Enhanced Heterogeneous Fenton-like Process for Sulfamethazine Removal via Dual-Reaction-Center Fe-Mo/rGO Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Catalysts
2.3. Material Characterization
2.4. Experimental Procedure
2.5. Calculation Method
3. Results and Discussion
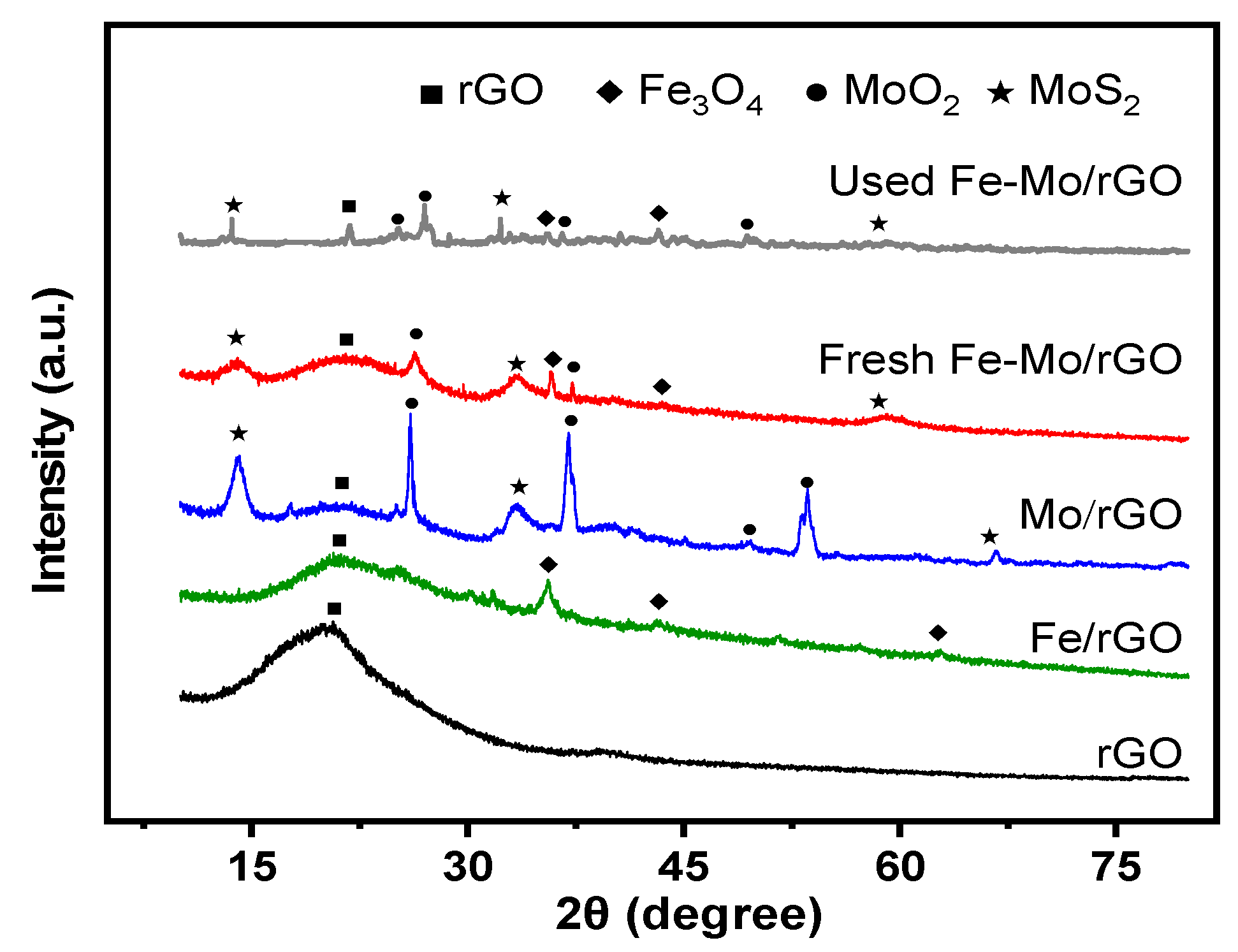
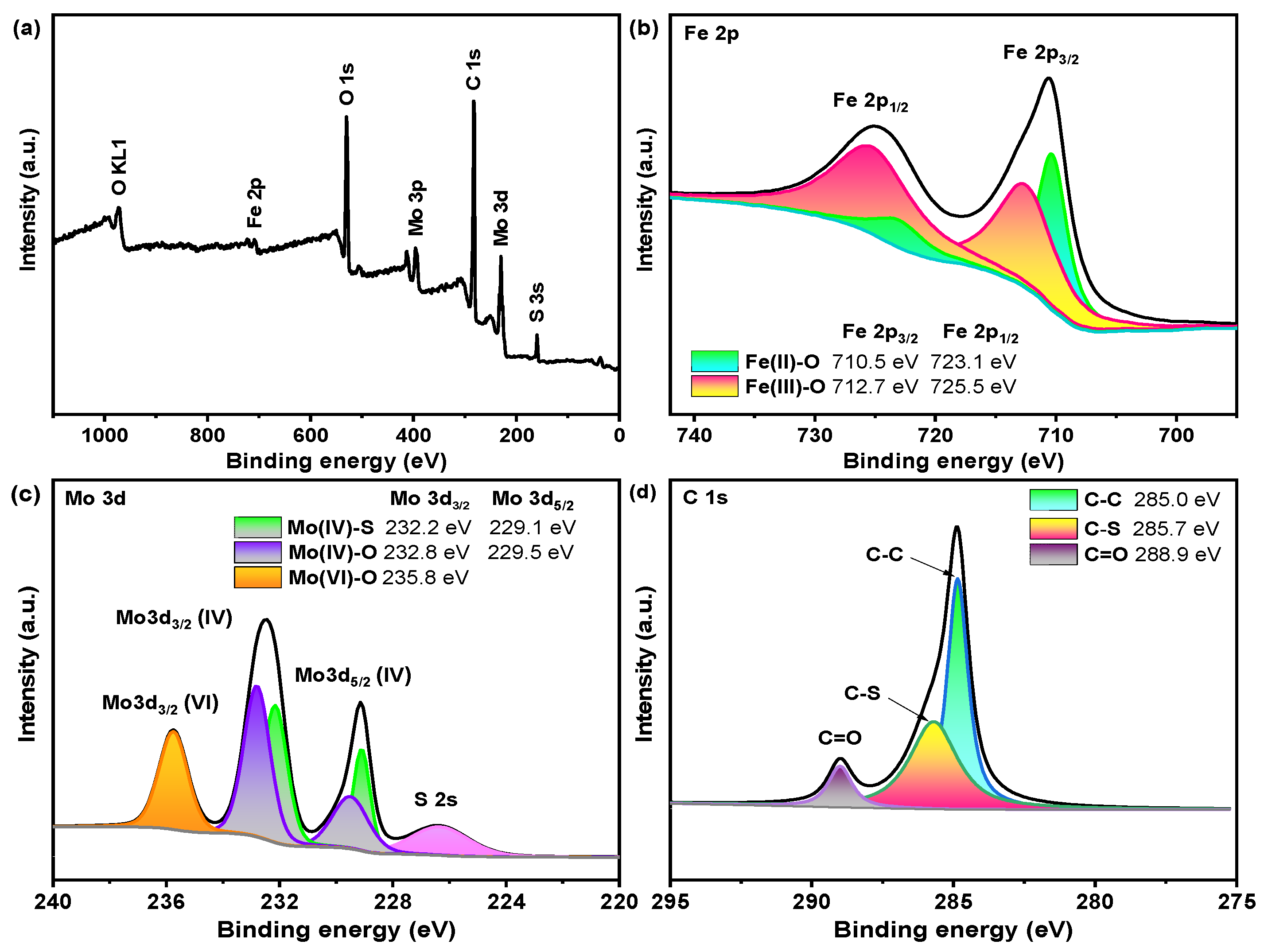
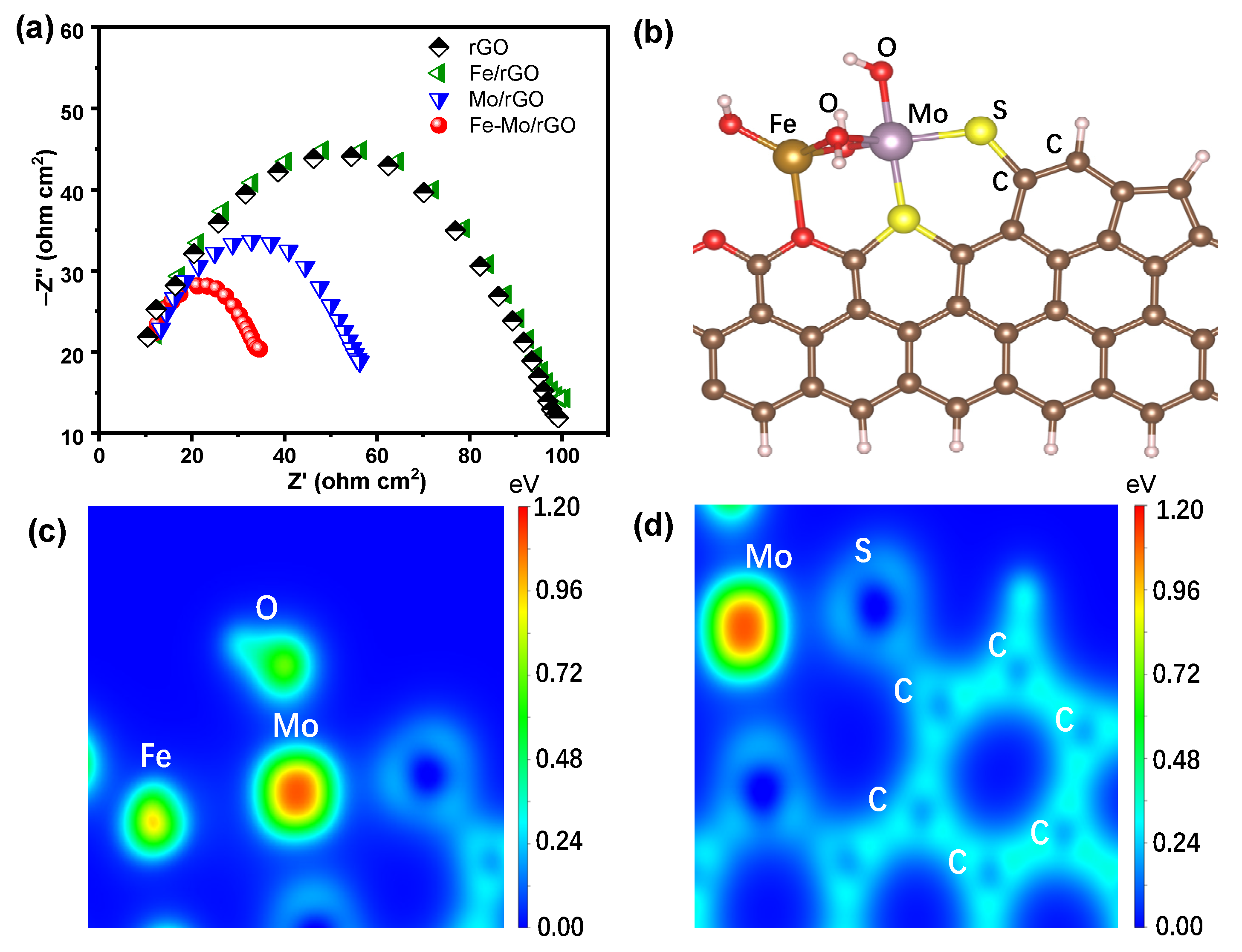
3.1. Characterization of the Catalyst
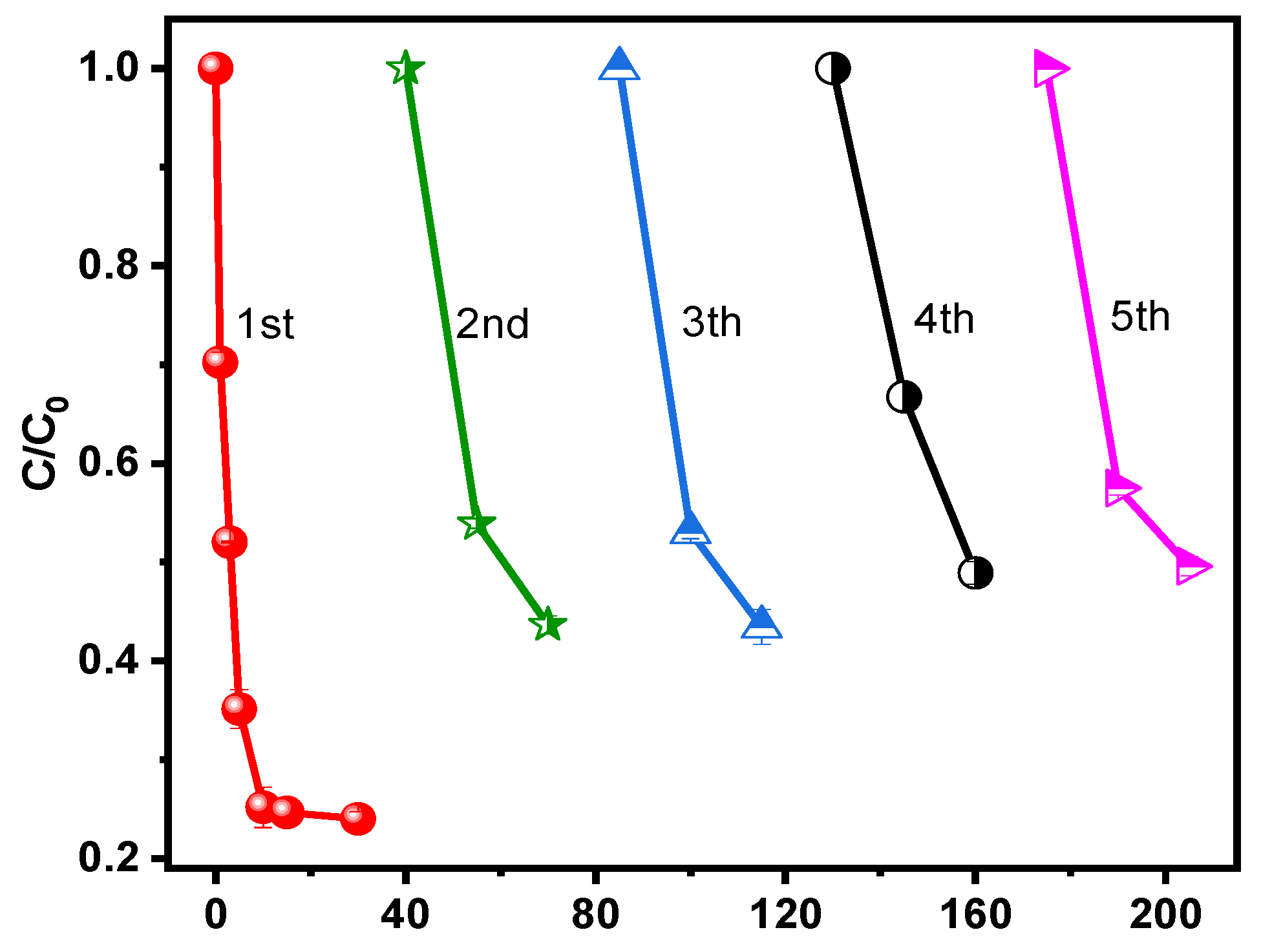
3.2. Catalytic Degradation of SMT
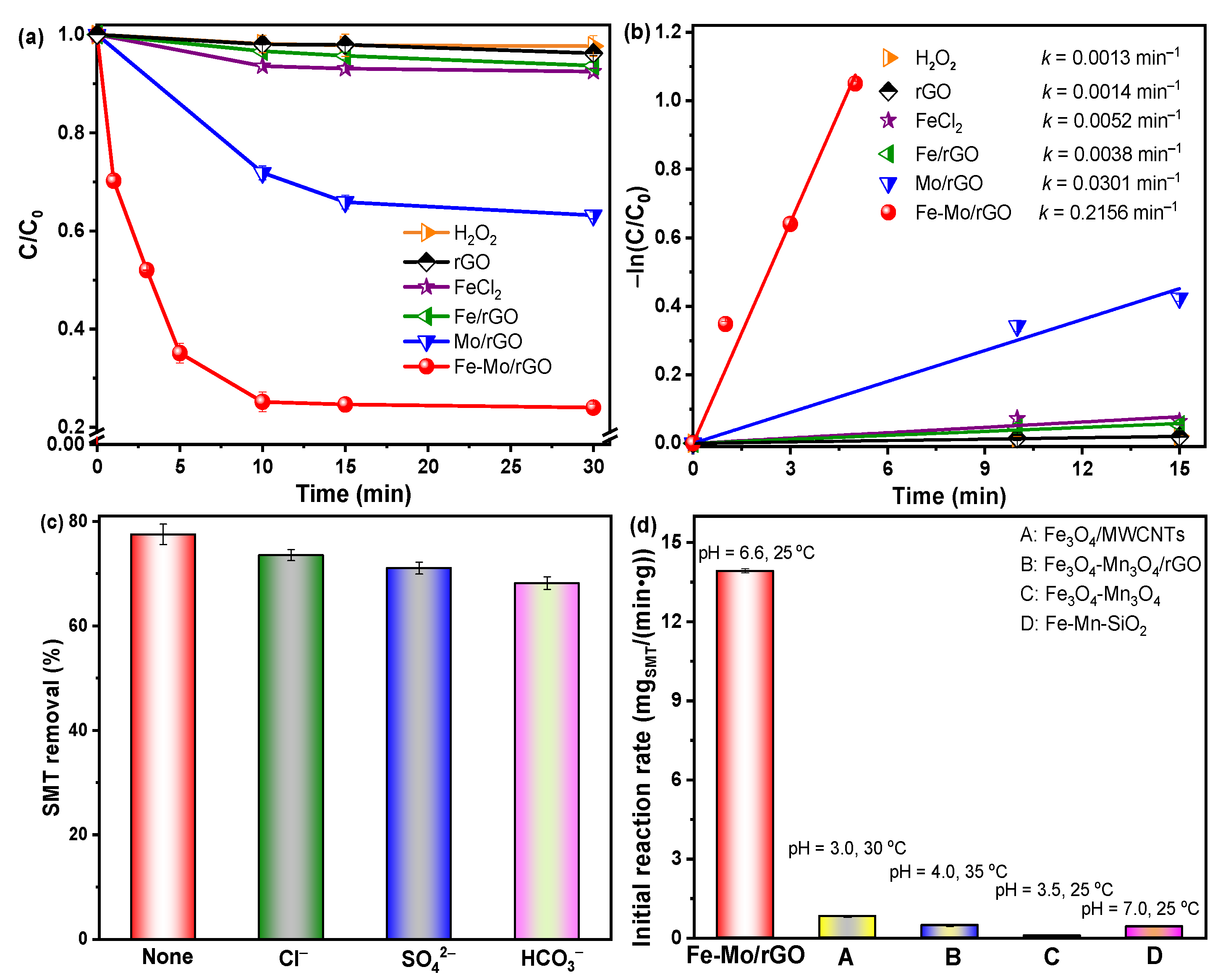
3.2.1. Effect of Different Catalysts
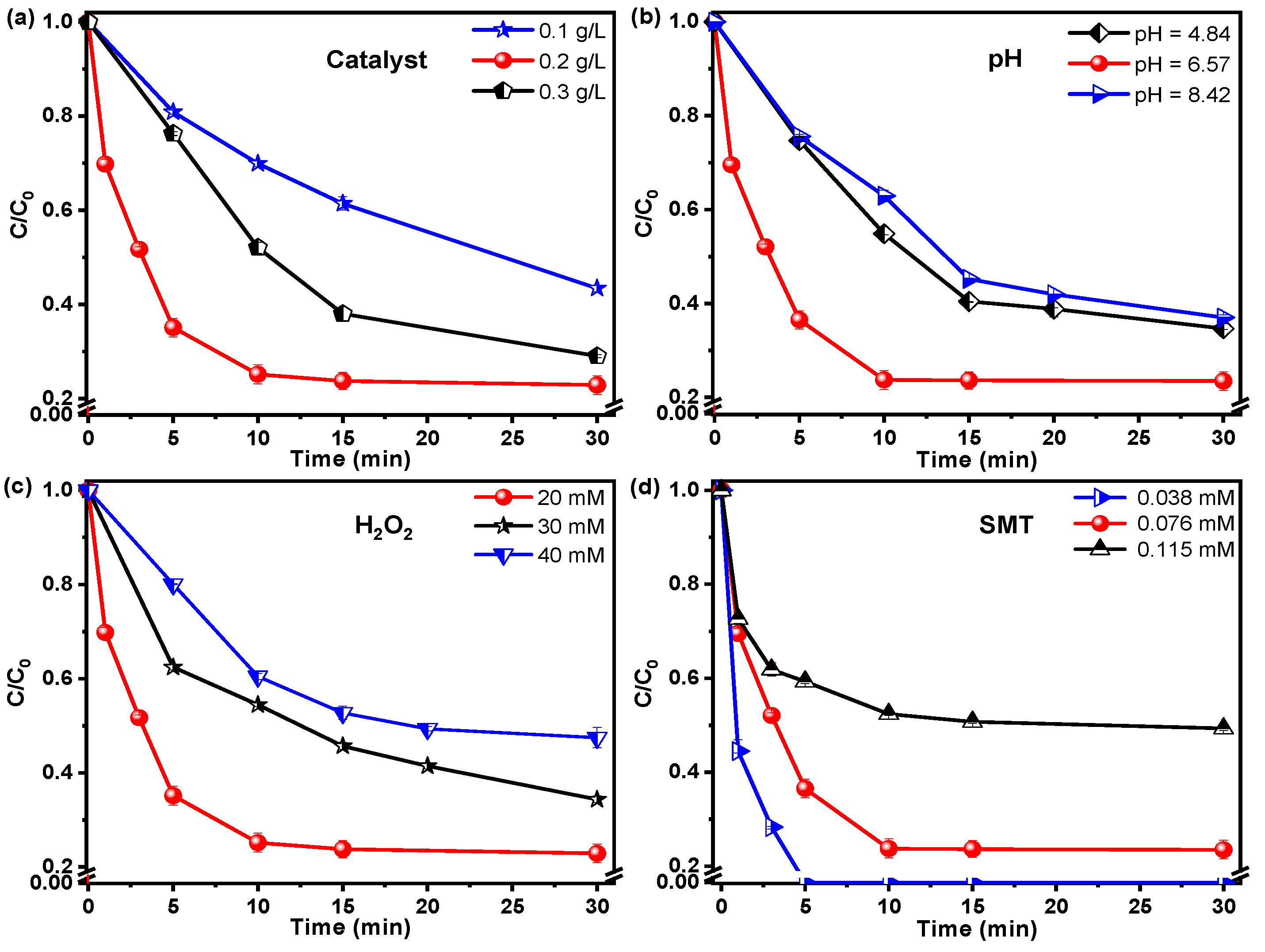
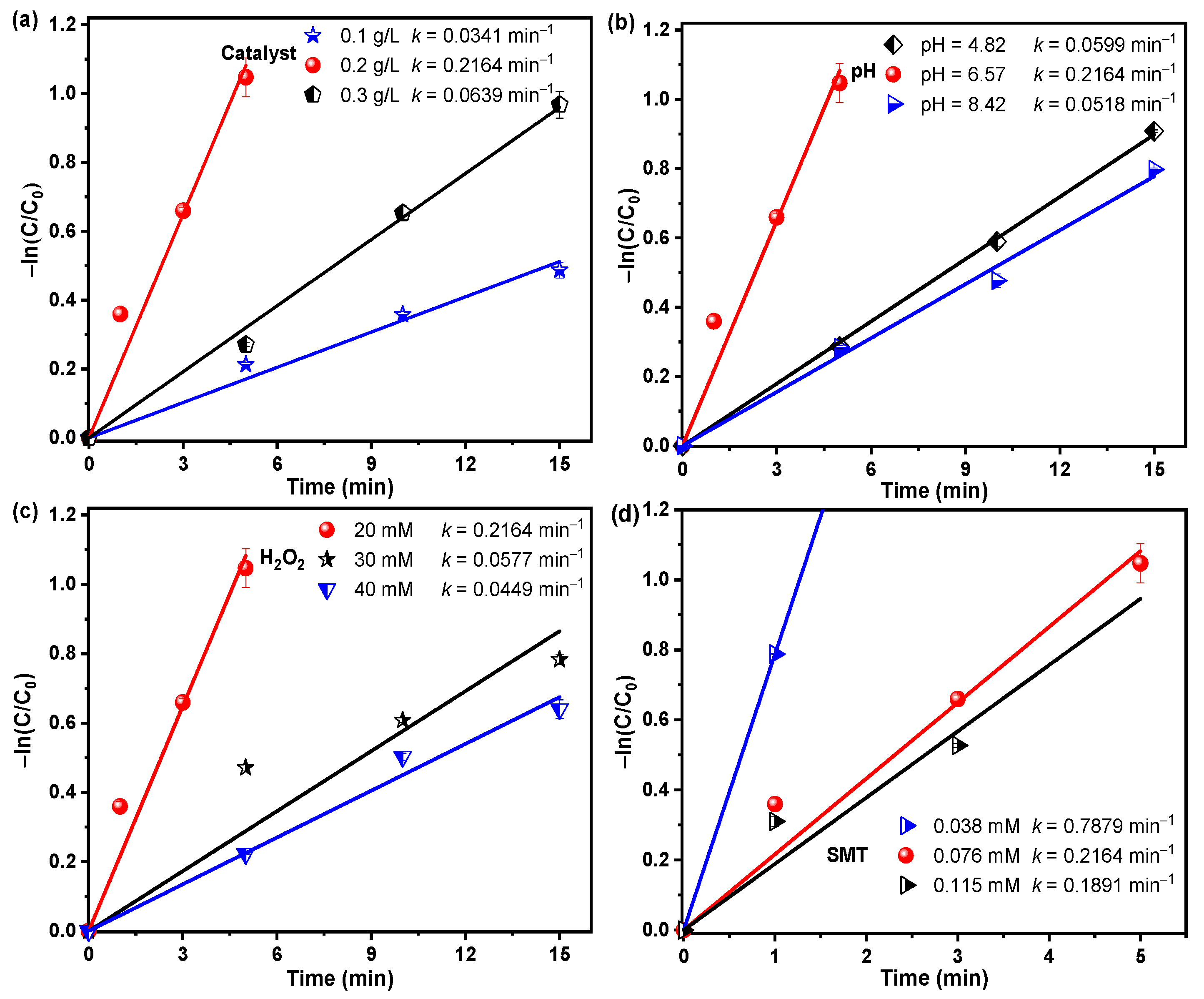
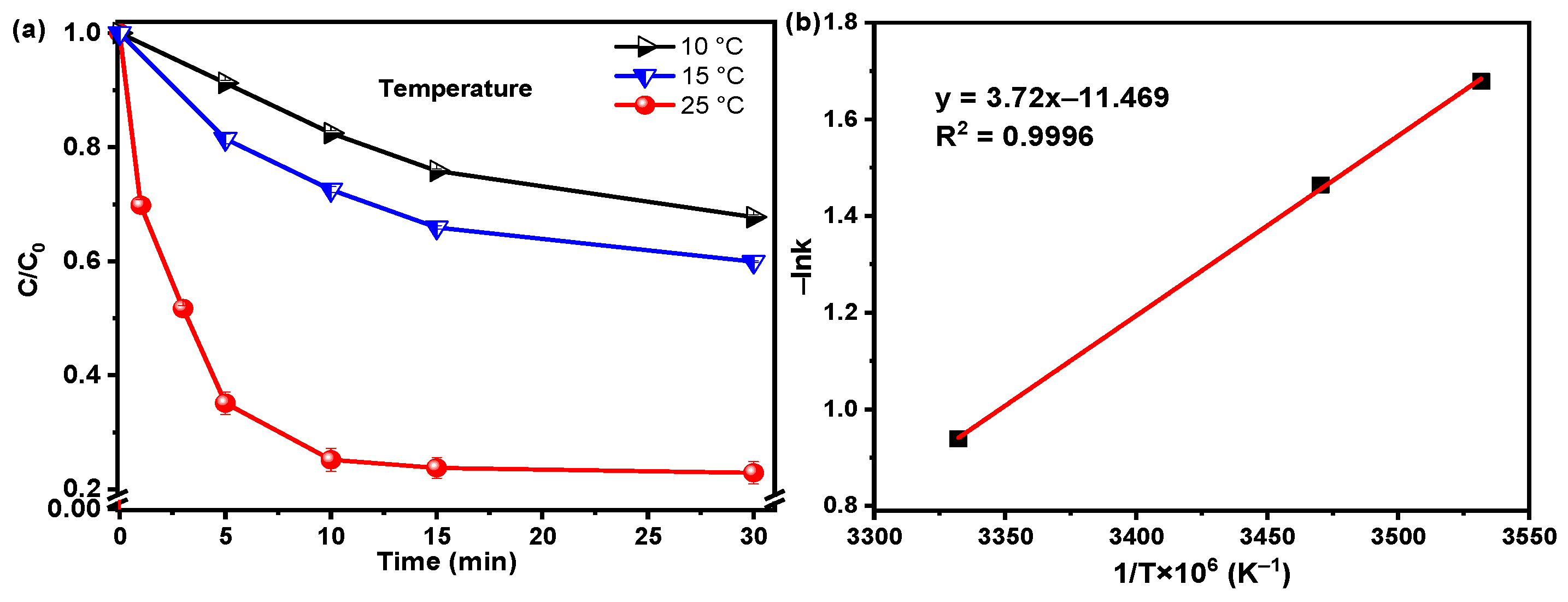
3.2.2. Effect of the Operating Condition
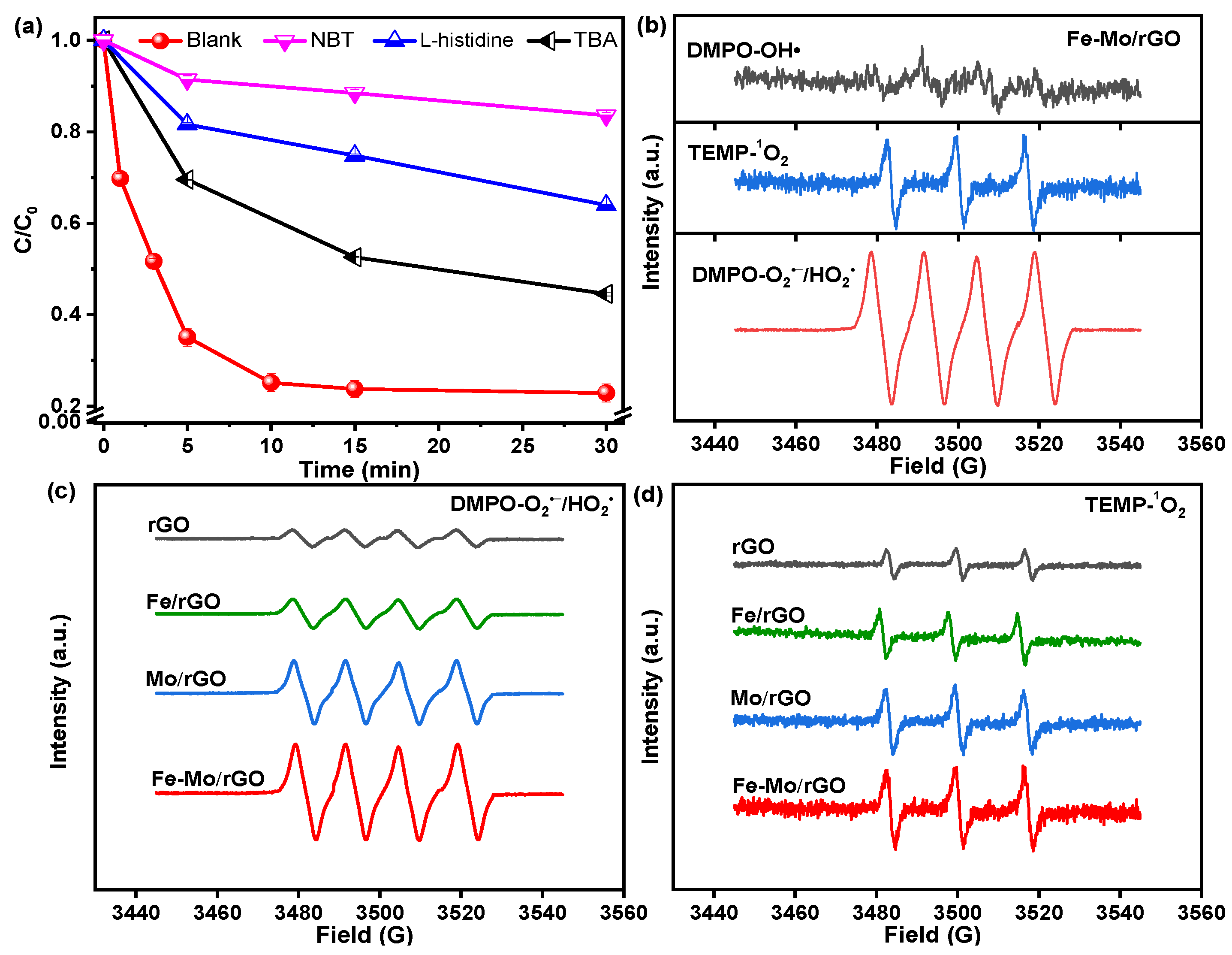
3.3. Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neafsey, K.; Zeng, X.; Lemley, A.T. Degradation of sulfonamides in aqueous solution by membrane anodic Fenton treatment. J. Agric. Food Chem. 2010, 58, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Wang, J.L. Degradation of sulfamethazine using Fe3O4-Mn3O4/reduced graphene oxide hybrid as Fenton-like catalyst. J. Hazard. Mater. 2017, 324, 653–664. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Singh, A.K.; Chakraborty, R.; Vidya, S.S.; Singh, A.; Carabineiro, S.A.C. Methionine-functionalized graphene oxide/sodium alginate bio-polymer nanocomposite hydrogel beads: Synthesis, isotherm