Adsorption of Carbon Dioxide with Ni-MOF-74 and MWCNT Incorporated Poly Acrylonitrile Nanofibers
Abstract
:1. Introduction
2. Experimental Section
2.1. Material
2.2. MOF Synthesis
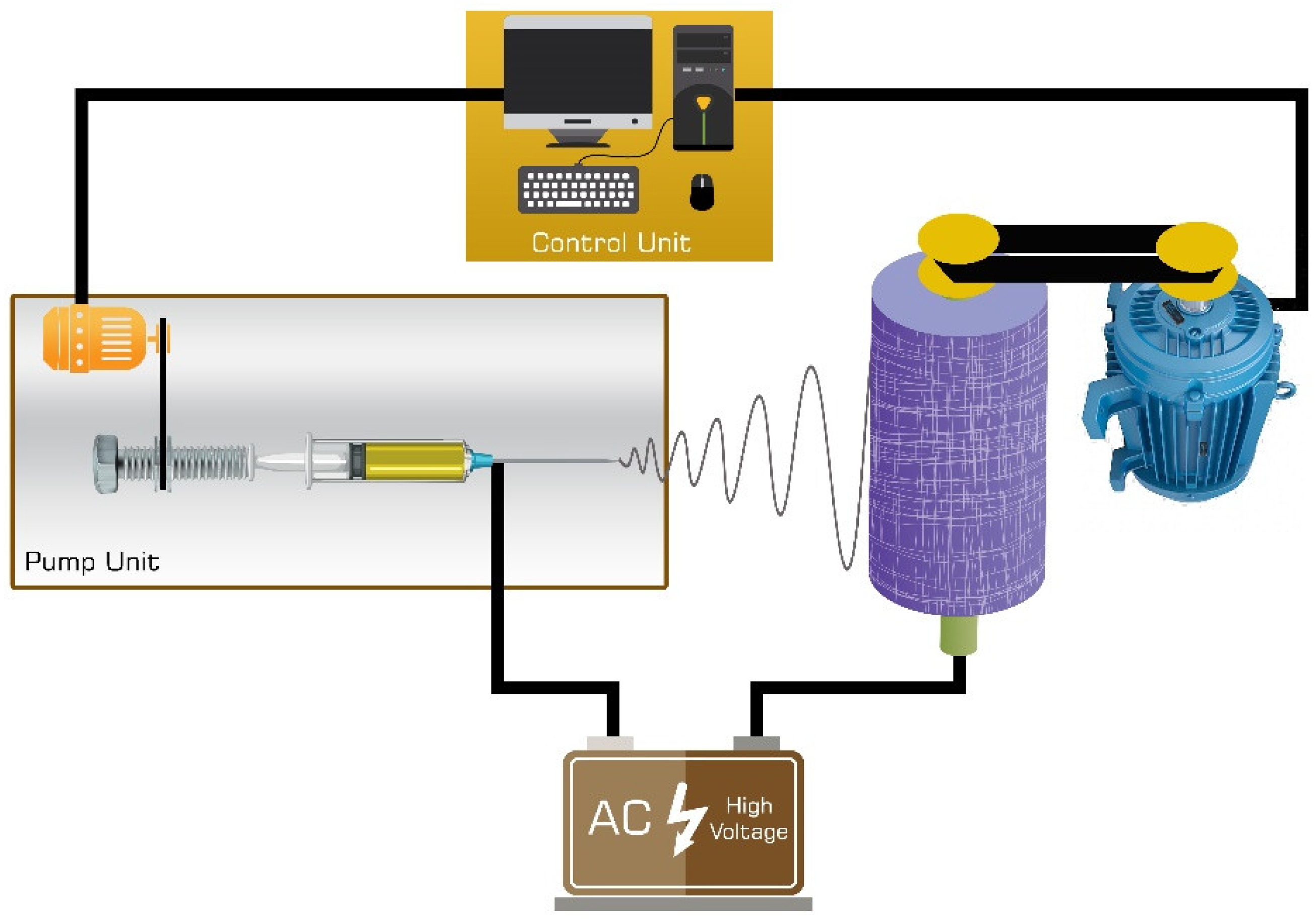
2.3. Electrospinning
2.4. Design of Experiment/Response Surface Methodology (RSM)
2.5. Secondary Growth of Ni-MOF-74 on the Nanofiber
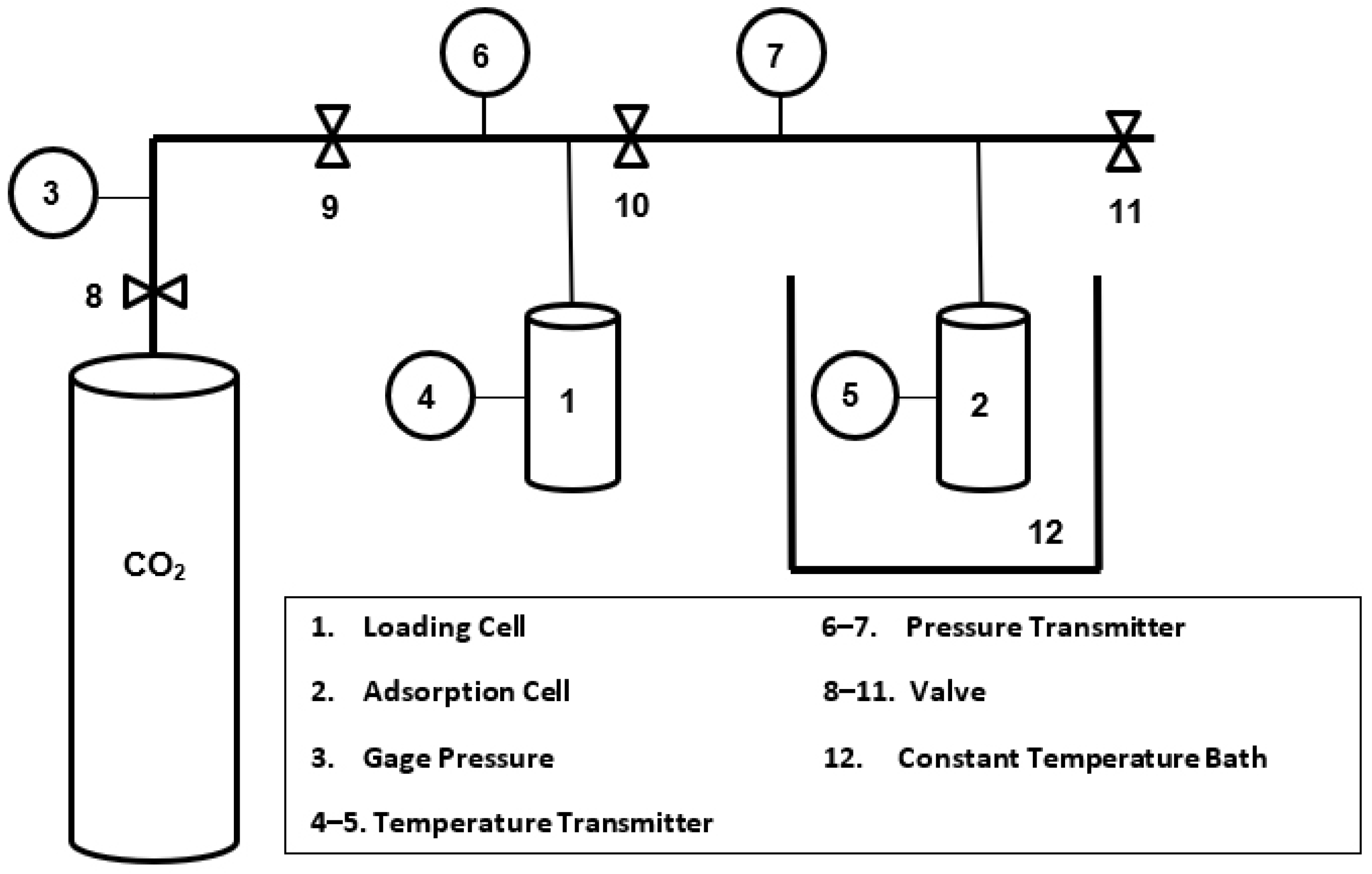
2.6. CO2 Adsorption Measurements
2.7. Characterizations
3. Results and Discussion
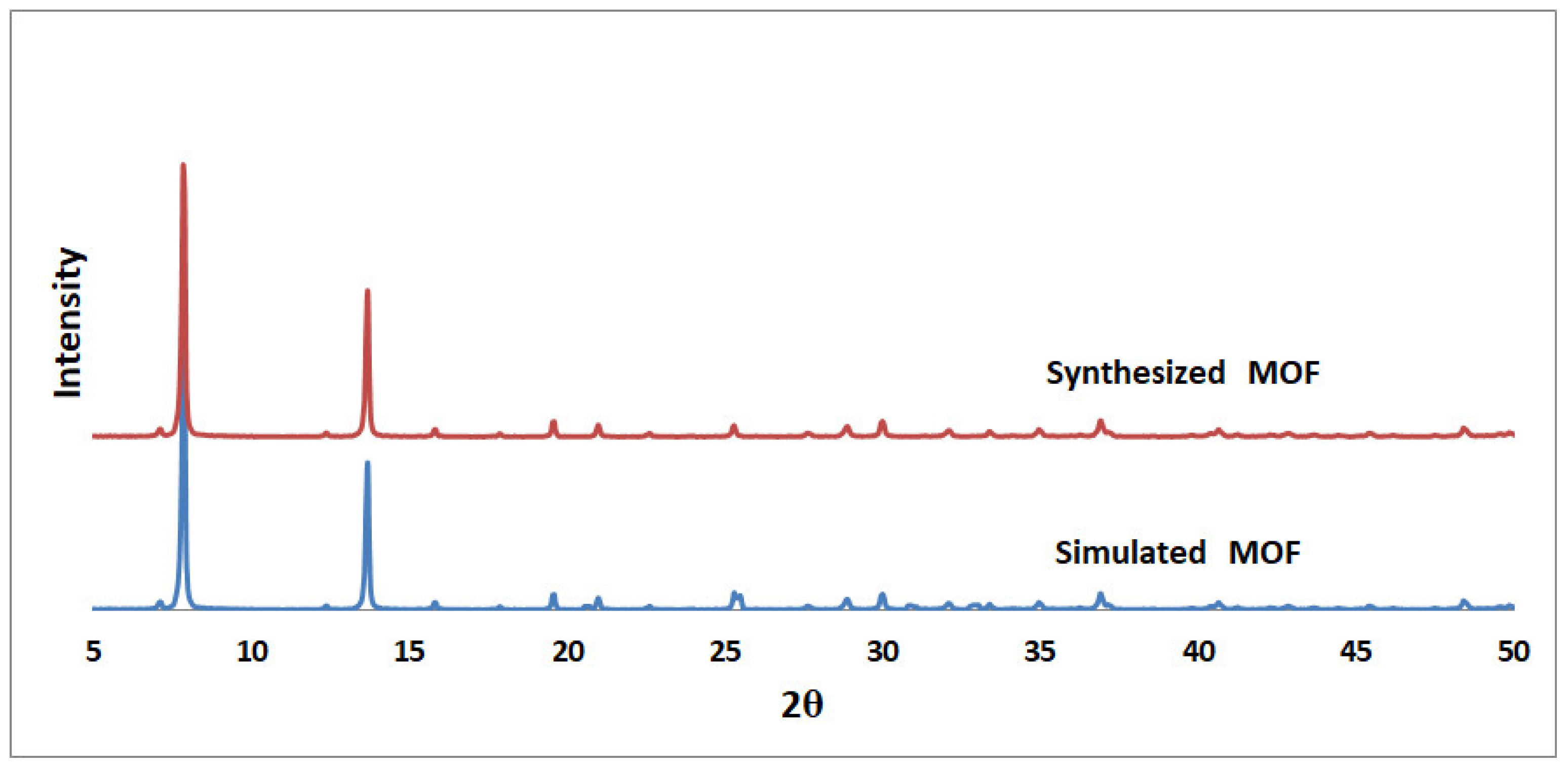
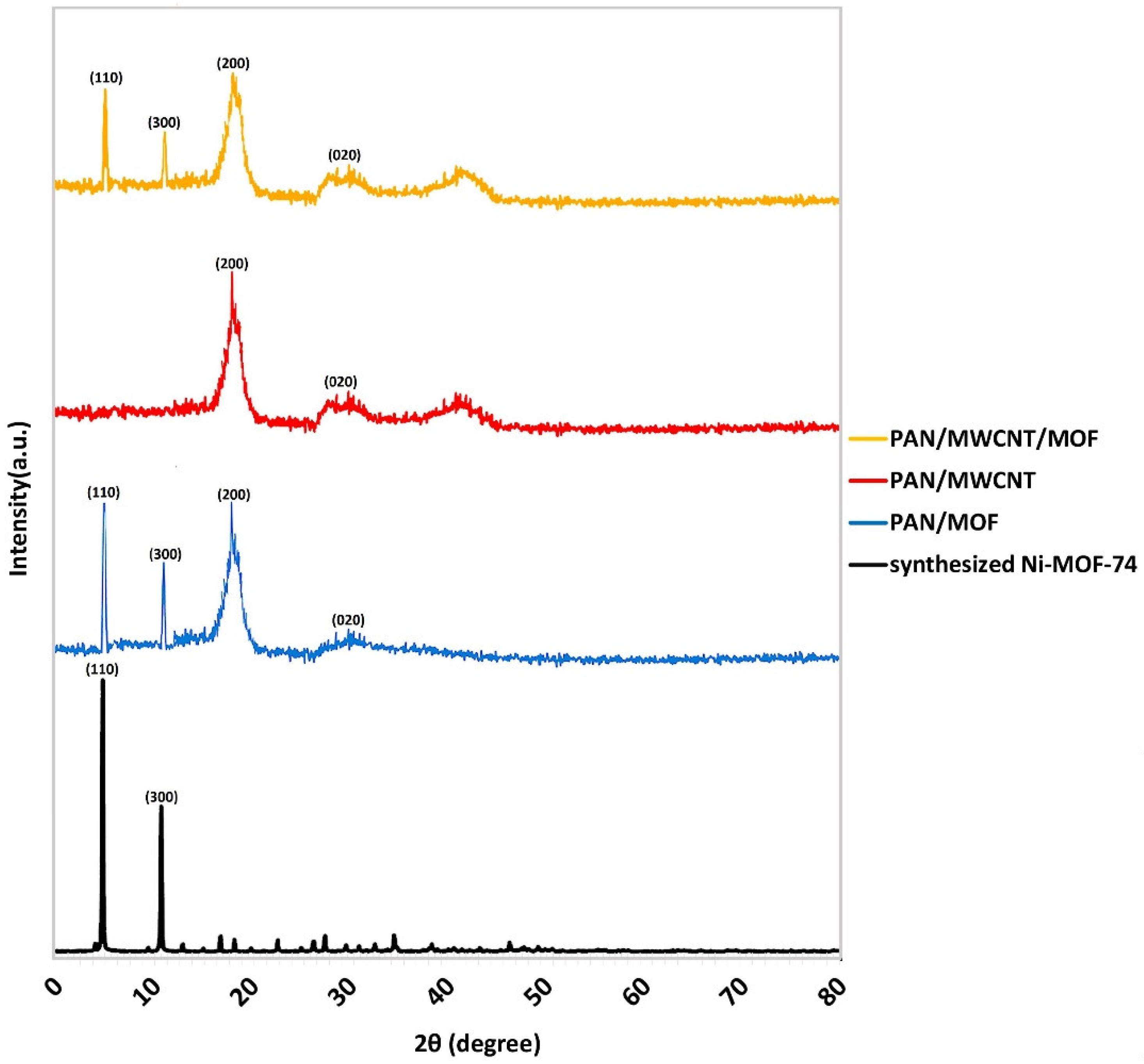
3.1. MOF Synthesis
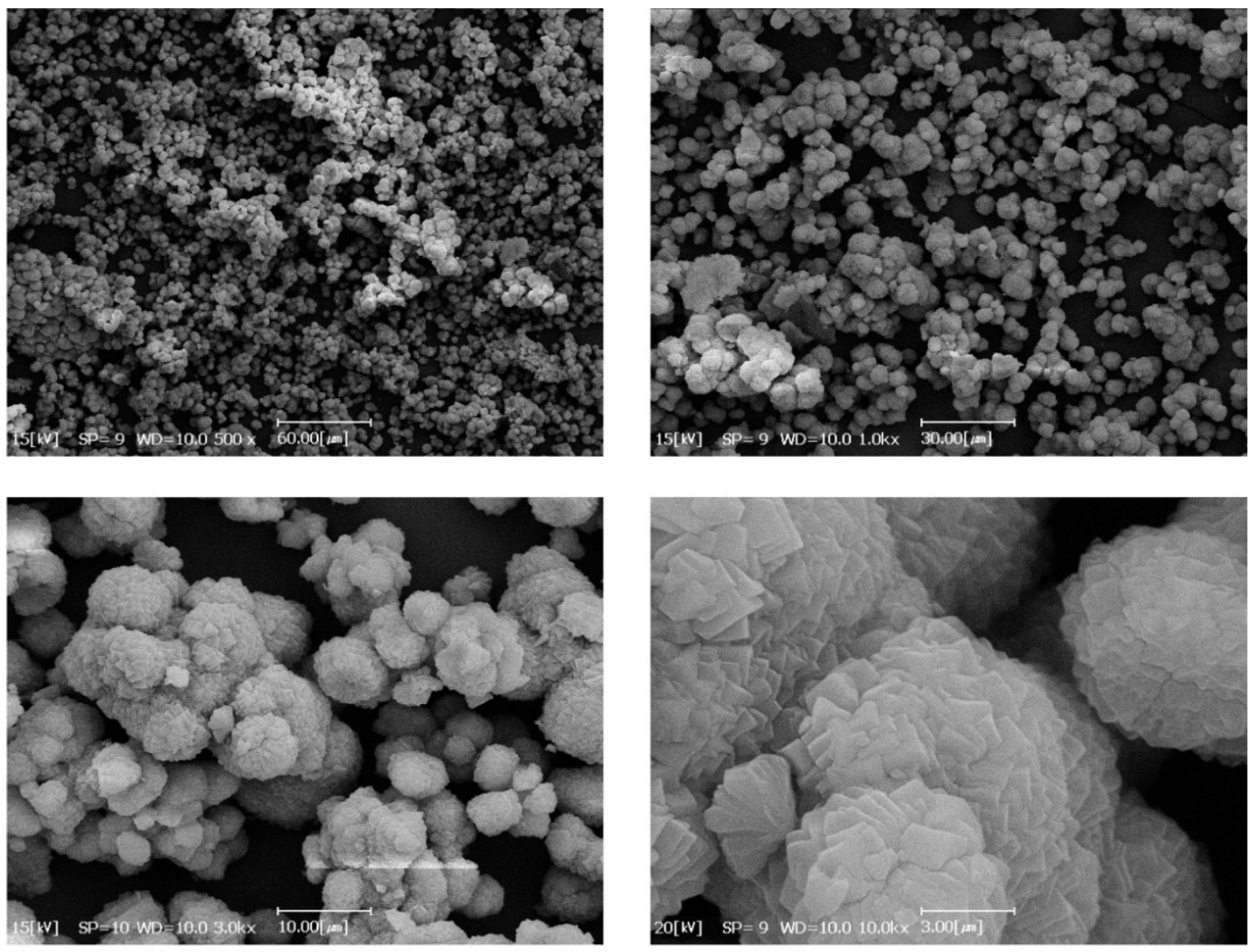
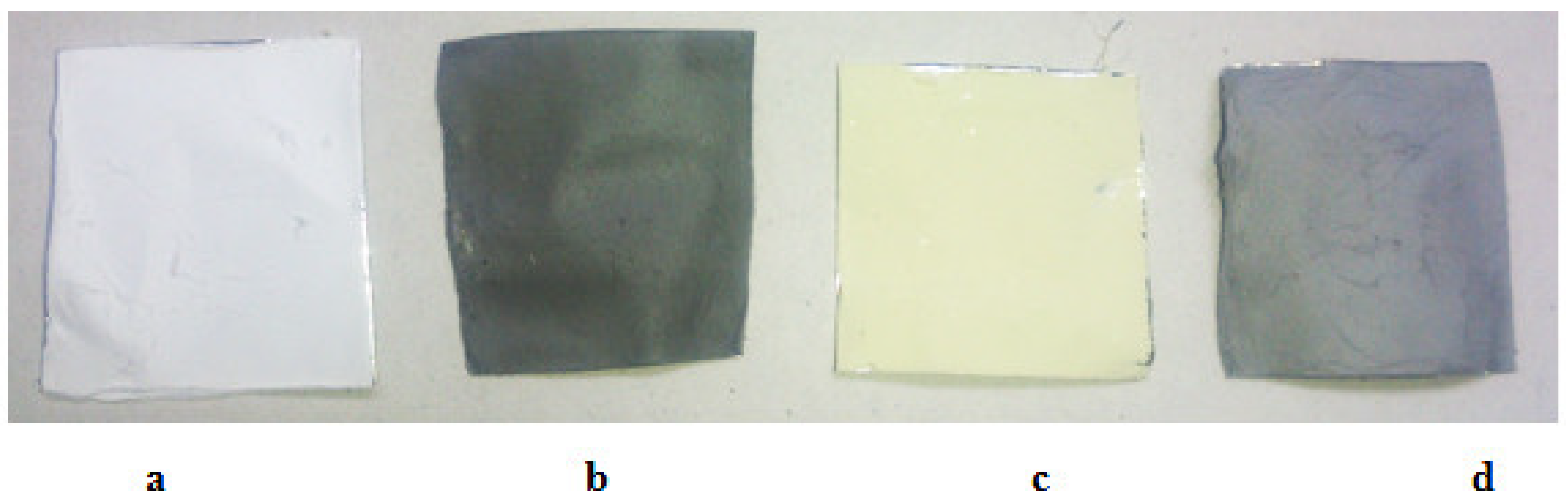
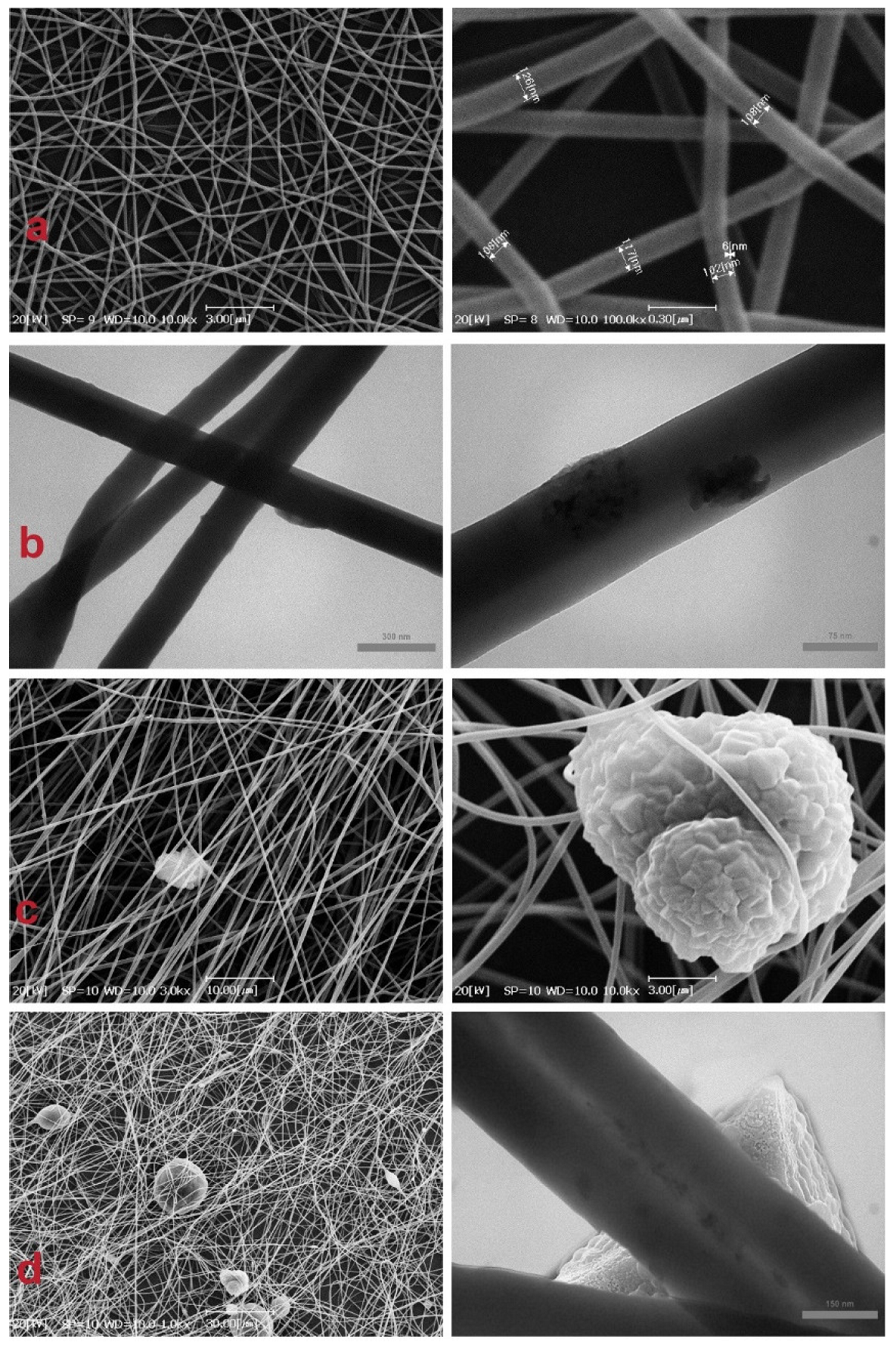
3.2. Nanofiber Characterizations
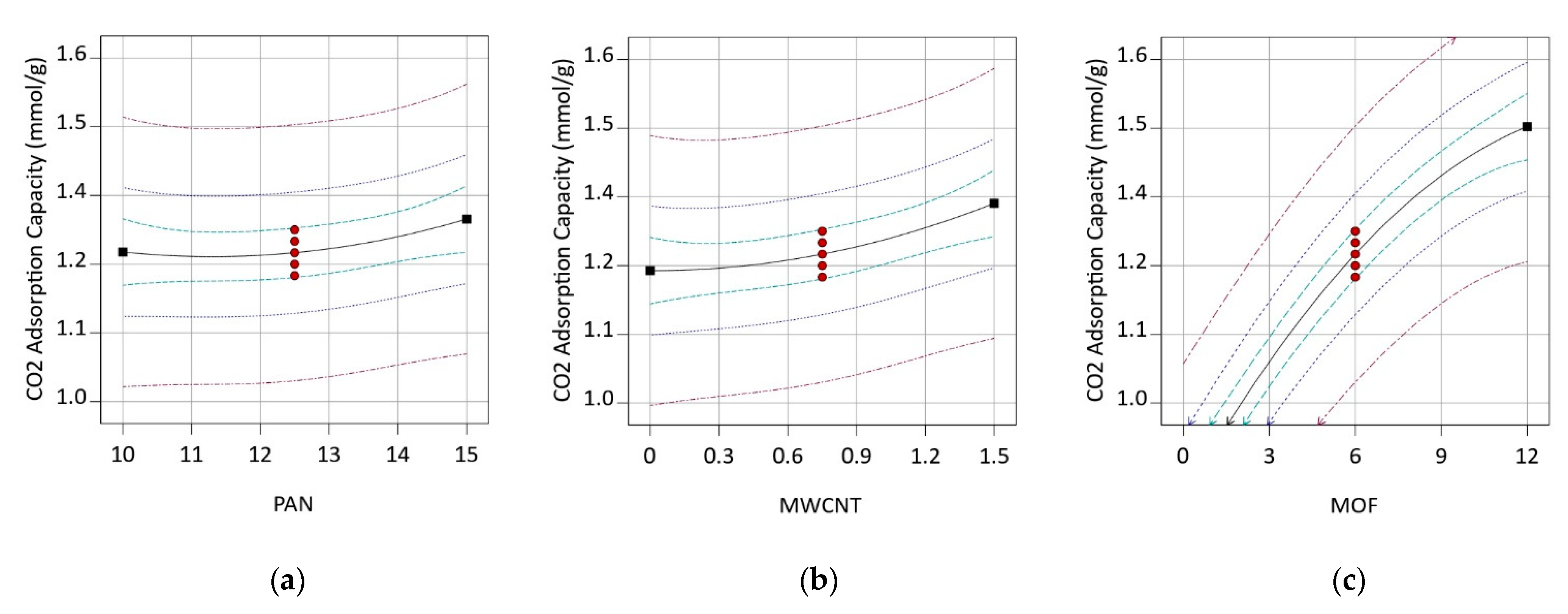
3.3. Box–Behnken Design
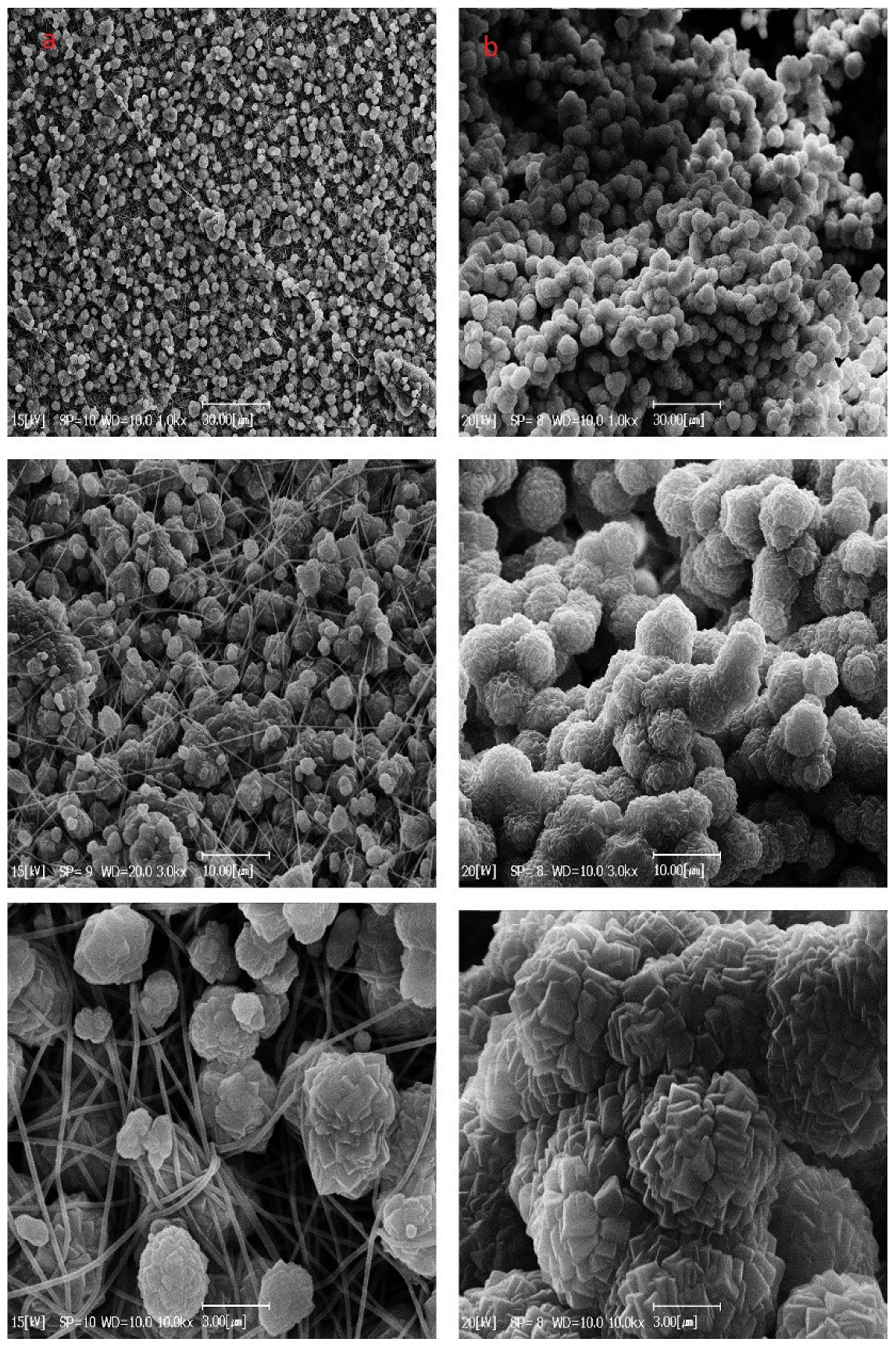
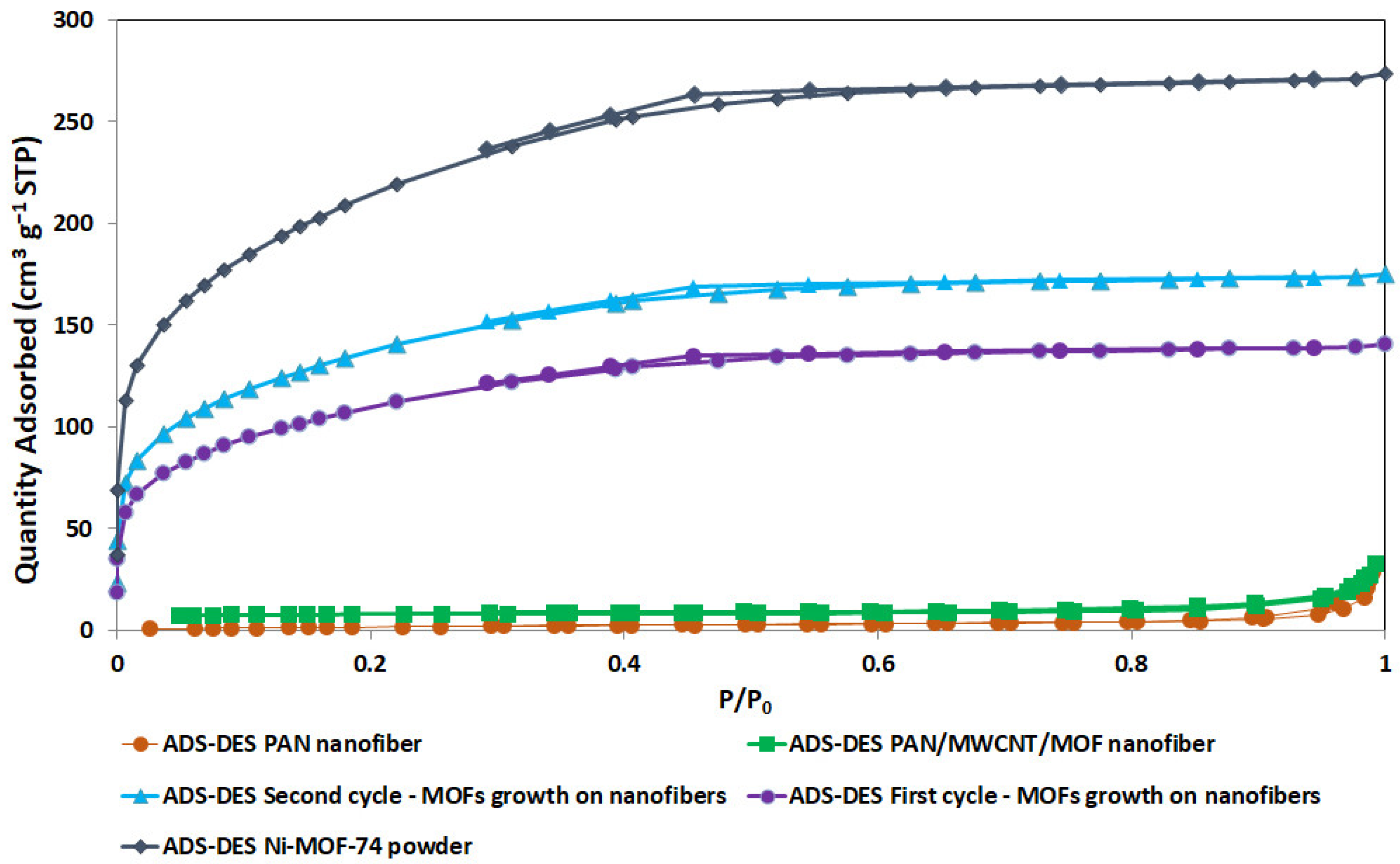
3.4. Characterization of MOF Secondary Growth on the PAN/MWCNT Nanofiber
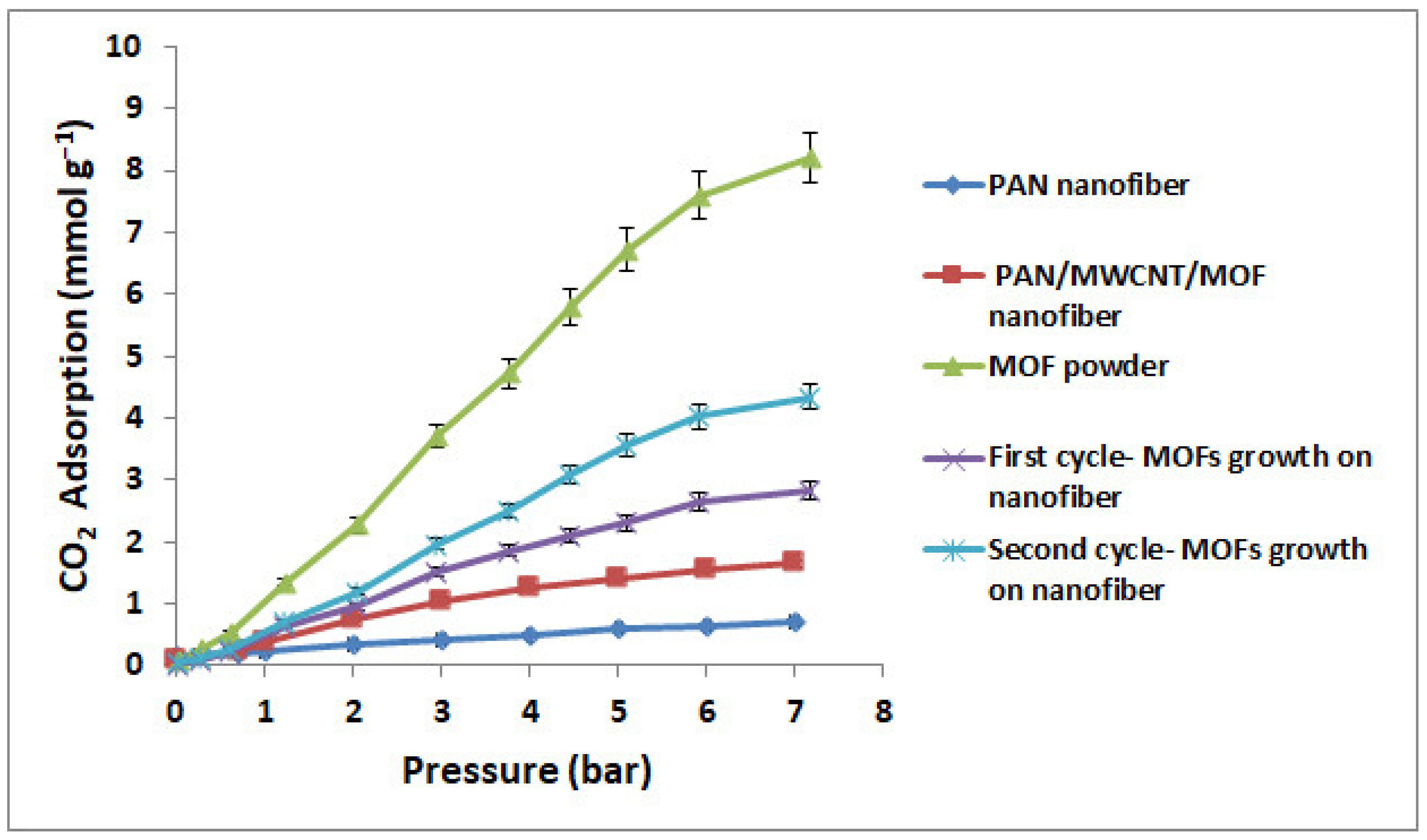
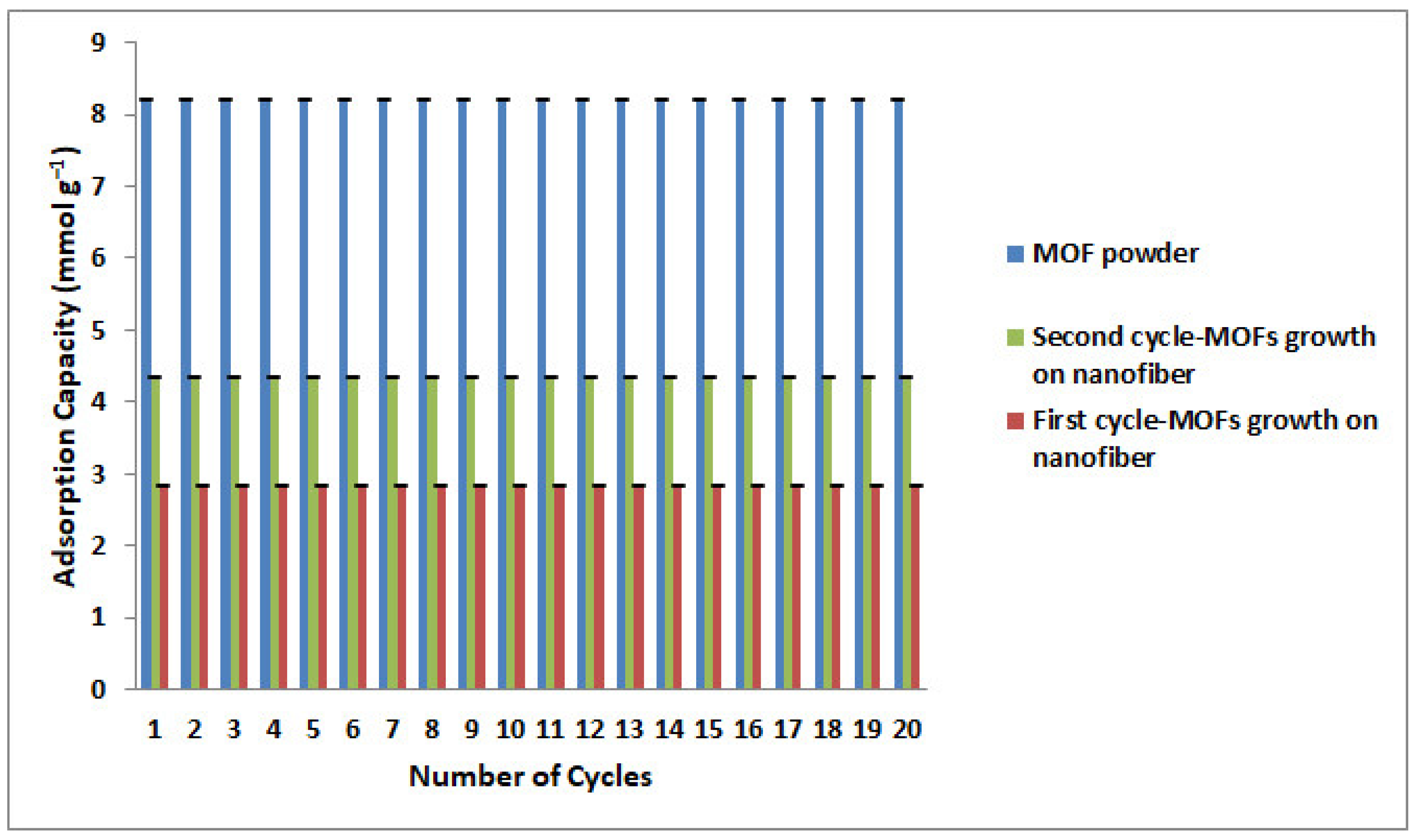
3.5. CO2 Adsorption Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K.; Pastré, J. Metal–organic frameworks—Prospective industrial applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Walton, K.S.; Millward, A.R.; Dubbeldam, D.; Frost, H.; Low, J.J.; Yaghi, O.M.; Snurr, R.Q. Understanding Inflections and Steps in Carbon Dioxide Adsorption Isotherms in Metal-Organic Frameworks. J. Am. Chem. Soc. 2008, 130, 406–407. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Webley, P. Optimum structured adsorbents for gas separation processes. Chem. Eng. Sci. 2009, 64, 5182–5191. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, F.; Webley, P. Structured adsorbents in gas separation processes. Sep. Purif. Technol. 2010, 70, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Amid, H.; Mazé, B.; Flickinger, M.C.; Pourdeyhimi, B. Hybrid adsorbent nonwoven structures: A review of current technologies. J. Mater. Sci. 2016, 51, 4173–4200. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, J.; Xue, Z.; Wang, X. Electrospun graphene oxide/carbon composite nanofibers with well-developed mesoporous structure and their adsorption performance for benzene and butanone. Chem. Eng. J. 2016, 306, 99–106. [Google Scholar] [CrossRef]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; CRC Press: Boca Raton, FL, USA, 2005; ISBN 9780429114182. [Google Scholar]
