Spontaneous Phase Segregation Enabling Clogging Aversion in Continuous Flow Microfluidic Synthesis of Nanocrystals Supported on Reduced Graphene Oxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Cu2O Nanocrystals on Reduced Graphene Oxide (rGO) Nanosheets
2.3. Characterization Methods
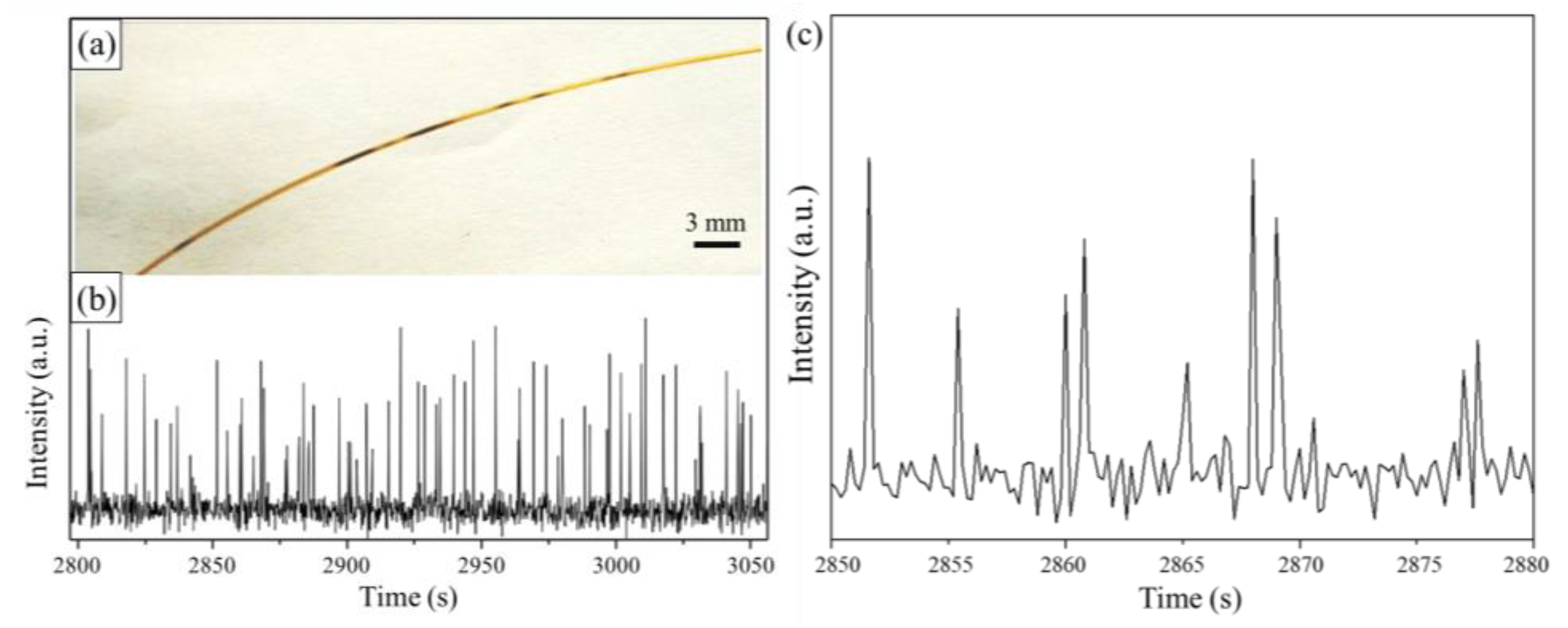
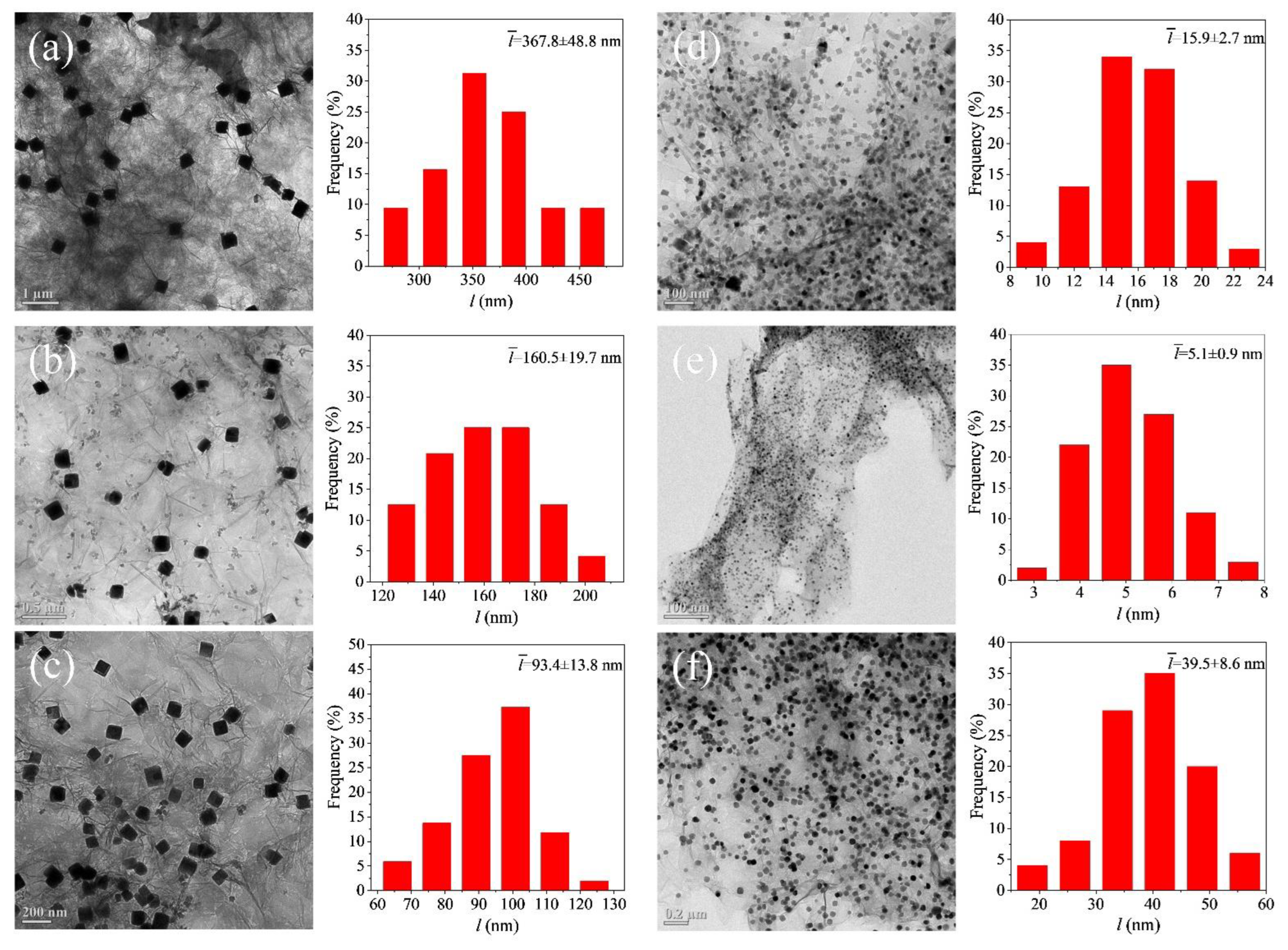
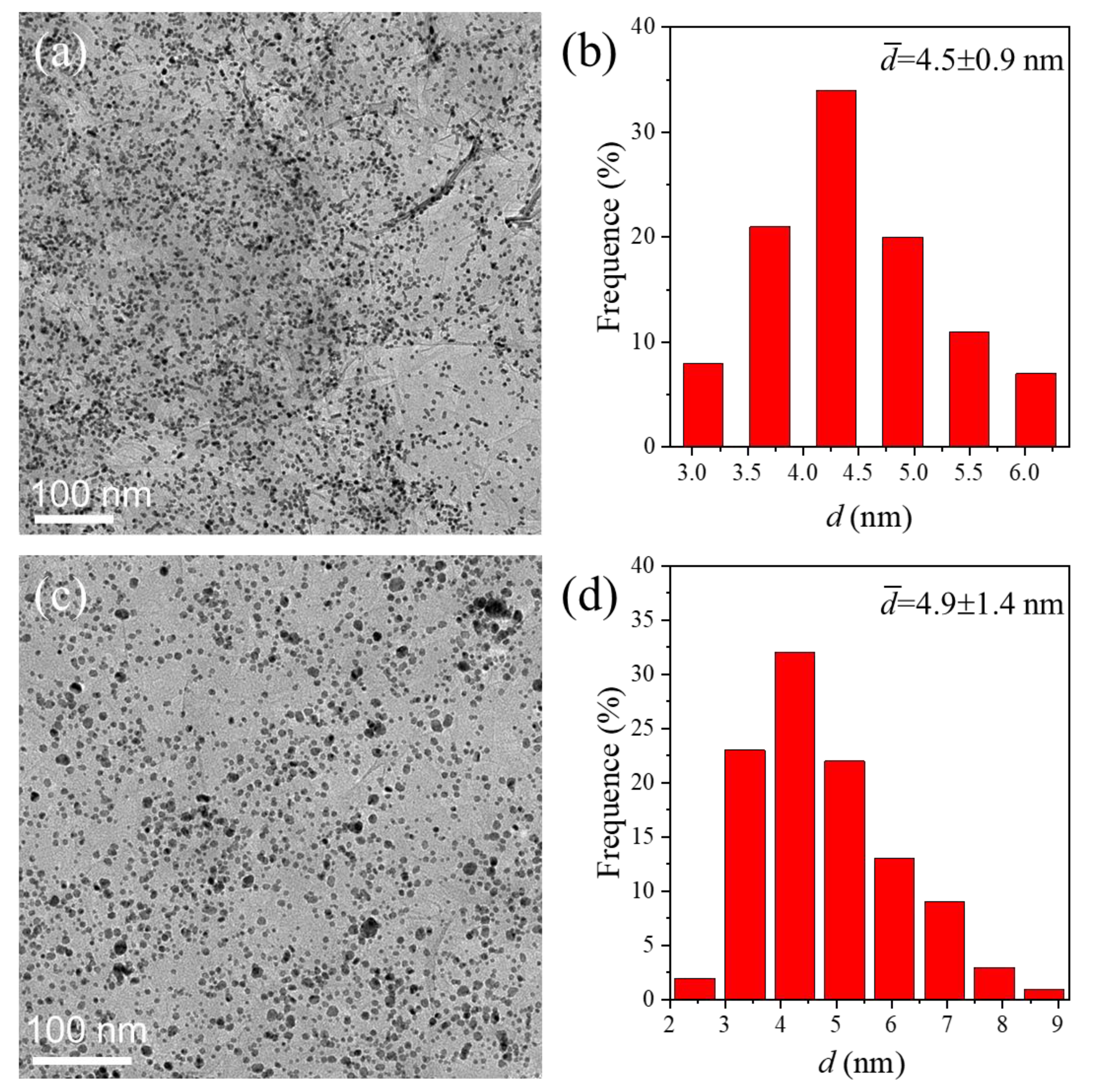
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munde, A.V.; Mulik, B.B.; Chavan, P.P.; Sapner, V.S.; Narwade, S.S.; Mali, S.M.; Sathe, B.R. Electrocatalytic ethanol oxidation on cobalt–bismuth nanoparticle-decorated reduced graphene oxide (Co–Bi@rGO): Reaction pathway investigation toward direct ethanol fuel cells. J. Phys. Chem. C 2021, 125, 2345–2356. [Google Scholar] [CrossRef]
- Li, J.; Ren, X.; Lv, H.; Wang, Y.; Li, Y.; Liu, B. Highly efficient hydrogen production from hydrolysis of ammonia borane over nanostructured Cu@CuCoOx supported on graphene oxide. J. Hazard. Mater. 2020, 391, 122199–122205. [Google Scholar] [CrossRef] [PubMed]
- Ranjith, K.S.; Raju, G.S.R.; Chodankar, N.R.; Ghoreishian, S.M.; Kwak, C.H.; Huh, Y.S.; Han, Y.K. Electroactive Ultra-Thin rGO-Enriched FeMoO4 Nanotubes and MnO2 Nanorods as Electrodes for High-Performance All-Solid-State Asymmetric Supercapacitors. Nanomaterials 2020, 10, 289. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.K.; Das, A. Graphene oxide surface chemistry regulated growth of SnO2 nanoparticles for electrochemical application. J. Alloys Compd. 2020, 834, 154901–154908. [Google Scholar] [CrossRef]
