Exposure to Nanoplastic Particles Enhances Acinetobacter Survival, Biofilm Formation, and Serum Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of NPs
2.2. Acinetobacter Strain
2.3. Analysis of A. johnsonii AC15 Growth
2.4. Adsorption Curve of NP during Bacterial Growth
2.5. Adsorption Curve of NP under the Condition of Non-Growth of Bacteria
2.6. Biofilm Formation of A. johnsonii AC15
2.7. Serum Resistance Analysis
2.8. Real-Time PCR (qRT-PCR)
2.9. Data Analysis
3. Results and Discussion
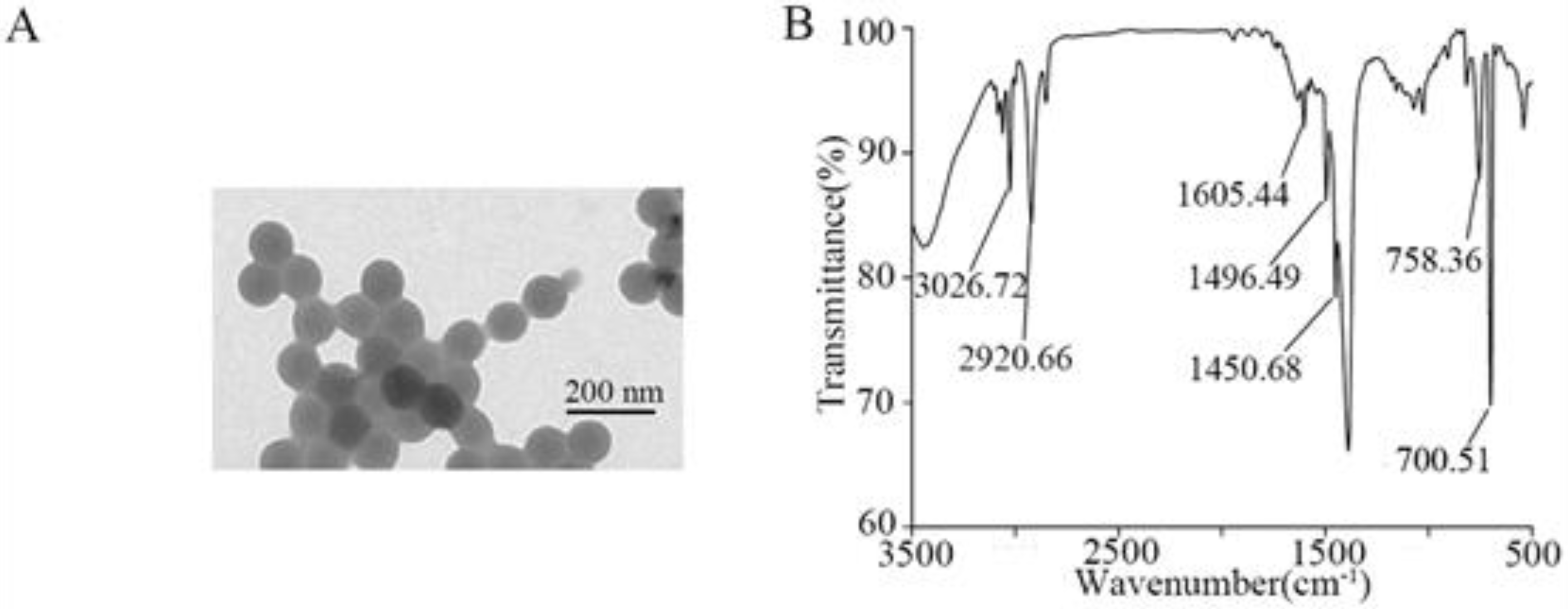
3.1. Characterization of NPs
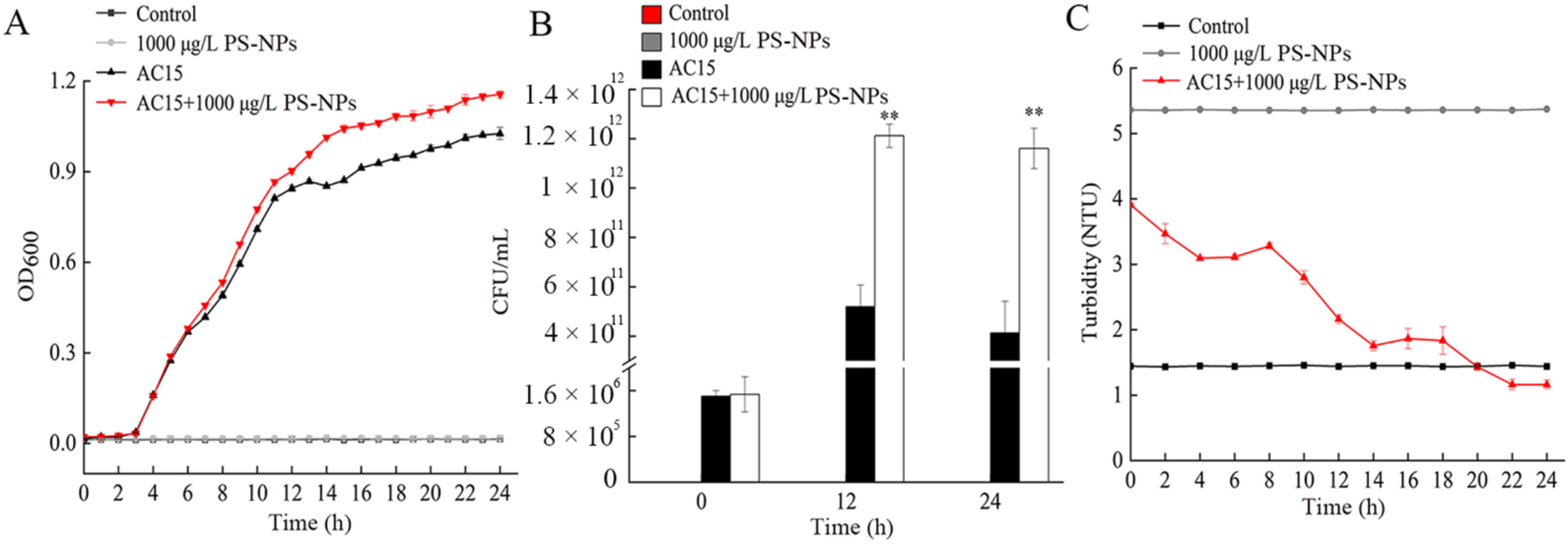
3.2. Interaction between NPs and A. johnsonii AC15
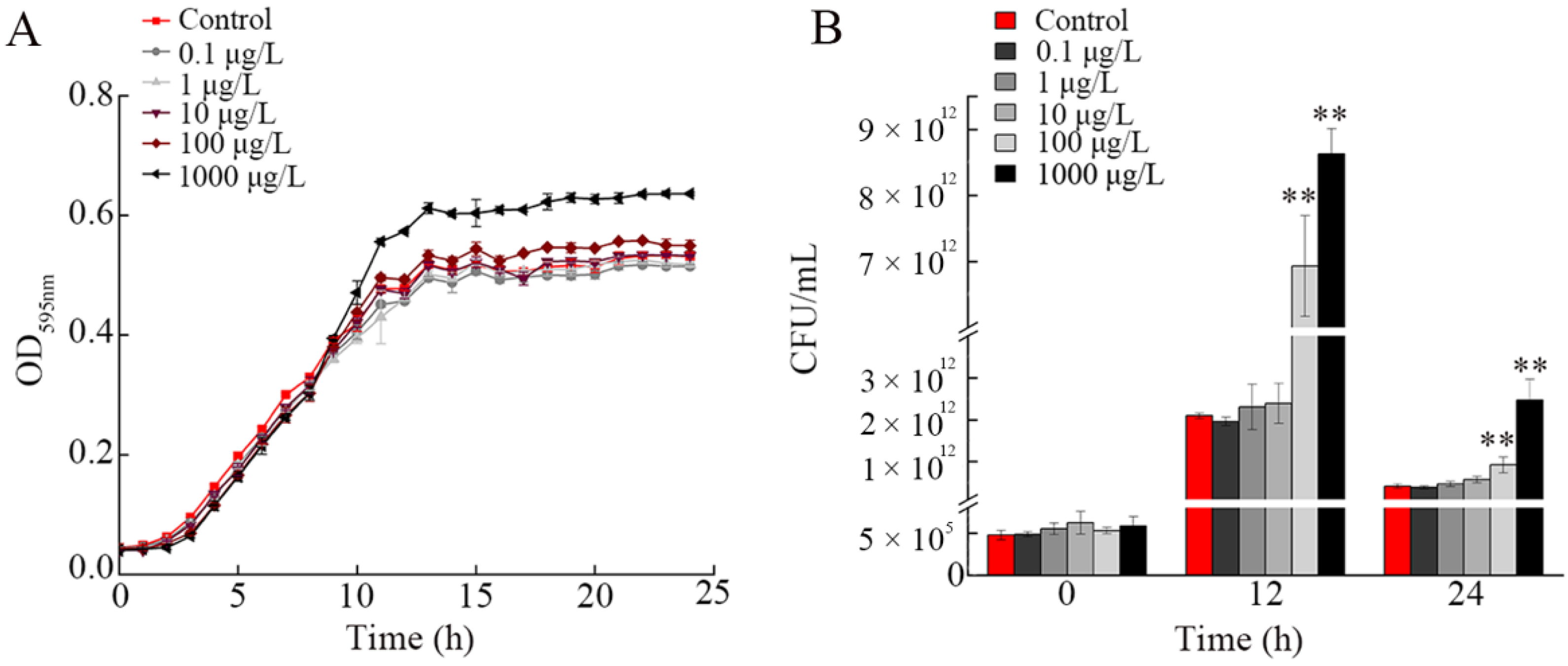
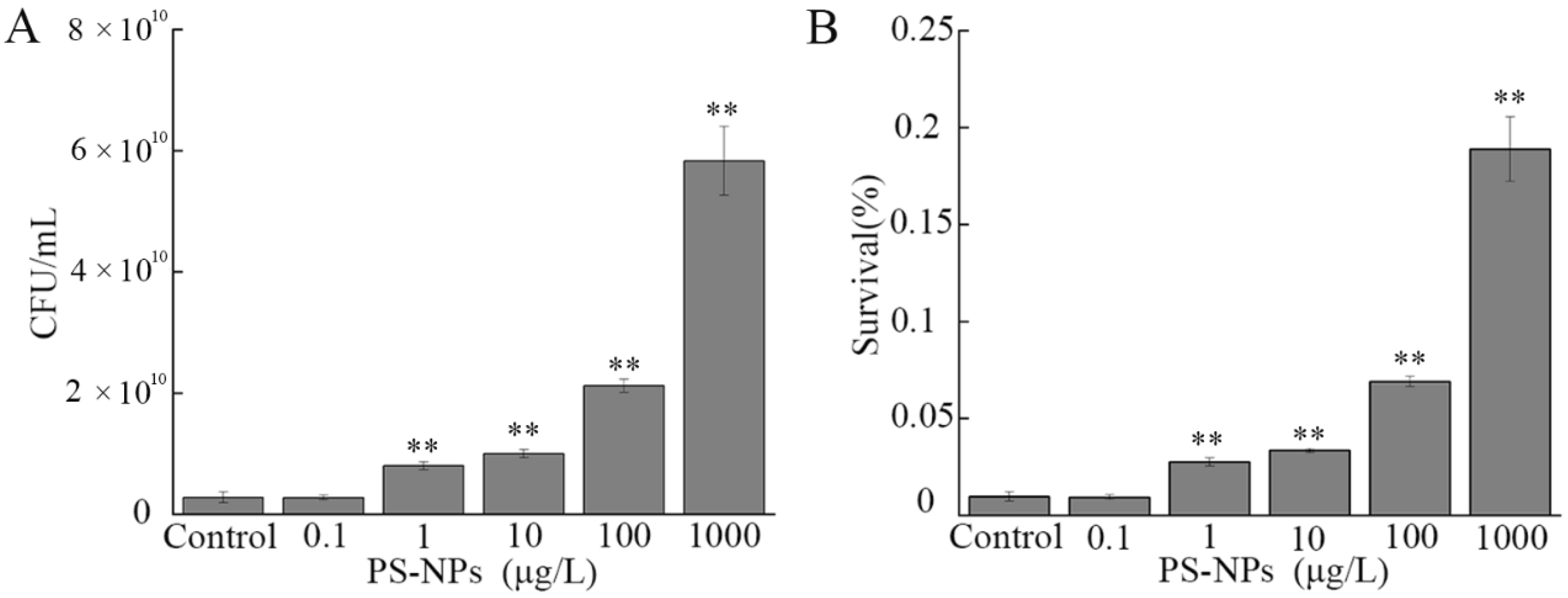
3.3. Effect of NP Exposure on Growth of A. johnsonii AC15
3.4. Adsorption Curve of NPs during Bacterial Growth
3.5. Adsorption Curve of NPs under the Condition of Non-Growth of Bacteria
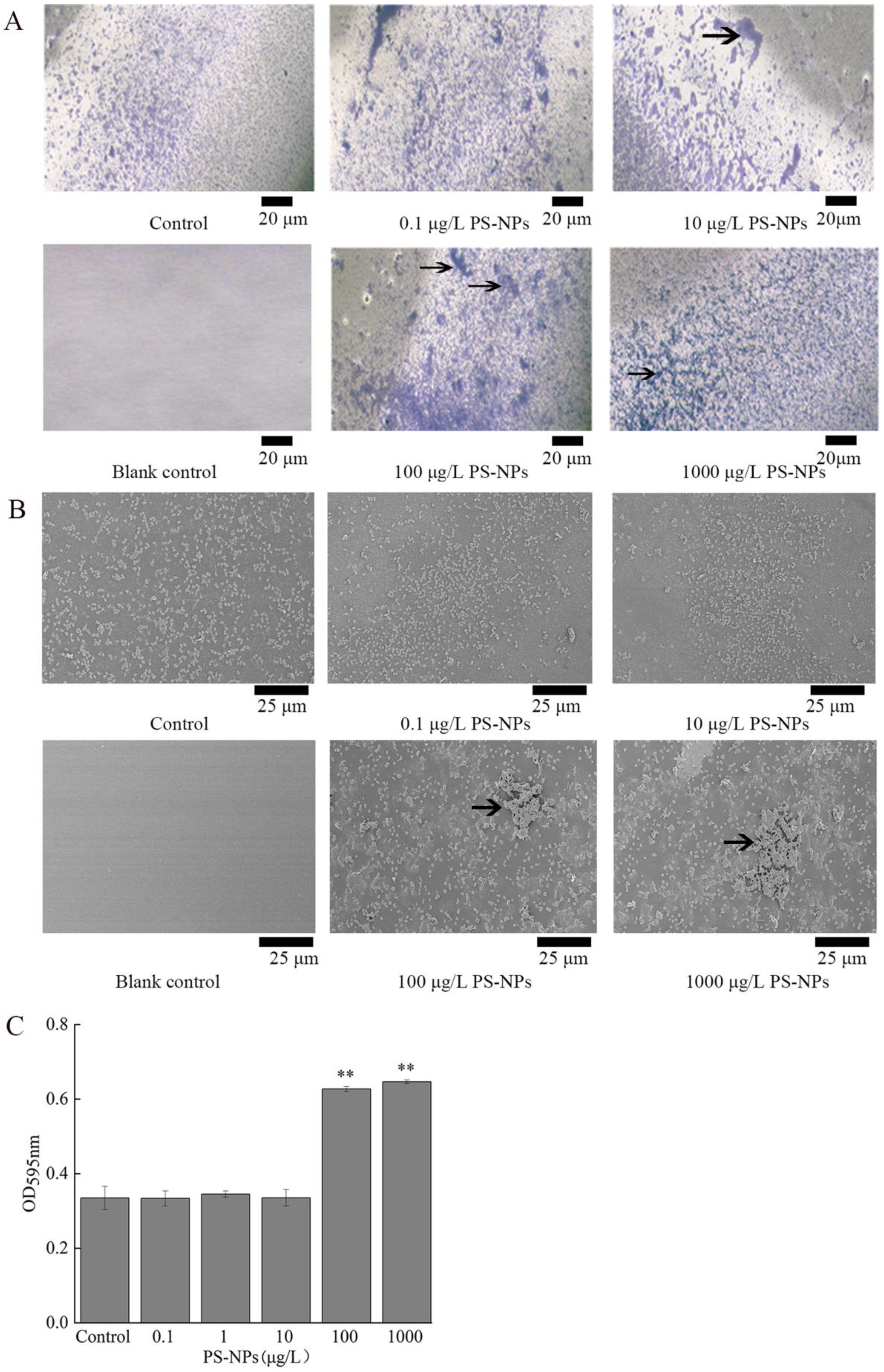
3.6. Effect of NP Exposure on Biofilm Formation of A. johnsonii AC15
3.7. Effect of NP Exposure on Serum Resistance of A. johnsonii AC15
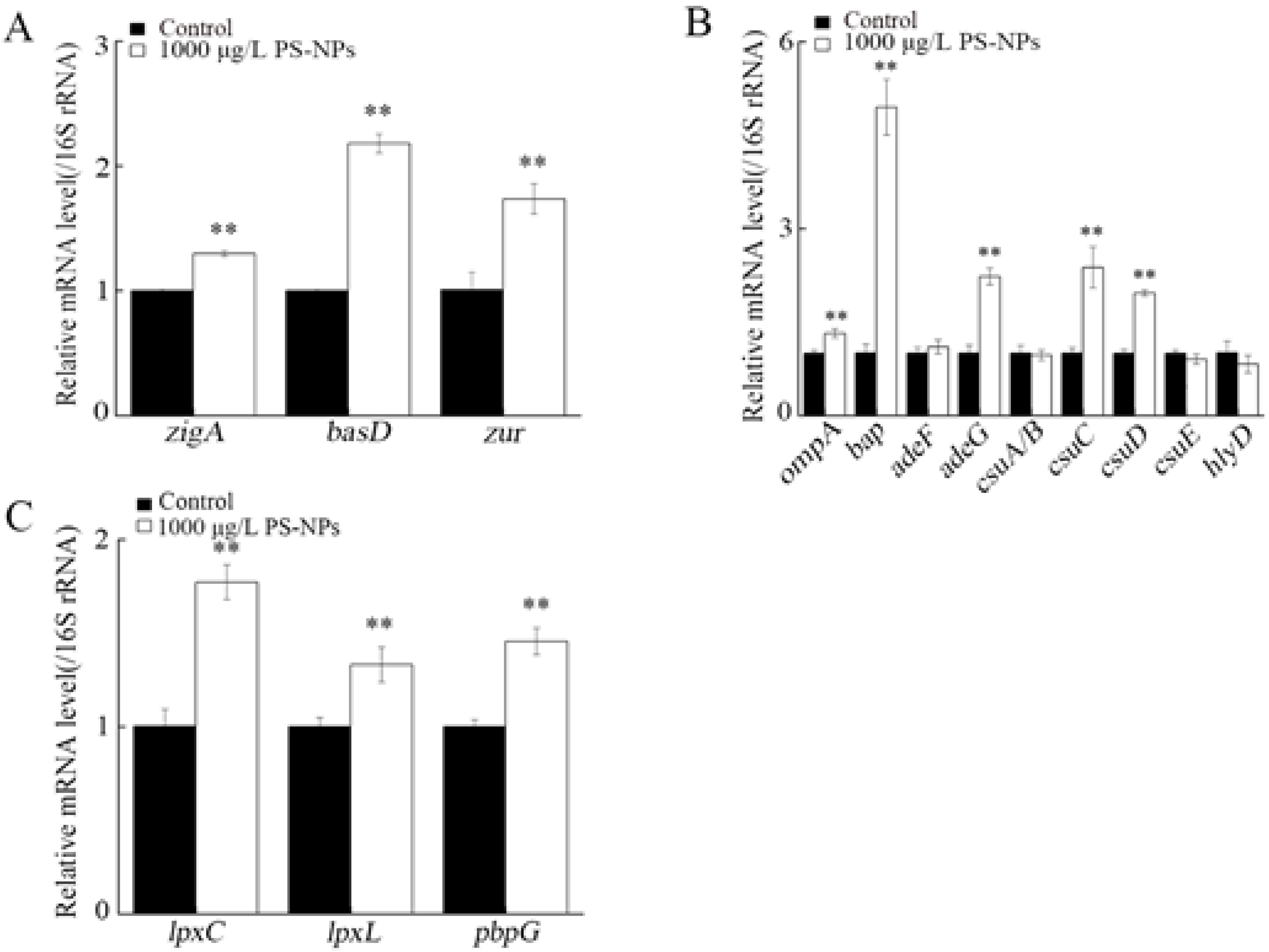
3.8. Effect of NP Exposure on Expression of Virulence Genes in A. johnsonii AC15
3.9. Effect of NP Exposure on Expression of Virulence Genes in A. johnsonii AC15 in NHS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, P.; Wang, X.; Su, M.; Zou, X.; Duan, L.; Zhang, H. Characteristics of plastic pollution in the environment: A review. Bull. Environ. Contam. Toxicol. 2021, 107, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.; An, Y.J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Z.; Chen, Y.; Yang, F.; Yao, W.; Xie, Y. Microplastics and nanoplastics: Emerging contaminants in food. J. Agric. Food Chem. 2021, 69, 10450–10468. [Google Scholar] [CrossRef]
- Da Costa, J.P.; Santos, P.S.M.; Duarte, A.C.; Rocha-Santos, T. Nanoplastics in the environment-sources, fates and effects. Sci. Total Environ. 2016, 566, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Kubowicz, S.; Booth, A.M. Biodegradability of plastics: Challenges and misconceptions. Environ. Sci. Technol. 2017, 51, 12058–12060. [Google Scholar] [CrossRef]
