Tuning the Reactivity of Perfluoropolyether-Functionalized Aluminum Nanoparticles by the Reaction Interface Fuel-Oxidizer Ratio
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
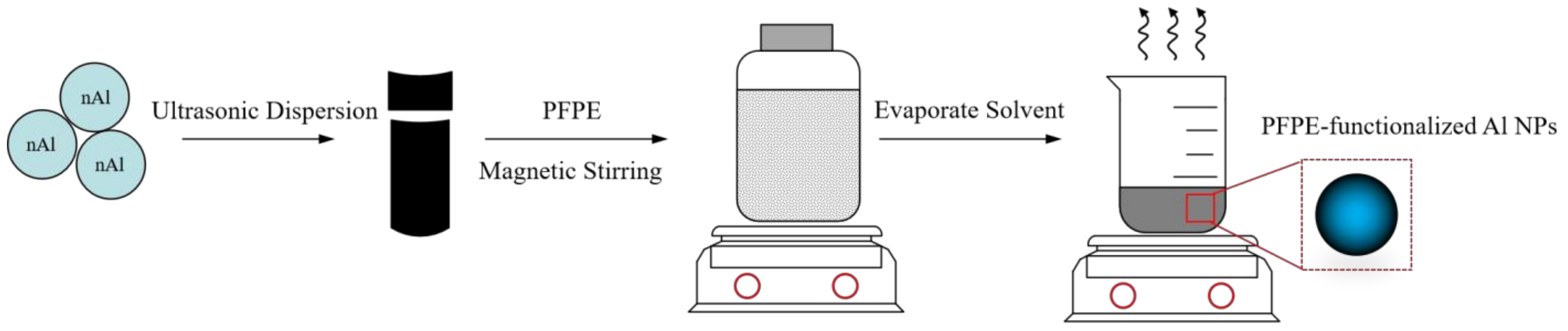
2.2. Preparations
