Collection of Controlled Nanosafety Data—The CoCoN-Database, a Tool to Assess Nanomaterial Hazard
Abstract
:1. Introduction
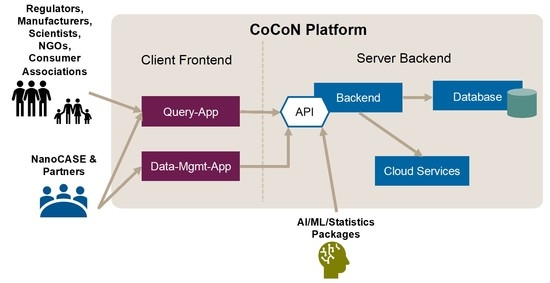
2. Materials and Methods
2.1. Literature Search and Study Selection
- The study deals with medical treatment or therapeutic application of the nanomaterial only, e.g., drug delivery or therapeutic application via injection.
- No real toxicological experiments have been shown; this is the case if a material- or engineering-oriented publication uses the buzz word “toxicity” or similar within the introduction or discussion, which led to the inclusion of the publication at the beginning.
- Neither animal studies nor cell- or tissue culture experiments have been described, which excludes reviews as well.
- The content deals exclusively with environmental issues, e.g., air pollution with diesel exhaust particles or ultrafine dust, or describes solely ecotoxicological topics such as plant research or distribution in environmental compartments such as water, soil or air.
- The publication is not available as pdf file and/or not written in English or German language.
2.2. Data Extraction and Collection
2.3. Quality Evaluation—Scoring
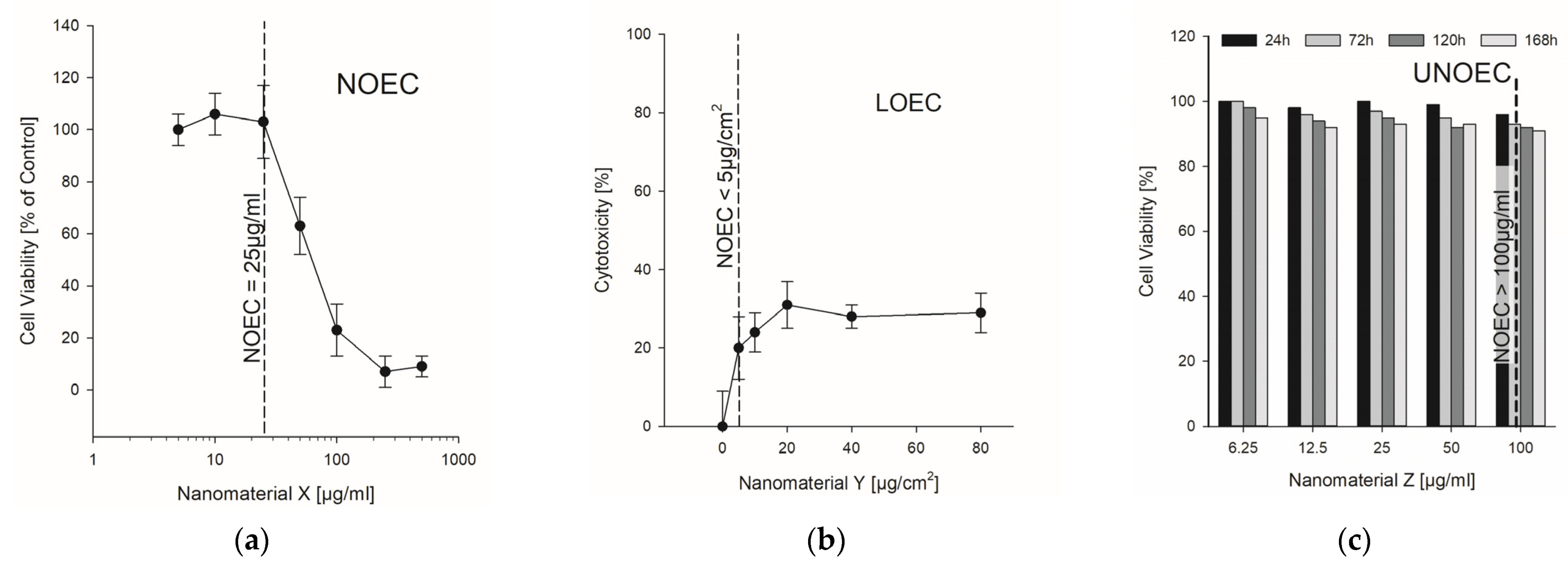
2.4. NOAEL/NOEC—Readout from Published Studies
3. Results
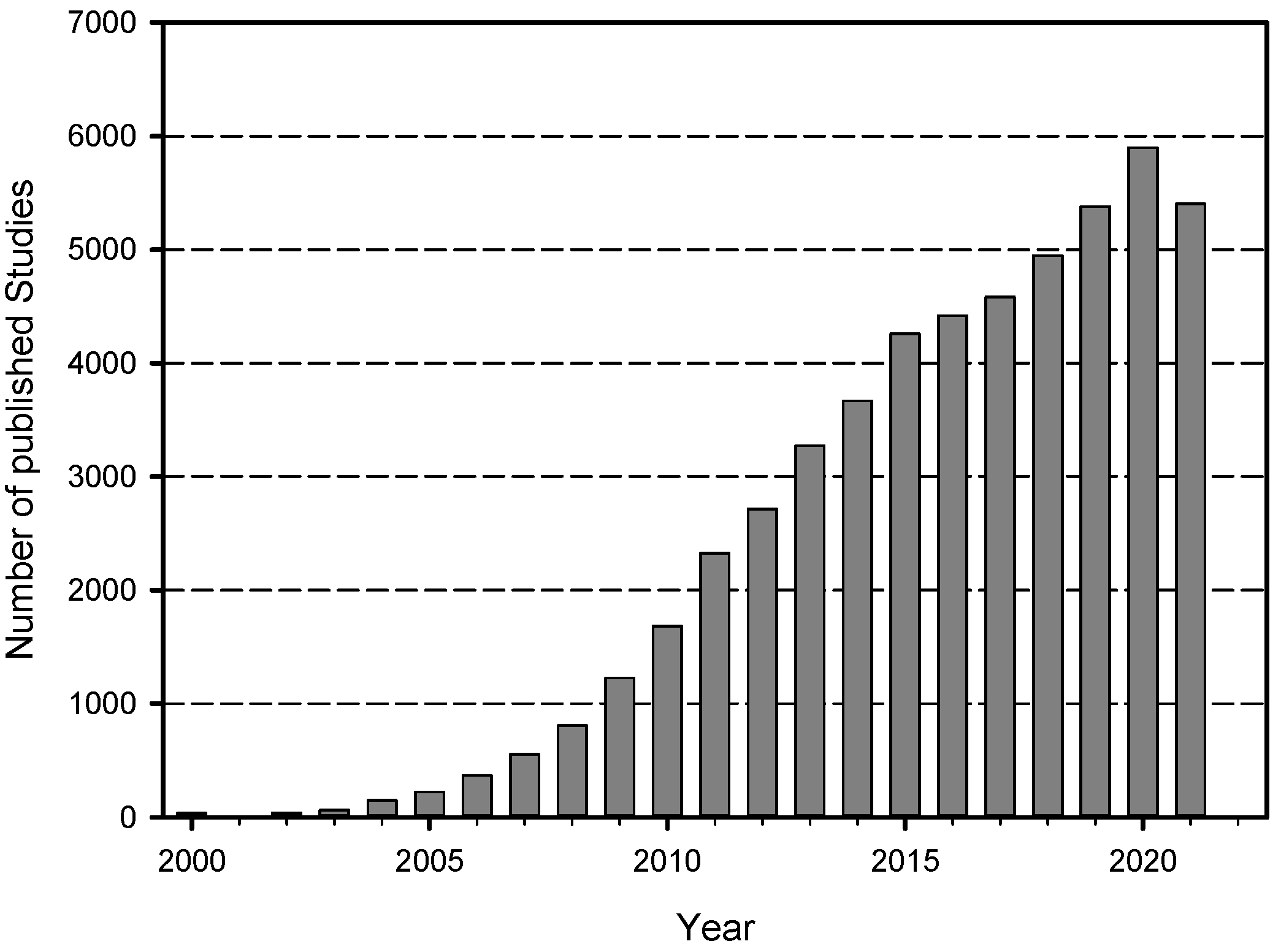
3.1. Key Figures of the Data Collection
3.2. Readout Results from the Data Collection
3.2.1. OECD-Project on Adverse Outcome Pathways for Nanomaterial Risk Assessment
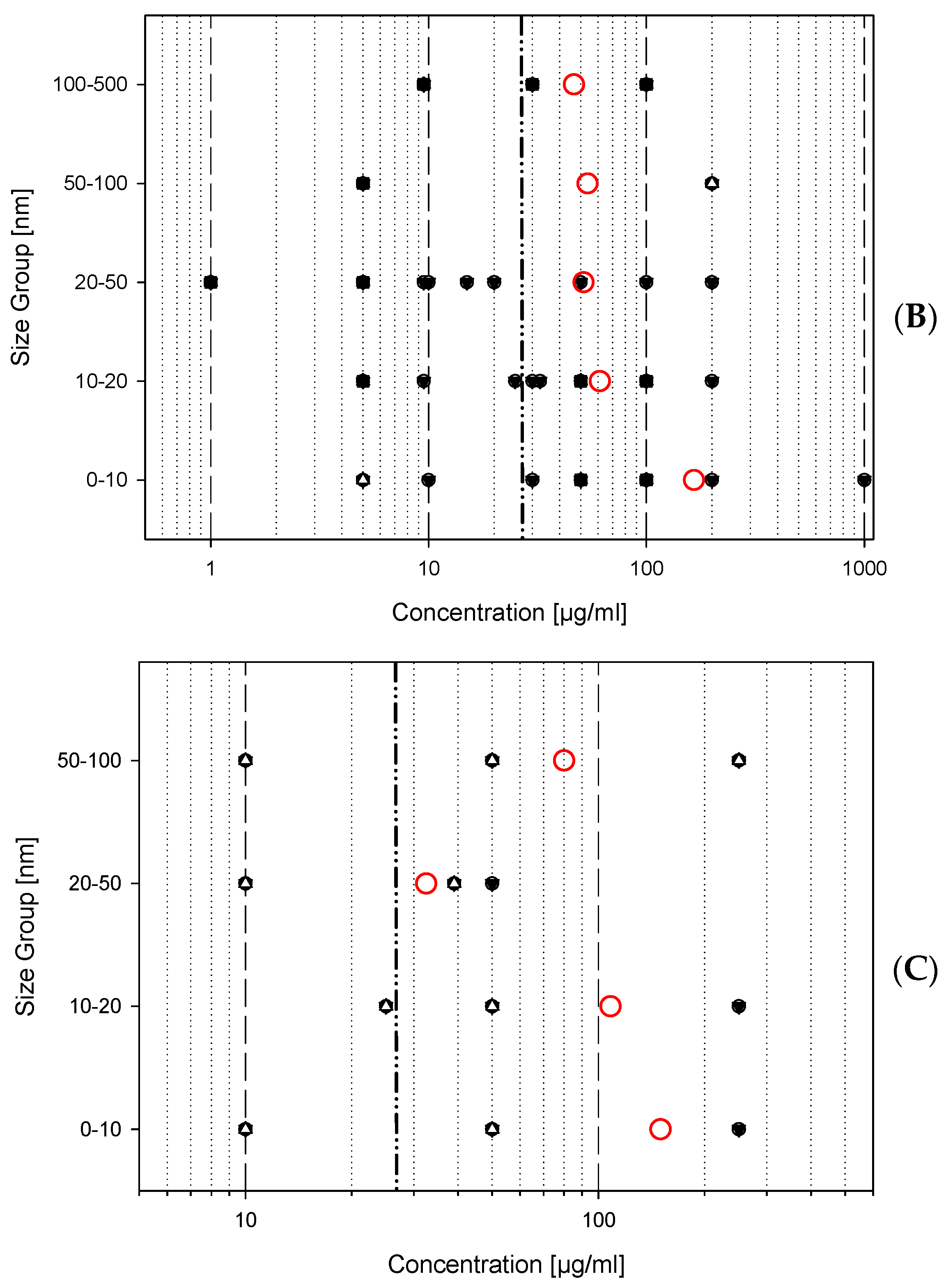
3.2.2. Evaluation of Titanium Dioxide Lung Toxicity
- Material = titanium dioxide;
- Type of study = in vitro studies;
- Cell type = mammalian lung cells (exclusively macrophages or immune cells);
- Concentration units = µg/mL (or µg/cm2, if recalculation is possible);
- Biological endpoints = acute cytotoxicity, formation of reactive oxygen species (ROS), cytokine production;
- Material = titanium dioxide;
- Type of study = in vivo studies;
- Species = rats or mice;
- Exposure = inhalation (mg/m3) or;
- Exposure = instillation or aspiration (µg/kg or µg/animal);
- Biological endpoint = immune cell migration (neutrophil or macrophage influx).
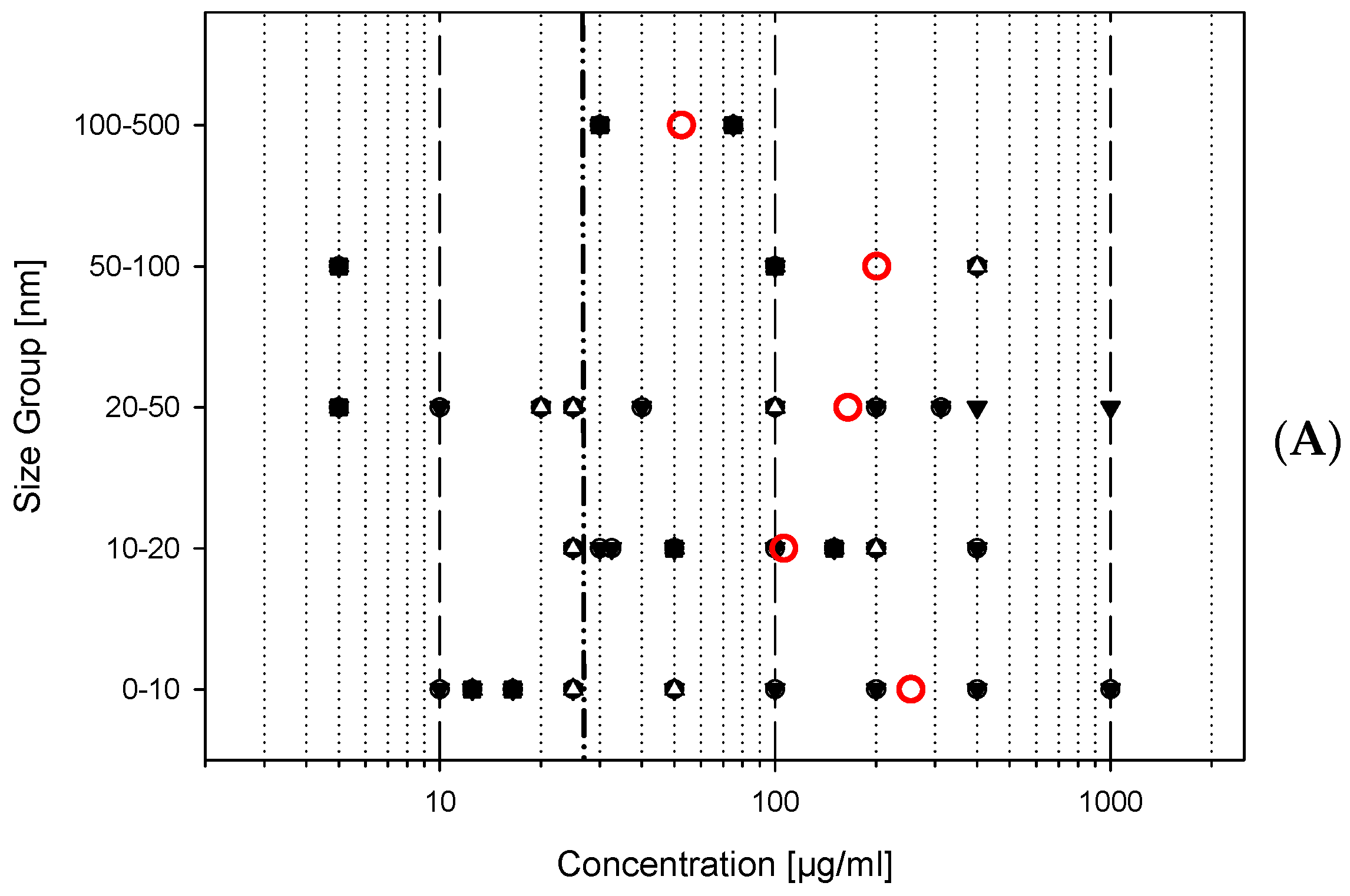
- 0–10 nm
- 11–20 nm
- 21–50 nm
- 51–100 nm
- 101–500 nm
- The overall number of studies with a comparable experimental study design is low; most toxicological studies do not yet use standardised protocols, e.g., OECD testing guidelines as suggested recently [28].