and kinetic studies for an adsorptive removal of fluoroquinolone antibiotics. Nanomaterials 2021, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Qian, L.; Guan, X.H.; Wu, D.L.; Zhao, G.H. Continuous bulk FeCuC aerogel with ultradispersed metal nanoparticles: An efficient 3D heterogeneous electro-Fenton cathode over a wide range of pH 3-9. Environ. Sci. Technol. 2016, 50, 5225–5233. [Google Scholar] [CrossRef]
- Guan, X.H.; Yang, H.Y.; Sun, Y.K.; Qiao, J.L. Enhanced immobilization of chromium (VI) in soil using sulfidated zero-valent iron. Chemosphere 2019, 228, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Xu, D.; Chen, Y.C.; Hu, Y.Y. Enhanced degradation of triclosan in heterogeneous E-Fenton process with MOF-derived hierarchical Mn/Fe@PC modified cathode. Chem. Eng. J. 2020, 384, 123324. [Google Scholar] [CrossRef]
- Lyu, L.; Zhang, L.L.; Hu, C. Galvanic-like cells produced by negative charge nonuniformity of lattice oxygen on d-TiCuAl-SiO2 nanospheres for enhancement of Fenton-catalytic efficiency. Environ. Sci. Nano 2016, 3, 1483–1492. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, Q.Z.; Kong, Y.; Shen, C.C.; Hao, H.T.; Dionysiou, D.D.; Shi, B.Y. Enhanced antibiotic removal through a dual-reaction-center Fenton-like process in 3D graphene based hydrogels. Environ. Sci. Nano 2019, 6, 388–398. [Google Scholar] [CrossRef]