- Čejka, J. Metal-Organic Frameworks. Applications from Catalysis to Gas Storage. Edited by David Farrusseng. Angew. Chem. Int. Ed. 2012, 51, 4782–4783. [Google Scholar] [CrossRef]
- Reitz, W. A Review of: “Nonwoven Fabrics”. Mater. Manuf. Process. 2006, 21, 427. [Google Scholar] [CrossRef]
- Rezaei, F.; Lawson, S.; Hosseini, H.; Thakkar, H.; Hajari, A.; Monjezi, S.; Rownaghi, A.A. MOF-74 and UTSA-16 film growth on monolithic structures and their CO2 adsorption performance. Chem. Eng. J. 2017, 313, 1346–1353. [Google Scholar] [CrossRef]
- Lin, R.; Ge, L.; Liu, S.; Rudolph, V.; Zhu, Z. Mixed-matrix membranes with metal-organic framework-decorated CNT fillers for efficient CO2 separation. ACS Appl. Mater. Interfaces 2015, 7, 14750–14757. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel. Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Wang, X.; Li, B. Electrospun Nanofibers for Energy and Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2014; pp. 249–263. [Google Scholar]
- Ali, N.; Babar, A.A.; Zhang, Y.; Iqbal, N.; Wang, X.; Yu, J.; Ding, B. Porous, flexible, and core-shell structured carbon nanofibers hybridized by tin oxide nanoparticles for efficient carbon dioxide capture. J. Colloid Interface Sci. 2019, 560, 379–387. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Yu, J.; Wang, M. Engineering biomimetic superhydrophobic surfaces of electrospun nanomaterials. Nano Today 2011, 6, 510–530. [Google Scholar] [CrossRef]
- Ding, B.; Wang, M.; Wang, X.; Yu, J.; Sun, G. Electrospun nanomaterials for ultrasensitive sensors. Mater. Today 2010, 13, 16–27. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, Z.; Wang, Y.; Shang, X.; Nie, P.; Liu, J. Electrospun polyacrylonitrile/β-cyclodextrin based porous carbon nanofiber self-supporting electrode for capacitive deionization. RSC Adv. 2017, 7, 55224–55231. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Tong, Y.; Zhang, M.; Zhang, Y.; Shao, C. TiO2 nanoparticles immobilized on polyacrylonitrile nanofibers mats: A flexible and recyclable photocatalyst for phenol degradation. RSC Adv. 2013, 3, 7503. [Google Scholar] [CrossRef]