- The variety of investigated titanium dioxide is high; in this period, not even two studies used the same material.
- In rats, NOAEL range from 200 µg/kg to 5000 µg/kg, which might represent the huge variety in the materials.
- Some studies found a response for the only investigated dose; in many cases the observation time was only 1 d and in this treatment period the influx of neutrophils can be expected after exposure with dust particles as a “normal defence response”.
- For mice, the NOAEL is between 30 µg/animal to 500 µg/animal, which represents the overload dose.
- In mice and rats, the inflammatory response is strongly dependent on the material tested.
- For the more realistic inhalation studies, 7 datasets out of 8 do not describe any cell migration into the lungs; the only study which found an inflammatory response used 100 mg/m3, 6 h per day for 5 days for the inhalation exposure.
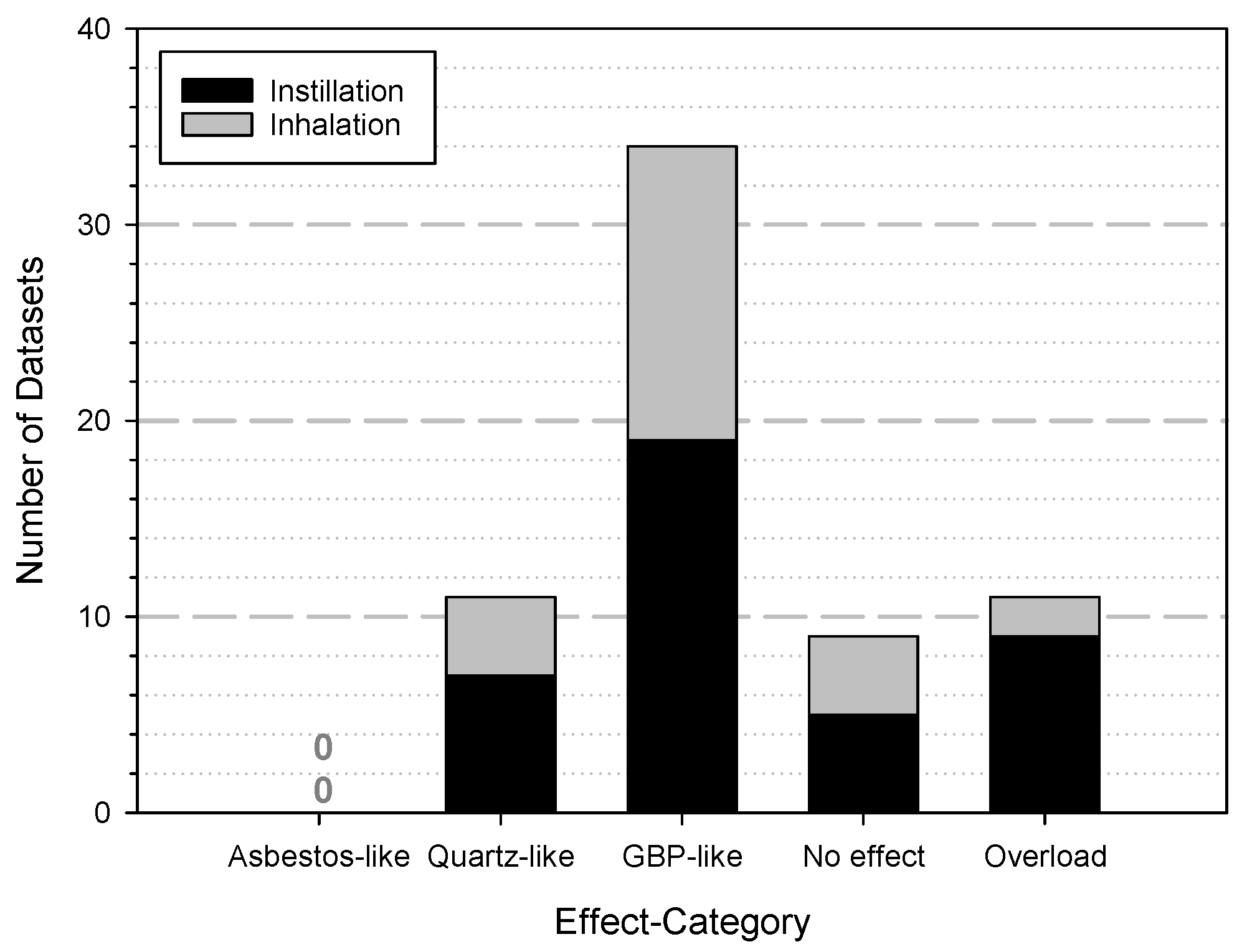
- Overload: this reflects the situation that for mice a higher dose than 500 µg/lung and for rats more than 2500 µg/lung were applied.
- No effect: describes the situation that even the highest dose used for the experiments did not induced any adverse effect in the animals during the observation time.
- GBP-like: the behaviour as a granular biopersistent particle (GBP) is described to induce a transient inflammatory response, which usually subsides after about 7 days and the animal recovers completely without permanent sequelae.
- Quartz-like: the relationship to quartz describes a more intense inflammation accompanied by oxidative stress, a formation of granuloma with signs of fibrosis.
- Asbestos-like: the effects which indicate quartz-like mechanisms and additionally genotoxicity and the induction of a higher tumour incidence.
4. Discussion
- A rigorous and adequate physicochemical characterization of the test materials is needed;
- Adequate particle controls must be included;
- Possible contaminations, such as endotoxins, should be analysed;
- Interferences of the tested material with the assay should be investigated;
- High-dose experiments designed to produce toxicological effects—which are publishable (and sensational)—should be avoided
- As far as possible, standardized protocols should be used to better compare results
