Triblock Copolymer Micelles with Tunable Surface Charge as Drug Nanocarriers: Synthesis and Physico-Chemical Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Hydrophobic Polyester Block (PLA)
2.3. Conversion of PLA into a Macroinitiator (PLA-Br)
2.4. Synthesis of Amphiphilic Diblock Copolymer (PLA-b-PDMAEMA)
2.5. Synthesis of Poly(D,L-Lactide)-b-Poly(N,N-Dimethylaminoethyl Methacrylate)-b-Poly(Oligo(Ethylene Glycol) Methyl Ether Methacrylate) (PLA-b-PDMAEMA-b-POEGMA) Amphiphilic Triblock Copolymer
2.6. Characterization
2.7. Copolymer Micelles Preparation
2.8. Evaluation of the Critical Micelle Concentration (CMC)
2.9. Drug Loading Procedure and In Vitro Drug Release Profiles
2.10. In Vitro Stability and Protein Adsorption
3. Results and Discussion
3.1. Synthesis and Characterization of Amphiphilic PLA-b-PDMAEMA-b-POEGMA Triblock Copolymers
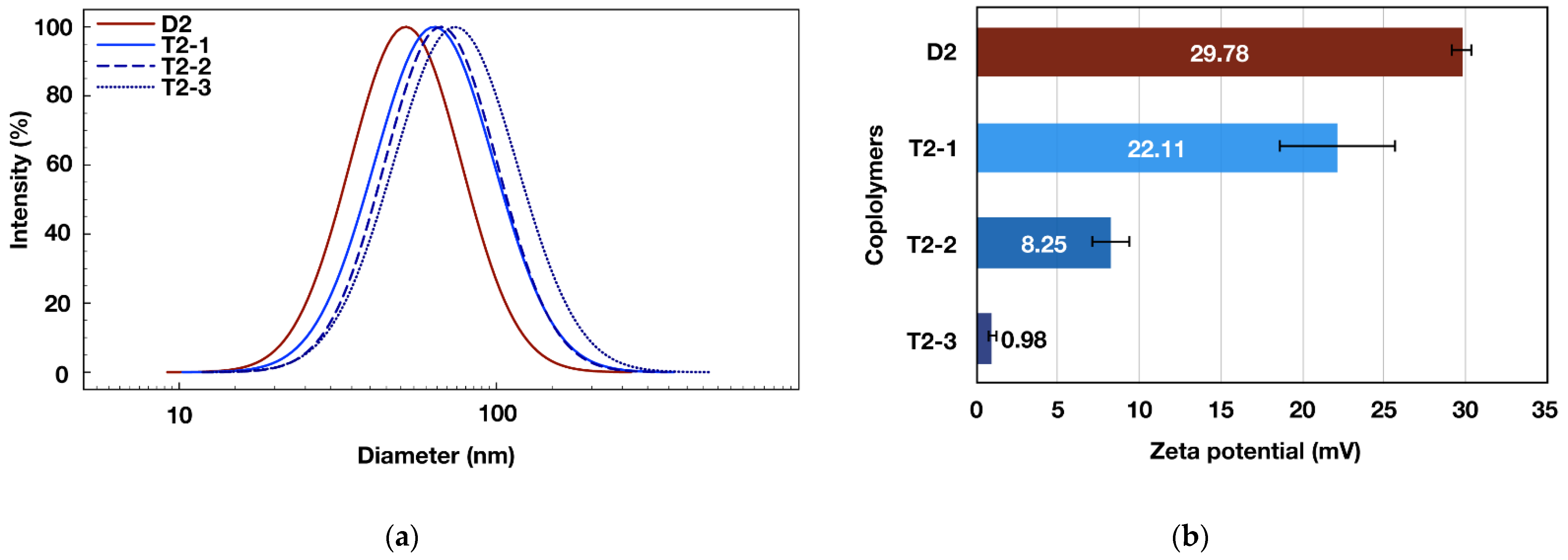
3.2. Triblock Copolymers Self-Assembly
3.3. Curcumin Loading into the Copolymer Micelles
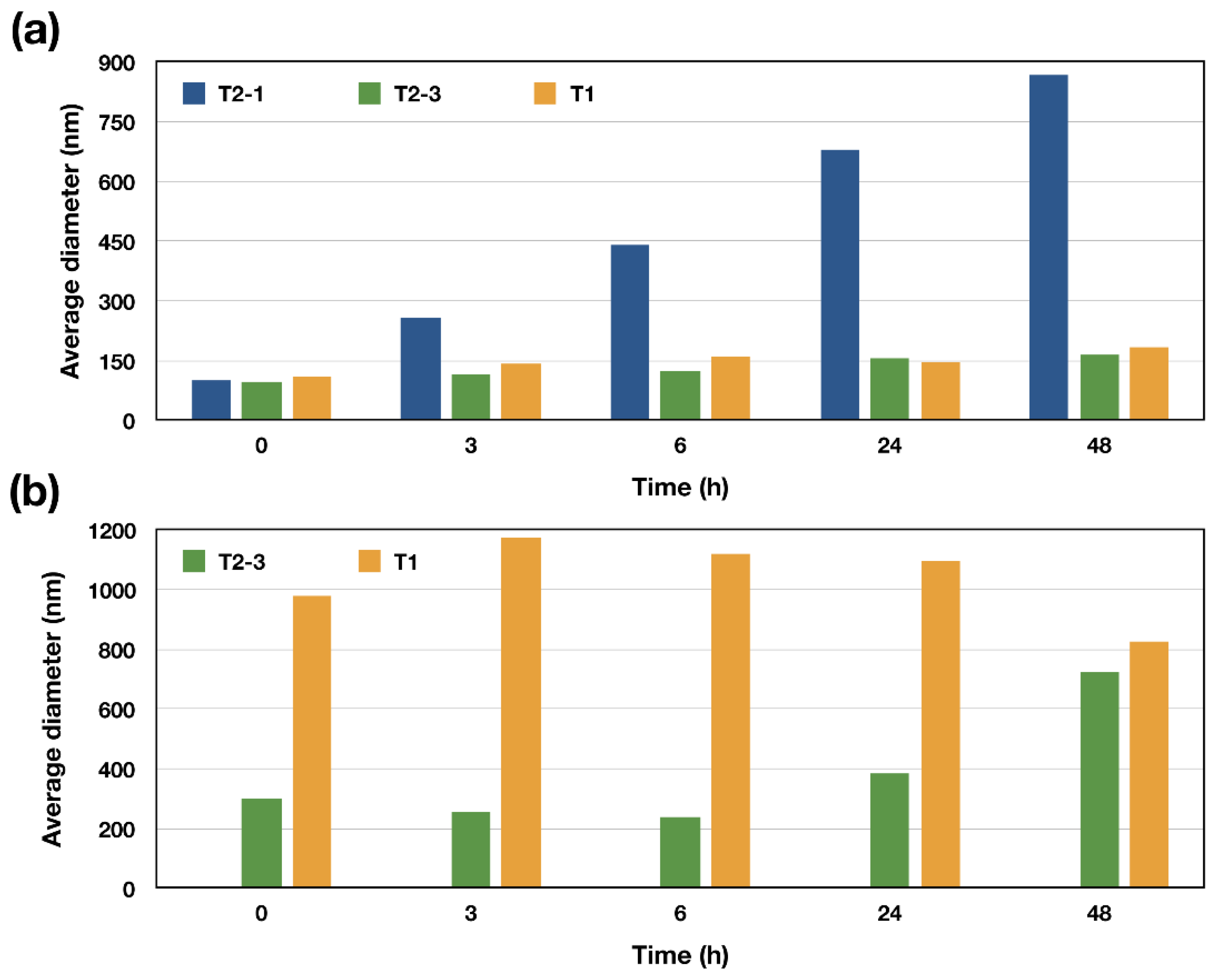
3.4. In Vitro Drug Release and Stability Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davit, B.; Conner, D.; Shargel, L. Drug product performance, in vivo: Bioavailability and bioequivalence. In Applied Biopharmaceutics & Pharmacokinetics, 7th ed.; Shargel, L., Yu, A., Eds.; McGraw-Hill Education: New York, NY, USA, 2016; Chapter 16; pp. 469–528. [Google Scholar]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hurst, S.; Loi, C.-M.; Brodfuehrer, J.; El-Kattan, A. Impact of physiological, Physicochemical and biopharmaceutical factors in absorption and metabolism mechanisms on the drug oral bioavailability of rats and humans. Expert Opin. Drug Metab. Toxicol. 2007, 3, 469–489. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.; Weyers, M.; Steenekamp, J.; Steyn, J.; Gouws, C.; Hamman, J. Drug bioavailability enhancing agents of natural origin (bioenhancers) that modulate drug membrane permeation and pre-systemic metabolism. Pharmaceutics 2019, 11, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Shrestha, N.; Préat, V.; Beloqui, A. Overcoming the intestinal barrier: A look into targeting approaches for improved oral drug delivery systems. J. Control. Release 2020, 322, 486–508. [Google Scholar] [CrossRef]