- Ra, E.J.; Kim, T.H.; Yu, W.J.; An, K.H.; Lee, Y.H. Ultramicropore formation in PAN/camphor-based carbon nanofiber paper. Chem. Commun. 2010, 46, 1320. [Google Scholar] [CrossRef]
- Rahimi, M.; Singh, J.K.; Babu, D.J.; Schneider, J.J.; Müller-Plathe, F. Understanding Carbon Dioxide Adsorption in Carbon Nanotube Arrays: Molecular Simulation and Adsorption Measurements. J. Phys. Chem. C 2013, 117, 13492–13501. [Google Scholar] [CrossRef]
- Wahiduzzaman; Khan, M.R.; Harp, S.; Neumann, J.; Sultana, Q.N. Processing and Performance of MOF (Metal Organic Framework)-Loaded PAN Nanofibrous Membrane for CO2 Adsorption. J. Mater. Eng. Perform. 2016, 25, 1276–1283. [Google Scholar] [CrossRef]
- Wu, Y.N.; Li, F.; Liu, H.; Zhu, W.; Teng, M.; Jiang, Y.; Li, W.; Xu, D.; He, D.; Hannam, P.; et al. Electrospun fibrous mats as skeletons to produce free-standing MOF membranes. J. Mater. Chem. 2012, 22, 16971–16978. [Google Scholar] [CrossRef]
- Peralta, D.; Chaplais, G.; Simon-Masseron, A.; Barthelet, K.; Chizallet, C.; Quoineaud, A.A.; Pirngruber, G.D. Comparison of the behavior of metal-organic frameworks and zeolites for hydrocarbon separations. J. Am. Chem. Soc. 2012, 134, 8115–8126. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Effects of Functionalization, Catenation, and Variation of the Metal Oxide and Organic Linking Units on the Low-Pressure Hydrogen Adsorption Properties of Metal−Organic Frameworks. J. Am. Chem. Soc. 2006, 128, 1304–1315. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Bao, Z.; Yu, L.; Ren, Q.; Lu, X.; Deng, S. Adsorption of CO2 and CH4 on a magnesium-based metal organic framework. J. Colloid Interface Sci. 2011, 353, 549–556. [Google Scholar] [CrossRef]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic Tuning of Carbon Dioxide Uptake via Metal Substitution in a Coordination Polymer with Cylindrical Pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef]