2.3. Characterizations
2.4. Calorific Value Measurement
2.5. Constant Volume Combustion Cell Test
3. Results and Discussion
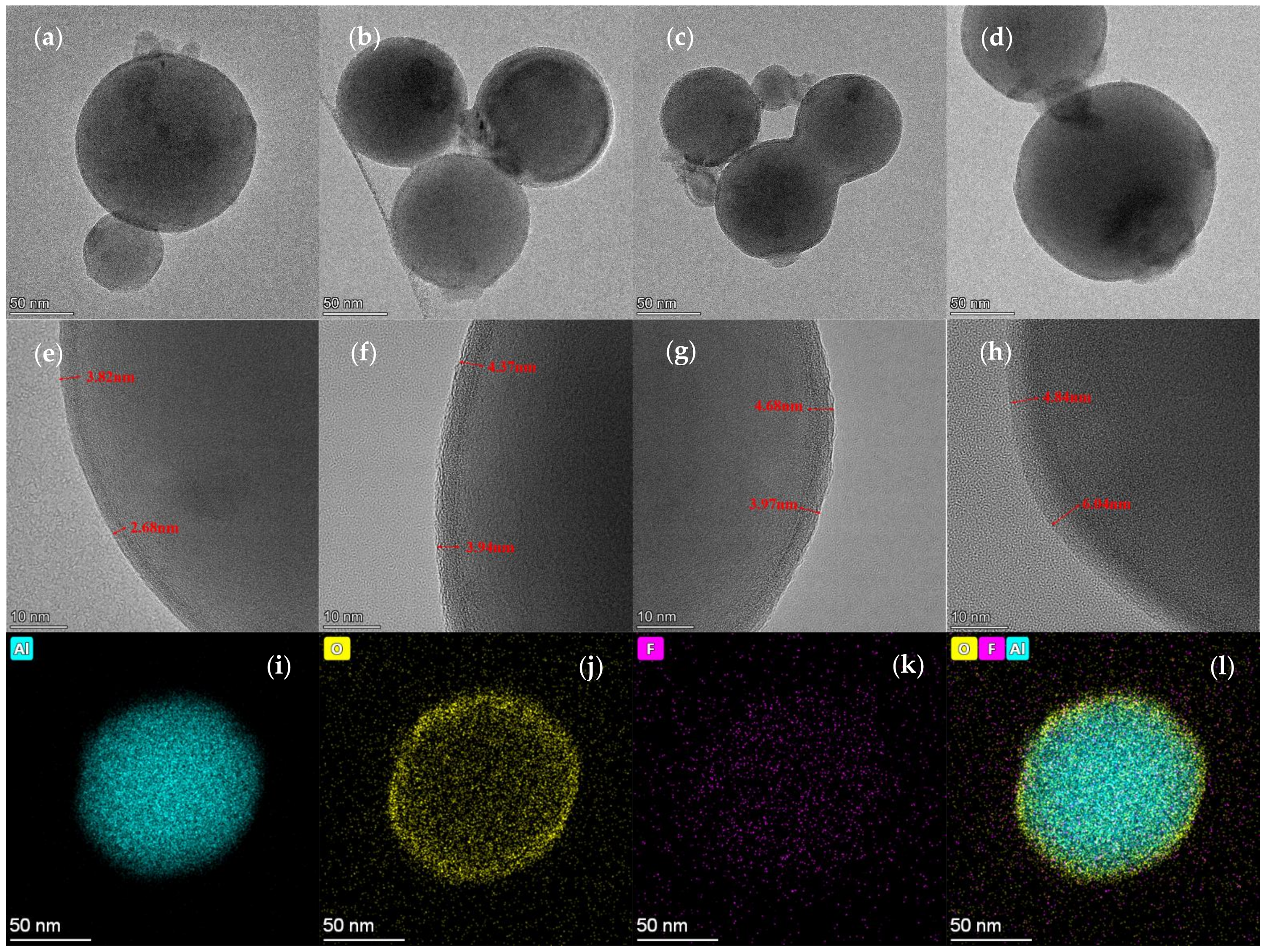
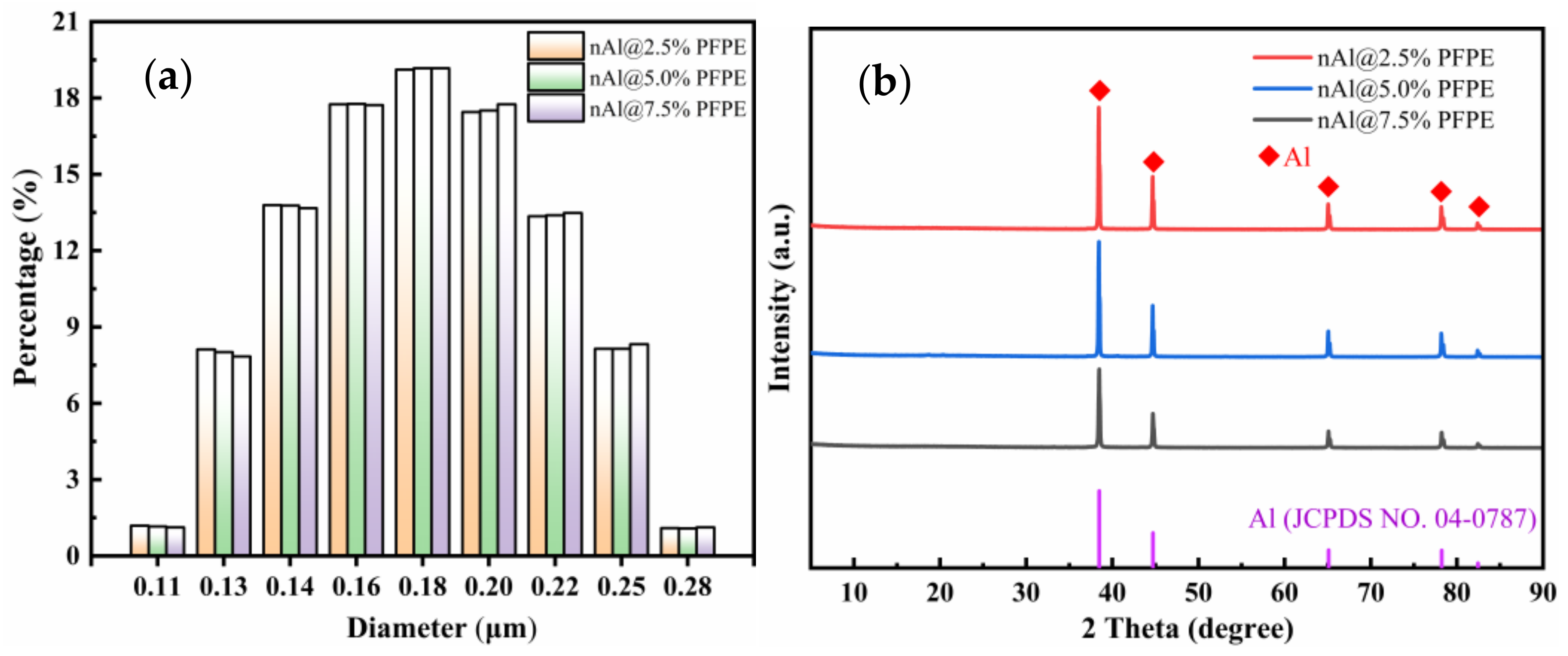
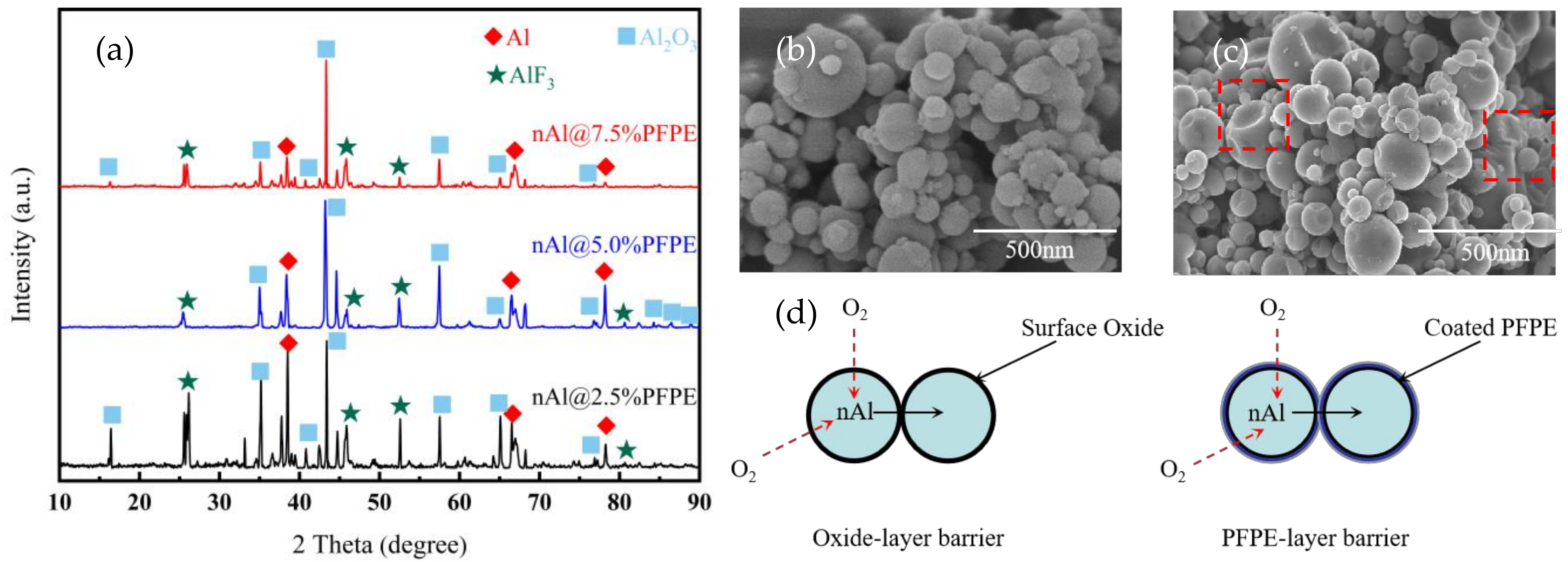
