Punicalagin and Ketogenic Amino Acids Loaded Organic Lipid Carriers Enhance the Bioavailability, Mitochondrial β-Oxidation, and Ketogenesis in Maturing Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture Materials and Chemicals
2.2. Synthesis of Nano Lipid Carrier (NLCs)
2.3. Physiochemical Characterization of NLCs-PUNI-KAA and NLCs-KAA
2.4. Human Mesenchymal Stem Cells (hMSCs) Culture and Induction of Adipocyte Differentiation
2.5. Cytotoxicity Analysis in hMSCs and Preadipocytes
2.6. Experimental Design for the Inhibition of Lipid Accumulation in Preadipocytes
2.7. Cell Membrane and Nuclear Damage Analysis in hMSCs
2.8. Oil Red O and Nile Red Staining Analysis to Determine Intracellular Lipid Levels in Maturing Adipocytes
2.9. Mitochondrial Membrane Potential (Δψm) (JC-1 Staining) Assay
2.10. Estimation of Triglyceride (TG), Total Cholesterol and Free Glycerol
2.11. Quantitative Polymerase Chain Reaction (qPCR) Analysis
2.12. Quantification of CREBp-1 and AMPK Protein by ELISA
2.13. Estimation of Acetoacetate (AcAc) and Beta-Hydroxybutyrate (β-HB)
2.14. Statistical Analysis
3. Results
3.1. Characterization of NLCs-PUNI-KAA and NLCs-KAA Using Zetasizer, FT-IR, and TEM Image Analysis
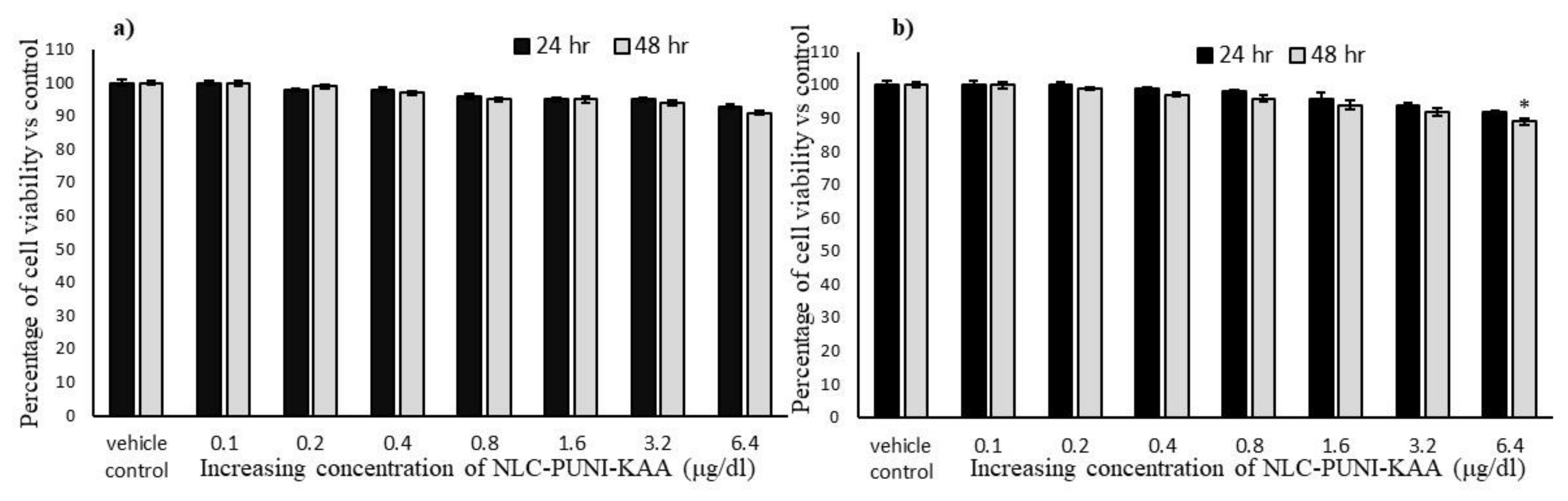
3.2. Effect of Punicalagin (PUNI), NLCs-PUNI-KAA, and NLCs-KAA on Cell Proliferation Potential in hMSCs and Adipocytes
3.3. Biosafety Analysis of NLCs-PUNI-KAA, NLCs-KAA, and PUNI in hMSCs Using Propidium Iodide (PI) Staining
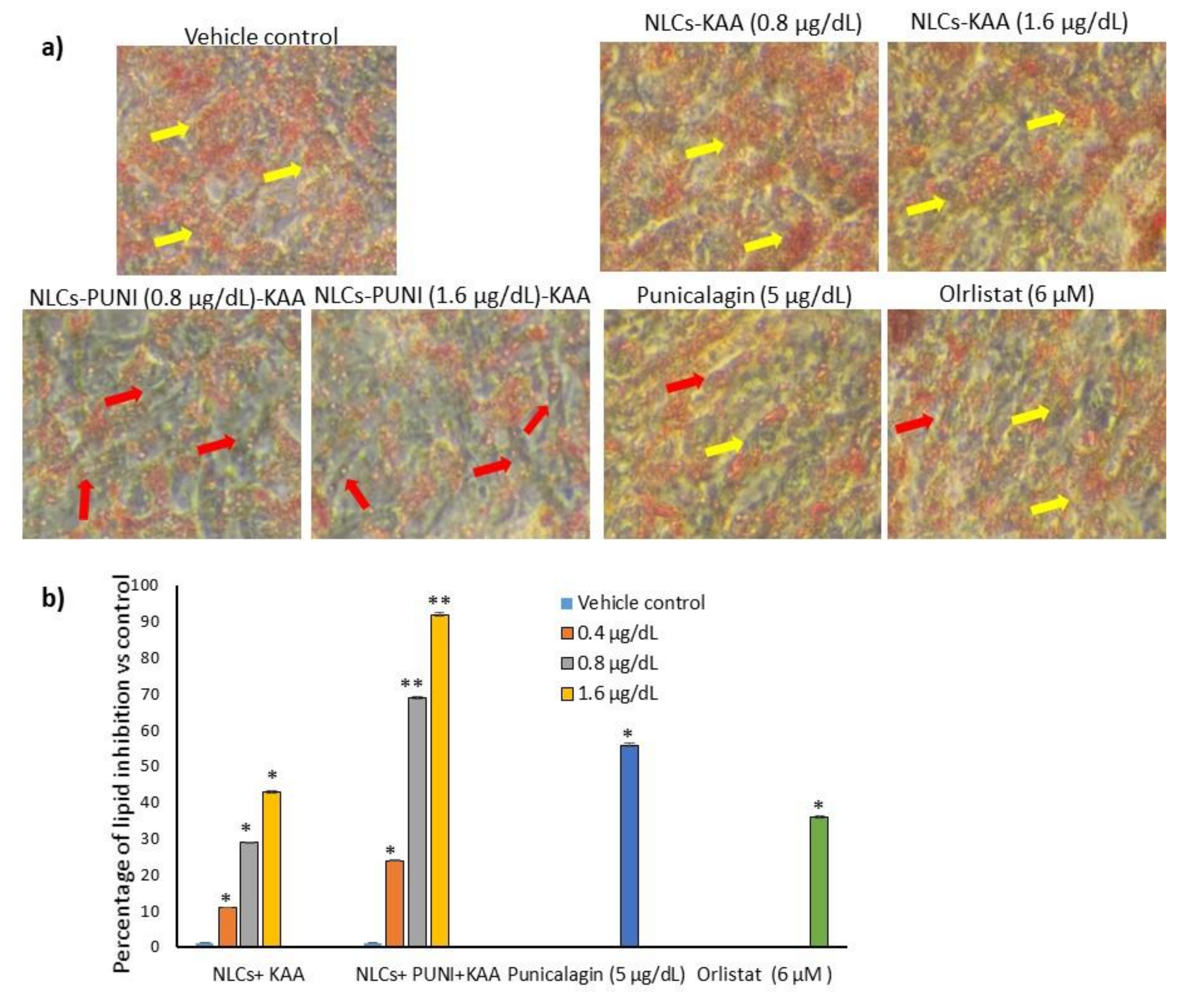
3.4. Effective Dose Determination Based on Lipid Accumulation Inhibitory Potential