- Karbalaei, S.; Hanachi, P.; Walker, T.R.; Cole, M. Occurrence, sources, human health impacts and mitigation of microplastic pollution. Environ. Sci. Pollut. Res. 2018, 25, 36046–36063. [Google Scholar] [CrossRef]
- Gaylarde, C.C.; Baptista Neto, J.A.; da Fonseca, E.M. Nanoplastics in aquatic systems—Are they more hazardous than microplastics? Environ. Pollut. 2021, 272, 115950. [Google Scholar] [CrossRef]
- Zhang, K.; Hamidian, A.H.; Tubić, A.; Zhang, Y.; Fang, J.K.H.; Wu, C.; Lam, P.K.S. Understanding plastic degradation and microplastic formation in the environment: A review. Environ. Pollut. 2021, 274, 116554. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, Y.; Zhu, Y.; Song, B.; Zeng, G.; Hu, D.; Wen, X.; Ren, X. Recent advances in toxicological research of nanoplastics in the environment: A review. Environ. Pollut. 2019, 252, 511–521. [Google Scholar] [CrossRef]
- Larue, C.; Sarret, G.; Castillo-Michel, H.; Pradas Del Real, A.E. A critical review on the impacts of nanoplastics and microplastics on aquatic and terrestrial photosynthetic organisms. Small 2021, 17, e2005834. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Zhao, Y.; Nurdebek, B.; Bu, Y.; Wang, D. Long-term exposure to polystyrene nanoparticles causes transgenerational toxicity by affecting the function and expression of MEV-1 and DAF-2 signals in Caenorhabditis elegans. NanoImpact 2022, 26, 100403. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Zhao, Y.; Yuan, Y.; Zhang, L.; Bian, Q.; Wang, D. Nanoplastics cause transgenerational toxicity through inhibiting germline microRNA mir-38 in C. elegans. J. Hazard. Mater. 2022, 437, 129302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, J.; Wang, R.; Pu, X.; Wang, D. A review of transgenerational and multigenerational toxicology in the in vivo model animal Caenorhabditis elegans. J. Appl. Toxicol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.P.; Duarte, A.C.; Santos-Echeandía, J.; Rocha-Santos, T. Significance of interactions between microplastics and POPs in the marine environment: A critical overview. TrAC Trends Anal. Chem. 2018, 111, 252–260. [Google Scholar] [CrossRef]