- Lyu, L.; Yan, D.B.; Yu, G.F.; Cao, W.R.; Hu, C. Efficient destruction of pollutants in water by a dual-reaction-center Fenton-like process over carbon nitride compounds-complexed Cu(II)-CuAlO2. Environ. Sci. Technol. 2018, 52, 4294–4304. [Google Scholar] [CrossRef]
- Li, J.C.; Li, X.H.; Han, J.D.; Meng, F.S.; Jiang, J.Y.; Li, J.; Xu, C.L.; Li, Y. Mesoporous bimetallic Fe/Co as highly active heterogeneous Fenton catalyst for the degradation of tetracycline hydrochlorides. Sci. Rep. 2019, 9, 15820. [Google Scholar] [CrossRef] [PubMed]
- Li, L.S.; Ye, W.Y.; Zhang, Q.Y.; Sun, F.Q.; Lu, P.; Li, X.K. Catalytic ozonation of dimethyl phthalate over cerium supported on activated carbon. J. Hazard. Mater. 2009, 170, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Wang, X.C.; Zhang, L.L.; Dionysiou, D.D.; Shi, B.Y. Fe-chelated polymer templated graphene aerogel with enhanced Fenton-like efficiency for water treatment. Environ. Sci. Nano 2019, 6, 3232–3241. [Google Scholar] [CrossRef]
- Lyu, L.; Yu, G.F.; Zhang, L.L.; Hu, C.; Sun, Y. 4-phenoxyphenol-functionalized reduced graphene oxide nanosheets: A metal-free Fenton-like catalyst for pollutant destruction. Environ. Sci. Technol. 2018, 52, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Xu, L.M.; Xu, X.Y.; Zhou, X.P.; Luo, J.; Zhang, L.L. Cobalt-doped edge-rich MoS2/nitrogenated graphene composite as an electrocatalyst for hydrogen evolution reaction. Mater. Sci. Eng. B Adv. 2016, 212, 30–38. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, Z.X.; Hussain, M.Z.; Jia, Q.L.; Xia, Y.D. Bimetallic Fe-Mo sulfide/carbon nanocomposites derived from phosphomolybdic acid encapsulated in MOF for efficient hydrogen generation. J. Mater. Sci. Technol. 2021, 84, 76–85. [Google Scholar] [CrossRef]
- Qu, S.Y.; Wang, W.H.; Pan, X.Y.; Li, C.L. Improving the Fenton catalytic performance of FeOCl using an electron mediator. J. Hazard. Mater. 2020, 384, 121494. [Google Scholar] [CrossRef]
- Han, J.; Jun, B.M.; Heo, J.; Lee, G.; Yoon, Y.; Park, C.M. Highly efficient organic dye removal from waters by magnetically recoverable La2O2CO3/ZnFe2O4-reduced graphene oxide nanohybrid. Ceram. Int. 2019, 45, 19247–19256. [Google Scholar] [CrossRef]
- Kharaji, A.G.; Shariati, A. Performance comparison of two newly developed bimetallic (X-Mo/Al2O3, X=Fe or Co) catalysts for reverse water gas shift reaction. China Pet. Process. Petrochem. Technol. 2016, 18, 51–58. [Google Scholar]
- Sun, S.H.; Ren, M.Z.; Pan, F.S.; Yuan, L.; Ning, P.G.; Sun, Z.; Xie, Y.B.; Cao, H.B. Degradation of potassium alkyl xanthogenate in wet air oxidation: Enhancement method, degradation mechanism and structure impact. J. Environ. Chem. Eng. 2022, 10, 107349. [Google Scholar] [CrossRef]
- Zhang, T.; Qian, C.Y.; Guo, P.R.; Gan, S.C.; Dong, L.Y.; Bai, G.; Guo, Q.Y. A novel reduced graphene oxide-attapulgite (RGO-ATP) supported Fe2O3 catalyst for heterogeneous Fenton-like oxidation of ciprofloxacin: Degradation mechanism and pathway. Catalysts 2020, 10, 189. [Google Scholar] [CrossRef]
