Abstract
The development of stimuli-responsive controlled release formulations is a potential method of improving pesticide utilization efficiency and alleviating current pesticide-related environmental pollution. In this study, a self-destruction redox-responsive pesticide delivery system using biodegradable disulfide-bond-bridged mesoporous organosilica (DMON) nanoparticles as the porous carriers and coordination complexes of gallic acid (GA) and Fe(III) ions as the capping agents were established for controlling prochloraz (PRO) release. The GA–Fe(III) complexes deposited onto the surface of DMON nanoparticles could effectively improve the light stability of prochloraz. Due to the decomposition of GA–Fe(III) complexes, the nano-vehicles had excellent redox-responsive performance under the reducing environments generated by the fungus. The spreadability of PRO@DMON–GA–Fe(III) nanoparticles on the rice leaves was increased due to the hydrogen bonds between GA and rice leaves. Compared with prochloraz emulsifiable concentrate, PRO@DMON–GA–Fe(III) nanoparticles showed better fungicidal activity against Magnaporthe oryzae with a longer duration under the same concentration of prochloraz. More importantly, DMON–GA–Fe(III) nanocarriers did not observe obvious toxicity to the growth of rice seedlings. Considering non-toxic organic solvents and excellent antifungal activity, redox-responsive pesticide controlled release systems with self-destruction properties have great application prospects in the field of plant disease management.
1. Introduction
In modern agriculture, pesticides play an irreplaceable role in crop yield and pest management [,]. However, about 0.1% of the traditionally used pesticides reach the desired target site of organisms, while 99.9% are lost into the surrounding environment []. Pesticide application in the field is inefficient due to runoff, biodegradation, ultraviolet (UV) degradation, leaching, and rapid evaporation, which not only increases the cost of pesticide application, but also has adverse effects on the environment [,]. At present, controlled release pesticide delivery systems have been developed to improve the utilization efficiency of active ingredients by increasing bioavailability, reducing photolysis, and prolonging durability [,,]. Conventional controlled release pesticide systems based on microencapsulation and clays can only release active ingredients slowly through molecular diffusion rather than achieve on-demand release at temporal and spatial resolutions [,,,]. Therefore, developing a precisely controlled release system is urgent to guarantee spatiotemporal pesticide release [].
In recent years, many efforts have been devoted to developing stimuli-responsive nanomaterials with the hope that they can be designed as effective pesticide delivery vehicles [,,]. A variety of endogenous (including pH values [], enzyme [], and redox []) or exogenous stimuli (including temperature [], light [], magnetic field [], and ultrasound []) have been used to induce chemical reactions in stimuli-responsive nanocarriers for realizing on-demand pesticide delivery. Among these various stimuli-responsive nanomaterials, disulfide-bond-bridged mesoporous organosilica (DMON) nanoparticles with redox-responsive properties are attracting much attention due to their excellent biocompatibility and degradable properties in reducing environments [,,]. The disulfide bond in the nanocarriers framework is one of the most biologically effective cleavable linkers in biology, which can be degraded and cleaved in the presence of glutathione []. The conventional mesoporous silica nanoparticles show a lower pesticide release rate and cannot degrade in the reduction environment in comparison with redox-responsive biodegradable DMON nanoparticles. Therefore, the DMON nanoparticles are optimal candidates as supporting materials to control pesticide release.
Gallic acid (GA) is a naturally occurring secondary metabolite that is widely present in plant tissues and plays an important role in plant defense against pathogens and insects [,,,]. As a water-soluble natural polyphenolic compound, the catechol groups of GA can react with Fe(III) ions to produce stable, surface-confined amorphous films on solid substrates [,,]. The metal–phenolic network formed by the self-assembly of the strong metal–phenol complex interface can effectively block porous pores of nanocarriers and inhibit the migration of pesticide molecules [,,]. In addition, the cross-linking process between GA and Fe(III) ions is rapid based on the wet chemistry method, the reaction process is fast and free of contaminants or toxic substances, which is a green and visible encapsulation strategy []. Therefore, GA–Fe(III) complexes can be employed as a supramolecular capping agent to block the pores of DMON nanoparticles.
Rice (Oryza sativa L.) is a crucial cultivated crop in the world, which supports over half of the global population []. As the most destructive disease on rice, rice blast caused by ascomycetous fungus (Magnaporthe oryzae) can induce an annual rice production loss of 10–30% (up to 50% in disease epidemics) [,]. It has been reported that Magnaporthe oryzae has an efficient antioxidant defense system, which can produce glutathione during host plant infection to neutralize reactive oxygen species produced by host defense responses [,]. Microenvironmental variations at disease-occurring sites facilitate the development of multiple-stimuli-responsive nanocarriers for on-demand and on-target pesticide release []. The loaded pesticide molecules can be precisely released to the target site due to the influence of the microenvironment generated by the pathogen infection site []. Thus, combining the pathogen infection process with the targeted pesticide release technique is an efficient approach to manage plant diseases.
As an inhibitor of the enzyme lanosterol 14α-demethylase, prochloraz (N-propyl-N-[2-(2,4,6-trichlorophenoxy)ethyl]imidazole-1-carboxamide) has excellent antifungal activity against Sclerotinia sclerotiorum [], Penicillium digitatum [], Lasiodiplodia theobromae [], Rhizoctonia solani [], Fusarium fujikuroi [], and Magnaporthe oryzae []. The most commonly used prochloraz formulation types include emulsifiable concentrate and oil-in-water emulsion because of its low solubility in water (about 26.5 μg/mL) [,]. However, prochloraz is unstable under UV and natural light, which leads to its short-term efficiency and low utilization rate []. To address these issues, prochloraz can be incorporated into the stable and biocompatible nanoparticles, thus enhancing photostability, increasing water solubility, and prolonging the effective duration of active ingredients.
Herein, the self-destructive redox-responsive biodegradable nano-vehicles were established using DMON nanoparticles as porous carriers and metal–phenolic networks as gatekeepers to deliver prochloraz for rice disease management. The redox environment caused by Magnaporthe oryzae infection could trigger degradation of the DMON nanoparticles and metal–phenolic networks, and the prochloraz released from PRO@DMON–GA–Fe(III) nanoparticles would induce the death of the pathogen. The characterization, release profiles, release mechanism, UV-shielding properties, spreadability, fungicidal activities, and biosafety of the prepared PRO@DMON–GA–Fe(III) nanoparticles were comprehensively investigated.
2. Experimental
2.1. Materials
Prochloraz technical (97% purity) was purchased from Hubei Jiufenglong Chemical Co., Ltd. (Wuhan, China). The 25% prochloraz emulsifiable concentrate was supplied by Sichuan Runer Technology Co., Ltd. (Chengdu, China). Triethylamine (TEA), tetraethyl orthosilicate (TEOS), and glutathione were received from Sinopharm Chemical Reagent Beijing Co., Ltd. (Beijing, China). Bis(triethoxysilyl propyl) disulfide (BTESPT), cetyltrimethylammonium bromide (CTAB), gallic acid (GA), sodium hydroxide (NaOH), and ferric chloride hexahydrate (FeCl3·6H2O) were supplied by Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Methanol, ethanol, acetic acid, and concentrated hydrochloric acid (HCl, 37%) were purchased from Sinopharm Chemical Reagent Beijing Co., Ltd. (Beijing, China). Methanol (HPLC grade) was ordered from Adamas Beta Co., Ltd. (Shanghai, China). Deionized water (18 MΩ/cm) was prepared using a Milli-Q water purification system (Millipore, Milford, MA, USA).