- Qu, M.; Nida, A.; Kong, Y.; Du, H.-H.; Xiao, G.-S.; Wang, D.-Y. Nanopolystyrene at predicted environmental concentration enhances microcystin-LR toxicity by inducing intestinal damage in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2019, 183, 109568. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.A.B.; Gaylarde, C.; Beech, I.; Bastos, A.C.; da Silva Quaresma, V.; de Carvalho, D.G. Microplastics and attached microorganisms in sediments of the Vitória bay estuarine system in SE Brazil. Ocean Coast. Manag. 2019, 169, 247–253. [Google Scholar] [CrossRef]
- Lu, L.; Luo, T.; Zhao, Y.; Cai, C.; Fu, Z.; Jin, Y. Interaction between microplastics and microorganism as well as gut microbiota: A consideration on environmental animal and human health. Sci. Total Environ. 2019, 667, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Yu, Y.; Li, X.; Wang, D.; Wang, D. Molecular control of innate immune response to Pseudomonas aeruginosa infection by intestinal let-7 in Caenorhabditis elegans. PLoS Pathog. 2017, 13, e1006152. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhi, L.; Wu, Q.; Jing, L.; Wang, D. NPR-9 regulates innate immune response in Caenorhabditis elegans by antagonizing activity of AIB interneurons. Cell. Mol. Immunol. 2018, 15, 27–37. [Google Scholar] [CrossRef]
- Sun, X.; Chen, B.; Li, Q.; Liu, N.; Xia, B.; Zhu, L.; Qu, K. Toxicities of polystyrene nanoand microplastics toward marine bacterium Halomonas alkaliphila. Sci. Total Environ. 2018, 642, 1378–1385. [Google Scholar] [CrossRef]
- Zhang, K.; Xiong, X.; Hu, H.; Wu, C.; Bi, Y.; Wu, Y.; Zhou, B.; Lam, P.K.; Liu, J. Occurrence and characteristics of microplastic pollution in Xiangxi Bay of Three Gorges Reservoir, China. Environ. Sci. Technol. 2017, 51, 3794–3801. [Google Scholar] [CrossRef] [PubMed]
