Adsorption and Gas-Sensing Properties of Agn (n = 1–4) Cluster Doped GeSe for CH4 and CO Gases in Oil-Immersed Transformer
Abstract
1. Introduction
2. Computational Details and Methods
3. Results and Discussion
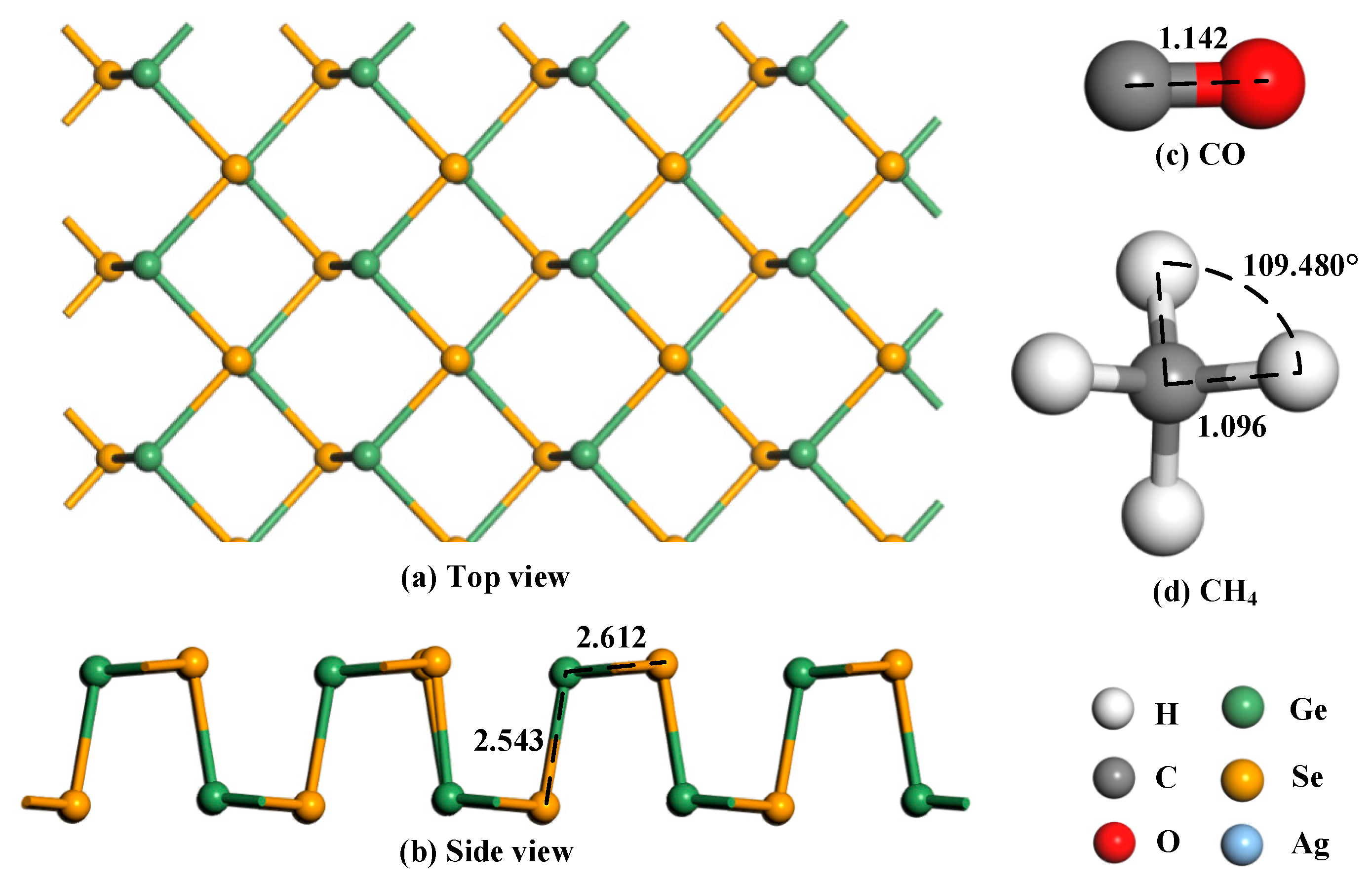
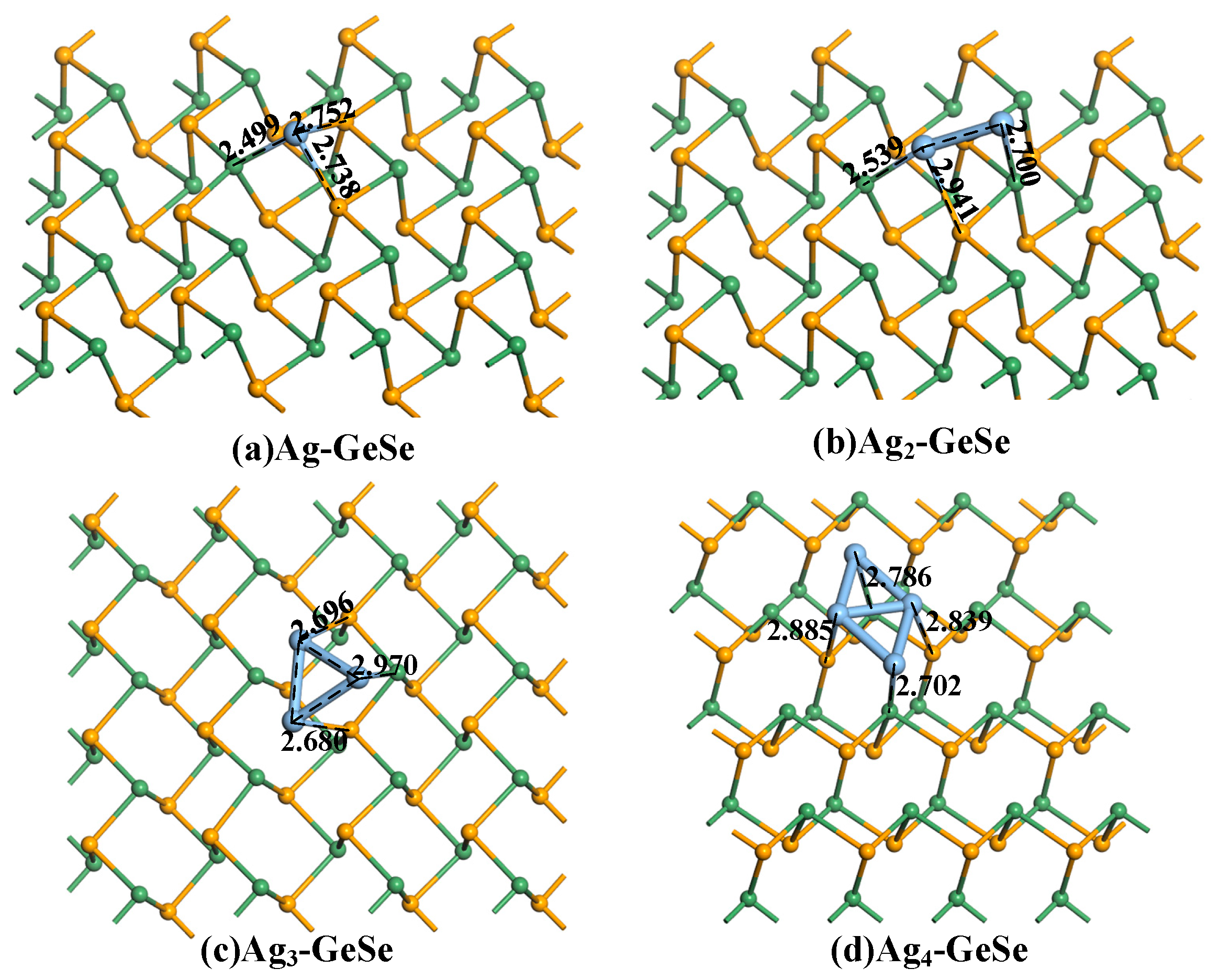
3.1. Geometry Optimization
3.2. Analysis of CO Gas Adsorption on Agn-GeSe Surface
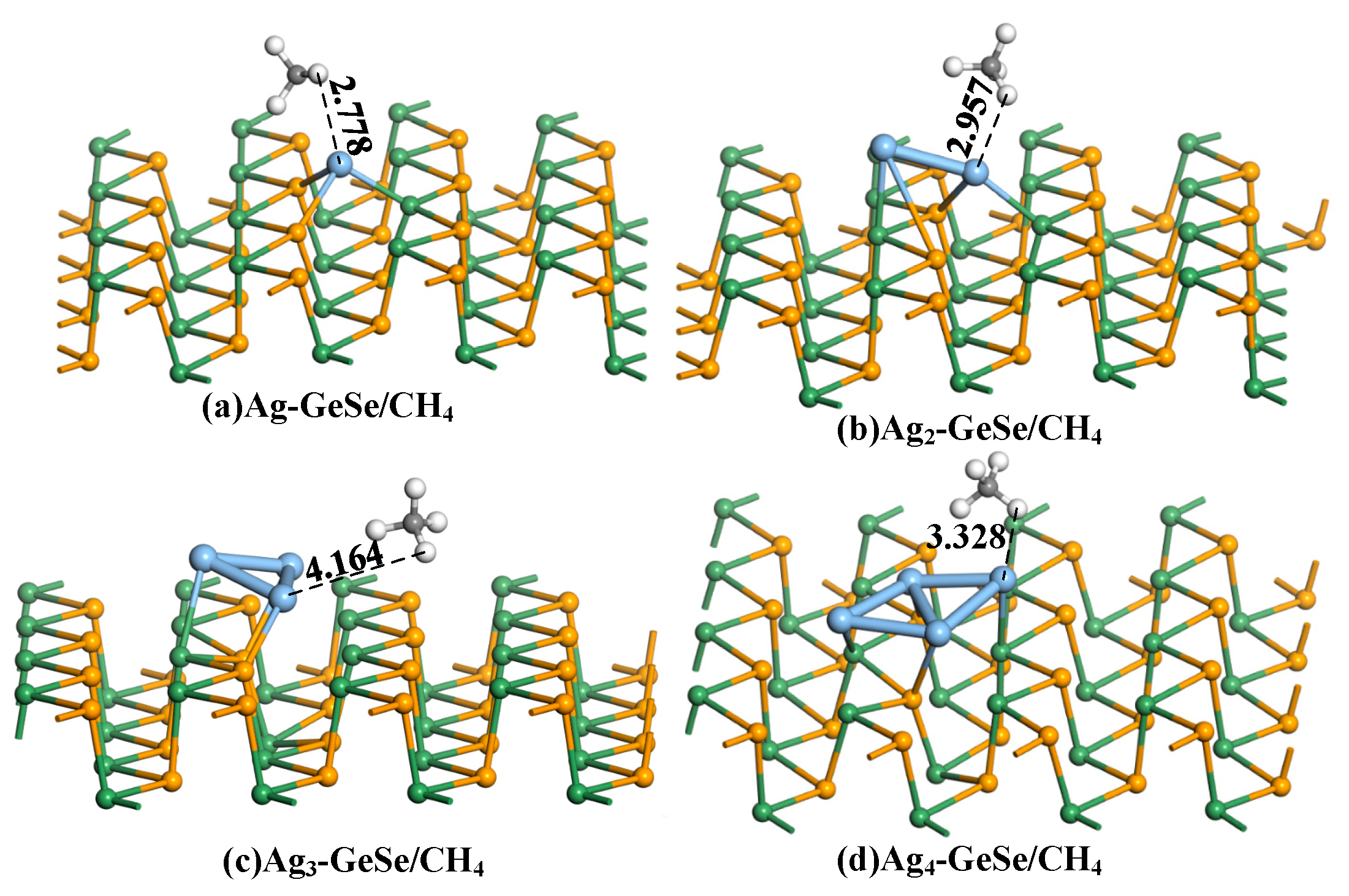
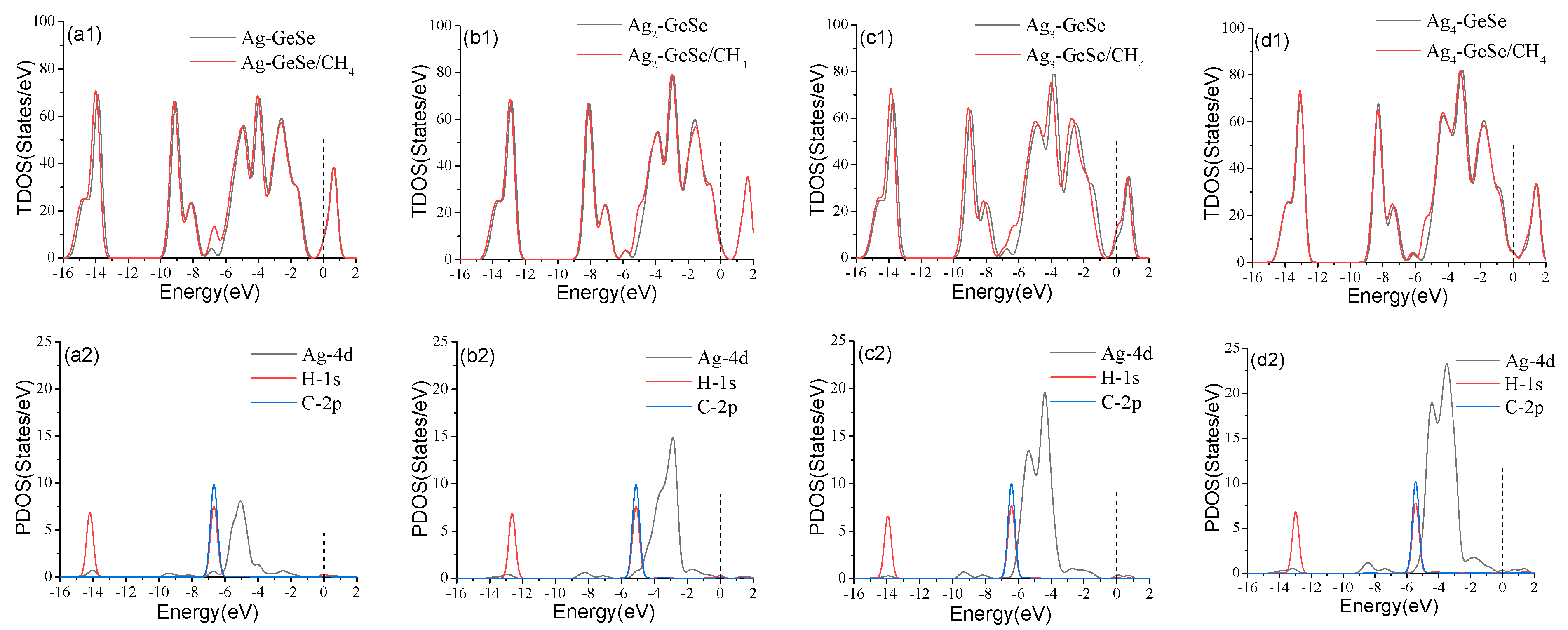
3.3. Analysis of CH4 Gas Adsorption on Agn-GeSe Surface
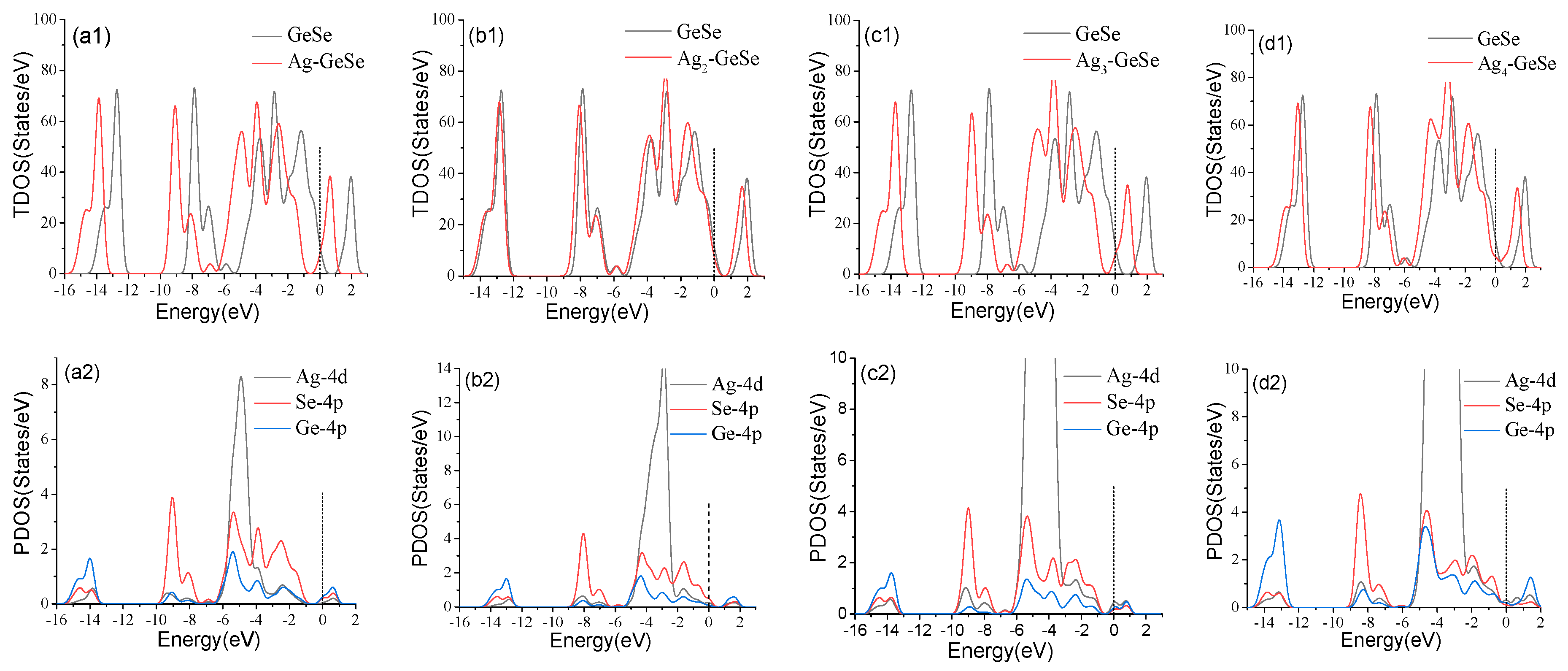
3.4. Molecular Orbital Theory Analysis of Gases Adsorption on Agn-GeSe
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, Y.; Ruan, J.; Shi, Y.; Jin, S.; Tian, Y.; Zhu, L. Inversion Detection Method for Resistivity of Oil-Immersed Paper in Transformer. IEEE Trans. Power Deliv. 2019, 34, 1757–1765. [Google Scholar] [CrossRef]
- Zhang, X.; Gui, Y.; Xiao, H.; Zhang, Y. Analysis of adsorption properties of typical partial discharge gases on Ni-SWCNTs using density functional theory. Appl. Surf. Sci. 2016, 379, 47–54. [Google Scholar] [CrossRef]
- Lin, M.-J.; Chen, L.-B.; Yu, C.-T. A Methodology for Diagnosing Faults in Oil-Immersed Power Transformers Based on Minimizing the Maintenance Cost. IEEE Access 2020, 8, 209570–209578. [Google Scholar] [CrossRef]
- Liu, J.; Fan, X.; Zhang, Y.; Zheng, H.; Zhang, C. Condition prediction for oil-immersed cellulose insulation in field transformer using fitting fingerprint database. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 279–287. [Google Scholar] [CrossRef]