- Qin, J.X.; Dai, L.; Shi, P.H.; Fan, J.C.; Min, Y.L.; Xu, Q.J. Rational design of efficient metal-free catalysts for peroxymonosulfate activation: Selective degradation of organic contaminants via a dual nonradical reaction pathway. J. Hazard. Mater. 2020, 398, 122808. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, J.L. Degradation of sulfamethazine antibiotics using Fe3O4-Mn3O4 nanocomposite as a Fenton-like catalyst. J. Chem. Technol. Biotechnol. 2017, 92, 874–883. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, Z.; Liu, J.; Deng, Y.; Zhang, D.X.; Du, P.Y.; Zhang, S.T.; Lu, X.Q. Novel Fe-Mn-O nanosheets/wood carbon hybrid with tunable surface properties as a superior catalyst for Fenton-like oxidation. Appl. Catal. B Environ. 2019, 259, 118058. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, S.Z. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Panjwani, M.K.; Wang, Q.; Ma, Y.M.; Lin, Y.X.; Xiao, F.; Yang, S.X. High degradation efficiency of sulfamethazine with the dual-reaction-center Fe-Mn-SiO2 Fenton-like nanocatalyst in a wide pH range. Environ. Sci. Nano 2021, 8, 2204–2213. [Google Scholar] [CrossRef]
- Tang, J.T.; Wang, J.L. Fe3O4-MWCNT magnetic nanocomposites as efficient Fenton-like catalysts for degradation of sulfamethazine in aqueous solution. Chemistryselect 2017, 2, 10727–10735. [Google Scholar] [CrossRef]
