Cu-labeled gold nanoparticles for dual imaging with positron emission tomography and optical imaging. J. Label. Compd. Radiopharm. 2019, 62, 471–482. [Google Scholar] [CrossRef]
- Pang, B.; Zhao, Y.; Luehmann, H.; Yang, M.; Detering, L.; Younan, X.; Zhang, C.; Zhang, L.; Lisa, Z.-Y.; Ren, Q.; et al. 64Cu-Doped PdCu@Au Tripods: A Multifunctional Nanomaterial for Positron Emission Tomography and Image-Guided Photothermal Cancer Treatment. ACS Nano 2016, 10, 3121–3131. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, P.; Jacobson, O.; Wang, Z.; Liu, Y.; Lin, L.; Lin, J.; Lu, N.; Zhang, H.; Tian, R.; et al. Biomineralization-Inspired Synthesis of Copper Sulfide–Ferritin Nanocages as Cancer Theranostics. ACS Nano 2016, 10, 3453–3460. [Google Scholar] [CrossRef]
- Phillips, E.; Penate-Medina, O.; Zanzonico, P.B.; Carvajal, R.D.; Mohan, P.; Ye, Y.; Humm, J.; Gönen, M.; Kalaigian, H.; Schöder, H.; et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014, 6, ra149. [Google Scholar] [CrossRef]
- Ni, D.; Jiang, D.; Valdovinos, H.F.; Ehlerding, E.B.; Yu, B.; Barnhart, T.E.; Huang, P.; Cai, W. Bioresponsive Polyoxometalate Cluster for Redox-Activated Photoacoustic Imaging-Guided Photothermal Cancer Therapy. Nano Lett. 2017, 17, 3282–3289. [Google Scholar] [CrossRef]
- Forte, E.; Fiorenza, D.; Torino, E.; Costagliola di Polidoro, A.; Cavaliere, C.; Netti, P.A.; Salvatore, M.; Aiello, M. Radiolabeled PET/MRI Nanoparticles for Tumor Imaging. J. Clin. Med. 2020, 9, 89. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Yang, C.-T.; Ghosh, K.K.; Padmanabhan, P.; Langer, O.; Liu, J.; Halldin, C.; Gulyás, B.Z. PET-MR and SPECT-MR multimodality probes: Development and challenges. Theranostics 2018, 8, 6210–6232. [Google Scholar] [CrossRef]
- Madru, R.; Budassi, M.; Benveniste, H.; Lee, H.; Smith, S.D.; Schlyer, D.J.; Vaska, P.; Knutsson, L.; Strand, S.-E. Simultaneous Preclinical Positron Emission Tomography-Magnetic Resonance Imaging Study of Lymphatic Drainage of Chelator-Free Cu-64-Labeled Nanoparticles. Cancer Biother. Radiopharm. 2018, 33, 213–220. [Google Scholar] [CrossRef]
- Malinge, J.; Géraudie, B.; Savel, P.; Nataf, V.; Prignon, A.; Provost, C.; Zhang, Y.; Ou, P.; Kerrou, K.; Talbot, J.-N.; et al. Liposomes for PET and MR Imaging and for Dual Targeting (Magnetic Field/Glucose Moiety): Synthesis, Properties, and in Vivo Studies. Mol. Pharm. 2017, 14, 406–414. [Google Scholar] [CrossRef]
- Vecchione, D.; Aiello, M.; Cavaliere, C.; Nicolai, E.; Netti, P.A.; Torino, E. Hybrid core shell nanoparticles entrapping Gd-DTPA and 18F-FDG for simultaneous PET/MRI acquisitions. Nanomedicine 2017, 12, 2223–2231. [Google Scholar] [CrossRef]
- Makino, A.; Kimura, S. Solid Tumor-Targeting Theranostic Polymer Nanoparticle in Nuclear Medicinal Fields. Sci. World J. 2014, 2014, 424513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abou, D.S.; Pickett, J.E.; Thorek, D.L.J. Nuclear molecular imaging with nanoparticles: Radiochemistry, applications and translation. Br. J. Radiol. 2015, 88, 20150185. [Google Scholar] [CrossRef] [PubMed]
- Boulos, J.C.; Rahama, M.; Hegazy, M.F.; Efferth, T. Shikonin derivatives for cancer prevention and therapy. Cancer Lett. 2019, 459, 248–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Pozo, F.M.; Tian, D.; Geng, X.; Yao, X.; Zhang, Y.; Tang, J. Shikonin Inhibits Cancer Through P21 Upregulation and Apoptosis Induction. Front. Pharmacol. 2020, 11, 861. [Google Scholar] [CrossRef] [PubMed]
