Easy Handling and Cost-Efficient Processing of a Tb3+-MOF: The Emissive Capacity of the Membrane-Immobilized Material, Water Vapour Adsorption and Proton Conductivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Complexes
2.2. Synthesis of {[Tb5L6(OH)3(H2O)3]·5DMF}n
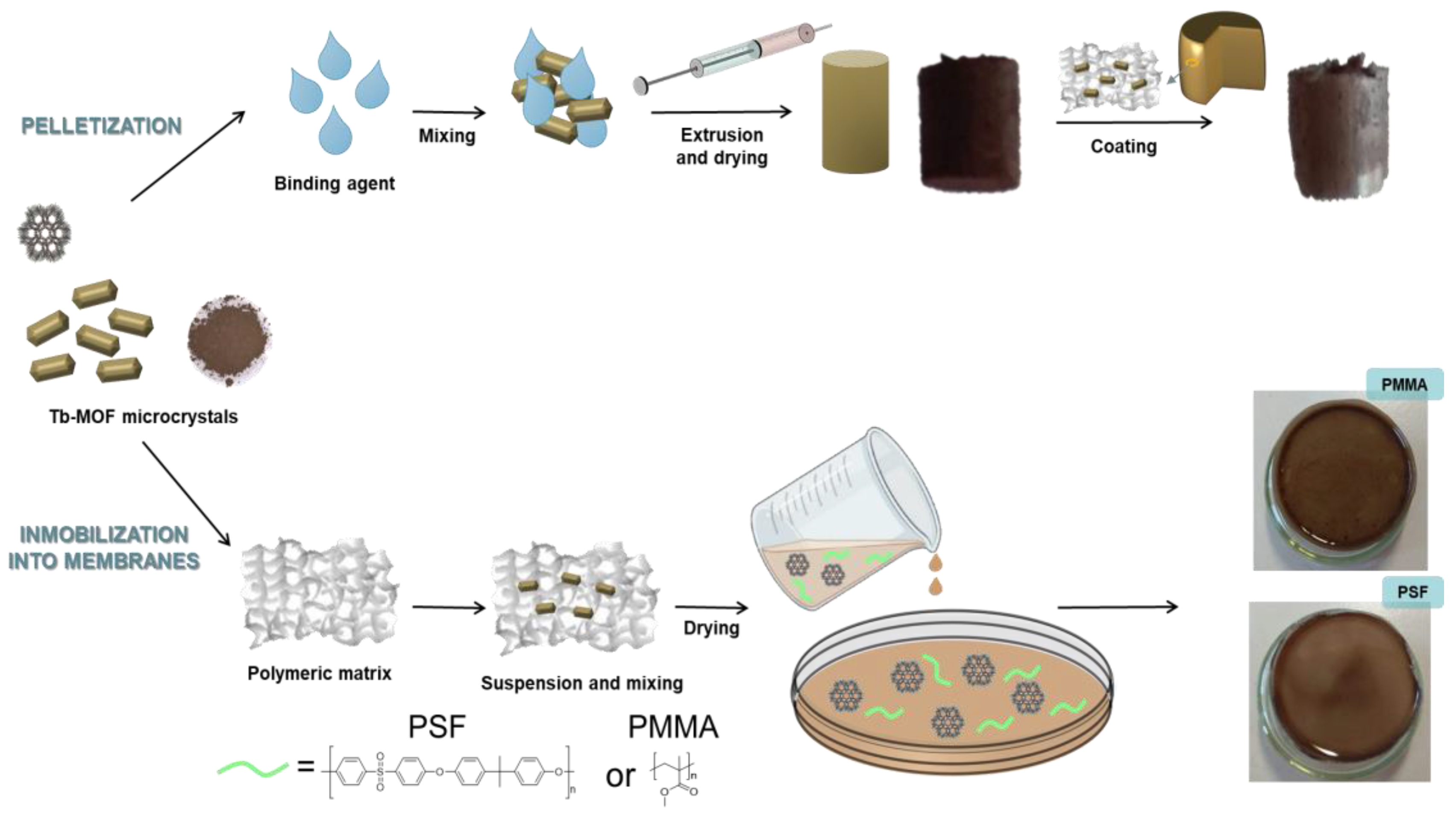
2.3. MOF Processing into Pellets and Membranes
2.3.1. Membrane’s Preparation and Characterization
2.3.2. Pellets Preparation and Characterization
3. Results
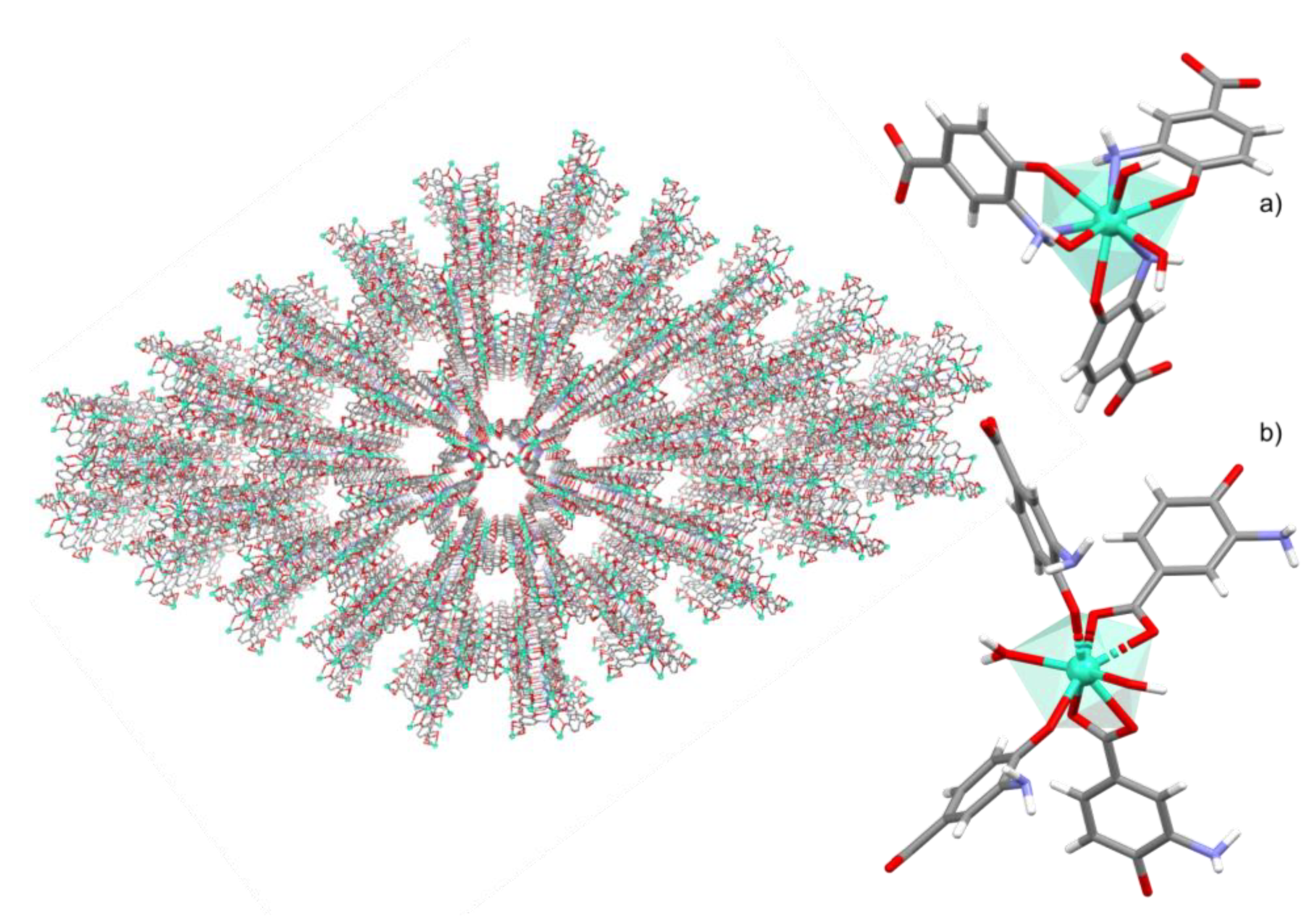
3.1. Crystal Structure Details
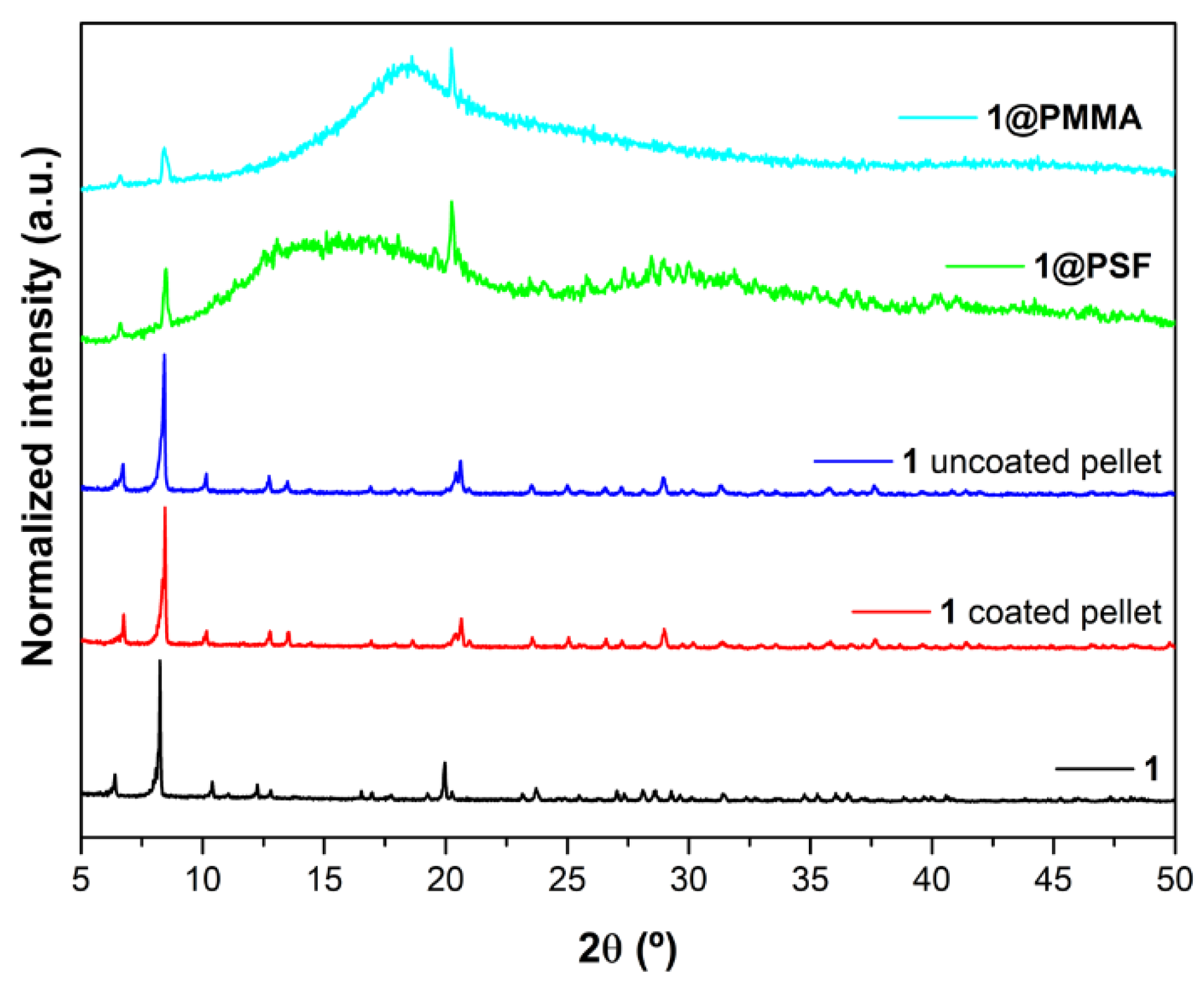
3.2. Pellets and Membranes Stability Studies
3.2.1. Moisture Stability
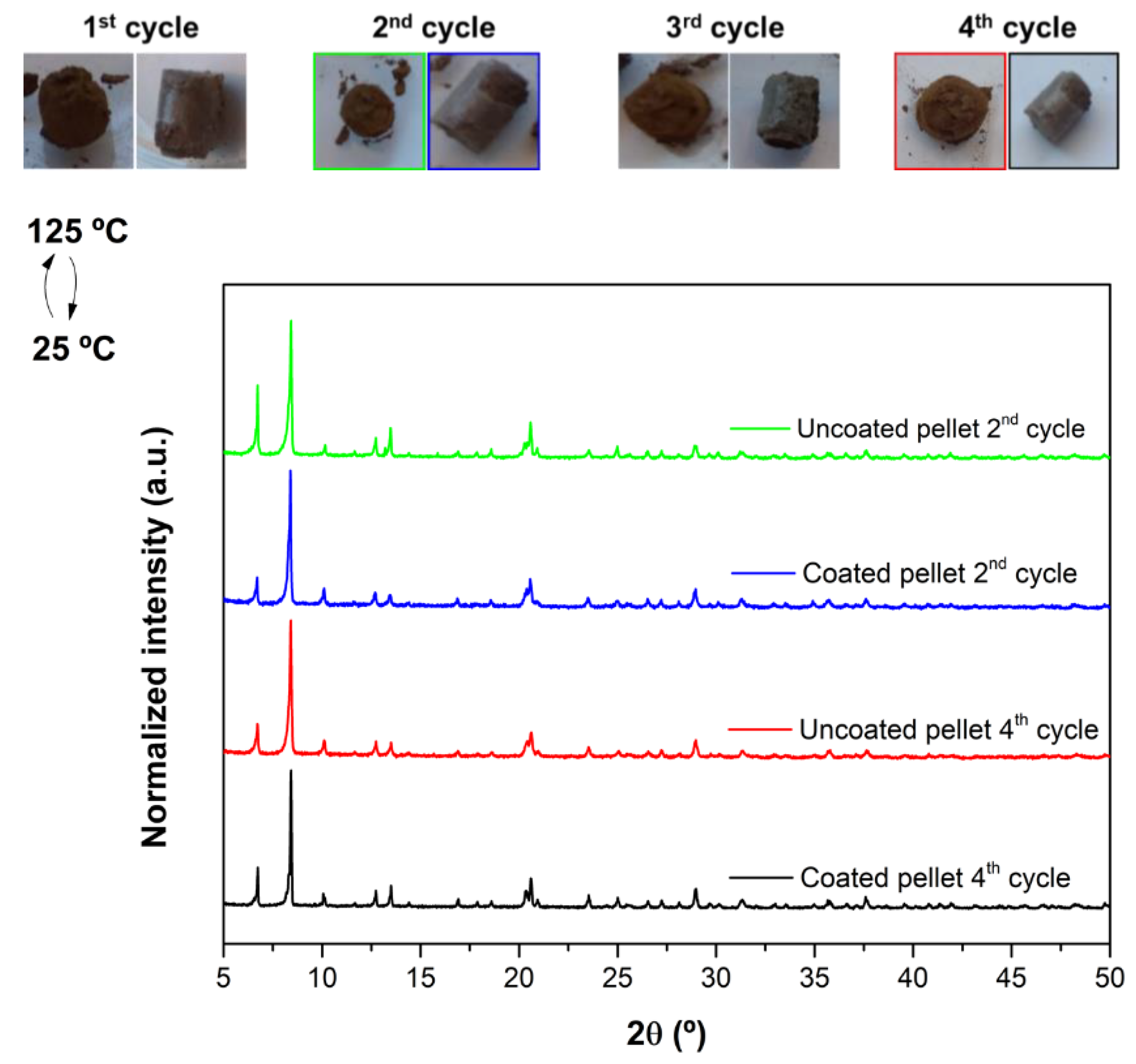
3.2.2. Temperature Stability
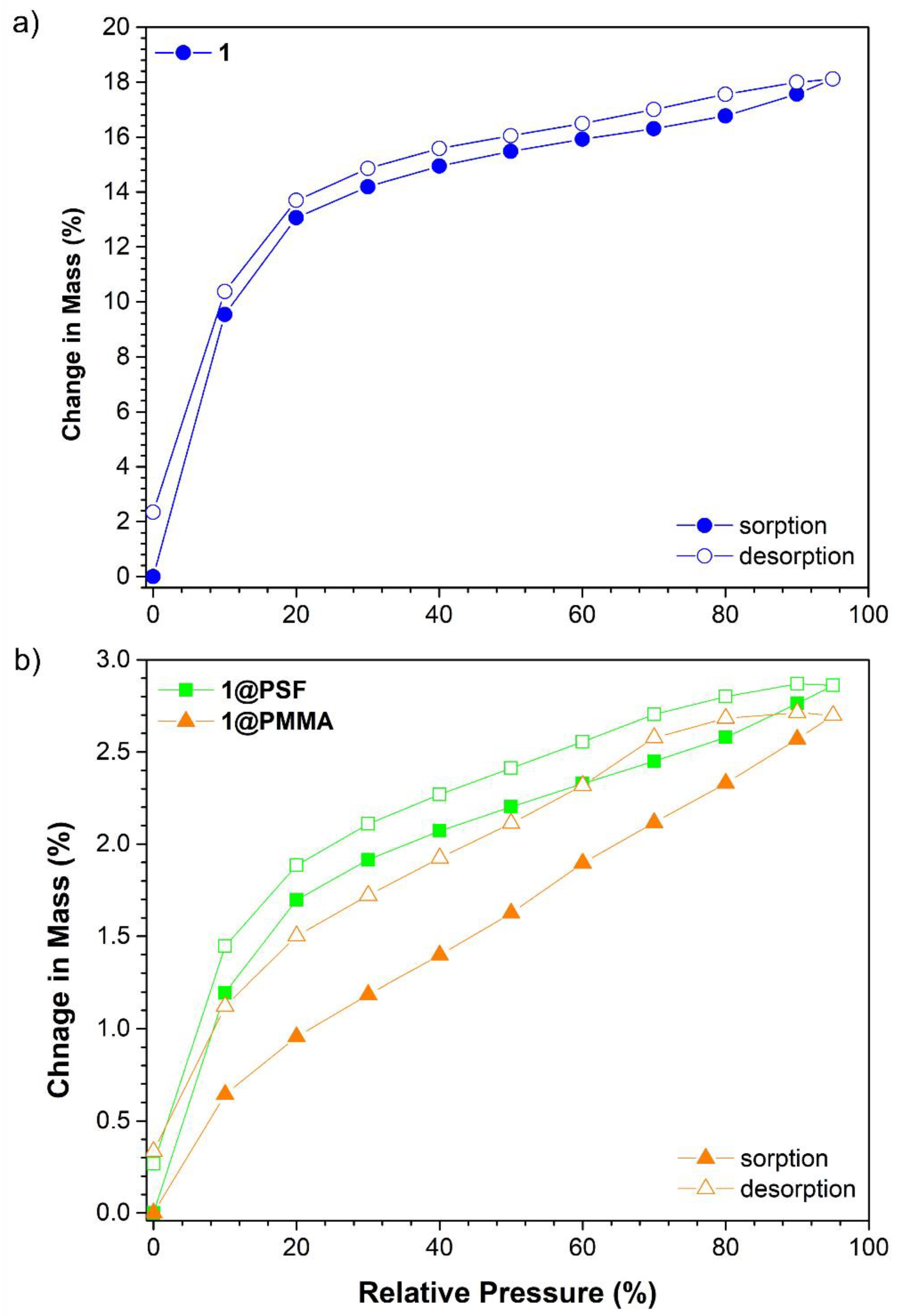
3.3. Water Vapour Adsorption Studies
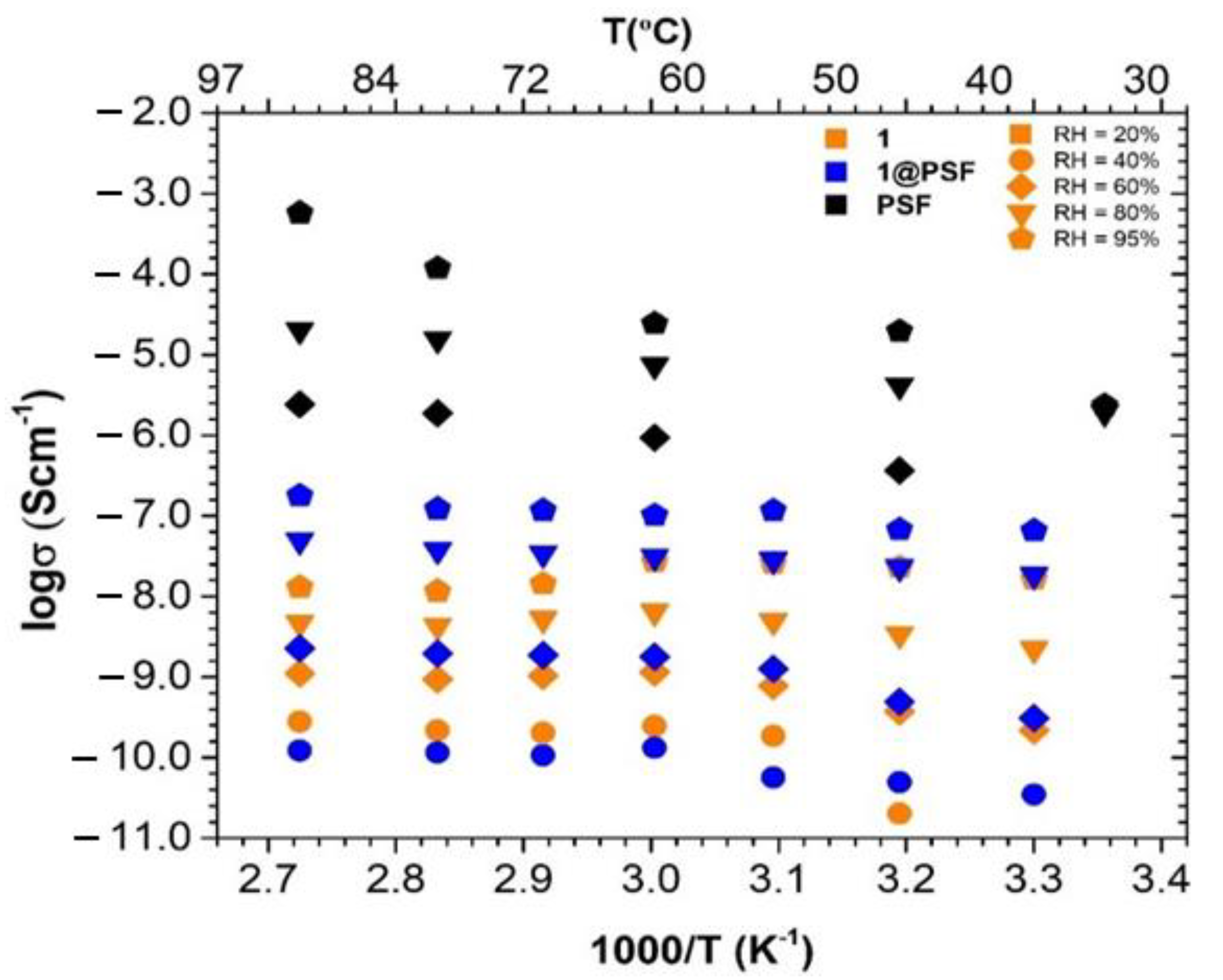
3.4. Electrical Conductivity
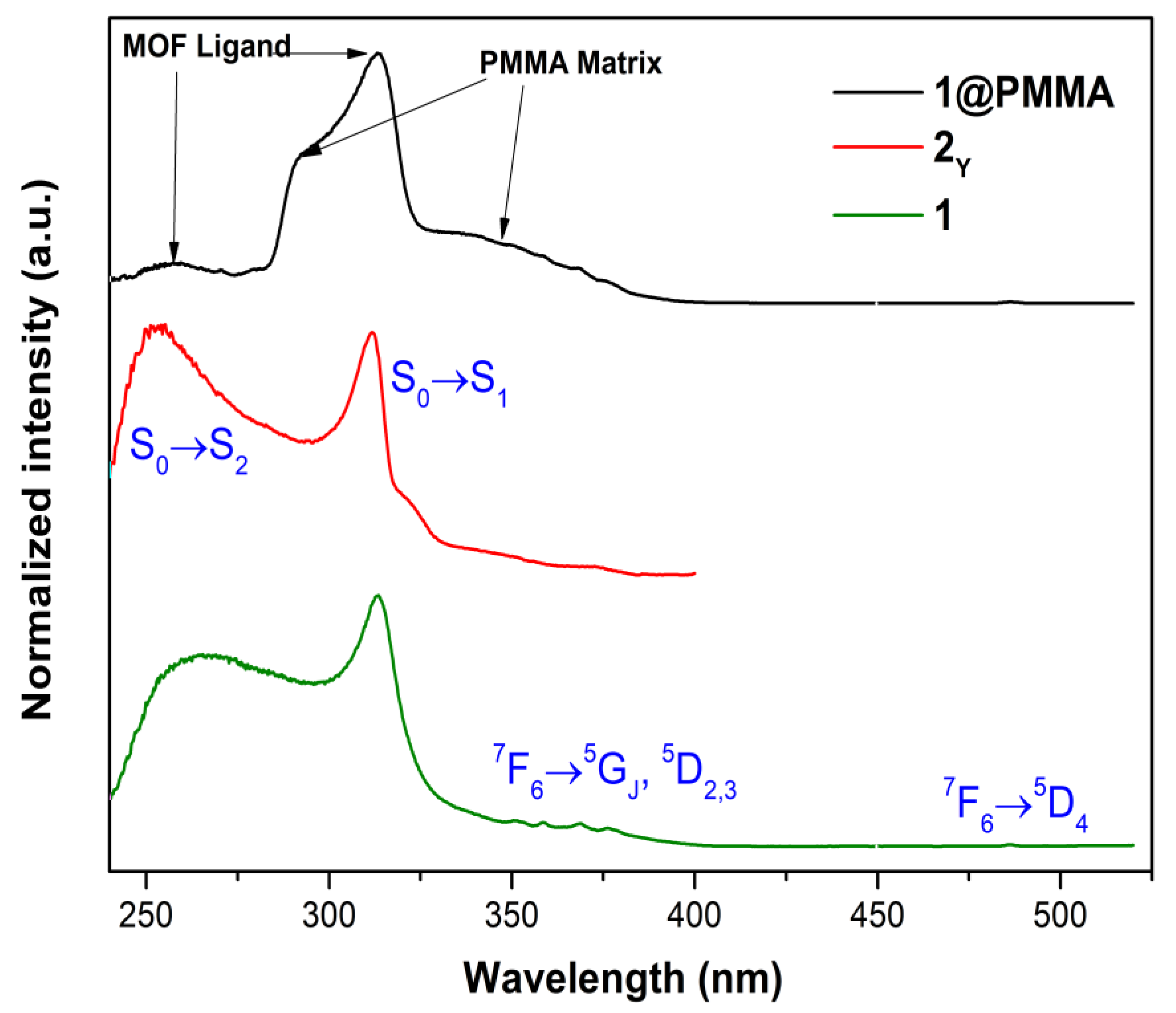
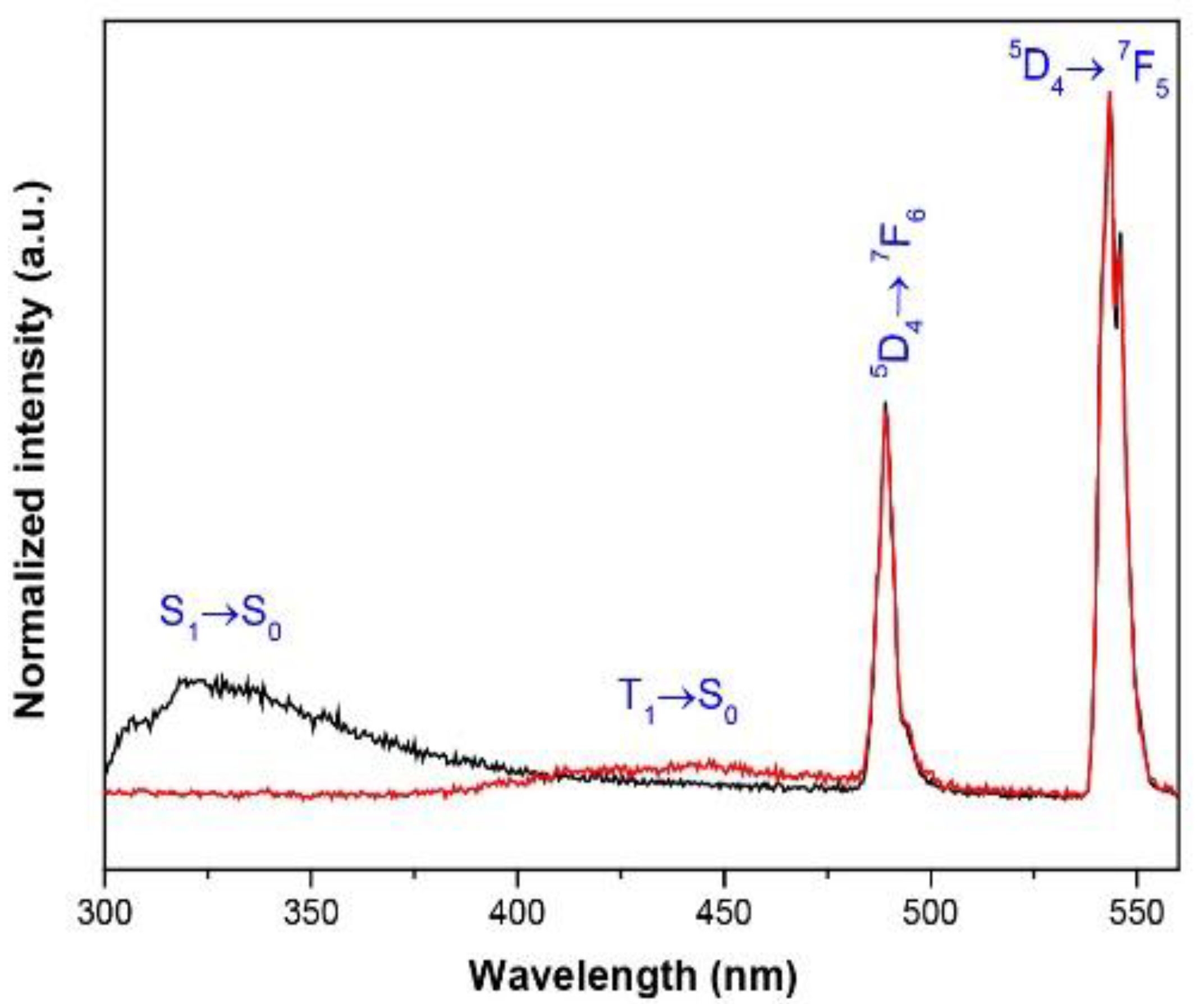
3.5. Photoluminescent Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, P.; Vilela, S.M.F.; Tomé, J.P.C.; Almeida Paz, F.A. Multifunctional metal-organic frameworks: From academia to industrial applications. Chem. Soc. Rev. 2015, 44, 6774–6803. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal–Organic Frameworks. Chem. Rev. 2011, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wang, J.; Li, Y.; He, C.; Xie, Z.; Duan, C. Luminescent Sensing and Catalytic Performances of a Multifunctional Lanthanide-Organic Framework Comprising a Triphenylamine Moiety. Adv. Funct. Mater. 