- Farokhzad, O.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Maeda, H.; Tsukigawa, K.; Fang, J. A retrospective 30 years after discovery of the enhanced permeability and retention effect of solid tumors: Next-generation chemotherapeutics and photodynamic therapy-problems, solutions, and prospects. Microcirculation 2016, 23, 173–182. [Google Scholar] [CrossRef]
- Allen, T.; Culli, P. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Mitchell, M.; Billingsley, M.; Haley, R.; Wechsler, M.; Peppas, N.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Majumder, N.; Das, N.; Das, S. Polymeric micelles for anticancer drug delivery. Ther. Deliv. 2020, 11, 613–635. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L. Micellar drug delivery systems based on natural biopolymers. Polymers 2021, 13, 477. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Haponiuk, J.; Thomas, S.; Gopi, S. Biopolymer based nanomaterials in drug delivery systems: A review. Mater. Today Chem. 2018, 9, 43–55. [Google Scholar] [CrossRef]
- Palmiero, U.; Sponchioni, M.; Manfredini, N.; Maraldi, M.; Moscatelli, D. Strategies to combine ROP with ATRP or RAFT polymerization for the synthesis of biodegradable polymeric nanoparticles for biomedical applications. Polym. Chem. 2018, 9, 4084–4099. [Google Scholar] [CrossRef]
- Lallana, E.; Sousa-Herves, A.; Fernandez-Trillo, F.; Riguera, R.; Fernandez-Megia, E. Click chemistry for drug delivery nanosystems. Pharm. Res. 2012, 29, 1–34. [Google Scholar] [CrossRef]
- Kakkar, A.; Traverso, G.; Farokhzad, O.; Weissleder, R.; Langer, R. Evolution of macromolecular complexity in drug delivery systems. Nat. Rev. Chem. 2017, 1, 0063. [Google Scholar] [CrossRef]