2.2. Preparation of PRO@DMON–GA–Fe(III) Nanoparticles
2.2.1. Synthesis of Disulfide-Bridged Mesoporous Organosilica (DMON) Nanoparticles
In a typical synthesis, 0.25 mL of TEA and 0.48 g of CTAB were dissolved in 15.5 mL of ethanol. This solution was then introduced into 94 mL of deionized water and mixed for 30 min at 80 °C. Then, another mixture (2.0 mL of TEOS and 0.8 mL of BTESPT) was added to the surfactant solution dropwise. The obtained mixture was heated at 80 °C for another 6 h. Next, under centrifugation at 20,000 rpm for 15 min, the resulting white products were obtained and fully washed using ethanol and deionized water. To remove the surfactant in the pores of DMON nanoparticles, the obtained products were redispersed in the acidic ethanol (3 mL of HCl and 100 mL of ethanol) and then refluxed at 80 °C for 12 h. The reflux was repeated at least four times. The CTAB-free DMON nanoparticles were collected by centrifugation, washed with ethanol, and dried under vacuum at 60 °C for 12 h.
2.2.2. Synthesis of PRO@DMON–GA–Fe(III) Nanoparticles
Approximately 400 mg of prochloraz was added to 10 mL of methanol, and then 200 mg of DMON nanoparticles were suspended in the prepared pesticide solution. The obtained prochloraz-loaded hybrid nanoparticles (PRO@DMON nanoparticles) were washed with deionized water several times after stirring in the dark at room temperature for 48 h. The precipitate was vacuum-dried at 60 °C for 12 h. After that, 20 mg of PRO@DMON nanoparticles were dispersed in 2 mL of deionized water. Approximately 400 μL of FeCl3·6H2O solution (30 mM) and 800 μL of GA solution (15 mM) were also added. After vortexing for 30 s, the suspension pH was adjusted using 40 μL of NaOH solution (0.5 M), which was followed by vortexing for 60 s. The PRO@DMON–GA–Fe(III) nanoparticles were collected by centrifugation, washed with deionized water, and dried under vacuum at 60 °C for 12 h.
2.3. Characterization
The morphology of the porous structure of the prepared nanoparticles was characterized using a Philips Tecnai 12 transmission electron microscope (TEM) at an acceleration voltage of 120 kV (Phillips, Eindhoven, The Netherlands). The elemental mapping of the as-synthesized samples was conducted using high-resolution TEM (HRTEM, Tecnai G2 F30, FEI, Hillsboro, Oregon USA) equipped with an energy-dispersive X-ray spectroscopy (EDX) system. The elemental composition and chemical state of the samples were characterized by X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250Xi, Thermo-Fisher, Waltham, MA, USA). A Tensor 27 Fourier transform spectrometer (FTIR) (Thermo-Fisher, Waltham, MA, USA) was used to analyze various functionalized nanoparticles. A TriStar II 3020 analyzer was used to conduct the nitrogen adsorption/desorption experiments (Micromeritics Instrument Corporation, Norcross, USA). The Brunauer–Emmett–Teller (BET) theory and Barret–Joyner–Halenda (BJH) were adopted to calculate the surface area and pore size of the samples, respectively. The contact angle and adhesion work on rice blades were measured using an SL-200B optical contact-angle goniometer (Kino Industrial Co., Ltd., New York, NY, USA).
2.4. Biodegradation Assays of DMON Nanoparticles
Typically, approximately 2 mg of DMON nanoparticles was dispersed in deionized water with 10 mM of glutathione. The mixture was stirred at room temperature for 10 d. At pre-determined time points (day 0, 2, 5, and 10), the samples were obtained to investigate the structural changes using TEM.
2.5. Pesticide Loading Content and Controlled Release Kinetics
2.5.1. Pesticide Loading Content
In order to evaluate the prochloraz content in the PRO@DMON–GA–Fe(III) nanoparticles, the nanoparticles were added to a methanol–water mixture solution (50:50, v/v), which contains 100 mM of glutathione. The concentration of prochloraz released from PRO@DMON–GA–Fe(III) nanoparticles was evaluated using a Waters high-performance liquid chromatography (HPLC) (Milford, MA, USA). The HPLC was equipped with a diode array detector with an InertSustain C18 analytical column (5 μm, 250 × 4.6 mm, GL Sciences Inc., Tokyo, Japan) maintained at 35 °C. The aqueous mobile phase contained 70% methanol and 30% ultra-pure water with 0.1% acetic acid. The injection volume of the samples was 20 μL and the mobile phase flow rate was 1 mL/min. The solvents were then filtered using a 0.45 μm membrane filter for HPLC measurements. The prochloraz loading efficiency of the nanoparticles was calculated according to Equation (1).
2.5.2. Controlled Release Kinetics
The influence of glutathione on the prochloraz release behavior of PRO@DMON–GA–Fe(III) nanoparticles was analyzed using the HPLC method. Approximately 1.5 mL of PRO@DMON–GA–Fe(III) nanoparticles dispersion solution at a concentration of 10 mg/mL was transferred into the dialysis bag. Then, the dialysis bag was immersed in 48.5 mL of methanol–water mixture (30:70, v/v) with different concentrations of glutathione (0, 2, and 10 mM) under slow magnetic stirring in the dark condition. At each specific time, 1 mL of the solution was extracted and an equal volume of methanol–water mixture (30:70, v/v) containing glutathione was replenished. Each treatment was repeated three times.
The release behaviors of prochloraz from the PRO@DMON–GA–Fe(III) nanoparticles suspension were evaluated using the zero-order (Equation (2)), first-order (Equation (3)), Ritger–Peppas (Equation (4)) and Higuchi (Equation (5)) models, respectively.
where k is the kinetic constant and relies on the combined characteristics of prochloraz and DMON–GA–Fe(III) nanoparticles system; Mt/Mz represents the percentage of prochloraz released from PRO@DMON–GA–Fe(III) nanoparticles at time t; n is a diffusional exponent to characterize the release mechanism of prochloraz from the nanoparticles.
2.6. Photolysis Test
To investigate the differences in photo-stability between the samples, PRO@DMON–GA–Fe(III) nanoparticles, prochloraz emulsifiable concentrate and prochloraz technical at the same prochloraz concentration were dispersed in a water–methanol mixture (70:30, v/v), respectively. The resultant solutions were transferred into quartz tubes at a distance of 20 cm from a 32 W germicidal UV lamp (254 nm). At 0, 0.5, 1, 2, 3, 5, 7, 10, 13, 16, 20, 24, 36, and 48 h, the prochloraz content in each quartz tube was analyzed using HPLC. The collected samples were filtered through a 0.45 μm membrane filter. Each treatment was repeated three times. The cumulative retention rate of prochloraz in the samples was calculated using Equation (6).
The prochloraz photodegradation of the samples was described using the pseudo-first-order kinetics Equation (7).
where C0 is the initial amount prochloraz in PRO@DMON–GA–Fe(III) nanoparticles; Ct is the retention amount of prochloraz in the system at time t; and k is the rate constant.
2.7. Spreadability of PRO@DMON–GA–Fe(III) Nanoparticles on Rice Blades
The adhesion work and contact angle were investigated in order to reveal the spreadability of PRO@DMON–GA–Fe(III) nanoparticles on rice blades. The rice leaf was sliced and securely attached to glass slides. Then, a microinject was used to deposit 5 μL of PRO@DMON–GA–Fe(III) nanoparticle aqueous suspension on the rice blade. The contact angle images and values were recorded by the sessile-drop method after 5 s. PRO@DMON nanoparticles were used as controls.