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Gottardo, S.; Mech, A.; Drbohlavova, J.; Malyska, A.; Bowadt, S.; Riego Sintes, J.; Rauscher, H. Towards safe and sustainable innovation in nanotechnology: State-of-play for smart nanomaterials. NanoImpact 2021, 21, 100297. [Google Scholar] [CrossRef] [PubMed]
- Tavernaro, I.; Dekkers, S.; Soeteman-Hernandez, L.G.; Herbeck-Engel, P.; Noorlander, C.; Kraegeloh, A. Safe-by-design part ii: A strategy for balancing safety and functionality in the different stages of the innovation process. Nanoimpact 2021, 24, 100354. [Google Scholar] [CrossRef]
- Soeteman-Hernández, L.G.; Bekker, C.; Groenewold, M.; Jantunen, P.; Mech, A.; Rasmussen, K.; Sintes, J.R.; Sips, A.J.A.M.; Noorlander, C.W. Perspective on how regulators can keep pace with innovation: Outcomes of a european regulatory preparedness workshop on nanomaterials and nano-enabled products. NanoImpact 2019, 14, 100166. [Google Scholar] [CrossRef]
- Hristozov, D.R.; Gottardo, S.; Critto, A.; Marcomini, A. Risk assessment of engineered nanomaterials: A review of available data and approaches from a regulatory perspective. Nanotoxicology 2012, 6, 880–898. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Guo, W.; Sakkiah, S.; Liu, J.; Patterson, T.A.; Hong, H. Nanomaterial databases: Data sources for promoting design and risk assessment of nanomaterials. Nanomaterials 2021, 11, 1599. [Google Scholar] [CrossRef]
- Karcher, S.C.; Harper, B.J.; Harper, S.L.; Hendren, C.O.; Wiesner, M.R.; Lowry, G.V. Visualization tool for correlating nanomaterial properties and biological responses in zebrafish. Environ. Sci. Nano 2016, 3, 1280–1292. [Google Scholar] [CrossRef]
- Juganson, K.; Ivask, A.; Blinova, I.; Mortimer, M.; Kahru, A. Nanoe-tox: New and in-depth database concerning ecotoxicity of nanomaterials. Beilstein J. Nanotechnol. 2015, 6, 1788–1804. [Google Scholar] [CrossRef]
- Comandella, D.; Gottardo, S.; Rio-Echevarria, I.M.; Rauscher, H. Quality of physicochemical data on nanomaterials: An assessment of data completeness and variability. Nanoscale 2020, 12, 4695–4708. [Google Scholar] [CrossRef] [Green Version]
- Romeo, D.; Salieri, B.; Hischier, R.; Nowack, B.; Wick, P. An integrated pathway based on in vitro data for the human hazard assessment of nanomaterials. Environ. Int. 2020, 137, 105505. [Google Scholar] [CrossRef]
- Krug, H.F. Nanosafety research—Are we on the right track? Angew Chem. Int. Ed. Engl. 2014, 53, 12304–12319. [Google Scholar] [CrossRef] [Green Version]
