Insight into the Roles of Metal Loading on CO2 Photocatalytic Reduction Behaviors of TiO2
Abstract
1. Introduction
2. Materials and Methods
2.1. Metal Deposited-Semiconductor Preparation
2.2. Characterization of Photocatalysts
2.3. CO2 Photoreduction
2.4. Density Functional Theory (DFT) Calculations
3. Results and Discussion
3.1. Characterization of Photocatalysts
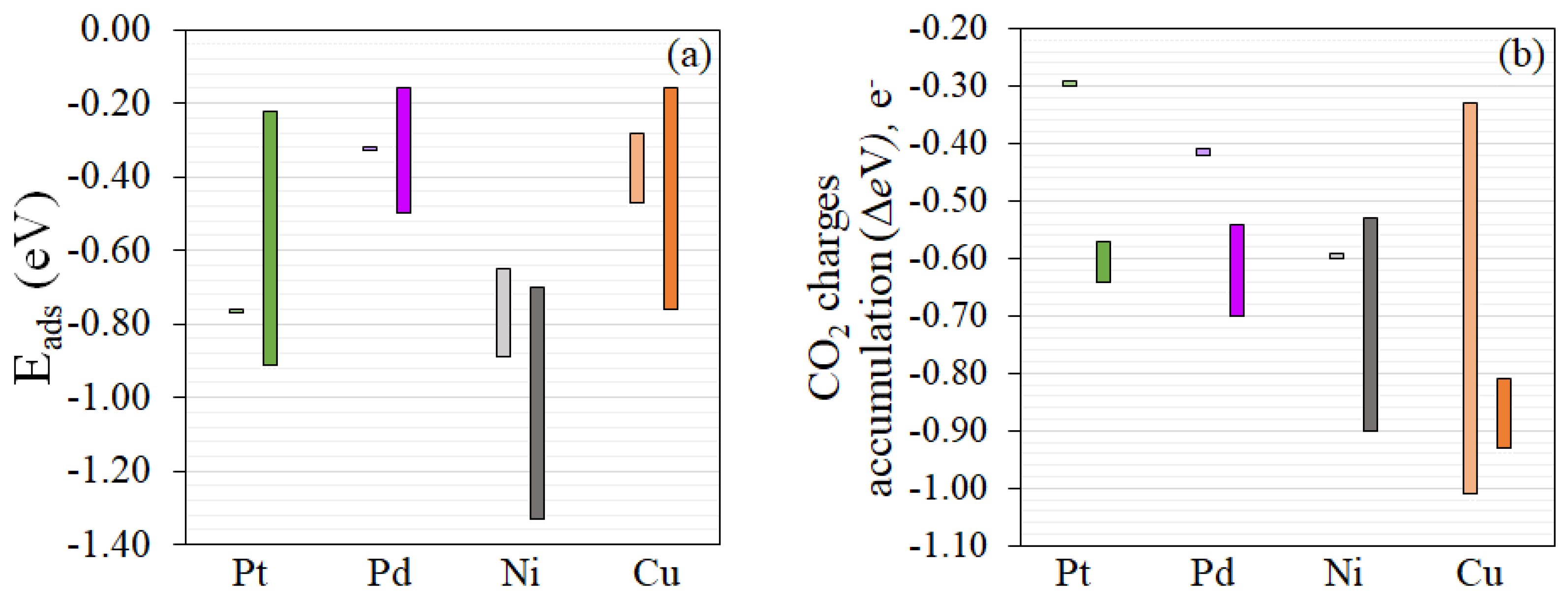
3.2. CO2 Photocatalytic Reduction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duraccio, V.; Gnoni, M.G.; Elia, V. Carbon capture and reuse in an industrial district: A technical and economic feasibility study. J. CO2 Util. 2015, 10, 23–29. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Abanades, S.; Zhang, Z. Enhanced activity of TiO2 by concentrating light for photoreduction of CO2 with H2O to CH4. Catal. Commun. 2018, 113, 6–9. [Google Scholar] [CrossRef]
- Su, K.Y.; Chen, C.Y.; Wu, R.J. Preparation of Pd/TiO2 nanowires for the photoreduction of CO2 into renewable hydrocarbon fuels. J. Taiwan Inst. Chem. Eng. 2019, 96, 409–418. [Google Scholar] [CrossRef]
- Xie, M.; Qiu, Y.; Song, C.; Qi, Y.; Li, Y.; Kitamura, Y. Optimization of Chlorella sorokiniana cultivation condition for simultaneous enhanced biomass and lipid production via CO2 fixation. Biores. Technol. Rep. 2018, 2, 15–20. [Google Scholar]
- Luo, Y.; Xia, C.; Abulizi, R.; Feng, Q.; Liu, W.; Zhang, A. Electrocatalysis of CO2 reduction on nano silver cathode in ionic liquid BMIMBF4: Synthesis of dimethylcarbonate. Int. J. Electrochem. Sci. 2017, 12, 4828–4834. [Google Scholar] [CrossRef]
- Gust, D.; Moore, T.A.; Moore, A.L. Solar fuels via artificial photosynthesis. Acc. Chem. Res. 2009, 42, 1890–1898. [Google Scholar] [CrossRef]
- Su, J.; Vayssieres, L. A place in the sun for artificial photosynthesis? ACS Energy Lett. 2016, 1, 121–135. [Google Scholar] [CrossRef]
- Butburee, T.; Chakthranont, P.; Phawa, C.; Faungnawakij, K. Beyond artificial photosynthesis: Prospects on photobiorefinery. ChemCatChem 2020, 12, 1873–1890. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Ahamad, T.; Veses, R.C.; Stadler, F.J. Highly visible active Ag2CrO4/Ag/BiFeO3@RGO nano-junction for photoreduction of CO2 and photocatalytic removal of ciprofloxacin and bromate ions: The triggering effect of Ag and RGO. Chem. Eng. J. 2019, 370, 148–165. [Google Scholar] [CrossRef]
- Butburee, T.; Kotchasarn, P.; Hirunsit, P.; Sun, Z.; Tang, Q.; Khemthong, P.; Sangkhun, W.; Thongsuwan, W.; Kumnorkaew, P.; Wang, H. New understanding of crystal control and facet selectivity of titanium dioxide ruling photocatalytic performance. J. Mater. Chem. A 2019, 7, 8156–8166. [Google Scholar] [CrossRef]
