Preclinical PET and MR Evaluation of 89Zr- and 68Ga-Labeled Nanodiamonds in Mice over Different Time Scales
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Coating of the Nanodiamonds (NDs)
2.2. Radiolabeling and Stability Testing
2.3. Cell Culture
2.4. Mouse Models
2.5. In Vivo Measurements
2.5.1. MR Measurements
2.5.2. PET Measurements
2.6. Gamma Counter Measurements
2.7. Data Evaluation and Statistics
3. Results
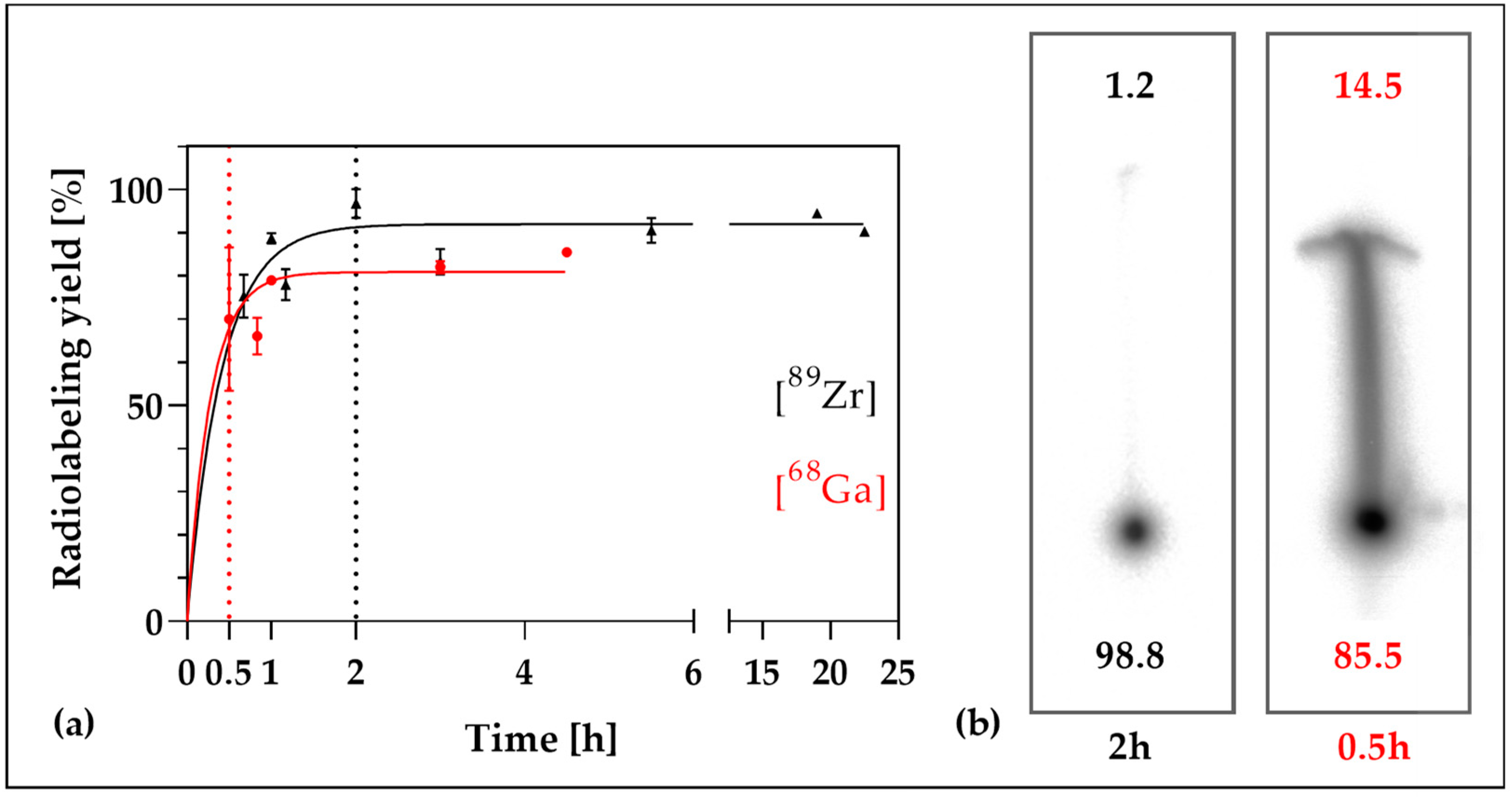
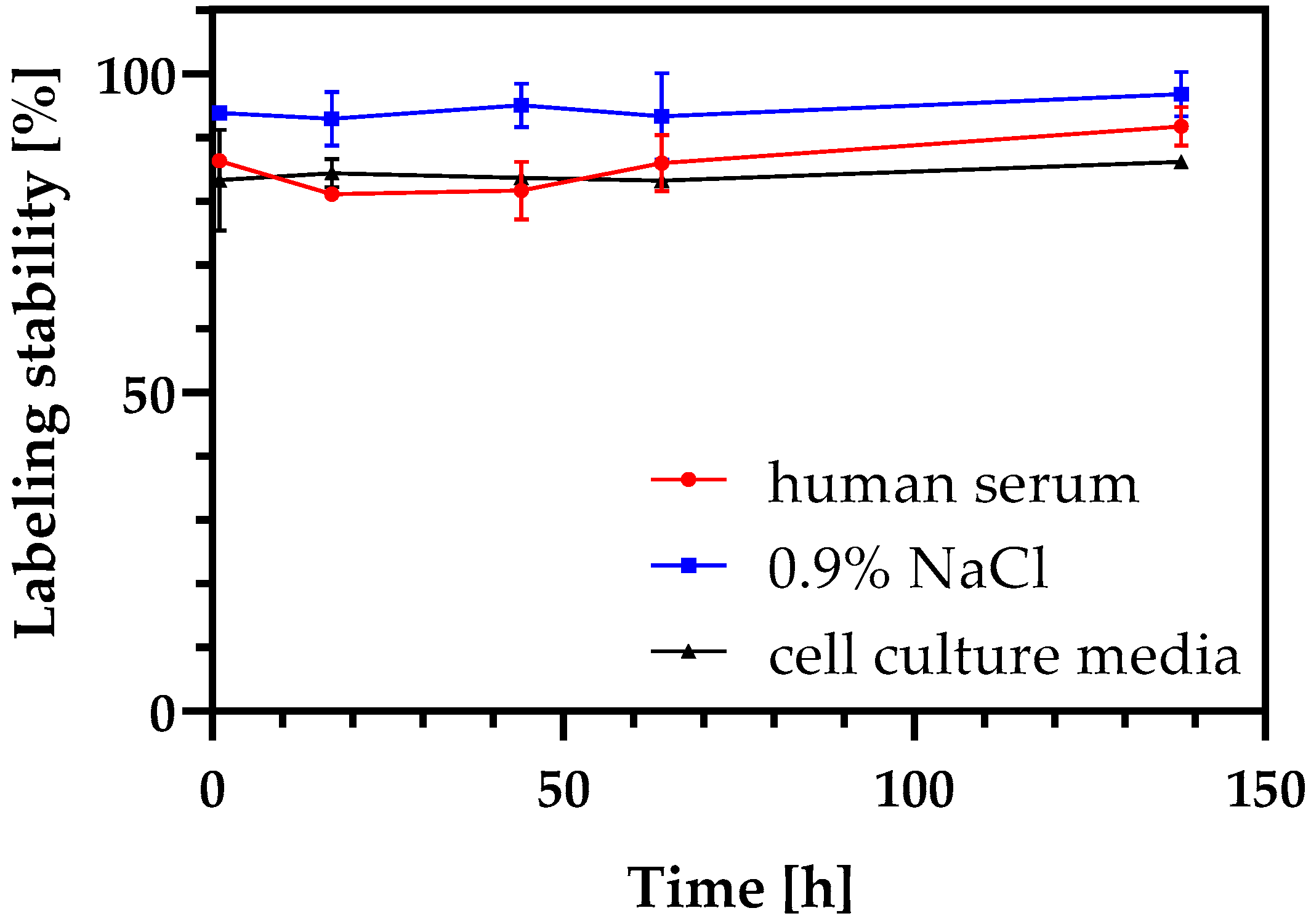
3.1. Radiolabeling of DFO-NDs and Stability of Radiolabeled DFO-NDs
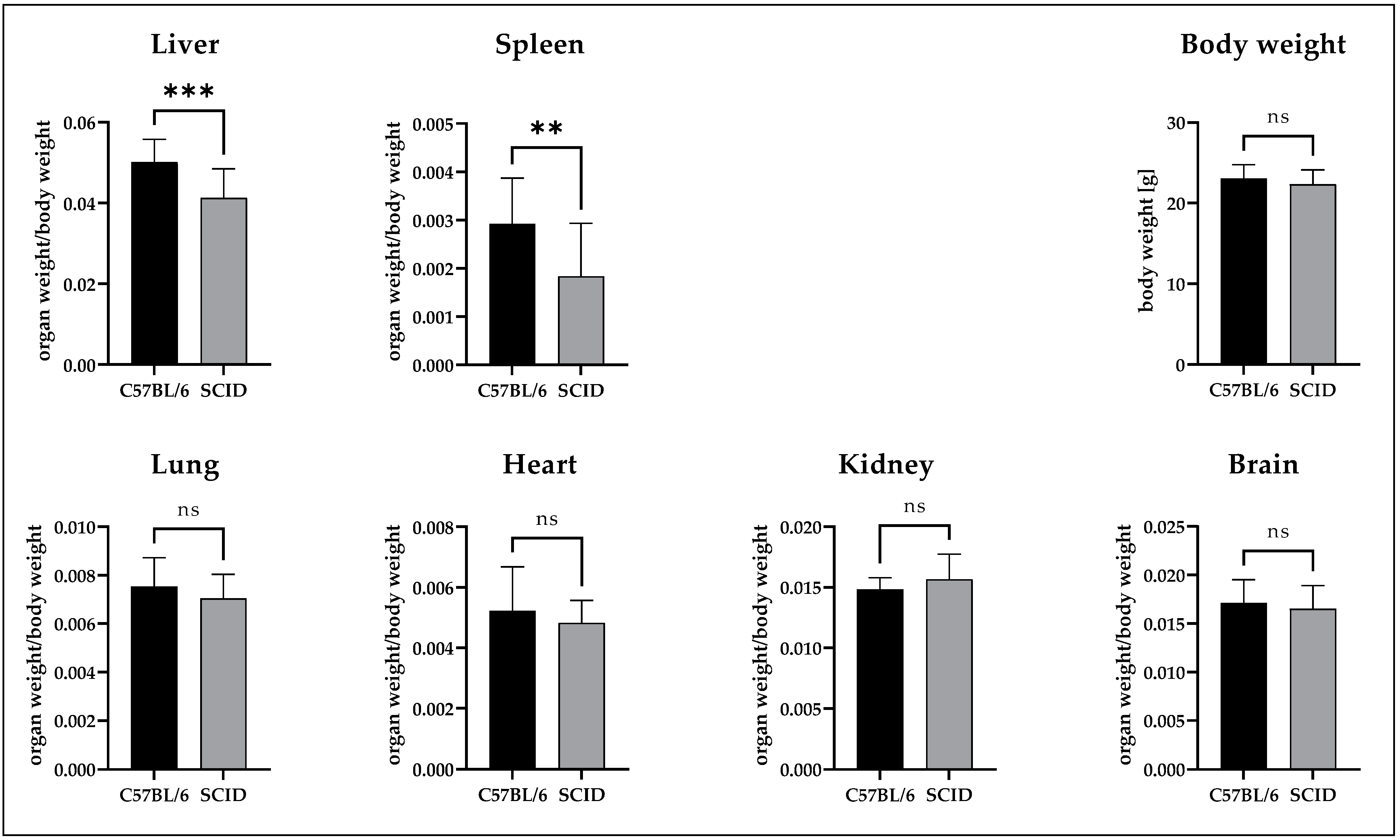
3.2. Comparison of the Organ Weights
3.3. Evaluation of PET and MR-Based Imaging
3.4. Ex Vivo Biodistribution Validation Based on Gamma-Counter Data
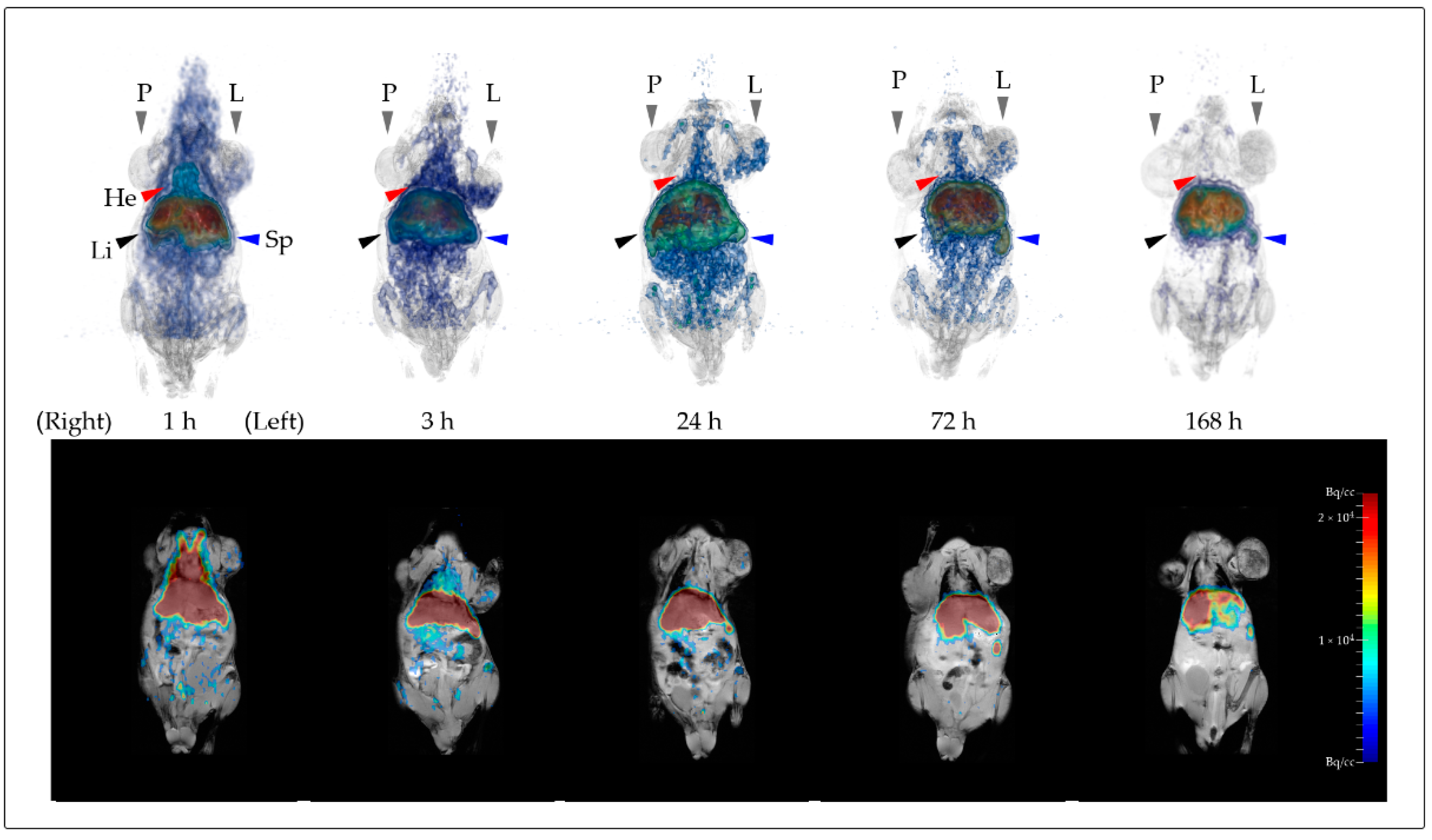
3.5. In Vivo Biodistribution Validation Based on PET and MRI Data
3.6. Correlation Analysis
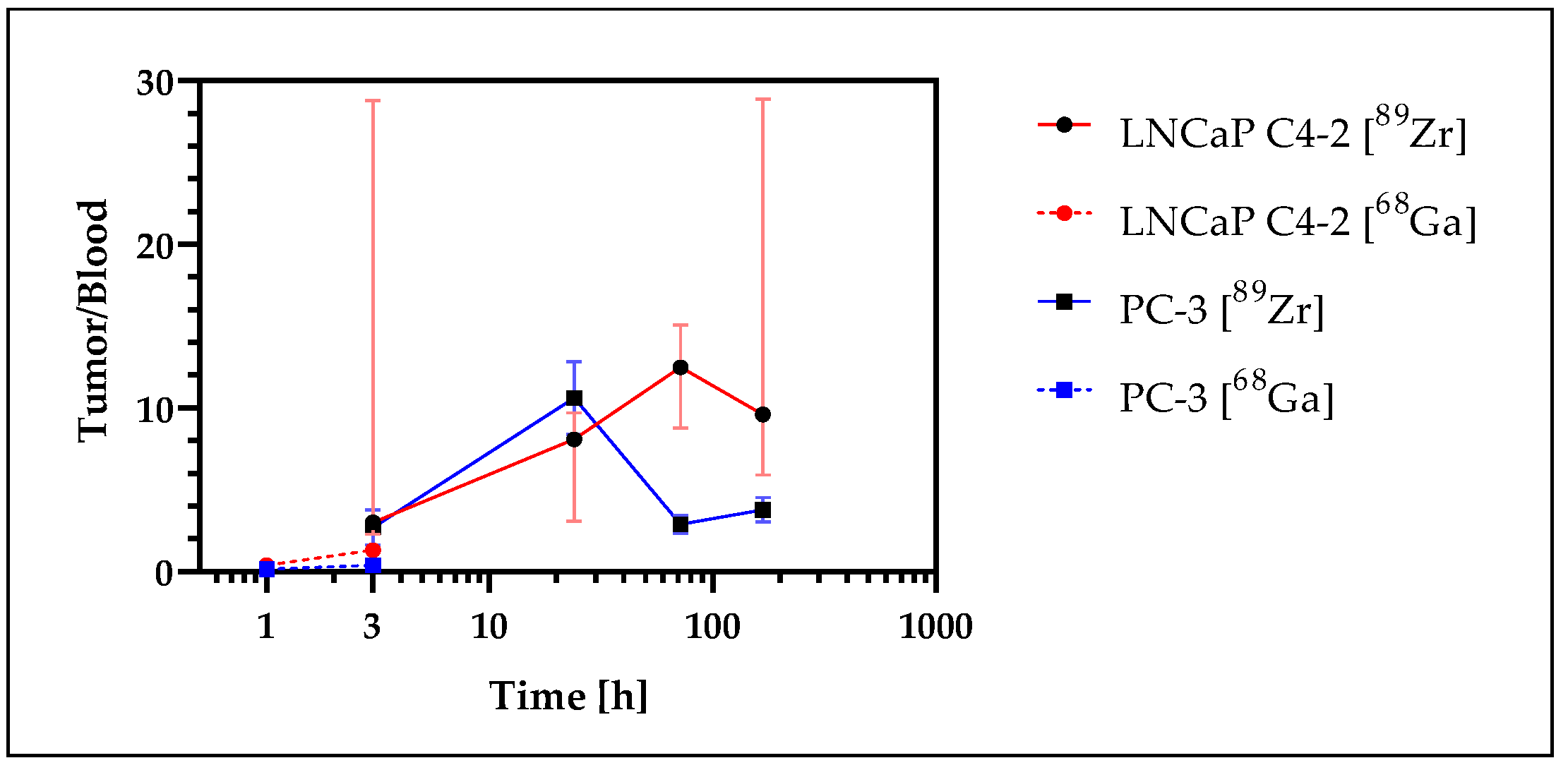
3.7. Tumor Uptake Analysis
4. Discussion
4.1. Radiolabeling of NDs
4.2. Biodistribution of Nanodiamonds in Normal Organs
4.3. Accumulation of NDs in Tumor Xenografts
4.4. Variance in Mouse Models
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwiatkowski, G.; Jahnig, F.; Steinhauser, J.; Wespi, P.; Ernst, M.; Kozerke, S. Direct hyperpolarization of micro- and nanodiamonds for bioimaging applications—Considerations on particle size, functionalization and polarization loss. J. Magn. Reson. 2018, 286, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Rej, E.; Gaebel, T.; Boele, T.; Waddington, D.E.; Reilly, D.J. Hyperpolarized nanodiamond with long spin-relaxation times. Nat. Commun. 2015, 6, 8459. [Google Scholar] [CrossRef] [PubMed]
- Rej, E.; Gaebel, T.; Waddington, D.E.; Reilly, D.J. Hyperpolarized Nanodiamond Surfaces. J. Am. Chem. Soc. 2017, 139, 193–199. [Google Scholar] [CrossRef]
- Wu, Y.; Jelezko, F.; Plenio, M.B.; Weil, T. Diamond Quantum Devices in Biology. Angew. Chem. Int. Ed. Engl. 2016, 55, 6586–6598. [Google Scholar] [CrossRef]
- Schwartz, I.; Scheuer, J.; Tratzmiller, B.; Muller, S.; Chen, Q.; Dhand, I.; Wang, Z.Y.; Muller, C.; Naydenov, B.; Jelezko, F.; et al. Robust optical polarization of nuclear spin baths using Hamiltonian engineering of nitrogen-vacancy center quantum dynamics. Sci. Adv. 2018, 4, eaat8978. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Martinez, G.V.; Gillies, R.J. Nanodiamond as a New Hyperpolarizing Agent and Its 13C MRS. J. Phys. Chem. Lett. 2014, 5, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Mindarava, Y.; Blinder, R.; Liu, Y.; Scheuer, J.; Lang, J.; Agafonov, V.; Davydov, V.A.; Laube, C.; Knolle, W.; Abel, B.; et al. Synthesis and coherent properties of 13C-enriched sub-micron diamond particles with nitrogen vacancy color centers. Carbon 2020, 165, 395–403. [Google Scholar] [CrossRef]
