Photochemical Method for Laser Absorption
Abstract
:1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Characterization
2.3. Electrochemical Measurements and Computational Details
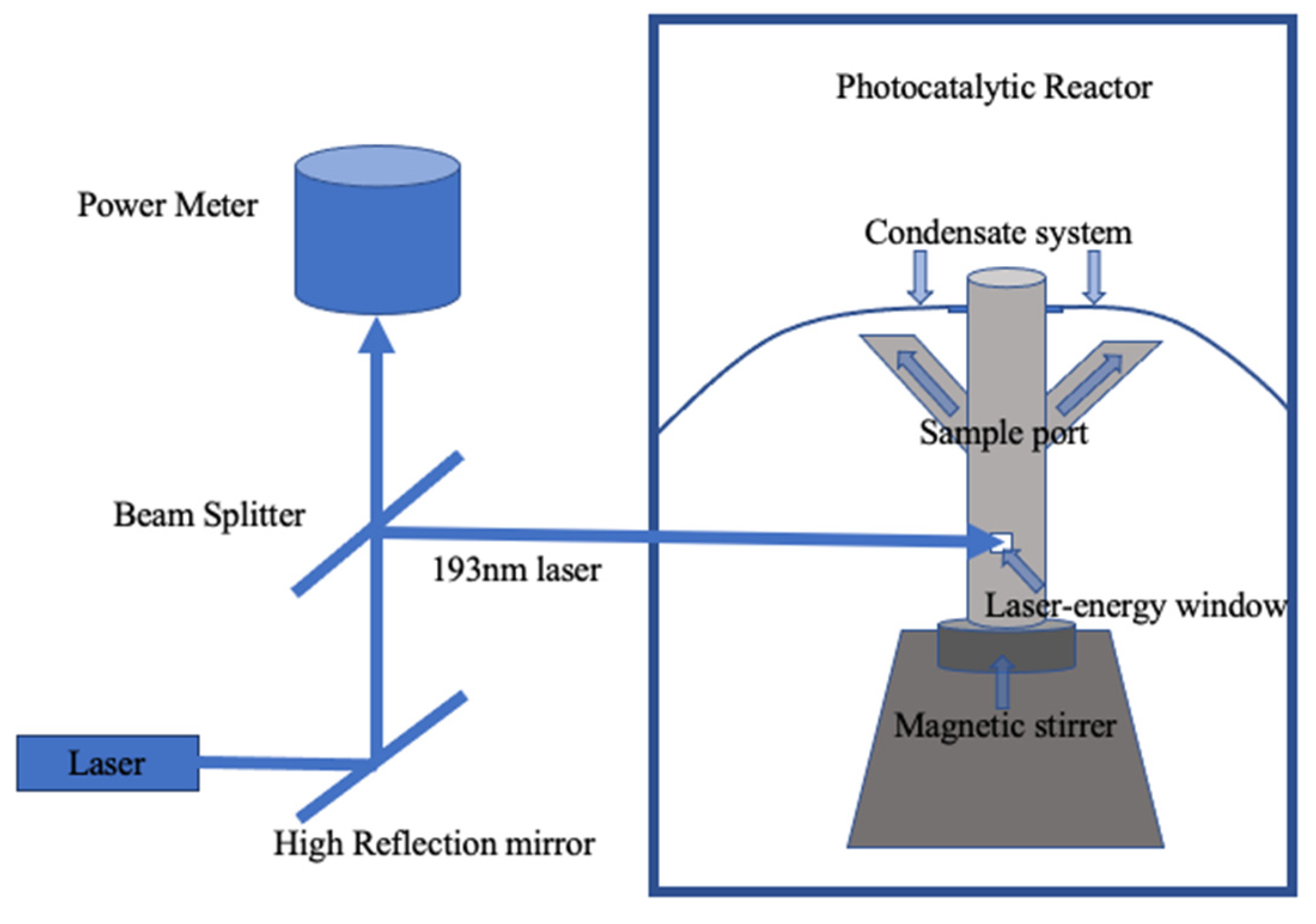
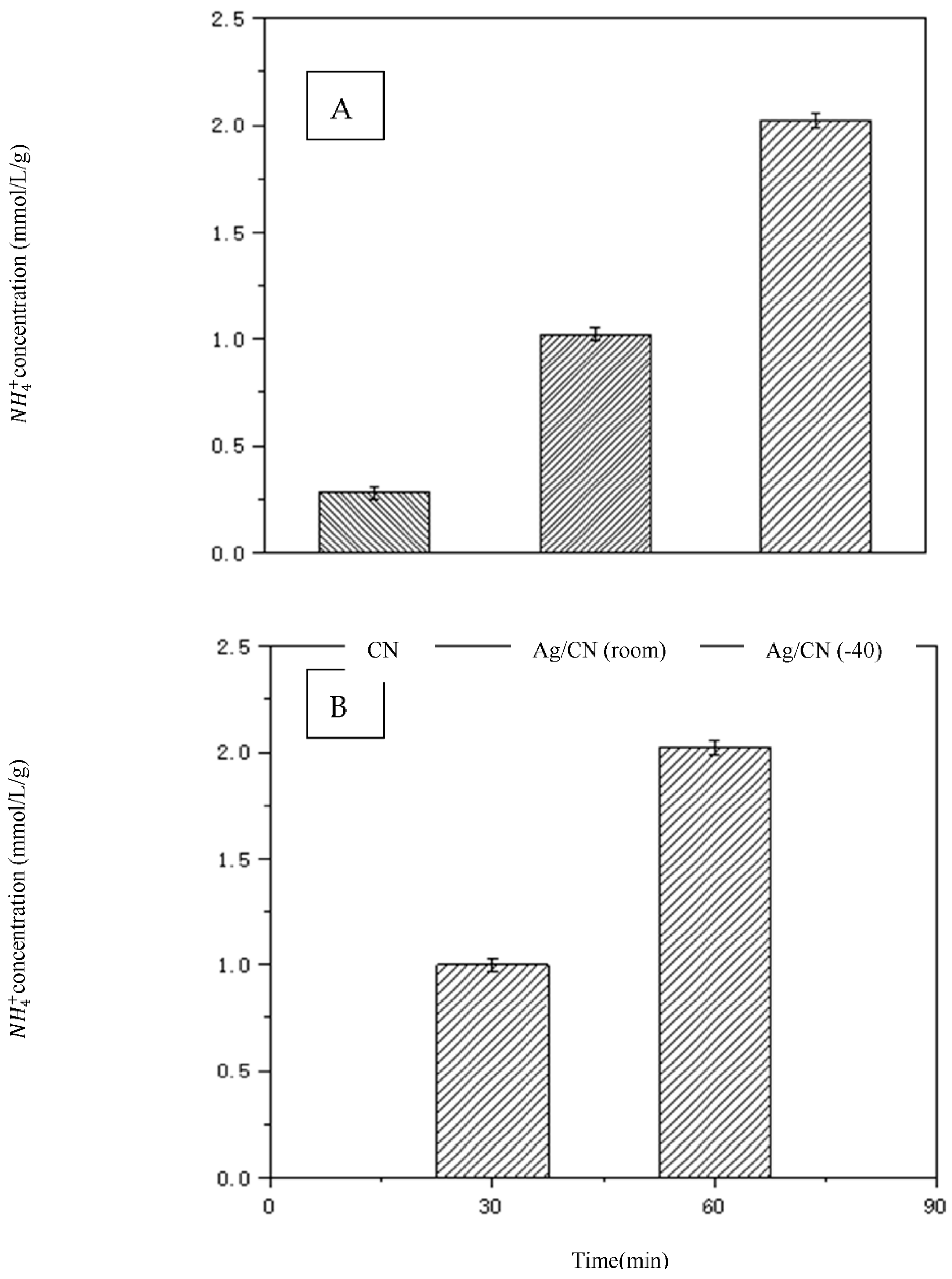
2.4. Photocatalytic Performance with Laser
3. Results and Discussion
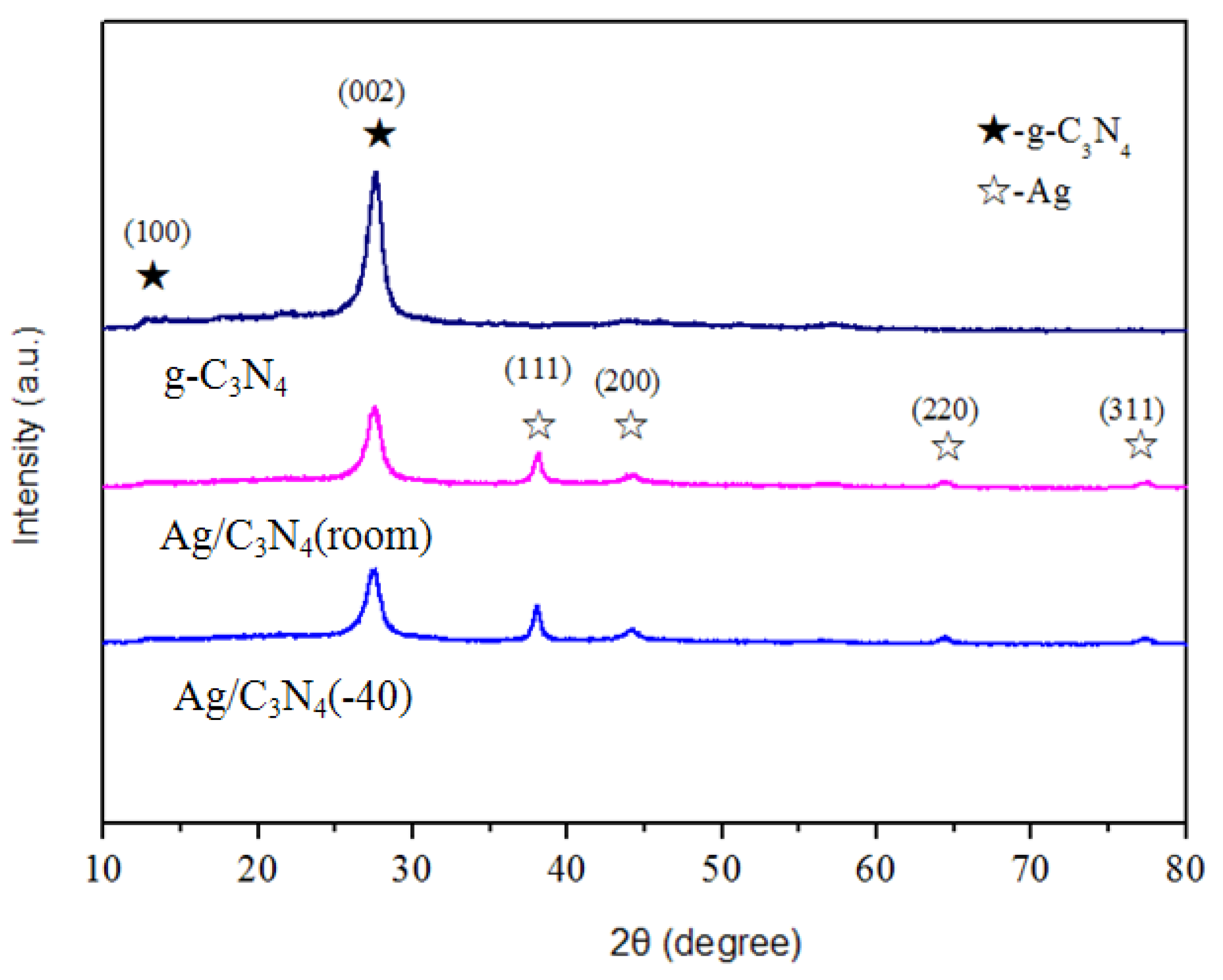
3.1. XRD
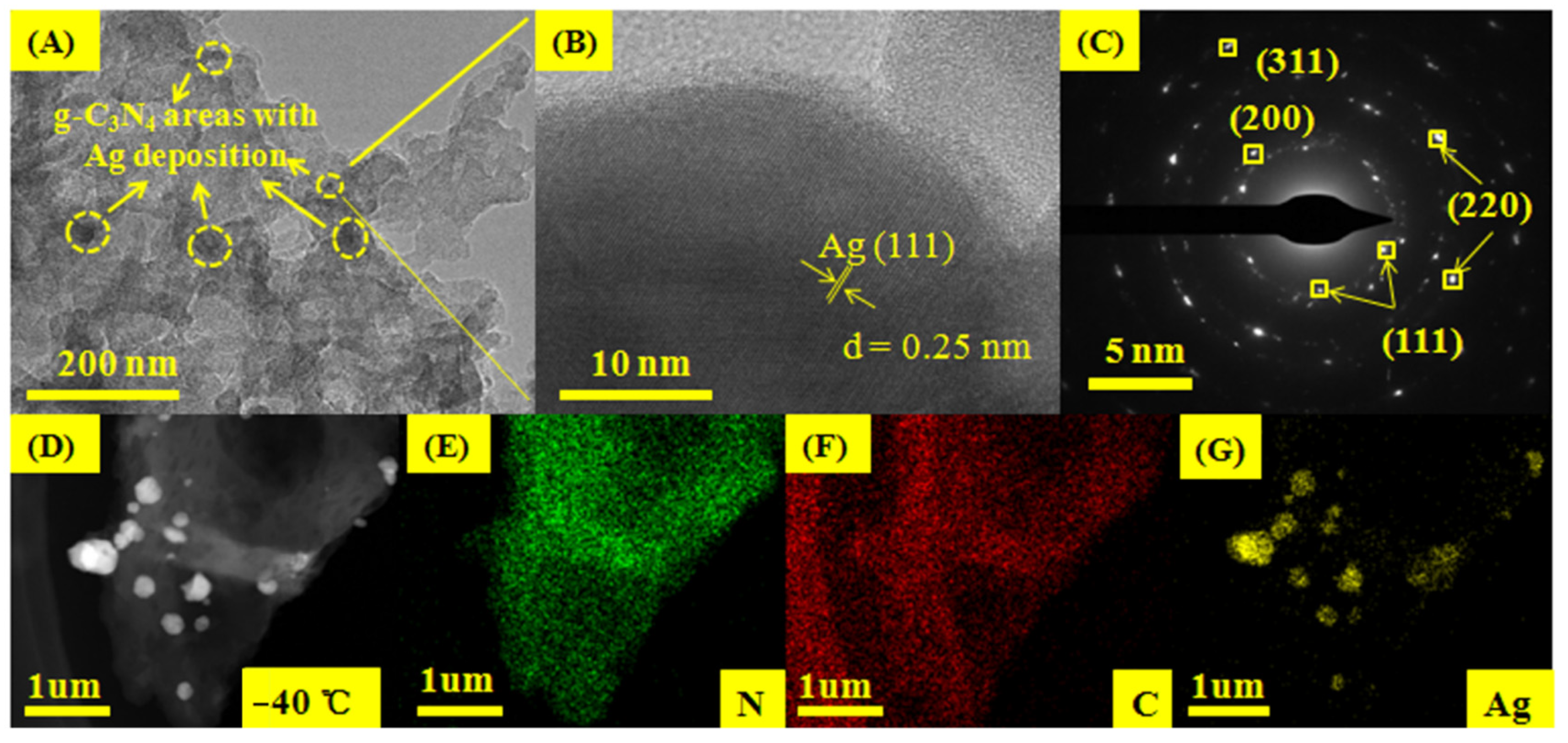
3.2. The Morphology of Ag/CN (−40)
3.3. Energy Absorption Calculation
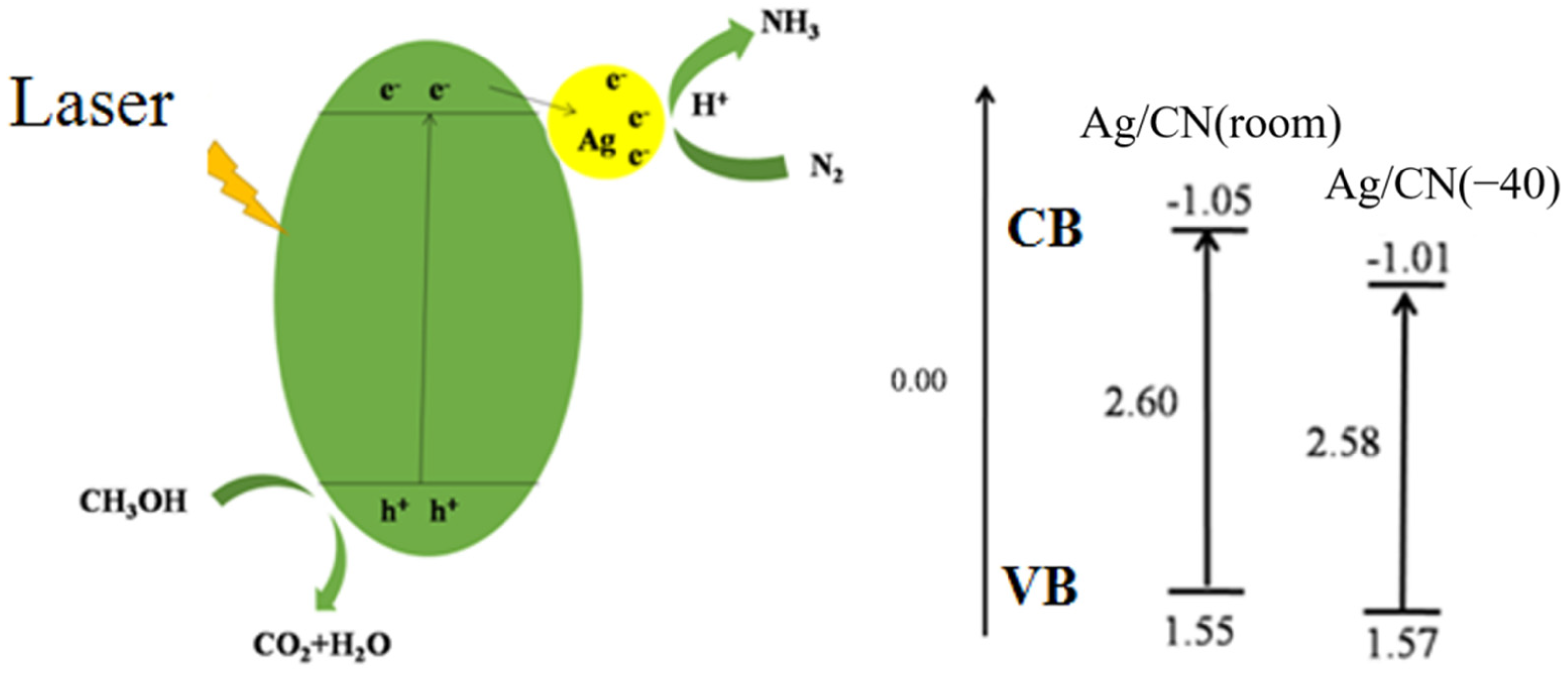
3.3.1. Analysis of the Energy Gaps of Ag/CN
3.3.2. Analysis of the Charge Density
3.3.3. Activity
3.4. Relation between Lifetime, Charge Density, and Energy Absorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.Y.; Guo, L.; Wang, Y.X.; Wang, T.Y.; Ma, T.X.; Zhang, Z.Z.; Wang, D.J.; Xu, B.; Fu, F. In-situ anion exchange based Bi2S3/OV-Bi2MoO6 heterostructure for efficient ammonia production: A synchronized approach to strengthen NRR and OER reactions. J. Mater. Sci. Technol. 2022, 110, 152–160. [Google Scholar] [CrossRef]
- Wang, F.; Mao, J. Effect of N-doping on graphene: NRR activity and N-source. Diam. Relat. Mater. 2021, 118, 108494. [Google Scholar] [CrossRef]