- Butburee, T.; Sun, Z.; Centeno, A.; Xie, F.; Zhao, Z.; Wu, D.; Peerakiatkhajohn, P.; Thaweesak, S.; Wang, H.; Wang, L. Improved CO2 photocatalytic reduction using a novel 3-component heterojunction. Nano Energy 2019, 62, 426–433. [Google Scholar] [CrossRef]
- You-Ji, L.; Wei, C. Photocatalytic degradation of rhodamine B using nanocrystalline TiO2-zeolite surface composite catalysts: Effects of photocatalytic condition on degradation efficiency. Catal. Sci. Technol. 2011, 1, 802–809. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, P.; Sharma, O.P.; Jain, S.L.; Khatri, O.P. Reduced graphene oxide-CuO nanocomposites for photocatalytic conversion of CO2 into methanol under visible light irradiation. Appl. Catal. B 2016, 181, 352–362. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, S.; Li, Q.; Lyu, M.; Butburee, T.; Luo, B.; Wang, H.; Fischer JM, T.A.; Zhang, C.; Wu, Z. Enriching CO2 activation sites on graphitic carbon nitride with simultaneous introduction of electron-transfer promoters for superior photocatalytic CO2-to-fuel conversion. Adv. Sustain. Syst. 2017, 1, 1700003. [Google Scholar] [CrossRef]
- Xiao, L.; Lin, R.; Wang, J.; Cui, C.; Wang, J.; Li, Z. A novel hollow-hierarchical structured Bi2WO6 with enhanced photocatalytic activity for CO2 photoreduction. J. Colloid Interface Sci. 2018, 523, 151–158. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, Q.; Wang, H.; Liu, Z.; Zhao, Z. Unraveling the role of surface property in the photoreduction performance of CO2 and H2O catalyzed by the modified ZnO. Mol. Catal. 2017, 436, 19–28. [Google Scholar] [CrossRef]
- Shao, K.; Wang, Y.; Iqbal, M.; Lin, L.; Wang, K.; Zhang, X.; He, M.; He, T. Modification of Ag nanoparticles on the surface of SrTiO3 particles and resultant influence on photoreduction of CO2. Appl. Surf. Sci. 2018, 434, 717–724. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Wang, T.; Li, Y.; Li, X.; Yin, J.; Wang, C. CO2 photoreduction with H2O vapor on highly dispersed CeO2/TiO2 catalysts: Surface species and their reactivity. J. Catal. 2016, 337, 293–302. [Google Scholar] [CrossRef]
- Sarkar, A.; Gracia-Espino, E.; Wågberg, T.; Shchukarev, A.; Mohl, M.; Rautio, A.-R.; Pitkänen, O.; Sharifi, T.; Kordas, K.; Mikkola, J.P. Photocatalytic reduction of CO2 with H2O over modified TiO2 nanofibers: Understanding the reduction pathway. Nano Res. 2016, 9, 1956–1968. [Google Scholar] [CrossRef]
- Phawa, C.; Prayoonpokarach, S.; Sinthiptharakoon, K.; Chakthranont, P.; Sangkhun, W.; Faungnawakij, K.; Butburee, T. Effects of Matching Facet Pairs of TiO2 on Photoelectrochemical Water Splitting Behaviors. ChemCatChem 2020, 12, 2116–2124. [Google Scholar] [CrossRef]