- Chu, J.; Li, W.; Yang, X.; Wu, Y.; Wang, D.; Yang, A.; Yuan, H.; Wang, X.; Li, Y.; Rong, M. Identification of gas mixtures via sensor array combining with neural networks. Sens. Actuators B Chem. 2021, 329, 129090. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Gui, Y.; Hu, W. First-principles study of SF6 decomposed gas adsorbed on Au-decorated graphene. Appl. Surf. Sci. 2016, 367, 259–269. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Wu, X.; Hu, W. Experimental Sensing and Density Functional Theory Study of H2S and SOF2 Adsorption on Au-Modified Graphene. Adv. Sci. 2015, 2, 1500101. [Google Scholar] [CrossRef]
- Cho, B.H.; Chino, H.; Tsuji, H.; Kunito, T.; Makishima, H.; Uchida, H.; Matsumoto, S.; Oyaizu, H. Analysis of oil components and hydrocarbon-utilizing microorganisms during laboratory-scale bioremediation of oil-contaminated soil of Kuwait. Chemosphere 1997, 35, 1613–1621. [Google Scholar] [CrossRef]
- Verma, A.M.; Agrawal, K.; Kawale, H.D.; Kishore, N. Production of Toluene by Decomposition of 2-Hydroxy-6-methylbenzaldehyde: A DFT Study. Chemistryselect 2018, 3, 220–229. [Google Scholar] [CrossRef]
- Gui, Y.; Li, T.; He, X.; Ding, Z.; Yang, P. Pt Cluster Modified h-BN for Gas Sensing and Adsorption of Dissolved Gases in Transformer Oil: A Density Functional Theory Study. Nanomaterials 2019, 9, 1746. [Google Scholar] [CrossRef]
- He, X.; Gui, Y.; Xie, J.; Liu, X.; Wang, Q.; Tang, C. A DFT study of dissolved gas (C2H2, H2, CH4) detection in oil on CuO-modified BNNT. Appl. Surf. Sci. 2020, 500, 144030. [Google Scholar] [CrossRef]