2011, 21, 2788–2794. [Google Scholar] [CrossRef]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.-C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef] [PubMed]
- García, H.; Navalon, S. Metal-Organic Frameworks: Applications in Separations and Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2018; ISBN 978-3-527-80910-3. [Google Scholar]
- Chen, Y.; Guerin, S.; Yuan, H.; O’donnell, J.; Xue, B.; Cazade, P.-A.; Haq, U.; Shimon, L.J.W.; Rencus-Lazar, S.; Tofail, S.A.M.; et al. Guest Molecule-Mediated Energy Harvesting in a Conformationally Sensitive Peptide−Metal Organic Framework. J. Am. Chem. Soc. 2022, 2022, 3468–3476. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Orr, A.A.; Makam, P.; Redko, B.; Haimov, E.; Wang, Y.; Shimon, L.J.W.; Rencus-Lazar, S.; Ju, M.; et al. Self-Assembled Peptide Nano-Superstructure towards Enzyme Mimicking Hydrolysis. Angew. Chem. 2021, 133, 17301–17307. [Google Scholar] [CrossRef]
- Chen, Z.; Wasson, M.C.; Drout, R.J.; Robison, L.; Idrees, K.B.; Knapp, J.G.; Son, F.A.; Zhang, X.; Hierse, W.; Kühn, C.; et al. The state of the field: From inception to commercialization of metal-organic frameworks. Faraday Discuss. 2021, 225, 9–69. [Google Scholar] [CrossRef] [PubMed]
- Casaban, J.; Zhang, Y.; Pacheco, R.; Coney, C.; Holmes, C.; Sutherland, E.; Hamill, C.; Breen, J.; James, S.L.; Tufano, D.; et al. Towards MOFs’ mass market adoption: MOF Technologies’ efficient and versatile one-step extrusion of shaped MOFs directly from raw materials. Faraday Discuss. 2021, 231, 312–325. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef]