- Gothwal, A.; Khan, I.; Gupta, U. Polymeric micelles: Recent advancements in the delivery of anticancer drugs. Pharm. Res. 2016, 33, 18–39. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Shukla, H.; Kalia, K.; Torchilin, V.; Tekade, R. Multifunctional polymeric micellar nanomedicine in the diagnosis and treatment of cancer. Mater. Sci. Eng. C 2021, 126, 112186. [Google Scholar] [CrossRef]
- Owens, D., III; Peppas, N. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Rahme, K.; Dagher, N. Chemistry routes for copolymer synthesis containing PEG for targeting, imaging, and drug delivery purposes. Pharmaceutics 2019, 11, 327. [Google Scholar] [CrossRef] [Green Version]
- Hama, S.; Itakura, S.; Nakai, M.; Nakayama, K.; Morimoto, S.; Suzuki, S.; Kogure, K. Overcoming the polyethylene glycol dilemma via pathological environment-sensitive change of the surface property of nanoparticles for cellular entry. J. Control. Release 2015, 206, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Appold, M.; Mari, C.; Lederle, C.; Elbert, J.; Schmidt, C.; Ott, I.; Stühn, B.; Gasser, G.; Gallei, M. Multi-stimuli responsive block copolymers as a smart release platform for a polypyridyl ruthenium complex. Polym. Chem. 2017, 8, 890–900. [Google Scholar] [CrossRef] [Green Version]
- Luss, A.; Kulikov, P.; Romme, S.; Andersen, C.; Pennisi, C.; Docea, A.; Kuskov, A.; Velonia, K.; Mezhuev, Y.; Shtilman, M.; et al. Nanosized carriers based on amphiphilic poly-N-vinyl-2-pyrrolidone for intranuclear drug delivery. Nanomedicine 2018, 13, 703–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Li, Y.; Fu, Y.; Hou, Y.; Chen, Y.; Luo, X. The synthesis and co-micellization of PCL-P(HEMA/HEMA-LA) and PCL-P(HEMA/HEMA-FA) as shell cross-linked drug carriers with target/redox properties. J. Biomater. Sci. Polym. Ed. 2019, 30, 276–294. [Google Scholar] [CrossRef]
- Lorson, T.; Lübtow, M.; Wegener, E.; Haider, M.; Borova, S.; Nahm, D.; Jordan, R.; Sokolski-Papkov, M.; Kabanov, A.; Luxenhofer, R. Poly(2-oxazoline)s based biomaterials: A comprehensive and critical update. Biomaterials 2018, 178, 204–280. [Google Scholar] [CrossRef]
