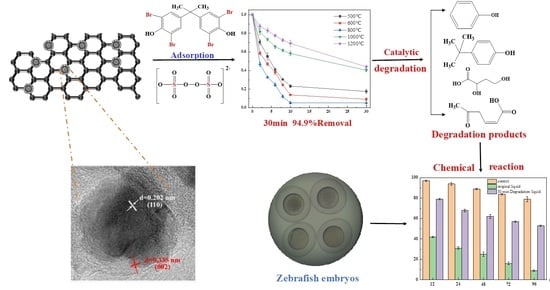
One-Step Synthesized Iron-Carbon Core-Shell Nanoparticles to Activate Persulfate for Effective Degradation of Tetrabromobisphenol A: Performance and Activation Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Materials
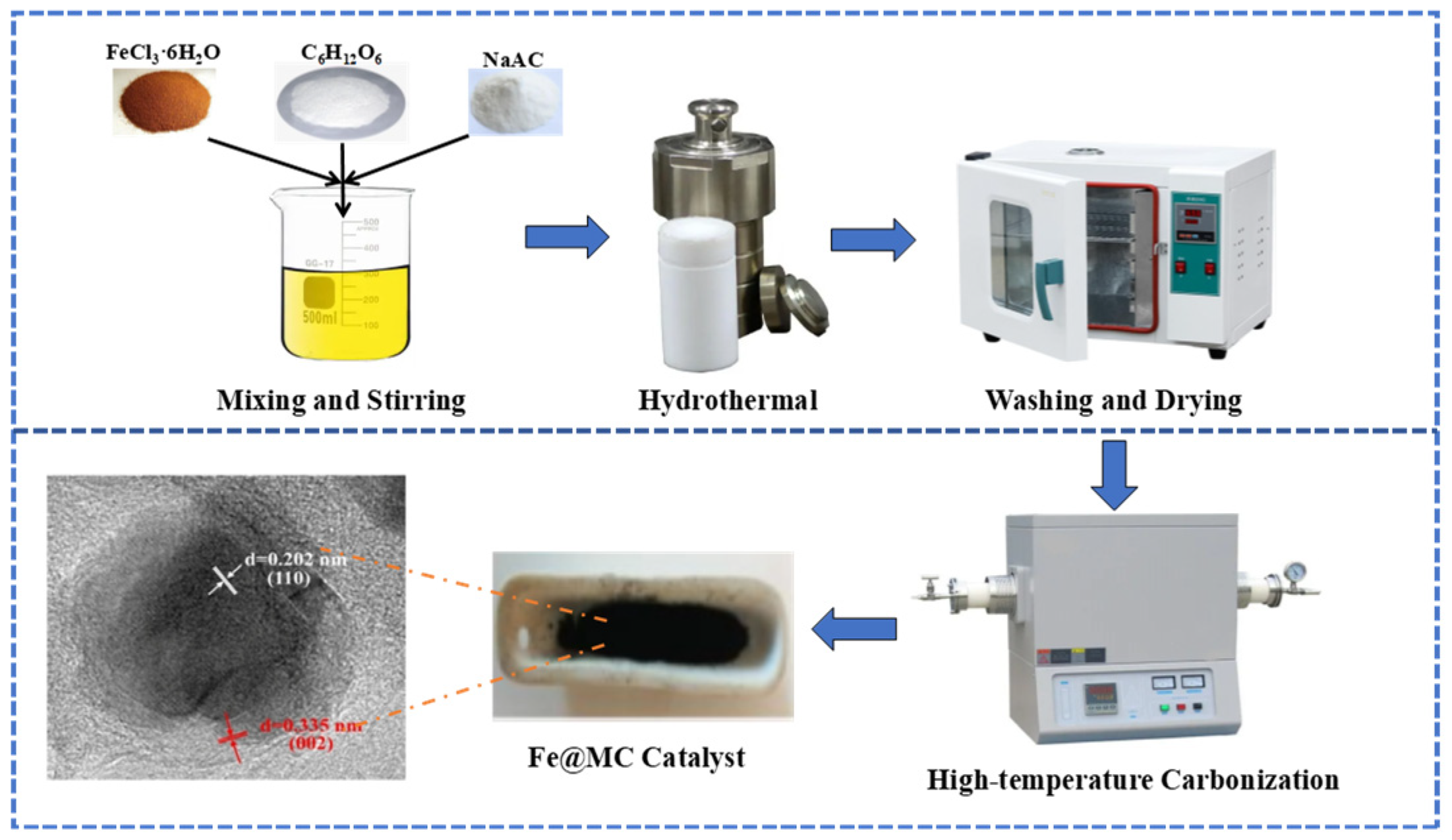
2.2. Material Synthesis
2.3. Material Characterization
2.4. Batch Experiments
2.5. Identification of Transformation Products
2.6. DFT Computational Details
2.7. Acute Toxicity Evaluation of Reaction Solutions
3. Results and Discussion
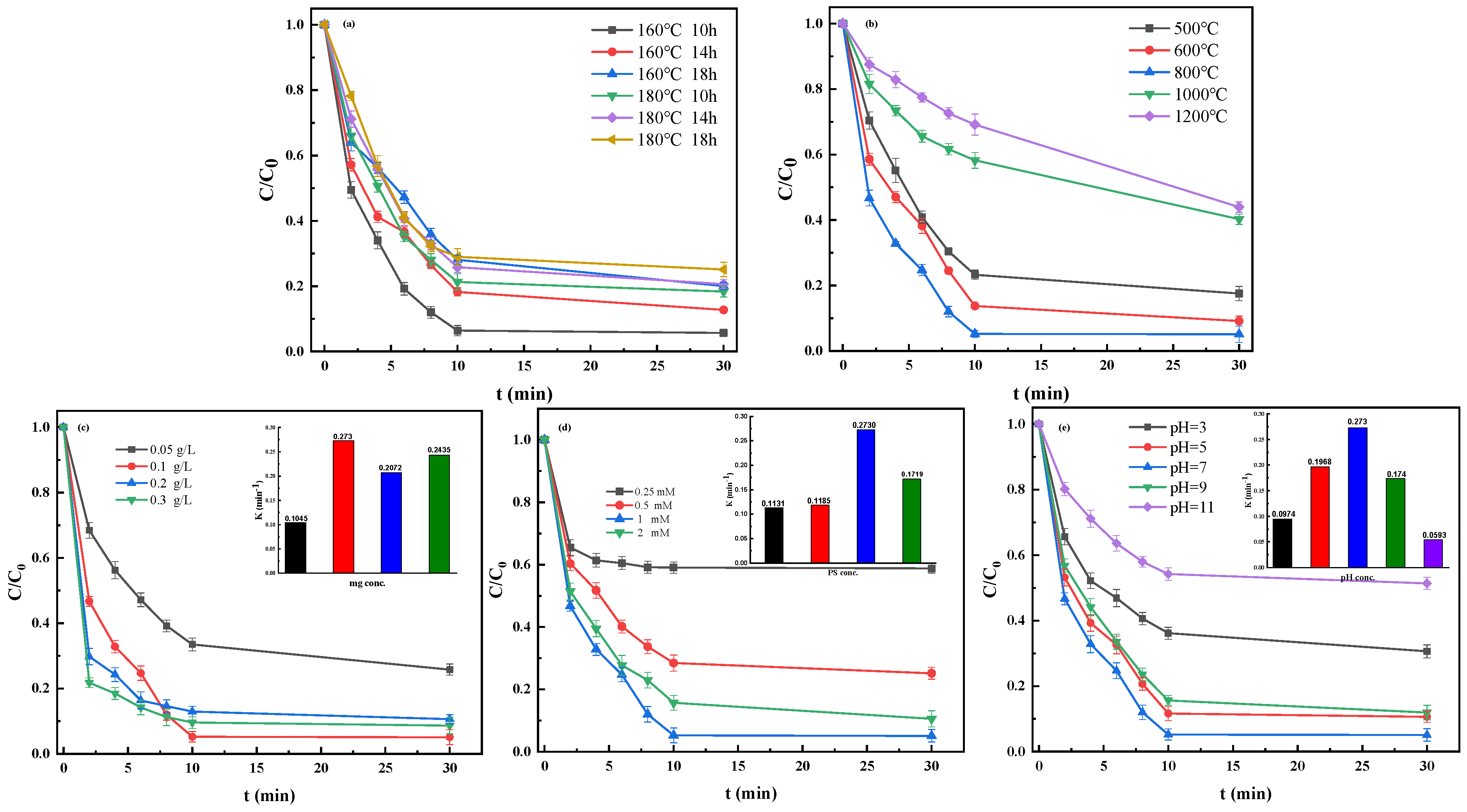
3.1. Performance of Materials Prepared with Different Conditions
3.2. Optimal Removal of TBBPA
3.2.1. Effect of Fe@MC Dosage, PS Dosage, and Initial pH
3.2.2. Effect of Co-Existing Substrates
3.3. Stability and Reusability
3.4. Mechanism Study
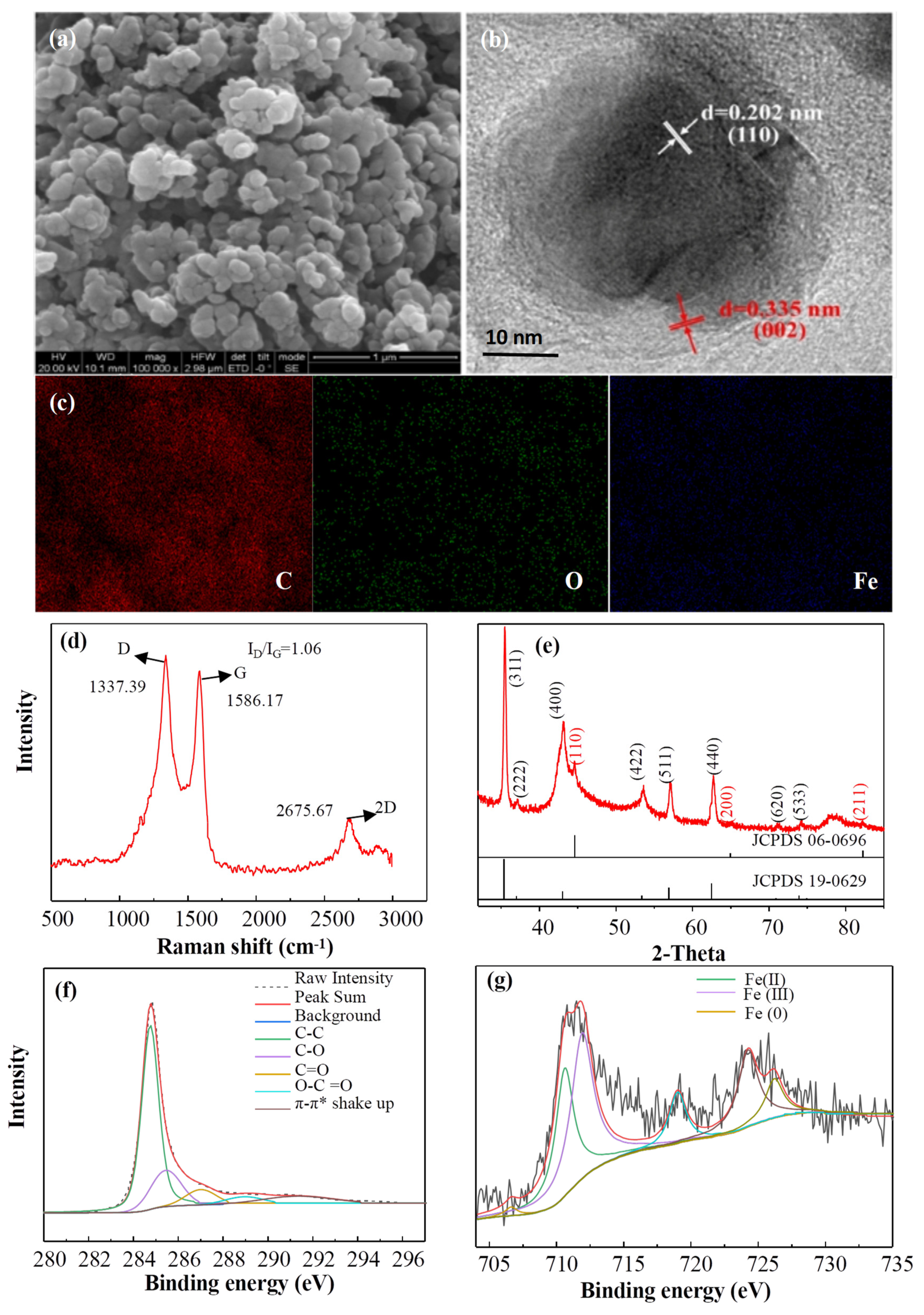
3.4.1. Characterization of Materials
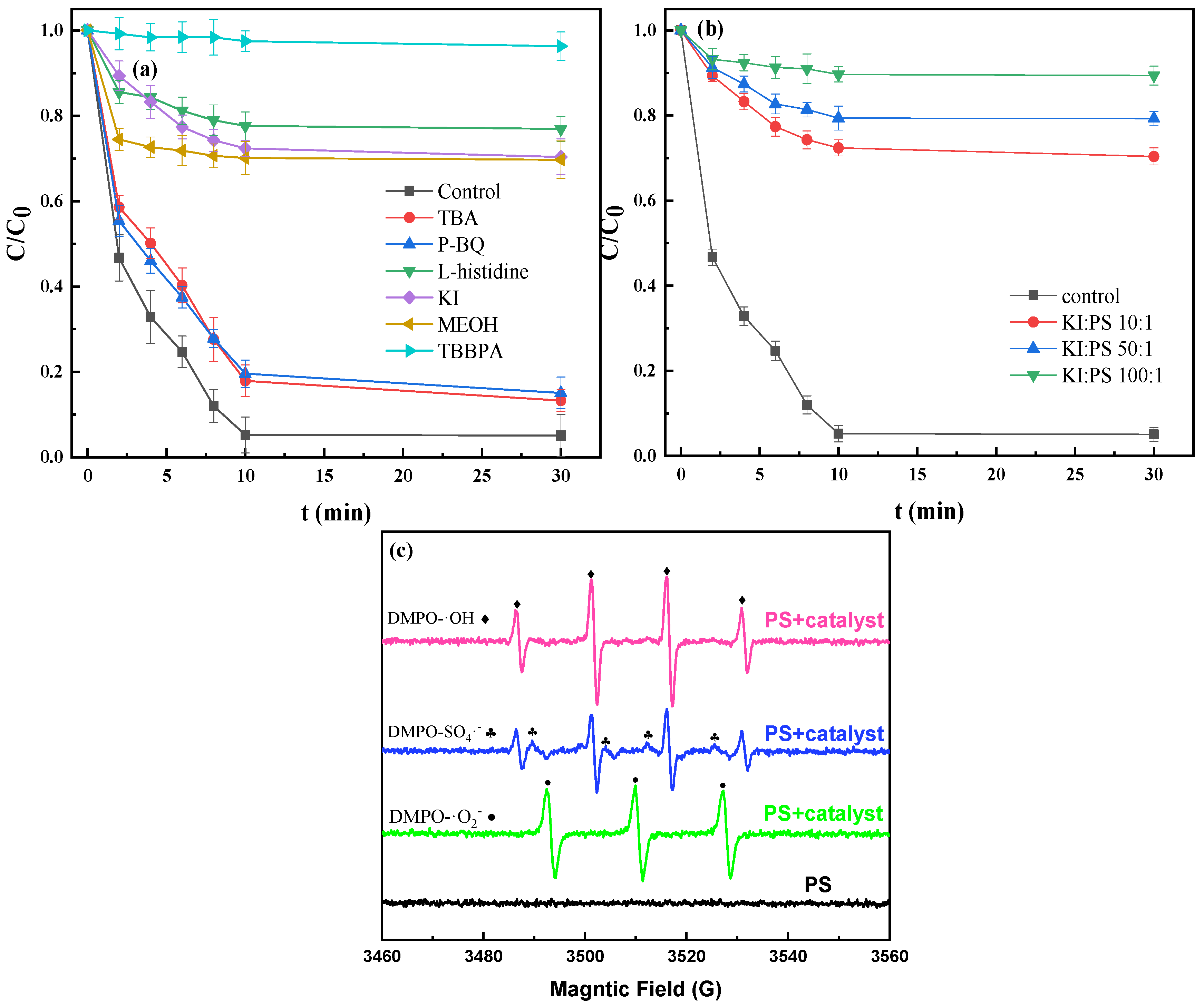
3.4.2. Identification of Free Radicals
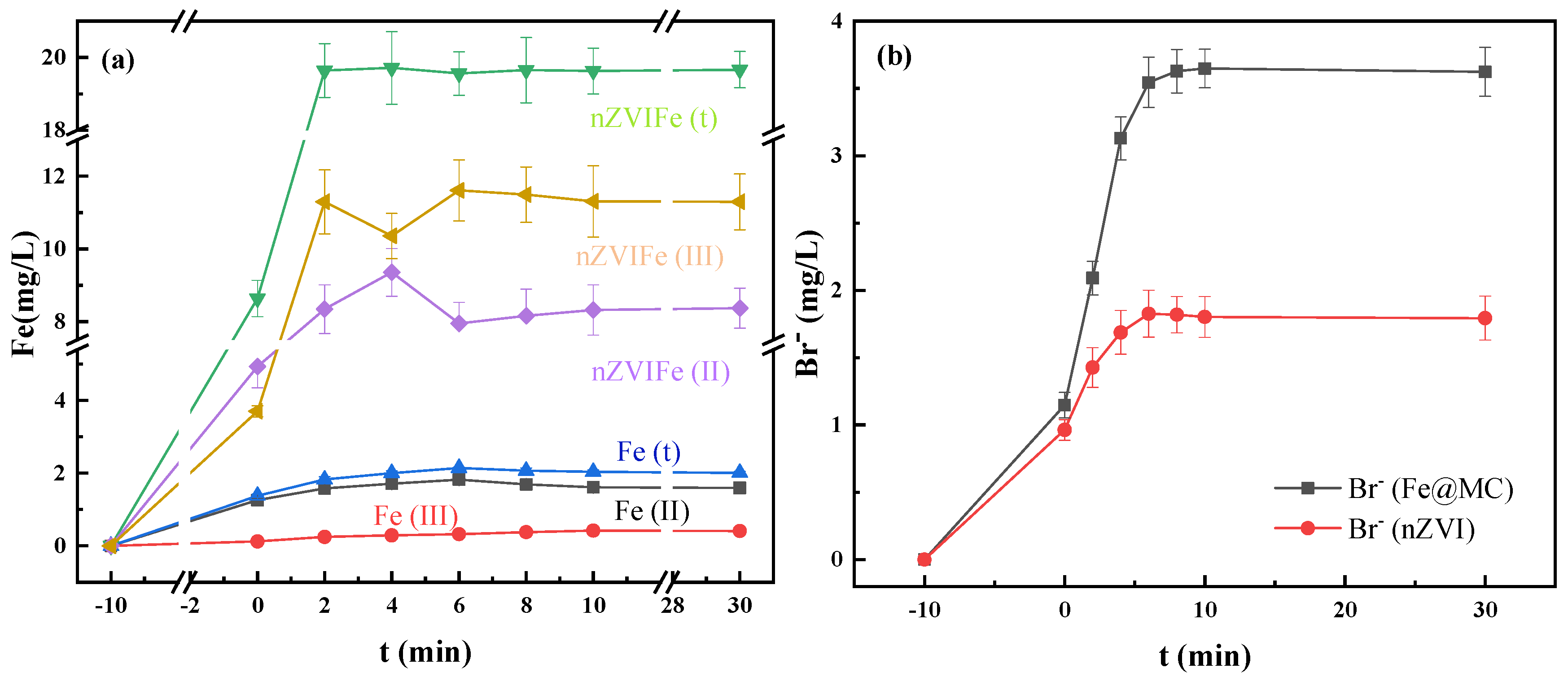
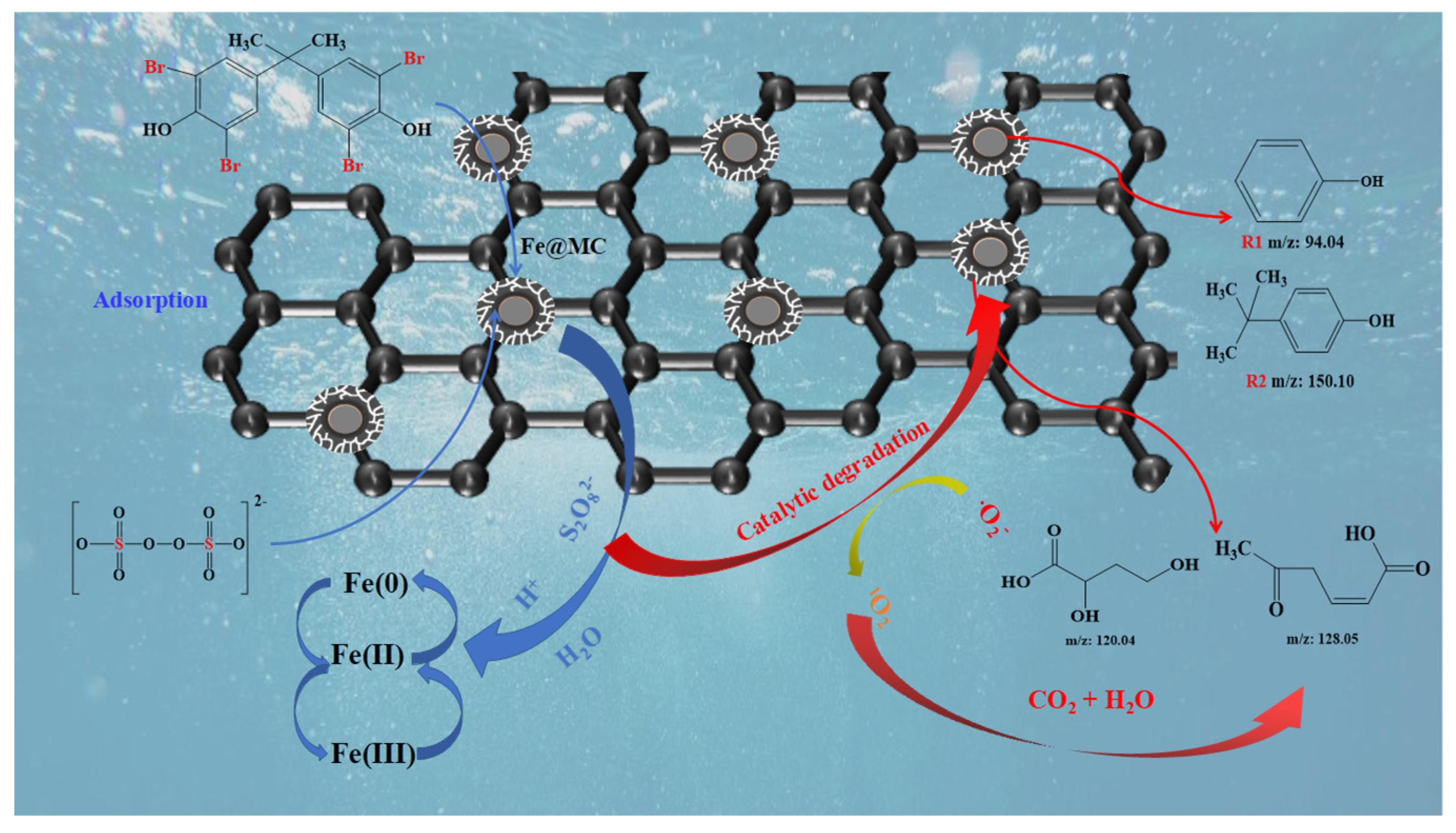
3.4.3. TBBPA Degradation Mechanism
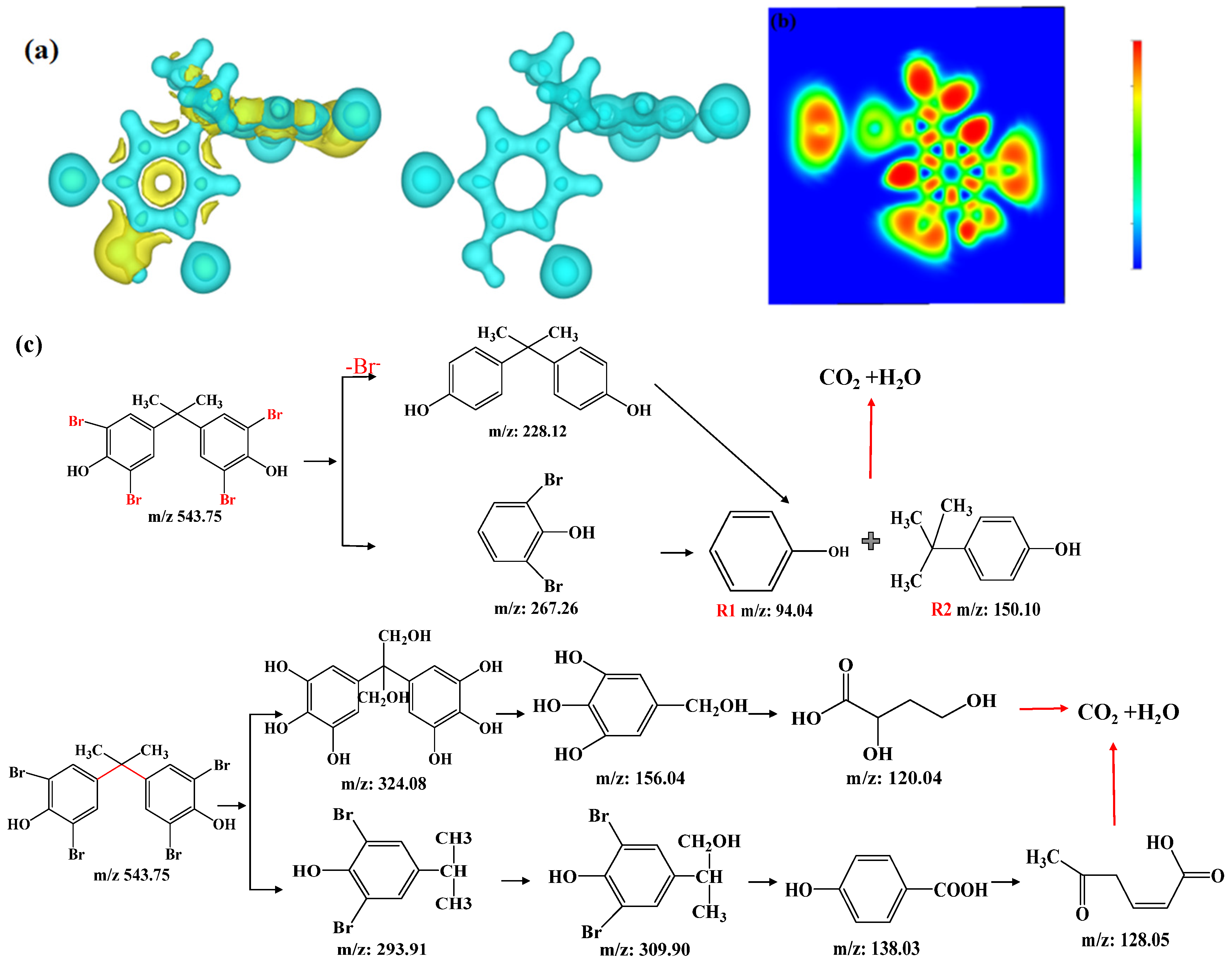
3.4.4. Intermediates and Degradation Pathways
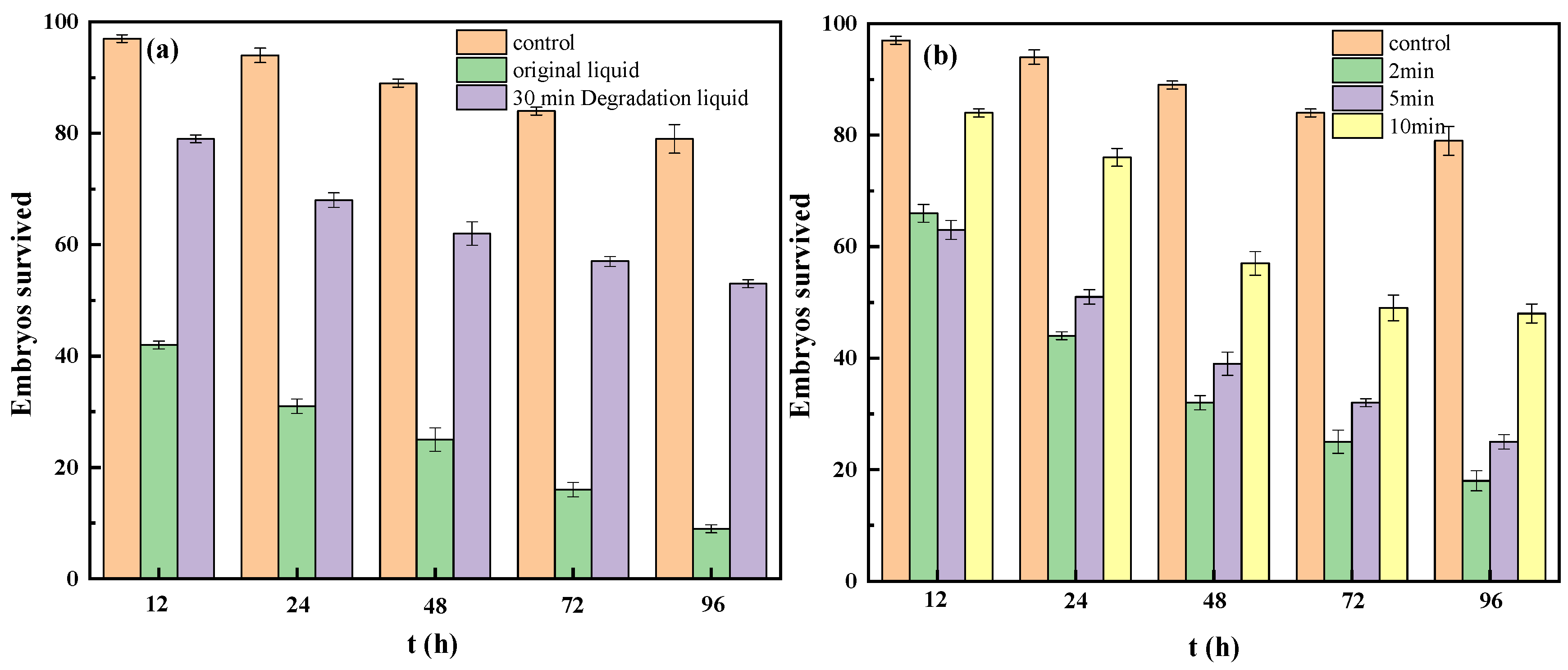
3.5. Toxicity of the Reaction Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, T.Y.; He, Y.Z.; Peng, X.X. Efficient Removal of Tetrabromobisphenol a (Tbbpa) Using Sewage Sludge-Derived Biochar: Adsorptive Effect and Mechanism. Chemosphere 2020, 251, 126370. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.L.; Lin, Q.; Gu, Y.G.; Du, F.Y.; Wang, X.H.; Shi, F.Q.; Ke, C.L.; Xiang, M.D.; Yu., Y.J. Bioaccumulation of Polycyclic Aromatic Hydrocarbons (Pahs) in Wild Marine Fish from the Coastal Waters of the Northern South China Sea: Risk Assessment for Human Health. Ecotoxicol. Environ. Saf. 2019, 180, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Yu., Y.J.; Liu, L.T.; Chen, X.C.; Xiang, M.D.; Li, Z.R.; Liu, Y.; Zeng, Y.; Han, Y.J.; Yu, Z.L. Brominated Flame Retardants and Heavy Metals in Common Aquatic Products from the Pearl River Delta, South China: Bioaccessibility Assessment and Human Health Implications. J. Hazard. Mater. 2021, 403, 124036. [Google Scholar] [PubMed]
- Yu, Y.J.; Yu, Z.L.; Chen, H.B.; Han, Y.J.; Xiang, M.D.; Chen, X.C.; Ma, R.X.; Wang, Z.D. Tetrabromobisphenol A: Disposition, Kinetics and Toxicity in Animals and Humans. Environ. Pollut. 2019, 253, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Do, Q.C.; Kim, D.G.; Ko, S.O. Nonsacrificial Template Synthesis of Magnetic-Based Yolk-Shell Nanostructures for the Removal of Acetaminophen in Fenton-Like Systems. ACS Appl. Mater. Interfaces 2017, 9, 28508–28518. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, M.G.; De La Cruz, A.A.; Dionysiou, D.D. Degradation of Microcystin-Lr Using Sulfate Radicals Generated through Photolysis, Thermolysis and e(-) Transfer Mechanisms. Appl. Catal. B Environ. 2010, 96, 290–298. [Google Scholar] [CrossRef]
- Kong, Z.; Lu, L.; Zhu, C.; Xu, J.; Fang, Q.; Liu, R.; Shen, Y. Enhanced adsorption and photocatalytic removal of PFOA from water by F-functionalized MOF with in-situ-growth TiO2: Regulation of electron density and bandgap. Sep. Purif. Technol. 2022, 297, 121449. [Google Scholar] [CrossRef]