- Halappanavar, S.; Ede, J.D.; Shatkin, J.A.; Krug, H.F. A systematic process for identifying key events for advancing the development of nanomaterial relevant adverse outcome pathways. NanoImpact 2019, 15, 100178. [Google Scholar] [CrossRef]
- Kroll, A.; Dierker, C.; Rommel, C.; Hahn, D.; Wohlleben, W.; Schulze-Isfort, C.; Gobbert, C.; Voetz, M.; Hardinghaus, F.; Schnekenburger, J. Cytotoxicity screening of 23 engineered nanomaterials using a test matrix of ten cell lines and three different assays. Part Fibre Toxicol. 2011, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Krug, H.F.; Bohmer, N.; Kühnel, D.; Marquardt, C.; Nau, K.; Steinbach, C. The dana(2.0) knowledge base nanomaterials-an important measure accompanying nanomaterials development. Nanomaterials 2018, 8, 204. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Cruz, M.L.; Hernández-Moreno, D.; Catalán, J.; Cross, R.K.; Stockmann-Juvala, H.; Cabellos, J.; Lopes, V.R.; Matzke, M.; Ferraz, N.; Izquierdo, J.J.; et al. Quality evaluation of human and environmental toxicity studies performed with nanomaterials—The guidenano approach. Environ. Sci. Nano 2018, 5, 381–397. [Google Scholar] [CrossRef]
- Schneider, K.; Schwarz, M.; Burkholder, I.; Kopp-Schneider, A.; Edler, L.; Kinsner-Ovaskainen, A.; Hartung, T.; Hoffmann, S. “Toxrtool”, a new tool to assess the reliability of toxicological data. Toxicol. Lett. 2009, 189, 138–144. [Google Scholar] [CrossRef]
- Krug, H.F. The uncertainty with nanosafety: Validity and reliability of published data. Colloids Surf. B Biointerfaces 2018, 172, 113–117. [Google Scholar] [CrossRef]
- Ghosh, M.; Bandyopadhyay, M.; Mukherjee, A. Genotoxicity of titanium dioxide (tio2) nanoparticles at two trophic levels: Plant and human lymphocytes. Chemosphere 2010, 81, 1253–1262. [Google Scholar] [CrossRef]
- Schanen, B.C.; Karakoti, A.S.; Seal, S.; Drake, D.R., 3rd; Warren, W.L.; Self, W.T. Exposure to titanium dioxide nanomaterials provokes inflammation of an in vitro human immune construct. ACS Nano 2009, 3, 2523–2532. [Google Scholar] [CrossRef] [Green Version]
- Panessa-Warren, B.J.; Warren, J.B.; Wong, B.A.; Misewich, J.A. Biological cellular response to carbon nanoparticle toxicity. J. Phys. Condens. Matter 2006, 18, S2185–S2201. [Google Scholar] [CrossRef]