- Xing, Z.; Zong, X.; Butburee, T.; Pan, J.; Bai, Y.; Wang, L. Nanohybrid materials of titania nanosheets and plasmonic gold nanoparticles for effective hydrogen evolution. Appl. Catal. A 2016, 521, 96–103. [Google Scholar] [CrossRef]
- Xiong, Z.; Lei, Z.; Chen, X.; Gong, B.; Zhao, Y.; Zhang, J.; Zheng, C.; Wu, J.C. CO2 photocatalytic reduction over Pt deposited TiO2 nanocrystals with coexposed {101} and {001} facets: Effect of deposition method and Pt precursors. Catal. Commun. 2017, 96, 1–5. [Google Scholar] [CrossRef]
- Abdullah, H.; Khan, M.M.R.; Ong, H.R.; Yaakob, Z. Modified TiO2 photocatalyst for CO2 photocatalytic reduction: An overview. J. CO2 Util. 2017, 22, 15–32. [Google Scholar] [CrossRef]
- Schoonen, M.A.; Xu, Y.; Strongin, D.R. An introduction to geocatalysis. J. Geochem. Explor. 1998, 62, 201–215. [Google Scholar] [CrossRef]
- Chong, R.; Su, C.; Du, Y.; Fan, Y.; Ling, Z.; Chang, Z.; Li, D. Insights into the role of MgAl layered double oxides interlayer in Pt/TiO2 toward photocatalytic CO2 reduction. J. Catal. 2018, 363, 92–101. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, Y.; Lawes, D.J.; Ball, G.E.; Zhou, C.; Liu, Z.; Amal, R. Analysis of the promoted activity and molecular mechanism of hydrogen production over fine Au-Pt alloyed TiO2 photocatalysts. ACS Catal. 2015, 5, 3924–3931. [Google Scholar] [CrossRef]
- Peerakiatkhajohn, P.; Butburee, T.; Yun, J.-H.; Chen, H.; Richards, R.M.; Wang, L. A hybrid photoelectrode with plasmonic Au@TiO2 nanoparticles for enhanced photoelectrochemical water splitting. J. Mater. Chem. A 2015, 3, 20127–20133. [Google Scholar] [CrossRef]
- Butburee, T.; Bai, Y.; Pan, J.; Zong, X.; Sun, C.; Liu, G.; Wang, L. Step-wise controlled growth of metal@TiO2 core–shells with plasmonic hot spots and their photocatalytic properties. J. Mater. Chem. A 2014, 2, 12776–12784. [Google Scholar] [CrossRef]
- Wang, Y.; Zu, M.; Li, S.; Butburee, T.; Wang, L.; Peng, F.; Zhang, S. Dual modification of TiO2 nanorods for selective photoelectrochemical detection of organic compounds. Sens. Actuators B 2017, 250, 307–314. [Google Scholar] [CrossRef][Green Version]
- Bai, Y.; Butburee, T.; Yu, H.; Li, Z.; Amal, R.; Lu, G.M.; Wang, L. Controllable synthesis of concave cubic gold core–shell nanoparticles for plasmon-enhanced photon harvesting. J. Colloid Interface Sci. 2015, 449, 246–251. [Google Scholar] [CrossRef]
- khalilzadeh, A.; Shariati, A. Fe-N-TiO2/CPO-Cu-27 nanocomposite for superior CO2 photoreduction performance under visible light irradiation. Sol. Energy 2019, 186, 166–174. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T., Jr. Band bending in semiconductors: Chemical and physical consequences at surfaces and interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Kočí, K.; Matějů, K.; Obalová, L.; Krejčíková, S.; Lacný, Z.; Plachá, D.; Čapek, L.; Hospodková, A.; Šolcová, O. Effect of silver doping on the TiO2 for photocatalytic reduction of CO2. Appl. Catal. B 2010, 96, 239–244. [Google Scholar] [CrossRef]