2.8. Fungicidal Activity
The antifungal activities of PRO@DMON–GA–Fe(III) nanoparticles and prochloraz emulsifiable concentrate against Magnaporthe oryzae were assayed by mycelium growth rate method. Different concentrations of PRO@DMON–GA–Fe(III) nanoparticles and prochloraz emulsifiable concentrate were added into the molten potato dextrose agar (PDA) medium, where all the concentrations were determined by the mass of prochloraz technical. Then, 20 mL of the medium containing prochloraz (0.125, 0.25, 0.5, 1.0, 2.0, and 4.0 mg/L) was placed into the sterilized Petri dish with a diameter of 9 cm. A 6 mm diameter mycelium disc which was 10 days old was cut from the edge of the culture media and placed in the center of the above medicated medium after solidification for 2 h. The medium containing sterile water or blank DMON–GA–Fe(III) nanoparticles was also inoculated and used as controls. The PDA agar plates were incubated at 25 °C in the dark condition for 14 days. Finally, the mean diameter of the radial mycelial growth was measured three times in different perpendicular directions. All treatments were performed in triplicate. The percentage of mycelium growth inhibition rate was calculated according to the Equation (8).
where Dt and Dc are the colony diameter of the treatment group and the control group at day 14, respectively.
2.9. Safety Assay
Rice seeds (Oryza sativa L. cv Xiangliangyou 900) were surface sterilized with 75% ethanol (v/v) for 5 min, then treated with 5% sodium hypochlorite (5% available chlorine) for 5 min and washed with deionized water several times. After soaking for 48 h in ultra-pure water, fifteen seeds were placed onto the sterilized Petri dishes (9 cm in diameter) containing double filter papers. Approximately 5 mL of blank DMON–GA–Fe(III) nanoparticles with different concentrations (0, 50, 100, 200, 400, and 800 mg/L) were added into each Petri dish. The treated rice seeds were cultivated in a growth incubator in the dark. The germination rate, root length, shoot length, root number, and fresh weight of the rice seedling were recorded after 7 days. Each treatment was repeated five times.
2.10. Data Analysis
SPSS 23.0 was used to conduct the statistical analyzes (SPSS, Chicago, IL, USA). The TEM images were analyzed using ImageJ software to determine nanoparticle diameter. The probit regression model was used to evaluate EC50 values. The Duncan’s multiple range test (p < 0.05) was used for data analysis, and the experimental data were expressed as the mean ± standard error (SE). The graphs were obtained using Origin 2022b (learning edition). Each experiment was conducted at least three times.
3. Results and Discussion
3.1. Preparation and Characterization of PRO@DMON–GA–Fe(III) Nanoparticles
Figure 1A shows the detailed preparation procedures of PRO@DMON–GA–Fe(III) nanoparticles. Firstly, the DMON nanoparticle was synthesized using a modified sol–gel method (with TEA as the catalyst, TEOS and BTESPT as the silicon source, and CTAB as the structure-directing agents). After extracting CTAB from the pores of the DMON nanoparticle, prochloraz molecules were loaded into the DMON nanoparticle to prepare a pesticide-loaded hybrid nanoparticle (PRO@DMON nanoparticle). Finally, the (GA–Fe(III)) complexes were deposited on the surface of the hybrid nanoparticle through the coordination between GA and Fe(III) ions, and the PRO@DMON–GA–Fe(III) nanoparticle was obtained.
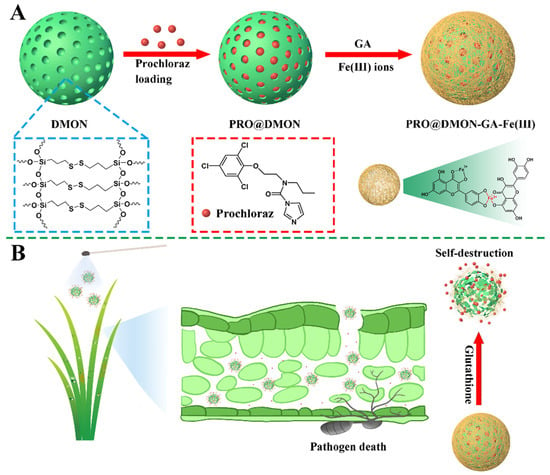
Figure 1.
The mechanism for preparation of PRO@DMON–GA–Fe(III) nanoparticle (A). The triggered release mechanism of prochloraz from PRO@DMON–GA–Fe(III) nanoparticles (B).
The EDX spectroscopy and TEM mapping were used to analyze the elemental composition differences among DMON nanoparticles, PRO@DMON nanoparticles, and DMON–GA–Fe(III) nanoparticles. Compared with blank DMON nanoparticles, a new peak attributed to chlorine can be observed in PRO@DMON nanoparticles, proving the successful loading of prochloraz. After further deposition of GA–Fe(III) complexes on the surface of PRO@DMON nanoparticles, the Fe peak was observed at the binding energy around 6.4 keV (Figure 2A). In addition, the element mappings (Figure 2B–F) of DMON–GA–Fe(III) nanoparticles demonstrate that the O, Si, S, Fe, and C elements were uniformly distributed in the nanoparticles. The above results indicate the successful loading of pesticide and the deposition of GA–Fe(III) complexes in DMON nanoparticles.
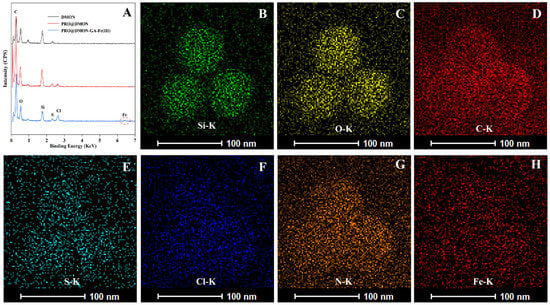
Figure 2.
EDX spectra of DMON nanoparticles, PRO@DMON nanoparticles and PRO@DMON–GA–Fe(III) nanoparticles (A). TEM mapping of PRO@DMON–GA–Fe(III) nanoparticles (B–H).
The prepared DMON nanoparticles had a homogeneous spherical morphology with good symmetry, and a uniform diameter of approximately 80 nm (Figure 3A). The enlarged TEM image indicated that the pores of the sphere were well-defined central-radial structures (Figure 3C). After prochloraz loading and GA–Fe(III) complexes deposition, the mesoporous DMON nanoparticles arrays were partly hidden indicating the successful preparation of PRO@DMON–GA–Fe(III) nanoparticles (Figure 3B,D).
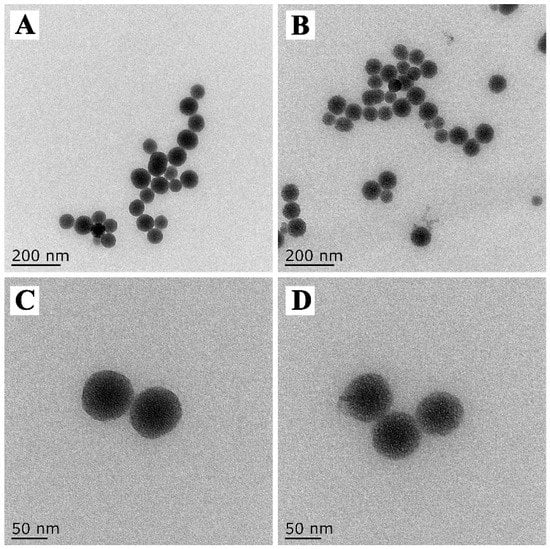
Figure 3.
TEM images of DMON nanoparticles (A,C) and PRO@DMON–GA–Fe(III) nanoparticles (B,D).
