Identification of Zirconia Particle Uptake in Human Osteoblasts by ToF-SIMS Analysis and Particle-Size Effects on Cell Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Particle Solutions
2.2. Isolation and Cultivation of Primary Human Osteoblsts
2.3. Scanning Electron Microscopy (SEM)
2.4. Secondary Ion Mass Spectrometry (SIMS) Analysis
2.5. Analysis of the Metabolic Activity of Osteoblasts
2.6. Statistical Analysis
3. Results and Discussion
3.1. Scanning Electron Microscopy
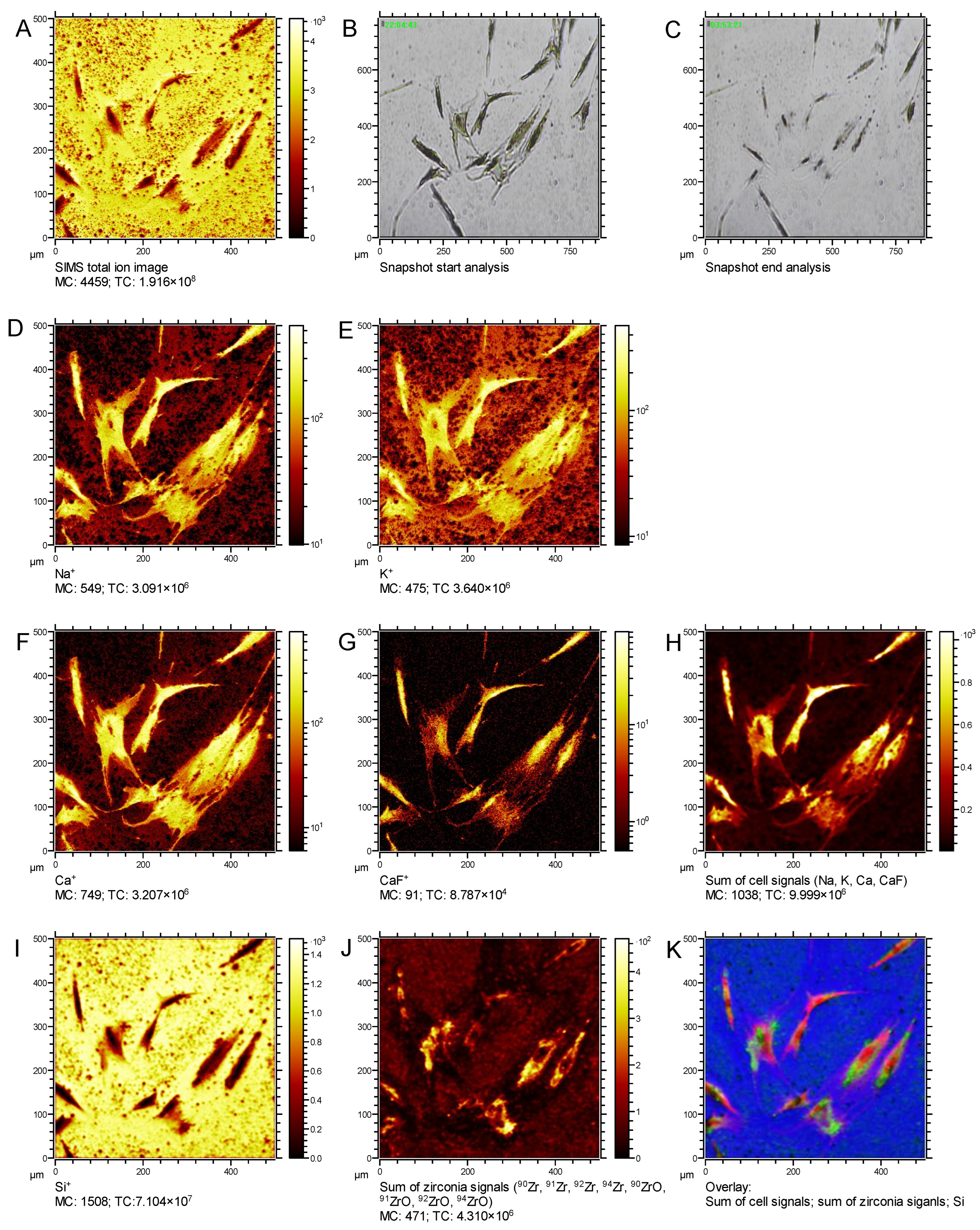
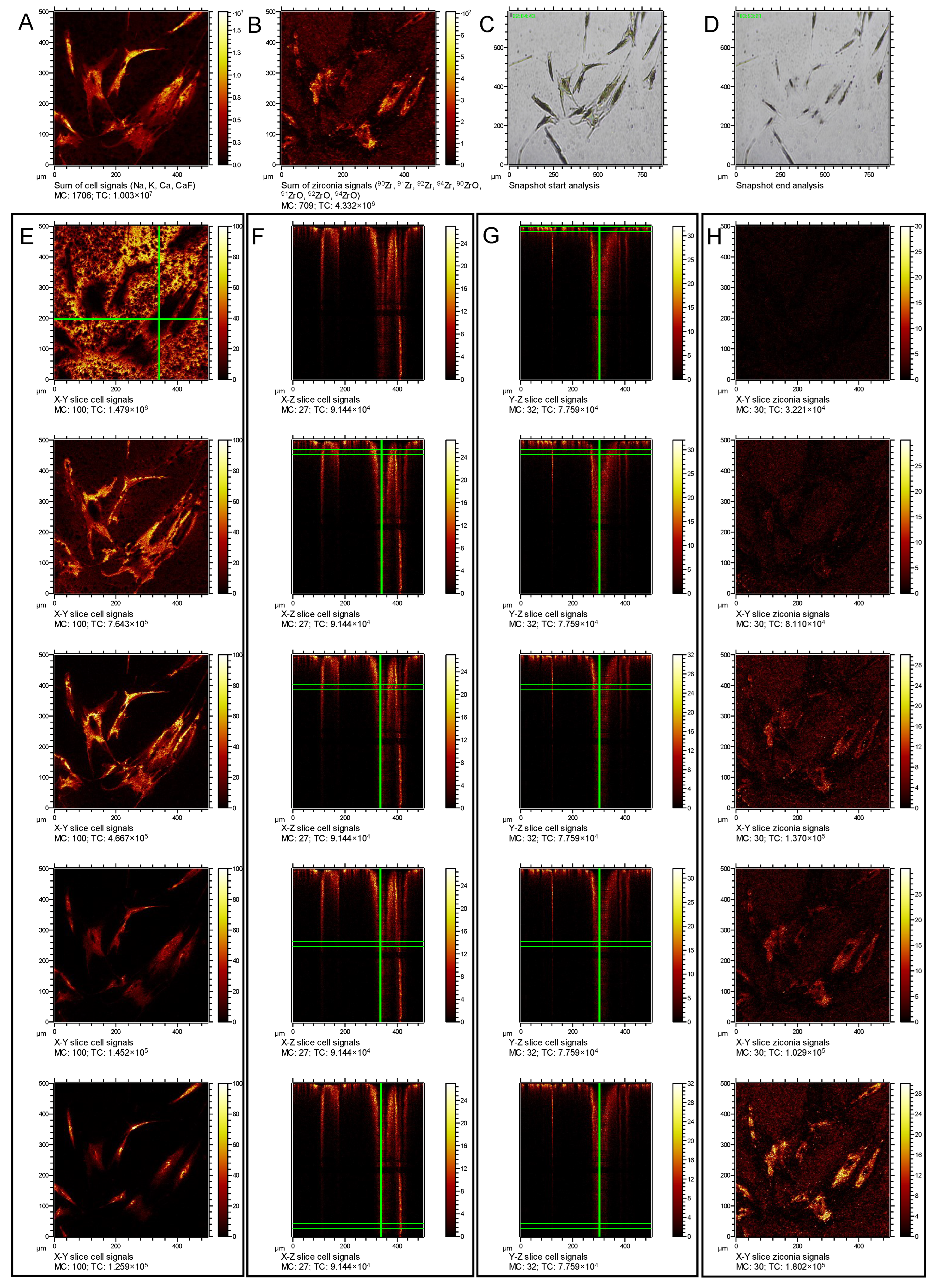
3.2. Establishment of ToF-SIMS for the Detection of Intracellular ZrNP in Human Osteoblasts
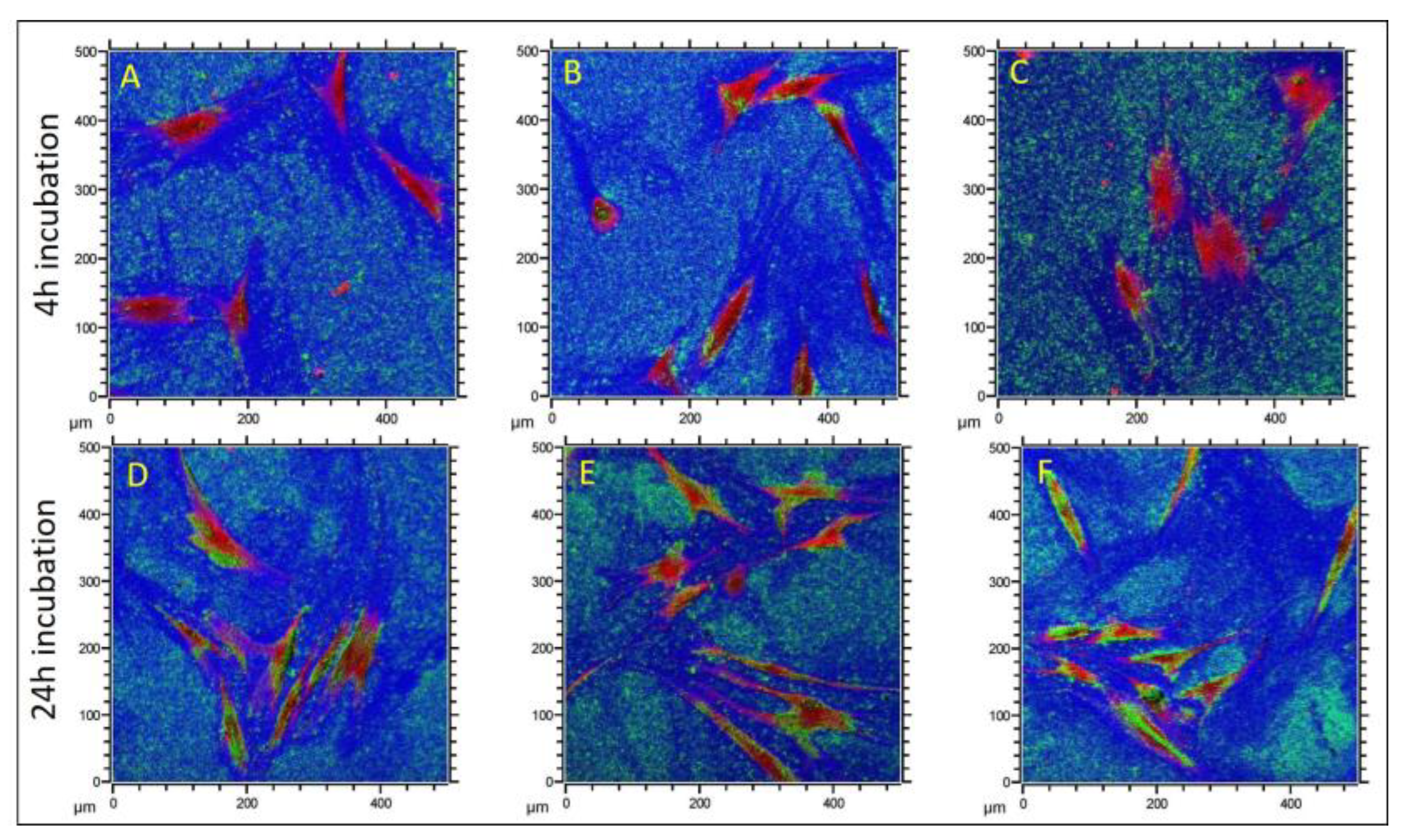
3.3. Identification of Size- and Time-Dependent ZrNP Internalization into Osteoblasts by ToF-SIMS
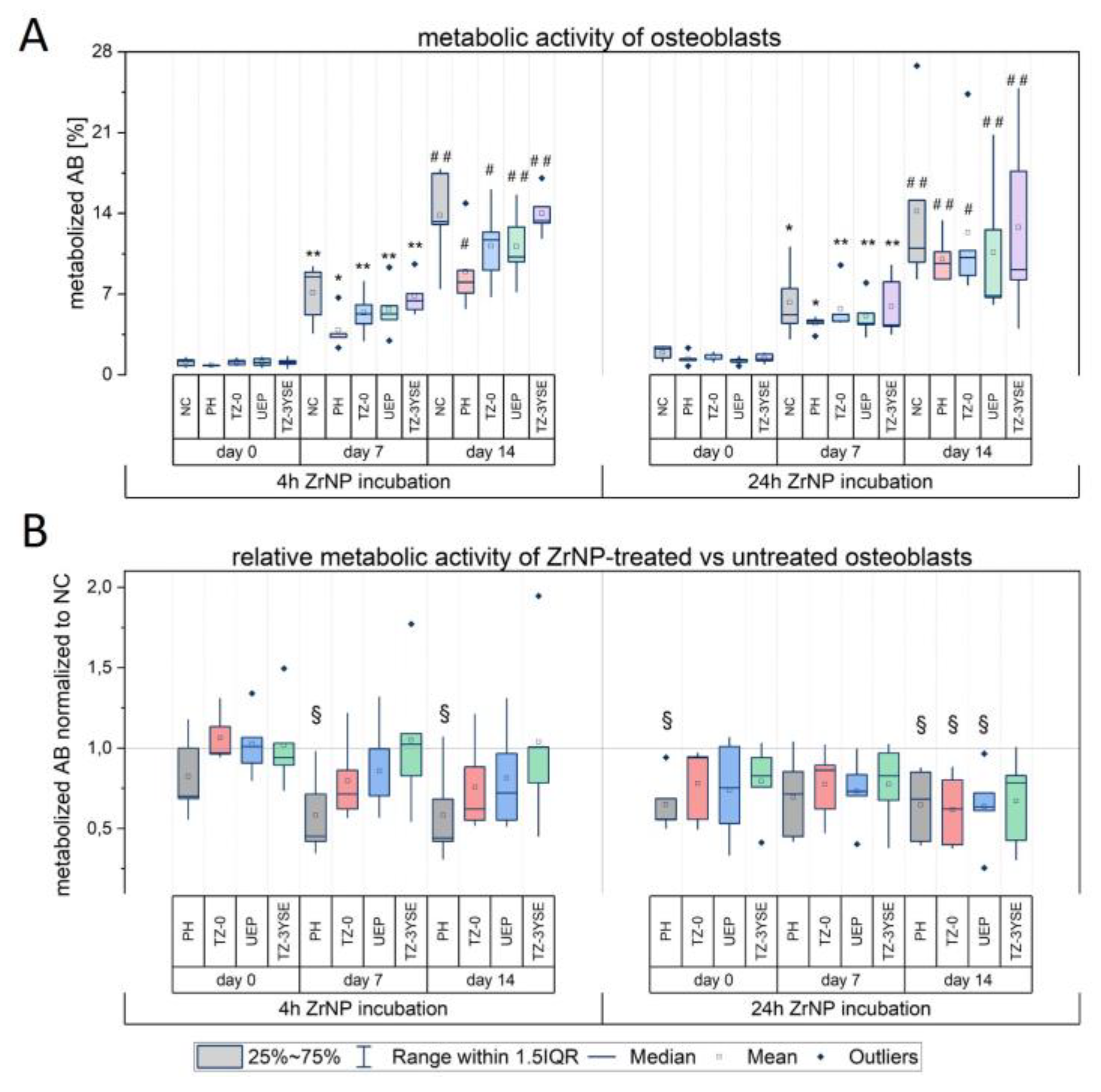
3.4. Effects of ZrNP on the Metabolic Activity of Osteoblasts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arena, A.; Prete, F.; Rambaldi, E.; Bignozzi, M.C.; Monaco, C.; Di Fiore, A.; Chevalier, J. Nanostructured Zirconia-Based Ceramics and Composites in Dentistry: A State-of-the-Art Review. Nanomaterials 2019, 9, 1393. [Google Scholar] [CrossRef] [PubMed]
- Bapat, R.A.; Yang, H.J.; Chaubal, T.V.; Dharmadhikari, S.; Abdulla, A.M.; Arora, S.; Rawal, S.; Kesharwani, P. Review on synthesis, properties and multifarious therapeutic applications of nanostructured zirconia in dentistry. RSC Adv. 2022, 12, 12773–12793. [Google Scholar] [CrossRef]