- Gui, Y.; Peng, X.; Liu, K.; Ding, Z. Adsorption of C2H2, CH4 and CO on Mn-doped graphene: Atomic, electronic, and gas-sensing properties. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 119, 113959. [Google Scholar] [CrossRef]
- Chen, W.; Gui, Y.; Li, T.; Zeng, H.; Xu, L.; Ding, Z. Gas-sensing properties and mechanism of Pd-GaNNTs for air decomposition products in ring main unit. Appl. Surf. Sci. 2020, 531, 147293. [Google Scholar] [CrossRef]
- Zhou, Q.; Zeng, W.; Chen, W.; Xu, L.; Kumar, R.; Umar, A. High sensitive and low-concentration sulfur dioxide (SO2) gas sensor application of heterostructure NiO-ZnO nanodisks. Sens. Actuators B Chem. 2019, 298, 126870. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Q.; Xu, L.; Gao, X.; Zeng, W. Gas sensing mechanism of dissolved gases in transformer oil on Ag-MoS2 monolayer: A DFT study. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 118, 113947. [Google Scholar] [CrossRef]
- Yang, A.; Wang, D.; Lan, T.; Chu, J.; Li, W.; Pan, J.; Liu, Z.; Wang, X.; Rong, M. Single ultrathin WO3 nanowire as a superior gas sensor for SO2 and H2S:Selective adsorption and distinct I-V response. Mater. Chem. Phys. 2020, 240, 122165. [Google Scholar] [CrossRef]
- Li, P.; Hong, Q.; Wu, T.; Cui, H. SOF2 sensing by Rh-doped PtS2 monolayer for early diagnosis of partial discharge in the SF6 insulation device. Mol. Phys. 2021, 119, e1919774. [Google Scholar] [CrossRef]
- Gui, Y.; Zhang, X.; Lv, P.; Wang, S.; Tang, C.; Zhou, Q. Ni-CNT Chemical Sensor for SF6 Decomposition Components Detection: A Combined Experimental and Theoretical Study. Sensors 2018, 18, 3493. [Google Scholar] [CrossRef]