- Shu, H.Y.; Chang, M.C.; Huang, S.W. Decolorization and Mineralization of Azo Dye Acid Blue 113 by the Uv/Oxone Process and Optimization of Operating Parameters. Desalin. Water Treat. 2016, 57, 7951–7962. [Google Scholar] [CrossRef]
- Zou, L.; Zhu, X.; Lu, L.; Xu, Y.; Chen, B. Bimetal organic framework/graphene oxide derived magnetic porous composite catalyst for peroxymonosulfate activation in fast organic pollutant degradation. J. Hazard. Mater. 2021, 419, 126427. [Google Scholar] [CrossRef]
- Xie, W.P.; Dong, W.; Kong, D.; Ji, Y.; Lu, J.; Yin, X. Formation of Halogenated Disinfection by-Products in Cobalt-Catalyzed Peroxymonosulfate Oxidation Processes in the Presence of Halides. Chemosphere 2016, 154, 613–619. [Google Scholar] [CrossRef]
- Oh, W.D.; Dong, Z.L.; Lim, T.T. Generation of Sulfate Radical through Heterogeneous Catalysis for Organic Contaminants Removal: Current Development, Challenges and Prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Liu, H.Z.; Bruton, T.A.; Li, W.; Van, B.J.; Prasse, C.; Doyle, F.M.; Sedlak, D.L. Oxidation of Benzene by Persulfate in the Presence of Fe(III)- and Mn(IV)-Containing Oxides: Stoichiometric Efficiency and Transformation Products. Environ. Sci. Technol. 2016, 50, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, N.; Liu, S.Q.; Asadi, A.; Shahlaei, M.; Moradi, S. Enhanced Heterogeneous Fenton Oxidation of Organic Pollutant Via Fe-Containing Mesoporous Silica Composites: A Review. J. Mol. Liq. 2021, 321, 114896. [Google Scholar] [CrossRef]
