MoO3 Nanoparticle Coatings on High-Voltage 5 V LiNi0.5Mn1.5O4 Cathode Materials for Improving Lithium-Ion Battery Performance
Abstract
:1. Introduction
2. Experimental Method
2.1. Synthesis of a Pristine LNMO Material
2.2. Preparation of MoO3-Caoted LNMO Materials
2.3. Materials Characterization
2.4. Electrode Preparation and Measurements
3. Results and Discussion
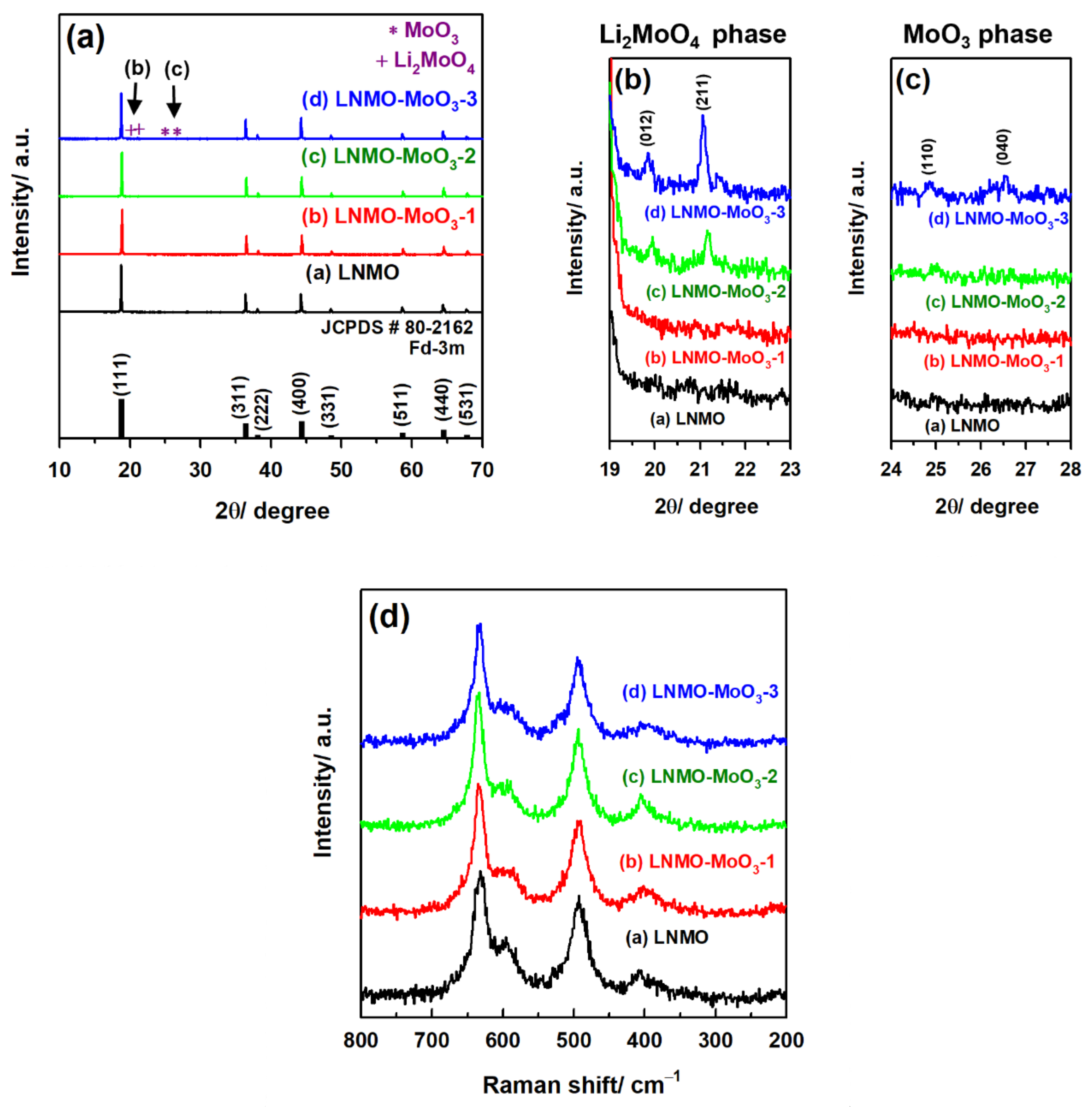
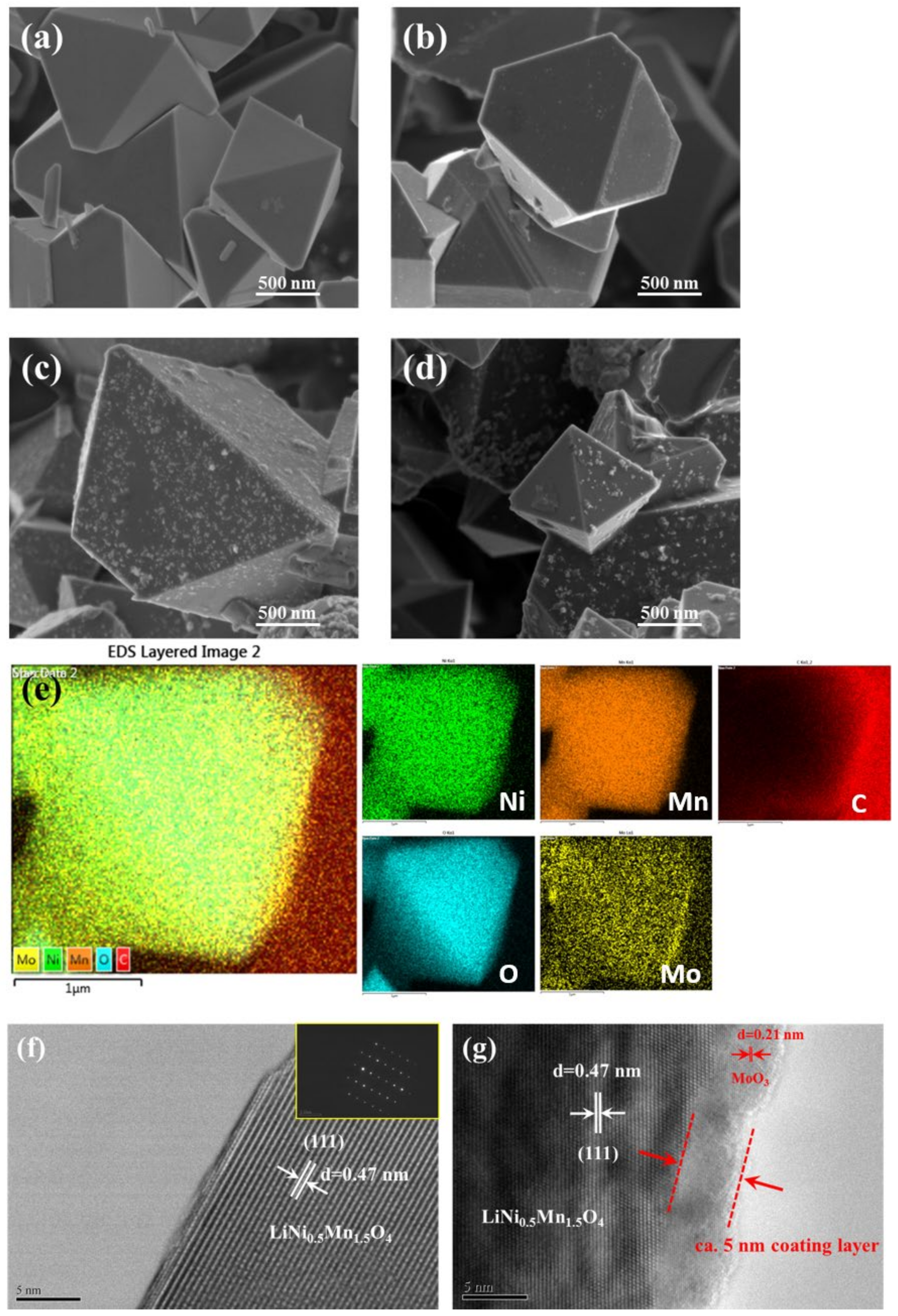
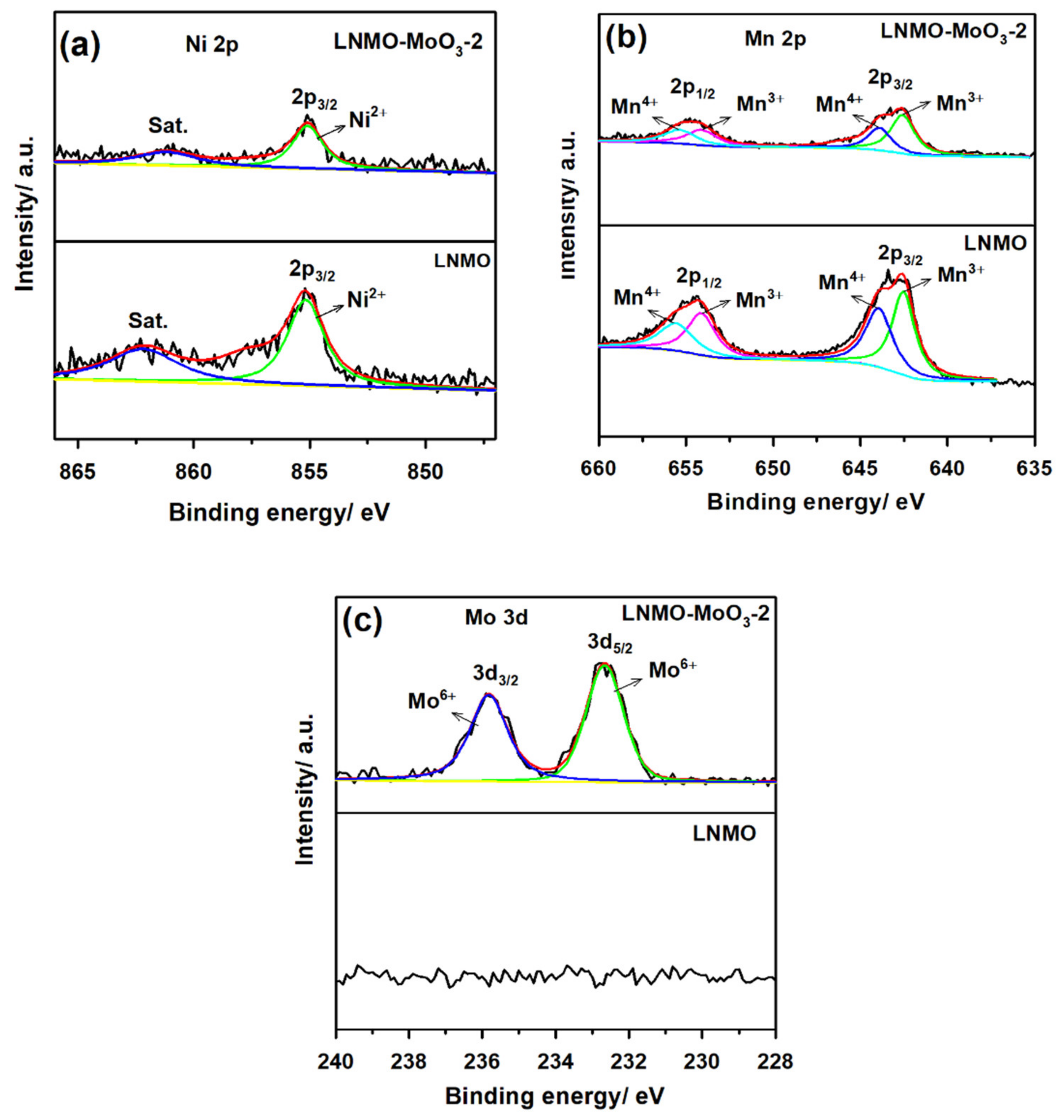
3.1. Structural and Morphological Characterization
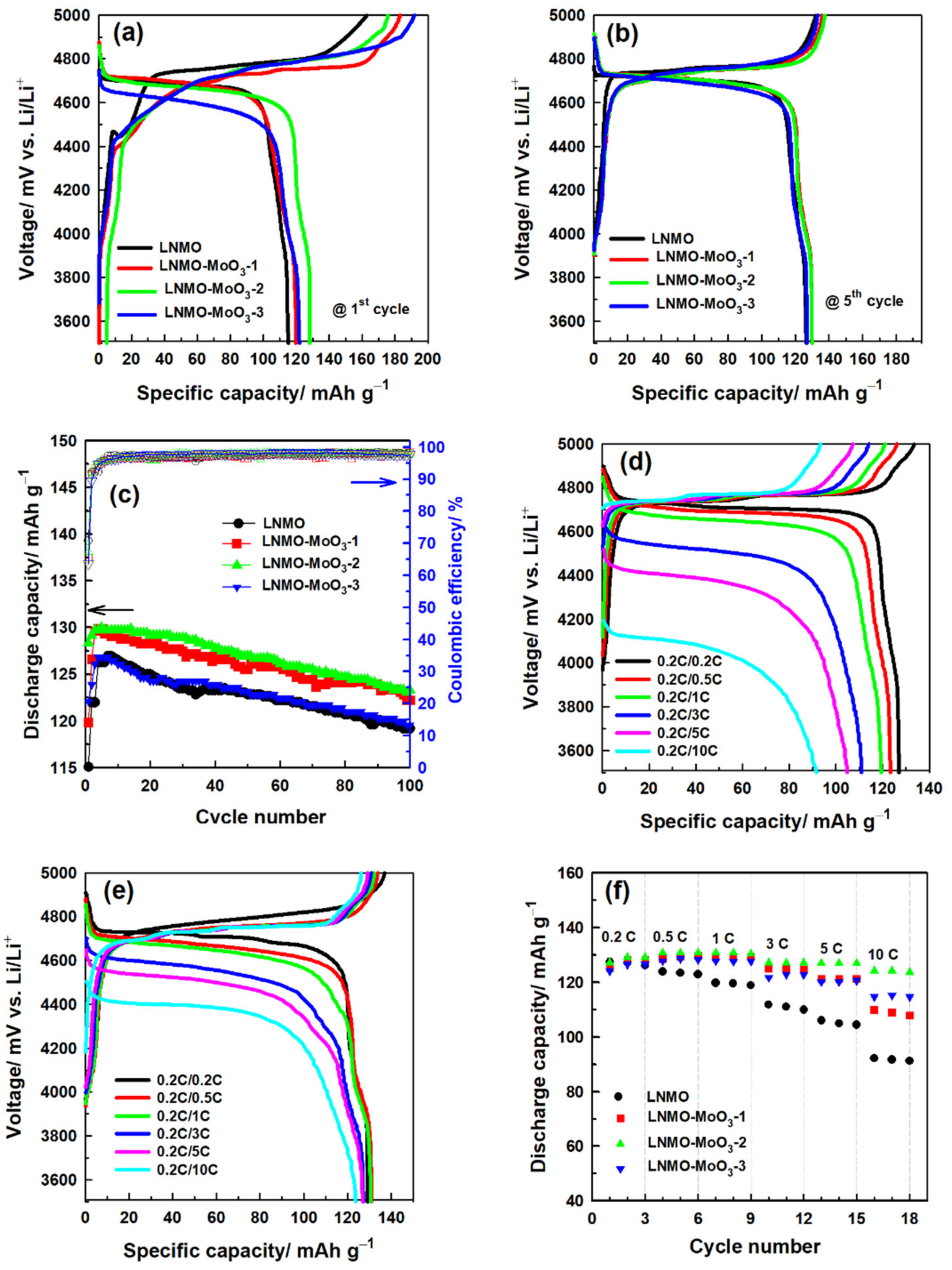
3.2. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, J.; Kintnermeyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Kraytsberg, A.; Ein-Eli, Y. Higher, stronger, better: A review of 5 volt cathode materials for advanced lithium-ion batteries. Adv. Energy. Mater. 2012, 2, 922–939. [Google Scholar] [CrossRef]
- Kosova, N.V.; Devyatkina, E.T. Comparative study of LiCoO2 surface modified with different oxides. J. Power Sources 2007, 174, 959–964. [Google Scholar] [CrossRef]
- Park, O.K.; Cho, Y.; Lee, S.; Yoo, H.-C.; Song, H.-K.; Cho, J. Who will drive electric vehicles, olivine or spinel? Energy Environ. Sci. 2011, 4, 1621–1633. [Google Scholar] [CrossRef]
- Ohzuku, T.; Takeda, S.; Iwanaga, M. Solid-state redox potentials for Li[Me1/2Mn3/2]O4 (Me: 3d-transition metal) having spinel-framework structures: A series of 5 volt materials for advanced lithium-ion batteries. J. Power Sources 1999, 81, 90–94. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, W.; Trottier, J.; Gagnon, C.; Barray, F.; Guerfi, A.; Mauger, A.; Groult, H.; Julien, C.M.; Goodenough, J.B.; et al. Spinel materials for high-voltage cathodes in Li-ion batteries. RSC Adv. 2014, 4, 154–167. [Google Scholar] [CrossRef]
- Yi, T.-F.; Mei, J.; Zhu, Y.-R. Key strategies for enhancing the cycling stability and rate capacity of LiNi0.5Mn1.5O4 as high-voltage cathode materials for high power lithium-ion batteries. J. Power Sources 2016, 316, 85–105. [Google Scholar] [CrossRef]