- Ren, J.; Langmi, H.W.; North, B.C.; Mathe, M. Review on processing of metal-organic framework (MOF) materials towards system integration for hydrogen storage. Int. J. Energy Res. 2015, 39, 607–620. [Google Scholar] [CrossRef]
- Qiu, S.; Xue, M.; Zhu, G. Metal-organic framework membranes: From synthesis to separation application. Chem. Soc. Rev. 2014, 43, 6116–6140. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Ye, Y.; Liu, X.; Cui, H.; Li, Z.; Zhang, Y.; Liang, B.; Li, H.; Chen, B. A Robust Mixed-Lanthanide PolyMOF Membrane for Ratiometric Temperature Sensing. Angew. Chem.-Int. Ed. 2020, 59, 21752–21757. [Google Scholar] [CrossRef] [PubMed]
- Dechnik, J.; Gascon, J.; Doonan, C.J.; Janiak, C.; Sumby, C.J. Mixed-Matrix Membranes. Angew. Chem.-Int. Ed. 2017, 56, 9292–9310. [Google Scholar] [CrossRef]
- Lim, D.W.; Kitagawa, H. Rational strategies for proton-conductive metal-organic frameworks. Chem. Soc. Rev. 2021, 50, 6349–6368. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Kitagawa, H. Proton Transport in Metal-Organic Frameworks. Chem. Rev. 2020, 120, 8416–8467. [Google Scholar] [CrossRef] [PubMed]
- Canivet, J.; Fateeva, A.; Guo, Y.; Coasne, B.; Farrusseng, D. Water adsorption in MOFs: Fundamentals and applications. Chem. Soc. Rev. 2014, 43, 5594–5617. [Google Scholar] [CrossRef]