- Welch, M.J.; Hawker, C.J.; Wooley, K.L. The advantages of nanoparticles for PET. J. Nucl. Med. 2009, 50, 1743–1746. [Google Scholar] [CrossRef]
- Goel, S.; England, C.G.; Chen, F.; Cai, W. Positron emission tomography and nanotechnology: A dynamic duo for cancer theranostics. Adv. Drug Deliv. Rev. 2017, 113, 157–176. [Google Scholar] [CrossRef]
- Kim, J.; Lee, N.; Hyeon, T. Recent development of nanoparticles for molecular imaging. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2017, 375, 20170022. [Google Scholar] [CrossRef]
- Arms, L.; Smith, D.W.; Flynn, J.; Palmer, W.; Martin, A.; Woldu, A.; Hua, S. Advantages and Limitations of Current Techniques for Analyzing the Biodistribution of Nanoparticles. Front. Pharmacol. 2018, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Boudon, J.; Maurizi, L.; Moreau, M.; Walker, P.; Severin, I.; Oudot, A.; Goze, C.; Poty, S.; Vrigneaud, J.M.; et al. Innovative Magnetic Nanoparticles for PET/MRI Bimodal Imaging. ACS Omega 2019, 4, 2637–2648. [Google Scholar] [CrossRef] [PubMed]
- Perez-Medina, C.; Teunissen, A.J.P.; Kluza, E.; Mulder, W.J.M.; van der Meel, R. Nuclear imaging approaches facilitating nanomedicine translation. Adv. Drug Deliv. Rev. 2020, 154–155, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Valdovinos, H.F.; Chen, F.; Lewis, C.M.; Ellison, P.A.; Luo, H.; Meyerand, M.E.; Nickles, R.J.; Cai, W. Intrinsically germanium-69-labeled iron oxide nanoparticles: Synthesis and in-vivo dual-modality PET/MR imaging. Adv. Mater. 2014, 26, 5119–5123. [Google Scholar] [CrossRef] [PubMed]
- Forte, E.; Fiorenza, D.; Torino, E.; Costagliola di Polidoro, A.; Cavaliere, C.; Netti, P.A.; Salvatore, M.; Aiello, M. Radiolabeled PET/MRI Nanoparticles for Tumor Imaging. J. Clin. Med. 2019, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, R.; Goel, S.; Dash, A.; Cai, W. Radiolabeled inorganic nanoparticles for positron emission tomography imaging of cancer: An overview. Q. J. Nucl. Med. Mol. Imaging 2017, 61, 181–204. [Google Scholar] [CrossRef]
- Xing, Y.; Zhao, J.; Conti, P.S.; Chen, K. Radiolabeled nanoparticles for multimodality tumor imaging. Theranostics 2014, 4, 290–306. [Google Scholar] [CrossRef]