- Wang, C.; Jian, K.; Ping, L.; Huayang, Z.; Hongqi, S.; Tade Moses, O.; Shaobin, W. Ferric Carbide Nanocrystals Encapsulated in Nitrogen-Doped Carbon Nanotubes as an Outstanding Environmental Catalyst. Environ. Sci. Nano 2017, 4, 170–179. [Google Scholar] [CrossRef]
- Sun, Z.K.; Sun, B.; Qiao, M.H.; Wei, J.; Yue, Q.; Wang, C.; Deng, Y.H.; Serge, K.; Zhao, D.Y. A General Chelate-Assisted Co-Assembly to Metallic Nanoparticles-Incorporated Ordered Mesoporous Carbon Catalysts for Fischer-Tropsch Synthesis. J. Am. Chem. Soc. 2012, 134, 17653–17660. [Google Scholar] [CrossRef]
- Wu, Y.W.; Chen, X.T.; Han, Y.; Yue, D.T.; Cao, X.D.; Zhao, Y.X.; Qian, X.F. Highly Efficient Utilization of Nano-Fe(0) Embedded in Mesoporous Carbon for Activation of Peroxydisulfate. Environ. Sci. Technol. 2019, 53, 9081–9090. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (Dft-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, W.; Xu, Y.; Zhu, Z.; Cui, F. Iron Sludge-Derived Magnetic Fe0/Fe3C Catalyst for Oxidation of Ciprofloxacin Via Peroxymonosulfate Activation. Chem. Eng. J. 2019, 365, 99–110. [Google Scholar] [CrossRef]
- Liu, L.; Xing, L.; Li, Y.W.; Su, R.D.; Li, Q.; Zhou, W.Z.; Gao, B.Y.; Yue, Q.Y. One-Step Synthesis of “Nuclear-Shell” Structure Iron-Carbon Nanocomposite as a Persulfate Activator for Bisphenol a Degradation. Chem. Eng. J. 2020, 382, 122780. [Google Scholar] [CrossRef]
- Lv, Y.C.; Zhang, Z.; Chen, Y.C.; Hu, Y.Y. A Novel Three-Stage Hybrid Nano Bimetallic Reduction/Oxidation/Biodegradation Treatment for Remediation of 2,2′4,4′-Tetrabromodiphenyl Ether. Chem. Eng. J. 2016, 289, 382–390. [Google Scholar] [CrossRef]