- Kuppan, S.; Duncan, H.; Chen, G. Controlling side reactions and self-discharge in high-voltage spinel cathodes: The critical role of surface crystallographic facets. Phys. Chem. Chem. Phys. 2015, 17, 26471–26481. [Google Scholar] [CrossRef]
- Wu, B.; Ren, Y.; Mu, D.; Liu, X.; Wu, F. Electrochemical performance of 5 V LiNi0.5Mn1.5O4 cathode modified with lithium carbonate addition in electrolyte. J. Power Sources 2014, 272, 183–189. [Google Scholar] [CrossRef]
- Qiao, R.; Wang, Y.; Olalde-Velasco, P.; Li, H.; Hu, Y.-S.; Yang, W. Direct evidence of gradient Mn(II) evolution at charged states in LiNi0.5Mn1.5O4 electrodes with capacity fading. J. Power Sources 2015, 273, 1120–1126. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Pieczonka, N.P.W.; Li, Z.; Wu, Y.; Harris, S.; Powell, B.R. Understanding the capacity fading mechanism in LiNi0.5Mn1.5O4/graphite Li-ion batteries. Electrochim. Acta 2013, 90, 556–562. [Google Scholar] [CrossRef]
- Hu, M.; Pang, X.; Zhou, Z. Recent progress in high-voltage lithium ion batteries. J. Power Sources 2013, 237, 229–242. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novak, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Huang, B.; Li, X.; Wang, Z.; Guo, H.; Xiong, X.; Wang, J. A novel carbamide-assistant hydrothermal process for coating Al2O3 onto LiMn1.5Ni0.5O4 particles used for cathode material of lithium-ion batteries. J. Alloy Compd. 2014, 583, 313–319. [Google Scholar] [CrossRef]
- Shin, W.-K.; Lee, Y.-S.; Kim, D.-W. Study on the cycling performance of LiNi0.5Mn1.5O4 electrodes modified by reactive SiO2 nanoparticles. J. Mater. Chem. 2014, 2, 6863–6869. [Google Scholar] [CrossRef]
- Wang, J.; Yao, S.; Lin, W.; Wu, B.; He, X.; Li, J.; Zhao, J. Improving the electrochemical properties of high-voltage lithium nickel manganese oxide by surface coating with vanadium oxides for lithium ion batteries. J. Power Sources 2015, 280, 114–124. [Google Scholar] [CrossRef]