- Cao, N.; Liu, Y.H.; Xu, X.; Xu, Y.S.; Wang, X.F.; Bi, L. Ambient electrosynthesis of NH3 from N2 using Bi-doped CeO2 cube as electrocatalyst. Int. J. Hydrogen Energy 2021, 46, 31523–31532. [Google Scholar] [CrossRef]
- Shen, Y.F.; Chen, L.C.; Zhang, L.P.; Han, W.H.; Jiang, M.; Zheng, H. Nitrogen fixation from air at normal temperature and pressure via Cobalt-iron photocatalyst day and night. Mol. Catal. 2022, 518, 112091. [Google Scholar] [CrossRef]
- Mansingh, S.; Das, K.K.; Sultana, S.; Parida, K. Recent advances in wireless photofixation of dinitrogen to ammonia under the ambient condition: A review. J. Photochem. Photobiol. C Photochem. Rev. 2021, 47, 100402. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Zhao, R.; Wang, F.; Liu, Y.; Yu, X.H.; Chen, L.G.; Yao, Y.; Lu, S.X.; Liao, X.Y. Ultralow- temperature synthesis of small Ag-doped carbon nitride for nitrogen photofixation. Catal. Sci. Technol. 2020, 10, 7652–7660. [Google Scholar] [CrossRef]
- Viviani, A.; Aprovitola, A.; Pezzella, G.; Rainone, C. CFD design capabilities for next generation high-speed aircraft. Acta Astronaut. 2021, 178, 143–158. [Google Scholar] [CrossRef]