- Kanda, S.; Kenichi, Y.; Kuwako, O. A Proton Conductive Coordination Polymer. I. [N,N′-Bis(2-hydroxyethyl)dithiooxamido]copper(II). Bull. Chem. Soc. Jpn. 1979, 52, 3296–3301. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.S.; Li, B.; You, H.; Zhang, L.; Zhang, Z.; Zang, H.Y.; Zheng, Q.; Xuan, W. Sulfonate-Functionalized Polyoxovanadate-Based Metal-Organic Polyhedra for Enhanced Proton Conduction via the Synergy of Linker and Metal Cluster Vertex. Chin. J. Struct. Chem. 2022, 41, 2208012–2208017. [Google Scholar] [CrossRef]
- Guo, S.S.; Huang, L.L.; Ye, Y.X.; Liu, L.Z.; Yao, Z.Z.; Xiang, S.C.; Zhang, J.D.; Zhang, Z.J. Carbazole based anionic MOF for proton conductivity. Chin. J. Struct. Chem. 2021, 40, 55–60. [Google Scholar] [CrossRef]
- Pérez, J.M.; Echenique-Errandonea, E.; Rojas, S.; Choquesillo-Lazarte, D.; Seco, J.M.; López-Vargas, M.E.; Rodríguez-Diéguez, A.; Fernández, I. Improved Performance of a Europium-based Metal-Organic Framework for Cyanosilylation of Demanding Ketones. ChemCatChem 2022, 14, e202200967. [Google Scholar] [CrossRef]
- Dechnik, J.; Mühlbach, F.; Dietrich, D.; Wehner, T.; Gutmann, M.; Lühmann, T.; Meinel, L.; Janiak, C.; Müller-Buschbaum, K. Luminescent Metal–Organic Framework Mixed-Matrix Membranes from Lanthanide Metal–Organic Frameworks in Polysulfone and Matrimid. Eur. J. Inorg. Chem. 2016, 2016, 4408–4415. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Bofill, J.M.; Alemany, P.; Alvarez, S.; Pinsky, M.; Avnir, D. Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; SHAPE, v1.1b; University of Barcelona: Barcelona, Spain, 2005; pp. 1–35. [Google Scholar]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package topospro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- O’Brien, F.E.M. The Control of Humidity by Saturated Salt Solutions. J. Sci. Instrum. 1948, 25, 73–76. [Google Scholar] [CrossRef]
- Schult, K.A.; Paul, D.R. Water Sorption and Transport in a Series of Polysulfones. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 2805–2817. [Google Scholar] [CrossRef]
- Thijs, H.M.L.; Becer, C.R.; Guerrero-Sanchez, C.; Fournier, D.; Hoogenboom, R.; Schubert, U.S. Water uptake of hydrophilic polymers determined by a thermal gravimetric analyzer with a controlled humidity chamber. J. Mater. Chem. 2007, 17, 4864–4871. [Google Scholar] [CrossRef]
- Xing, X.S.; Fu, Z.H.; Zhang, N.N.; Yu, X.Q.; Wang, M.S.; Guo, G.C. High proton conduction in an excellent water-stable gadolinium metal–organic framework. Chem. Commun. 2019, 55, 1241–1244. [Google Scholar] [CrossRef]
- Thammakan, S.; Rodlamul, P.; Semakul, N.; Yoshinari, N.; Konno, T.; Ngamjarurojana, A.; Rujiwatra, A. Gas Adsorption, Proton Conductivity, and Sensing Potential of a Nanoporous Gadolinium Coordination Framework. Inorg. Chem. 2020, 59, 3053–3061. [Google Scholar] [CrossRef]
- Zhai, L.; Yu, J.W.; Zhang, J.; Zhang, W.W.; Wang, L.; Ren, X.M. High quantum yield pure blue emission and fast proton conduction from an indium–metal–organic framework. Dalton Trans. 2019, 48, 12088–12095. [Google Scholar] [CrossRef]