- Li, X.; Zhuang, Z.; Li, W.; Pan, H. Photocatalytic reduction of CO2 over noble metal-loaded and nitrogen-doped mesoporous TiO2. Appl. Catal. A 2012, 429, 31–38. [Google Scholar] [CrossRef]
- Liu, D.; Fernández, Y.; Ola, O.; Mackintosh, S.; Maroto-Valer, M.; Parlett, C.M.; Lee, A.F.; Wu, J.C. On the impact of Cu dispersion on CO2 photoreduction over Cu/TiO2. Catal. Commun. 2012, 25, 78–82. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, X.; Zhao, Y.; Wang, L.; Zhao, Z.; Huang, X.; Liu, J.; Li, J. Efficient photocatalysts of TiO2 nanocrystals-supported PtRu alloy nanoparticles for CO2 reduction with H2O: Synergistic effect of Pt-Ru. Appl. Catal. B 2018, 236, 445–457. [Google Scholar] [CrossRef]
- King, D.M.; Du, X.; Cavanagh, A.S.; Weimer, A.W. Quantum confinement in amorphous TiO2 films studied via atomic layer deposition. Nanotechnology 2008, 19, 445401. [Google Scholar] [CrossRef]
- Digdaya, I.A.; Han, L.; Buijs, T.W.F.; Zeman, M.; Dam, B.; Smets, A.H.M.; Smith, W.A. Extracting large photovoltages from a-SiC photocathodes with an amorphous TiO2 front surface field layer for solar hydrogen evolution. Energy Environ. Sci. 2015, 8, 1585–1593. [Google Scholar] [CrossRef]
- Enright, B.; Fitzmaurice, D. Spectroscopic determination of electron and hole effective masses in a nanocrystalline semiconductor film. J. Phys. Chem. 1996, 100, 1027–1035. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Zaanen, J.; Andersen, O.K. Band theory and Mott insulators: Hubbard U instead of stoner I. Phys. Rev. B 1991, 44, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Dudarev, S.; Botton, G. Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B—Condens. Matter Mater. Phys. 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metalamorphous- semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Yang, C.T.; Wood, B.C.; Bhethanabotla, V.R.; Joseph, B. CO2 adsorption on anatase TiO2 (101) surfaces in the presence of subnanometer Ag/Pt clusters: Implications for CO2 photoreduction. J. Phys. Chem. C 2014, 118, 26236–26248. [Google Scholar] [CrossRef]
- Barcaro, G.; Thomas, I.O.; Fortunelli, A. Validation of density-functional versus density-functional+U approaches for oxide ultrathin films. J. Chem. Phys. 2010, 132. [Google Scholar] [CrossRef]