- Zhang, Y.; Lawn, B.R. Novel Zirconia Materials in Dentistry. J. Dent. Res. 2018, 97, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Fretwurst, T.; Nelson, K.; Tarnow, D.P.; Wang, H.L.; Giannobile, W.V. Is Metal Particle Release Associated with Peri-implant Bone Destruction? An Emerging Concept. J. Dent. Res. 2018, 97, 259–265. [Google Scholar] [CrossRef]
- Zhang, L.; Haddouti, E.; Welle, K.; Burger, C.; Wirtz, D.C.; Schildberg, F.A.; Kabir, K. The Effects of Biomaterial Implant Wear Debris on Osteoblasts. Front. Cell Dev. Biol. 2020, 8, 352. [Google Scholar] [CrossRef]
- He, X.; Reichl, F.X.; Milz, S.; Michalke, B.; Wu, X.; Sprecher, C.M.; Yang, Y.; Gahlert, M.; Rohling, S.; Kniha, H.; et al. Titanium and zirconium release from titanium- and zirconia implants in mini pig maxillae and their toxicity in vitro. Dent. Mater. 2020, 36, 402–412. [Google Scholar] [CrossRef]
- Houshmand, A.; Donkiewicz, P.; Smeets, R.; Jung, O.; Barbeck, M. Incidental finding of a degrading zirconia dental implant 29 months after implantation: Histological and histomorphometrical analysis. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2919–2923. [Google Scholar] [CrossRef]
- Nelson, K.; Hesse, B.; Addison, O.; Morrell, A.P.; Gross, C.; Lagrange, A.; Suarez, V.I.; Kohal, R.; Fretwurst, T. Distribution and Chemical Speciation of Exogenous Micro- and Nanoparticles in Inflamed Soft Tissue Adjacent to Titanium and Ceramic Dental Implants. Anal. Chem. 2020, 92, 14432–14443. [Google Scholar] [CrossRef] [PubMed]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Hou, Y.; Cai, K.; Li, J.; Chen, X.; Lai, M.; Hu, Y.; Luo, Z.; Ding, X.; Xu, D. Effects of titanium nanoparticles on adhesion, migration, proliferation, and differentiation of mesenchymal stem cells. Int. J. Nanomed. 2013, 8, 3619–3630. [Google Scholar] [CrossRef]