- Krug, H.F.; Nau, K. Reliability for nanosafety research—Considerations on the basis of a comprehensive literature review. ChemBioEng Rev. 2017, 4, 331–338. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Halappanavar, S.; Ede, J.D.; Mahapatra, I.; Krug, H.F.; Kuempel, E.D.; Lynch, I.; Vandebriel, R.J.; Shatkin, J.A. A methodology for developing key events to advance nanomaterial-relevant adverse outcome pathways to inform risk assessment. Nanotoxicology 2021, 15, 289–310. [Google Scholar] [CrossRef]
- Halappanavar, S.; Nymark, P.; Krug, H.F.; Clift, M.J.D.; Rothen-Rutishauser, B.; Vogel, U. Non-animal strategies for toxicity assessment of nanoscale materials: Role of adverse outcome pathways in the selection of endpoints. Small 2021, 17, e2007628. [Google Scholar] [CrossRef]
- Luechtefeld, T.; Marsh, D.; Rowlands, C.; Hartung, T. Machine learning of toxicological big data enables read-across structure activity relationships (rasar) outperforming animal test reproducibility. Toxicol. Sci. 2018, 165, 198–212. [Google Scholar] [CrossRef]
- Wittmaack, K. Excessive delivery of nanostructured matter to submersed cells caused by rapid gravitational settling. ACS Nano 2011, 5, 3766–3778. [Google Scholar] [CrossRef]
- Wittmaack, K. Novel dose metric for apparent cytotoxicity effects generated by in vitro cell exposure to silica nanoparticles. Chem. Res. Toxicol. 2011, 24, 150–158. [Google Scholar] [CrossRef]
- Oberdorster, G. Safety assessment for nanotechnology and nanomedicine: Concepts of nanotoxicology. J. Intern. Med. 2010, 267, 89–105. [Google Scholar] [CrossRef]
- Rasmussen, K.; Rauscher, H.; Kearns, P.; Gonzalez, M.; Riego Sintes, J. Developing oecd test guidelines for regulatory testing of nanomaterials to ensure mutual acceptance of test data. Regul. Toxicol. Pharmacol. 2019, 104, 74–83. [Google Scholar] [CrossRef]
- Warheit, D.B.; Donner, E.M. How meaningful are risk determinations in the absence of a complete dataset? Making the case for publishing standardized test guideline and ’no effect’ studies for evaluating the safety of nanoparticulates versus spurious ’high effect’ results from single investigative studies. Sci. Technol. Adv. Mater. 2015, 16, 034603. [Google Scholar] [CrossRef] [Green Version]
- Powers, C.M.; Mills, K.A.; Morris, S.A.; Klaessig, F.; Gaheen, S.; Lewinski, N.; Ogilvie Hendren, C. Nanocuration workflows: Establishing best practices for identifying, inputting, and sharing data to inform decisions on nanomaterials. Beilstein J. Nanotechnol. 2015, 6, 1860–1871. [Google Scholar] [CrossRef] [Green Version]