- Li, K.; Zang, X.; Cheng, M.; Chen, X. Stimuli-responsive nanoparticles based on poly acrylic derivatives for tumor therapy. Int. J. Pharm. 2021, 601, 120506. [Google Scholar] [CrossRef]
- Bilensoy, E. Cationic nanoparticles for cancer therapy. Expert Opin. Drug Deliv. 2010, 7, 795–809. [Google Scholar] [CrossRef]
- Pan, Z.; He, X.; Song, N.; Fang, D.; Li, Z.; Li, J.; Luo, F.; Li, J.; Tan, H.; Fu, Q. Albumin modified cationic nanocarriers to potentially create a new platform for drug delivery systems. ACS Appl. Mater. Interfaces 2019, 11, 16421–16429. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.; Chin, D.; Wagner, A.; Rimbach, G. Curcumin—From molecule to biological function. Angew. Chem. Int. Ed. 2012, 51, 5308–5332. [Google Scholar] [CrossRef]
- Stohs, S.; Chen, O.; Ray, S.; Ji, J.; Bucci, L.; Preuss, H. Highly bioavailable forms of curcumin and promising avenues for curcumin-based research and application: A review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef] [Green Version]
- Marquardt, J.; Gomez-Quiroz, L.; Camacho, L.; Pinna, F.; Lee, Y.-H.; Kitade, M.; Domínguez, M.; Castven, D.; Breuhahn, K.; Conner, E.; et al. Curcumin effectively inhibits oncogenic NF-kB signaling and restrains stemness features in liver cancer. J. Hepatol. 2015, 63, 661–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iurciuc-Tincu, C.-E.; Atanase, L.; Ochiuz, L.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M. Curcumin-loaded polysaccharides-based complex particles obtained by polyelectrolyte complexation and ionic gelation. I-Particles obtaining and characterization. Int. J. Biol. Macromol. 2020, 147, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Long, X.; Du, J.; Teng, M.; Zhang, W.; Wang, Y.; Wang, X.; Wang, Z.; Zhang, P.; Li, J. Enhanced curcumin solubility and antibacterial activity by encapsulation in PLGA oily core nanocapsules. Food Funct. 2020, 11, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Pietkiewicz, J.; Wilk, K.; Bazylinska, U. In vitro studies of serum albumin interaction with poly(D,L-lactide)nanospheres loaded by hydrophobic cargo. J. Pharm. Biomed. Anal. 2016, 117, 426–435. [Google Scholar] [CrossRef]