- Sorescu, D.C.; Al-Saidi, W.A.; Jordan, K.D. CO2 adsorption on TiO2 (101) anatase: A dispersion-corrected density functional theory study. J. Chem. Phys. 2011, 135, 124701. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B—Condens. Matter Mater. Phys. 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillonin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Sanville, E.; Kenny, S.D.; Smith, R.; Henkelman, G. Improved grid-based algorithm for bader charge allocation. J. Comput. Chem. 2007, 28, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef]
- Portillo-Vélez, N.S.; Olvera-Neria, O.; Hernández-Pérez, I.; Rubio-Ponce, A. Localized electronic states induced by oxygen vacancies on anatase TiO2 (101) surface. Surf. Sci. 2013, 616, 115–119. [Google Scholar] [CrossRef]
- He, H.; Zapol, P.; Curtiss, L.A. A theoretical study of CO2 anions on anatase (101) surface. J. Phys. Chem. C 2010, 114, 21474–21481. [Google Scholar] [CrossRef]
- Yang, C.T.; Balakrishnan, N.; Bhethanabotla, V.R.; Joseph, B. Interplay between subnanometer Ag and Pt clusters and anatase TiO2 (101) surface: Implications for catalysis and photocatalysis. J. Phys. Chem. C 2014, 118, 4702–4714. [Google Scholar] [CrossRef]
- Yang, C.T.; Wood, B.C.; Bhethanabotla, V.R.; Joseph, B. The effect of the morphology of supported subnanometer Pt clusters on the first and key step of CO2 photoreduction. Phys. Chem. Chem. Phys. 2015, 17, 25379–25392. [Google Scholar] [CrossRef]
- Meng, L.D.; Wang, G.C. A DFT + U study of acetylene selective hydrogenation over anatase supported PdaAgb (a + b = 4) cluster. Phys. Chem. Chem. Phys. 2014, 16, 17541–17550. [Google Scholar] [CrossRef]
- Iyemperumal, S.K.; Deskins, N.A. Activation of CO2 by supported Cu clusters. Phys. Chem. Chem. Phys. 2017, 19, 28788–28807. [Google Scholar] [CrossRef] [PubMed]
- Bearden, J.A.; Burr, A. Reevaluation of X-ray atomic energy levels. Rev. Mod. Phys. 1967, 39, 125–142. [Google Scholar] [CrossRef]
- Dann, E.K.; Gibson, E.K.; Blackmore, R.H.; Catlow CR, A.; Collier, P.; Chutia, A.; Erden, T.E.; Hardacre, C.; Kroner, A.; Nachtegaal, M. Structural selectivity of supported Pd nanoparticles for catalytic NH3 oxidation resolved using combined operando spectroscopy. Nat. Catal. 2019, 2, 157–163. [Google Scholar] [CrossRef]
- Wirick, S.; Flynn, G.; Sutton, S.; Zolensky, M. Comparison of nickel XANES spectra and elemental maps from a ureilite, a LL3. 8 ordinary chondrite, two carbonaceous chondrites and two large cluster IDPs. In Proceedings of the 45th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 17–21 March 2014. [Google Scholar]
- Kunphonoi, R.; Afanasiev, P.; Geantet, C.; Puzenat, E. Investigation on electron transfer from semiconductor to metal in photocatalytic H2 production. In Proceedings of the 9th European meeting on Solar Chemistry and Photocatalysis: Environmental Applications (SPEA), Strasbourg, France, 13–17 June 2016. [Google Scholar]