- Yazaydin, A.O.; Snurr, R.Q.; Park, T.-H.; Koh, K.; Liu, J.; LeVan, M.D.; Benin, A.I.; Jakubczak, P.; Lanuza, M.; Galloway, D.B.; et al. Screening of Metal−Organic Frameworks for Carbon Dioxide Capture from Flue Gas Using a Combined Experimental and Modeling Approach. J. Am. Chem. Soc. 2009, 131, 18198–18199. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.U.; Zhou, H.-C. Recent advances in carbon dioxide capture with metal-organic frameworks. Greenh. Gases Sci. Technol. 2012, 2, 239–259. [Google Scholar] [CrossRef]
- Liu, J.; Benin, A.I.; Furtado, A.M.B.; Jakubczak, P.; Willis, R.R.; LeVan, M.D. Stability Effects on CO2 Adsorption for the DOBDC Series of Metal–Organic Frameworks. Langmuir 2011, 27, 11451–11456. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Benin, A.I.; Jakubczak, P.; Willis, R.R.; LeVan, M.D. CO2/H2O Adsorption Equilibrium and Rates on Metal−Organic Frameworks: HKUST-1 and Ni/DOBDC. Langmuir 2010, 26, 14301–14307. [Google Scholar] [CrossRef]
- Du, P.; Dong, Y.; Liu, C.; Wei, W.; Liu, D.; Liu, P. Fabrication of hierarchical porous nickel based metal-organic framework (Ni-MOF) constructed with nanosheets as novel pseudo-capacitive material for asymmetric supercapacitor. J. Colloid Interface Sci. 2018, 518, 57–68. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, W.; Kaiser, A. Electrospinning of Metal–Organic Frameworks for Energy and Environmental Applications. Adv. Sci. 2020, 7, 1902590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.J.; Li, Q.; Kim, H.; Lee, K. Preparation of Ni-MOF-74 membrane for CO2 separation by layer-by-layer seeding technique. Microporous Mesoporous Mater. 2012, 163, 169–177. [Google Scholar] [CrossRef]
- Meng, L.-Y.Y.; Park, S.-J.J. Effect of heat treatment on CO2 adsorption of KOH-activated graphite nanofibers. J. Colloid Interface Sci. 2010, 352, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Wang, X.; Ahmed Babar, A.; Yu, J.; Ding, B. Highly flexible NiCO2O4/CNTs doped carbon nanofibers for CO2 adsorption and supercapacitor electrodes. J. Colloid Interface Sci. 2016, 476, 87–93. [Google Scholar] [CrossRef]
- Lai, D.; Wei, Y.; Zou, L.; Xu, Y.; Lu, H. Wet spinning of PVA composite fibers with a large fraction of multi-walled carbon nanotubes. Prog. Nat. Sci. Mater. Int. 2015, 25, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Dalton, A.B.; Collins, S.; Muñoz, E.; Razal, J.M.; Ebron, V.H.; Ferraris, J.P.; Coleman, J.N.; Kim, B.G.; Baughman, R.H. Super-tough carbon-nanotube fibres. Nature 2003, 423, 703. [Google Scholar] [CrossRef]