- Jeliazkova, N.; Apostolova, M.D.; Andreoli, C.; Barone, F.; Barrick, A.; Battistelli, C.; Bossa, C.; Botea-Petcu, A.; Chatel, A.; De Angelis, I.; et al. Towards fair nanosafety data. Nat. Nanotechnol. 2021, 16, 644–654. [Google Scholar] [CrossRef]
- Higgins, S.G.; Nogiwa-Valdez, A.A.; Stevens, M.M. Considerations for implementing electronic laboratory notebooks in an academic research environment. Nat. Protoc. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Solle, D. Be fair to your data. Anal. Bioanal. Chem. 2020, 412, 3961–3965. [Google Scholar] [CrossRef]
- Rovida, C.; Barton-Maclaren, T.; Benfenati, E.; Caloni, F.; Chandrasekera, P.C.; Chesne, C.; Cronin, M.T.D.; De Knecht, J.; Dietrich, D.R.; Escher, S.E.; et al. Internationalization of read-across as a validated new approach method (nam) for regulatory toxicology. Altern. Anim. Exp. ALTEX 2020, 37, 579–606. [Google Scholar] [CrossRef]
- Wigger, H.; Nowack, B. Material-specific properties applied to an environmental risk assessment of engineered nanomaterials—Implications on grouping and read-across concepts. Nanotoxicology 2019, 13, 623–643. [Google Scholar] [CrossRef]
- Hristozov, D.; Gottardo, S.; Semenzin, E.; Oomen, A.; Bos, P.; Peijnenburg, W.; van Tongeren, M.; Nowack, B.; Hunt, N.; Brunelli, A.; et al. Frameworks and tools for risk assessment of manufactured nanomaterials. Environ. Int. 2016, 95, 36–53. [Google Scholar] [CrossRef]
- Henschler, D. Toxicological problems relating to changes in the environment. Angew. Chem. Int. Ed. Engl. 1973, 12, 274–282. [Google Scholar] [CrossRef]
- Wörle-Knirsch, J.M.; Pulskamp, K.; Krug, H.F. Oops they did it again! Carbon nanotubes hoax scientists in viability assays. Nano Lett. 2006, 6, 1261–1268. [Google Scholar] [CrossRef]
- Faria, M.; Bjornmalm, M.; Thurecht, K.J.; Kent, S.J.; Parton, R.G.; Kavallaris, M.; Johnston, A.P.R.; Gooding, J.J.; Corrie, S.R.; Boyd, B.J.; et al. Minimum information reporting in bio-nano experimental literature. Nat. Nanotechnol. 2018, 13, 777–785. [Google Scholar] [CrossRef]
- Hirsch, C.; Roesslein, M.; Krug, H.F.; Wick, P. Nanomaterial cell interactions: Are current in vitro tests reliable? Nanomedicine 2011, 6, 837–847. [Google Scholar] [CrossRef]
- Schulze, C.; Kroll, A.; Lehr, C.M.; Sch„fer, U.F.; Becker, K.; Schnekenburger, J.; Schulze-Isfort, C.; Landsiedel, R.; Wohlleben, W. Not ready to use—Overcoming pitfalls when dispersing nanoparticles in physiological media. Nanotoxicology 2008, 2, 51–61. [Google Scholar] [CrossRef]