- Li, H.C.; Chao, S.; Bingcai, P. Fe(III)-Doped g-C3N4 Mediated Peroxymonosulfate Activation for Selective Degradation of Phenolic Compounds via High-Valent Iron-Oxo Species. Environ. Sci. Technol. 2018, 52, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, N.; Du, Y.C.; Tong, T.Z.; Zhang, L.; Lin, K.; Han, X.J. One-Step Synthesis of Novel Fe3C@Nitrogen-Doped Carbon Nanotubes/Graphene Nanosheets for Catalytic Degradation of Bisphenol a in the Presence of Peroxymonosulfate. Chem. Eng. J. 2019, 356, 1022–1031. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Y.; Jiang, X.; Xie, Z.; Li, X.; Chen, C.; Chen, H. Impacts of Inorganic Anions and Natural Organic Matter on Thermally Activated Persulfate Oxidation of Btex in Water. Chemosphere 2018, 190, 296–306. [Google Scholar] [CrossRef]
- Lou, X.Y.; Wu, L.X.; Guo, Y.G.; Chen, C.C.; Wang, Z.H.; Xiao, D.X.; Fang, C.G.; Liu, J.S.; Zhao, J.C.; Lu, S.Y. Peroxymonosulfate Activation by Phosphate Anion for Organics Degradation in Water. Chemosphere 2014, 117, 582–585. [Google Scholar] [CrossRef]
- Shon, H.K.; Vigneswaran, S.; Snyder, S.A. Effluent Organic Matter (Efom) in Wastewater: Constituents, Effects, and Treatment. Crit. Rev. Environ. Sci. Technol. 2006, 36, 327–374. [Google Scholar] [CrossRef]
- Yu, F.; Chen, J.H.; Chen, L.; Jing, H.; Gong, W.Y.; Yuan, Z.W.; Wang, J.H.; Ma, J. Magnetic Carbon Nanotubes Synthesis by Fenton’s Reagent Method and Their Potential Application for Removal of Azo Dye from Aqueous Solution. J. Colloid Interface Sci. 2012, 378, 175–183. [Google Scholar] [CrossRef]
- Grierson, D.S.; Sumant, A.V.; Konicek, A.R.; Friedmann, T.A.; Sullivan, J.P.; Carpick, R.W. Thermal Stability and Rehybridization of Carbon Bonding in Tetrahedral Amorphous Carbon. J. Appl. Phys. 2010, 107, 033523. [Google Scholar] [CrossRef]