- He, F.A.; Fan, J.T.; Song, F.; Zhang, L.M.; Chan, H. Fabrication of hybrids based on graphene and metal nanoparticles by in situ and self-assembled methods. Nanoscale 2011, 3, 1182–1188. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Bagheri, S. Graphene supported heterogeneous catalysts: An overview. Int. J. Hydrog. Energy 2015, 40, 948–979. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, F.; Peng, M.; Wang, X.; Xia, D.; Guo, G. One-step, facile and ultrafast synthesis of phase- and size-controlled Pt-Bi intermetallic nanocatalysts through continuous-flow microfluidics. J. Am. Chem. Soc. 2015, 137, 6263–6269. [Google Scholar] [CrossRef]
- Hao, N.; Xu, Z.; Nie, Y.; Jin, C.; Closson, A.B.; Zhang, M.; Zhang, J.X.J. Microfluidics-enabled rational design of ZnO micro-/nanoparticles with enhanced photocatalysis, cytotoxicity, and piezoelectric properties. Chem. Eng. J. 2019, 378, 122222–122231. [Google Scholar] [CrossRef]
- Lignos, I.; Stavrakis, S.; Nedelcu, G.; Protesescu, L.; deMello, A.J.; Kovalenko, M.V. Synthesis of cesium lead halide perovskite nanocrystals in a droplet-based microfluidic platform: Fast parametric space mapping. Nano Lett. 2016, 16, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Thiele, M.; Soh, J.Z.E.; Knauer, A.; Malsch, D.; Stranik, O.; Müller, R.; Csáki, A.; Henkel, T.; Köhler, J.M.; Fritzsche, W. Gold