- Keshtkar, S.; Rashidi, A.; Kooti, M.; Askarieh, M.; Pourhashem, S.; Ghasemy, E.; Izadi, N. A novel highly sensitive and selective H2S gas sensor at low temperatures based on SnO2 quantum dots-C(60)nanohybrid: Experimental and theory study. Talanta 2018, 188, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Yang, J.; Xu, C.; Wang, J.; Xue, J. Temperature-programmed desorption/pulse surface reaction (TPD/TPSR) studies of CH4, C2H6, C2H4, and CO over a cobalt/MWNTS catalyst. React. Kinet. Catal. Lett. 2006, 89, 209–217. [Google Scholar] [CrossRef]
- Shin, H.; Krogel, J.T.; Gasperich, K.; Kent, P.R.C.; Benali, A.; Heinonen, O. Optimized structure and electronic band gap of monolayer GeSe from quantum Monte Carlo methods. Phys. Rev. Mater. 2021, 5, 024002. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Q.; Wang, Z.; Ye, H.; Chen, X.; Fan, X.; Zhang, G. High Selective Gas Detection for small molecules based on Germanium selenide monolayer. Appl. Surf. Sci. 2018, 433, 575–581. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Peng, Y.; Gui, Y.; Sun, H. Full Length Article Pd and Pt decorated GeSe monolayers as promising materials for SOF2 and SO2F2 sensing. Appl. Surf. Sci. 2021, 560, 150028. [Google Scholar] [CrossRef]
- Li, X.; Tang, C.; Wang, J.; Tian, W.; Hu, D. Analysis and mechanism of adsorption of naphthenic mineral oil, water, formic acid, carbon dioxide, and methane on meta-aramid insulation paper. J. Mater. Sci. 2019, 54, 8556–8570. [Google Scholar] [CrossRef]
- Wang, X.; Tan, J.; Han, C.; Wang, J.-J.; Lu, L.; Du, H.; Jia, C.-L.; Deringer, V.L.; Zhou, J.; Zhang, W. Sub-Angstrom Characterization of the Structural Origin for High In-Plane Anisotropy in 2D GeS2. ACS Nano 2020, 14, 4456–4462. [Google Scholar] [CrossRef]
