Highly Efficient Visible Light Active Doped ZnO Photocatalysts for the Treatment of Wastewater Contaminated with Dyes and Pathogens of Emerging Concern
Abstract
:1. Introduction
2. Structure and Properties of ZnO
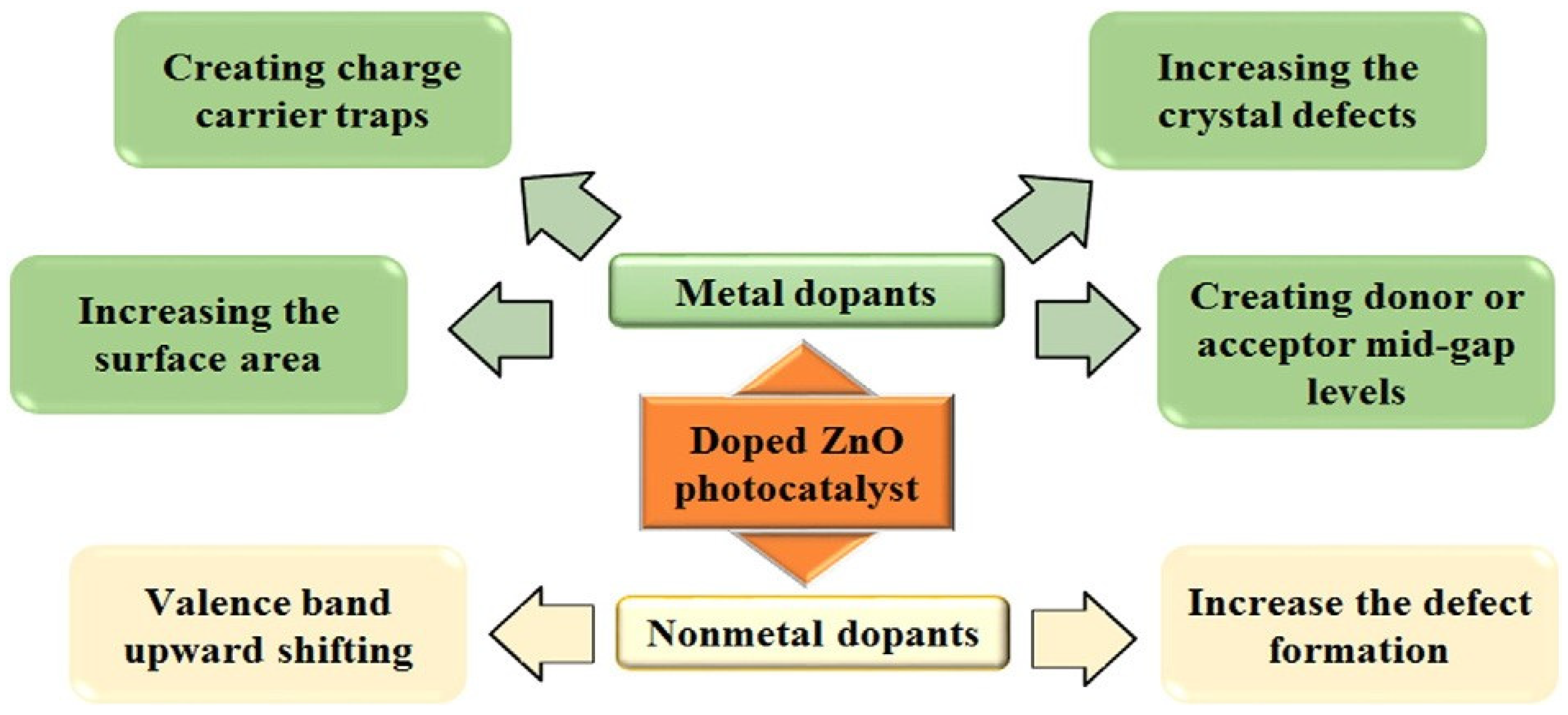
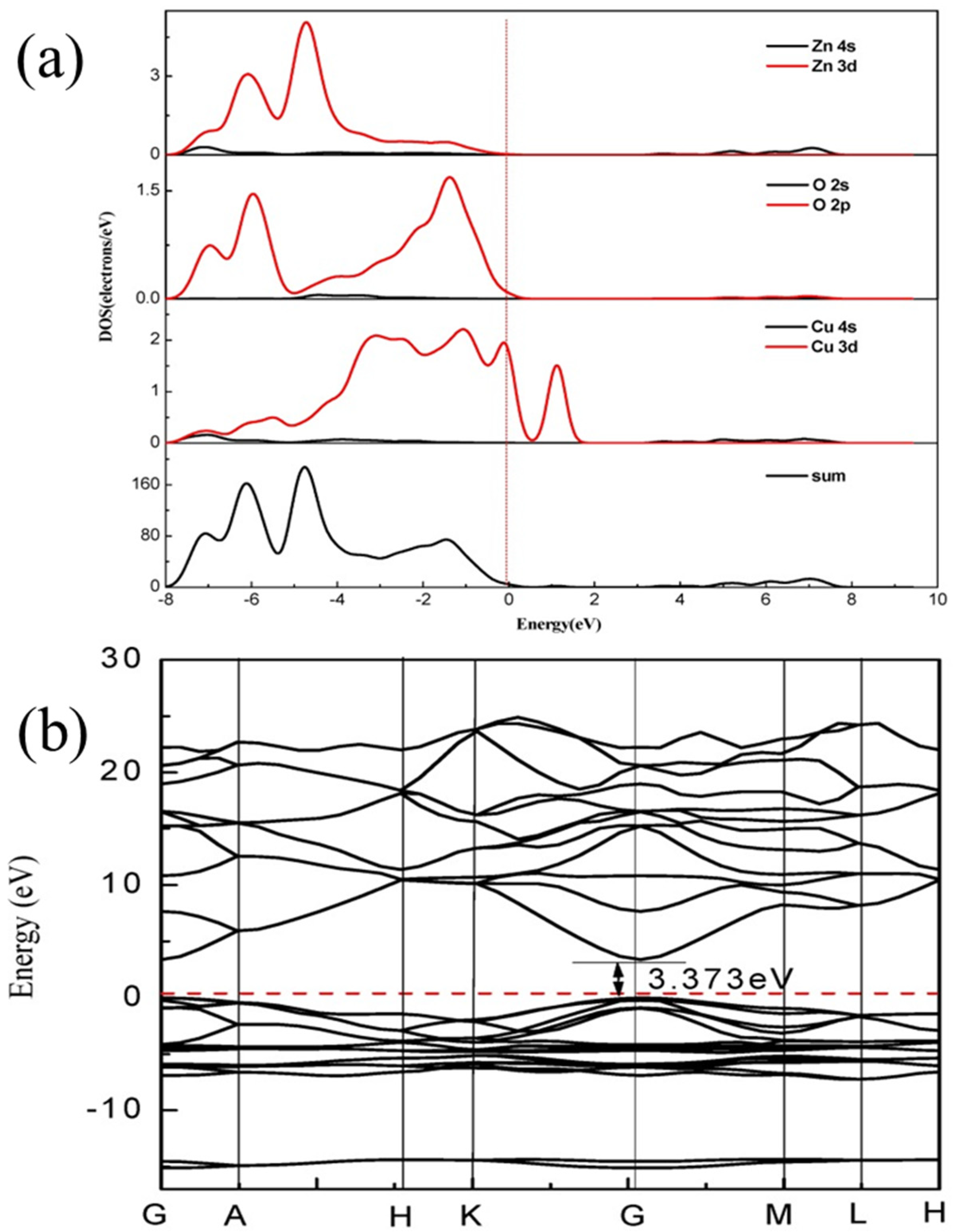
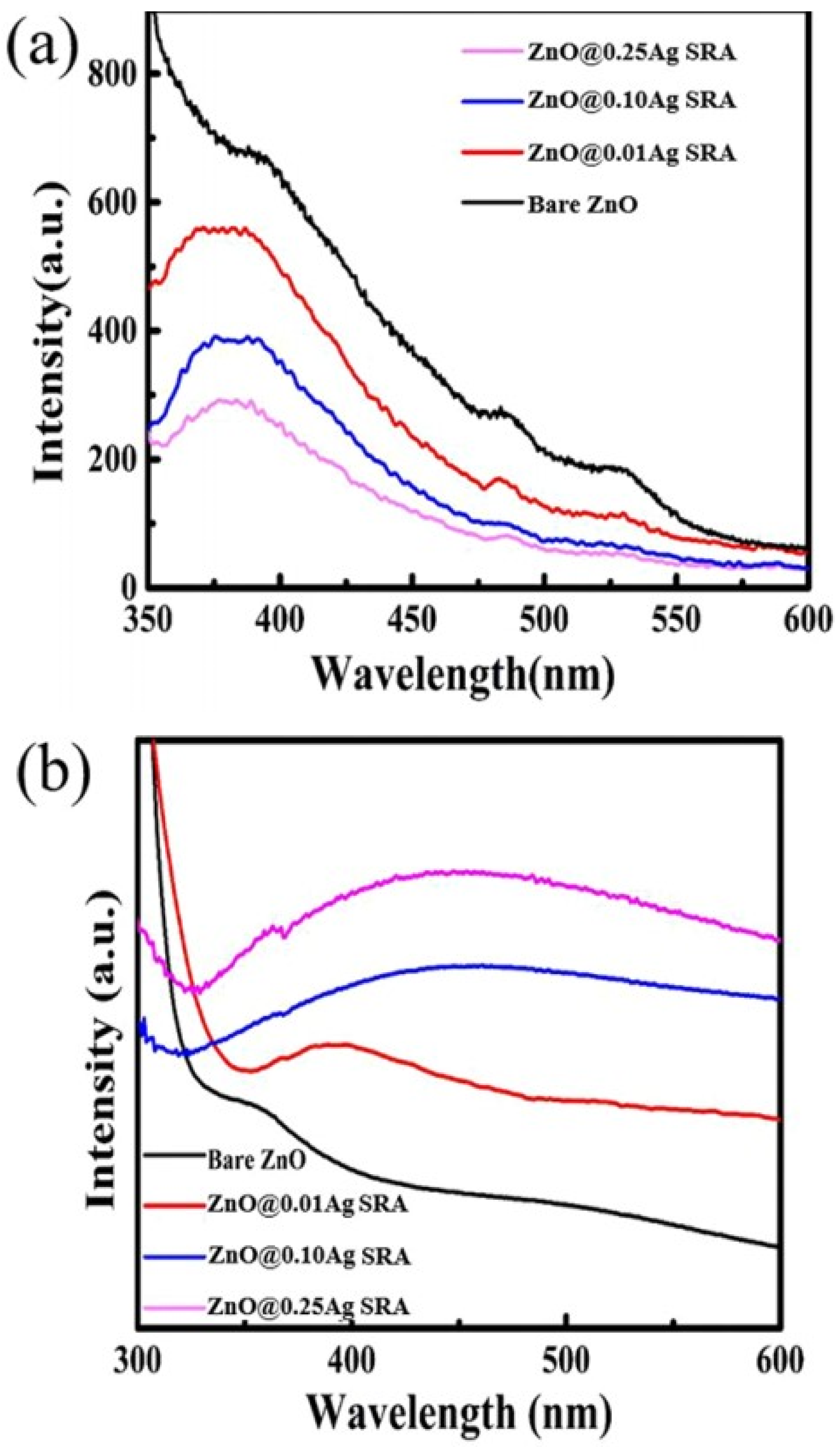
3. Visible Light Active (VLA) ZnO Photocatalysts
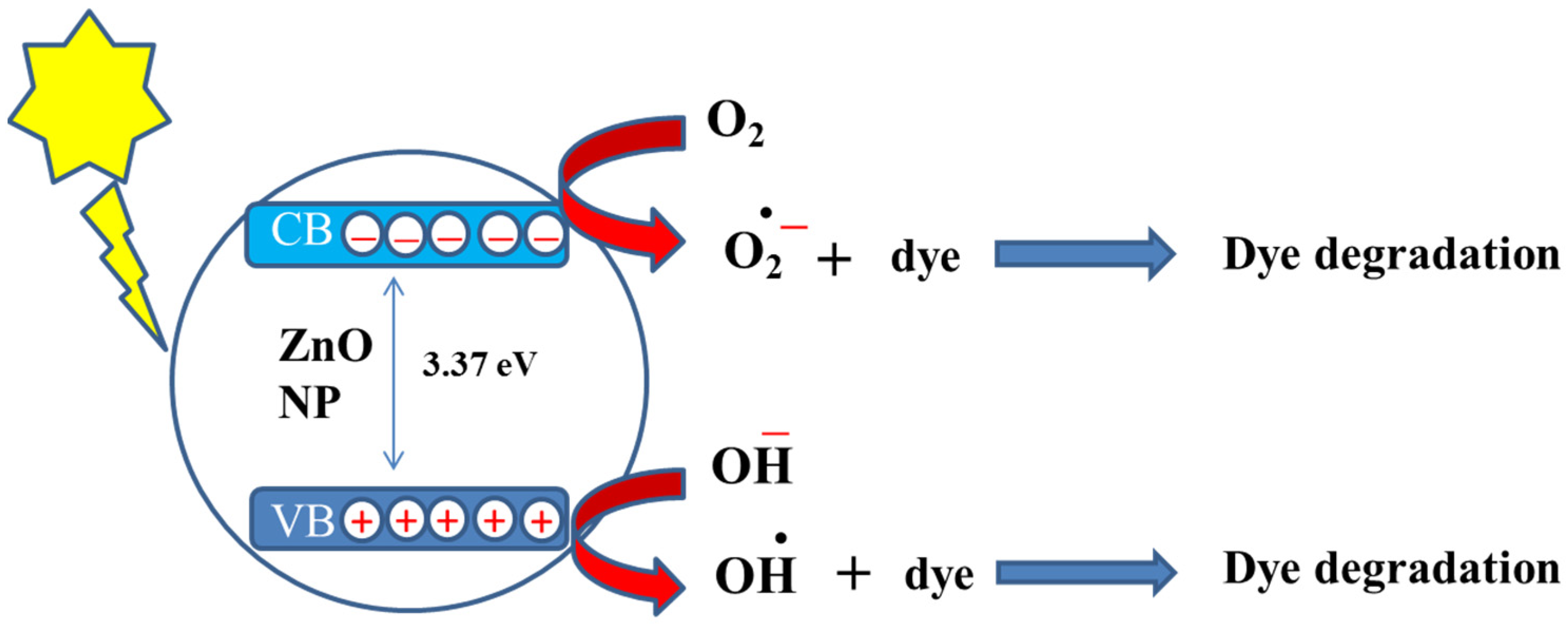
4. Use of ZnO for the Breakdown of Dyes
5. Water Disinfection with Visible Light Active ZnO-Based Photocatalysts
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, B.; Viphavakit, C.; Chitaree, R.; Ghosh, S.; Pathak, A.K.; Verma, S.; Sakda, N. Optical Fiber, Nanomaterial, and THz-Metasurface-Mediated Nano-Biosensors: A Review. Biosensors 2022, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Zafar, N.; Madni, A.; Khalid, A.; Khan, T.; Kousar, R.; Naz, S.S.; Wahid, F. Pharmaceutical and Biomedical Applications of Green Synthesized Metal and Metal Oxide Nanoparticles. Curr. Pharm. Des. 2020, 26, 5844–5865. [Google Scholar] [CrossRef] [PubMed]
- Annu, A.A.; Ahmed, S. Green synthesis of metal, metal oxide nanoparticles, and their various applications. Handb. Ecomater. 2018, 1–45. [Google Scholar] [CrossRef]
- Musa, I.; Qamhieh, N. Study of optical energy gap and quantum confinement effects in zinc oxide nanoparticles and nanorods. Dig. J. Nanomater. Biostruct. 2019, 14, 119–125. [Google Scholar]
- Kim, K.J.; Kreider, P.B.; Choi, C.; Chang, C.H.; Ahn, H.G. Visible-light-sensitive Na-doped p-type flower-like ZnO photocatalysts synthesized via a continuous flow microreactor. RSC Adv. 2013, 3, 12702–12710. [Google Scholar] [CrossRef]
- Fortunato, E.; Gonçalves, A.; Pimentel, A.; Barquinha, P.; Gonçalves, G.; Pereira, L.; Ferreira, I.; Martins, R. Zinc oxide, a multifunctional material: From material to device applications. Appl. Phys. A 2009, 96, 197–205. [Google Scholar] [CrossRef]
- Ates, T.; Tatar, C.; Yakuphanoglu, F. Preparation of semiconductor ZnO powders by sol–gel method: Humidity sensors. Sens. Actuators A Phys. 2013, 190, 153–160. [Google Scholar] [CrossRef]
- Naidu, R.; Espana, V.A.A.; Liu, Y.; Jit, J. Emerging contaminants in the environment: Risk-based analysis for better management. Chemosphere 2016, 154, 350–357. [Google Scholar] [CrossRef]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Castellote, M.; Bengtsson, N. Principles of TiO2 photocatalysis. In Applications of Titanium Dioxide Photocatalysis to Construction Materials; Springer: Berlin/Heidelberg, Germany, 2011; pp. 5–10. [Google Scholar]
- Xie, J.; Wang, H.; Duan, M.; Zhang, L. Synthesis and photocatalysis properties of ZnO structures with different morphologies via hydrothermal method. Appl. Surf. Sci. 2011, 257, 6358–6363. [Google Scholar] [CrossRef]
- Kajbafvala, A.; Ghorbani, H.; Paravar, A.; Samberg, J.P.; Kajbafvala, E.; Sadrnezhaad, S. Effects of morphology on photocatalytic performance of Zinc oxide nanostructures synthesized by rapid microwave irradiation methods. Superlattices Microstruct. 2012, 51, 512–522. [Google Scholar] [CrossRef]
