Decadal Journey of CNT-Based Analytical Biosensing Platforms in the Detection of Human Viruses
Abstract
1. Introduction
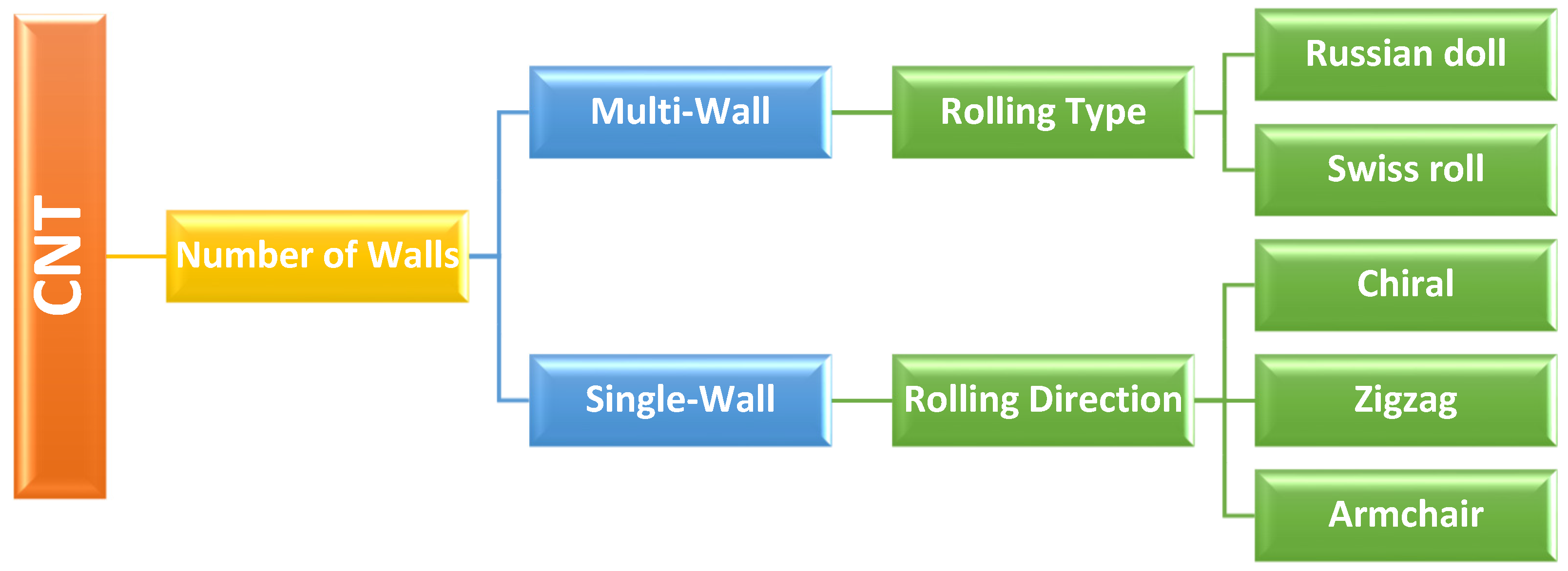
1.1. Carbon Nanotubes and Their Applicability in Biosensing
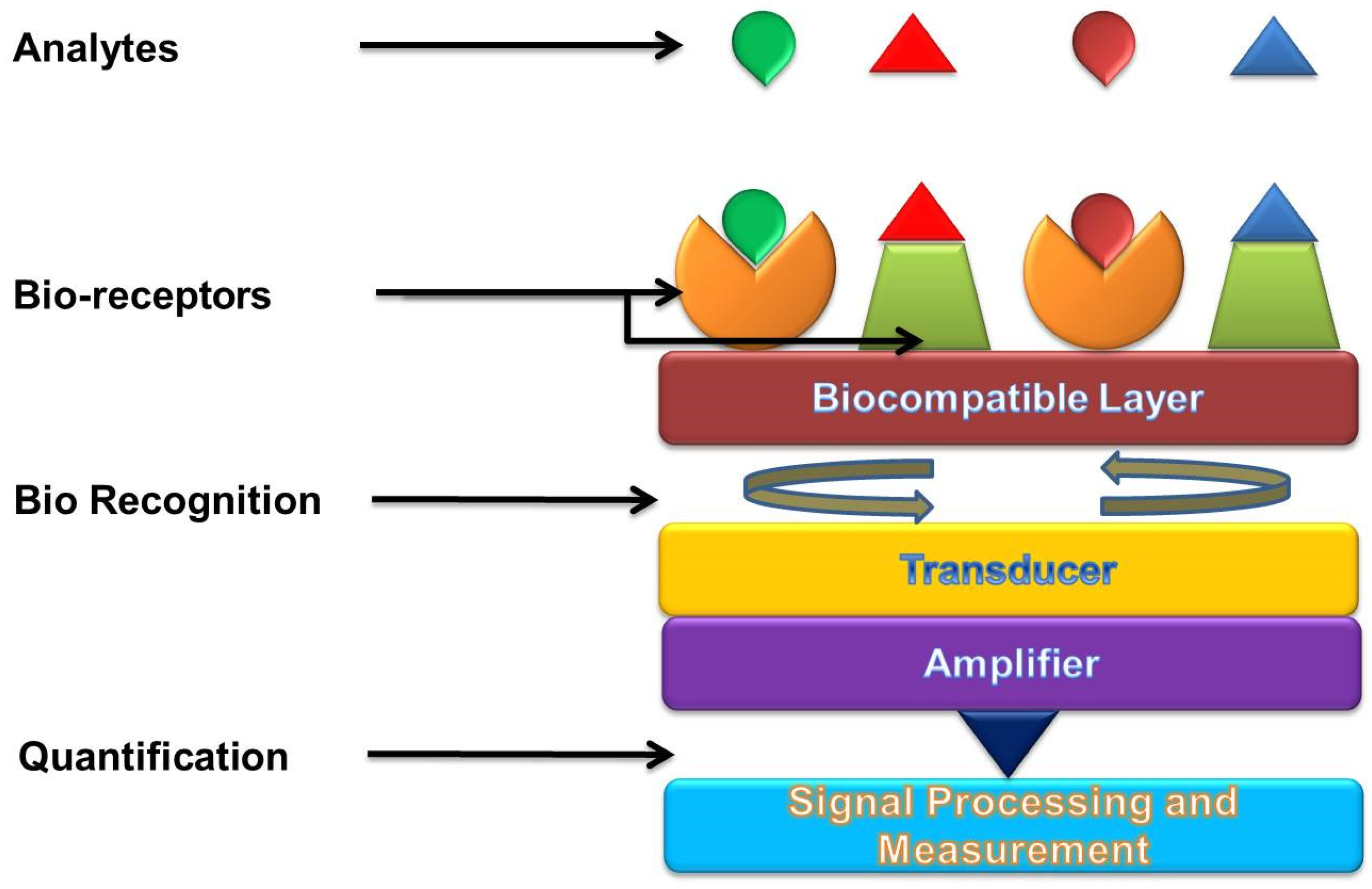
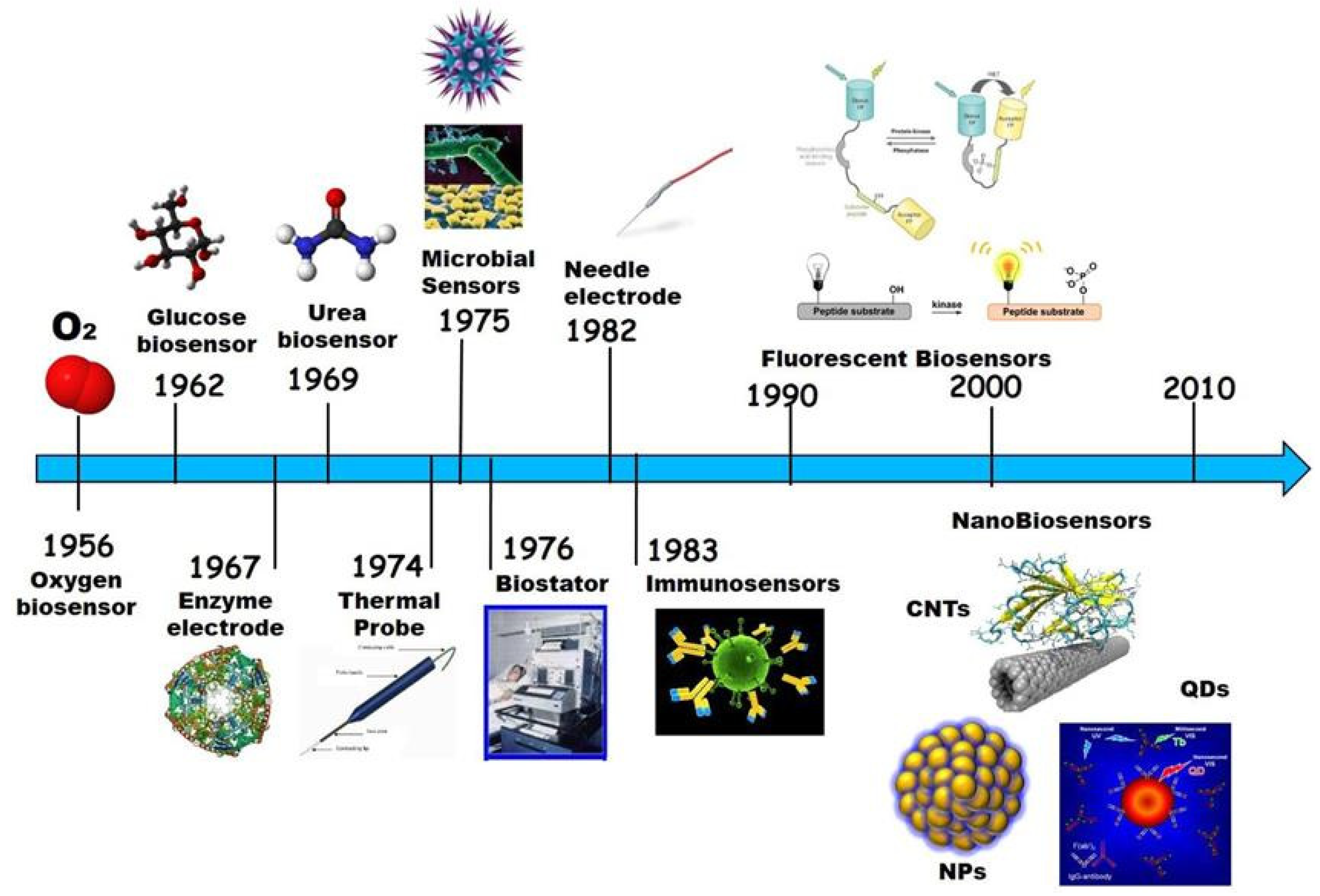
1.2. Biosensors
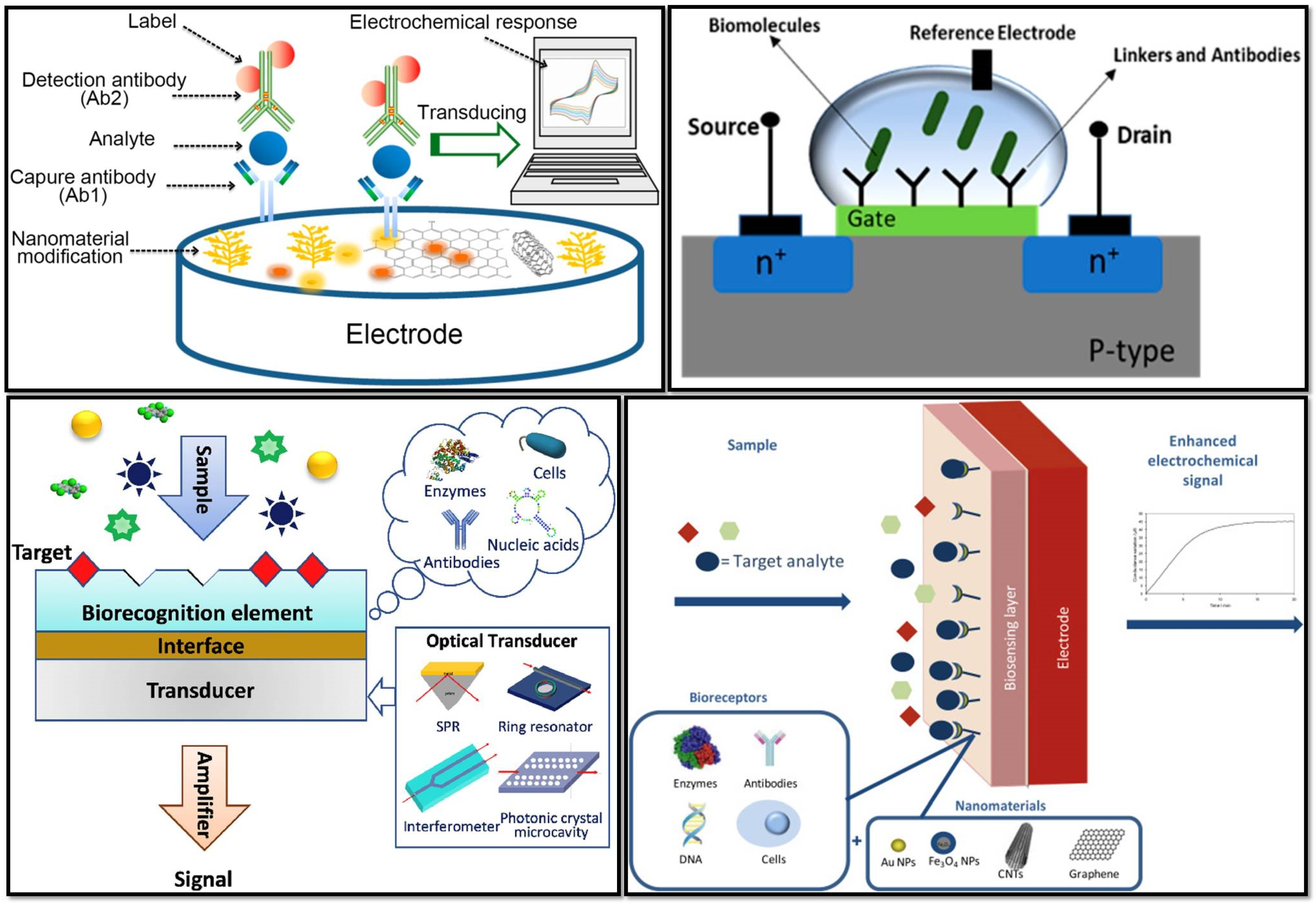