- Pretze, M.; van der Meulen, N.P.; Wangler, C.; Schibli, R.; Wangler, B. Targeted 64Cu-labeled gold nanoparticles for dual imaging with positron emission tomography and optical imaging. J. Label. Comp. Radiopharm. 2019, 62, 471–482. [Google Scholar] [CrossRef]
- Purtov, K.; Petunin, A.; Inzhevatkin, E.; Burov, A.; Ronzhin, N.; Puzyr, A.; Bondar, V. Biodistribution of Different Sized Nanodiamonds in Mice. J. Nanosci. Nanotechnol. 2015, 15, 1070–1075. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Kang, C.; Li, J.; Zhu, Y.; Li, W.; Huang, Q.; Zhu, Z. Biodistribution and toxicity of nanodiamonds in mice after intratracheal instillation. Toxicol. Lett. 2010, 198, 237–243. [Google Scholar] [CrossRef]
- Wei, Q.; Zhan, L.; Juanjuan, B.; Jing, W.; Jianjun, W.; Taoli, S.; Yi’an, G.; Wangsuo, W. Biodistribution of co-exposure to multi-walled carbon nanotubes and nanodiamonds in mice. Nanoscale Res. Lett. 2012, 7, 473. [Google Scholar] [CrossRef] [PubMed]
- Rojas, S.; Gispert, J.D.; Martin, R.; Abad, S.; Menchon, C.; Pareto, D.; Victor, V.M.; Alvaro, M.; Garcia, H.; Herance, J.R. Biodistribution of amino-functionalized diamond nanoparticles. In vivo studies based on 18F radionuclide emission. ACS Nano 2011, 5, 5552–5559. [Google Scholar] [CrossRef] [PubMed]
- Madamsetty, V.S.; Sharma, A.; Toma, M.; Samaniego, S.; Gallud, A.; Wang, E.; Pal, K.; Mukhopadhyay, D.; Fadeel, B. Tumor selective uptake of drug-nanodiamond complexes improves therapeutic outcome in pancreatic cancer. Nanomedicine 2019, 18, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Inzhevatkin, E.V.; Baron, A.V.; Volkova, M.B.; Maksimov, N.G.; Golubenko, N.K.; Loshkareva, M.V.; Puzyr, A.P.; Ronzhin, N.O.; Bondar, V.S. Biodistribution of Detonation Synthesis Nanodiamonds in Mice after Intravenous Administration and Some Biochemical Changes in Blood Plasma. Bull. Exp. Biol. Med. 2021, 172, 77–80. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, Y.W.; Lui, J.H.; Wang, H.F.; Liu, Y.F. Biodistribution and fate of nanodiamonds in vivo. Diam. Relat. Mater. 2009, 18, 95–100. [Google Scholar] [CrossRef]
- Kumar, K. The Current Status of the Production and Supply of Gallium-68. Cancer Biother. Radiopharm. 2020, 35, 163–166. [Google Scholar] [CrossRef]