- Jiang, B.; Dai, D.J.; Yao, Y.Y.; Xu, T.F.; Li, R.H.; Xie, R.J.; Chen, L.K.; Chen, W.X. The Coupling of Hemin with Persistent Free Radicals Induces a Nonradical Mechanism for Oxidation of Pollutants. Chem. Commun. 2016, 52, 9566–9569. [Google Scholar] [CrossRef]
- Xu, L.J.; Wang, J.L. Degradation of Chlorophenols Using a Novel Fe-0/CeO2 Composite. Appl. Catal. B Environ. 2013, 142, 396–405. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Wang, L.Y.; Xu, H.D.; Wen, Q.X. Efficient Heterogeneous Activation of Peroxymonosulfate by Modified CuFe2O4 for Degradation of Tetrabromobisphenol A. Chem. Eng. J. 2020, 389, 7951–7962. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.F.; Hu, Y.Y.; Fang, Z.; Cheng, J.H.; Chen, Y.C. Adsorption Characteristics of Tetrabromobisphenol a onto Sodium Bisulfite Reduced Graphene Oxide Aerogels. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 781–788. [Google Scholar] [CrossRef]
- Li, H.X.; Wan, J.Q.; Ma, Y.W.; Wang, Y.; Chen, X.; Guan, Z.Y. Degradation of Refractory Dibutyl Phthalate by Peroxymonosulfate Activated with Novel Catalysts Cobalt Metal-Organic Frameworks: Mechanism, Performance, and Stability. J. Hazard. Mater. 2016, 318, 154–163. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M.A. Application of Peroxymonosulfate and Its Activation Methods for Degradation of Environmental Organic Pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Ahn, Y.Y.; Bae, H.; Kim, H.I.; Kim, S.H.; Kim, J.H.; Lee, S.G.; Lee, J. Surface-Loaded Metal Nanoparticles for Peroxymonosulfate Activation: Efficiency and Mechanism Reconnaissance. Appl. Catal. B Environ. 2019, 241, 561–569. [Google Scholar] [CrossRef]