3.1. Micro-Structure Analysis
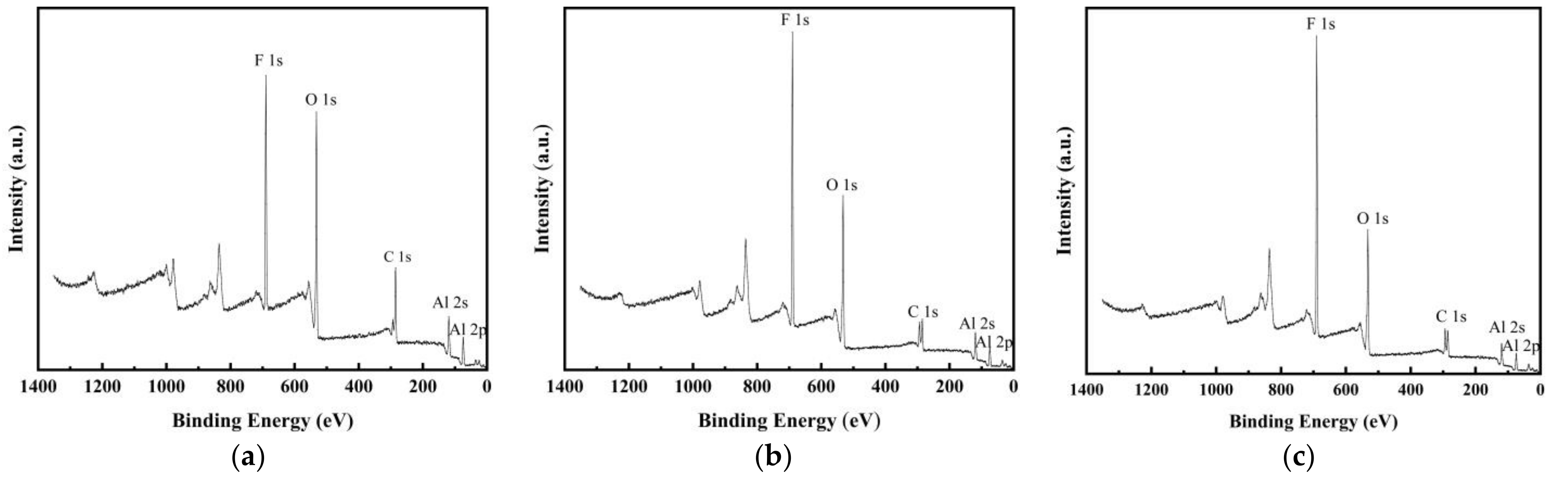
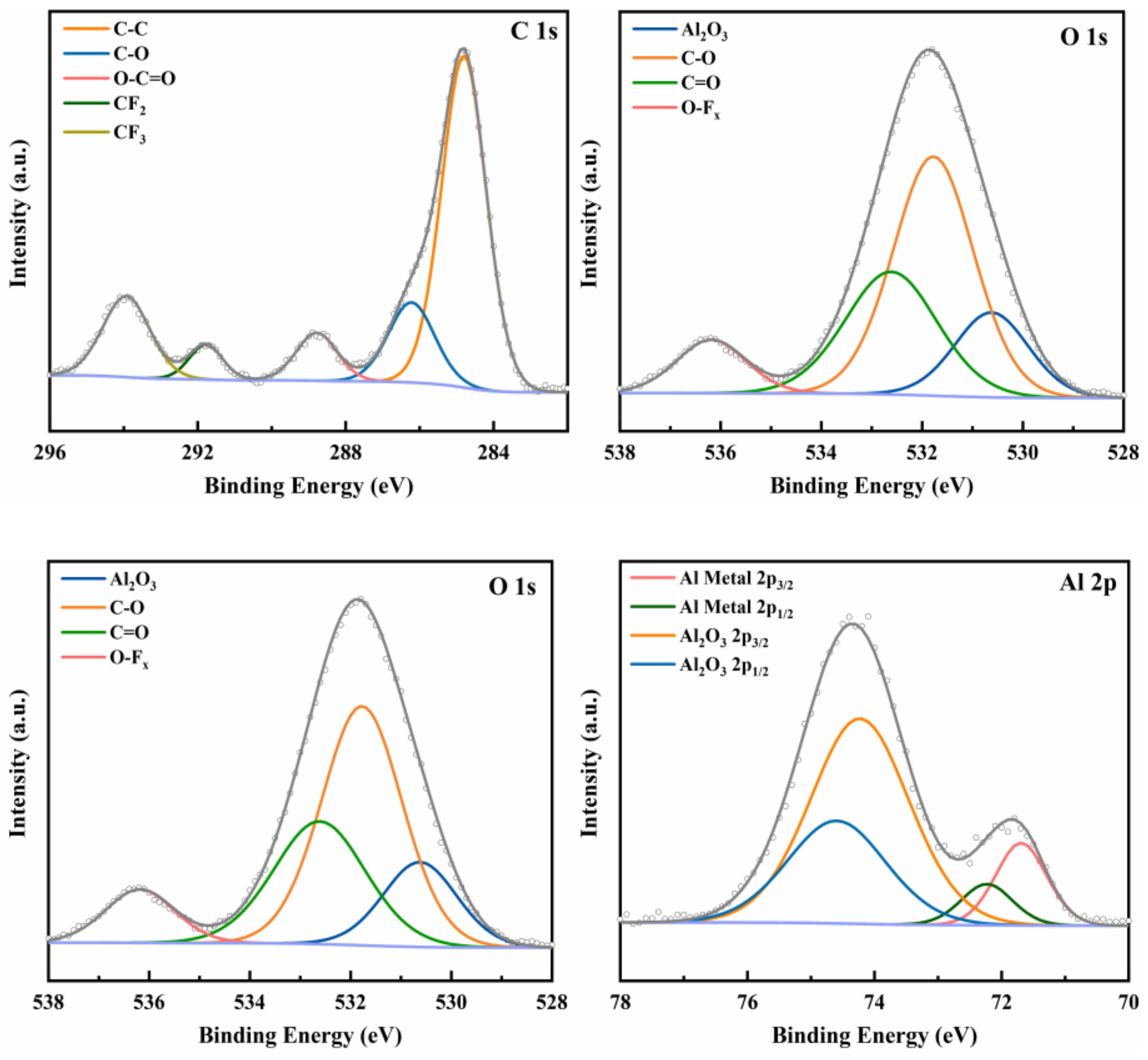
3.2. X-ray Photoelectron Spectroscopy Analysis
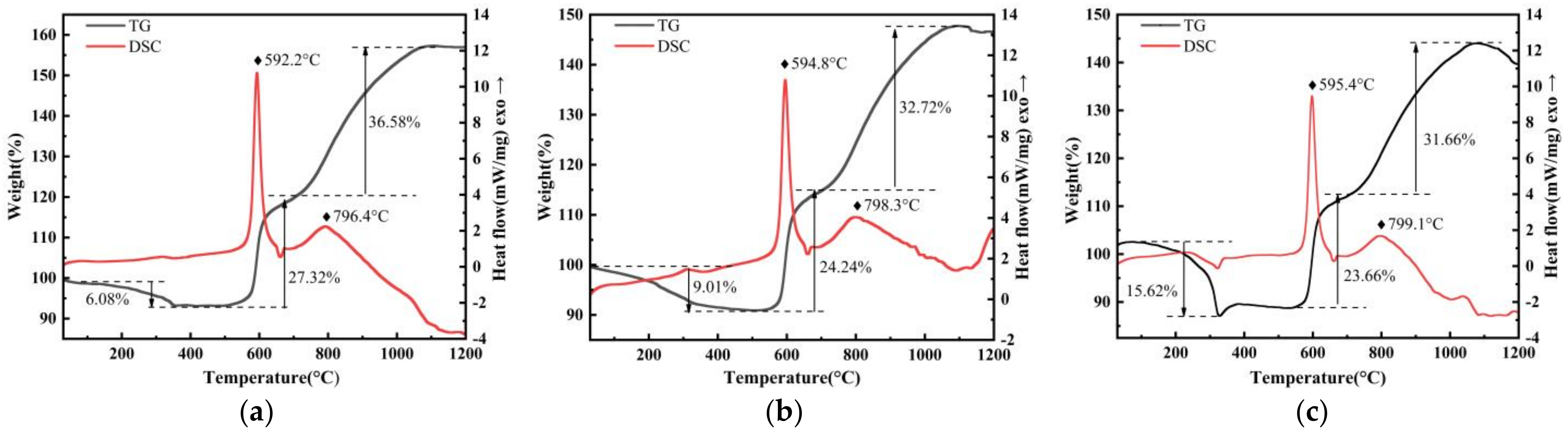
3.3. Oxidation Mechanism Analysis