- Letchford, K.; Liggins, R.; Burt, H. Solubilization of hydrophobic drugs by methoxy poly(ethylene glycol)-block-polycaprolactone diblock copolymer micelles: Theoretical and experimental data and correlations. J. Pharm. Sci. 2008, 97, 1179–1190. [Google Scholar] [CrossRef]
- Kumari, P.; Swami, M.; Nadipalli, S.; Myneni, S.; Ghosh, B.; Biswas, S. Curcumin delivery by poly(lactide)-based co-polymeric micelles: An in vitro anticancer study. Pharm. Res. 2016, 33, 826–841. [Google Scholar] [CrossRef]
- Chang, T.; Trench, D.; Putnam, J.; Stenzel, M.; Lord, M. Curcumin-loading-dependent stability of PEGMEMA-based micelles affects endocytosis and exocytosis in colon carcinoma cells. Mol. Pharm. 2016, 13, 924–932. [Google Scholar] [CrossRef]
- Vaidya, F.; Sharma, R.; Shaikh, S.; Ray, D.; Aswal, V.; Pathak, C. Pluronic micelles encapsulated curcumin manifests apoptotic cell death and inhibits pro-inflammatory cytokines in human breast adenocarcinoma cells. Cancer Rep. 2019, 2, e1133. [Google Scholar] [CrossRef]
- Bagheri, M.; Fens, M.; Kleijn, T.; Capomaccio, R.; Mehn, D.; Krawczyk, P.; Scutigliani, E.; Gurinov, A.; Baldus, M.; van Kronenburg, N.; et al. In vitro and in vivo studies on HPMA-based polymeric micelles loaded with curcumin. Mol. Pharm. 2021, 18, 1247–1263. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, W.; Gao, M.; Yang, Y. Development of curcumin-loaded methoxy poly(ethylene glycol)-block-poly(caprolactone)-block-poly(1,4,8-Trioxa [4.6] spiro-9-undecanone) nanoparticles and studies on their in vitro anti-tumor activities. Colloids Surf. B Biointerfaces 2019, 184, 110525. [Google Scholar] [CrossRef]
- Kulkarni, B.; Qutub, S.; Ladelta, V.; Khashab, N.; Hadjichristidis, N. AIE-Based fluorescent triblock copolymer micelles for simultaneous drug delivery and intracellular imaging. Biomacromolecules 2021, 22, 5243–5255. [Google Scholar] [CrossRef] [PubMed]