- Di, M.; Wang, J. Microplastics in surface waters and sediments of the Three Gorges Reservoir, China. Sci. Total Environ. 2018, 616–617, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Du, H.; Tang, M.; Wang, Q.; Huang, Q.; He, Y.; Cheng, F.; Zhao, F.; Wang, D.; Xiao, G. Biosafety assessment of Acinetobacter strains isolated from the Three Gorges Reservoir region in nematode Caenorhabditis elegans. Sci. Rep. 2021, 11, 19721. [Google Scholar] [CrossRef] [PubMed]
- Fiester, S.E.; Arivett, B.A.; Beckett, A.C.; Wagner, B.R.; Ohneck, E.J.; Schmidt, R.E.; Grier, J.T.; Actis, L.A. Miltefosine reduces the cytolytic activity and virulence of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2018, 63, e01409-18. [Google Scholar] [CrossRef]
- Grier, J.T.; Arivett, B.A.; Ramírez, M.S.; Chosed, R.J.; Bigner, J.A.; Ohneck, E.J.; Metz, M.L.; Wood, C.R.; Arce, S.; Tartaro, A.; et al. Two Acinetobacter baumannii isolates obtained from a fatal necrotizing fasciitis infection display distinct genomic and phenotypic characteristics in comparison to type strains. Front. Cell. Infect. Microbiol. 2021, 11, 635673. [Google Scholar] [CrossRef]
- Yu, K.; Zeng, W.; Xu, Y.; Liao, W.; Xu, W.; Zhou, T.; Cao, J.; Chen, L. Bloodstream infections caused by ST2 Acinetobacter baumannii: Risk factors, antibiotic regimens, and virulence over 6 years period in China. Antimicrob. Resist. Infect. Control 2021, 10, 16. [Google Scholar] [CrossRef]
- Sarshar, M.; Behzadi, P.; Scribano, D.; Palamara, A.T.; Ambrosi, C. Acinetobacter baumannii: An ancient commensal with weapons of a pathogen. Pathogens 2021, 10, 387. [Google Scholar] [CrossRef]
- Martinez, J.; Razo-Gutierrez, C.; Le, C.; Courville, R.; Pimentel, C.; Liu, C.; Fung, S.E.; Tuttobene, M.R.; Phan, K.; Vila, A.J.; et al. Cerebrospinal fluid (CSF) augments metabolism and virulence expression factors in Acinetobacter baumannii. Sci. Rep. 2021, 11, 4737. [Google Scholar] [CrossRef]
- Pitt, J.A.; Kozal, J.S.; Jayasundara, N.; Massarsky, A.; Trevisan, R.; Geitner, N.; Wiesner, M.; Levin, E.D.; Di Giulio, R.T. Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquat. Toxicol. 2018, 194, 185–194. [Google Scholar] [CrossRef]