- Karageorgou, M.A.; Vranješ-Djurić, S.; Radović, M.; Lyberopoulou, A.; Antić, B.; Rouchota, M.; Gazouli, M.; Loudos, G.; Xanthopoulos, S.; Sideratou, Z.; et al. Gallium-68 Labeled Iron Oxide Nanoparticles Coated with 2,3-Dicarboxypropane-1,1-diphosphonic Acid as a Potential PET/MR Imaging Agent: A Proof-of-Concept Study. Contrast Media Mol. Imaging 2017, 2017, 6951240. [Google Scholar] [CrossRef]
- Hajiramezanali, M.; Atyabi, F.; Mosayebnia, M.; Akhlaghi, M.; Geramifar, P.; Jalilian, A.R.; Mazidi, S.M.; Yousefnia, H.; Shahhosseini, S.; Beiki, D. 68Ga-radiolabeled bombesin-conjugated to trimethyl chitosan-coated superparamagnetic nanoparticles for molecular imaging: Preparation, characterization and biological evaluation. Int. J. Nanomed. 2019, 14, 2591–2605. [Google Scholar] [CrossRef]
- Miller, L.; Winter, G.; Baur, B.; Witulla, B.; Solbach, C.; Reske, S.; Linden, M. Synthesis, characterization, and biodistribution of multiple 89Zr-labeled pore-expanded mesoporous silica nanoparticles for PET. Nanoscale 2014, 6, 4928–4935. [Google Scholar] [CrossRef]
- McDonagh, P.R.; Sundaresan, G.; Yang, L.; Sun, M.; Mikkelsen, R.; Zweit, J. Biodistribution and PET imaging of 89-zirconium labeled cerium oxide nanoparticles synthesized with several surface coatings. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1429–1440. [Google Scholar] [CrossRef]
- Choi, P.S.; Lee, J.Y.; Yang, S.D.; Park, J.H. Biological behavior of nanoparticles with Zr-89 for cancer targeting based on their distinct surface composition. J. Mater. Chem. B 2021, 9, 8237–8245. [Google Scholar] [CrossRef] [PubMed]
- Baur, B.; Andreolli, E.; Al-Momani, E.; Malik, N.; Machulla, H.-J.; Reske, S.N.; Solbach, C. Synthesis and labelling of Df-DUPA-Pep with gallium-68 and zirconium-89 as new PSMA ligands. J. Radioanal. Nucl. Chem. 2014, 299, 1715–1721. [Google Scholar] [CrossRef]
- Gradishar, W.J. Albumin-bound paclitaxel: A next-generation taxane. Expert Opin. Pharmacother. 2006, 7, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ermakova, A.; Liu, W.; Pramanik, G.; Vu, T.M.; Kurz, A.; McGuinness, L.; Naydenov, B.; Hafner, S.; Reuter, R.; et al. Programmable Biopolymers for Advancing Biomedical Applications of Fluorescent Nanodiamonds. Adv. Funct. Mater. 2015, 25, 6576–6585. [Google Scholar] [CrossRef]
- Hoonjan, M.; Sachdeva, G.; Chandra, S.; Kharkar, P.S.; Sahu, N.; Bhatt, P. Investigation of HSA as a biocompatible coating material for arsenic trioxide nanoparticles. Nanoscale 2018, 10, 8031–8041. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Raabe, M.; Hodgson, L.; Mantell, J.; Verkade, P.; Lasser, T.; Landfester, K.; Weil, T.; Lieberwirth, I. High-Contrast Imaging of Nanodiamonds in Cells by Energy Filtered and Correlative Light-Electron Microscopy: Toward a Quantitative Nanoparticle-Cell Analysis. Nano Lett. 2019, 19, 2178–2185. [Google Scholar] [CrossRef]