- Fakhari, S.; Jamzad, M.; Fard, H.K. Green synthesis of zinc oxide nanoparticles: A comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Sangeetha, G.; Rajeshwari, S.; Venckatesh, R. Green synthesis of zinc oxide nanoparticles by aloe barbadensis miller leaf extract: Structure and optical properties. Mater. Res. Bull. 2011, 46, 2560–2566. [Google Scholar] [CrossRef]
- Rekha, K.; Nirmala, M.; Nair, M.G.; Anukaliani, A. Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles. Phys. B Condens. Matter 2010, 405, 3180–3185. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Shuaib, D.T.; Ajala, A.O.; Mohammed, A.K. Application of TiO2 and ZnO nanoparticles immobilized on clay in wastewater treatment: A review. Appl. Water Sci. 2020, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Kumbhakar, P. Observation of exciton–phonon coupling and enhanced photoluminescence emission in ZnO nanotwins synthesized by a simple wet chemical approach. Mater. Lett. 2013, 100, 40–43. [Google Scholar] [CrossRef]
- Gionco, C.; Fabbri, D.; Calza, P.; Paganini, M.C. Synthesis, characterization, and photocatalytic tests of N-doped zinc oxide: A new interesting photocatalyst. J. Nanomater. 2016, 2016, 4129864. [Google Scholar] [CrossRef] [Green Version]
- Rodnyi, P.; Khodyuk, I. Optical and luminescence properties of zinc oxide. Opt. Spectrosc. 2011, 111, 776–785. [Google Scholar] [CrossRef] [Green Version]
- Sorbiun, M.; Mehr, E.S.; Ramazani, A.; Fardood, S.T. Green synthesis of zinc oxide and copper oxide nanoparticles using aqueous extract of oak fruit hull (jaft) and comparing their photocatalytic degradation of basic violet 3. Int. J. Environ. Res. 2018, 12, 29–37. [Google Scholar] [CrossRef]
- Lu, J.; Ali, H.; Hurh, J.; Han, Y.; Batjikh, I.; Rupa, E.J.; Anandapadmanaban, G.; Park, J.K.; Yang, D.C. The assessment of photocatalytic activity of zinc oxide nanoparticles from the roots of Codonopsis lanceolata synthesized by one-pot green synthesis method. Optik 2019, 184, 82–89. [Google Scholar] [CrossRef]
- Madhumitha, G.; Fowsiya, J.; Gupta, N.; Kumar, A.; Singh, M. Green synthesis, characterization and antifungal and photocatalytic activity of Pithecellobium dulce peel–mediated ZnO nanoparticles. J. Phys. Chem. Solids 2019, 127, 43–51. [Google Scholar] [CrossRef]
- Heiland, G.; Mollwo, E.; Stöckmann, F. Electronic processes in zinc oxide. In Solid State Physics; Elsevier: Amsterdam, The Netherlands, 1959; pp. 191–323. [Google Scholar]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Film. 2016, 605, 2–19. [Google Scholar] [CrossRef] [Green Version]
- Farooq, M.H.; Aslam, I.; Anam, H.S.; Tanveer, M.; Ali, Z.; Ghani, U.; Boddula, R. Improved photocatalytic performance of reduced zinc oxide (ZnO) novel morphology of astray like microstructure under solar light irradiation. Mater. Sci. Energy Technol. 2019, 2, 181–186. [Google Scholar] [CrossRef]
- Kumari, V.; Mittal, A.; Jindal, J.; Yadav, S.; Kumar, N. S-, N-and C-doped ZnO as semiconductor photocatalysts: A review. Front. Mater. Sci. 2019, 13, 1–22. [Google Scholar] [CrossRef]