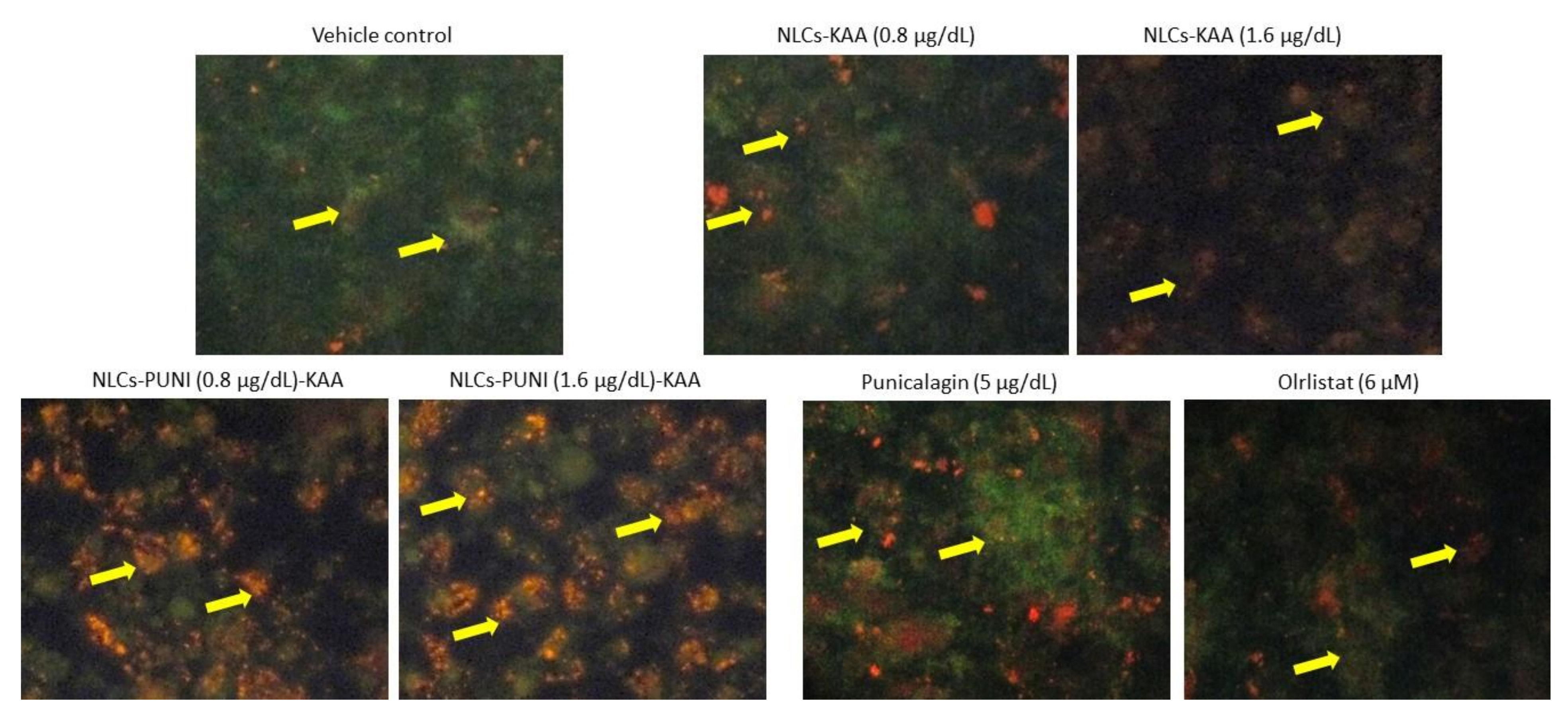
3.5. Identification of Hypertrophic Adipocyte Using Nile Red Fluorescence Staining Analysis
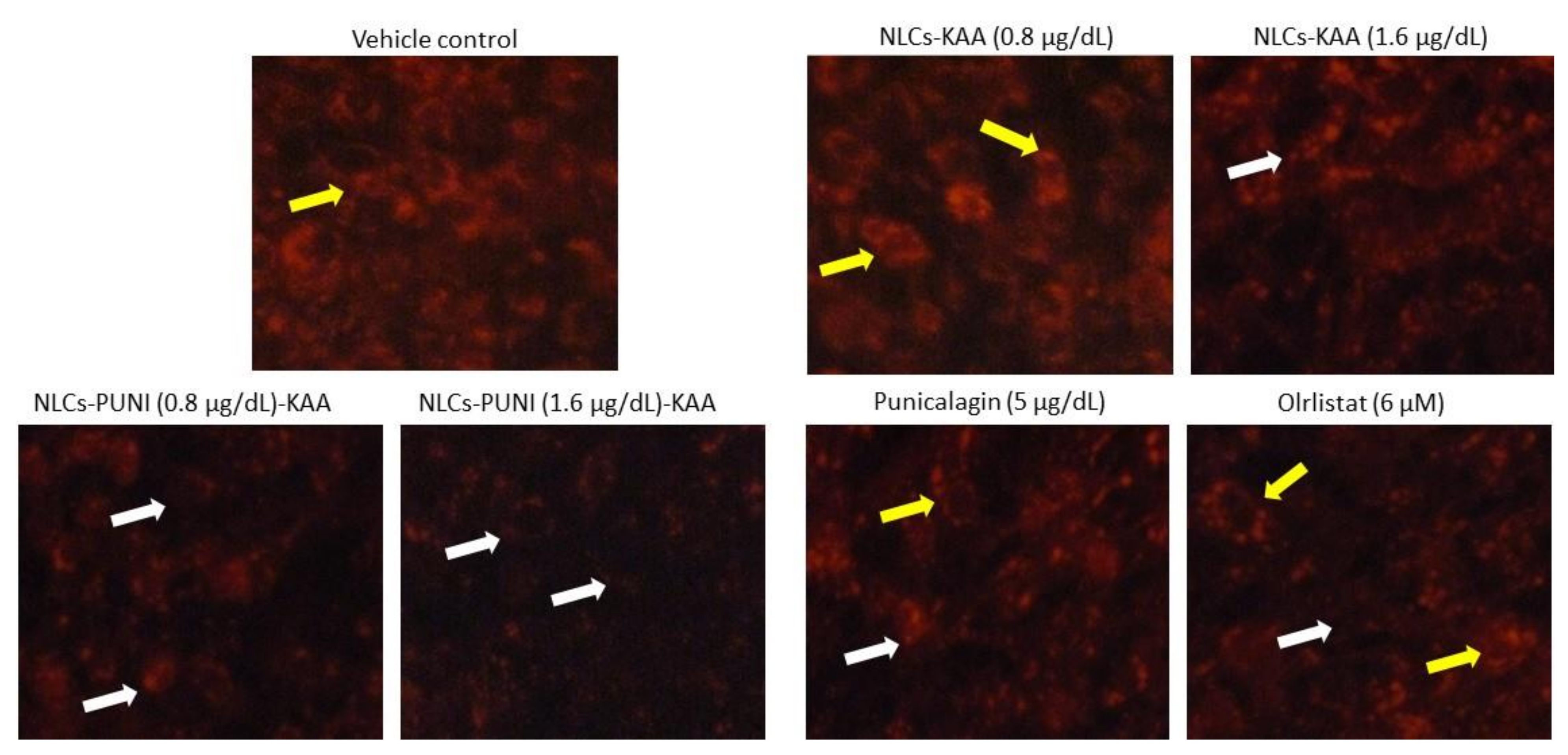
3.6. Mitochondrial Membrane Potential (Δψm, JC-1) and Oxidative Capacity Analysis
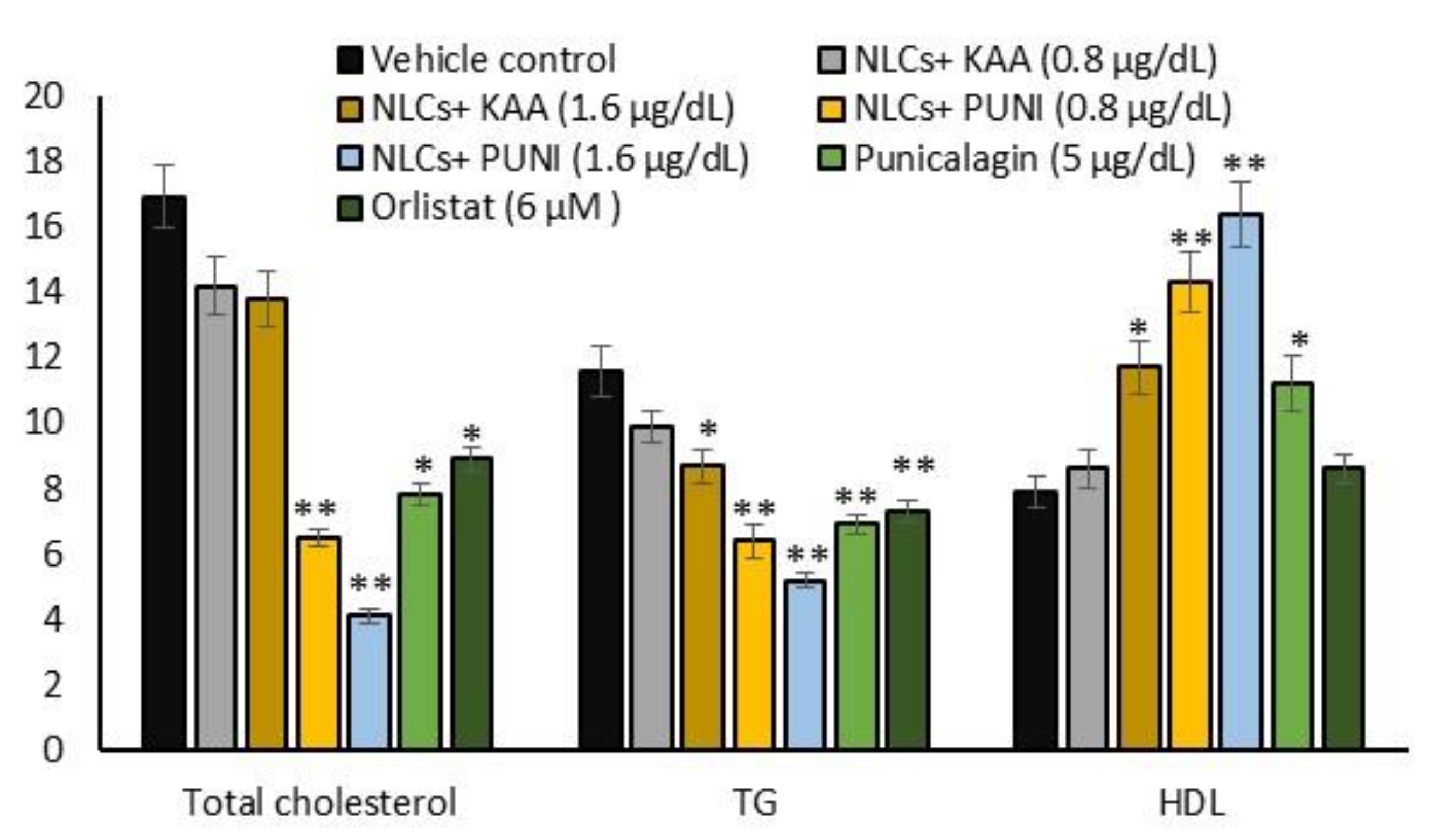
3.7. Intracellular Total Cholesterol, TG, and HDL Levels
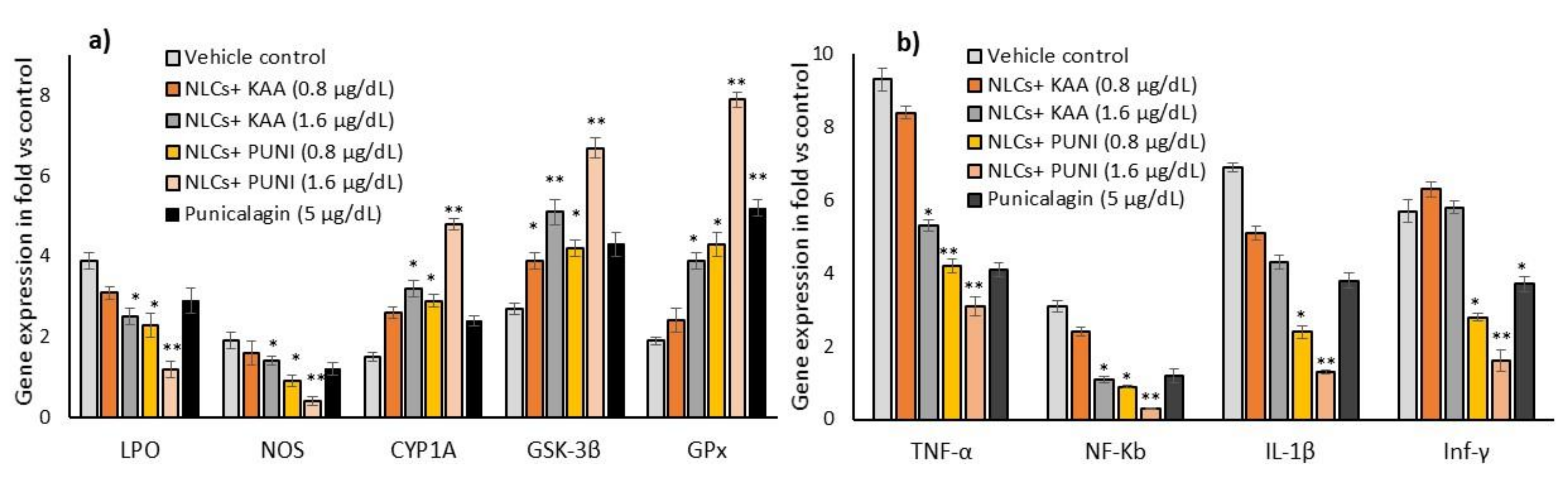
3.8. Oxidative Stress, Antioxidant and Tissue Damage Related Gene Expressions in NLCs-PUNI-KAA, NLCs-KAA, and PUNI Treated hMSCs
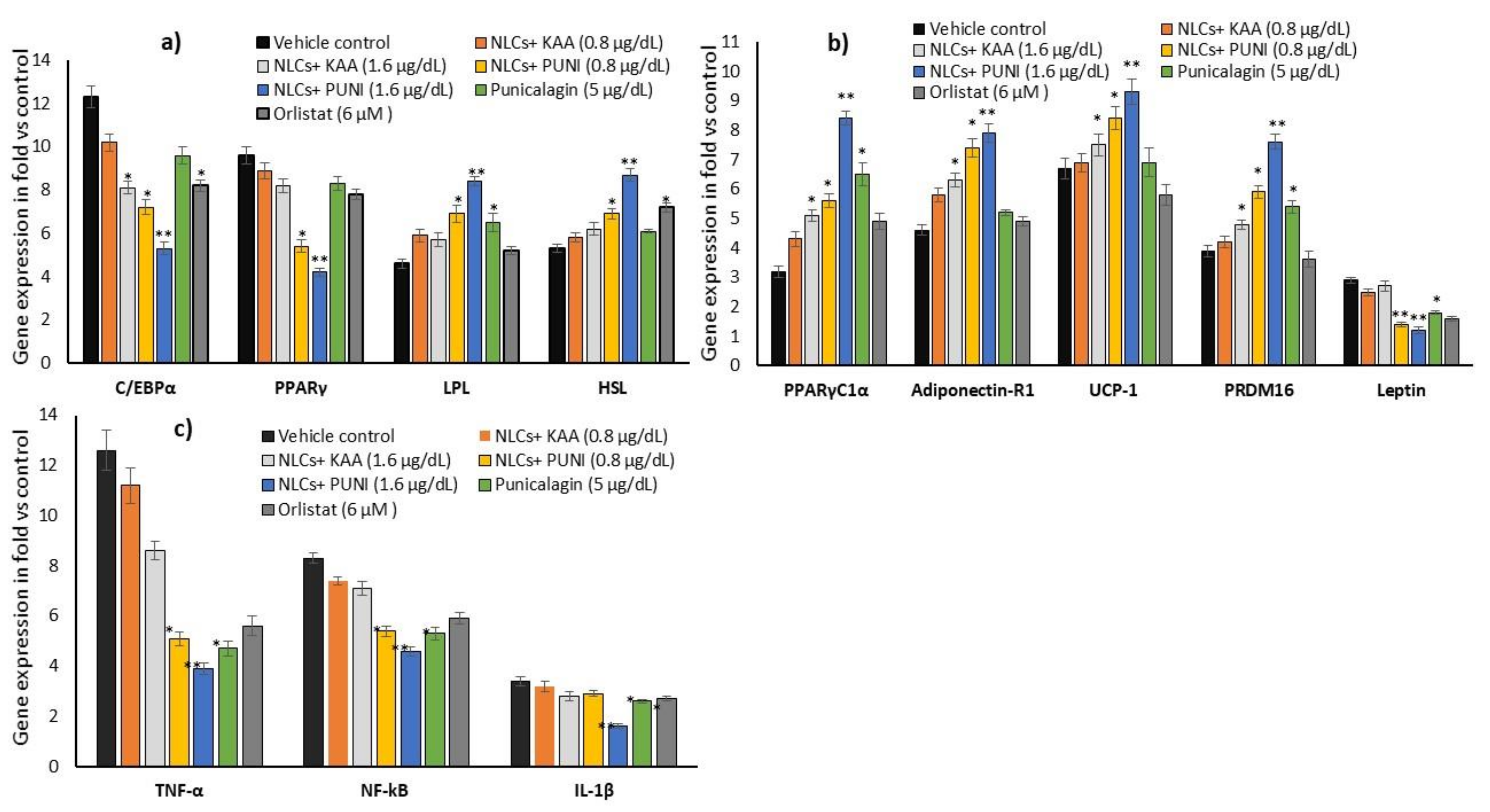
3.9. Adipogenesis, Mitochondrial Thermogenesis and Inflammatory Gene Expression Analysis in NLCs-PUNI-KAA, NLCs-KAA, PUNI, and Orlistat Treated Maturing Adipocytes
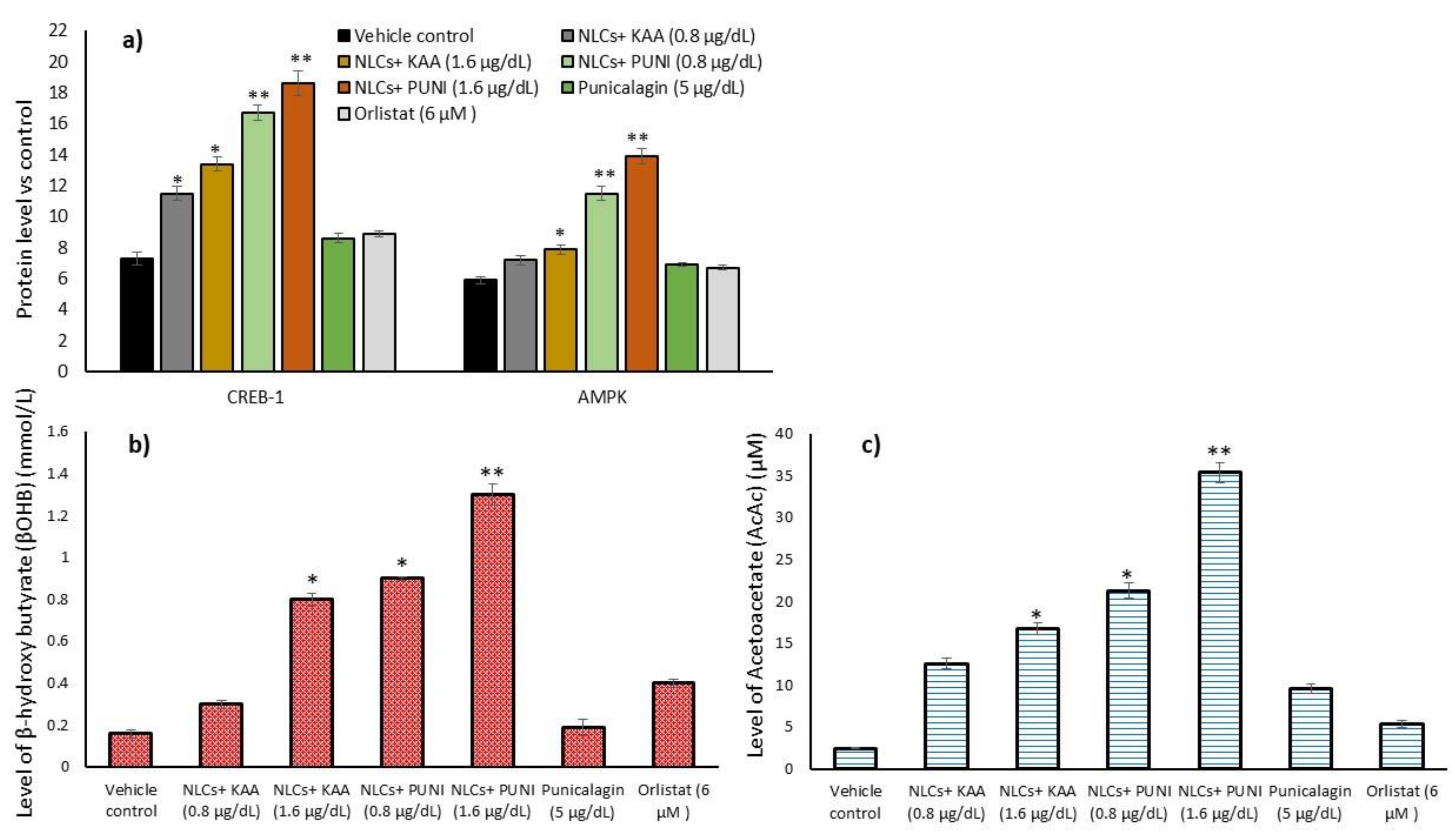
3.10. Intracellular Protein Levels in Adipocyte’s Stromal Vascular Fraction (SVF)
3.11. Acetoacetate and Beta-Hydroxybutyrate Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scherer, T.; Lindtner, C.; O’Hare, J.; Hackl, M.; Zielinski, E.; Freudenthaler, A.; Baumgartner-Parzer, S.; Tödter, K.; Heeren, J.; Krššák, M.; et al. Insulin regulates hepatic triglyceride secretion and lipid content via signaling in the brain. Diabetes 2016, 65, 1511–1520. [Google Scholar] [CrossRef] [Green Version]
- Konstantynowicz, K.; Mikłosz, A.; Stepek, T.; Chabowski, A. The role of hepatic lipid accumulation in the development of insulin resistance in the liver. Postepy Hig. Med. Dosw. 2011, 65, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Korenblat, K.M.; Fabbrini, E.; Mohammed, B.S.; Klein, S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 2008, 34, 1369–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krssak, M.; Brehm, A.; Bernroider, E.; Anderwald, C.; Nowotny, P.; Dalla Man, C.; Cobelli, C.; Cline, G.W.; Shulman, G.I.; Waldhäusl, W.; et al. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes 2004, 53, 3048–3056. [Google Scholar] [CrossRef] [Green Version]
- Petersen, M.C.; Shulman, G.I. Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol. Sci. 2017, 38, 649–665. [Google Scholar] [CrossRef]
- Chibalin, A.V.; Leng, Y.; Vieira, E.; Krook, A.; Björnholm, M.; Long, Y.C.; Kotova, O.; Zhong, Z.; Sakane, F.; Steiler, T.; et al. Downregulation of diacylglycerol kinase delta contributes to hyperglycemia induced insulin resistance. Cell 2008, 132, 375–386. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, Y.; Nishikata, N.; Shikata, N.; Kimura, Y.; Aleman, J.O.; Young, J.D.; Koyama, N.; Kelleher, J.K.; Takahashi, M.; Stephanopoulos, G. Ketogenic essential amino acids modulate lipid synthetic pathways and prevent hepatic steatosis in mice. PLoS ONE 2010, 5, e12057. [Google Scholar] [CrossRef] [Green Version]
- Cotter, D.G.; Schugar, R.C.; Crawford, P.A. Ketone body metabolism and cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1060–H1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frago, L.M.; Chowen, J.A. Involvement of Astrocytes in Mediating the Central Effects of Ghrelin. Int. J. Mol. Sci. 2017, 18, 536. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.; Du, Y.; Lv, Z.; Chen, S.; Zhu, J.; Sheng, H.; Guo, F. Effects of essential amino acids on lipid metabolism in mice and humans. J. Mol. Endocrinol. 2016, 57, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Guo, K.; LeBlanc, R.E.; Loh, D.; Schwartz, G.J.; Yu, Y.H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar] [CrossRef] [Green Version]