- Sandler, J.K.W.; Kirk, J.E.; Kinloch, I.A.; Shaffer, M.S.P.; Windle, A.H. Ultra-low electrical percolation threshold in carbon-nanotube-epoxy composites. Polymer 2003, 44, 5893–5899. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Wang, L.; Wang, A. Manipulated dispersion of carbon nanotubes with derivatives of chitosan. Carbon N. Y. 2007, 45, 1917–1920. [Google Scholar] [CrossRef]
- Clavé, G.; Campidelli, S. Efficient covalent functionalisation of carbon nanotubes: The use of “click chemistry”. Chem. Sci. 2011, 2, 1887. [Google Scholar] [CrossRef]
- Dyke, C.A.; Tour, J.M. Covalent Functionalization of Single-Walled Carbon Nanotubes for Materials Applications. J. Phys. Chem. A 2004, 108, 11151–11159. [Google Scholar] [CrossRef]
- Li, X.; Wong, S.Y.; Tjiu, W.C.; Lyons, B.P.; Oh, S.A.; He, C. Bin Non-covalent functionalization of multi walled carbon nanotubes and their application for conductive composites. Carbon N. Y. 2008, 46, 829–831. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Sreekumar, T.V.; Kumar, S.; Moore, V.C.; Hauge, R.H.; Smalley, R.E. Poly(vinyl alcohol)/SWNT Composite Film. Nano Lett. 2003, 3, 1285–1288. [Google Scholar] [CrossRef]
- Ghosal, K.; Ghosh, D.; Das, S.K. Preparation and evaluation of naringin-loaded polycaprolactone microspheres based oral suspension using Box-Behnken design. J. Mol. Liq. 2018, 256, 49–57. [Google Scholar] [CrossRef]
- Heidari, M.; Bahrami, H.; Ranjbar-Mohammadi, M. Fabrication, optimization and characterization of electrospun poly(caprolactone)/gelatin/graphene nanofibrous mats. Mater. Sci. Eng. C 2017, 78, 218–229. [Google Scholar] [CrossRef]
- Díaz-García, M.; Mayoral, Á.; Díaz, I.; Sánchez-Sánchez, M. Nanoscaled M-MOF-74 Materials Prepared at Room Temperature. Cryst. Growth Des. 2014, 14, 2479–2487. [Google Scholar] [CrossRef]
- Xu, T.; Hou, X.; Liu, S.; Liu, B. One-step synthesis of magnetic and porous Ni@MOF-74(Ni) composite. Microporous Mesoporous Mater. 2018, 259, 178–183. [Google Scholar] [CrossRef]