- Papageorgiou, I.; Brown, C.; Schins, R.; Singh, S.; Newson, R.; Davis, S.; Fisher, J.; Ingham, E.; Case, C.P. The effect of nano- and micron-sized particles of cobalt-chromium alloy on human fibroblasts in vitro. Biomaterials 2007, 28, 2946–2958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Lykotrafitis, G.; Bao, G.; Suresh, S. Size-Dependent Endocytosis of Nanoparticles. Adv. Mater. 2009, 21, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Wang, Q.; Li, J.; Liu, H.; Zhao, Q.; Huang, X.; Dong, H.; Chen, W.; Gui, R.; Nie, X. Zirconia Nanoparticles Induce HeLa Cell Death through Mitochondrial Apoptosis and Autophagy Pathways Mediated by ROS. Front. Chem. 2021, 9, 522708. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, F.M.; Katubi, K.M.S.; Ali, D.; Alarifi, S. Apoptotic and DNA-damaging effects of yttria-stabilized zirconia nanoparticles on human skin epithelial cells. Int. J. Nanomed. 2019, 14, 7003–7016. [Google Scholar] [CrossRef]
- Suh, K.S.; Lee, Y.S.; Seo, S.H.; Kim, Y.S.; Choi, E.M. Effect of zinc oxide nanoparticles on the function of MC3T3-E1 osteoblastic cells. Biol. Trace Elem. Res. 2013, 155, 287–294. [Google Scholar] [CrossRef]
- Sun, G.J.; Yang, S.F.; Ti, Y.F.; Guo, G.D.; Fan, G.T.; Chen, F.R.; Xu, S.G.; Zhao, J.N. Influence of Ceramic Debris on Osteoblast Behaviors: An In Vivo Study. Orthop. Surg. 2019, 11, 770–776. [Google Scholar] [CrossRef]