- Liu, H.-L.; Tian, L.-J.; Wang, S.-T.; Wang, D.-Y. Size-dependent transgenerational toxicity induced by nanoplastics in nematode Caenorhabditis elegans. Sci. Total Environ. 2021, 790, 148217. [Google Scholar] [CrossRef]
- Cedervall, T.; Hansson, L.A.; Lard, M.; Frohm, B.; Linse, S. Food chain transport of nanoparticles affects behaviour and fat metabolism in fish. PLoS ONE 2012, 7, e32254. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Lee, D.K.; Long, N.P.; Kwon, S.W.; Park, J.H. Uptake of nanopolystyrene particles induces distinct metabolic profiles and toxic effects in Caenorhabditis elegans. Environ. Pollut. 2019, 246, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, H.; Qu, M.; Wang, D. Response of tyramine and glutamate related signals to nanoplastic exposure in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2021, 217, 112239. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Q.; Wang, D. Neuronal Gα subunits required for the control of response to polystyrene nanoparticles in the range of μg/L in C. elegans. Ecotoxicol. Environ. Saf. 2021, 225, 112732. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.-T.; Zhao, Y.; Bi, K.; Wang, D.-Y. Increase in germline methyltransferases governing methylation of histone H3K9 is associated with transgenerational nanoplastic toxicity in Caenorhabditis elegans. Environ. Sci. Nano 2022, 9, 265–274. [Google Scholar] [CrossRef]
- Xu, R.; Hua, X.; Rui, Q.; Wang, D. Polystyrene nanoparticles caused dynamic alteration in mitochondrial unfolded protein response from parents to the offspring in C. elegans. Chemosphere 2022, 308, 136154. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, Y.; Hua, X.; Wang, D. Induction of transgenerational toxicity is associated with the activated germline insulin signals in nematodes exposed to nanoplastic at predicted environmental concentrations. Ecotoxicol. Environ. Saf. 2022, 243, 114022. [Google Scholar] [CrossRef]
- Xu, R.; Hua, X.; Rui, Q.; Wang, D. Alteration in Wnt signaling mediates induction of transgenerational toxicity of polystyrene nanoplastics in C. elegans. NanoImpact 2022, 28, 100425. [Google Scholar] [CrossRef]