- Li, X.; Guo, W.; Liu, Y.; He, W.; Xiao, Z. Spinel LiNi0.5Mn1.5O4 as superior electrode materials for lithium-ion batteries: Ionic liquid assisted synthesis and the effect of CuO coating. Electrochim. Acta 2014, 116, 278–283. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Yoon, C.S.; Oh, I.-H. Surface structural change of ZnO-coated LiNi0.5Mn1.5O4 spinel as 5 V cathode materials at elevated temperatures. Electrochim. Acta 2003, 48, 503–506. [Google Scholar] [CrossRef]
- Nisar, U.; Amin, R.; Essehli, R.; Shakoor, R.A.; Kahraman, R.; Kim, D.K.; Khaleel, M.A.; Belharouak, I. Extreme fast charging characteristics of zirconia modified LiNi0.5Mn1.5O4 cathode for lithium ion batteries. J. Power Sources 2018, 396, 774–781. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, D.H.; Brüner, P.; Lee, H.; Hah, H.J.; Kim, S.K.; Jung, Y.S. Extremely conductive RuO2-coated LiNi0.5Mn1.5O4 for lithium-ion batteries. Electrochim. Acta 2017, 232, 236–243. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.; Su, Y.; Yan, N.; Bao, L.; Chen, S. Li[Li0.2Mn0.54Ni0.13Co0.13]O2–MoO3 composite cathodes with low irreversible capacity loss for lithium ion batteries. J. Power Sources 2014, 247, 20–25. [Google Scholar] [CrossRef]
- Wu, F.; Tian, J.; Su, Y.; Guan, Y.; Jin, Y.; Wang, Z.; He, T.; Bao, L.; Chen, S. Lithium-active molybdenum trioxide coated LiNi0.5Co0.2Mn0.3O2 cathode material with enhanced electrochemical properties for lithium-ion batteries. J. Power Sources 2014, 269, 747–754. [Google Scholar] [CrossRef]
- Kim, J.-H.; Myung, S.-T.; Yoon, C.S.; Kang, S.G.; Sun, Y.-K. Comparative study of LiNi0.5Mn1.5O4-δ and LiNi0.5Mn1.5O4 cathodes having two crystallographic structures: Fd3m and P4332. Chem. Mater. 2004, 16, 906–914. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, D.; Yan, H.; Li, W. Precise preparation of high performance spherical hierarchical LiNi0.5Mn1.5O4 for 5 V lithium ion secondary batteries. J. Mater. Chem. A 2013, 1, 5492–5496. [Google Scholar] [CrossRef]