- Yoncheva, K.; Kamenova, K.; Perperieva, T.; Hadjimitova, V.; Donchev, P.; Kaloyanov, K.; Konstantinov, S.; Kondeva- Burdina, M.; Tzankova, V.; Petrov, P. Cationic triblock copolymer micelles enhance antioxidant activity, intracellular uptake and cytotoxicity of curcumin. Int. J. Pharm. 2015, 490, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Grancharov, G.; Gancheva, V.; Kyulavska, M.; Momekova, D.; Momekov, G.; Petrov, P. Functional multilayered polymeric nanocarriers for delivery of mitochondrial targeted anticancer drug curcumin. Polymer 2016, 84, 27–37. [Google Scholar] [CrossRef]
- Babikova, D.; Kalinova, R.; Zhelezova, I.; Momekova, D.; Konstantinov, S.; Momekov, G.; Dimitrov, I. Functional block copolymer nanocarriers for anticancer drug delivery. RSC Adv. 2016, 6, 84634–84644. [Google Scholar] [CrossRef]
- Skandalis, A.; Selianitis, D.; Pispas, S. PnBA-b-PNIPAM-b-PDMAEA thermo-responsive triblock terpolymers and their quaternized analogs as gene and drug delivery vectors. Polymers 2021, 13, 2361. [Google Scholar] [CrossRef]
- Itzinger, R.; Schwarzinger, C.; Paulik, C. Investigation of the influence of impurities on the ring-opening polymerisation of L-Lactide from biogenous feedstock. J. Polym. Res. 2020, 27, 383. [Google Scholar] [CrossRef]
- Spasova, M.; Mespouille, L.; Coulembier, O.; Paneva, D.; Manolova, N.; Rashkov, I.; Dubois, P. Amphiphilic poly(D- or L-lactide)-b-poly(N,N-dimethylamino-2-ethyl methacrylate) block copolymers: Controlled synthesis, characterization, and stereocomplex formation. Biomacromolecules 2009, 10, 1217–1223. [Google Scholar] [CrossRef]
- Mao, J.; Ji, X.; Bo, S. Synthesis and pH/temperature-responsive behavior of PLLA-b-PDMAEMA block polyelectrolytes prepared via ROP and ATRP. Macromol. Chem. Phys. 2011, 212, 744–752. [Google Scholar] [CrossRef]