2. Types of Biosensors Used for Virus Detection
2.1. Immunosensors
2.2. Optical Biosensor
2.3. Electrochemical Biosensor
2.4. Field-Effect Transistor (FET)-Based Biosensor
3. CNTs-Based Biosensors for the Detection of Human Viruses
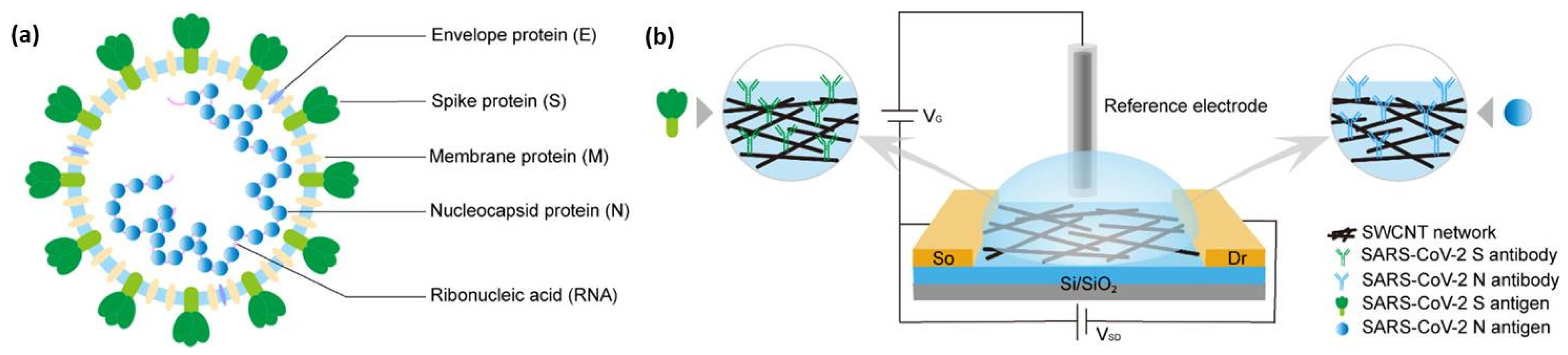
3.1. SARS-CoV-2
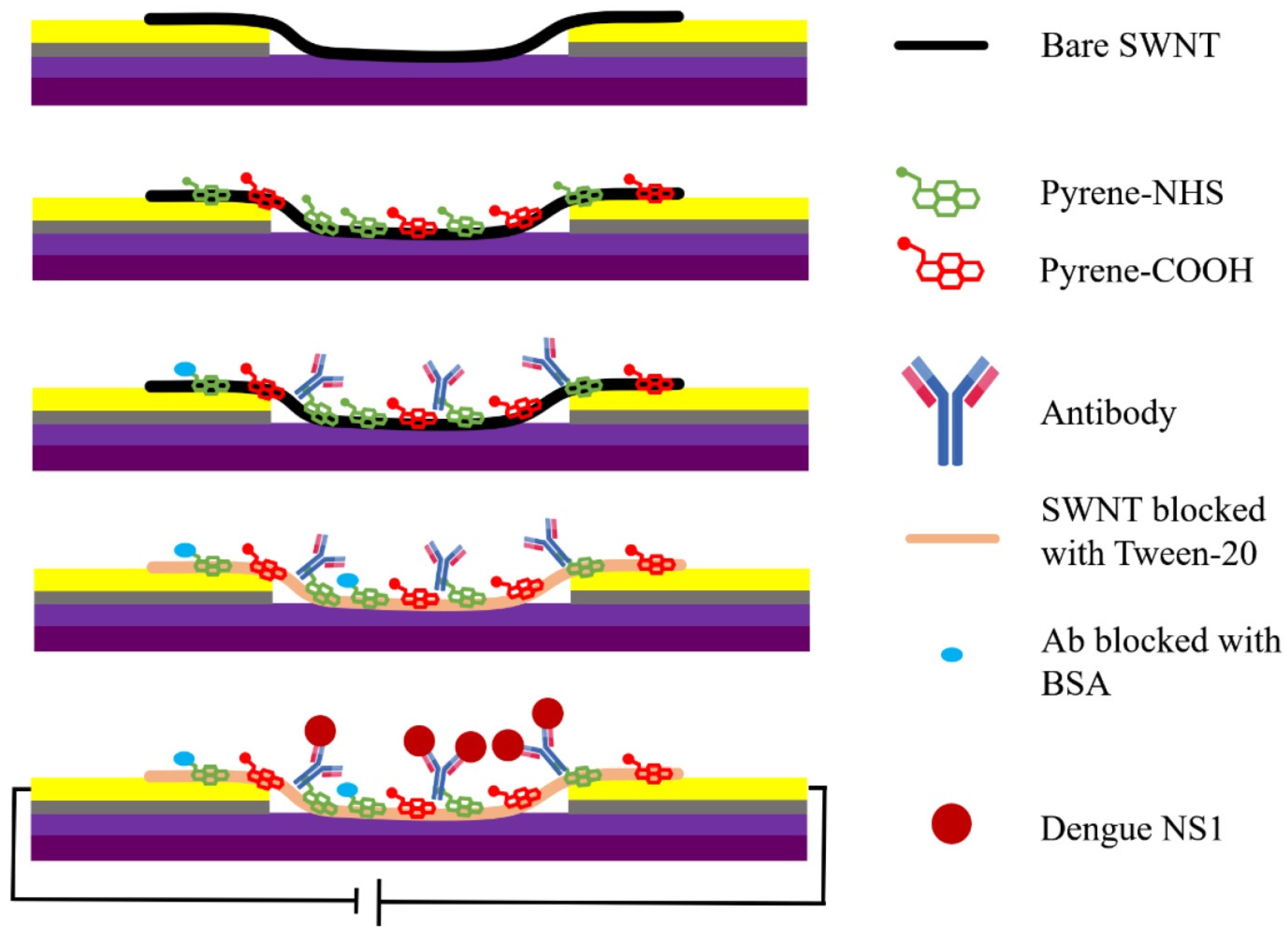
3.2. Dengue Virus (DNV)
3.3. Influenza Virus