- Liu, S.J.; Cao, C.; Yang, F.; Yu, M.H.; Yao, S.L.; Zheng, T.F.; He, W.W.; Zhao, H.X.; Hu, T.L.; Bu, X.H. High Proton Conduction in Two CoII and MnII Anionic Metal-Organic Frameworks Derived from 1,3,5-Benzenetricarboxylic Acid. Cryst. Growth Des. 2016, 16, 6776–6780. [Google Scholar] [CrossRef]
- Meng, X.; Wang, H.N.; Wang, L.S.; Zou, Y.H.; Zhou, Z.Y. Enhanced proton conductivity of a MOF-808 framework through anchoring organic acids to the zirconium clusters by post-synthetic modification. CrystEngComm 2019, 21, 3146–3150. [Google Scholar] [CrossRef]
- Vilela, S.M.F.; Devic, T.; Várez, A.; Salles, F.; Horcajada, P. A new proton-conducting Bi-carboxylate framework. Dalton Trans. 2019, 48, 11181–11185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.M.; Dong, L.Z.; Qin, J.S.; Guan, W.; Liu, J.; Li, S.L.; Lu, M.; Lan, Y.Q.; Su, Z.M.; Zhou, H.C. Effect of Imidazole Arrangements on Proton-Conductivity in Metal-Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 6183–6189. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, X.; Li, X.; Fu, Z.; Su, Y.; Xu, G. Porous Cadmium(II) Anionic Metal-Organic Frameworks Based on Aromatic Tricarboxylate Ligands: Encapsulation of Protonated Flexible Bis(2-methylimidazolyl) Ligands and Proton Conductivity. Cryst. Growth Des. 2015, 15, 4543–4548. [Google Scholar] [CrossRef]