- Kim, B.; Jeong, J.; Mohanty, A.; Lee, T.; Han, S.; Heo, J.; Jung, K.-S.; Kim, J.; Paik, H. Quaternized poly(poly(ethylene glycol)methyl ether methacrylate)-b-poly(2-(dimethylamino)ethyl methacrylate) as block copolymers by sequential monomer addition: Dispersion of copper phthalocyanine. React. Funct. Polym. 2017, 120, 147–152. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.; Hatton, T. Micellization of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymers in aqueous solutions: Thermodynamics of copolymer association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Griffin, W. Classification of Surface-Active Agents by “HLB”. J. Soc. Cosmet. Chem. 1949, 1, 31–326. [Google Scholar]
- Wiradharma, N.; Zhang, Y.; Venkataraman, S.; Hedrick, J.; Yanga, Y. Self-assembled polymer nanostructures for delivery of anticancer therapeutics. Nano Today 2009, 4, 302–317. [Google Scholar] [CrossRef]
- Tang, L.; Yang, X.; Yin, Q.; Cai, K.; Wang, H.; Chaudhury, I.; Yao, C.; Zhou, Q.; Kwon, M.; Hartman, J.; et al. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. USA 2014, 111, 15344–15349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, D.; Pygall, S.; Cooper, V.; Mann, J. Overcoming sink limitations in dissolution testing: A review of traditional methods and the potential utility of biphasic systems. J. Pharm. Pharmacol. 2012, 64, 1549–1559. [Google Scholar] [CrossRef]
- Abouelmagd, S.; Sun, B.; Chang, A.; Ku, Y.; Yeo, Y. Release kinetics study of poorly water-soluble drugs from nanoparticles: Are we doing it right? Mol. Pharm. 2015, 12, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Lo, C.-L.; Chou, M.-H.; Lu, P.-L.; Lo, I.; Chiang, Y.-T.; Hung, S.-Y.; Yang, C.-Y.; Lin, S.-Y.; Wey, S.-P.; Lo, J.-M.; et al. The effect of PEG-5K grafting level and particle size on tumor accumulation and cellular uptake. Int. J. Pharm. 2013, 456, 424–431. [Google Scholar] [CrossRef]

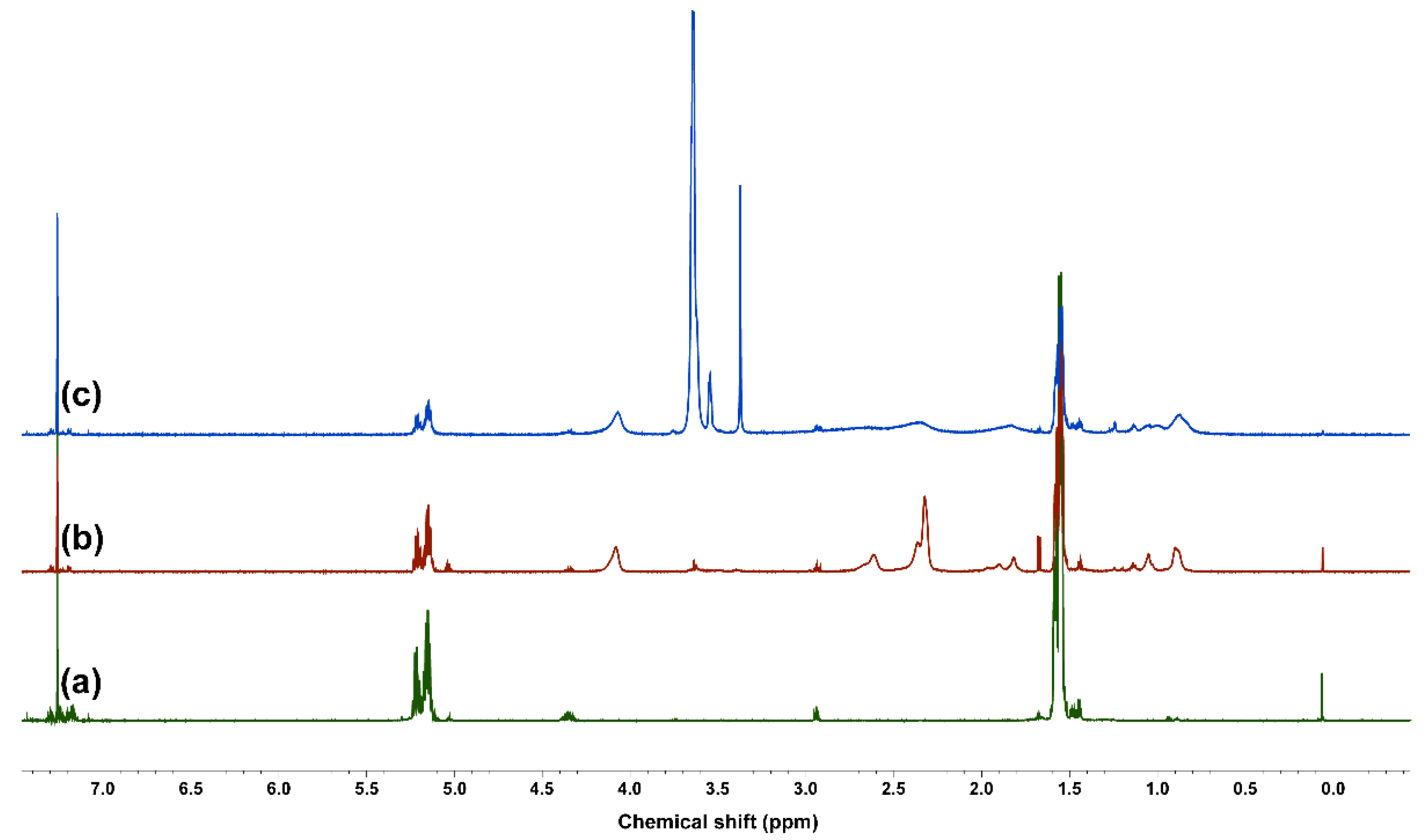
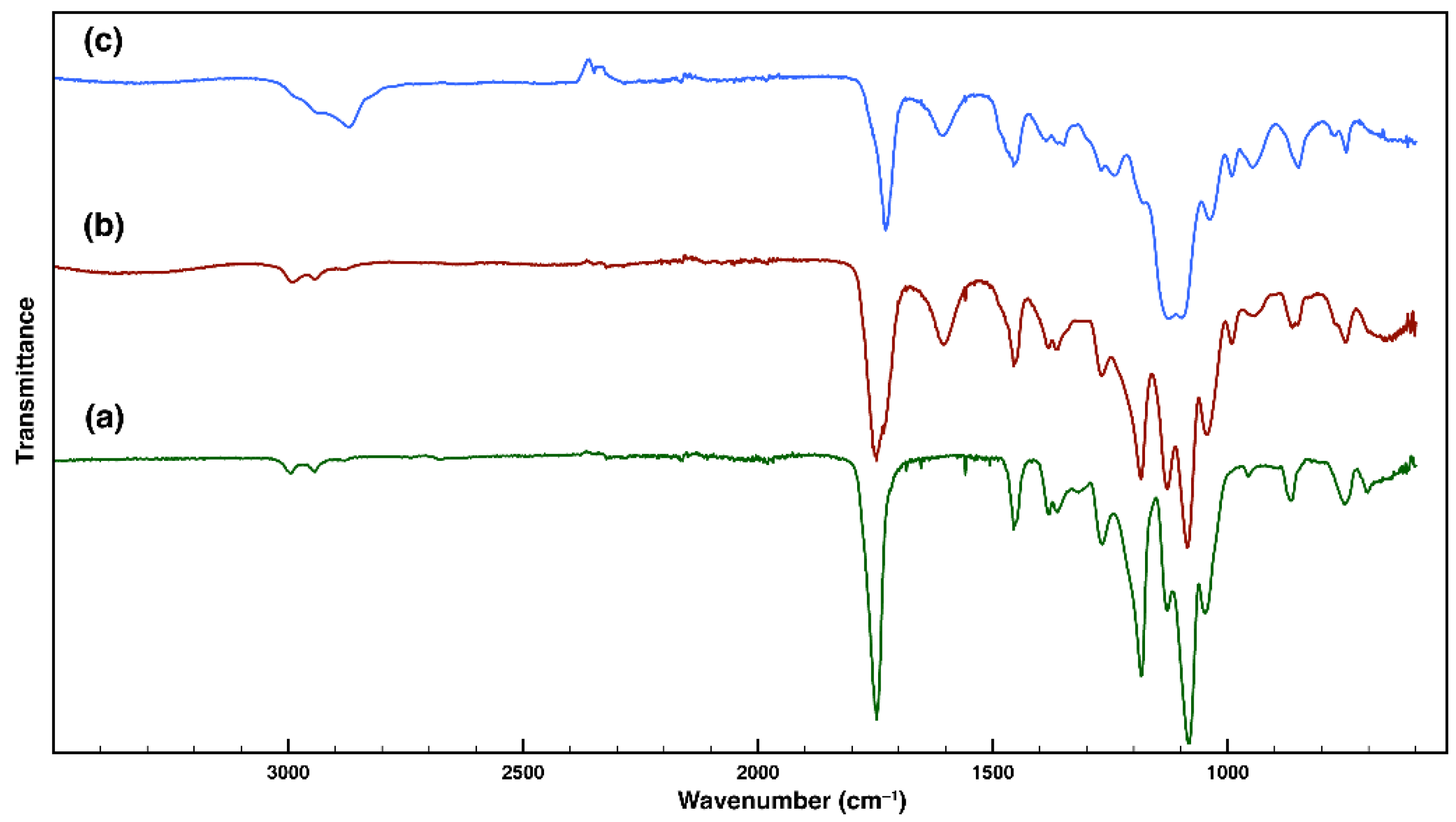
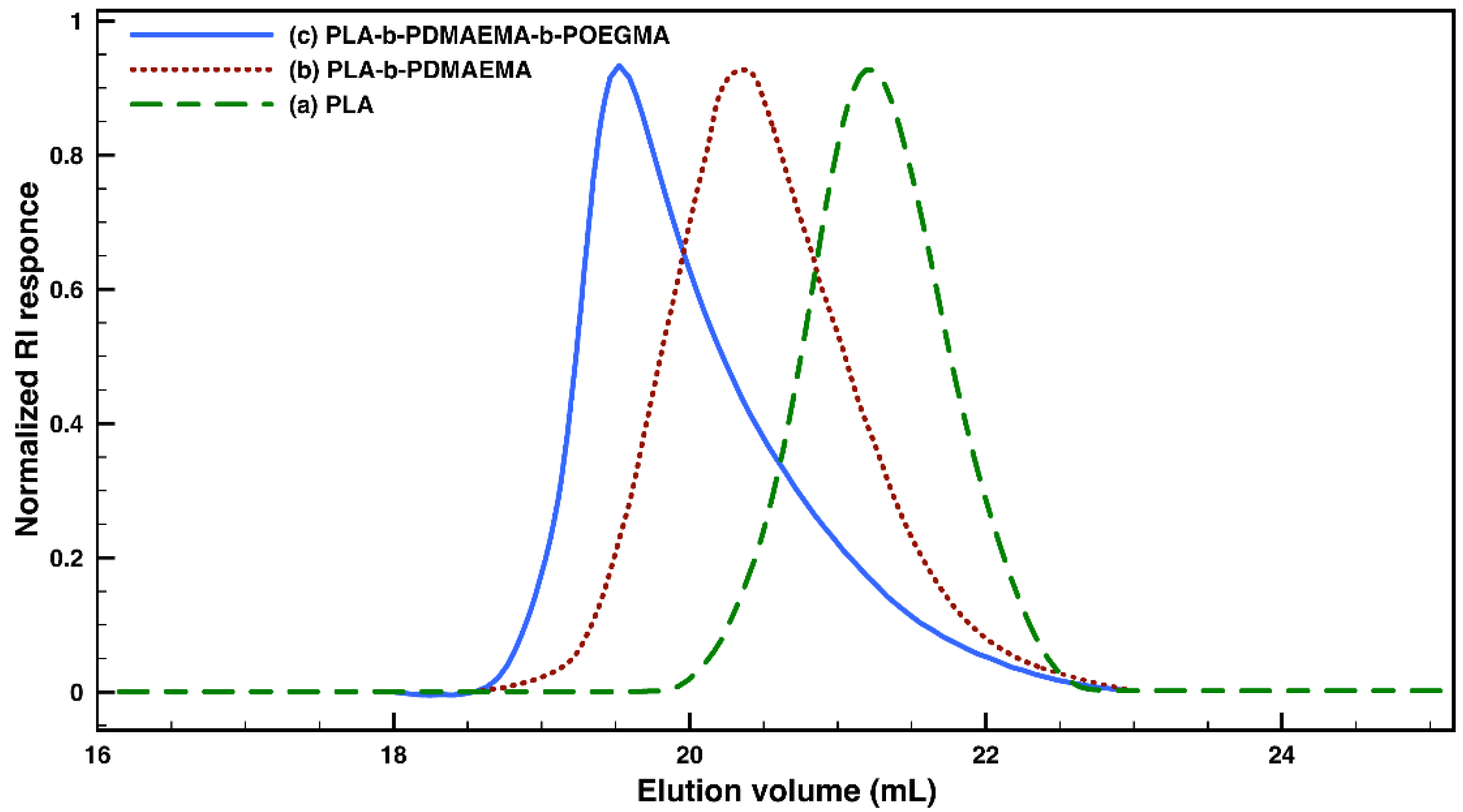
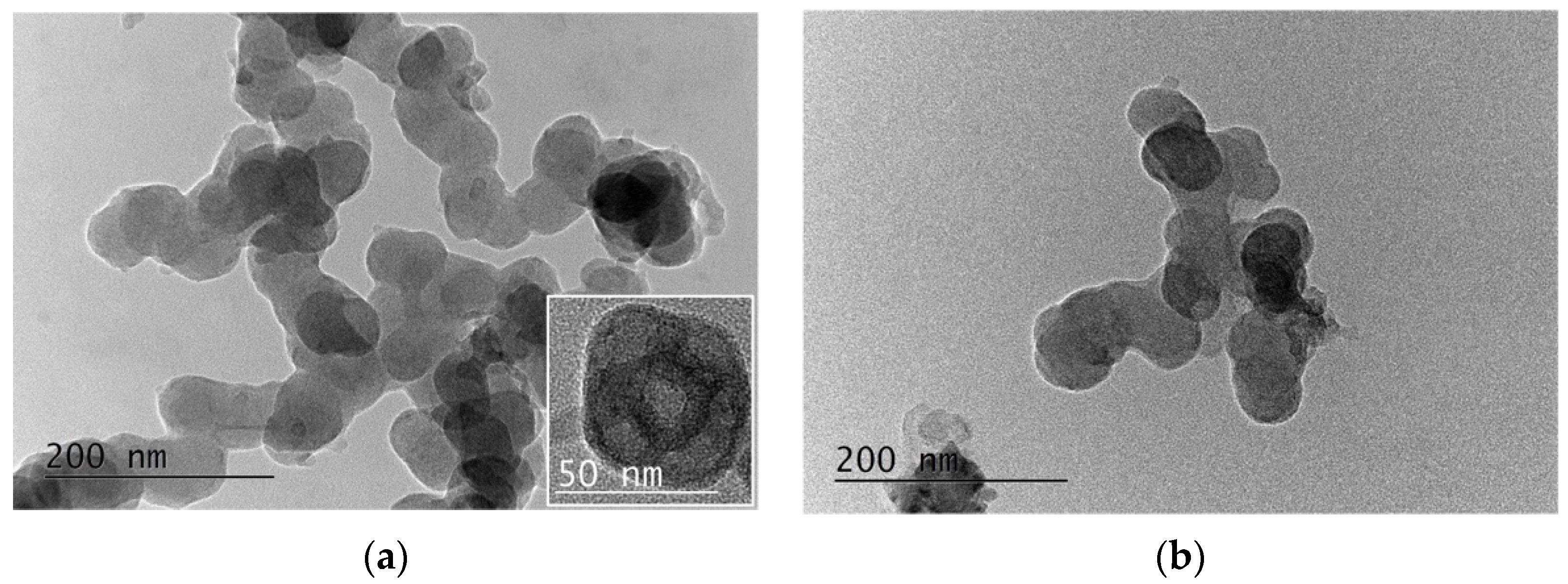