nanocubes—Direct comparison of synthesis approaches reveals the need for a microfluidic synthesis setup for a high reproducibility. Chem. Eng. J. 2016, 288, 432–440. [Google Scholar] [CrossRef]
- Shin, Y.; Lim, Y.; Kwak, T.; Hwang, J.H.; Georgescu, A.; Huh, D.; Kim, D.; Kang, T. Microfluidic multi-scale homogeneous mixing with uniform residence time distribution for rapid production of various metal core–shell nanoparticles. Adv. Funct. Mater. 2020, 31, 2007856–2007865. [Google Scholar] [CrossRef]
- Wang, L.; Karadaghi, L.R.; Brutchey, R.L.; Malmstadt, N. Self-optimizing parallel millifluidic reactor for scaling nanoparticle synthesis. Chem. Commun. 2020, 56, 3745–3748. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, D.; Kang, X.; Zhang, D.; Dou, X.; Wang, X.; Guo, G. A scalable synthesis of ternary nanocatalysts for a high-efficiency electrooxidation catalysis by microfluidics. Nanoscale 2020, 12, 12647–12654. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, A.M.; deMello, J.C. Segmented flow reactors for nanocrystal synthesis. Adv. Mater. 2013, 25, 1813–1821. [Google Scholar] [CrossRef]
- Niu, G.; Ruditskiy, A.; Vara, M.; Xia, Y. Toward continuous and scalable production of colloidal nanocrystals by switching from batch to droplet reactors. Chem. Soc. Rev. 2015, 44, 5806–5820. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Sebastian Cabeza, V. Insights in the diffusion controlled interfacial flow synthesis of Au nanostructures in a microfluidic system. Langmuir 2017, 33, 14315–14324. [Google Scholar] [CrossRef]