- Gui, Y.; Liu, Z.; Ji, C.; Xu, L.; Chen, X. Adsorption behavior of metal oxides (CuO, NiO, Ag2O) modified GeSe monolayer towards dissolved gases (CO, CH4, C2H2, C2H4) in transformer oil. J. Ind. Eng. Chem. 2022, 112, 134–145. [Google Scholar] [CrossRef]
- Guo, L.-Y.; Liang, S.; Yang, Z.; Jin, L.; Tan, Y.; Huang, Z. Gas-Sensing Properties of Dissolved Gases in Insulating Material Adsorbed on SnO2–GeSe Monolayer. Chemosensors 2022, 10, 212. [Google Scholar] [CrossRef]
- He, X.; Gui, Y.; Liu, K.; Xu, L. Comparison of sensing and electronic properties of C2H2 on different transition metal oxide nanoparticles (Fe2O3, NiO, TiO2) modified BNNT (10,0). Appl. Surf. Sci. 2020, 521, 146463. [Google Scholar] [CrossRef]
- Cao, W.; Gui, Y.; Chen, T.; Xu, L.; Ding, Z. Adsorption and gas-sensing properties of Pt2–GaNNTs for SF6 decomposition products. Appl. Surf. Sci. 2020, 524, 146570. [Google Scholar] [CrossRef]
- Li, T.; Gui, Y.; Zhao, W.; Tang, C.; Dong, X. Palladium modified MoS2 monolayer for adsorption and scavenging of SF6 decomposition products: A DFT study. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 123, 114178. [Google Scholar] [CrossRef]
- Gui, Y.; Shi, J.; Yang, P.; Li, T.; Tang, C.; Xu, L. Platinum modified MoS2 monolayer for adsorption and gas sensing of SF6 decomposition products: A DFT study. High Volt. 2020, 5, 454–462. [Google Scholar] [CrossRef]
- Xu, L.; Gui, Y.; Li, W.; Li, Q.; Chen, X. Gas-sensing properties of Ptn-doped WSe2 to SF6 decomposition products. J. Ind. Eng. Chem. 2021, 97, 452–459. [Google Scholar] [CrossRef]