- Song, J.; Shin, D.W.; Lu, Y.; Amos, C.D.; Manthiram, A.; Goodenough, J.B. Role of oxygen vacancies on the performance of Li[Ni0.5-xMn1.5+x]O4 (x = 0, 0.05, and 0.08) spinel cathodes for lithium-ion batteries. Chem. Mater. 2012, 24, 3101–3109. [Google Scholar] [CrossRef]
- Qian, Y.; Deng, Y.; Wan, L.; Xu, H.; Qin, X.; Chen, G. Investigation of the effect of extra lithium addition and post annealing on the electrochemical performance of high-voltage spinel LiNi0.5Mn1.5O4 cathode material. J. Phys. Chem. C 2014, 118, 15581–15589. [Google Scholar] [CrossRef]
- Amdouni, N.; Zaghib, K.; Gendron, F.; Mauger, A.; Julien, C.M. Structure and insertion properties of disordered and ordered LiNi0.5Mn1.5O4 spinels prepared by wet chemistry. Ionics 2006, 12, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Yi, T.-F.; Hu, X.-G. Preparation and characterization of sub-micro LiNi0.5-xMn1.5+xO4 for 5 V cathode materials synthesized by an ultrasonic-assisted co-precipitation method. J. Power Sources 2007, 167, 185–191. [Google Scholar] [CrossRef]
- Jafta, C.J.; Mathe, M.K.; Manyala, N.; Roos, W.D.; Ozoemena, K.I. Microwave-assisted synthesis of high-voltage nanostructured LiMn1.5Ni0.5O4 spinel: Tuning the Mn3+ content and electrochemical performance. ACS Appl. Mater. Interfaces 2013, 5, 7592–7598. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Bai, Y.; Zhao, S.; Zhang, W. Improved cycling performance of 5 V spinel LiMn1.5Ni0.5O4 by amorphous FePO4 coating. J. Power Sources 2012, 219, 333–338. [Google Scholar] [CrossRef]
- Khyzhun, O.Y.; Strunskus, T.; Solonin, Y.M. XES, XPS and NEXAFS studies of the electronic structure of cubic MoO1.9 and H1.63MoO3 thick films. J. Alloys Compd. 2004, 366, 54–60. [Google Scholar] [CrossRef]