- Rought, P.; Marsh, C.; Pili, S.; Silverwood, I.P.; Sakai, V.G.; Li, M.; Brown, M.S.; Argent, S.P.; Vitorica-Yrezabal, I.; Whitehead, G.; et al. Modulating proton diffusion and conductivity in metal–organic frameworks by incorporation of accessible free carboxylic acid groups. Chem. Sci. 2019, 10, 1492–1499. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, X.F.; Zhu, H.B.; Zhao, Y.; Li, W.S. A unique 3D metal–organic framework based on a 12-connected pentanuclear Cd(II) cluster exhibiting proton conduction. Dalton Trans. 2015, 44, 14741–14746. [Google Scholar] [CrossRef]
- Deepa, K.; Kesava, M.; Sureshkumar, R.; Dinakaran, K.; Arthanareeswaran, G. Synthesis and electrochemical properties of blend membranes of polysulfone and poly (acrylic acid-co-2-(2-(piperazin-1-yl) ethylamino)-2-hydroxyethyl methacrylate) for proton exchange membrane fuel cell. Int. J. Hydrog. Energy 2018, 43, 21760–21768. [Google Scholar] [CrossRef]
- Echenique-Errandonea, E.; Mendes, R.F.; Figueira, F.; Choquesillo-Lazarte, D.; Beobide, G.; Cepeda, J.; Ananias, D.; Rodríguez-Diéguez, A.; Almeida Paz, F.A.; Seco, J.M. Multifunctional Lanthanide-Based Metal−Organic Frameworks Derived from 3-Amino-4-hydroxybenzoate: Single-Molecule Magnet Behavior, Luminescent Properties for Thermometry, and CO2 Adsorptive Capacity. Inorg. Chem. 2022, 61, 12977–12990. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. FULLPROF 2000, version 2.5d; Lab. Léon Brillouin (CEA-CNRS), Cent. d’Études Saclay: Gif sur Yvette, France, 2000.