- Lyu, L.; Cao, W.R.; Yu, G.F.; Yan, D.B.; Deng, K.L.; Lu, C.; Hu, C. Enhanced polarization of electron-poor/rich micro-centers over nZVCu-Cu(II)-rGO for pollutant removal with H2O2. J. Hazard. Mater. 2020, 383, 121182. [Google Scholar] [CrossRef]
- Indrawirawan, S.; Sun, H.Q.; Duan, X.G.; Wang, S.B. Low temperature combustion synthesis of nitrogen-doped graphene for metal-free catalytic oxidation. J. Mater. Chem. A 2014, 3, 3432–3440. [Google Scholar] [CrossRef]
- Gao, P.; Chen, X.J.; Hao, M.J.; Xiao, F.; Yang, S.X. Oxygen vacancy enhancing the Fe2O3-CeO2 catalysts in Fenton-like reaction for the sulfamerazine degradation under O2 atmosphere. Chemosphere 2019, 228, 521–527. [Google Scholar] [CrossRef]
- Liu, Y.D.; Li, J.S.; Wu, L.R.; Wan, D.J.; Shi, Y.H.; He, Q.C.; Chen, J. Synergetic adsorption and Fenton-like degradation of tetracycline hydrochloride by magnetic spent bleaching earth carbon: Insights into performance and reaction mechanism. Sci. Total Environ. 2021, 761, 143956. [Google Scholar] [CrossRef] [PubMed]
- Su, C.J.; Li, R.H.; Li, C.L.; Wang, W.H. Piezo-promoted regeneration of Fe2+ boosts peroxydisulfate activation by Bi2Fe4O9 nanosheets. Appl. Catal. B Environ. 2022, 310, 121330. [Google Scholar] [CrossRef]
- Qin, W.H.; Ren, M.Y.; Lu, Y.W.; Yang, S.X. High effective degradation of phenol with Cu/Bi-Ce/Al2O3 heterogeneous Fenton-like catalyst in a two-stage fixed-bed reactor. Sep. Purif. Technol. 2022, 299, 121733. [Google Scholar] [CrossRef]