- Jiao, Z.X.; Zhang, H.; Shang, Y.X.; Liu, X.C.; Wu, S. A power-by-wire aircraft brake system based on high-speed on-off valves. Aerosp. Sci. Technol. 2020, 106, 1061. [Google Scholar] [CrossRef]
- Sabatini, R.; Richardson, M.A. A new approach to eye-safety analysis for airborne laser systems flight test and training operations. Opt. Laser Technol. 2003, 35, 191–198. [Google Scholar] [CrossRef]
- Wang, Z.H.; Wang, Y.Z.; He, H.B.; Shen, Z.C.; Sytchkova, A.; Chen, R.Y.; Zhang, Y.H.; Li, D.W.; Hu, G.H.; Zheng, Y.H.; et al. Laser-induced damage of 1064 nm multilay-er antireflection coatings after exposure to gamma rays. Opt. Mater. 2021, 122, Part B, 111580. [Google Scholar] [CrossRef]
- Schulmeister, K.; Jean, M. The risk of retinal injury from Class 2 and visible Class 3R la-sers, including medical laser aiming beams. Med. Laser Appl. 2010, 25, 99–110. [Google Scholar] [CrossRef]
- Nazeer, Z.; Bibi, I.; Majid, F.; Kamal, S.; Ibrahim, S.M.; Iqbal, M. Energy band gap tuning of Ba1−xZnxFe12−yCryO19 doped with Cr and Zn ions to enhance the optical, dielectric, ferroelectric, and photocatalytic properties. Opt. Mater. 2022, 125, 112090. [Google Scholar] [CrossRef]