XPS spectra showed that the peaks of DMON nanoparticles at binding energies of 532.79, 399.94, 285.02, and 103.25 eV corresponded to Si 2p, C 1s, N 1s, and O 1s, respectively. This result indicates the successful synthesis of disulfide-bridged mesoporous organosilica nanoparticles (Figure 4A). After prochloraz loading, two new characteristic peaks attributed to N and Cl elements appeared at 399.90 and 200.47 eV, respectively, indicating the existence of prochloraz in the DMON nanocarriers (Figure 4B). For PRO@DMON–GA–Fe(III) nanoparticles, the major peak at 711.19 eV in the Fe 2p spectra confirmed the presence of Fe(III) ions (Figure 4C). The high-resolution Fe 2d XPS spectrum of PRO@DMON–GA–Fe(III) nanoparticles in Figure 4C indicated that the two main peaks at 711.35 and 724.35 eV belonged to the binding energies of 2p3/2 and the 2p1/2 region of Fe(III) ions, respectively, suggesting the successful deposition of the GA–Fe(III) complexes on the surface of the PRO@DMON nanoparticles [].
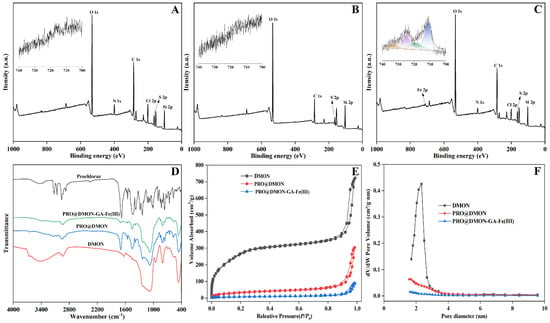
Figure 4.
XPS spectra of DMON nanoparticles (A), PRO@DMON nanoparticles (B), and PRO@DMON–GA–Fe(III) nanoparticles (C). FTIR spectra of prochloraz, DMON nanoparticles, PRO@DMON nanoparticles, and PRO@DMON–GA–Fe(III) nanoparticles (D). Nitrogen adsorption–desorption isotherms (E), and BJH pore size (F) of DMON nanoparticles, PRO@DMON nanoparticles, and PRO@DMON–GA–Fe(III) nanoparticles.
To further verify the formation processes of PRO@DMON–GA–Fe(III) nanoparticles, FTIR spectra of the samples were analyzed. The DMON nanoparticles exhibited strong absorption peaks at 1084, 803, and 464 cm−1, respectively (Figure 4D). These peaks can be attributed to asymmetric stretching and the stretching of Si−O−Si bonds. The peaks at 3434 and 1633 cm−1 were due to adsorbed water molecules or –OH groups on the surface of DMON nanoparticles. After loading prochloraz to the nanocarriers, two new peaks were displayed at 1698 and 1451 cm−1, which were attributed to the stretching vibrations of C=O and C=N [,]. Next, the resultant hybrid nanoparticles were deposited with GA–Fe(III) complexes. The peak intensities of the prochloraz in PRO@DMON–GA–Fe(III) nanoparticles at 1698 and 1451 cm−1 were decreased, indicating the GA–Fe(III) complexes wrapped on the surface of the PRO@DMON nanoparticles successfully.
Nitrogen adsorption–desorption isotherms during pesticide loading and modification were characterized to investigate the variations of pore size distribution and BET-specific surface area. The DMON nanoparticles showed a typical type IV isotherm based on the hysteresis loop (Figure 4E), indicating their well-defined mesoporous structure. The specific surface area, average pore size, and pore volume were 1020.81 m2/g, 2.33 nm, and 1.13 cm3/g, respectively. After prochloraz molecules loading and GA–Fe(III) complexes deposition, the nanoparticles showed decreasing pore volume and pore size (Figure 4F and Table 1). These results validate that the pesticide loading was successful, and the pores were completely blocked by GA–Fe(III) complexes.

Table 1.
Surface and porosity properties of the samples.
3.2. Reducibility-Responsive Biodegradability Assay
To investigate the reducibility-responsive biodegradability of the as-synthesized DMON nanoparticles, the morphological changes of the samples over time under the reducing environment were also monitored (Figure 5). The glutathione was adopted to simulate the reduction condition at the localized necrosis site of plants. On the second day of the experiment, the spherical morphologies were mainly retained. With the incubation time extended to five days, a slight number of DMON nanoparticles had spherical morphologies. On day 10, all DMON nanoparticles were severely damaged. These results indicated that the gradual degradation of DMON could be achieved by extending the incubation time in reduction environments [,]. The reducibility-responsive property of the nanoparticles facilitates their use as pesticide carriers for the on-demand release of active ingredients.

Figure 5.
Degradation behaviors of DMON nanoparticles in 10 mM glutathione solution at 0, 2, 5, and 10 days.
3.3. Pesticide Loading and Controlled Release Kinetics
The prochloraz loading efficiency of PRO@DMON–GA–Fe(III) nanoparticles was determined as 13.8% using HPLC. The mechanism of prochloraz-triggered release from PRO@DMON–GA–Fe(III) nanoparticles is shown in Figure 1B. When rice plants were infected by pathogens, the reductive environment secreted by Magnaporthe oryzae could induce the decomposition of the capped materials (GA–Fe(III) complexes) and the porous carriers (DMON nanoparticles), which would trigger the release of prochloraz and lead to the death of Magnaporthe oryzae.
Figure 6 shows the release manner of prochloraz from PRO@DMON–GA–Fe(III) nanoparticles in different glutathione concentrations (0, 2, and 10 mM) at room temperature. Only a small amount of prochloraz (19.34%) leaked out from PRO@DMON–GA–Fe(III) nanoparticles in the absence of glutathione after 120 h, indicating the good sealing effect of GA–Fe((III) complexes on prochloraz-loaded DMON nanocarriers. In contrast, the cumulative release amount of prochloraz increased to 41.66% at a glutathione concentration of 2 mM after 120 h. With the glutathione concentration increasing to 10 mM, the cumulative release amount of prochloraz from PRO@DMON–GA–Fe(III) nanoparticles was up to 91.96% at the same time. This redox-responsive release property is primarily ascribed to the decompositions of the GA–Fe(III) complexes and the organosilica frameworks in PRO@DMON–GA–Fe(III) nanoparticles. The results of pesticide controlled release experiment indicated that PRO@DMON–GA–Fe(III) nanoparticles could intelligently release prochloraz in response to a reductive environment produced by Magnaporthe oryzae.
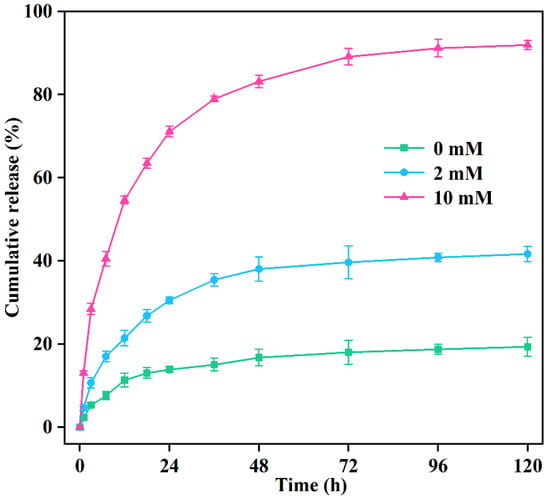
Figure 6.
Effect of glutathione on the release performance of prochloraz from PRO@DMON–GA–Fe(III) nanoparticles.