- Ji, W.; Shen, R.; Yang, R.; Yu, G.; Guo, X.; Peng, L.; Ding, W. Partially nitrided molybdenum trioxide with promoted performance as an anode material for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 699–704. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, X.; Sushko, P.V.; Sushko, M.L.; Kovarik, L.; Feng, J.; Deng, Z.; Zheng, J.; Graff, G.L.; Nie, Z. High-performance LiNi0.5Mn1.5O4 spinel controlled by Mn3+ concentration and site disorder. Adv. Mater. 2012, 24, 2109–2116. [Google Scholar] [CrossRef]
- Hong, S.-K.; Mho, S.-I.; Yeo, I.-H.; Kang, Y.; Kim, D.-W. Structural and electrochemical characteristics of morphology-controlled Li[Ni0.5Mn1.5]O4 cathodes. Electrochim. Acta 2015, 156, 29–37. [Google Scholar] [CrossRef]
- Lu, D.; Xu, M.; Zhou, L.; Garsuch, A.; Lucht, B.L. Failure mechanism of graphite/LiNi0.5Mn1.5O4 cells at high voltage and elevated temperature. J. Electrochem. Soc. 2013, 160, A3138–A3143. [Google Scholar] [CrossRef]
- Deng, Y.; Mou, J.; Wu, H.; Jiang, N.; Zheng, Q.; Lam, K.H.; Xu, C.; Lin, D. A superior Li2SiO3-composited LiNi0.5Mn1.5O4 cathode for high-voltage and high-performance lithium-ion batteries. Electrochim. Acta 2017, 235, 19–31. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, Y.; Zhang, S.; Tang, P.; Xiao, X.; Ma, M.; Zhang, H.; Yin, Y.; Wang, D.; Yang, S. Improved high temperature performance of a spinel LiNi0.5Mn1.5O4 cathode for high-voltage lithium-ion batteries by surface modification of a flexible conductive nanolayer. ACS Omega 2019, 4, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Chang, Q.; Wei, A.; Li, W.; Bai, X.; Zhang, L.; He, R.; Liu, Z. Structural and electrochemical characteristics of Al2O3-modified LiNi0.5Mn1.5O4 cathode materials for lithium-ion batteries. Ceram. Int. 2019, 45, 5100–5110. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; He, Y.; Lu, K. Li4Ti5O12 epitaxial coating on LiNi0.5Mn1.5O4 surface for improving the electrochemical performance through solvothermal-assisted processing. J. Alloy. Compd. 2019, 779, 978–984. [Google Scholar] [CrossRef]