- Fu, S.-F.; Ding, J.-N.; Zhang, Y.; Li, Y.-F.; Zhu, R.; Yuan, X.-Z.; Zou, H. Exposure to polystyrene nanoplastic leads to inhibition of anaerobic digestion system. Sci. Total Environ. 2018, 625, 64–70. [Google Scholar] [CrossRef]
- Nomura, T.; Tani, S.; Yamamoto, M.; Nakagawa, T.; Toyoda, S.; Fujisawa, E.; Yasui, A.; Konishi, Y. Cytotoxicity and colloidal behavior of polystyrene latex nanoparticles toward filamentous fungi in isotonic solutions. Chemosphere 2016, 149, 84–90. [Google Scholar] [CrossRef]
- Tomaras, A.P.; Flagler, M.J.; Dorsey, C.W.; Gaddy, J.A.; Actis, L.A. Characterization of a two-component regulatory system from Acinetobacter baumannii that controls biofilm formation and cellular morphology. Microbiology 2008, 154, 3398–3409. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.M.; Pulido, M.R.; Di Venanzio, G.; Kinsella, R.L.; Webb, A.I.; Scott, N.E.; Pachón, J.; Feldman, M.F. Pathogenic Acinetobacter species have a functional type I secretion system and contact-dependent inhibition systems. J. Biol. Chem. 2017, 292, 9075–9087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, J.; Wu, J.; Wang, J.; Luo, Y. Potential risks of microplastics combined with superbugs: Enrichment of antibiotic resistant bacteria on the surface of microplastics in mariculture system. Ecotoxicol. Environ. Saf. 2020, 187, 109852. [Google Scholar] [CrossRef] [PubMed]
- Enright, M.C.; McKenzie, H. Moraxella (Branhamella) catarrhalis—clinical and molecular aspects of a rediscovered pathogen. J. Med. Microbiol. 1997, 46, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.K.; Adams, F.G.; Brown, M.H. Diversity and function of capsular polysaccharide in Acinetobacter baumannii. Front. Microbiol. 2019, 9, 3301. [Google Scholar] [CrossRef]
- Liao, C.H.; Sheng, W.H.; Chen, Y.C.; Hung, C.C.; Wang, J.T.; Chang, S.C. Predictive value of the serum bactericidal test for mortality in patients infected with multidrug-resistant Acinetobacter baumannii. J. Infect. 2007, 55, 149–157. [Google Scholar] [CrossRef]