- Lomis, N.; Westfall, S.; Farahdel, L.; Malhotra, M.; Shum-Tim, D.; Prakash, S. Human Serum Albumin Nanoparticles for Use in Cancer Drug Delivery: Process Optimization and In Vitro Characterization. Nanomaterials 2016, 6, 116. [Google Scholar] [CrossRef]
- Harding, C.V.; Heuser, J.E.; Stahl, P.D. Exosomes: Looking back three decades and into the future. J. Cell Biol. 2013, 200, 367–371. [Google Scholar] [CrossRef]
- Pan, B.T.; Teng, K.; Wu, C.; Adam, M.; Johnstone, R.M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 1985, 101, 942–948. [Google Scholar] [CrossRef]
- Isola, A.L.; Chen, S. Exosomes: The Messengers of Health and Disease. Curr. Neuropharmacol. 2017, 15, 157–165. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.M.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, P.; Tan, H.; Chen, X.; Wang, Q.; Chen, T. Exosomes as Smart Nanoplatforms for Diagnosis and Therapy of Cancer. Front. Oncol. 2021, 11, 3364. [Google Scholar] [CrossRef]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell derived exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef]
- Deep, G. Exosomes-based biomarker discovery for diagnosis and prognosis of prostate cancer. Front. Biosci. 2017, 22, 1682–1696. [Google Scholar] [CrossRef]
- Tai, Y.-L.; Chen, K.-C.; Hsieh, J.-T.; Shen, T.-L. Exosomes in cancer development and clinical applications. Cancer Sci. 2018, 109, 2364–2374. [Google Scholar] [CrossRef]
- Khan, A.A.; de Rosales, R.T.M. Radiolabelling of Extracellular Vesicles for PET and SPECT imaging. Nanotheranostics 2021, 5, 256–274. [Google Scholar] [CrossRef]
- Yi, Y.; Lee, J.H.; Kim, S.-Y.; Pack, C.-G.; Ha, D.H.; Park, S.R.; Youn, J.; Cho, B.S. Advances in Analysis of Biodistribution of Exosomes by Molecular Imaging. Int. J. Mol. Sci. 2020, 21, 665. [Google Scholar] [CrossRef]
- Barberis, E.; Vanella, V.V.; Falasca, M.; Caneapero, V.; Cappellano, G.; Raineri, D.; Ghirimoldi, M.; De Giorgis, V.; Puricelli, C.; Vaschetto, R.; et al. Circulating exosomes are strongly involved in SARS-CoV-2 infection. Front. Mol. Biosci. 2021, 8, 632290. [Google Scholar] [CrossRef]
- Abdelgawad, M.; Bakry, N.S.; Farghali, A.A.; Abdel-Latif, A.; Lotfy, A. Mesenchymal stem cell-based therapy and exosomes in COVID-19: Current trends and prospects. Stem Cell Res. Ther. 2021, 12, 469. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.H.; Kim, J.-H. Diverse Effects of Exosomes on COVID-19: A Perspective of Progress from Transmission to Therapeutic Developments. Front. Immunol. 2021, 12, 716407. [Google Scholar] [CrossRef] [PubMed]
- McGough, I.; Vincent, J.-P. Exosomes in developmental signalling. Development 2016, 143, 2482–2493. [Google Scholar] [CrossRef]
- Junker, K.; Heinzelmann, J.; Beckham, C.; Ochiya, T.; Jenster, G. Extracellular Vesicles and Their Role in Urologic Malignancies. Eur. Urol. 2016, 70, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Cheng, X.; Pan, X.; Li, J. Emerging role of exosomes in liver physiology and pathology. Hepatol. Res. 2017, 47, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Lorenc, T.; Chrzanowski, J.; Olejarz, W. Current Perspectives on Clinical Use of Exosomes as a Personalized Contrast Media and Theranostics. Cancers 2020, 12, 3386. [Google Scholar] [CrossRef] [PubMed]