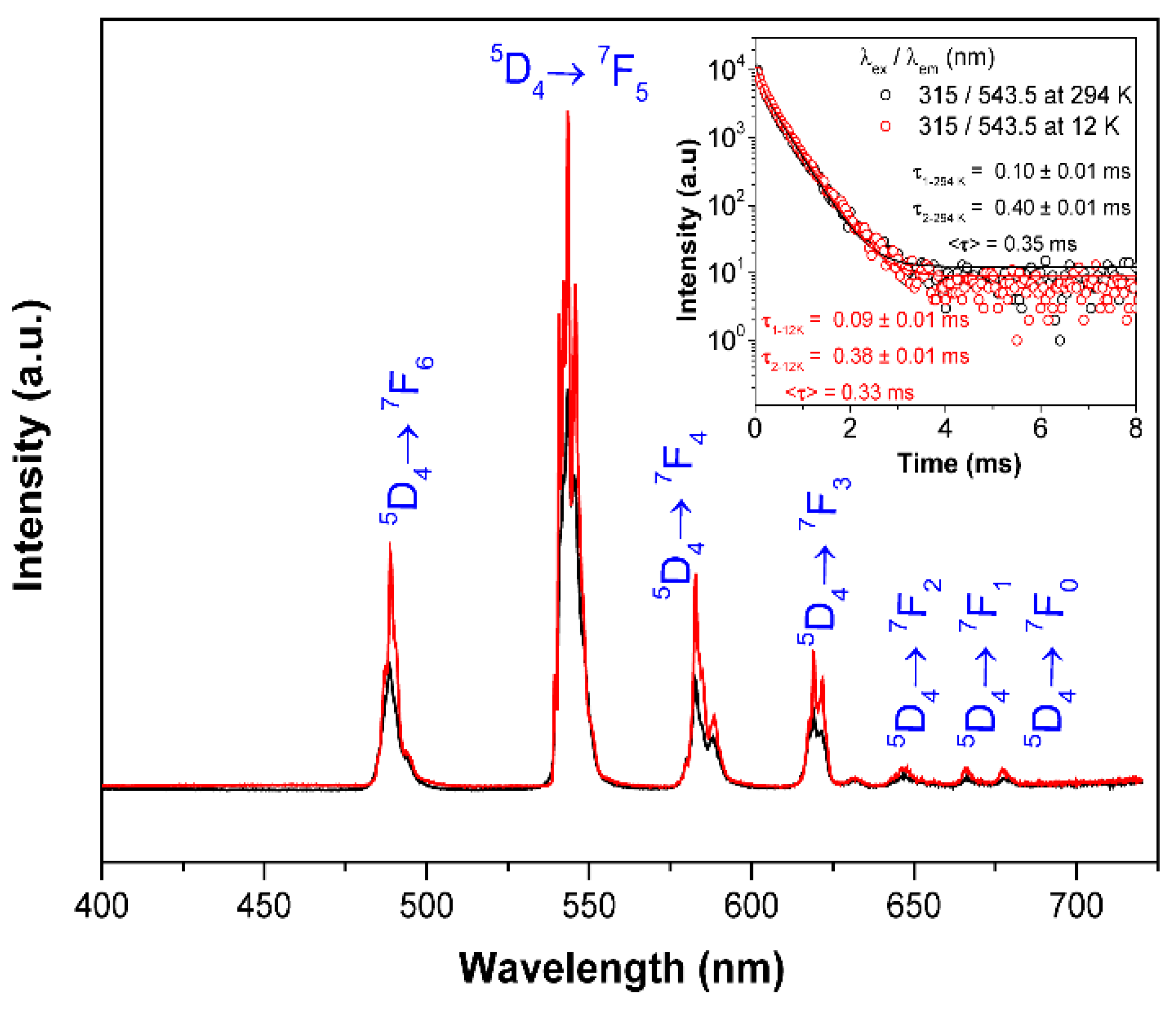
| Temperature (K) | λex/λem (nm) | τ1 (ms) | τ2 (ms) | <τ> (ms) |
|---|---|---|---|---|
| 294 | 315/543.5 | 0.10 ± 0.01 | 0.40 ± 0.01 | 0.35 |
| 12 | 315/543.5 | 0.09 ± 0.01 | 0.38 ± 0.01 | 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echenique-Errandonea, E.; Mendes, R.F.; Figueira, F.; Barbosa, P.; Rojas, S.; Choquesillo-Lazarte, D.; Cepeda, J.; Ananias, D.; Figueiredo, F.; Almeida Paz, F.A.; et al. Easy Handling and Cost-Efficient Processing of a Tb3+-MOF: The Emissive Capacity of the Membrane-Immobilized Material, Water Vapour Adsorption and Proton Conductivity. Nanomaterials 2022, 12, 4380. https://doi.org/10.3390/nano12244380
Echenique-Errandonea E, Mendes RF, Figueira F, Barbosa P, Rojas S, Choquesillo-Lazarte D, Cepeda J, Ananias D, Figueiredo F, Almeida Paz FA, et al. Easy Handling and Cost-Efficient Processing of a Tb3+-MOF: The Emissive Capacity of the Membrane-Immobilized Material, Water Vapour Adsorption and Proton Conductivity. Nanomaterials. 2022; 12(24):4380. https://doi.org/10.3390/nano12244380
Chicago/Turabian StyleEchenique-Errandonea, Estitxu, Ricardo Faria Mendes, Flávio Figueira, Paula Barbosa, Sara Rojas, Duane Choquesillo-Lazarte, Javier Cepeda, Duarte Ananias, Filipe Figueiredo, Filipe A. Almeida Paz, and et al. 2022. "Easy Handling and Cost-Efficient Processing of a Tb3+-MOF: The Emissive Capacity of the Membrane-Immobilized Material, Water Vapour Adsorption and Proton Conductivity" Nanomaterials 12, no. 24: 4380. https://doi.org/10.3390/nano12244380
APA StyleEchenique-Errandonea, E., Mendes, R. F., Figueira, F., Barbosa, P., Rojas, S., Choquesillo-Lazarte, D., Cepeda, J., Ananias, D., Figueiredo, F., Almeida Paz, F. A., Rodríguez-Diéguez, A., & Seco, J. M. (2022). Easy Handling and Cost-Efficient Processing of a Tb3+-MOF: The Emissive Capacity of the Membrane-Immobilized Material, Water Vapour Adsorption and Proton Conductivity. Nanomaterials, 12(24), 4380. https://doi.org/10.3390/nano12244380