- Nymark, P.; Bakker, M.; Dekkers, S.; Franken, R.; Fransman, W.; Garcia-Bilbao, A.; Greco, D.; Gulumian, M.; Hadrup, N.; Halappanavar, S.; et al. Toward rigorous materials production: New approach methodologies have extensive potential to improve current safety assessment practices. Small 2020, 16, e1904749. [Google Scholar] [CrossRef]
- Yazdi, A.S.; Guarda, G.; Riteau, N.; Drexler, S.K.; Tardivel, A.; Couillin, I.; Tschopp, J. Nanoparticles activate the nlr pyrin domain containing 3 (nlrp3) inflammasome and cause pulmonary inflammation through release of il-1alpha and il-1beta. Proc. Natl. Acad. Sci. USA 2010, 107, 19449–19454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielinska, A.; Costa, B.; Ferreira, M.V.; Migueis, D.; Louros, J.M.S.; Durazzo, A.; Lucarini, M.; Eder, P.; Chaud, M.V.; Morsink, M.; et al. Nanotoxicology and nanosafety: Safety-by-design and testing at a glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. [Google Scholar] [CrossRef]



| Databases | Website | Remark |
|---|---|---|
| caNanoLab | https://cananolab.nci.nih.gov/ | Nanotechnology in biomedical research on cancer |
| eNanoMapper | https://data.enanomapper.net/ | Ontology and safety assessment of nanomaterials |
| NR | https://nanomaterialregistry.net/ | Physicochemical properties of selected nanomaterials |
| NanoData | https://nanodata.echa.europa.eu/ | Nanotechnology in products in 8 different sectors |
| Nanodatabase | https://nanodb.dk/en/ | Nanomaterials in products |
| NanoE-Tox | http://www.beilstein-journals.org/bjnano/content/supplementary/2190-4286-6-183-S2.xls | Ecotoxicological data available as an excel sheet which will not be updated anymore |
| NanoNature | https://nano.nature.com/ | Literature database on nanomaterials; will be retired in June 2022 |
| Nanowatch | https://www.bund.net/themen/chemie/nanotechnologie/nanoprodukte-im-alltag/nanoproduktdatenbank/ | Commercially available products containing nanomaterials; available in German only |
| Nanowerk | https://www.nanowerk.com/ | Database on suppliers of commercially available nanomaterials |
| NBIK | http://nbi.oregonstate.edu/ no safe connection on access day | Database on study results of nanomaterial exposure effects in embryo zebrafish |