- Dietzel, P.D.C.C.; Panella, B.; Hirscher, M.; Blom, R.; Fjellvåg, H. Hydrogen adsorption in a nickel based coordination polymer with open metal sites in the cylindrical cavities of the desolvated framework. Chem. Commun. 2006, 1, 959–961. [Google Scholar] [CrossRef]
- Thakkar, H.; Eastman, S.; Al-Naddaf, Q.; Rownaghi, A.A.; Rezaei, F. 3D-Printed Metal–Organic Framework Monoliths for Gas Adsorption Processes. ACS Appl. Mater. Interfaces 2017, 9, 35908–35916. [Google Scholar] [CrossRef]
- Scherrer, P. Göttinger Nachrichten Gesell; Mathematisch-Physikalische Klasse; Springer: Berlin, Germany, 1918; Volume 2. [Google Scholar]
- Bae, Y.-S.; Liu, J.; Wilmer, C.E.; Sun, H.; Dickey, A.N.; Kim, M.B.; Benin, A.I.; Willis, R.R.; Barpaga, D.; LeVan, M.D.; et al. The effect of pyridine modification of Ni–DOBDC on CO2 capture under humid conditions. Chem. Commun. 2014, 50, 3296–3298. [Google Scholar] [CrossRef]
- Grant Glover, T.; Peterson, G.W.; Schindler, B.J.; Britt, D.; Yaghi, O. MOF-74 building unit has a direct impact on toxic gas adsorption. Chem. Eng. Sci. 2011, 66, 163–170. [Google Scholar] [CrossRef]
- Yu, X.; Wang, J.; Huang, Z.-H.; Shen, W.; Kang, F. Ordered mesoporous carbon nanospheres as electrode materials for high-performance supercapacitors. Electrochem. Commun. 2013, 36, 66–70. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, J.; Lv, R.; Liang, Q.; Zhan, C.; Bai, Y.; Huang, Z.-H.; Shen, W.; Kang, F. Facile synthesis of nitrogen-doped carbon nanosheets with hierarchical porosity for high performance supercapacitors and lithium–sulfur batteries. J. Mater. Chem. A 2015, 3, 18400–18405. [Google Scholar] [CrossRef]
- Do, D.D. Adsorption Analysis: Equilibria and Kinetics; (Series on Chemical Engineering); Imperial College Press: London, UK, 1998; Volume 2, ISBN 978-1-86094-130-6. [Google Scholar]
- Ge, J.J.; Hou, H.; Li, Q.; Graham, M.J.; Greiner, A.; Reneker, D.H.; Harris, F.W.; Cheng, S.Z.D. Assembly of Well-Aligned Multiwalled Carbon Nanotubes in Confined Polyacrylonitrile Environments: Electrospun Composite Nanofiber Sheets. J. Am. Chem. Soc. 2004, 126, 15754–15761. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Zhang, D.; Zhao, J. Dry-jet wet-spun PAN/MWCNT composite fibers with homogeneous structure and circular cross-section. J. Appl. Polym. Sci. 2012, 125, E58–E66. [Google Scholar] [CrossRef]