- Ghobadi, N.; Sohrabi, P.; Hatami, H.R. Correlation between the photocatalytic activity of CdSe nanostructured thin films with optical band gap and Urbach energy. Chem. Phys. 2020, 538, 10911. [Google Scholar] [CrossRef]
- Gondal, M.A.; Siddiqui, M.N. Al-Hooshani, Removal of sulfur compounds from diesel u-sing ArF laser and oxygen. J. Environ. Sci. Health 2013, 48, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Li, Q.; Chen, Y.; Lu, S.X.; Liao, X.Y.; Yao, Y. Size effects of Ag nanoparticle for N2 photofxation over Ag/g-C3N4: Built-in electric fields determine photocatalytic performance. Colloid Surf. A 2021, 626, 127053. [Google Scholar] [CrossRef]
- Wu, D.P.; Tian, J.F.; Xing, Y.L.; Jin, X.Y.; Ni, G. Fabrication of Z-scheme ZnO/Bi2O4 heterojunction photocatalyst with superior photocatalytic nitrogen fixation under visible light irradiation. Solid State Sci. 2021, 119, 106709. [Google Scholar] [CrossRef]
- Li, Y.F.; Yang, M.; Xing, Y.; Liu, X.; Yang, Y.; Wang, X.; Song, S. Preparation of Carbon-Richg-C3N4 Nanosheets with Enhanced Visible Light Utilization for Efficient Photocatalytic Hydrogen Production. Small 2017, 13, 1701552. [Google Scholar] [CrossRef]