- Wen, J.Q.; Chen, G.X.; Zhang, J.M.; Li, D.M.; Zhang, X.Z. Magnetic transition in nonmetal N-and F-doping g-ZnO monolayer with different concentrations. J. Supercond. Nov. Magn. 2018, 31, 3133–3139. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Zhang, Q.; Yin, S.; Sato, T.; Saito, F. Mechanochemical doping of a non-metal element into zinc oxide. Chem. Sustain. Dev. 2007, 15, 249–253. [Google Scholar]
- Ma, Z.; Ren, F.; Ming, X.; Long, Y.; Volinsky, A.A. Cu-doped ZnO electronic structure and optical properties studied by first-principles calculations and experiments. Materials 2019, 12, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Zhu, L.; Li, Y.; Zhang, X.; Niu, W.; Guo, Y.; Ye, Z. Highly conductive thin films of nonmetal F and B co-doped ZnO on flexible substrates: Experiment and first-principles calculations. J. Alloy. Compd. 2017, 697, 156–160. [Google Scholar] [CrossRef]
- Boonchun, A.; Lambrecht, W.R. Electronic structure of defects and doping in ZnO: Oxygen vacancy and nitrogen doping. Phys. Status Solidi (B) 2013, 250, 2091–2101. [Google Scholar] [CrossRef]
- Byzynski, G.; Melo, C.; Volanti, D.P.; Ferrer, M.M.; Gouveia, A.F.; Ribeiro, C.; Andrés, J.; Longo, E. The interplay between morphology and photocatalytic activity in ZnO and N-doped ZnO crystals. Mater. Des. 2017, 120, 363–375. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.A.; Nogueira, A.E.; Goncalves, M.C.; Paris, E.C.; Ribeiro, C.; Poirier, G.Y.; Giraldi, T.R. Photoactivity of N-doped ZnO nanoparticles in oxidative and reductive reactions. Appl. Surf. Sci. 2018, 433, 879–886. [Google Scholar] [CrossRef]
- Kumari, R.; Sahai, A.; Goswami, N. Effect of nitrogen doping on structural and optical properties of ZnO nanoparticles. Prog. Nat. Sci. Mater. Int. 2015, 25, 300–309. [Google Scholar] [CrossRef] [Green Version]
- Soöllradl, S.; Greiwe, M.; Bukas, V.J.; Buchner, M.R.; Widenmeyer, M.; Kandemir, T.; Zweifel, T.; Senyshyn, A.; Guünther, S.; Nilges, T. Nitrogen-doping in ZnO via combustion synthesis? Chem. Mater. 2015, 27, 4188–4195. [Google Scholar] [CrossRef]
- Zeuner, A.; Alves, H.; Sann, J.; Kriegseis, W.; Neumann, C.; Hofmann, D.; Meyer, B.; Hoffmann, A.; Haboeck, U.; Straßburg, M. Nitrogen doping in bulk and epitaxial ZnO. Phys. Status Solidi (C) 2004, 1, 731–734. [Google Scholar] [CrossRef]
- Qiu, Y.; Yan, K.; Deng, H.; Yang, S. Secondary branching and nitrogen doping of ZnO nanotetrapods: Building a highly active network for photoelectrochemical water splitting. Nano Lett. 2012, 12, 407–413. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, L.-C.; Xie, Y.; Liu, G.; Ma, X.; Cheng, H.-M. Crystallinity-dependent substitutional nitrogen doping in ZnO and its improved visible light photocatalytic activity. J. Colloid Interface Sci. 2013, 400, 18–23. [Google Scholar] [CrossRef]
- Balcha, A.; Yadav, O.P.; Dey, T. Photocatalytic degradation of methylene blue dye by zinc oxide nanoparticles obtained from precipitation and sol-gel methods. Environ. Sci. Pollut. Res. 2016, 23, 25485–25493. [Google Scholar] [CrossRef]
- Irani, M.; Mohammadi, T.; Mohebbi, S. Photocatalytic degradation of methylene blue with ZnO nanoparticles; a joint experimental and theoretical study. J. Mex. Chem. Soc. 2016, 60, 218–225. [Google Scholar] [CrossRef]