- Lenz, R.; Enders, K.; Nielsen, T.G. Microplastic exposure studies should be environmentally realistic. Proc. Natl. Acad. Sci. USA 2016, 113, E4121–E4122. [Google Scholar] [CrossRef]
- Hood, M.I.; Mortensen, B.L.; Moore, J.L.; Zhang, Y.; Kehl-Fie, T.E.; Sugitani, N.; Chazin, W.J.; Caprioli, R.M.; Skaar, E.P. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog. 2012, 8, e1003068. [Google Scholar] [CrossRef]
- Fiester, S.E.; Actis, L.A. Stress responses in the opportunistic pathogen Acinetobacter baumannii. Future Microbiol. 2013, 8, 353–365. [Google Scholar] [CrossRef]
- Gaddy, J.A.; Arivett, B.A.; McConnell, M.J.; López-Rojas, R.; Pachón, J.; Actis, L.A. Role of acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, Galleria mellonella caterpillars, and mice. Infect. Immun. 2012, 80, 1015–1024. [Google Scholar] [CrossRef]
- Nairn, B.L.; Lonergan, Z.R.; Wang, J.; Braymer, J.J.; Zhang, Y.; Calcutt, M.W.; Lisher, J.P.; Gilston, B.A.; Chazin, W.J.; de Crécy-Lagard, V.; et al. The response of Acinetobacter baumannii to zinc starvation. Cell Host Microbe 2016, 19, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Su, P.W.; Moi, S.H.; Chuang, L.Y. Biofilm formation in Acinetobacter Baumannii: Genotype-phenotype correlation. Molecules 2019, 24, 1849. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J.A.; Tomaras, A.P.; Actis, L.A. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect. Immun. 2009, 77, 3150–3160. [Google Scholar] [CrossRef] [PubMed]
- Fattahian, Y.; Rasooli, I.; Mousavi Gargari, S.L.; Rahbar, M.R.; Darvish Alipour Astaneh, S.; Amani, J. Protection against Acinetobacter baumannii infection via its functional deprivation of biofilm associated protein (Bap). Microb. Pathog. 2011, 51, 402–406. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lu, F.; Yuan, F.; Jiang, D.; Zhao, P.; Zhu, J.; Cheng, H.; Cao, J.; Lu, G. Biofilm formation caused by clinical Acinetobacter baumannii isolates is associated with overexpression of the AdeFGH efflux pump. Antimicrob. Agents Chemother. 2015, 59, 4817–4825. [Google Scholar] [CrossRef]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat. Rev. Microbiol. 2018, 16, 91–102. [Google Scholar] [CrossRef]