- Pan, J.J.; Chen, B.; Xie, Y.; Ren, N.; Yi, T.F. V2O5 modified LiNi0.5Mn1.5O4 as cathode material for high-performance Li-ion battery. Mater. Lett. 2019, 253, 136–139. [Google Scholar] [CrossRef]
- Deng, S.; Wang, B.; Yuan, Y.; Li, X.; Sun, Q.; Doyle-Davis, K.; Banis, M.N.; Liang, J.; Zhao, Y.; Li, J.; et al. Manipulation of an ionic and electronic conductive interface for highly stable high-voltage cathodes. Nano Energy 2019, 65, 103988. [Google Scholar] [CrossRef]
- Zhang, D.; Hu, L.L.; Sun, Y.G.; Piao, J.Y.; Tao, X.S.; Xu, Y.S.; Cao, A.M.; Wan, L.J. Construction of uniform transition-metal phosphate nanoshells and its potential for improving li-ion battery performance. J. Mater. Chem. A 2018, 6, 8992–8999. [Google Scholar] [CrossRef]
- Kuenzel, M.; Kim, G.T.; Zarrabeitia, M.; Lin, S.D.; Schuer, A.R.; Geiger, D.; Kaiser, U.; Bresser, D.; Passerini, S. Crystal engineering of TMPOx-coated LiNi0.5Mn1.5O4 cathodes for high performance lithium-ion batteries. Mater. Today 2020, 39, 127–136. [Google Scholar] [CrossRef]
- Chu, C.T.; Mondal, A.; Kosova, N.V.; Lin, J.Y. Improved high-temperature cyclability of AlF3 modified spinel LiNi0.5Mn1.5O4 cathode for lithium-ion batteries. Appl. Surf. Sci. 2020, 530, 147169. [Google Scholar] [CrossRef]
- Yu, C.; Dong, L.; Zhang, Y.; Du, K.; Gao, M.; Zhao, H.; Bai, Y. Promoting electrochemical performances of LiNi0.5Mn1.5O4 cathode via YF3 surface coating. Solid State Ion. 2020, 357, 115464. [Google Scholar] [CrossRef]
- Ma, C.; Wen, Y.; Qiao, Q.; He, P.; Ren, S.; Li, M.; Zhao, P.; Qiu, J.; Tang, G. Improving electrochemical performance of high-voltage spinel LiNi0.5Mn1.5O4 cathodes by silicon oxide surface modification. ACS Appl. Energy Mater. 2021, 4, 12201–12210. [Google Scholar] [CrossRef]
- Bai, N.; Ma, Y.J.; Wang, A.M.; Luo, X. Effects of MoO3 coating on the structure and electrochemical performance of high-voltage spinel LiNi0.5Mn1.5O4. Ionics 2021, 27, 469–478. [Google Scholar] [CrossRef]