- Ahn, S.; Peterson, T.D.; Righter, J.; Miles, D.M.; Tratnyek, P.G. Disinfection of Ballast Water with Iron Activated Persulfate. Environ. Sci. Technol. 2013, 47, 11717–11725. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Yang, S.; Sun, C.; Wang, X. Feasibility of a Two-Stage Reduction/Subsequent Oxidation for Treating Tetrabromobisphenol a in Aqueous Solutions. Water Res. 2011, 45, 1519–1528. [Google Scholar] [CrossRef]
- Zeng, C.; Yan, W.; Tong, X.; Zhicheng, Y.; Jinquan, W.; Quanmo, X. Targeted Degradation of TBBPA Using Novel Molecularly Imprinted Polymer Encapsulated C-Fe-Nx Nanocomposite Driven from Mofs. J. Hazard. Mater. 2022, 424, 127499. [Google Scholar] [CrossRef]
- Yu., Y.J.; Hou, Y.B.; Dang, Y.; Zhu, X.H.; Li, Z.C.; Chen, H.B.; Xiang, M.D.; Li, Z.R.; Hu, G.C. Exposure of Adult Zebrafish (Danio Rerio) to Tetrabromobisphenol a Causes Neurotoxicity in Larval Offspring, an Adverse Transgenerational Effect. J. Hazard. Mater. 2021, 414, 125408. [Google Scholar] [CrossRef]
- Xiang, M.H.; Huang, M.F.; Li, H.; Wang, W.B.; Huang, Y.; Lu, Z.; Wang, C.; Si, R.F.; Cao, W. Nanoscale zero-valent iron/cobalt@mesoporous hydrated silica core–shell particles as a highly active heterogeneous Fenton catalyst for the degradation of tetrabromobisphenol A. Chem. Eng. J. 2021, 417, 129208. [Google Scholar] [CrossRef]
- Li, H.; Wan, J.; Ma, Y.; Yan, W. Synthesis of novel core–shell Fe@Fe3O4 as heterogeneous activator of persulfate for oxidation of dibutyl phthalate under neutral conditions. Chem. Eng. J. 2016, 301, 315–324. [Google Scholar] [CrossRef]