- Ye, M.; Shi, B. Zirconia Nanoparticles-Induced Toxic Effects in Osteoblast-Like 3T3-E1 Cells. Nanoscale Res. Lett. 2018, 13, 353. [Google Scholar] [CrossRef]
- Zhou, G.; Gu, G.; Li, Y.; Zhang, Q.; Wang, W.; Wang, S.; Zhang, J. Effects of cerium oxide nanoparticles on the proliferation, differentiation, and mineralization function of primary osteoblasts in vitro. Biol. Trace Elem. Res. 2013, 153, 411–418. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef]
- Priest, L.; Peters, J.S.; Kukura, P. Scattering-based Light Microscopy: From Metal Nanoparticles to Single Proteins. Chem. Rev. 2021, 121, 11937–11970. [Google Scholar] [CrossRef]
- Friedrich, R.P.; Kappes, M.; Cicha, I.; Tietze, R.; Braun, C.; Schneider-Stock, R.; Nagy, R.; Alexiou, C.; Janko, C. Optical Microscopy Systems for the Detection of Unlabeled Nanoparticles. Int. J. Nanomed. 2022, 17, 2139–2163. [Google Scholar] [CrossRef] [PubMed]
- Malmberg, P.; Nygren, H.; Sjovall, P.; Lausmaa, J. Subcellular localisation of cholesterol and phosphocholine with pattern-recognition-imaging-TOF-SIMS. Spectrosc.-Int. J. 2004, 18, 503–511. [Google Scholar] [CrossRef]
- Nygren, H.; Malmberg, P.; Kriegeskotte, C.; Arlinghaus, H.F. Bioimaging TOF-SIMS: Localization of cholesterol in rat kidney sections. FEBS Lett. 2004, 566, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Veith, L.; Bottner, J.; Vennemann, A.; Breitenstein, D.; Engelhard, C.; Meijer, J.; Estrela-Lopis, I.; Wiemann, M.; Hagenhoff, B. Detection of ZrO2 Nanoparticles in Lung Tissue Sections by Time-of-Flight Secondary Ion Mass Spectrometry and Ion Beam Microscopy. Nanomaterials 2018, 8, 44. [Google Scholar] [CrossRef]