- Moscariello, P.; Raabe, M.; Liu, W.N.; Bernhardt, S.; Qi, H.Y.; Kaiser, U.; Wu, Y.Z.; Weil, T.; Luhmann, H.J.; Hedrich, J. Unraveling In Vivo Brain Transport of Protein-Coated Fluorescent Nanodiamonds. Small 2019, 15, 1902992. [Google Scholar] [CrossRef]
- Moya, C.; Escudero, R.; Malaspina, D.C.; de la Mata, M.; Hernandez-Saz, J.; Faraudo, J.; Roig, A. Insights into Preformed Human Serum Albumin Corona on Iron Oxide Nanoparticles: Structure, Effect of Particle Size, Impact on MRI Efficiency, and Metabolization. ACS Appl. Bio Mater. 2019, 2, 3084–3094. [Google Scholar] [CrossRef]
- Chubarov, A.S. Serum Albumin for Magnetic Nanoparticles Coating. Magnetochemistry 2022, 8, 13. [Google Scholar] [CrossRef]
- Suckow, C.E.; Stout, D.B. MicroCT liver contrast agent enhancement over time, dose, and mouse strain. Mol. Imaging Biol. 2008, 10, 114–120. [Google Scholar] [CrossRef]
- Price, J.E. Xenograft models in immunodeficient animals: I. Nude mice: Spontaneous and experimental metastasis models. Methods Mol. Med. 2001, 58, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M. Laboratory Mice and Rats. Mater Methods 2012, 2, 125–132. [Google Scholar] [CrossRef]
- Chen, J.; Liao, S.; Xiao, Z.; Pan, Q.; Wang, X.; Shen, K.; Wang, S.; Yang, L.; Guo, F.; Liu, H.-f.; et al. The development and improvement of immunodeficient mice and humanized immune system mouse models. Front. Immunol. 2022, 13, 1007579. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.K.; Lee, S.C.; Han, B.; Park, K. Analysis on the current status of targeted drug delivery to tumors. J. Control. Release 2012, 164, 108–114. [Google Scholar] [CrossRef]
- Maeda, H.; Tsukigawa, K.; Fang, J. A Retrospective 30 Years After Discovery of the Enhanced Permeability and Retention Effect of Solid Tumors: Next-Generation Chemotherapeutics and Photodynamic Therapy—Problems, Solutions, and Prospects. Microcirculation 2016, 23, 173–182. [Google Scholar] [CrossRef]
- Ngoune, R.; Peters, A.; von Elverfeldt, D.; Winkler, K.; Pütz, G. Accumulating nanoparticles by EPR: A route of no return. J. Control. Release 2016, 238, 58–70. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef]