| NECID | https://necid.ifa.dguv.de/Login.aspx?ReturnUrl=%2fUser%2fFirstpage.aspx | Data on occupational nanomaterial exposure during various exposure scenarios |
| NIL | http://nanoparticlelibrary.net/ | Physicochemical characteristics of very specifically produced nanomaterials |
| NKB | https://ssl.biomax.de/nanocommons/ | Nano-safety knowledge infrastructure |
| PubVINAS | http://www.pubvinas.com/ no safe connection on access day | Virtual nanostructure simulation tool |
| PaFTox | https://publica.fraunhofer.de/dokumente/N-277711.html | Data on genotoxicity of nanomaterials; the database is not available anymore although funded by government money |
| StatNano | https://statnano.com/ | Applications and properties of nanomaterials |
| Field Delimiter | Where | How | What |
|---|---|---|---|
| All Fields | Contains | nanotox * | |
| Or | All Fields | Contains | fulleren * AND toxic * |
| Or | All Fields | Contains | carbo nanotube * AND toxic * |
| Or | All Fields | Contains | bucky ball * AND toxic |
| Or | All Fields | Contains | nanotube * AND toxic |
| Or | All Fields | Contains | nanoparticle * AND toxic * |
| Or | All Fields | Contains | nanomat * AND toxic * |
| Or | All Fields | Contains | Nano * AND toxic |
| Or | Year | Contains | 2021 1 |
| Given Concentration Units for In Vitro Studies | Given Dose Units for In Vivo Studies | |
|---|---|---|
| µg/mL | oral exposure | inhalation/instillation |
| µg/cm2 | µg/animal | µg/m3 |
| cm2/mL | µg/kg BW and | mg/m3 |
| m2/cm2 | mg/kg BW | cm2/m3 |
| mM/µM/nM | dermal or intradermal | /cm3 |
| ppm | µg/µL or mL | µg/animal |
| /mL | g or mg/ear | µg/g lung tissue |
| /cm2 | mg/animal | µg/lung |
| /cell | mg/kg | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krug, H.F. Collection of Controlled Nanosafety Data—The CoCoN-Database, a Tool to Assess Nanomaterial Hazard. Nanomaterials 2022, 12, 441. https://doi.org/10.3390/nano12030441
Krug HF. Collection of Controlled Nanosafety Data—The CoCoN-Database, a Tool to Assess Nanomaterial Hazard. Nanomaterials. 2022; 12(3):441. https://doi.org/10.3390/nano12030441
Chicago/Turabian StyleKrug, Harald F. 2022. "Collection of Controlled Nanosafety Data—The CoCoN-Database, a Tool to Assess Nanomaterial Hazard" Nanomaterials 12, no. 3: 441. https://doi.org/10.3390/nano12030441
APA StyleKrug, H. F. (2022). Collection of Controlled Nanosafety Data—The CoCoN-Database, a Tool to Assess Nanomaterial Hazard. Nanomaterials, 12(3), 441. https://doi.org/10.3390/nano12030441