The pesticide release is commonly controlled by diffusion or dissolution mechanisms. The experimental data were fitted using the four different kinetic models in order to further investigate the prochloraz release kinetics of PRO@DMON–GA–Fe(III) nanoparticles. As shown in Table 2, the controlled release kinetics of prochloraz were best described by the Higuchi model (r2 > 0.95). Thus, the prochloraz release was dominated by Fickian diffusion.

Table 2.
Determined parameters of prochloraz released from PRO@DMON–GA–Fe(III) nanoparticles by fitting several kinetic equations.
3.4. Photostability of the PRO@DMON–GA–Fe(III) Nanoparticles
The photostability of PRO@DMON–GA–Fe(III) nanoparticles, prochloraz emulsifiable concentrate, and prochloraz technical were evaluated under UV light. As shown in Figure 7A, prochloraz emulsifiable concentrate and prochloraz technical dispersing in deionized water decomposed rapidly after being exposed to UV irradiation. The residual rates of prochloraz in the emulsifiable concentrate and technical were only 10.36% and 8.23% after 24 h irradiation, respectively. The above results indicated that emulsifiable concentrate in the water cannot protect the active ingredients of pesticides from photolysis. In contrast, the prochloraz encapsulated with DMON–GA–Fe(III) nanoparticles exhibited better light stability, and the remaining rate of prochloraz in the nanocarriers was more than 80% after being exposed for 48 h. The photodegradation data of the PRO@DMON–GA–Fe(III) nanoparticles, prochloraz emulsifiable concentrate, and prochloraz technical could be satisfactorily fitted with the pseudo-first-order kinetics (Figure 7B). The apparent rate constants and the half-lives are fully provided in Table 3. The dissipation half-life (DT50) values of prochloraz technical, prochloraz emulsifiable concentrate, and PRO@DMON–GA–Fe(III) nanoparticles were calculated to be 7.79 h, 7.37 h, and 154.03 h, respectively. The improved photostability of the nanoparticles was attributed to the absorption or reflection of UV light by the DMON–GA–Fe(III) nanocarriers, which could reduce the absorption intensity of UV light by the loaded pesticide molecules [].
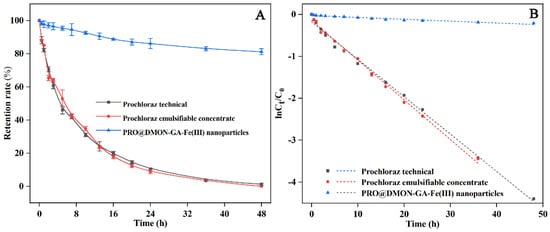
Figure 7.
Stabilities of prochloraz technical, prochloraz emulsifiable concentrate, and PRO@DMON–GA–Fe(III) nanoparticles under UV radiation (A). Pseudo-first-order models of prochloraz photodegradation for prochloraz technical, prochloraz emulsifiable concentrate, and PRO@DMON–GA–Fe(III) nanoparticles (B).

Table 3.
Modeling parameters for photodegradation of prochloraz technical, prochloraz emulsifiable concentrate, and PRO@DMON–GA–Fe(III) nanoparticles under UV radiation.
3.5. Spreadability of DMON-GA-Fe(III) Nanoparticles on Rice Blades
The spreadability of pesticides on the surface of plant leaves is closely related to the contact angle and adhesion work, which can effectively affect the utilization rate of active ingredients in the field []. The adhesion work of PRO@DMON–GA–Fe(III) nanoparticles was within 46–49 mJ/ m2 and the contact angle of PRO@DMON nanoparticles was within 108–110° over 60 s (Figure 8). Due to the coverage by GA–Fe(III) complex shells, PRO@DMON–GA–Fe(III) nanoparticles showed an increase in the adhesion work from 60.4 to 87.7 mJ/m2, while the contact angle of PRO@DMON–GA–Fe(III) nanoparticles decreased from 98.7° to 76.6° within 60 s under the same conditions. The increased adhesion work and decreased contact angle showed that PRO@DMON–GA–Fe(III) nanoparticles had a higher wettability and adhesion ability than PRO@DMON nanoparticles on the rice blades, indicating that GA–Fe(III) complex shells could effectively improve the utilization and deposition of active ingredients. The differences in the spreadability between PRO@DMON nanoparticles and PRO@DMON–GA–Fe(III) nanoparticles could be attributed to the hydrogen bonding, which is mainly generated by the interaction between the polyphenol groups of GA and fatty alcohol or fatty acid of rice leaves. The above findings were consistent with previous studies of polyphenol adhesive chemistry [,].
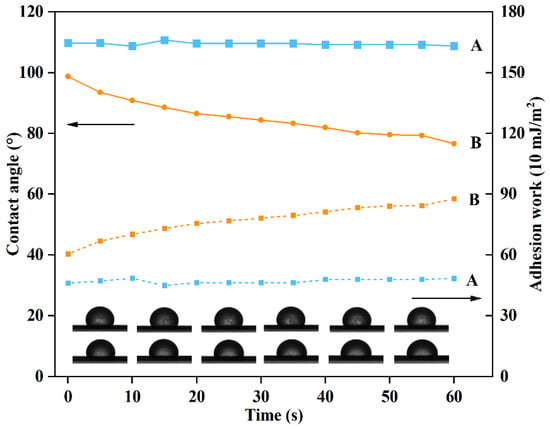
Figure 8.
Contact angles and adhesion works of PRO@DMON nanoparticles and PRO@DMON–GA–Fe(III) nanoparticles on rice blades.
3.6. Bioactivity
The mycelium growth rate method was adopted to investigate the fungicidal activities of prochloraz emulsifiable concentrate and PRO@DMON–GA–Fe(III) nanoparticles against Magnaporthe oryzae. The prochloraz emulsifiable concentrate and PRO@DMON–GA–Fe(III) nanoparticles inhibited the Magnaporthe oryzae colony growth in a typical dose-dependent manner (Figure 9). The coefficient of determination (r2), 95% confidence interval, EC50, and toxicity regression equations of different prochloraz formulations are shown in Table 4. Prochloraz emulsifiable concentrate and PRO@DMON–GA–Fe(III) nanoparticles had an EC50 of 2.39 and 0.81 mg/L, respectively. This indicates that PRO@DMON–GA–Fe(III) nanoparticles showed higher fungicidal activity than prochloraz emulsifiable concentrate. In addition, blank DMON–GA–Fe(III) nanoparticles did not show inhibitory effects at a concentration of 100 mg/L based on the mycelial growth diameter. Attributed to their sustained-release property and small particle size, PRO@DMON–GA–Fe(III) nanoparticles showed stronger fungicidal activity against Magnaporthe oryzae compared with prochloraz emulsifiable concentrate. Therefore, the prepared biodegradable nanoparticles will facilitate a highly potential approach for plant disease prevention and control in modern agriculture.
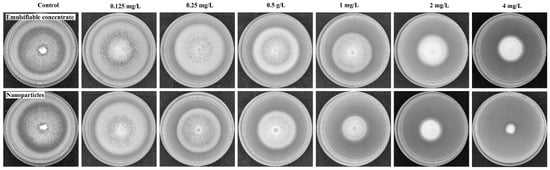
Figure 9.
Fungicidal activities of prochloraz emulsifiable concentrate and PRO@DMON–GA–Fe(III) nanoparticles evaluated with Magnaporthe oryzae.

Table 4.
Regression equation models obtained from prochloraz emulsifiable concentrate and PRO@DMON–GA–Fe(III) nanoparticles against Magnaporthe oryzae.