- Isakov, V.A.; Bogdanova, A.A.; Bessonov, V.V.; Sentsova, T.B.; Tutelyan, V.A.; Lin, Y.; Kazlova, V.; Hong, J.; Velliquette, R.A. Effects of multivitamin, multimineral and phytonutrient supplementation on nutrient status and biomarkers of heart health risk in a russian population: A Randomized, Double Blind, Placebo Controlled Study. Nutrients 2018, 10, 120. [Google Scholar] [CrossRef] [Green Version]
- Bagherniya, M.; Nobili, V.; Blesso, C.N.; Sahebkar, A. Medicinal plants and bioactive natural compounds in the treatment of non-alcoholic fatty liver disease: A clinical review. Pharmacol. Res. 2018, 130, 213–240. [Google Scholar] [CrossRef]
- Kang, B.; Kim, C.Y.; Hwang, J.; Jo, K.; Kim, S.; Suh, H.J.; Choi, H.S. Punicalagin, a Pomegranate-derived Ellagitannin, suppresses obesity and obesity-induced inflammatory responses via the Nrf2/Keap1 signaling pathway. Mol. Nutr. Food Res. 2019, 63, e1900574. [Google Scholar] [CrossRef]
- Zou, X.; Yan, C.; Shi, Y.; Cao, K.; Xu, J.; Wang, X.; Chen, C.; Luo, C.; Li, Y.; Gao, J.; et al. Mitochondrial dysfunction in obesity-associated non-alcoholic fatty liver disease: The protective effects of pomegranate with its active component punicalagin. Antioxid. Redox Signal. 2014, 21, 1557–1570. [Google Scholar] [CrossRef]