- Pu, Y.; Luo, Y.; Wei, X.; Sun, J.; Li, L.; Zou, W.; Dong, L. Synergistic effects of Cu2O-decorated CeO2 on photocatalytic CO2 reduction: Surface Lewis acid/base and oxygen defect. Appl. Catal. B 2019, 254, 580–586. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, L.; Su, Y.; Sun, S.; Wang, Q.; Wang, H.; Wang, W. Boosted CO2 photoreduction to methane: Via Co doping in bismuth vanadate atomic layers. Catal. Sci. Technol. 2018, 8, 3115–3122. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, W.; Wang, X.; Zhang, X.; Chen, H.; Hu, H.; Liu, L.; Ye, J.; Wang, D. Enhanced photocatalytic CO2 reduction over TiO2 using metalloporphyrin as the cocatalyst. Catalysts 2020, 10, 654. [Google Scholar] [CrossRef]
- Cai, S.; Wang, L.; Heng, S.; Li, H.; Bai, Y.; Dang, D.; Wang, Q.; Zhang, P.; He, C. Interaction of single-atom platinum-oxygen vacancy defects for the boosted photosplitting water H2 evolution and CO2 photoreduction: Experimental and theoretical study. J. Phys. Chem. C 2020, 124, 24566–24579. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef]
- Usubharatana, P.; Mcmartin, D.; Veawab, A.; Tontiwachwuthikul, P. Photocatalytic process for CO2 emission reduction from industrial flue gas streams. Ind. Eng. Chem. Res. 2006, 45, 2558–2568. [Google Scholar] [CrossRef]
- Gattrell, M.; Gupta, N.; Co, A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 2006, 594, 1–19. [Google Scholar] [CrossRef]
- Mori, K.; Yamashita, H.; Anpo, M. Photocatalytic reduction of CO2 with H2O on various titanium oxide photocatalysts. RSC Adv. 2012, 2, 3165–3172. [Google Scholar] [CrossRef]
- Gurunathan, K. Photocatalytic hydrogen production using transition metal ions-doped γ-Bi2O3 semiconductor particles. Int. J. Hydrogen Energy 2004, 29, 933–940. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, J.; Liu, D.; Wei, W.; Xie, J.; Chen, M. In situ synthesis of bimetallic Ag/Pt loaded single-crystalline anatase TiO2 hollow nano-hemispheres and their improved photocatalytic properties. CrystEngComm 2014, 16, 2384–2394. [Google Scholar] [CrossRef]
- Umh, H.N.; Song, C.K.; Lee, S.Y.; Bae, S.; Kim, T.Y.; Kim, Y.H.; Joo, J.B.; Yi, J. Band alignment modulations of metal-semiconductor system for enhanced charge separation directly related to a photocatalytic performance. Catal. Commun. 2020, 136, 105921. [Google Scholar] [CrossRef]
- Butburee, T.; Bai, Y.; Wang, H.; Chen, H.; Wang, Z.; Liu, G.; Zou, J.; Khemthong, P.; Lu GQ, M.; Wang, L. 2D porous TiO2 single-crystalline nanostructure demonstrating high photo-electrochemical water splitting performance. Adv. Mater. 2018, 30, 1705666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Wang, Y.; Zhang, P.; Liu, D.; Li, Y.; Jin, Z.; Mamba, B.B.; Kuvarega, A.T.; Gui, J. Insight into l-cysteine-assisted growth of Cu2S nanoparticles on exfoliated MoS2 nanosheets for effective photoreduction removal of Cr (VI). Appl. Surface Sci. 2020, 518, 146191. [Google Scholar] [CrossRef]