- Luo, L.; Yang, M.; Chen, G. Continuous synthesis of reduced graphene oxide-supported bimetallic NPs in liquid–liquid segmented flow. Ind. Eng. Chem. Res. 2020, 59, 8456–8468. [Google Scholar] [CrossRef]
- Stolzenburg, P.; Lorenz, T.; Dietzel, A.; Garnweitner, G. Microfluidic synthesis of metal oxide nanoparticles via the nonaqueous method. Chem. Eng. Sci. 2018, 191, 500–510. [Google Scholar] [CrossRef]
- Richard, C.; McGee, R.; Goenka, A.; Mukherjee, P.; Bhargava, R. On-demand milifluidic synthesis of quantum dots in digital droplet reactors. Ind. Eng. Chem. Res. 2020, 59, 3730–3735. [Google Scholar] [CrossRef]
- Sun, Y.; Alimohammadi, F.; Zhang, D.; Guo, G. Enabling colloidal synthesis of edge-oriented MoS2 with expanded interlayer spacing for enhanced HER catalysis. Nano Lett. 2017, 17, 1963–1969. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Zhang, Q.; Zhang, D.; Hu, Q.; Wang, Y.; Wang, X.; Pu, Q.; Guo, G. Visual and real-time imaging focusing for highly sensitive laser-induced fluorescence detection at yoctomole levels in nanocapillaries. Chem. Commun. 2020, 56, 2423–2426. [Google Scholar] [CrossRef] [PubMed]