- Leite, M.; Quinta-Costa, M.; Leite, P.S.; Guimaraes, J.E. Critical evaluation of techniques to detect and measure cell death—Study in a model of UV radiation of the leukaemic cell line HL60. Anal. Cell. Pathol. 1999, 19, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Subash-Babu, P.; Al-Maiman, S.A.; Al-Harbi, L.N.; Alshatwi, A.A. Beneficial fatty acid ratio of Salvia hispanica L. (Chia Seed) potentially inhibits adipocyte hypertrophy, and decreases adipokines expression and inflammation in macrophage. Foods 2020, 9, 368. [Google Scholar] [CrossRef] [Green Version]
- Cloey, T.; Bachorik, P.S.; Becker, D.; Finney, C.; Lowry, D.; Sigmund, W. Re-evaluation of serum-plasma differences in total cholesterol concentration. JAMA 1990, 263, 2788–2789. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1967, 72, 248–254. [Google Scholar] [CrossRef]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef] [Green Version]
- Williamson, D.H.; Mellanby, J.; Krebs, H.A. Enzymatic determination of D (-) β-hydroxybutyric acid and acetoacetic acid in blood. Biochem. J. 1962, 82, 90–94. [Google Scholar] [CrossRef]
- Kim, H.Y. Analysis of variance (ANOVA) comparing means of more than two groups. Restor. Dent. Endod. 2014, 39, 74–77. [Google Scholar] [CrossRef] [Green Version]
- Solerte, S.B.; Gazzaruso, C.; Schifino, N.; Locatelli, E.; Destro, T.; Ceresini, G.; Ferrari, E.; Fioravanti, M. Metabolic effects of orally administered amino acid mixture in elderly subjects with poorly controlled type 2 diabetes mellitus. Am. J. Cardiol. 2004, 93, 23A–29A. [Google Scholar] [CrossRef]