- Jin, H.; Tian, X.K.; Nie, Y.L.; Zhou, Z.X.; Yang, C.; Li, Y.; Lu, L.K. Oxygen vacancy promoted heterogeneous Fenton-like degradation of ofloxacin at pH 3.2-9.0 by Cu substituted magnetic Fe3O4@FeOOH nanocomposite. Environ. Sci. Technol. 2017, 51, 12699–12706. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Quan, X. Carbon-based materials for electrochemical reduction of CO2 to C2+ oxygenates: Recent progress and remaining challenges. ACS Catal. 2021, 11, 2076–2097. [Google Scholar] [CrossRef]
- Chen, Y.J.; Ji, S.F.; Zhao, S.; Chen, W.X.; Dong, J.C.; Cheong, W.C.; Shen, R.A.; Wen, X.D.; Zheng, L.R.; Rykov, A.I.; et al. Enhanced oxygen reduction with single-atomic-site iron catalysts for a zinc-air battery and hydrogen-air fuel cell. Nat. Commun. 2018, 9, 5244. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.Z.; Gu, Y.; Yang, Z.S.; Zhou, L.X. Synthesis and assessment of schwertmannite/few-layer graphene composite for the degradation of sulfamethazine in heterogeneous Fenton-like reaction. R. Soc. Open Sci. 2020, 7, 191977. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Luo, H.; Huang, D.L.; Zhang, C. Biochar-mediated Fenton-like reaction for the degradation of sulfamethazine: Role of environmentally persistent free radicals. Chemosphere 2020, 255, 126975. [Google Scholar] [CrossRef]


| Catalyst | C0 (mM) | Catalyst Dosage (g/L) | [H2O2]0 (mM) | pH | T (°C) | Reaction Time (min) | Removal Efficiency (%) | Initial Reaction Rate (mgSMT/min/gcat) 1 | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Fe-Mo/rGO | 0.076 | 0.2 | 20.0 | 6.6 | 25 | 15 | 80 | 13.9 | This work |