- Shinde, D.R.; Tambade, P.S.; Chaskar, M.G.; Gadave, K.M. Photocatalytic degradation of dyes in water by analytical reagent grades ZnO, TiO2 and SnO2: A comparative study. Drink. Water Eng. Sci. 2017, 10, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Rakibuddin, M.; Ananthakrishnan, R. Novel nano coordination polymer based synthesis of porous ZnO hexagonal nanodisk for higher gas sorption and photocatalytic activities. Appl. Surf. Sci. 2016, 362, 265–273. [Google Scholar] [CrossRef]
- Bora, T.; Sathe, P.; Laxman, K.; Dobretsov, S.; Dutta, J. Defect engineered visible light active ZnO nanorods for photocatalytic treatment of water. Catal. Today 2017, 284, 11–18. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, L.; Luo, X. ZIF-8 derived Ag-doped ZnO photocatalyst with enhanced photocatalytic activity. RSC Adv. 2018, 8, 4890–4894. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Singh, V.; Tanwar, A. Structural, morphological, optical and photocatalytic properties of Ag-doped ZnO nanoparticles. J. Mater. Sci. Mater. Electron. 2016, 27, 2166–2173. [Google Scholar] [CrossRef]
- Ashebir, M.E.; Tesfamariam, G.M.; Nigussie, G.Y.; Gebreab, T.W. Structural, optical, and photocatalytic activities of Ag-doped and Mn-doped ZnO nanoparticles. J. Nanomater. 2018, 2018, 9425938. [Google Scholar] [CrossRef]
- Vallejo, W.; Cantillo, A.; Díaz-Uribe, C. Methylene blue photodegradation under visible irradiation on Ag-Doped ZnO thin films. Int. J. Photoenergy 2020, 2020, 1627498. [Google Scholar] [CrossRef] [Green Version]
- Sabry, R.S.; Rahmah, M.I.; Aziz, W.J. A systematic study to evaluate effects of stearic acid on superhydrophobicity and photocatalytic properties of Ag-doped ZnO nanostructures. J. Mater. Sci. Mater. Electron. 2020, 31, 13382–13391. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Wei, F.; Zhao, X.; Su, Y.; Han, X. Ag–ZnO submicrometer rod arrays for high-efficiency photocatalytic degradation of Congo red and disinfection. ACS Sustain. Chem. Eng. 2019, 7, 11258–11266. [Google Scholar] [CrossRef]
- Saffari, R.; Shariatinia, Z.; Jourshabani, M. Synthesis and photocatalytic degradation activities of phosphorus containing ZnO microparticles under visible light irradiation for water treatment applications. Environ. Pollut. 2020, 259, 113902. [Google Scholar] [CrossRef]
- Kuriakose, S.; Satpati, B.; Mohapatra, S. Highly efficient photocatalytic degradation of organic dyes by Cu doped ZnO nanostructures. Phys. Chem. Chem. Phys. 2015, 17, 25172–25181. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Rizzo, L. Cu-doped ZnO as efficient photocatalyst for the oxidation of arsenite to arsenate under visible light. Appl. Catal. B Environ. 2018, 238, 471–479. [Google Scholar] [CrossRef]
- Chandekar, K.V.; Shkir, M.; Al-Shehri, B.M.; AlFaify, S.; Halor, R.G.; Khan, A.; Al-Namshah, K.S.; Hamdy, M.S. Visible light sensitive Cu doped ZnO: Facile synthesis, characterization and high photocatalytic response. Mater. Charact. 2020, 165, 110387. [Google Scholar] [CrossRef]
- Etacheri, V.; Roshan, R.; Kumar, V. Mg-doped ZnO nanoparticles for efficient sunlight-driven photocatalysis. ACS Appl. Mater. Interfaces 2012, 4, 2717–2725. [Google Scholar] [CrossRef]