- Capelle, K.; Gross, E.K.U. Spin-density functionals from current-density functional theory and vice versa: A road towards new approximations. Phys. Rev. Lett. 1997, 78, 1872–1875. [Google Scholar] [CrossRef]
- Gui, Y.; Shi, J.; Xu, L.; Ran, L.; Chen, X. Aun (n = 1–4) cluster doped MoSe2 nanosheet as a promising gas-sensing material for C2H4 gas in oil-immersed transformer. Appl. Surf. Sci. 2021, 541, 148356. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Q.; Zeng, W. Competitive adsorption of SF6 decompositions on Ni-doped ZnO (100) surface: Computational and experimental study. Appl. Surf. Sci. 2019, 479, 185–197. [Google Scholar] [CrossRef]
- Zheng, W.; Tang, C.; Xie, J.; Gui, Y. Micro-scale effects of nano-SiO2 modification with silane coupling agents on the cellulose/nano-SiO2 interface. Nanotechnology 2019, 30, 445701. [Google Scholar] [CrossRef] [PubMed]
- Saidi, W.A.; Feng, H.; Fichthorn, K.A. Binding of Polyvinylpyrrolidone to Ag Surfaces: Insight into a Structure-Directing Agent from Dispersion-Corrected Density Functional Theory. J. Phys. Chem. C 2013, 117, 1163–1171. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Comment on “Generalized gradient approximation made simple”. Reply Phys. Rev. Lett. 1998, 80, 891. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- White, J.A.; Bird, D.M. Implementation of gradient-corrected exchange-correlation potentials in Car-Parrinello total-energy calculations. Phys. Rev. B 1994, 50, 4954–4957. [Google Scholar] [CrossRef] [PubMed]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Z. Convergence analysis of generalized nonlinear inexact Uzawa algorithm for stabilized saddle point problems. Front. Math. China 2011, 6, 473–492. [Google Scholar] [CrossRef]