3.4. The Calorific Value Analysis
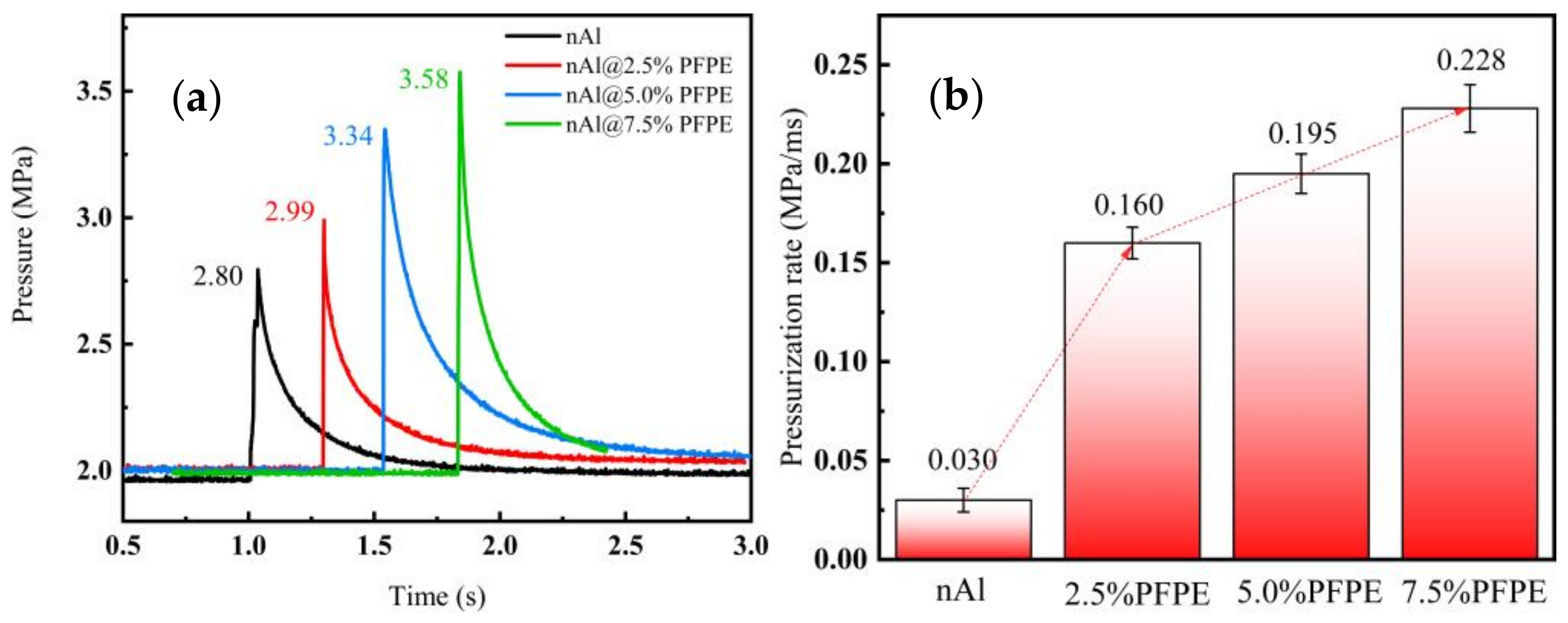
3.5. Constant Volume Combustion Characteristics
4. Conclusions
- (1)
- PFPE-functionalized Al NPs with varying percentages of PFPE were constructed using the solvent suspension method. The micro-structure of the PFPE-functionalized Al NPs basically had a normal distribution with no obvious agglomeration. PFPE dispersed homogeneously throughout Al NPs by means of physical adsorption.