- Adam, R.E.; Alnoor, H.; Pozina, G.; Liu, X.; Willander, M.; Nur, O. Synthesis of Mg-doped ZnO NPs via a chemical low-temperature method and investigation of the efficient photocatalytic activity for the degradation of dyes under solar light. Solid State Sci. 2020, 99, 106053. [Google Scholar] [CrossRef]
- Adeel, M.; Saeed, M.; Khan, I.; Muneer, M.; Akram, N. Synthesis and characterization of Co–ZnO and evaluation of its photocatalytic activity for photodegradation of methyl orange. ACS Omega 2021, 6, 1426–1435. [Google Scholar] [CrossRef]
- Hernández-Carrillo, M.; Torres-Ricárdez, R.; García-Mendoza, M.; Ramírez-Morales, E.; Rojas-Blanco, L.; Díaz-Flores, L.; Sepúlveda-Palacios, G.; Paraguay-Delgado, F.; Pérez-Hernández, G. Eu-modified ZnO nanoparticles for applications in photocatalysis. Catal. Today 2020, 349, 191–197. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.S.; Ahmed, E.; Ahmad, M.; Keller, V.; Khan, W.Q.; Khalid, N. Rare earth co-doped ZnO photocatalysts: Solution combustion synthesis and environmental applications. Sep. Purif. Technol. 2020, 237, 116328. [Google Scholar] [CrossRef]
- Pascariu, P.; Tudose, I.V.; Suchea, M.; Koudoumas, E.; Fifere, N.; Airinei, A. Preparation and characterization of Ni, Co doped ZnO nanoparticles for photocatalytic applications. Appl. Surf. Sci. 2018, 448, 481–488. [Google Scholar] [CrossRef]
- Shanmugam, V.; Jeyaperumal, K.S. Investigations of visible light driven Sn and Cu doped ZnO hybrid nanoparticles for photocatalytic performance and antibacterial activity. Appl. Surf. Sci. 2018, 449, 617–630. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, X.; Yang, C.; Cao, M.; Ma, M.; Liu, R. ZnO microspheres-reduced graphene oxide nanocomposite for photocatalytic degradation of methylene blue dye. Appl. Surf. Sci. 2017, 392, 196–203. [Google Scholar] [CrossRef]
- Ullah, R.; Dutta, J. Photocatalytic degradation of organic dyes with manganese-doped ZnO nanoparticles. J. Hazard. Mater. 2008, 156, 194–200. [Google Scholar] [CrossRef]
- Mohamed, R.; Shawky, A. CNT supported Mn-doped ZnO nanoparticles: Simple synthesis and improved photocatalytic activity for degradation of malachite green dye under visible light. Appl. Nanosci. 2018, 8, 1179–1188. [Google Scholar] [CrossRef]
- Labhane, P.; Patle, L.; Sonawane, G.; Sonawane, S. Fabrication of ternary Mn doped ZnO nanoparticles grafted on reduced graphene oxide (RGO) sheet as an efficient solar light driven photocatalyst. Chem. Phys. Lett. 2018, 710, 70–77. [Google Scholar] [CrossRef]
- Ghosh, M.; Manoli, K.; Shen, X.; Wang, J.; Ray, A.K. Solar photocatalytic degradation of caffeine with titanium dioxide and zinc oxide nanoparticles. J. Photochem. Photobiol. A Chem. 2019, 377, 1–7. [Google Scholar] [CrossRef]
- Subhan, M.A.; Uddin, N.; Sarker, P.; Azad, A.K.; Begum, K. Photoluminescence, photocatalytic and antibacterial activities of CeO2· CuO· ZnO nanocomposite fabricated by co-precipitation method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Tahir, M.; Sagir, M.; Bamufleh, H.S. Improved photocatalytic performance of Gd and Nd co-doped ZnO nanorods for the degradation of methylene blue. Ceram. Int. 2020, 46, 11955–11961. [Google Scholar] [CrossRef]