3.4. Human Immunodeficiency Virus (HIV)
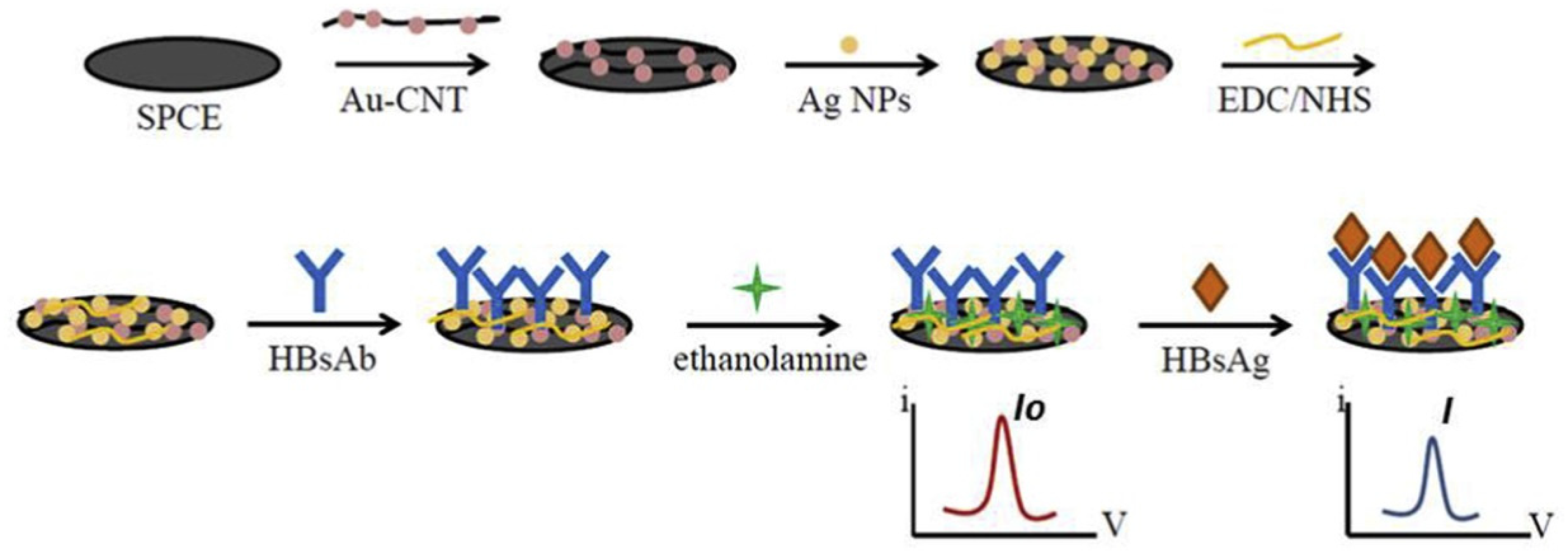
3.5. Hepatitis Virus
| Virus. | Target | Sensor Type | Limit of Detection (LOD) | Detection Range | Detection Platform | Ref. No | |
|---|---|---|---|---|---|---|---|
| SARS-CoV-2 | Antigen | FET-based | 4.12 fg/mL | 0.1 fg/mL to 5.0 pg/mL | CNT surface | [18] | |