- Jonidi, J.A.; Kakavandi, B.; Jaafarzadeh, N.; Rezaei, K.R.; Ahmadi, M.; Akbar, B.A. Fenton-like catalytic oxidation of tetracycline by AC@Fe3O4 as a heterogeneous persulfate activator: Adsorption and degradation studies. Ind. Eng. Chem. 2017, 45, 323–333. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Liu, C.; Yang, C.; Yu, Y.; Lu, L.; Ma, R.; Li, L. One-Step Synthesized Iron-Carbon Core-Shell Nanoparticles to Activate Persulfate for Effective Degradation of Tetrabromobisphenol A: Performance and Activation Mechanism. Nanomaterials 2022, 12, 4483. https://doi.org/10.3390/nano12244483
Yu Y, Liu C, Yang C, Yu Y, Lu L, Ma R, Li L. One-Step Synthesized Iron-Carbon Core-Shell Nanoparticles to Activate Persulfate for Effective Degradation of Tetrabromobisphenol A: Performance and Activation Mechanism. Nanomaterials. 2022; 12(24):4483. https://doi.org/10.3390/nano12244483
Chicago/Turabian StyleYu, Yunjiang, Chang Liu, Chenyu Yang, Yang Yu, Lun Lu, Ruixue Ma, and Liangzhong Li. 2022. "One-Step Synthesized Iron-Carbon Core-Shell Nanoparticles to Activate Persulfate for Effective Degradation of Tetrabromobisphenol A: Performance and Activation Mechanism" Nanomaterials 12, no. 24: 4483. https://doi.org/10.3390/nano12244483
APA StyleYu, Y., Liu, C., Yang, C., Yu, Y., Lu, L., Ma, R., & Li, L. (2022). One-Step Synthesized Iron-Carbon Core-Shell Nanoparticles to Activate Persulfate for Effective Degradation of Tetrabromobisphenol A: Performance and Activation Mechanism. Nanomaterials, 12(24), 4483. https://doi.org/10.3390/nano12244483