- Di Rocco, G.; Baldari, S.; Toietta, G. Towards Therapeutic Delivery of Extracellular Vesicles: Strategies for In Vivo Tracking and Biodistribution Analysis. Stem Cells Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Kim, D.H.; Kothandan, V.K.; Kim, H.W.; Kim, K.S.; Kim, J.Y.; Cho, H.J.; Lee, Y.-K.; Lee, D.-E.; Hwang, S.R. Noninvasive Assessment of Exosome Pharmacokinetics In Vivo: A Review. Pharmaceutics 2019, 11, 649. [Google Scholar] [CrossRef]
- Almeida, S.; Santos, L.; Falcão, A.; Gomes, C.; Abrunhosa, A. In Vivo Tracking of Extracellular Vesicles by Nuclear Imaging: Advances in Radiolabeling Strategies. Int. J. Mol. Sci. 2020, 21, 9443. [Google Scholar] [CrossRef]
- Rashid, M.H.; Borin, T.F.; Ara, R.; Angara, K.; Cai, J.; Achyut, B.R.; Liu, Y.; Arbab, A.S. Differential in vivo biodistribution of 131I-labeled exosomes from diverse cellular origins and its implication for theranostic application. Nanomedicine 2019, 21, 102072. [Google Scholar] [CrossRef]
| Nanomaterial Probe | Isotope | Morphology, Coating, Modifications, Chelator, and Hydrodynamic Diameter, etc. | Applications and Research Outcomes | Ref. |
|---|---|---|---|---|
| 68Ga-NOTA-MSA (human) | 68Ga | NPs, SCN-mannose modified HSA, NOTA | Diagnostic PET imaging for SLN | [16] |
| [64Cu]DO3A-KRAS PNA-peptide | 64Cu | NPs, DO3A | Diagnostic PET imaging, specific genetic characteristics of radiolabeled-PNA-peptide NPs | [17] |
| HDL | 89Zr | NPs, PL or apoA-I conjugated, 8.6 ± 1.3 nm | Diagnostic PET imaging, 89Zr-labeled TAM imaging using HDL, specific for macrophages, quantitative macrophage PET | [18] |
| DFB | 89Zr | Nanocarriers, PEGylated, ~15 nm | Diagnostic PET imaging, ~15 nm PEG40kDa-89Zr-radiolabeled surrogates of PEG-prodrugs of SN-38 (PLX038) | [19] |
| BPT-DPP | 64Cu | NPs, spherical, PEGlyated, NOTA, 31.3 ± 2.8 mm | PET/PAI dual modality imaging probe | [20] |
| SNs and SNs-RPM | 18F | Nanometric emulsions, [18F]FBEM conjugated, PEGylated, ~130–150 nm | Diagnostic PET imaging, 18F-radiolabeled technique for lipid-based nanocarriers | [21] |
| MNPs | 64Cu | NPs, PEGylated, ~11 nm | Diagnostic PET imaging, good imaging, therapeutic effects on A431 tumors, potential in targeted radiotherapy | [22] |
| CBT-68Ga-NPs | 68Ga | NPs, 258.3 ± 127.85 nm | Diagnostic PET imaging, tumor-targeted imaging probe | [23] |
| Micelles | 67Ga | Nanocarriers, α-alkyl chain, NOTA or DOTA | Diagnostic PET imaging, higher uptake in liver of micelles due to increased lipophilicity of Ga(III) chelates | [24,25] |
| Nano-GO sheets | 66Ga 64Cu | PEGlylated, NOTA, 10–50 nm | Diagnostic PET imaging, site-specific tumor neovasculature targeting through functionalization of the TRC105 antibody | [26,27] |
| Zeolite Y | 68Ga | Nanocrystalline, azide functionalized, µm to ~55 nm | Diagnostic PET imaging, incorporation of 68Ga- to pores of azide-functionalized NaY zeolite as a bifunctional molecular targeting vector | [28] |
| QDs | 64Cu | Amine functionalized, DOTA, ~20–25 nm | PET/NIRF, dual modality imaging QD-based nanoprobe for tumor VEGFR expression | [29] |
| Metal oxides | 89Zr | Nanorods, nanospheres, NPs, PEGylated, ~140 nm | Diagnostic PET/MRI imaging, chelator-free radiolabeling of 89Zr- on metal oxide for multimodal imaging | [30] |
| Dendrimer-based single molecular platform | 67/68Ga | NPs, NOTA and DOTA | PET/MRI or SPECT/MRI dual modality imaging probe with quantifiable radioisotopes chelated in NOTA or DOTA | [31] |