- Arshad, M.; Qayyum, A.; Abbas, G.; Haider, R.; Iqbal, M.; Nazir, A. Influence of different solvents on portrayal and photocatalytic activity of tin-doped zinc oxide nanoparticles. J. Mol. Liq. 2018, 260, 272–278. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Nguyen, L.T.; Duong, A.T.; Nguyen, B.D.; Hai, N.Q.; Chu, V.H.; Nguyen, T.D.; Bach, L.G. Preparation, characterization and photocatalytic activity of La-doped zinc oxide nanoparticles. Materials 2019, 12, 1195. [Google Scholar] [CrossRef] [Green Version]
- Babajani, N.; Jamshidi, S. Investigation of photocatalytic malachite green degradation by iridium doped zinc oxide nanoparticles: Application of response surface methodology. J. Alloy. Compd. 2019, 782, 533–544. [Google Scholar] [CrossRef]
- Yousefi, R.; Jamali-Sheini, F.; Cheraghizade, M.; Khosravi-Gandomani, S.; Saáaedi, A.; Huang, N.M.; Basirun, W.J.; Azarang, M. Enhanced visible-light photocatalytic activity of strontium-doped zinc oxide nanoparticles. Mater. Sci. Semicond. Processing 2015, 32, 152–159. [Google Scholar] [CrossRef]
- Khezami, L.; Taha, K.K.; Ghiloufi, I.; El Mir, L. Adsorption and photocatalytic degradation of malachite green by vanadium doped zinc oxide nanoparticles. Water Sci. Technol. 2016, 73, 881–889. [Google Scholar] [CrossRef]
- Goswami, M. Enhancement of photocatalytic activity of synthesized Cobalt doped Zinc Oxide nanoparticles under visible light irradiation. Opt. Mater. 2020, 109, 110400. [Google Scholar] [CrossRef]
- Ebrahimi, R.; Hossienzadeh, K.; Maleki, A.; Ghanbari, R.; Rezaee, R.; Safari, M.; Shahmoradi, B.; Daraei, H.; Jafari, A.; Yetilmezsoy, K. Effects of doping zinc oxide nanoparticles with transition metals (Ag, Cu, Mn) on photocatalytic degradation of Direct Blue 15 dye under UV and visible light irradiation. J. Environ. Health Sci. Eng. 2019, 17, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Alshamsi, H.A.H.; Hussein, B.S. Hydrothermal Preparation of Silver Doping Zinc Oxide Nanoparticles: Study the Characterization and Photocatalytic Activity. Orient. J. Chem. 2018, 34, 1898. [Google Scholar] [CrossRef]
- Saber, O.; El-Brolossy, T.A.; Al Jaafari, A.A. Improvement of photocatalytic degradation of naphthol green B under solar light using aluminum doping of zinc oxide nanoparticles. Water Air Soil Pollut. 2012, 223, 4615–4626. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, S.; Liu, S.; Chen, J.; Han, B.; Wang, Y.; Wang, Y. Preparation and photocatalytic activity of Nd doped ZnO nanoparticles. Mater. Technol. 2014, 29, 262–268. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Asal, S. Physicochemical and photocatalytic studies of Ln3+-ZnO for water disinfection and wastewater treatment applications. J. Mol. Struct. 2017, 1149, 404–413. [Google Scholar] [CrossRef]
- Yi, G.; Yuan, Y.; Li, X.; Zhang, Y. ZnO Nanopillar Coated Surfaces with Substrate-Dependent Superbactericidal Property. Small 2018, 14, 1703159. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.H.; Misra, A.J.; Das, S.; Das, B.; Jayabalan, R.; Suar, M.; Mishra, A.; Tamhankar, A.J.; Lundborg, C.S.; Tripathy, S.K. Mechanistic insight into the disinfection of Salmonella sp. by sun-light assisted sonophotocatalysis using doped ZnO nanoparticles. Chem. Eng. J. 2018, 336, 476–488. [Google Scholar] [CrossRef]