3.7. Safety of DMON–GA–Fe(III) Nanoparticles on Rice Seedlings
Several physiological and biochemical parameters were used to evaluate the biosafety of DMON–GA–Fe(III) nanoparticles against rice growth []. These parameters sensitive to adverse environments included germination rate, fresh weight, shoot length, and root length []. Compared with the water (control) treatments, DMON–GA–Fe(III) nanoparticle treatments at different concentrations did not significantly affect seed germination rate, root length, shoot length, fresh weight, and root number of 7-day-old seedlings (Table 5). Therefore, these nanoparticles were non-phytotoxic, which will further improve their application to rice plant disease control in modern agriculture.

Table 5.
The germination rate, root length, shoot length, root number, and fresh weight, of 7-day-old seedlings of rice germinated from seeds treated with different concentration of blank DMON–GA–Fe(III) nanoparticles.
4. Conclusions
In this work, a redox-responsive pesticide delivery system based on metal–polyphenol-network-coated biodegradable DMON nanoparticles was established by non-covalent bonding to manage rice diseases. The prepared PRO@DMON–GA–Fe(III) nanoparticles could effectively reduce the photolysis of prochloraz by absorbing or reflecting UV light and preventing its premature leakage. In addition, the DMON–GA–Fe(III) nanocarriers with self-destruction properties could be decomposed by glutathione, realizing the on-demand release of prochloraz. The release mechanism explored by the Higuchi model presented that a Fickian diffusion controlled the release of prochloraz from PRO@DMON–GA–Fe(III) nanoparticles. The measurement of leaf contact angle and adhesion work demonstrated that GA–Fe(III) complex coating could enhance the deposition and adhesion of the nanoparticles on the rice leaves. The biological activity experiments showed that PRO@DMON–GA–Fe(III) nanoparticles had good antifungal activity and long-term efficacy against Magnaporthe oryzae. More importantly, the blank DMON–GA–Fe(III) nanoparticles did not show obvious toxicity to rice seedlings. Therefore, the redox-responsive biodegradable pesticide delivery system provided a promising strategy for enhancing pesticide targeting and realizing sustainable plant diseases management.
Author Contributions
Y.L.: data curation, writing—original draft, funding acquisition. S.W.: investigation, data curation. Y.Y.: writing—review and editing, formal analysis. S.Y.: visualization. A.L.: formal analysis. Y.W.: validation. J.S.: investigation. Z.H.: supervision, funding acquisition, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge financial support of this work by the Postdoctoral Science Foundation of Jiangsu Province (2021K279B), the Jiangsu Provincial Key Research and Development Program (BE2020319), the Carbon Peak Carbon Neutral Science and Technology Innovation Special Fund of Jiangsu Province (BE2022424), the Jiangsu Agricultural Science and Technology Innovation Fund (CX [20]1012 and CX(22)1001), the Jiangsu Modern Agricultural Machinery Equipment and Technology Demonstration and Promotion Project (NJ2020-58), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, D.; Saleh, N.B.; Byro, A.; Zepp, R.; Sahle-Demessie, E.; Luxton, T.P.; Ho, K.T.; Burgess, R.M.; Flury, M.; White, J.C.; et al. Nano-enabled pesticides for sustainable agriculture and global food security. Nat. Nanotechnol. 2022, 17, 347–360. [Google Scholar] [CrossRef]
- Bhatt, D.; Srivastava, A.; Srivastava, P.C.; Sharma, A. Evaluation of three novel soil bacterial strains for efficient biodegradation of persistent boscalid fungicide: Kinetics and identification of microbial biodegradation intermediates. Environ. Pollut. 2023, 316, 120484. [Google Scholar] [CrossRef]
- Wang, X.; Yan, M.; Zhou, J.; Song, W.; Xiao, Y.; Cui, C.; Gao, W.; Ke, F.; Zhu, J.; Gu, Z. Delivery of acetamiprid to tea leaves enabled by porous silica nanoparticles: Efficiency, distribution and metabolism of acetamiprid in tea plants. BMC Plant Biol. 2021, 21, 337. [Google Scholar] [CrossRef] [PubMed]
- Foong, S.Y.; Ma, N.L.; Lam, S.S.; Peng, W.; Low, F.; Lee, B.H.K.; Alstrup, A.K.O.; Sonne, C. A recent global review of hazardous chlorpyrifos pesticide in fruit and vegetables: Prevalence, remediation and actions needed. J. Hazard. Mater. 2020, 400, 123006. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Ramadass, K.; Sooriyakumar, P.; Hettithanthri, O.; Vithange, M.; Bolan, N.; Tavakkoli, E.; van Zwieten, L.; Vinu, A. Nanoporous materials for pesticide formulation and delivery in the agricultural sector. J. Control. Release 2022, 343, 187–206. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.-H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef]
- Singh, A.; Dhiman, N.; Kar, A.K.; Singh, D.; Purohit, M.P.; Ghosh, D.; Patnaik, S. Advances in controlled release pesticide formulations: Prospects to safer integrated pest management and sustainable agriculture. J. Hazard. Mater. 2020, 385, 121525. [Google Scholar] [CrossRef]
- Liu, T.; Luo, J.; Liu, S.; Li, T.; Li, H.; Zhang, L.; Mu, W.; Zou, N. Clothianidin loaded TA/Fe (III) controlled-release granules: Improve pesticide bioavailability and alleviate oxidative stress. J. Hazard. Mater. 2021, 416, 125861. [Google Scholar] [CrossRef]
- Hedaoo, R.K.; Gite, V.V. Renewable resource-based polymeric microencapsulation of natural pesticide and its release study: An alternative green approach. RSC Adv. 2014, 4, 18637–18644. [Google Scholar] [CrossRef]
- Zeng, X.; Zhong, B.; Jia, Z.; Zhang, Q.; Chen, Y.; Jia, D. Halloysite nanotubes as nanocarriers for plant herbicide and its controlled release in biodegradable polymers composite film. Appl. Clay Sci. 2019, 171, 20–28. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, Y.; Xu, X.; Zhang, M.; Ma, L. Structure regulation of bentonite-alginate nanocomposites for controlled release of imidacloprid. ACS Omega 2020, 5, 10068–10076. [Google Scholar] [CrossRef] [PubMed]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Soroush, F.; Varma, R.S. Nano/microencapsulation of plant biocontrol agents by chitosan, alginate, and other important biopolymers as a novel strategy for alleviating plant biotic stresses. Int. J. Biol. Macromol. 2022, 222, 1589–1604. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Lowry, G.V.; Ghoshal, S.; Tufenkji, N.; Brambilla, D.; Dutcher, J.R.; Gilbertson, L.M.; Giraldo, J.P.; Kinsella, J.M.; Landry, M.P.; et al. Technology readiness and overcoming barriers to sustainably implement nanotechnology-enabled plant agriculture. Nat. Food 2020, 1, 416–425. [Google Scholar] [CrossRef]