| MDIO-64Cu-DOTA | 64Cu | IONPs, dextran coated, DOTA, ~62.7 nm | PET/MRI dual-modality imaging probe, Anionic charges on surface of nanoparticulate MDIO-64Cu-DOTA to facilitate recognition by SR-A on macrophages for VAP | [32] |
| 89Zr/Fe-DFO micelles | 89Zr | Micelles, ~25–50 nm | PET/MRI dual modality imaging probe, with Fe-DFO for MRI, high tumor-to-blood and tumor-to-muscle ratio, on EPR-based tumor imaging | [33] |
| HAS-IONPs | 64Cu | Dopamine and Cy5.5 coating, DOTA, ~29.4 ± 1.2 nm | PET/NIRF/MRI triple-modality imaging probe, dopamine and Cy5.5coating IONPs encapsulated in HSA matrices | [34] |
| TCL-SPIOs | 124I | IONPs, PEGylated, tyramine coating, 39 ± 8 nm | Optical/PET/MRI triple-modality imaging probe through adaptation of Cerenkov radiation | [35] |
| APAS-131I-PNPs/DOX | 131I | Nanoparticulate platform, PEGylated, 241.16 ± 13.57 nm | Theranostic (SPECT, chemotherapy), enhanced cellular uptake in cancer cells by smart theranostic system, enhanced SPECT imaging and chemo/radioactive combination cancer therapy | [36] |
| Shikonin-AgNPs | 131I | NPs, spherical, modified by shikonin, ~106 nm | Theranostic (Gamma-counter, therapy), cell viability and proliferation of human lung carcinoma cell inhibited by synergistic antitumor combinatorial therapy | [37] |
| 123I-61-B-AuNPs | 123I | NPs, PEGylated, ~54.48 ± 14.72 nm | Theranostic (SPECT/CT, BNCT) HER2-targeting boron-containing AuNPs for specific tumor localization and tracking, antibody modified boron containing AuNPs for BNCT | [38] |
| 125I-HSA | 125I | Nanodrugs, Cy5.5 | Theranostics (SPECT/CT, radiation-based therapy), enhanced cell uptake under X-ray exposure, prolonged tumor retention time, positive correlation between cell uptake and Caveolin-1 expression, albumin-based combination therapy | [39] |
| Exosomes/Extracellular Vesicles (EV) | 124I | Nanovesicles, neuraminidase modified, ~100 nm | Diagnostic PET imaging, tracking quantitatively of radiolabeled EVs with neuraminidase modification on EV surface | [40] |
| 131I/125I | Nanovesicles, SULFO-SHPP conjugated, 233.8 ± 32.7 nm) | PET imaging, surface modification of EVs with linker SULFO-SHPP and radioiodine labeling on linker | [41] | |
| 64Cu/68Ga | Nanovesicles, Cy7, NOTA, ~100 nm | Optical/PET dual imaging probe, less dependency on cell type for exosome biodistribution in mice | [42] | |
| 89Zr | Nanovesicles, <~150 nm | Diagnostic imaging (PET), direct radiolabeling of [89Zr]Zr(oxinate)4 to target internal components of EVs without surface modifications | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phua, V.J.X.; Yang, C.-T.; Xia, B.; Yan, S.X.; Liu, J.; Aw, S.E.; He, T.; Ng, D.C.E. Nanomaterial Probes for Nuclear Imaging. Nanomaterials 2022, 12, 582. https://doi.org/10.3390/nano12040582
Phua VJX, Yang C-T, Xia B, Yan SX, Liu J, Aw SE, He T, Ng DCE. Nanomaterial Probes for Nuclear Imaging. Nanomaterials. 2022; 12(4):582. https://doi.org/10.3390/nano12040582
Chicago/Turabian StylePhua, Vanessa Jing Xin, Chang-Tong Yang, Bin Xia, Sean Xuexian Yan, Jiang Liu, Swee Eng Aw, Tao He, and David Chee Eng Ng. 2022. "Nanomaterial Probes for Nuclear Imaging" Nanomaterials 12, no. 4: 582. https://doi.org/10.3390/nano12040582
APA StylePhua, V. J. X., Yang, C.-T., Xia, B., Yan, S. X., Liu, J., Aw, S. E., He, T., & Ng, D. C. E. (2022). Nanomaterial Probes for Nuclear Imaging. Nanomaterials, 12(4), 582. https://doi.org/10.3390/nano12040582