- Veith, L.; Dietrich, D.; Vennemann, A.; Breitenstein, D.; Engelhard, C.; Karst, U.; Sperling, M.; Wiemann, M.; Hagenhoff, B. Combination of micro X-ray fluorescence spectroscopy and time-of-flight secondary ion mass spectrometry imaging for the marker-free detection of CeO2 nanoparticles in tissue sections. J. Anal. Atom. Spectrom. 2018, 33, 491–501. [Google Scholar] [CrossRef]
- Veith, L.; Vennemann, A.; Breitenstein, D.; Engelhard, C.; Hagenhoff, B.; Wiemann, M. Distribution of Paramagnetic Fe2O3/SiO2-Core/Shell Nanoparticles in the Rat Lung Studied by Time-of-Flight Secondary Ion Mass Spectrometry: No Indication for Rapid Lipid Adsorption. Nanomaterials 2018, 8, 571. [Google Scholar] [CrossRef]
- Brunelle, A.; Laprévote, O. Recent advances in biological tissue imaging with Time-of-flight Secondary Ion Mass Spectrometry: Polyatomic ion sources, sample preparation, and applications. Curr. Pharm. Des. 2007, 13, 3335–3343. [Google Scholar] [CrossRef]
- Gamble, L.J.; Graham, D.J.; Bluestein, B.; Whitehead, N.P.; Hockenbery, D.; Morrish, F.; Porter, P. ToF-SIMS of tissues: “Lessons learned” from mice and women. Biointerphases 2015, 10, 019008. [Google Scholar] [CrossRef]
- Möller, J.; Beumer, A.; Lipinsky, D.; Arlinghaus, H.F. Introduction of a cryosectioning-ToF-SIMS instrument for analysis of non-dehydrated biological samples. Appl. Surf. Sci. 2006, 252, 6709–6711. [Google Scholar] [CrossRef]