| Macroinitiators (PLA-Br) | Diblock Copolymers (PLA-b-PDMAEMA) | Triblock Copolymers (PLA-b-PDMAEMA-b-POEGMA) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | DPna (LA) | Mna (g mol−1) | ÐMb | Code | DPna (DMAEMA) | Mna (g mol−1) | ÐMb | Code | DPna (OEGMA) | Mna (g mol−1) | ÐMb |
| M1 | 34 | 5000 | 1.08 | D1 | 20 | 8150 | 1.27 | T1 | 2 | 9150 | 1.32 |
| M2 | 22 | 3300 | 1.15 | D2 | 16 | 5800 | 1.22 | T2-1 | 2 | 6800 | 1.28 |
| T2-2 | 6 | 8800 | 1.33 | ||||||||
| T2-3 | 12 | 11,800 | 1.38 | ||||||||
| Empty Micelles | Drug Loaded Micelles | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Code | HLB a | CMC b (mg mL−1) | d c (nm) | PdI c | ζ c (mV) | d c (nm) | PdI c | DLE d (%) | DLC d (%) |
| T1 | 9.07 | 0.069 | 72.26 ± 0.21 | 0.158 | 21.49 ± 4.17 | 74.14 ± 0.21 | 0.135 | 75 | 7.3 |
| T2-1 | 10.29 | 0.088 | 65.42 ± 1.01 | 0.198 | 22.11 ± 3.60 | 82.88 ± 1.14 | 0.170 | 60 | 5.9 |
| T2-2 | 12.50 | 0.129 | 66.83 ± 0.22 | 0.193 | 8.25 ± 1.25 | 71.46 ± 0.70 | 0.156 | 67 | 6.5 |
| T2-3 | 14.41 | 0.243 | 71.73 ± 0.29 | 0.200 | 0.98 ± 0.34 | 73.94 ± 0.63 | 0.217 | 63 | 6.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinova, R.; Dimitrov, I. Triblock Copolymer Micelles with Tunable Surface Charge as Drug Nanocarriers: Synthesis and Physico-Chemical Characterization. Nanomaterials 2022, 12, 434. https://doi.org/10.3390/nano12030434
Kalinova R, Dimitrov I. Triblock Copolymer Micelles with Tunable Surface Charge as Drug Nanocarriers: Synthesis and Physico-Chemical Characterization. Nanomaterials. 2022; 12(3):434. https://doi.org/10.3390/nano12030434
Chicago/Turabian StyleKalinova, Radostina, and Ivaylo Dimitrov. 2022. "Triblock Copolymer Micelles with Tunable Surface Charge as Drug Nanocarriers: Synthesis and Physico-Chemical Characterization" Nanomaterials 12, no. 3: 434. https://doi.org/10.3390/nano12030434
APA StyleKalinova, R., & Dimitrov, I. (2022). Triblock Copolymer Micelles with Tunable Surface Charge as Drug Nanocarriers: Synthesis and Physico-Chemical Characterization. Nanomaterials, 12(3), 434. https://doi.org/10.3390/nano12030434