- (2)
- The oxidation mechanism of PFPE-functionalized Al NPs at a slow heating rate was regulated by the reaction interface Fuel-Oxidizer ratio. Under the effect of an exothermic surface reaction between the PFPE and Al2O3 shell, the active aluminum contained in the aluminum core was more likely to participate in the vigorous oxidation reaction.
- (3)
- After adding different contents of PFPE, the calorific value of the modified Al NPs was increased by 7.22%, 4.80%, and 3.21%, respectively. Simultaneously, compared with Al NPs, the exothermic reaction rate and the exothermic reaction energy were all increased because of the decomposition products participating in the interface reaction.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohkura, Y.; Rao, P.M.; Zheng, X.L. Flash ignition of Al nanoparticles: Mechanism and applications. Combust. Flame 2011, 158, 2544–2548. [Google Scholar] [CrossRef]
- Davari, S.A.; Gottfried, J.L.; Liu, C.; Ribeiro, E.L.; Duscher, G.; Mukherjee, D. Graphitic coated Al nanoparticles manufactured as superior energetic materials via laser ablation synthesis in organic solvents. Appl. Surf. Sci. 2019, 473, 156–163. [Google Scholar] [CrossRef]
- Gnanaprakash, K.; Chakravarthy, S.R.; Sarathi, R. Combustion mechanism of composite solid propellant sandwiches containing nano-aluminium. Combust. Flame 2017, 182, 64–75. [Google Scholar] [CrossRef]
- Kumar, A.S.; Rao, V.B.; Sinha, R.K.; Rao, A.S. Evaluation of Plastic Bonded Explosive (PBX) Formulations Based on RDX, Aluminum, and HTPB for Underwater Applications. Propellants Explos. Pyrotech. 2010, 35, 359–364. [Google Scholar] [CrossRef]
- Hasani, S.; Panjepour, M.; Shamanian, M. The Oxidation Mechanism of Pure Aluminum Powder Particles. Oxid. Met. 2012, 78, 179–195. [Google Scholar] [CrossRef]
- Jeurgens, L.P.H.; Sloof, W.G.; Tichelaar, F.D.; Mittemeijer, E.J. Growth kinetics and mechanisms of aluminum-oxide films formed by thermal oxidation of aluminum. J. Appl. Phys. 2002, 92, 1649–1656. [Google Scholar] [CrossRef]
- Zhao, W.J.; Ren, H.; Ou, Y.P.; Jiao, Q.J. Nanocomposites with Al and Ti binary fuels and potassium oxysalts for energetic applications. Mater. Lett. 2020, 262, 127189. [Google Scholar] [CrossRef]
- Foley, T.J.; Johnson, C.E.; Higa, K.T. Inhibition of oxide formation on aluminum nanoparticles by transition metal coating. Chem. Mater. 2005, 17, 4086–4091. [Google Scholar] [CrossRef]
- Terry, B.C.; Sippel, T.R.; Pfeil, M.A.; Gunduz, I.E.; Son, S.F. Removing hydrochloric acid exhaust products from high performance solid rocket propellant using aluminum-lithium alloy. J. Hazard. Mater. 2016, 317, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.J.; Wang, X.Z.; Wang, H.Y.; Wu, T.; Kline, D.J.; Rehwoldt, M.; Ren, H.; Zachariah, M.R. Titanium enhanced ignition and combustion of Al/I2O5 mesoparticle composites. Combust. Flame 2020, 212, 245–251. [Google Scholar] [CrossRef]
- Wang, H.X.; Ren, H.; Yan, T.; Li, Y.R.; Zhao, W.J. A latent highly activity energetic fuel: Thermal stability and interfacial reaction kinetics of selected fluoropolymer encapsulated sub-micron sized Al particles. Sci. Rep. 2021, 11, 738. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Risha, G.A.; Yang, V.; Yetter, R.A. Effect of particle size on combustion of aluminum particle dust in air. Combust. Flame 2009, 156, 5–13. [Google Scholar] [CrossRef]
- Freedman, O.E. Oxidation of Aluminum. J. Natl. Dent. Assoc. 1977, 95, 187. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.J.; Aral, G.; Ogata, S.; Kalia, R.K.; Nakano, A.; Vashishta, P. Oxidation of aluminum nanoclusters. Phys. Rev. B 2005, 71, 205413. [Google Scholar] [CrossRef] [Green Version]