- Camara, M.C.; Monteiro, R.A.; Carvalho, L.B.; Oliveira, J.L.; Fraceto, L.F. Enzyme stimuli–responsive nanoparticles for bioinsecticides: An emerging approach for uses in crop protection. ACS Sustain. Chem. Eng. 2021, 9, 106–112. [Google Scholar] [CrossRef]
- Monteiro, R.A.; Camara, M.C.; de Oliveira, J.L.; Campos, E.V.R.; Carvalho, L.B.; Proença, P.L.d.F.; Guilger-Casagrande, M.; Lima, R.; Nascimento, J.d.; Gonçalves, K.C.; et al. Zein based-nanoparticles loaded botanical pesticides in pest control: An enzyme stimuli-responsive approach aiming sustainable agriculture. J. Hazard. Mater. 2021, 417, 126004. [Google Scholar] [CrossRef]
- Zhao, M.; Li, P.; Zhou, H.; Hao, L.; Chen, H.; Zhou, X. pH/redox dual responsive from natural polymer-based nanoparticles for on-demand delivery of pesticides. Chem. Eng. J. 2022, 435, 134861. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, S.; Dong, H.; Yu, S.; Jia, H.; Wang, J.; Yao, Y.; Wang, Y.; Song, J.; Huo, Z. Zeolitic imidazole framework-90-based pesticide smart-delivery system with enhanced antimicrobial performance. Nanomaterials 2022, 12, 3622. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, Y.; Wang, W.; Dong, H.; Tang, R.; Yang, J.; Niu, J.; Zhou, Z.; Jiang, N.; Cao, Y. Fabrication of smart stimuli-responsive mesoporous organosilica nano-vehicles for targeted pesticide delivery. J. Hazard. Mater. 2020, 389, 122075. [Google Scholar] [CrossRef]
- Guan, M.; Li, Z.; Hao, L.; Zhou, M.; Chen, L.; Chen, H.; Zhou, H.; Zhou, X. Functionalization of boron nitride nanosheets via thiol terminated polyethyleneimine to enhance aqueous dispersibility and efficiency as carriers for essential oils and pesticides. Chem. Eng. J. 2021, 423, 130166. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, Z.; Hao, L.; Chen, H.; Zhou, X.; Zhou, H. CMC based microcapsules for smart delivery of pesticides with reduced risks to the environment. Carbohydr. Polym. 2023, 300, 120260. [Google Scholar] [CrossRef]
- Liang, W.; Xie, Z.; Cheng, J.; Xiao, D.; Xiong, Q.; Wang, Q.; Zhao, J.; Gui, W. A light-triggered pH-responsive metal–organic framework for smart delivery of fungicide to control Sclerotinia diseases of oilseed rape. ACS Nano 2021, 15, 6987–6997. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C.; Zhang, J.; Liu, B.; Teng, G.; Wang, J.; Zhang, X.; Cai, D.; Wu, Z. Alternating magnetic field-responsive nanoplatforms for controlled imidacloprid release and sustainable pest control. ACS Sustain. Chem. Eng. 2021, 9, 10491–10502. [Google Scholar] [CrossRef]
- Li, X.; Han, J.; Wang, X.; Zhang, Y.; Jia, C.; Qin, J.; Wang, C.; Wu, J.; Fang, W.; Yang, Y. A triple-stimuli responsive hormone delivery system equipped with pillararene magnetic nanovalves. Mater. Chem. Front. 2019, 3, 103–110. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, Y.; Dong, H.; Niu, J.; Tang, J.; Yang, J.; Tang, G.; Zhou, Z.; Tang, R.; Shi, X.; et al. A bioresponsive system based on mesoporous organosilica nanoparticles for smart delivery of fungicide in response to pathogen presence. ACS Sustain. Chem. Eng. 2020, 8, 5716–5723. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Y.; Abbaraju, P.L.; Azimi, I.; Lei, C.; Tang, J.; Jambhrunkar, M.; Fu, J.; Zhang, M.; Liu, Y.; et al. Stepwise degradable nanocarriers enabled cascade delivery for synergistic cancer therapy. Adv. Funct. Mater. 2018, 28, 1800706. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, W. Recent advances in redox-responsive nanoparticles for combined cancer therapy. Nanoscale Adv. 2022, 4, 3504–3516. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Montemayor, A.M.; Wimsatt, S.T.; Tikekar, R.V. Effect of combination of UV-A light and chitosan-gallic acid coating on microbial safety and quality of fresh strawberries. Food Control. 2022, 140, 109106. [Google Scholar] [CrossRef]
- El-Nagar, A.; Elzaawely, A.A.; Taha, N.A.; Nehela, Y. The antifungal activity of gallic acid and its derivatives against Alternaria solani, the causal agent of tomato early blight. Agronomy 2020, 10, 1402. [Google Scholar] [CrossRef]
- Luzi, F.; Puglia, D.; Dominici, F.; Fortunati, E.; Giovanale, G.; Balestra, G.M.; Torre, L. Effect of gallic acid and umbelliferone on thermal, mechanical, antioxidant and antimicrobial properties of poly (vinyl alcohol-co-ethylene) films. Polym. Degrad. Stab. 2018, 152, 162–176. [Google Scholar] [CrossRef]
- Cherepanov, P.V.; Rahim, M.A.; Bertleff-Zieschang, N.; Sayeed, M.A.; O’Mullane, A.P.; Moulton, S.E.; Caruso, F. Electrochemical behavior and redox-dependent disassembly of gallic acid/FeIII metal–phenolic networks. ACS Appl. Mater. Interfaces 2018, 10, 5828–5834. [Google Scholar] [CrossRef] [PubMed]
- Maerten, C.; Lopez, L.; Lupattelli, P.; Rydzek, G.; Pronkin, S.; Schaaf, P.; Jierry, L.; Boulmedais, F. Electrotriggered confined self-assembly of metal–polyphenol nanocoatings using a morphogenic approach. Chem. Mater. 2017, 29, 9668–9679. [Google Scholar] [CrossRef]
- Fan, G.; Cottet, J.; Rodriguez-Otero, M.R.; Wasuwanich, P.; Furst, A.L. Metal–phenolic networks as versatile coating materials for biomedical applications. ACS Appl. Bio Mater. 2022, 5, 4687–4695. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, C.; Jiang, S.; Zhang, C.; Tian, Y. pH-responsive hollow Fe–gallic acid coordination polymer for multimodal synergistic-therapy and MRI of cancer. Nanoscale Adv. 2022, 4, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.T.; Yehya, W.A.; Saad, O.; Simarani, K.; Chowdhury, Z.; Alhadi, A.A.; Al-Ani, L.A. Surface functionalization of iron oxide nanoparticles with gallic acid as potential antioxidant and antimicrobial agents. Nanomaterials 2017, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Dong, Z.; Wang, C.; Li, Q.; Hao, Y.; Yang, Z.; Zhu, W.; Zhang, Y.; Liu, Z.; Feng, L. Ferrous ions doped calcium carbonate nanoparticles potentiate chemotherapy by inducing ferroptosis. J. Control. Release 2022, 348, 346–356. [Google Scholar] [CrossRef]
- Guo, J.; Suma, T.; Richardson, J.J.; Ejima, H. Modular assembly of biomaterials using polyphenols as building blocks. ACS Biomater. Sci. Eng. 2019, 5, 5578–5596. [Google Scholar] [CrossRef]
- Gindri, R.G.; Navarro, B.B.; Dias, P.V.d.; Tarouco, C.P.; Nicoloso, F.T.; Brunetto, G.; Berghetti, Á.L.P.; da Silva, L.O.S.; Fett, J.P.; Menguer, P.K.; et al. Physiological responses of rice (Oryza sativa L.) oszip7 loss-of-function plants exposed to varying Zn concentrations. Physiol. Mol. Biol. Plants 2020, 26, 1349–1359. [Google Scholar] [CrossRef]