- Tyler, B.J.; Rangaranjan, S.; Möller, J.; Beumer, A.; Arlinghaus, H.F. TOF-SIMS imaging of chlorhexidine-digluconate transport in frozen hydrated biofilms of the fungus Candida albicans. Appl. Surf. Sci. 2006, 252, 6712–6715. [Google Scholar] [CrossRef]
- Schaepe, K.; Kokesch-Himmelreich, J.; Rohnke, M.; Wagner, A.-S.; Schaaf, T.; Wenisch, S.; Janek, J. Assessment of different sample preparation routes for mass spectrometric monitoring and imaging of lipids in bone cells via ToF-SIMS. Biointerphases 2015, 10, 019016. [Google Scholar] [CrossRef]
- Altmann, B.; Kohal, R.J.; Steinberg, T.; Tomakidi, P.; Bachle-Haas, M.; Wennerberg, A.; Att, W. Distinct cell functions of osteoblasts on UV-functionalized titanium- and zirconia-based implant materials are modulated by surface topography. Tissue Eng. Part C Methods 2013, 19, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, S.; Kudo, M.; Hayama, M.; Hasegawa, U.; Sakai, K.; Tozu, M.; Hoshi, T. TOF-SIMS Imaging of Protein Adsorption on Dialysis Membrane by means of Information Entropy. E-J. Surf. Sci. Nanotechnol. 2003, 1, 67–71. [Google Scholar] [CrossRef][Green Version]
- Bellion, M.; Santen, L.; Mantz, H.; Hähl, H.; Quinn, A.; Nagel, A.; Gilow, C.; Weitenberg, C.; Schmitt, Y.; Jacobs, K. Protein adsorption on tailored substrates: Long-range forces and conformational changes. J. Phys. Condens. Matter 2008, 20, 404226. [Google Scholar] [CrossRef]
- Ekblad, T.; Liedberg, B. Protein adsorption and surface patterning. Curr. Opin. Colloid Interface Sci. 2010, 15, 499–509. [Google Scholar] [CrossRef]