- Rai, A.; Lee, D.; Park, K.H.; Zachariah, M.R. Importance of phase change of aluminum in oxidation of aluminum nanoparticles. J. Phys. Chem. B 2004, 108, 14793–14795. [Google Scholar] [CrossRef]
- Zhang, X.F.; Fu, C.R.; Xia, Y.J.; Duan, Y.R.; Li, Y.F.; Wang, Z.C.; Jiang, Y.Y.; Li, H. Atomistic Origin of the Complex Morphological Evolution of Aluminum Nanoparticles during Oxidation: A Chain-like Oxide Nucleation and Growth Mechanism. ACS Nano 2019, 13, 3005–3014. [Google Scholar] [CrossRef]
- Laboureur, D.; Glabeke, G.; Gouriet, J.B. Validation of Cabrera-Mott model for low-temperature oxidation of aluminum nanoparticles. J. Nanopart. Res. 2021, 23, 71. [Google Scholar] [CrossRef]
- Rai, A.; Park, K.; Zhou, L.; Zachariah, M.R. Understanding the mechanism of aluminium nanoparticle oxidation. Combust. Theory Model. 2006, 10, 843–859. [Google Scholar] [CrossRef]
- McCollum, J.; Pantoya, M.L.; Iacono, S.T. Catalyzing aluminum particle reactivity with a fluorine oligomer surface coating for energy generating applications. J. Fluor. Chem. 2015, 180, 265–271. [Google Scholar] [CrossRef]
- Valluri, S.K.; Schoenitz, M.; Dreizin, E. Fluorine-containing oxidizers for metal fuels in energetic formulations. Def. Technol. 2019, 15, 1–22. [Google Scholar] [CrossRef]
- Kappagantula, K.; Pantoya, M.L.; Hunt, E.M. Impact ignition of aluminum-teflon based energetic materials impregnated with nano-structured carbon additives. J. Appl. Phys. 2012, 112, 024902. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, K.T.; Min, T.S.; Kim, K.J.; Kim, S.H. Improved Energetic-Behaviors of Spontaneously Surface-Mediated Al Particles. Sci. Rep. 2017, 7, 4659. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, L.; Mao, Y.F.; Gong, F.Y. An effective way to enhance energy output and combustion characteristics of Al/PTFE. Combust. Flame 2020, 214, 419–425. [Google Scholar] [CrossRef]
- He, W.; Liu, P.J.; Gong, F.Y.; Tao, B.W.; Gu, J.; Yang, Z.J.; Yan, Q.L. Tuning the Reactivity of Metastable Intermixed Composite n-Al/PTFE by Polydopamine Interfacial Control. ACS Appl. Mater. Interfaces 2018, 10, 32849–32858. [Google Scholar] [CrossRef] [PubMed]
- McCollum, J.; Morey, A.M.; Iacono, S.T. Morphological and combustion study of interface effects in aluminum-poly(vinylidene fluoride) composites. Mater. Des. 2017, 134, 64–70. [Google Scholar] [CrossRef]
- Wang, H.Y.; Rehwoldt, M.; Kline, D.J.; Wu, T.; Wang, P.; Zachariah, M.R. Comparison study of the ignition and combustion characteristics of directly-written Al/PVDF, Al/Viton and Al/THV composites. Combust. Flame 2019, 201, 181–186. [Google Scholar] [CrossRef]
- DeLisio, J.B.; Hu, X.L.; Wu, T.; Egan, G.C.; Young, G.; Zachariah, M.R. Probing the Reaction Mechanism of Aluminum/Poly(vinylidene fluoride) Composites. J. Phys. Chem. B 2016, 120, 5534–5542. [Google Scholar] [CrossRef]
- Kappagantula, K.S.; Pantoya, M.L.; Horn, J. Effect of surface coatings on aluminum fuel particles toward nanocomposite combustion. Surf. Coat. Technol. 2013, 237, 456–459. [Google Scholar] [CrossRef]
- Kappagantula, K.S.; Farley, C.; Pantoya, M.L.; Horn, J. Tuning Energetic Material Reactivity Using Surface Functionalization of Aluminum Fuels. J. Phys. Chem. C 2012, 116, 24469–24475. [Google Scholar] [CrossRef]
- Zhao, W.J.; Jiao, Q.J.; Ou, Y.P.; Yang, R.J.; Zhu, Y.L.; Wang, F. Perfluoroalkyl Acid-Functionalized Aluminum Nanoparticles for Fluorine Fixation and Energy Generation. ACS Appl. Nano Mater. 2021, 4, 6337–6344. [Google Scholar] [CrossRef]
- Bello, M.N.; Hill, K.J.; Pantoya, M.L.; Jouet, R.J.; Horn, J.M. Surface engineered nanoparticles dispersed in kerosene: The effect of oleophobicity on droplet combustion. Combust. Flame 2018, 188, 243–249. [Google Scholar] [CrossRef]