- Amini, S.; Ebrahimzdeh, H.; Seidi, S.; Jalilian, N. Preparation of electrospun polyacrylonitrile/Ni-MOF-74 nanofibers for extraction of atenolol and captopril prior to HPLC-DAD. Microchim. Acta 2020, 187, 508. [Google Scholar] [CrossRef]
- Villajos, J.A.; Orcajo, G.; Martos, C.; Botas, J.Á.; Villacañas, J.; Calleja, G. Co/Ni mixed-metal sited MOF-74 material as hydrogen adsorbent. Int. J. Hydrogen Energy 2015, 40, 5346–5352. [Google Scholar] [CrossRef]
- Sadegh, H.; Zare, K.; Maazinejad, B.; Shahryari-ghoshekandi, R.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Synthesis of MWCNT-COOH-Cysteamine composite and its application for dye removal. J. Mol. Liq. 2016, 215, 221–228. [Google Scholar] [CrossRef]
- Montazerolghaem, M.; Aghamiri, S.F.; Talaie, M.R.; Tangestaninejad, S. A comparative investigation of CO2 adsorption on powder and pellet forms of MIL-101. J. Taiwan Inst. Chem. Eng. 2017, 72, 45–52. [Google Scholar] [CrossRef]



| Variable | Name | Level and Quantities | ||
|---|---|---|---|---|
| X1 | PAN Concentration | 10 | 12.5 | 15 |
| X2 | MWCNT Concentration | 0 | 0.75 | 1.5 |
| X3 | MOF Concentration | 0 | 6 | 12 |
| RUN | Variables | Response | ||
|---|---|---|---|---|
| X1 (PAN,w/v%) | X2 (MWCNT,w/w%) | X3 (MOF,w/w%) | CO2 Adsorption (mmol CO2/g at 25 °C and 7 bar) | |
| 1 | 15 | 0 | 6 | 1.25 ± 0.03 |
| 2 | 12.5 | 0.75 | 6 | 1.28 ± 0.02 |
| 3 | 12.5 | 1.5 | 12 | 1.59 ± 0.02 |
| 4 | 15 | 0.75 | 12 | 1.63 ± 0.02 |
| 5 | 12.5 | 0.75 | 6 | 1.25 ± 0.02 |
| 6 | 12.5 | 0.75 | 6 | 1.22 ± 0.03 |
| 7 | 10 | 0 | 6 | 0.86 ± 0.03 |
| 8 | 12.5 | 0.75 | 6 | 1.39 ± 0.02 |
| 9 | 15 | 0.75 | 0 | 0.85 ± 0.02 |
| 10 | 10 | 1.5 | 6 | 1.39 ± 0.03 |
| 11 | 12.5 | 0 | 0 | 0.85 ± 0.04 |
| 12 | 10 | 0.75 | 0 | 0.86 ± 0.03 |
| 13 | 12.5 | 0 | 12 | 1.43 ± 0.01 |
| 14 | 12.5 | 0.75 | 6 | 1.26 ± 0.03 |
| 15 | 12.5 | 1.5 | 0 | 0.88 ± 0.02 |
| 16 | 10 | 0.75 | 12 | 1.40 ± 0.03 |
| 17 | 15 | 1.5 | 6 | 1.39 ± 0.03 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| model | 0.9463 | 9 | 0.1051 | 63.04 | 0.0001≥ | significant |
| A(PAN) | 0.0066 | 1 | 0.0066 | 3.96 | 0.0867 | |
| B (CNT) | 0.0276 | 1 | 0.0276 | 16.56 | 0.0048 | |
| C (MOF) | 0.8450 | 1 | 0.8450 | 506.64 | 0.0001≥ | |