- Krukowski, P.; Hattori, T.; Okada, M.; Piskorski, M.; Lutsyk, I.; Saito, A.; Osuga, H.; Kuwahara, Y. Study of Stereochemical Crystallization of Racemic Mixtures of thiaheterohelicene Molecules on Ag (111) Surface by Scanning Tunneling Microscopy and Raman Scattering Spectroscopy. Appl. Surf. Sci. 2022, 589, 152860. [Google Scholar] [CrossRef]
- Mun-oz-Batista, M.J.; Fontelles-Carceller, O.; Ferrer, M.; Fernndez-García, M.; Kubacka, A. Disinfection capability of Ag/g-C3N4 composite photocatalysts under UV and visible light illumination. Appl. Catal. B-Environ. 2016, 183, 86–95. [Google Scholar] [CrossRef]
- Yang, B.Q.; Huang, P.L.; An, Q. An efficient chalcopyrite depressant for Cu-Mo separation and its interaction mechanism: Adsorption configuration and DFT calculations. J. Mol. Liq. 2022, 345, 118171. [Google Scholar] [CrossRef]
- Boungou, C.C.; Bouka-Pivoteau, G.B.; Malonda-Boungou, B.R.; N’dollo, M.; Moussounda, P.S.; Raji, A.T.; Kanga, E. Adsorption and coadsorption of H and Li on Ag (100) surface: DFT studies including dispersion correction. Comput. Condens. Matte. 2021, 28, e00582. [Google Scholar] [CrossRef]