- Gustafsson, B. The Convergence Rate for Difference Approximations to General Mixed Initial Boundary-Value-Problems. Siam J. Numer. Anal. 1981, 18, 179–190. [Google Scholar] [CrossRef]
- Liu, S.H.; Tsai, H.M.; Pao, C.W.; Chiou, J.W.; Ling, D.C.; Pong, W.F.; Tsai, M.H.; Lin, H.J.; Jang, L.Y.; Lee, J.F.; et al. Electronic and magnetic properties of the Ag-doped Fe3O4 films studied by x-ray absorption spectroscopy. Appl. Phys. Lett. 2006, 89, 092112. [Google Scholar] [CrossRef]
- Cui, J.; He, J.; Chen, Y. Delocalized Carriers and the Electrical Transport Properties of n-Type GeSe Crystals. ACS Appl. Energy Mater. 2019, 2, 3703–3707. [Google Scholar] [CrossRef]
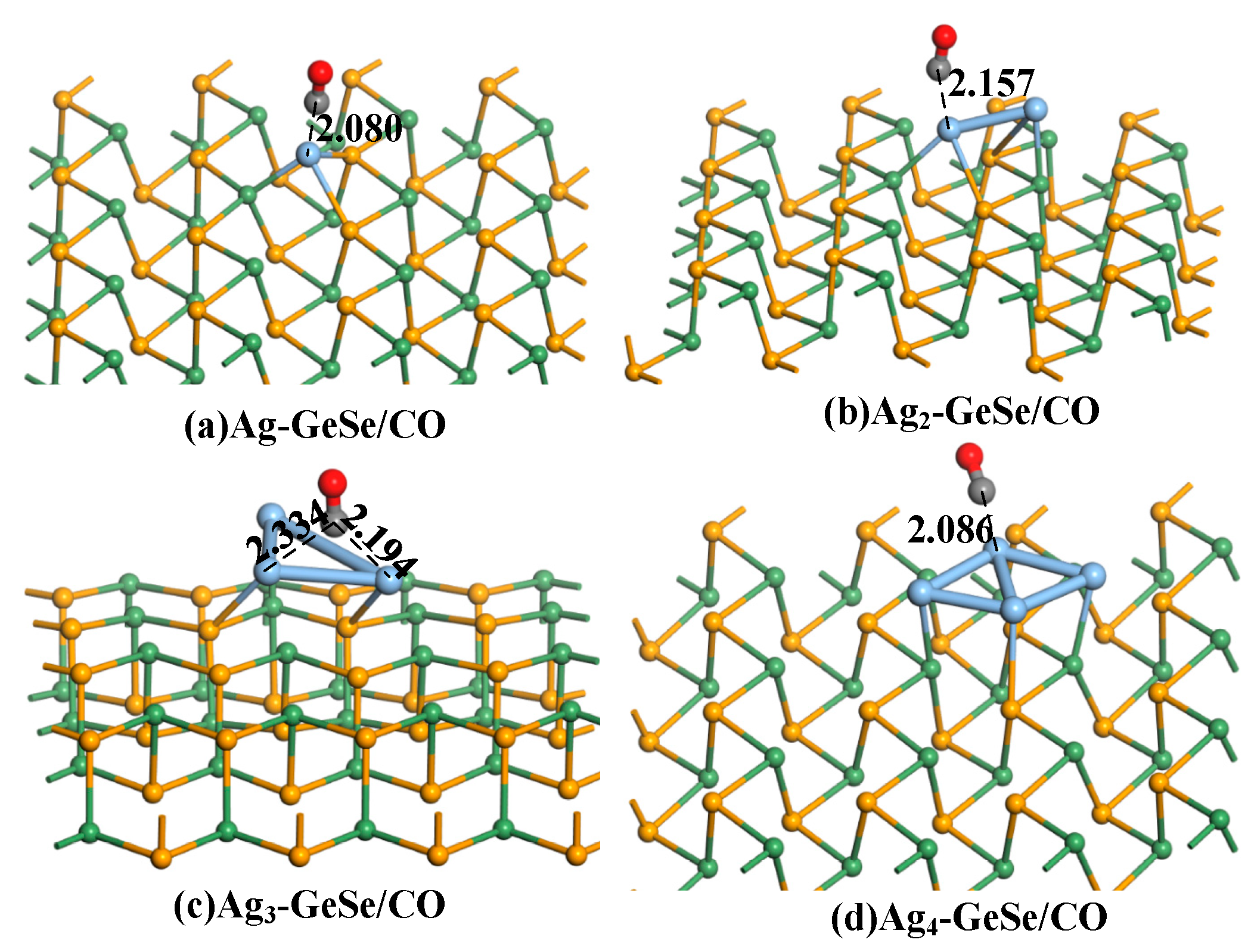
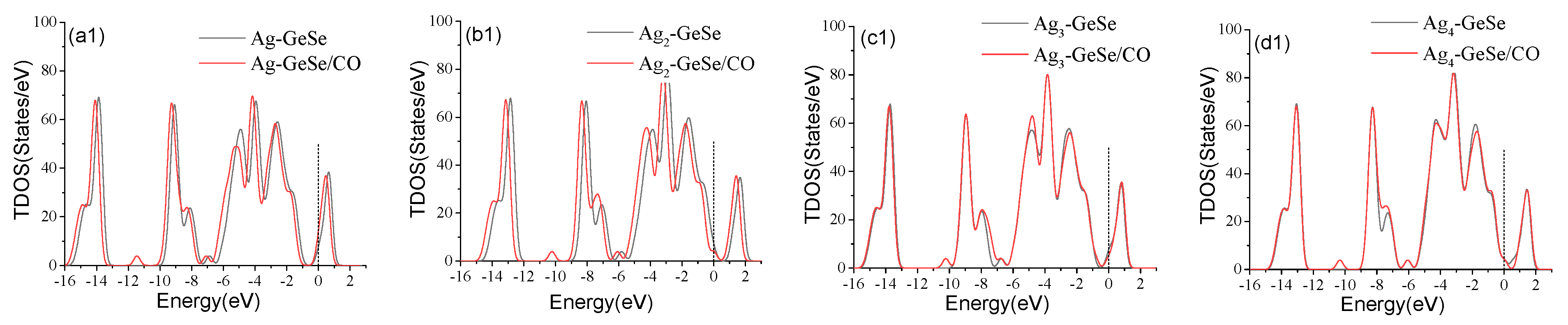
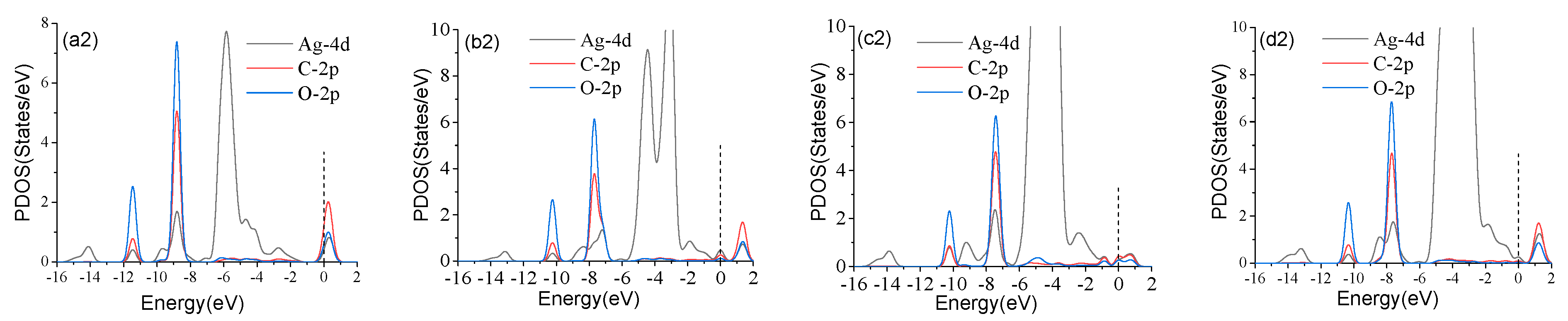
| Configuration | Structure | d (Å) | Eads (eV) | QT (e) |
|---|---|---|---|---|
| Ag-GeSe/CO | Figure 4a | 2.080 | −0.177 | 0.134 |
| Ag2-GeSe/CO | Figure 4b | 2.157 | −0.166 | 0.105 |
| Ag3-GeSe/CO | Figure 4c | 2.194 | −0.171 | −0.014 |
| Ag4-GeSe/CO | Figure 4d | 2.086 | −0.193 | −0.165 |
| Configuration | Structure | d (Å) | Eads (eV) | QT (e) |
|---|---|---|---|---|
| Ag-GeSe/CH4 | Figure 6a | 2.778 | −0.158 | 0.034 |
| Ag2-GeSe/CH4 | Figure 6b | 2.957 | −0.159 | 0.013 |
| Ag3-GeSe/CH4 | Figure 6c | 4.164 | −0.122 | −0.068 |
| Ag4-GeSe/CH4 | Figure 6d | 3.328 | −0.018 | −0.026 |
| Configuration | Structure | EHOMO (eV) | ELUMO (eV) | Eg (eV) |
|---|---|---|---|---|
| Ag-GeSe | \ | −4.707 | −3.483 | 1.224 |