- Höök, F.; Vörös, J.; Rodahl, M.; Kurrat, R.; Böni, P.; Ramsden, J.J.; Textor, M.; Spencer, N.D.; Tengvall, P.; Gold, J.; et al. A comparative study of protein adsorption on titanium oxide surfaces using in situ ellipsometry, optical waveguide lightmode spectroscopy, and quartz crystal microbalance/dissipation. Colloids Surf. B Biointerfaces 2002, 24, 155–170. [Google Scholar] [CrossRef]
- Scotchford, C.A.; Gilmore, C.P.; Cooper, E.; Leggett, G.J.; Downes, S. Protein adsorption and human osteoblast-like cell attachment and growth on alkylthiol on gold self-assembled monolayers. J. Biomed. Mater. Res. 2002, 59, 84–99. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, T.; Varela, J.; Lynch, I.; Salvati, A.; Dawson, K.A. Quantitative assessment of the comparative nanoparticle-uptake efficiency of a range of cell lines. Small 2011, 7, 3341–3349. [Google Scholar] [CrossRef]
- Huang, K.; Ma, H.; Liu, J.; Huo, S.; Kumar, A.; Wei, T.; Zhang, X.; Jin, S.; Gan, Y.; Wang, P.C.; et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano 2012, 6, 4483–4493. [Google Scholar] [CrossRef]
- Schübbe, S.; Schumann, C.; Cavelius, C.; Koch, M.; Müller, T.; Kraegeloh, A. Size-Dependent Localization and Quantitative Evaluation of the Intracellular Migration of Silica Nanoparticles in Caco-2 Cells. Chem. Mater. 2012, 24, 914–923. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]

| Particle Label | Composition in Addition to ZrO2 | Manufacturer |
|---|---|---|
| PH | No data | Tosoh Corporation, Tokyo, Japan |
| TZ-0 | No data | Tosoh Corporation, Tokyo, Japan |
| UEP | Fe2O3: 0.002%, SiO2: 0.01%, TiO2: 0.001% | Daiichi Kigenso Kagaku Kogyo Co., Ltd., Osaka, Japan |