- Dimapilis, E.A.S.; Hsu, C.-S.; Mendoza, R.M.O.; Lu, M.-C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Sultana, K.A.; Islam, M.T.; Silva, J.A.; Turley, R.S.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L.; Noveron, J.C. Sustainable synthesis of zinc oxide nanoparticles for photocatalytic degradation of organic pollutant and generation of hydroxyl radical. J. Mol. Liq. 2020, 307, 112931. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J. Hazard. Mater. 2021, 402, 123560. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.-N.; Rebia, R.A.; Saito, Y.; Kharaghani, D.; Khatri, M.; Tanaka, T.; Lee, H.; Kim, I.-S. Zinc oxide nanoparticles attached to polyacrylonitrile nanofibers with hinokitiol as gluing agent for synergistic antibacterial activities and effective dye removal. J. Ind. Eng. Chem. 2020, 85, 258–268. [Google Scholar] [CrossRef]
- Gancheva, M.; Markova-Velichkova, M.; Atanasova, G.; Kovacheva, D.; Uzunov, I.; Cukeva, R. Design and photocatalytic activity of nanosized zinc oxides. Appl. Surf. Sci. 2016, 368, 258–266. [Google Scholar] [CrossRef]
- Mahamuni, P.P.; Patil, P.M.; Dhanavade, M.J.; Badiger, M.V.; Shadija, P.G.; Lokhande, A.C.; Bohara, R.A. Synthesis and characterization of zinc oxide nanoparticles by using polyol chemistry for their antimicrobial and antibiofilm activity. Biochem. Biophys. Rep. 2019, 17, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.J.; Bellah, M.M.; Hassan, M.R.; Rahman, S. Synthesis of ZnO nanoparticles by two different methods & comparison of their structural, antibacterial, photocatalytic and optical properties. Nano Express 2020, 1, 010007. [Google Scholar]
- Ogunyemi, S.O.; Abdallah, Y.; Zhang, M.; Fouad, H.; Hong, X.; Ibrahim, E.; Masum, M.M.I.; Hossain, A.; Mo, J.; Li, B. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae, Artificial cells. Nanomed. Biotechnol. 2019, 47, 341–352. [Google Scholar]
- Suresh, J.; Pradheesh, G.; Alexramani, V.; Sundrarajan, M.; Hong, S.I. Green synthesis and characterization of zinc oxide nanoparticle using insulin plant (Costus pictus D. Don) and investigation of its antimicrobial as well as anticancer activities. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 015008. [Google Scholar] [CrossRef]
- Panchal, P.; Paul, D.R.; Sharma, A.; Choudhary, P.; Meena, P.; Nehra, S. Biogenic mediated Ag/ZnO nanocomposites for photocatalytic and antibacterial activities towards disinfection of water. J. Colloid Interface Sci. 2020, 563, 370–380. [Google Scholar] [CrossRef]
- He, W.; Kim, H.-K.; Wamer, W.G.; Melka, D.; Callahan, J.H.; Yin, J.-J. Photogenerated charge carriers and reactive oxygen species in ZnO/Au hybrid nanostructures with enhanced photocatalytic and antibacterial activity. J. Am. Chem. Soc. 2014, 136, 750–757. [Google Scholar] [CrossRef]
- Qamar, M.A.; Shahid, S.; Javed, M.; Iqbal, S.; Sher, M.; Akbar, M.B. Highly efficient g-C3N4/Cr-ZnO nanocomposites with superior photocatalytic and antibacterial activity. J. Photochem. Photobiol. A Chem. 2020, 401, 112776. [Google Scholar] [CrossRef]