- Laube, C.; Oeckinghaus, T.; Lehnert, J.; Griebel, J.; Knolle, W.; Denisenko, A.; Kahnt, A.; Meijer, J.; Wrachtrup, J.; Abel, B. Controlling the fluorescence properties of nitrogen vacancy centers in nanodiamonds. Nanoscale 2019, 11, 1770–1783. [Google Scholar] [CrossRef]
- Wu, H.C.; Hsieh, J.T.; Gleave, M.E.; Brown, N.M.; Pathak, S.; Chung, L.W. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: Role of bone stromal cells. Int. J. Cancer 1994, 57, 406–412. [Google Scholar] [CrossRef]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Investig. Urol. 1979, 17, 16–23. [Google Scholar]
- Gaupel, A.C.; Wang, W.L.W.; Mordan-McCombs, S.; Lee, E.C.Y.; Tenniswood, M. Xenograft, Transgenic, and Knockout Models of Prostate Cancer. In Animal Models for the study of Human Disease; Academic Press: Cambridge, MA, USA, 2013; pp. 973–995. [Google Scholar] [CrossRef]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.Z.; Huang, J. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.; Winter, G.; Baur, B.; Kuntz, A.J.; Kull, T.; Solbach, C.; Beer, A.J.; Linden, M. Quantitative and correlative biodistribution analysis of 89Zr-labeled mesoporous silica nanoparticles intravenously injected into tumor-bearing mice. Nanoscale 2017, 9, 9743–9753. [Google Scholar] [CrossRef]
- Vollmar, S.; Hampl, J.A.; Kracht, L.; Herholz, K. Integration of Functional Data (PET) into Brain Surgery Planning and Neuronavigation. In Advances in Medical Engineering; Springer Proceedings in Physics; Springer: Berlin/Heidelberg, Germany, 2007; Volume 114, pp. 98–103. [Google Scholar]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Kletting, P.; Schimmel, S.; Hanscheid, H.; Fernandez, M.; Broer, J.; Nosske, D.; Lassmann, M.; Glatting, G. The NUKDOS Software for Dosimetry in Molecular Radiotherapy. Eur. J. Nucl. Med. Mol. I 2013, 40, S175. [Google Scholar]
- Petrik, M.; Zhai, C.; Novy, Z.; Urbanek, L.; Haas, H.; Decristoforo, C. In Vitro and In Vivo Comparison of Selected Ga-68 and Zr-89 Labelled Siderophores. Mol. Imaging Biol. 2016, 18, 344–352. [Google Scholar] [CrossRef]
- Starmans, L.W.E.; Hummelink, M.; Rossin, R.; Kneepkens, E.C.M.; Lamerichs, R.; Donato, K.; Nicolay, K.; Grull, H. 89Zr- and Fe-Labeled Polymeric Micelles for Dual Modality PET and T1-Weighted MR Imaging. Adv. Healthc. Mater. 2015, 4, 2137–2145. [Google Scholar] [CrossRef]
- Li, N.; Yu, Z.; Pham, T.T.; Blower, P.J.; Yan, R. A generic 89Zr labeling method to quantify the in vivo pharmacokinetics of liposomal nanoparticles with positron emission tomography. Int. J. Nanomed. 2017, 12, 3281–3294. [Google Scholar] [CrossRef]