- Shenouda, A.Y.; Liu, H.K. Electrochemical behaviour of tin borophosphate negative electrodes for energy storage systems. J. Power Sources 2008, 185, 1386–1391. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, F.; Zhang, K.; Liang, Y.; Yang, S.; Liang, J.; Chen, J. Facile polymer-assisted synthesis of LiNi0.5Mn1.5O4 with a hierarchical micro–nano structure and high rate capability. RSC Adv. 2012, 2, 5669–5675. [Google Scholar] [CrossRef]
- Ding, Y.-L.; Goh, B.M.; Zhang, H.; Loh, K.P.; Lu, L. Single-crystalline nanotubes of spinel lithium nickel manganese oxide with lithium titanate anode for high-rate lithium ion batteries. J. Power Sources 2013, 236, 1–9. [Google Scholar] [CrossRef]
- Yang, C.-C.; Hu, H.-C.; Lin, S.J.; Chien, W.-C. Electrochemical performance of V-doped spinel Li4Ti5O12/C composite anode in Li-half and Li4Ti5O12/LiFePO4-full cell. J. Power Sources 2014, 258, 424–433. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Yamamoto, N. Zero-strain insertion material of Li[Li1/3Ti5/3]O4 for rechargeable lithium cells. J. Electrochem. Soc. 1995, 142, 1431–1435. [Google Scholar] [CrossRef]
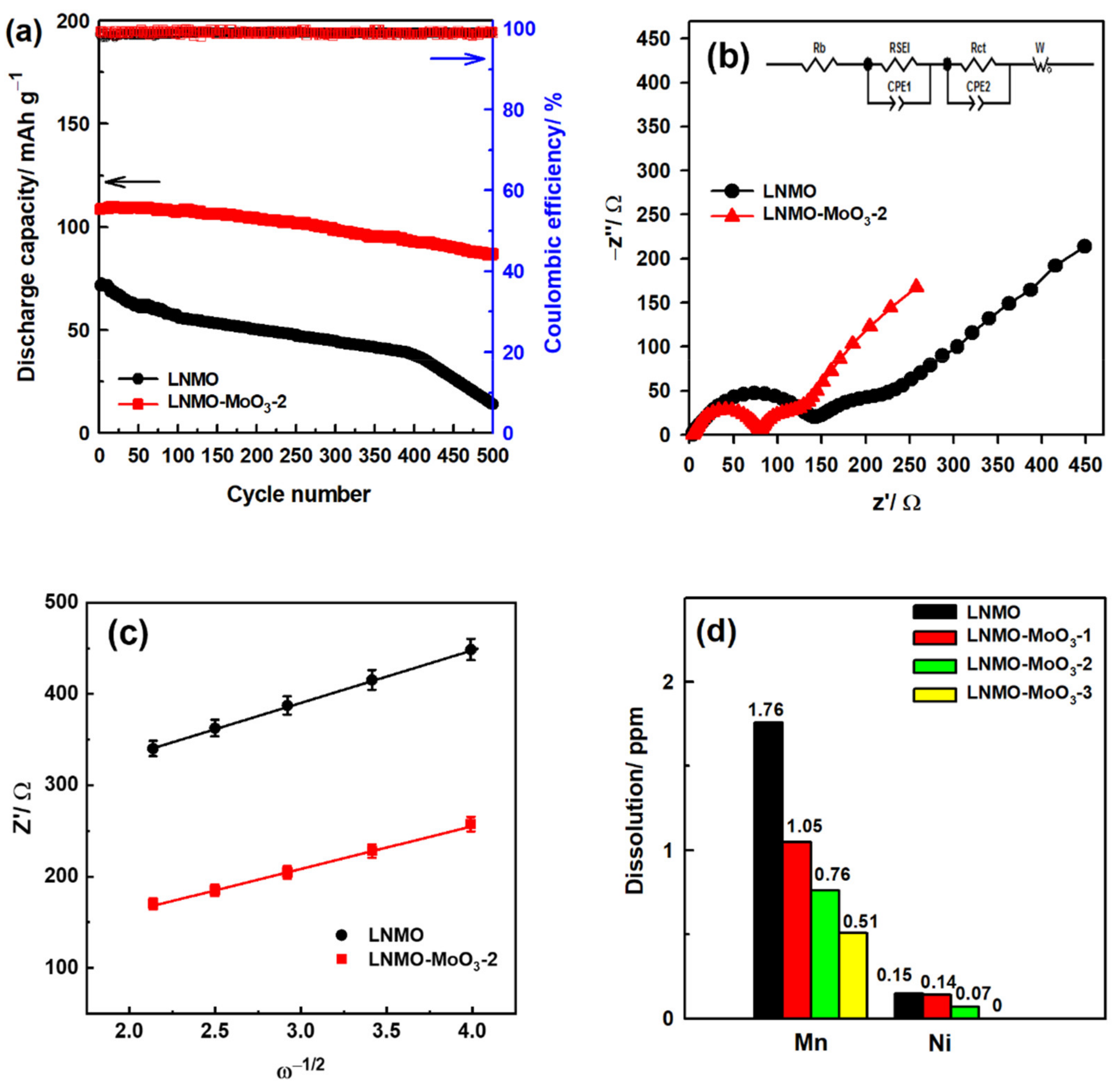
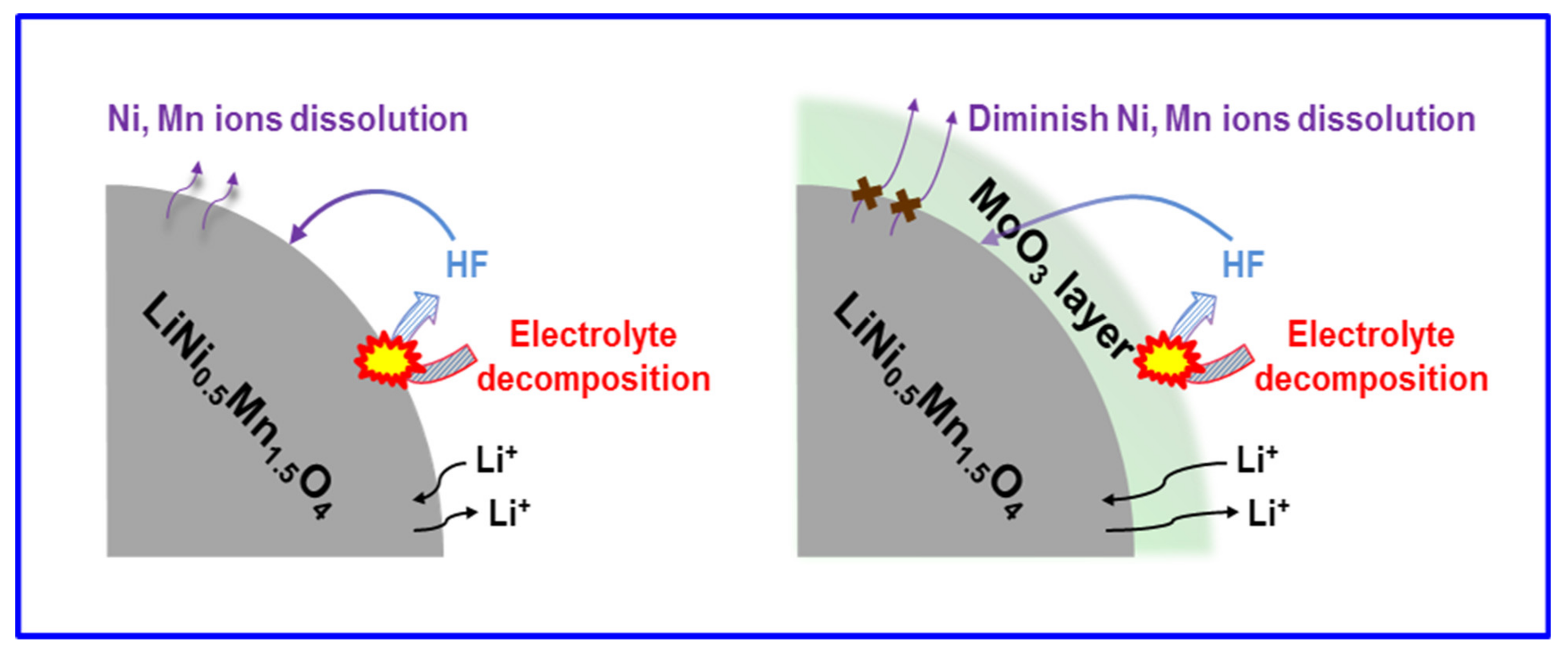
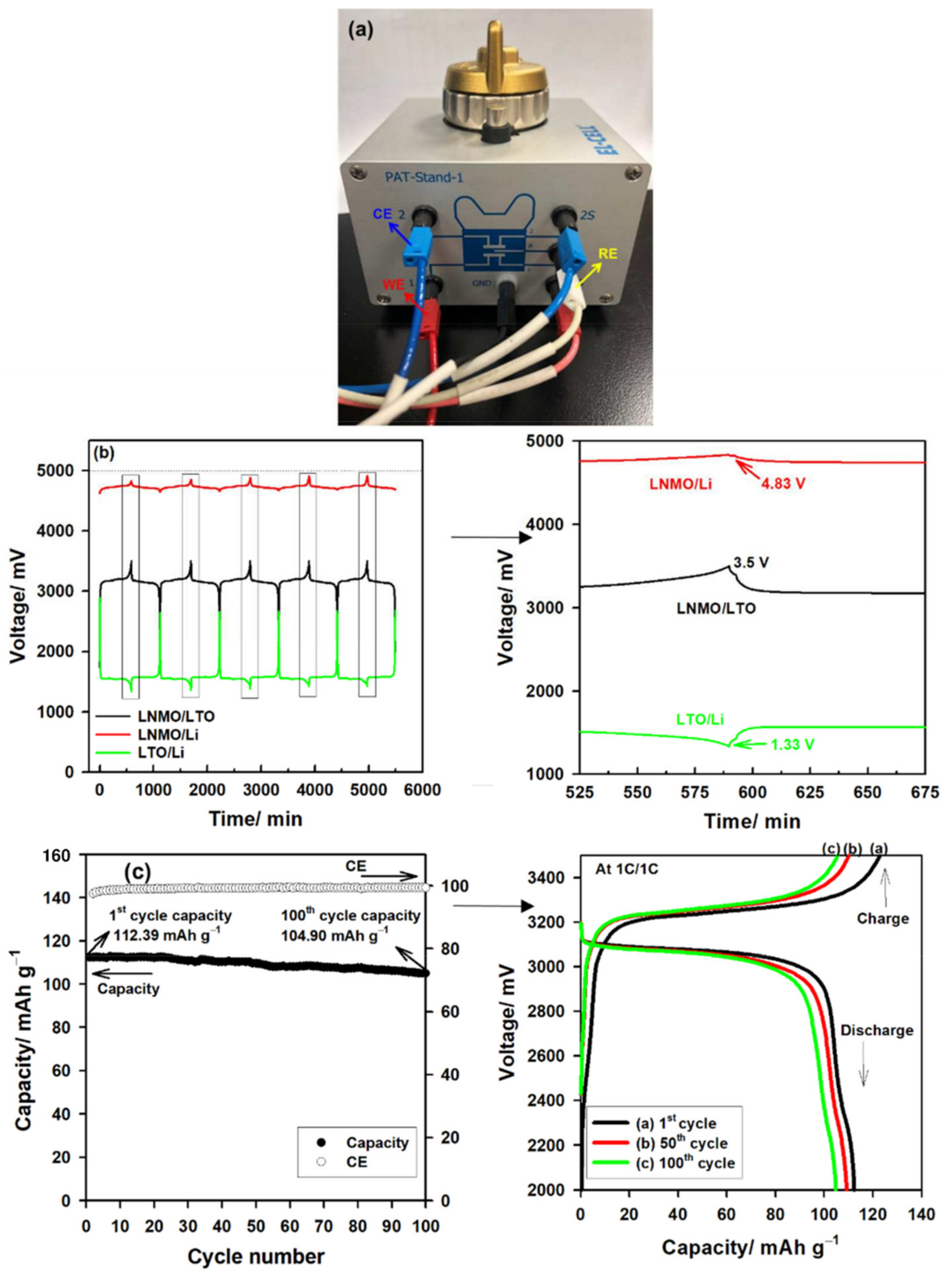
| Coating Materials | Rate Capability (mAh g−1 at C−Rate) | Capacity Retention (%@C−Rate)/Cycles | Ref. |
|---|---|---|---|
| CuO (3 wt.%) | 126 at 2 C; 99 at 10 C | 95.6 at 0.5 C/100 | [20] |
| ZrO2 (1 wt.%) | ~130 at 1 C; 128 at 10 C | 85.6% at 40 C/1200 | [22] |
| RuO2 (0.56 wt.%) | ~118 at 1 C; 20 at 10 C | 96.1% at 0.5 C/150 | [23] |
| Li2SiO3 (0.1 wt.%) | ~107 at 2 C | 85.5% at 1 C/300 | [39] |
| Polyaniline (1 wt.%) | 120 at 1 C; 66 at 5 C | 99.7% at 0.5 C/200 | [40] |
| Al2O3 (0.5 wt.%) | 101 at 5 C; 83 at 7 C | 92.6% at 1 C/200 | [41] |
| Li4Ti5O12 (LTO) | 114 at 1 C; 91 at 10 C | 93.6% at 0.5 C/100 | [42] |
| V2O5 | 111 at 1 C; 52 at 5 C | 96.6% at 1 C/100 | [43] |
| Li3PO4–TiO2 | ~100 at 1 C; 60 at 5 C | 81.2% at 0.5 C/300 | [44] |