- Hung, L.C.; Basri, M.; Tejo, B.; Ismail, R.; Nang, H.L.L.; Abu Hassan, H.; May, C.Y. An improved method for the preparations of nanostructured lipid carriers containing heat-sensitive bioactives. Colloids Surf. B Biointerfaces 2011, 87, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Tamjidi, F.; Nasirpour, A.; Varshosaz, J.; Shahedi, M. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov. Food Sci. Emerg. Technol. 2013, 19, 29–43. [Google Scholar] [CrossRef]
- McDonald, T.J.W.; Cervenka, M.C. Ketogenic diet therapies for seizures and status epilepticus. Semin. Neurol. 2020, 40, 719–729. [Google Scholar] [CrossRef]
- Binyamin, O.; Nitzan, K.; Frid, K.; Ungar, Y.; Rosenmann, H.; Gabizon, R. Brain targeting of 9c,11t-Conjugated Linoleic Acid, a natural calpain inhibitor, preserves memory and reduces Aβ and P25 accumulation in 5XFAD mice. Sci. Rep. 2019, 9, 18437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ok, E.; Do, G.M.; Lim, Y.; Park, J.E.; Park, Y.J.; Kwon, O. Pomegranate vinegar attenuates adiposity in obese rats through coordinated control of AMPK signaling in the liver and adipose tissue. Lipids Health Dis. 2013, 12, 163. [Google Scholar] [CrossRef] [Green Version]
- Xia, B.; Shi, X.C.; Xie, B.C.; Zhu, M.Q.; Chen, Y.; Chu, X.Y.; Cai, G.H.; Liu, M.; Yang, S.Z.; Mitchell, G.A.; et al. Urolithin A exerts antiobesity effects through enhancing adipose tissue thermogenesis in mice. PLoS Biol. 2020, 18, e3000688. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, M.S.; Miyoshi, H.; Souza, S.C.; Cacicedo, J.M.; Saha, A.K.; Greenberg, A.S.; Ruderman, N.B. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte: Potential mechanism and physiological relevance. J. Biol. Chem. 2008, 283, 16514–16524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, F.; Zhang, X.N.; Wang, W.; Xing, D.M.; Xie, W.D.; Su, H.; Du, L.J. Evidence of anti-obesity effects of the pomegranate leaf extract in high-fat diet induced obese mice. Int. J. Obes. 2007, 31, 1023–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Tang, H.; Deng, R.; Wang, N.; Zhang, Y.; Wang, Y.; Liu, Y.; Li, F.; Wang, X.; Zhou, L. Berberine suppresses adipocyte differentiation via decreasing CREB transcriptional activity. PLoS ONE 2015, 10, e0125667. [Google Scholar] [CrossRef]
- Cheng, K.T.; Wang, Y.S.; Chou, H.C.; Chang, C.C.; Lee, C.K.; Juan, S.H. Kinsenoside-mediated lipolysis through an AMPK-dependent pathway in C3H10T1/2 adipocytes: Roles of AMPK and PPARα in the lipolytic effect of kinsenoside. Phytomedicine 2015, 22, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Mu, J.; Birnbaum, M.J. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis in 3T3-L1 adipocytes. J. Biol. Chem. 2003, 278, 43074–43080. [Google Scholar] [CrossRef] [Green Version]
- Garcia-rodríguez, D.; Giménez-Cassina, A. Ketone bodies in the brain beyond fuel metabolism: From excitability to gene expression and cell signaling. Front. Mol. Neurosci. 2021, 14, 732120. [Google Scholar] [CrossRef]
- Hasegawa, S.; Ikeda, Y.; Yamasaki, M.; Fukui, T. The role of acetoacetyl-CoA synthetase, a ketone body-utilizing enzyme, in 3T3-L1 adipocyte differentiation. Biol. Pharm. Bull. 2012, 35, 1980–1985. [Google Scholar] [CrossRef] [Green Version]
- Puchalska, P.; Crawford, P.A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [Green Version]
- Yeh, Y.Y. Ketone body synthesis from leucine by adipose tissue from different sites in the rat. Arch. Biochem. Biophys. 1984, 233, 10–18. [Google Scholar] [CrossRef]