- Fernandez, J.; Orth, K. Rise of a cereal killer: The biology of Magnaporthe oryzae biotrophic growth. Trends Microbiol. 2018, 26, 582–597. [Google Scholar] [CrossRef]
- Salman, E.K.; Ghoniem, K.E.; Badr, E.S.; Emeran, A.A. The potential of dimetindene maleate inducing resistance to blast fungus Magnaporthe oryzae through activating the salicylic acid signaling pathway in rice plants. Pest Manag. Sci. 2022, 78, 633–642. [Google Scholar] [CrossRef]
- Samalova, M.; Meyer, A.J.; Gurr, S.J.; Fricker, M.D. Robust anti-oxidant defences in the rice blast fungus Magnaporthe oryzae confer tolerance to the host oxidative burst. New Phytol. 2014, 201, 556–573. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Fujita, Y.; Ashizawa, T.; Suzuki, F.; Nagamura, Y.; Hayano-Saito, Y. Serotonin attenuates biotic stress and leads to lesion browning caused by a hypersensitive response to Magnaporthe oryzae penetration in rice. Plant J. 2016, 85, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ban, Q.; Wang, W.; Hou, J.; Jiang, Z. Novel nano-encapsulated probiotic agents: Encapsulate materials, delivery, and encapsulation systems. J. Control. Release 2022, 349, 184–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Lou, X.-Y.; Cai, Z.; Zhang, M.-Z.; Jia, C.; Qin, J.-C.; Yang, Y.-W. Supramolecular nanoplatform based on mesoporous silica nanocarriers and pillararene nanogates for fungus control. ACS Appl. Mater. Interfaces 2021, 13, 32295–32306. [Google Scholar] [CrossRef]
- Xiao, D.; Cheng, J.; Liang, W.; Sun, L.; Zhao, J. Metal-phenolic coated and prochloraz-loaded calcium carbonate carriers with pH responsiveness for environmentally-safe fungicide delivery. Chem. Eng. J. 2021, 418, 129274. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Luo, C.; Fu, Y.; Zhu, F. Fungicidal actions and resistance mechanisms of prochloraz to Penicillium digitatum. Plant Dis. 2021, 105, 408–415. [Google Scholar] [CrossRef]
- Wang, C.; Xu, L.; Liang, X.; Zhang, Y.; Zheng, H.; Chen, J.; Yang, Y. Biochemical and molecular characterization of prochloraz resistance in Lasiodiplodia theobromae field isolates. Plant Dis. 2022. [Google Scholar] [CrossRef]
- Wu, L.; Pan, H.; Huang, W.; Wang, M.; Hu, Z.; Zhang, F. Self-assembled degradable iron-doped mesoporous silica nanoparticles for the smart delivery of prochloraz to improve plant protection and reduce environmental impact. Environ. Technol. Innov. 2022, 28, 102890. [Google Scholar] [CrossRef]
- Gao, X.; Peng, Q.; Yuan, K.; Li, Y.; Shi, M.; Miao, J.; Liu, X. Monitoring and characterization of prochloraz resistance in Fusarium fujikuroi in China. Pestic. Biochem. Physiol. 2022, 187, 105189. [Google Scholar] [CrossRef]
- Abdelrahman, T.M.; Qin, X.; Li, D.; Senosy, I.A.; Mmby, M.; Wan, H.; Li, J.; He, S. Pectinase-responsive carriers based on mesoporous silica nanoparticles for improving the translocation and fungicidal activity of prochloraz in rice plants. Chem. Eng. J. 2021, 404, 126440. [Google Scholar] [CrossRef]
- Liang, Y.; Fan, C.; Dong, H.; Zhang, W.; Tang, G.; Yang, J.; Jiang, N.; Cao, Y. Preparation of MSNs-chitosan@prochloraz nanoparticles for reducing toxicity and improving release properties of prochloraz. ACS Sustain. Chem. Eng. 2018, 6, 10211–10220. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Shi, L.; Liu, Q.; Gao, Y.; Chen, W.; Yang, J.; Yuan, X.; Feng, J. Fabrication and characterization of prochloraz nanoemulsion against Penicillium citrinum for the postharvest storage of navel oranges. Langmuir 2021, 37, 13757–13766. [Google Scholar] [CrossRef]
- Zhao, P.; Cao, L.; Ma, D.; Zhou, Z.; Huang, Q.; Pan, C. Translocation, distribution and degradation of prochloraz-loaded mesoporous silica nanoparticles in cucumber plants. Nanoscale 2018, 10, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Tóth, I.Y.; Szekeres, M.R.; Turcu, R.; Sáringer, S.R.; Illés, E.B.; Nesztor, D.N.; Tombácz, E. Mechanism of in situ surface polymerization of gallic acid in an environmental-inspired preparation of carboxylated core–shell magnetite nanoparticles. Langmuir 2014, 30, 15451–15461. [Google Scholar] [CrossRef]
- Ossai, V.; Obiefuna, A.P.; Laraps, B.C.; Okenyeka, O.U.; Ezeorah, J.C.; Dege, N.; Ibezim, A.; Lutter, M.; Jurkschat, K.; Obasi, N.L. Crystal structural and in silico studies of Schiff bases derived from 4-aminoantipyrine. Solid State Sci. 2020, 106, 106293. [Google Scholar] [CrossRef]
- Shi, L.; Liang, Q.; Zang, Q.; Lv, Z.; Meng, X.; Feng, J. Construction of prochloraz-loaded hollow mesoporous silica nanoparticles coated with metal–phenolic networks for precise release and improved biosafety of pesticides. Nanomaterials 2022, 12, 2885. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Jiao, X.; Fan, W.; Yang, Z.; Wen, Y.; Chen, X. Controllable synthesis of versatile mesoporous organosilica nanoparticles as precision cancer theranostics. Biomaterials 2020, 256, 120191. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Feng, J.; Fan, W.; Tang, W.; Rong, X.; Liao, W.; Wei, Z.; Xu, Y.; Wu, A.; Chen, X.; et al. Intelligent pore switch of hollow mesoporous organosilica nanoparticles for high contrast magnetic resonance imaging and tumor-specific chemotherapy. Nano Lett. 2021, 21, 9551–9559. [Google Scholar] [CrossRef]
- Duan, L.; Fang, Z.; Han, X.; Dou, Z.; Liu, Y.; Wen, M.; Hou, T.; Yang, D.; Wang, C.; Zhang, G. Study on droplet impact and spreading and deposition behavior of harvest aids on cotton leaves. Langmuir 2022, 38, 12248–12262. [Google Scholar] [CrossRef]
- Zheng, D.; Bai, B.; Zhao, H.; Xu, X.; Hu, N.; Wang, H. Stimuli-responsive Ca-alginate-based photothermal system with enhanced foliar adhesion for controlled pesticide release. Colloids Surf. B 2021, 207, 112004. [Google Scholar] [CrossRef]
- Liang, Y.; Song, J.; Dong, H.; Huo, Z.; Gao, Y.; Zhou, Z.; Tian, Y.; Li, Y.; Cao, Y. Fabrication of pH-responsive nanoparticles for high efficiency pyraclostrobin delivery and reducing environmental impact. Sci. Total Environ. 2021, 787, 147422. [Google Scholar] [CrossRef] [PubMed]
- Hassanisaadi, M.; Barani, M.; Rahdar, A.; Heidary, M.; Thysiadou, A.; Kyzas, G.Z. Role of agrochemical-based nanomaterials in plants: Biotic and abiotic stress with germination improvement of seeds. Plant Growth Regul. 2022, 97, 375–418. [Google Scholar] [CrossRef]
- Hatamleh, A.A.; Danish, M.; Al-Dosary, M.A.; El-Zaidy, M.; Ali, S. Physiological and oxidative stress responses of Solanum lycopersicum (L.) (tomato) when exposed to different chemical pesticides. RSC Adv. 2022, 12, 7237–7252. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).