| Co3(PO4)2 (2.87 wt.%) | ~125 at 1 C; 82 at 10 C | 94.8% at 1 C/100 (55 °C) | [45] |
| MnPOx (2 wt.%) NiPOx (2 wt.%) | 120 at 1 C; 100 at 10 C 128 at 1 C; 110 at 10 C | 69% at 10 C/1000 82% at 10 C/1000 | [46] |
| AlF3 (1 wt.%) | 121 at 0.2 C | 81.7% at 0.2 C/100 (55 °C) | [47] |
| YF3 | 95 at 1 C; 32 at 5 C | 84% at 0.1 C/100 | [48] |
| SiO2 (0.8 wt.%) | ~120 at 1 C; 90 at 5 C | 91.3% at 3 C/1000 | [49] |
| MoO3 (2 wt.%) | ~118 at 1 C; 96 at 2 C | 96% at 0.2 C/100 | [50] |
| MoO3 (2 wt.%) | 131 at 1 C; 124 at 10 C | 80.1% 10C/500 | This work |
| Sample | Rb/Ω | RSEI/Ω | Rct/Ω | DLi+/cm2 s−1 |
|---|---|---|---|---|
| LNMO | 3.92 | 139.79 | 74.20 | 1.04 × 10−14 |
| LNMO-MoO3-2 | 4.04 | 76.40 | 49.37 | 1.59 × 10−14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.-H.; Shih, J.-Y.; Li, Y.-J.J.; Tsai, Y.-D.; Hung, T.-F.; Karuppiah, C.; Jose, R.; Yang, C.-C. MoO3 Nanoparticle Coatings on High-Voltage 5 V LiNi0.5Mn1.5O4 Cathode Materials for Improving Lithium-Ion Battery Performance. Nanomaterials 2022, 12, 409. https://doi.org/10.3390/nano12030409
Wu Z-H, Shih J-Y, Li Y-JJ, Tsai Y-D, Hung T-F, Karuppiah C, Jose R, Yang C-C. MoO3 Nanoparticle Coatings on High-Voltage 5 V LiNi0.5Mn1.5O4 Cathode Materials for Improving Lithium-Ion Battery Performance. Nanomaterials. 2022; 12(3):409. https://doi.org/10.3390/nano12030409
Chicago/Turabian StyleWu, Zong-Han, Jeng-Ywan Shih, Ying-Jeng James Li, Yi-De Tsai, Tai-Feng Hung, Chelladurai Karuppiah, Rajan Jose, and Chun-Chen Yang. 2022. "MoO3 Nanoparticle Coatings on High-Voltage 5 V LiNi0.5Mn1.5O4 Cathode Materials for Improving Lithium-Ion Battery Performance" Nanomaterials 12, no. 3: 409. https://doi.org/10.3390/nano12030409
APA StyleWu, Z.-H., Shih, J.-Y., Li, Y.-J. J., Tsai, Y.-D., Hung, T.-F., Karuppiah, C., Jose, R., & Yang, C.-C. (2022). MoO3 Nanoparticle Coatings on High-Voltage 5 V LiNi0.5Mn1.5O4 Cathode Materials for Improving Lithium-Ion Battery Performance. Nanomaterials, 12(3), 409. https://doi.org/10.3390/nano12030409