| TZ-3YSE | Y2O3: 5.15%, Al2O3: 0.25%, SiO2: ≤0.02%, Fe2O3: ≤0.01%, Na2O: ≤0.04% | Tosoh Corporation, Tokyo, Japan |
| Particle Label | Exposure Time | Particle Internalization |
|---|---|---|
| PH | 4 h | No |
| 24 h | Yes | |
| TZ-0 | 4 h | No |
| 24 h | Yes | |
| UEP | 4 h | No |
| 24 h | partly | |
| TZ-3YSE | 4 h | No |
| 24 h | possibly |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welle, A.; Rabel, K.; Schwotzer, M.; Kohal, R.J.; Steinberg, T.; Altmann, B. Identification of Zirconia Particle Uptake in Human Osteoblasts by ToF-SIMS Analysis and Particle-Size Effects on Cell Metabolism. Nanomaterials 2022, 12, 4272. https://doi.org/10.3390/nano12234272
Welle A, Rabel K, Schwotzer M, Kohal RJ, Steinberg T, Altmann B. Identification of Zirconia Particle Uptake in Human Osteoblasts by ToF-SIMS Analysis and Particle-Size Effects on Cell Metabolism. Nanomaterials. 2022; 12(23):4272. https://doi.org/10.3390/nano12234272
Chicago/Turabian StyleWelle, Alexander, Kerstin Rabel, Matthias Schwotzer, Ralf Joachim Kohal, Thorsten Steinberg, and Brigitte Altmann. 2022. "Identification of Zirconia Particle Uptake in Human Osteoblasts by ToF-SIMS Analysis and Particle-Size Effects on Cell Metabolism" Nanomaterials 12, no. 23: 4272. https://doi.org/10.3390/nano12234272
APA StyleWelle, A., Rabel, K., Schwotzer, M., Kohal, R. J., Steinberg, T., & Altmann, B. (2022). Identification of Zirconia Particle Uptake in Human Osteoblasts by ToF-SIMS Analysis and Particle-Size Effects on Cell Metabolism. Nanomaterials, 12(23), 4272. https://doi.org/10.3390/nano12234272