- Sippel, T.R.; Son, S.F.; Groven, L.J. Altering Reactivity of Aluminum with Selective Inclusion of Polytetrafluoroethylene through Mechanical Activation. Propellants Explos. Pyrotech. 2013, 38, 286–295. [Google Scholar] [CrossRef]
- Padhye, R.; Aquino, A.J.A.; Tunega, D.; Pantoya, M.L. Fluorination of an Alumina Surface: Modeling Aluminum-Fluorine Reaction Mechanisms. ACS Appl. Mater. Interfaces 2017, 9, 24290–24297. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Quaglio, M.; Marasso, S.L.; Chiodoni, A.; Cocuzza, M.; Bongiovanni, R. Direct Photolithography of Perfluoropolyethers for Solvent-Resistant Microfluidics. Langmuir 2013, 29, 15711–15718. [Google Scholar] [CrossRef] [PubMed]
- Black, J.E.; Silva, G.M.C.; Klein, C.; Iacovella, C.R.; Morgado, P.; Martins, L.F.G.; Filipe, E.J.M.; McCabe, C. Perfluoropolyethers: Development of an All-Atom Force Field for Molecular Simulations and Validation with New Experimental Vapor Pressures and Liquid Densities. J. Phys. Chem. B 2017, 121, 6588–6600. [Google Scholar] [CrossRef] [Green Version]
- Clayton, N.A.; Kappagantula, K.S.; Pantoya, M.L.; Kettwich, S.C.; Iacono, S.T. Fabrication, Characterization, and Energetic Properties of Metallized Fibers. ACS Appl. Mater. Interfaces 2014, 6, 6049–6053. [Google Scholar] [CrossRef] [PubMed]
- Kettwich, S.C.; Kappagantula, K.; Kusel, B.S.; Avjian, E.K.; Danielson, S.T.; Miller, H.A.; Pantoya, M.L.; Iacono, S.T. Thermal investigations of nanoaluminum/perfluoropolyether core-shell impregnated composites for structural energetics. Thermochim. Acta 2014, 591, 45–50. [Google Scholar] [CrossRef]
- McCollum, J.; Pantoya, M.L.; Iacono, S.T. Activating Aluminum Reactivity with Fluoropolymer Coatings for Improved Energetic Composite Combustion. ACS Appl. Mater. Interfaces 2015, 7, 18742–18749. [Google Scholar] [CrossRef]
- Brooks, K.P.; Beckstead, M.W. Dynamics of aluminum combustion. J. Propuls. Power 1995, 11, 769–780. [Google Scholar] [CrossRef]
- Eisenreich, N.; Fietzek, H.; Juez-Lorenzo, M.D.; Kolarik, V.; Koleczko, A.; Weiser, V. On the mechanism of low temperature oxidation for aluminum particles down to the nano-scale. Propellants Explos. Pyrotech. 2004, 29, 137–145. [Google Scholar] [CrossRef]
- Zhou, Y.A.; Liu, J.Z.; Wang, J.R.; Xv, T.W.; Wang, J.H.; Zhou, J.H.; Cen, K.F. Experimental Study on Dynamic Combustion Characteristics of Aluminum Particles. Propellants Explos. Pyrotech. 2017, 42, 982–992. [Google Scholar] [CrossRef]

| Samples | Calorific Value (MJ·kg−1) | Energy Efficiency (%) |
|---|---|---|
| nAl | 23.95 | 89.86 |
| nAl@2.5%PFPE | 25.68 | 96.60 |
| nAl@5.0%PFPE | 25.10 | 97.48 |
| nAl@7.5%PFPE | 24.72 | 98.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Nie, J.; Li, S.; Wang, W.; Pan, Q.; Guo, X. Tuning the Reactivity of Perfluoropolyether-Functionalized Aluminum Nanoparticles by the Reaction Interface Fuel-Oxidizer Ratio. Nanomaterials 2022, 12, 530. https://doi.org/10.3390/nano12030530
Wu C, Nie J, Li S, Wang W, Pan Q, Guo X. Tuning the Reactivity of Perfluoropolyether-Functionalized Aluminum Nanoparticles by the Reaction Interface Fuel-Oxidizer Ratio. Nanomaterials. 2022; 12(3):530. https://doi.org/10.3390/nano12030530
Chicago/Turabian StyleWu, Chengcheng, Jianxin Nie, Shengwei Li, Wei Wang, Qi Pan, and Xueyong Guo. 2022. "Tuning the Reactivity of Perfluoropolyether-Functionalized Aluminum Nanoparticles by the Reaction Interface Fuel-Oxidizer Ratio" Nanomaterials 12, no. 3: 530. https://doi.org/10.3390/nano12030530
APA StyleWu, C., Nie, J., Li, S., Wang, W., Pan, Q., & Guo, X. (2022). Tuning the Reactivity of Perfluoropolyether-Functionalized Aluminum Nanoparticles by the Reaction Interface Fuel-Oxidizer Ratio. Nanomaterials, 12(3), 530. https://doi.org/10.3390/nano12030530