- Sariket, D.; Ray, D.; Baduri, S.; Ghosh, S.; Maity, A.; Bhattacharya, C. Synthesis of g-C3N4/InVO4 Semiconductor for Improved Photocatalytic and Photoelectrochemical Applications. Electroanalysis 2020, 32, 2535–2544. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 heterostructures for CO2 reduction through a direct Z-scheme: Protecting Cu2O from photocorrosion. Appl. Catal. B Environ. 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Maicu, M.; Hidalgo, M.; Colón, G.; Navío, J.A. Comparative study of the photodeposition of Pt, Au and Pd on pre-sulphated TiO2 for the photocatalytic decomposition of phenol. J. Photochem. Photobiol. A 2011, 217, 275–283. [Google Scholar] [CrossRef]
- Lu, J.; Jin, H.; Dai, Y.; Yang, K.; Huang, B. Effect of electronegativity and charge balance on the visible-light-responsive photocatalytic activity of nonmetal doped anatase TiO2. Int. J. Photoenergy 2011, 2012, 928503. [Google Scholar] [CrossRef]
- Lee, B.-Y.; Park, S.-H.; Lee, S.-C.; Kang, M.; Park, C.-H.; Choung, S.-J. Optical properties of Pt-TiO2 catalyst and photocatalytic activities for benzene decomposition. Korean J. Chem. Eng. 2003, 20, 812–818. [Google Scholar] [CrossRef]
- Khan, M.R.; Chuan, T.W.; Yousuf, A.; Chowdhury, M.; Cheng, C.K. Schottky barrier and surface plasmonic resonance phenomena towards the photocatalytic reaction: Study of their mechanisms to enhance photocatalytic activity. Catal. Sci. Technol. 2015, 5, 2522–2531. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Liu, G.; Wang, Y. Photocatalytic and photoelectrocatalytic reduction of CO2 using heterogeneous catalysts with controlled nanostructures. Chem. Commun. 2016, 52, 35–59. [Google Scholar] [CrossRef] [PubMed]
- Tseng, I.-H.; Chang, W.-C.; Wu, J.C.S. Photoreduction of CO2 using sol–gel derived titania and titania-supported copper catalysts. Appl. Catal. B Environ. 2002, 37, 37–48. [Google Scholar] [CrossRef]
- Wu, J.C.; Lin, H.-M.; Lai, C.-L. Photo reduction of CO2 to methanol using optical-fiber photoreactor. Appl. Catal. A Gen. 2005, 296, 194–200. [Google Scholar] [CrossRef]
- Wang, J.-J.; Jing, Y.-H.; Ouyang, T.; Zhang, Q.; Chang, C.-T. Photocatalytic reduction of CO2 to energy products using Cu–TiO2 /ZSM-5 and Co–TiO2/ZSM-5 under low energy irradiation. Catal. Commun. 2015, 59, 69–72. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Jiu, H.; Qi, G.; Huang, Y. ZnO-reduced graphene oxide nanocomposites as efficient photocatalysts for photocatalytic reduction of CO2. Ceram. Int. 2015, 41, 6256–6262. [Google Scholar] [CrossRef]