| Fe3O4-Mn3O4/rGO | 0.07 | 0.5 | 6.0 | 3.0 | 35 | 80 | 98 | 0.5 | [2] |
| Fe3O4-Mn3O4 | 0.07 | 0.5 | 6.0 | 3.0 | 45 | 50 | 100 | 0.05 | [23] |
| Fe-Mn-SiO2 | 0.07 | 1.0 | 60.0 | 7.0 | 25 | 180 | 100 | 0.4 | [26] |
| Fe3O4-MWCNTs | 0.07 | 0.5 | 6.0 | 3.0 | 30 | 180 | 98 | 0.9 | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, W.; Ma, Y.; He, T.; Hu, J.; Gao, P.; Yang, S. Enhanced Heterogeneous Fenton-like Process for Sulfamethazine Removal via Dual-Reaction-Center Fe-Mo/rGO Catalyst. Nanomaterials 2022, 12, 4138. https://doi.org/10.3390/nano12234138
Qin W, Ma Y, He T, Hu J, Gao P, Yang S. Enhanced Heterogeneous Fenton-like Process for Sulfamethazine Removal via Dual-Reaction-Center Fe-Mo/rGO Catalyst. Nanomaterials. 2022; 12(23):4138. https://doi.org/10.3390/nano12234138
Chicago/Turabian StyleQin, Weihua, Yueming Ma, Ting He, Jingbin Hu, Pan Gao, and Shaoxia Yang. 2022. "Enhanced Heterogeneous Fenton-like Process for Sulfamethazine Removal via Dual-Reaction-Center Fe-Mo/rGO Catalyst" Nanomaterials 12, no. 23: 4138. https://doi.org/10.3390/nano12234138
APA StyleQin, W., Ma, Y., He, T., Hu, J., Gao, P., & Yang, S. (2022). Enhanced Heterogeneous Fenton-like Process for Sulfamethazine Removal via Dual-Reaction-Center Fe-Mo/rGO Catalyst. Nanomaterials, 12(23), 4138. https://doi.org/10.3390/nano12234138