- Sarbisheh, E.K.; Salih, A.K.; Raheem, S.J.; Lewis, J.S.; Price, E.W. A High-Denticity Chelator Based on Desferrioxamine for Enhanced Coordination of Zirconium-89. Inorg. Chem. 2020, 59, 11715–11727. [Google Scholar] [CrossRef]
- Steinberg, J.D.; Raju, A.; Chandrasekharan, P.; Yang, C.T.; Khoo, K.; Abastado, J.P.; Robins, E.G.; Townsend, D.W. Negative contrast Cerenkov luminescence imaging of blood vessels in a tumor mouse model using [68Ga]gallium chloride. EJNMMI Res. 2014, 4, 15. [Google Scholar] [CrossRef]
- Lehnert, W.; Gregoire, M.C.; Reilhac, A.; Meikle, S.R. Characterisation of partial volume effect and region-based correction in small animal positron emission tomography (PET) of the rat brain. Neuroimage 2012, 60, 2144–2157. [Google Scholar] [CrossRef]
- Carter, L.M.; Kesner, A.L.; Pratt, E.C.; Sanders, V.A.; Massicano, A.V.F.; Cutler, C.S.; Lapi, S.E.; Lewis, J.S. The Impact of Positron Range on PET Resolution, Evaluated with Phantoms and PHITS Monte Carlo Simulations for Conventional and Non-conventional Radionuclides. Mol. Imaging Biol. 2020, 22, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Abou, D.S.; Ku, T.; Smith-Jones, P.M. In vivo biodistribution and accumulation of 89Zr in mice. Nucl. Med. Biol. 2011, 38, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Lazar, G.; Husztik, E.; Lazar, G. The Role of the Reticuloendothelial System in Natural Immunity. Neuroimmune 2005, 5, 95–101. [Google Scholar] [CrossRef]
- Golden-Mason, L.; O’Farrelly, C. Having it all? Stem cells, haematopoiesis and lymphopoiesis in adult human liver. Immunol. Cell Biol. 2002, 80, 45–51. [Google Scholar] [CrossRef]
- Muench, M.O.; Beyer, A.I.; Fomin, M.E.; Thakker, R.; Mulvaney, U.S.; Nakamura, M.; Suemizu, H.; Barcena, A. The adult livers of immunodeficient mice support human hematopoiesis: Evidence for a hepatic mast cell population that develops early in human ontogeny. PLoS ONE 2014, 9, e97312. [Google Scholar] [CrossRef]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Bronte, V.; Pittet, M.J. The spleen in local and systemic regulation of immunity. Immunity 2013, 39, 806–818. [Google Scholar] [CrossRef]