- Moller, T.; Scholten, F.; Thanh, T.N.; Sinev, I.; Timoshenko, J.; Wang, X.; Jovanov, Z.; Gliech, M.; Roldan Cuenya, B.; Varela, A.S.; et al. Electrocatalytic CO2 reduction on CuOx nanocubes: Tracking the evolution of chemical state, geometric structure, and catalytic selectivity using operando spectroscopy. Angew. Chem. Int. Edit. 2020, 59, 17974–17983. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhang, D.; Wang, Y.; Guo, G.; Wang, X.; Sun, Y. Spontaneous Phase Segregation Enabling Clogging Aversion in Continuous Flow Microfluidic Synthesis of Nanocrystals Supported on Reduced Graphene Oxide. Nanomaterials 2022, 12, 4315. https://doi.org/10.3390/nano12234315
Wang D, Zhang D, Wang Y, Guo G, Wang X, Sun Y. Spontaneous Phase Segregation Enabling Clogging Aversion in Continuous Flow Microfluidic Synthesis of Nanocrystals Supported on Reduced Graphene Oxide. Nanomaterials. 2022; 12(23):4315. https://doi.org/10.3390/nano12234315
Chicago/Turabian StyleWang, Dumei, Dongtang Zhang, Yanan Wang, Guangsheng Guo, Xiayan Wang, and Yugang Sun. 2022. "Spontaneous Phase Segregation Enabling Clogging Aversion in Continuous Flow Microfluidic Synthesis of Nanocrystals Supported on Reduced Graphene Oxide" Nanomaterials 12, no. 23: 4315. https://doi.org/10.3390/nano12234315
APA StyleWang, D., Zhang, D., Wang, Y., Guo, G., Wang, X., & Sun, Y. (2022). Spontaneous Phase Segregation Enabling Clogging Aversion in Continuous Flow Microfluidic Synthesis of Nanocrystals Supported on Reduced Graphene Oxide. Nanomaterials, 12(23), 4315. https://doi.org/10.3390/nano12234315