| Ag2-GeSe | \ | −4.555 | −3.311 | 1.244 |
| Ag3-GeSe | \ | −4.700 | −3.648 | 1.052 |
| Ag4-GeSe | \ | −4.329 | −3.716 | 0.613 |
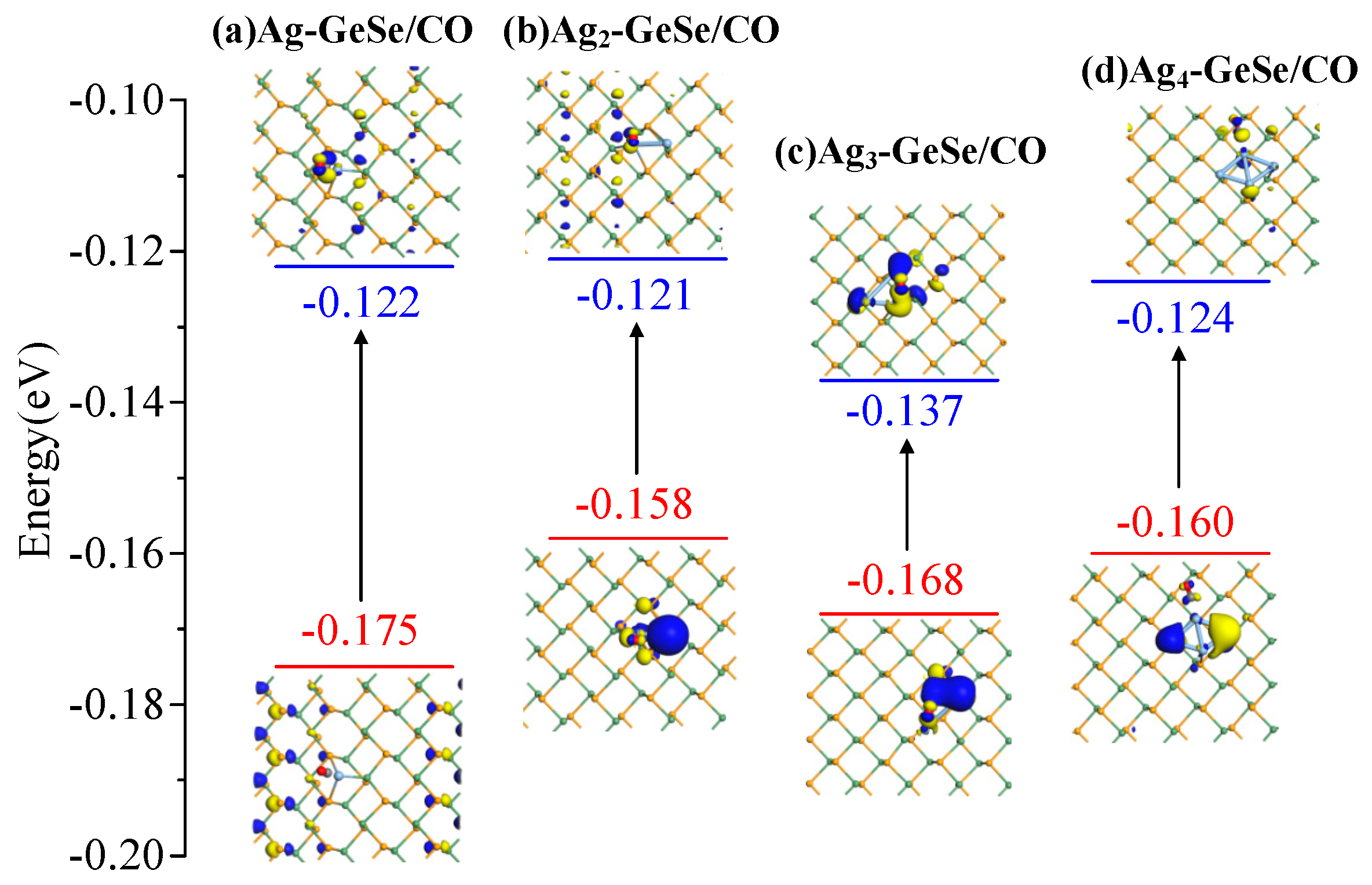
| Ag-GeSe/CO Ag2-GeSe/CO | Figure 8a Figure 8b | −0.175 −0.158 | −0.122 −0.121 | 0.053 0.037 |
| Ag3-GeSe/CO | Figure 8c | −0.168 | −0.137 | 0.031 |
| Ag4-GeSe/CO | Figure 8d | −0.160 | −0.124 | 0.036 |
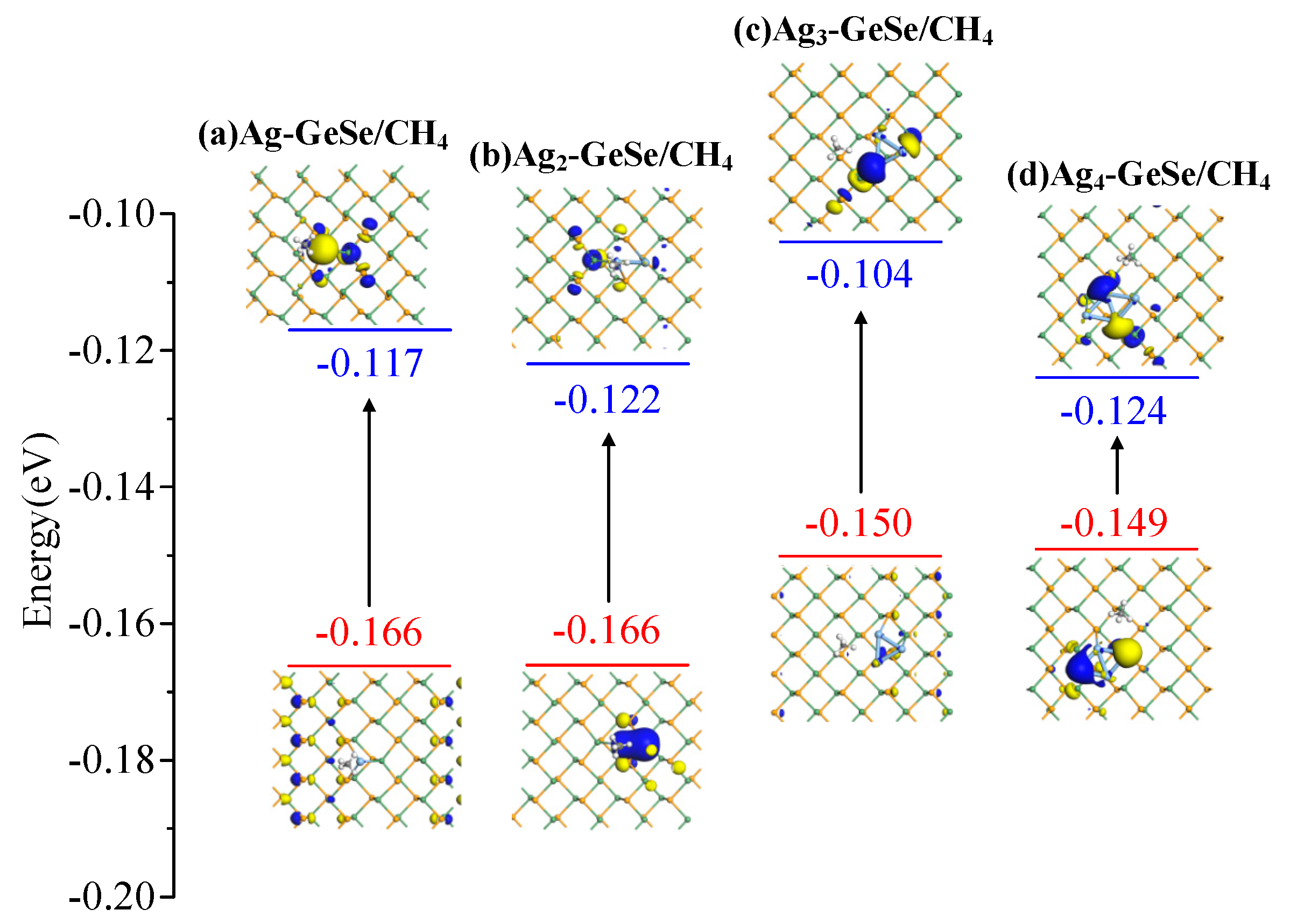
| Ag-GeSe/CH4 Ag2-GeSe/CH4 | Figure 9a Figure 9b | −0.166 −0.166 | −0.117 −0.122 | 0.049 0.044 |
| Ag3-GeSe/CH4 | Figure 9c | −0.150 | −0.104 | 0.046 |
| Ag4-GeSe/CH4 | Figure 9d | −0.149 | −0.124 | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, A.; Sun, M.; Gui, Y. Adsorption and Gas-Sensing Properties of Agn (n = 1–4) Cluster Doped GeSe for CH4 and CO Gases in Oil-Immersed Transformer. Nanomaterials 2022, 12, 4203. https://doi.org/10.3390/nano12234203
Dong A, Sun M, Gui Y. Adsorption and Gas-Sensing Properties of Agn (n = 1–4) Cluster Doped GeSe for CH4 and CO Gases in Oil-Immersed Transformer. Nanomaterials. 2022; 12(23):4203. https://doi.org/10.3390/nano12234203
Chicago/Turabian StyleDong, Aijuan, Meiling Sun, and Yingang Gui. 2022. "Adsorption and Gas-Sensing Properties of Agn (n = 1–4) Cluster Doped GeSe for CH4 and CO Gases in Oil-Immersed Transformer" Nanomaterials 12, no. 23: 4203. https://doi.org/10.3390/nano12234203
APA StyleDong, A., Sun, M., & Gui, Y. (2022). Adsorption and Gas-Sensing Properties of Agn (n = 1–4) Cluster Doped GeSe for CH4 and CO Gases in Oil-Immersed Transformer. Nanomaterials, 12(23), 4203. https://doi.org/10.3390/nano12234203