- Song, Y.; Li, J.; Wang, C. Modification of porphyrin/dipyridine metal complexes on the surface of TiO2 nanotubes with enhanced photocatalytic activity for photoreduction of CO2 into methanol. J. Mater. Res. 2018, 33, 2612–2620. [Google Scholar] [CrossRef]

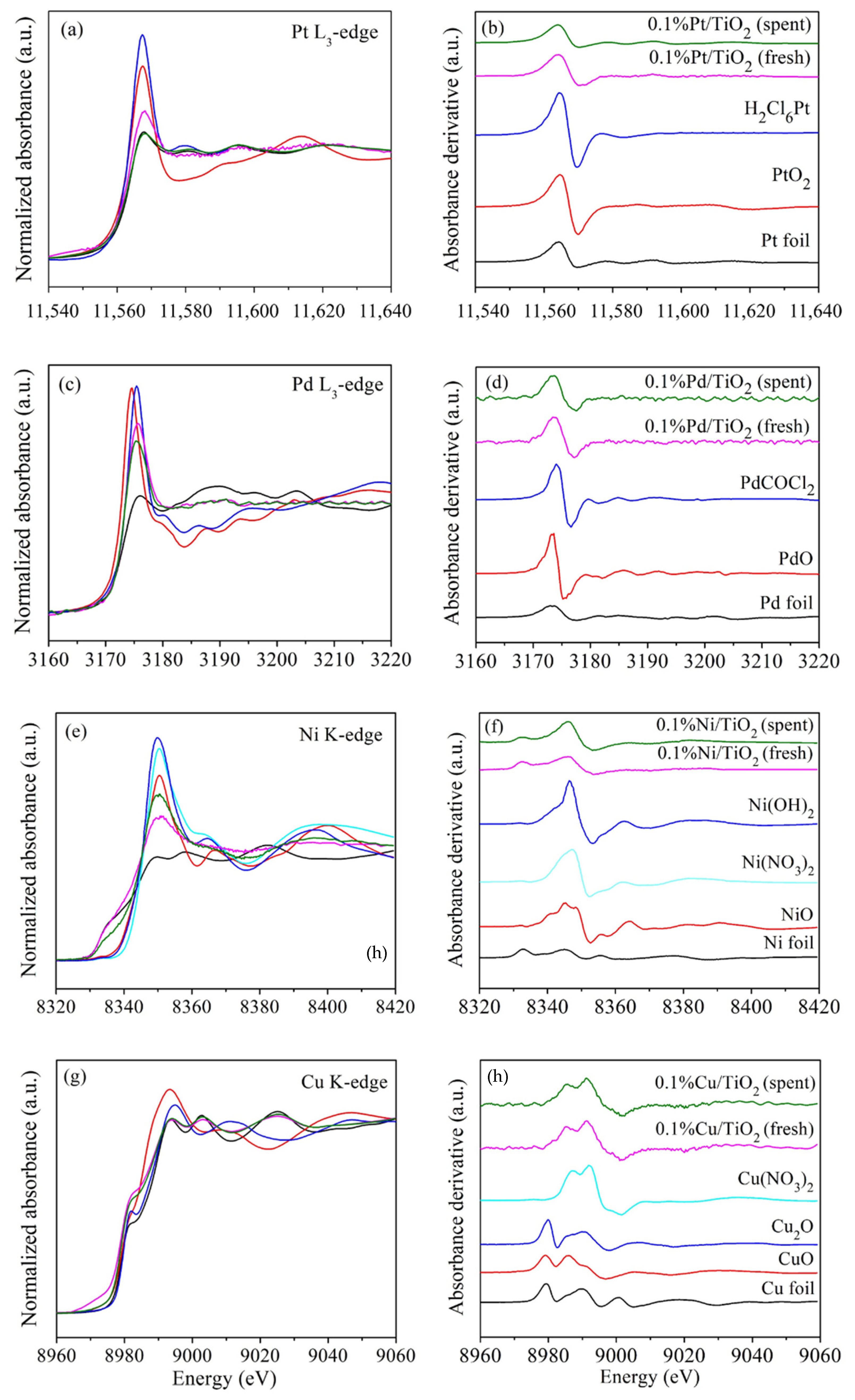



| Standards | Fresh (%) | Spent (%) |
|---|---|---|
| 0.1%Pt/TiO2 | ||
| Pt foil | 0.724 | 1.000 |
| PtO2 | 0.121 | 0.000 |
| H2Cl6Pt | 0.155 | 0.000 |
| 0.1%Pd/TiO2 | ||
| Pd foil | 0.822 | 0.905 |
| PdO | 0.178 | 0.095 |
| 0.1%Ni/TiO2 | ||
| Ni foil | 0.970 | 0.828 |
| NiO | 0.000 | 0.000 |
| Ni(OH)2 | 0.030 | 0.172 |
| 0.1%Cu/TiO2 | ||
| Cu foil | 0.000 | 0.000 |
| CuO | 0.399 | 0.068 |
| Cu2O | 0.601 | 0.932 |
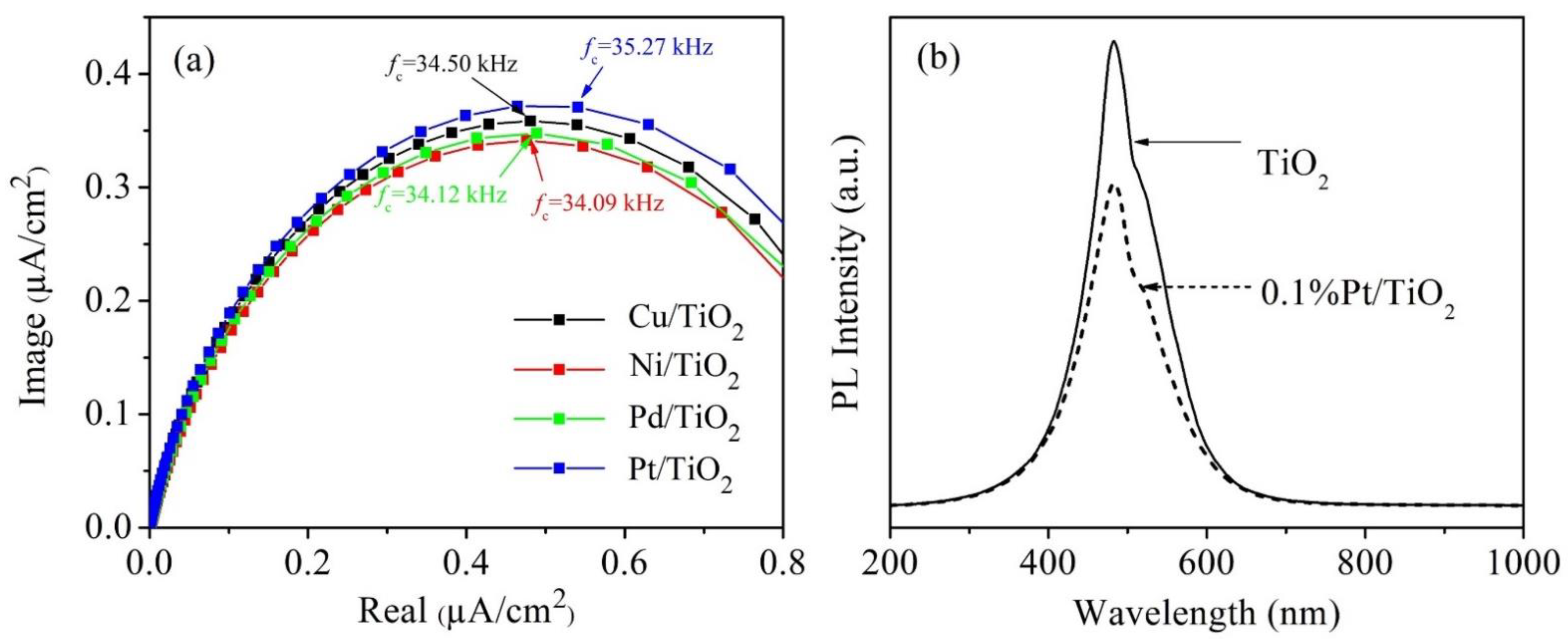
| Catalyst | Vfb (V vs. Ag/AgCl) | ΦM | EF | EC | EV | ND | ECB-EF |
|---|---|---|---|---|---|---|---|
| (eV) | (eV vs. Vacuum) | (cm−3) | (mV) | ||||
| TiO2 | −0.53 | - | −4.17 | −4.12 | −7.32 | 1.23 × 1020 | 47.83 |
| Pt/TiO2 | −0.23 | −5.65 (Pt) | −4.47 | −4.45 | −7.65 | 3.27 × 1020 | 22.60 |
| Pd/TiO2 | −0.34 | −5.22 (Pd) | −4.36 | −4.32 | −7.52 | 1.96 × 1020 | 35.72 |
| Ni/TiO2 | −0.53 | −5.04 (Ni) | −4.17 | −4.12 | −7.32 | 1.32 × 1020 | 45.98 |
| Cu/TiO2 | −0.23 | −4.65 (Cu) | −4.47 | −4.44 | −7.64 | 2.64 × 1020 | 28.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Permporn, D.; Khunphonoi, R.; Wilamat, J.; Khemthong, P.; Chirawatkul, P.; Butburee, T.; Sangkhun, W.; Wantala, K.; Grisdanurak, N.; Santatiwongchai, J.; et al. Insight into the Roles of Metal Loading on CO2 Photocatalytic Reduction Behaviors of TiO2. Nanomaterials 2022, 12, 474. https://doi.org/10.3390/nano12030474
Permporn D, Khunphonoi R, Wilamat J, Khemthong P, Chirawatkul P, Butburee T, Sangkhun W, Wantala K, Grisdanurak N, Santatiwongchai J, et al. Insight into the Roles of Metal Loading on CO2 Photocatalytic Reduction Behaviors of TiO2. Nanomaterials. 2022; 12(3):474. https://doi.org/10.3390/nano12030474
Chicago/Turabian StylePermporn, Darika, Rattabal Khunphonoi, Jetsadakorn Wilamat, Pongtanawat Khemthong, Prae Chirawatkul, Teera Butburee, Weradesh Sangkhun, Kitirote Wantala, Nurak Grisdanurak, Jirapat Santatiwongchai, and et al. 2022. "Insight into the Roles of Metal Loading on CO2 Photocatalytic Reduction Behaviors of TiO2" Nanomaterials 12, no. 3: 474. https://doi.org/10.3390/nano12030474
APA StylePermporn, D., Khunphonoi, R., Wilamat, J., Khemthong, P., Chirawatkul, P., Butburee, T., Sangkhun, W., Wantala, K., Grisdanurak, N., Santatiwongchai, J., Hirunsit, P., Klysubun, W., & de Luna, M. D. G. (2022). Insight into the Roles of Metal Loading on CO2 Photocatalytic Reduction Behaviors of TiO2. Nanomaterials, 12(3), 474. https://doi.org/10.3390/nano12030474