- Mao, Y.; Montoya-Castillo, A.; Markland, T.E. Excited state diabatization on the cheap using DFT: Photoinduced electron and hole transfer. J. Chem. Phys. 2020, 153, 244111. [Google Scholar] [CrossRef]
- Pomogaeva, A.; Filatov, M.; Choi, C.H. Manifestations of strong electron correlation in polyacene: Fundamental gap, density of states, and photoconductivity. Carbon Trends 2022, 7, 100146. [Google Scholar] [CrossRef]
- Bakr, A.; Rouby, W.; Khan, M.; Farghali, A.; Xulu, B. Synthesis and characterization of Z- scheme α-Fe2O3 NTs/ruptured tubular g-C3N4 for enhanced photoelectrochemical water oxidation. Sol. Energy 2019, 193, 403–412. [Google Scholar] [CrossRef]
- Meleppat, R.K.; Prabhathan, P.; Keey, S.L.; Matham, M.V. Plasmon Resonant Silica-Coated Silver Nanoplates as Contrast Agents for Optical Coherence Tomography. J. Biomed. Nanotechnol. 2016, 12, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Ratheesh, K.M.; Prabhathan, P.; Seah, L.K.; Murukeshan, V.M. Gold nanorods with higher aspect ratio as potential contrast agent in optical coherence tomography and for photothermal applications around 1300nm imaging window. Biomed. Phys. Eng. Express. 2016, 2, 055005. [Google Scholar] [CrossRef]