- Munawar, T.; Yasmeen, S.; Hussain, A.; Akram, M.; Iqbal, F. Novel direct dual-Z-scheme ZnO-Er2O3-Yb2O3 heterostructured nanocomposite with superior photocatalytic and antibacterial activity. Mater. Lett. 2020, 264, 127357. [Google Scholar] [CrossRef]
- Das, S.; Sinha, S.; Das, B.; Jayabalan, R.; Suar, M.; Mishra, A.; Tamhankar, A.J.; Lundborg, C.S.; Tripathy, S.K. Disinfection of multidrug resistant Escherichia coli by solar-photocatalysis using Fe-doped ZnO nanoparticles. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oquendo-Cruz, A.; Perales-Pérez, O. Synthesis, characterization and bactericide properties of pure and Li doped ZnO nanoparticles for alternative water disinfection methods. J. Electron. Mater. 2018, 47, 6260–6265. [Google Scholar] [CrossRef]
- Darwish, A.S.; Bayaumy, F.E.; Ismail, H.M. Photoactivated water-disinfecting, and biological properties of Ag NPs@ Sm-doped ZnO nanorods/cuttlefish bone composite: In-vitro bactericidal, cercaricidal and schistosomicidal studies. Mater. Sci. Eng. C 2018, 93, 996–1011. [Google Scholar] [CrossRef]



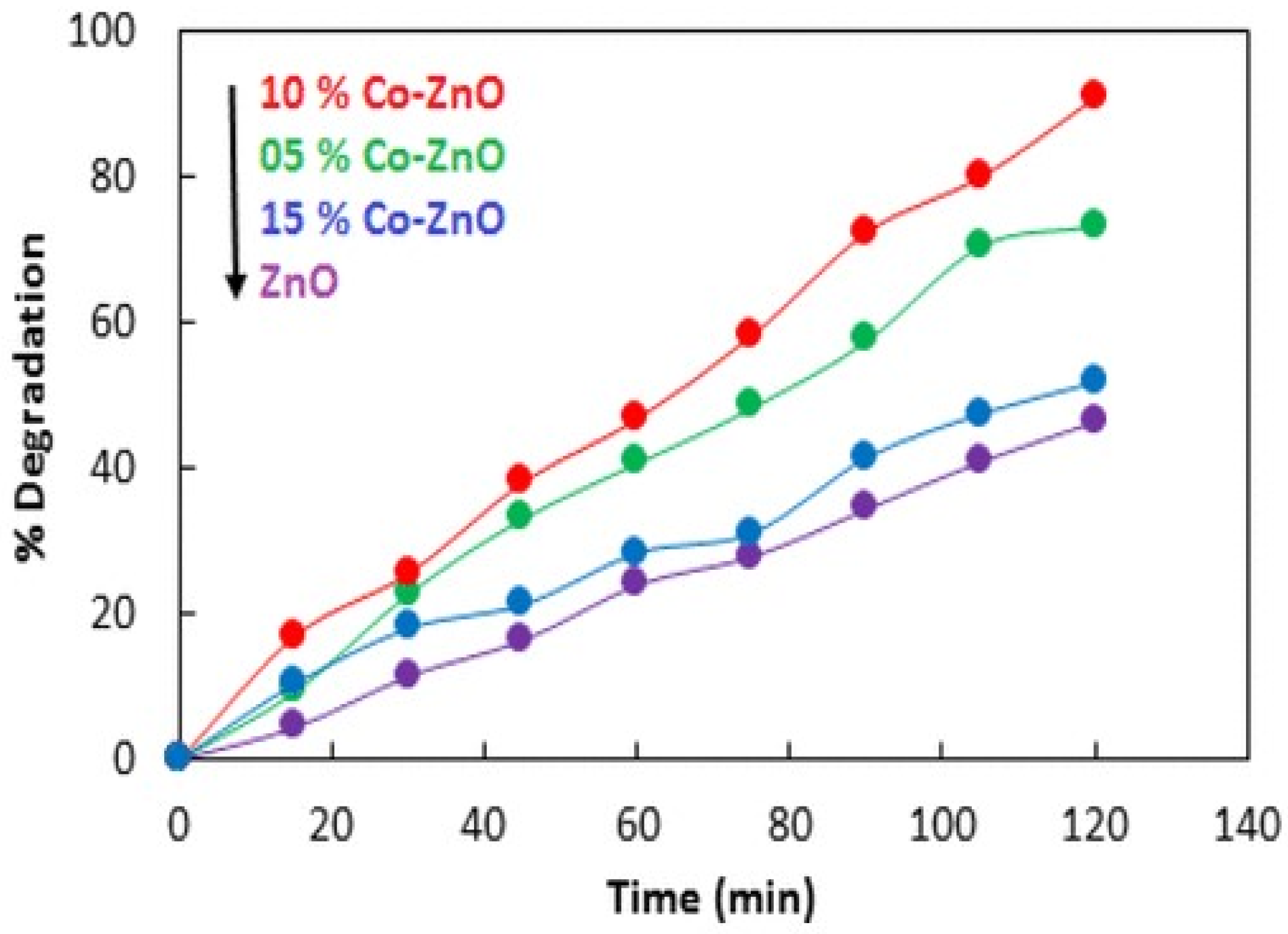
| Sr. No. | Doped ZnO | Pollutants | % Degradation | Light Source | References |
|---|---|---|---|---|---|
| 1. | Sn/ZnO | Methyl blue dye | 81 | 150 W Xe lamp | [68] |
| 2. | La/ZnO | Methyl orange dye | 85.86 | Visible light | [69] |
| 3. | Ir/ZnO | Malachite green | >90 | 9 W fluorescent visible lamp | [70] |
| 4. | Sr/ZnO | Methylene blue | 50 | 500 W Xe lamp | [71] |
| 5. | V/ZnO | Malachite green | >99 | Osram Lumilux daylight lamp | [72] |
| 6. | Co/ZnO | Methylene blue | 98 | % 500 W halogen lamp | [73] |
| 7. | Cu/ZnO | Direct blue 15 dye | 70 | Visible light | [74] |
| 8. | Ag/ZnO | Cibacron brilliant yellow 3G-B | 65 | 400 mW·cm−2 Xe lamp | [75] |
| 9. | Al/ZnO | Naphthol green B | 100 | 25 W·cm−2 sunlight | [76] |
| 10. | Nd/ZnO | Congo red | 93.7 | Visible light | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aftab, S.; Shabir, T.; Shah, A.; Nisar, J.; Shah, I.; Muhammad, H.; Shah, N.S. Highly Efficient Visible Light Active Doped ZnO Photocatalysts for the Treatment of Wastewater Contaminated with Dyes and Pathogens of Emerging Concern. Nanomaterials 2022, 12, 486. https://doi.org/10.3390/nano12030486
Aftab S, Shabir T, Shah A, Nisar J, Shah I, Muhammad H, Shah NS. Highly Efficient Visible Light Active Doped ZnO Photocatalysts for the Treatment of Wastewater Contaminated with Dyes and Pathogens of Emerging Concern. Nanomaterials. 2022; 12(3):486. https://doi.org/10.3390/nano12030486
Chicago/Turabian StyleAftab, Saima, Tayyaba Shabir, Afzal Shah, Jan Nisar, Iltaf Shah, Haji Muhammad, and Noor S. Shah. 2022. "Highly Efficient Visible Light Active Doped ZnO Photocatalysts for the Treatment of Wastewater Contaminated with Dyes and Pathogens of Emerging Concern" Nanomaterials 12, no. 3: 486. https://doi.org/10.3390/nano12030486
APA StyleAftab, S., Shabir, T., Shah, A., Nisar, J., Shah, I., Muhammad, H., & Shah, N. S. (2022). Highly Efficient Visible Light Active Doped ZnO Photocatalysts for the Treatment of Wastewater Contaminated with Dyes and Pathogens of Emerging Concern. Nanomaterials, 12(3), 486. https://doi.org/10.3390/nano12030486