- Luke, N.R.; Sauberan, S.L.; Russo, T.A.; Beanan, J.M.; Olson, R.; Loehfelm, T.W.; Cox, A.D.; St Michael, F.; Vinogradov, E.V.; Campagnari, A.A. Identification and characterization of a glycosyltransferase involved in Acinetobacter baumannii lipopolysaccharide core biosynthesis. Infect. Immun. 2010, 78, 2017–2023. [Google Scholar] [CrossRef]
- Lin, L.; Tan, B.; Pantapalangkoor, P.; Ho, T.; Baquir, B.; Tomaras, A.; Montgomery, J.I.; Reilly, U.; Barbacci, E.G.; Hujer, K.; et al. Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. mBio 2012, 3, e00312-12. [Google Scholar] [CrossRef]
- Russo, T.A.; MacDonald, U.; Beanan, J.M.; Olson, R.; MacDonald, I.J.; Sauberan, S.L.; Luke, N.R.; Schultz, L.W.; Umland, T.C. Penicillin-binding protein 7/8 contributes to the survival of Acinetobacter baumannii in vitro and in vivo. J. Infect. Dis. 2009, 199, 513–521. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.; Ding, G.; Lu, X.; Huang, Q.; Du, H.; Xiao, G.; Wang, D. Exposure to Nanoplastic Particles Enhances Acinetobacter Survival, Biofilm Formation, and Serum Resistance. Nanomaterials 2022, 12, 4222. https://doi.org/10.3390/nano12234222
Tang M, Ding G, Lu X, Huang Q, Du H, Xiao G, Wang D. Exposure to Nanoplastic Particles Enhances Acinetobacter Survival, Biofilm Formation, and Serum Resistance. Nanomaterials. 2022; 12(23):4222. https://doi.org/10.3390/nano12234222
Chicago/Turabian StyleTang, Mingfeng, Guoying Ding, Xiaoyu Lu, Qian Huang, Huihui Du, Guosheng Xiao, and Dayong Wang. 2022. "Exposure to Nanoplastic Particles Enhances Acinetobacter Survival, Biofilm Formation, and Serum Resistance" Nanomaterials 12, no. 23: 4222. https://doi.org/10.3390/nano12234222
APA StyleTang, M., Ding, G., Lu, X., Huang, Q., Du, H., Xiao, G., & Wang, D. (2022). Exposure to Nanoplastic Particles Enhances Acinetobacter Survival, Biofilm Formation, and Serum Resistance. Nanomaterials, 12(23), 4222. https://doi.org/10.3390/nano12234222