| Sample | Ag Particle Size (nm) | Life Time [15] (τ, ms) | Charge Density | BET Surface Area (m2/g) | Energy a Absorption Rate (%) |
|---|---|---|---|---|---|
| Ag/C3N4 (−40) | ~13.0 | 0.29 | 12 | 7.1 | |
| Ag/C3N4 (room) | ~33.2 | 0.20 | 13 | 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Zhang, Y.; Qi, X.; Duanmu, Y.; Yao, Y. Photochemical Method for Laser Absorption. Nanomaterials 2022, 12, 4384. https://doi.org/10.3390/nano12244384
Tang W, Zhang Y, Qi X, Duanmu Y, Yao Y. Photochemical Method for Laser Absorption. Nanomaterials. 2022; 12(24):4384. https://doi.org/10.3390/nano12244384
Chicago/Turabian StyleTang, Weiwei, Yinuo Zhang, Xingyu Qi, Yu Duanmu, and Yue Yao. 2022. "Photochemical Method for Laser Absorption" Nanomaterials 12, no. 24: 4384. https://doi.org/10.3390/nano12244384
APA StyleTang, W., Zhang, Y., Qi, X., Duanmu, Y., & Yao, Y. (2022). Photochemical Method for Laser Absorption. Nanomaterials, 12(24), 4384. https://doi.org/10.3390/nano12244384