| AB | 0.0000 | 1 | 0.0000 | 0.00 | 1.0000 | |
| AC | 0.0132 | 1 | 0.0132 | 7.93 | 0.0259 | |
| BC | 0.0042 | 1 | 0.0042 | 2.53 | 0.1555 | |
| A2 | 0.0038 | 1 | 0.0038 | 2.27 | 0.1755 | |
| B2 | 0.0038 | 1 | 0.0038 | 2.27 | 0.1755 | |
| C2 | 0.0442 | 1 | 0.0442 | 26.52 | 0.0013 | |
| Residual | 0.0117 | 7 | 0.0017 | |||
| Lack of fit | 0.0077 | 3 | 0.0026 | 2.56 | 0.1931 | Not significant |
| Pure error | 0.0040 | 4 | 0.0010 | |||
| Cor total | 0.9580 | 16 |
| Sum of Squares | Mean Squares | F-Value | Probe (p) > F | ||
|---|---|---|---|---|---|
| Model | 0.9463 | 0.1051 | 63.04 | 0.0001< | significant |
| Residual error | 0.0117 | 0.0017 | |||
| Lack of fit | 0.0077 | 0.0026 | 2.56 | 0.1931 | |
| Pure error | 0.0040 | 0.0010 |
| Samples | Mass Loading of MOF (wt.%) | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| PAN nanofiber | 0 | 6.75 | 0.044 | 26.1 |
| PAN/MWCNT/MOF nanofiber | 12 | 65 | 0.08 | 4.9 |
| First cycle-MOFs growth on nanofibers | 43 | 353 | 0.22 | 2.5 |
| Second cycle-MOFs growth on nanofibers | 65 | 493 | 0.27 | 2.2 |
| Ni-MOF-74 powder | 100 | 788 | 0.38 | 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harandizadeh, A.H.; Aghamiri, S.; Hojjat, M.; Ranjbar-Mohammadi, M.; Talaie, M.R. Adsorption of Carbon Dioxide with Ni-MOF-74 and MWCNT Incorporated Poly Acrylonitrile Nanofibers. Nanomaterials 2022, 12, 412. https://doi.org/10.3390/nano12030412
Harandizadeh AH, Aghamiri S, Hojjat M, Ranjbar-Mohammadi M, Talaie MR. Adsorption of Carbon Dioxide with Ni-MOF-74 and MWCNT Incorporated Poly Acrylonitrile Nanofibers. Nanomaterials. 2022; 12(3):412. https://doi.org/10.3390/nano12030412
Chicago/Turabian StyleHarandizadeh, Amir Hossein, Seyedfoad Aghamiri, Mohammad Hojjat, Marziyeh Ranjbar-Mohammadi, and Mohammad Reza Talaie. 2022. "Adsorption of Carbon Dioxide with Ni-MOF-74 and MWCNT Incorporated Poly Acrylonitrile Nanofibers" Nanomaterials 12, no. 3: 412. https://doi.org/10.3390/nano12030412
APA StyleHarandizadeh, A. H., Aghamiri, S., Hojjat, M., Ranjbar-Mohammadi, M., & Talaie, M. R. (2022). Adsorption of Carbon Dioxide with Ni-MOF-74 and MWCNT Incorporated Poly Acrylonitrile Nanofibers. Nanomaterials, 12(3), 412. https://doi.org/10.3390/nano12030412