- Timens, W. The human spleen and the immune system: Not just another lymphoid organ. Res. Immunol. 1991, 142, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Economopoulos, V.; Noad, J.C.; Krishnamoorthy, S.; Rutt, B.K.; Foster, P.J. Comparing the MRI appearance of the lymph nodes and spleen in wild-type and immuno-deficient mouse strains. PLoS ONE 2011, 6, e27508. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chang, B.; Li, Y.; Zhang, X.; Xue, K.; Gao, J.; Zhao, X.; Gu, P. Observation on husbandry and reproduction of mice with severe combined immunodeficiency and histological examination of their immune organs. Chin. J. Cancer Res. 1991, 3, 6–13. [Google Scholar] [CrossRef]

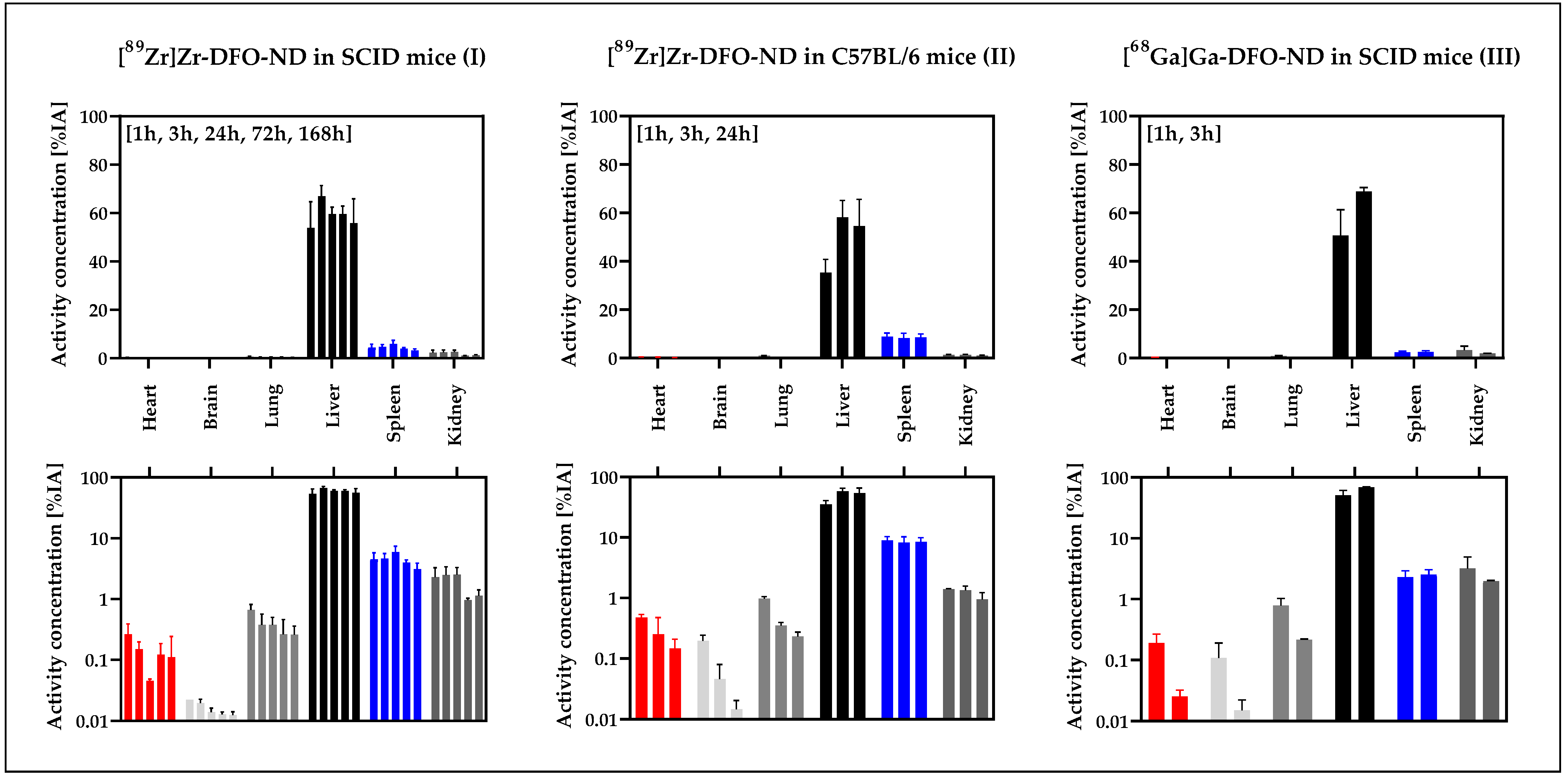
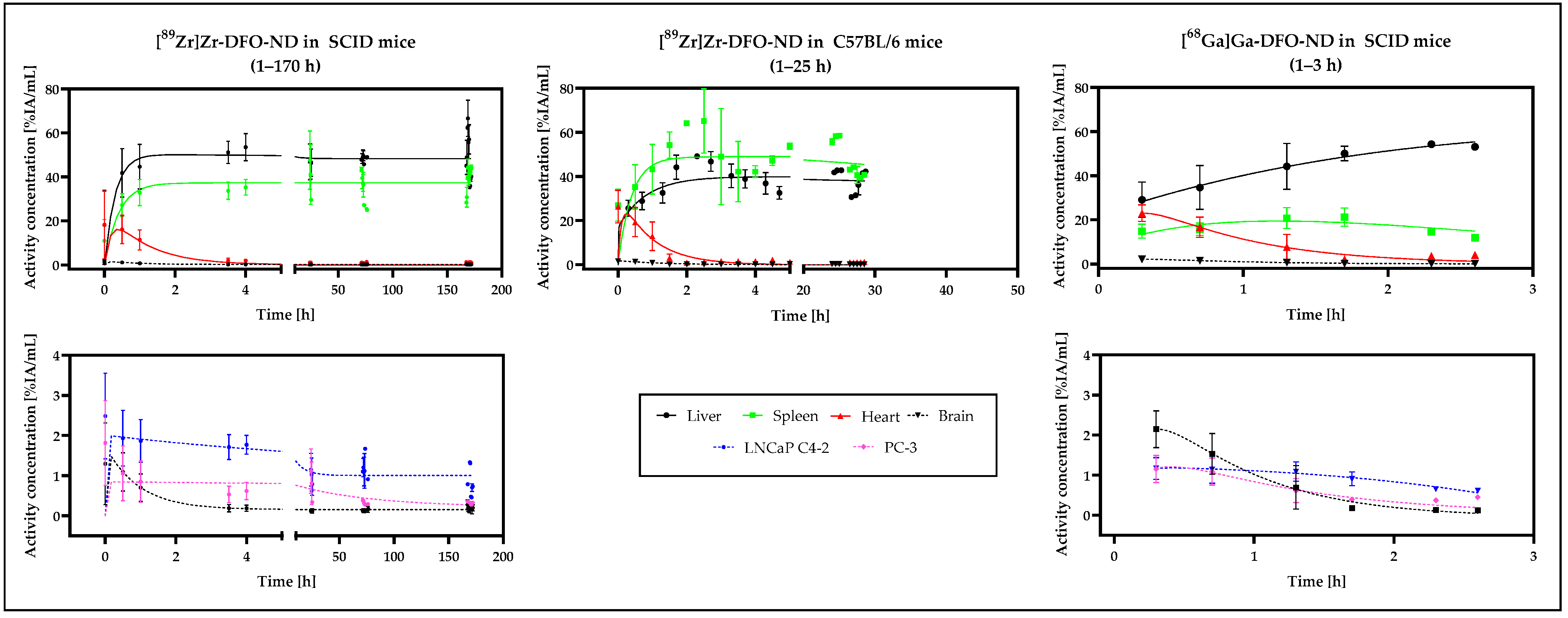
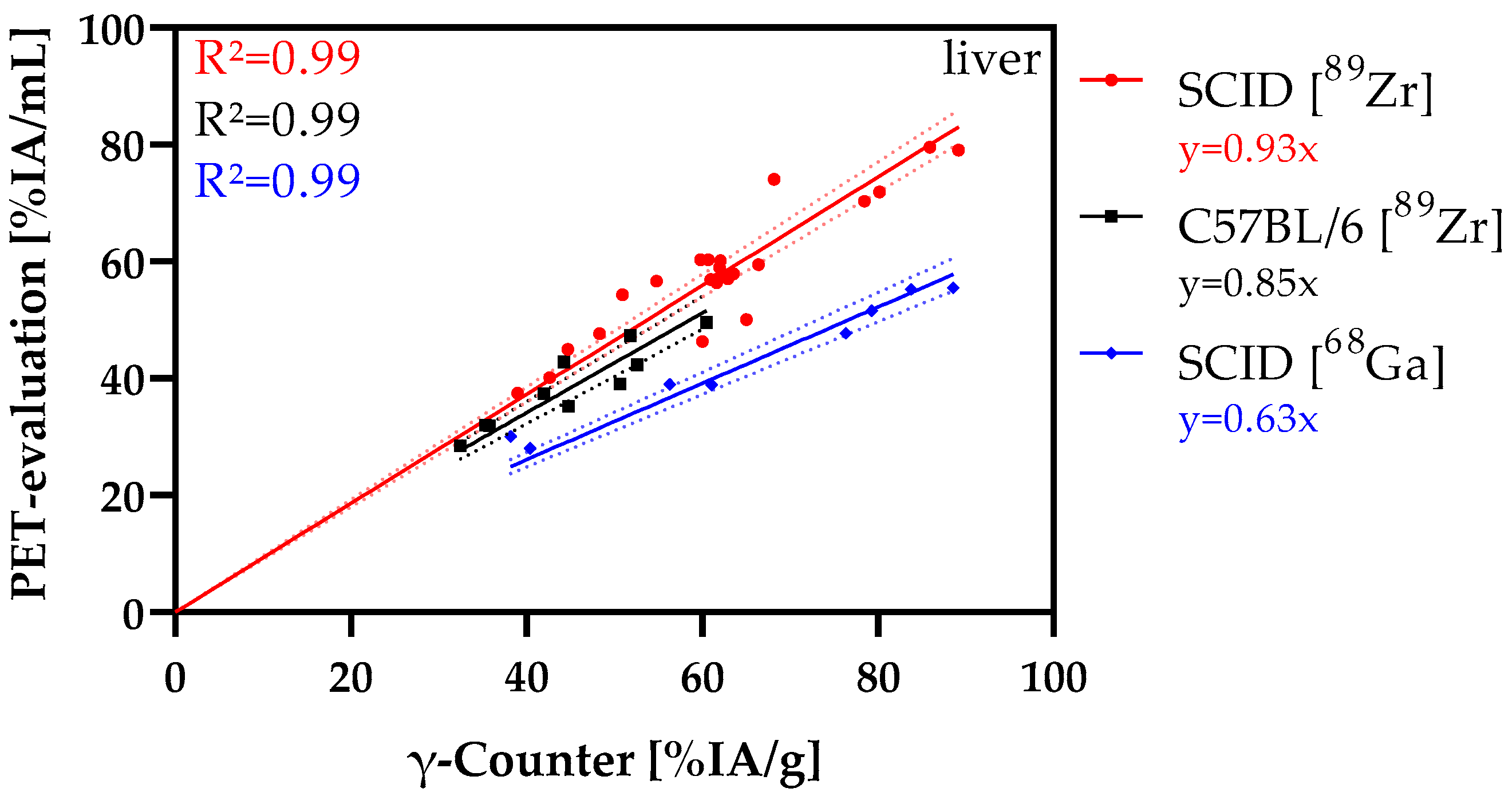
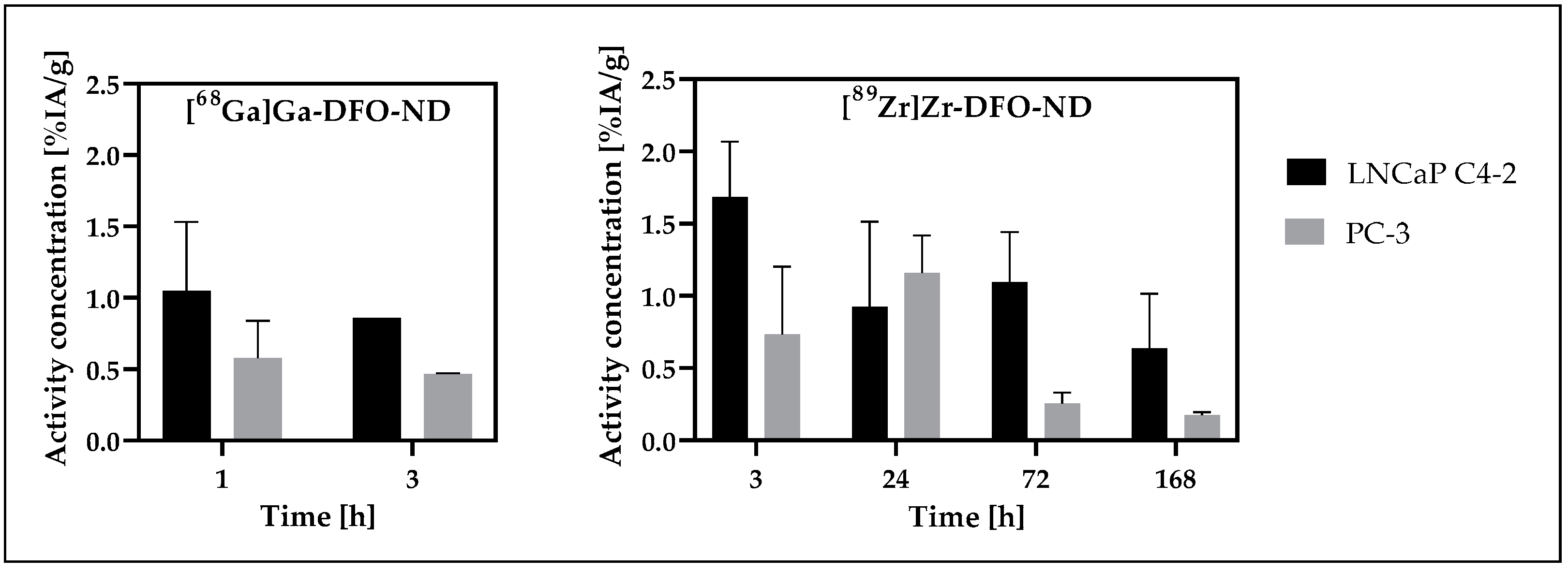
| Study Model | I | II | III |
|---|---|---|---|
| Radionuclide | 89Zr | 89Zr | 68Ga |
| Mouse strain | CB17/lcr-Prkdc scid/Crl | C57BL/6NCrl | CB17/lcr-Prkdc scid/Crl |
| Number of animals (n=) | 25 * | 10 | 8 † |
| Time Points | 1 h, 3 h, 24 h, 3 d, 7 d | 1 h, 3 h, 24 h | 1 h, 3 h |
| Injected activity [MBq] (avg. ± SD) | 1.41 ± 0.42 | 1.46 ± 0.39 | 6.08 ± 1.53 |
| ND [µg] | 40.4 ± 7.7 | 43.5 ± 4.8 | 34.7 ± 9.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winter, G.; Eberhardt, N.; Löffler, J.; Raabe, M.; Alam, M.N.A.; Hao, L.; Abaei, A.; Herrmann, H.; Kuntner, C.; Glatting, G.; et al. Preclinical PET and MR Evaluation of 89Zr- and 68Ga-Labeled Nanodiamonds in Mice over Different Time Scales. Nanomaterials 2022, 12, 4471. https://doi.org/10.3390/nano12244471
Winter G, Eberhardt N, Löffler J, Raabe M, Alam MNA, Hao L, Abaei A, Herrmann H, Kuntner C, Glatting G, et al. Preclinical PET and MR Evaluation of 89Zr- and 68Ga-Labeled Nanodiamonds in Mice over Different Time Scales. Nanomaterials. 2022; 12(24):4471. https://doi.org/10.3390/nano12244471
Chicago/Turabian StyleWinter, Gordon, Nina Eberhardt, Jessica Löffler, Marco Raabe, Md. Noor A. Alam, Li Hao, Alireza Abaei, Hendrik Herrmann, Claudia Kuntner, Gerhard Glatting, and et al. 2022. "Preclinical PET and MR Evaluation of 89Zr- and 68Ga-Labeled Nanodiamonds in Mice over Different Time Scales" Nanomaterials 12, no. 24: 4471. https://doi.org/10.3390/nano12244471
APA StyleWinter, G., Eberhardt, N., Löffler, J., Raabe, M., Alam, M. N. A., Hao, L., Abaei, A., Herrmann, H., Kuntner, C., Glatting, G., Solbach, C., Jelezko, F., Weil, T., Beer, A. J., & Rasche, V. (2022). Preclinical PET and MR Evaluation of 89Zr- and 68Ga-Labeled Nanodiamonds in Mice over Different Time Scales. Nanomaterials, 12(24), 4471. https://doi.org/10.3390/nano12244471