- Singal, S.A.; Hazan, S.J.; Sydenstricker, V.P.; Littlejohn, J.M. The production of fatty livers in rats on threonine-and lysine-deficient diets. J. Biol. Chem. 1953, 200, 867–874. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subash-Babu, P.; Al-Numair, N.; Almuzaini, T.; Athinarayanan, J.; Alshatwi, A.A. Punicalagin and Ketogenic Amino Acids Loaded Organic Lipid Carriers Enhance the Bioavailability, Mitochondrial β-Oxidation, and Ketogenesis in Maturing Adipocytes. Nanomaterials 2022, 12, 368. https://doi.org/10.3390/nano12030368
Subash-Babu P, Al-Numair N, Almuzaini T, Athinarayanan J, Alshatwi AA. Punicalagin and Ketogenic Amino Acids Loaded Organic Lipid Carriers Enhance the Bioavailability, Mitochondrial β-Oxidation, and Ketogenesis in Maturing Adipocytes. Nanomaterials. 2022; 12(3):368. https://doi.org/10.3390/nano12030368
Chicago/Turabian StyleSubash-Babu, Pandurangan, Nouf Al-Numair, Tahani Almuzaini, Jegan Athinarayanan, and Ali Abdullah Alshatwi. 2022. "Punicalagin and Ketogenic Amino Acids Loaded Organic Lipid Carriers Enhance the Bioavailability, Mitochondrial β-Oxidation, and Ketogenesis in Maturing Adipocytes" Nanomaterials 12, no. 3: 368. https://doi.org/10.3390/nano12030368
APA StyleSubash-Babu, P., Al-Numair, N., Almuzaini, T., Athinarayanan, J., & Alshatwi, A. A. (2022). Punicalagin and Ketogenic Amino Acids Loaded Organic Lipid Carriers Enhance the Bioavailability, Mitochondrial β-Oxidation, and Ketogenesis in Maturing Adipocytes. Nanomaterials, 12(3), 368. https://doi.org/10.3390/nano12030368