| Antigen | FET-based | 0.55 fg/mL | 5.5 fg/mL to 5.5 pg/mL | CNT surface | [17] | ||
| Gene | FET-based | 10 fM | NA | CNT surface | [19] | ||
| Protein | Optical | 12.6 nM | NA | SWCNT substrate | [16] | ||
| Antibody | Electrochemical | 0.7 pg/mL | 0.7 pg/mL and 10.0 ng/mL | Electrode | [20] | ||
| Protein | Electrochemical | 7 nM | NA | Electrode | [21] | ||
| Protein | Immunosensor | 350 genome equivalents/mL | NA | Electrode | [22] | ||
| Protein | Immunosensor | 5.62 fg/mL | NA | CNT thin-film | [23] | ||
| Dengue | Antibody | Immunosensor | 10−13 g/mL | 10−13 to 10−5 g/mL | Electrode | [29] | |
| Protein | Immunosensor | 12 ng/mL | 40 ng/mL to 2 µg/mL | Electrode | [24] | ||
| Protein | Immunosensor | 6.8 ng/mL | 20 to 800 ng/mL | CNT surface | [31] | ||
| Protein | Immunosensor | 0.035 μg/mL | 0.1 to 2.5 μg/mL | Electrode | [25] | ||
| Virus | Immunosensor | 8 DNV /chip | NA | Electrode | [26] | ||
| Antibody | Immunosensor | 0.09 ng/mL | 0.03 to 1200 ng/mL | Electrode | [27] | ||
| Protein | Immunosensor | 1 ng/mL | 1 to 1000 ng/mL | Electrode | [28] | ||
| Toxin | Electrochemical | 3 × 10−13 g/mL | 0.001 to 2 μg/mL | Electrode | [30] | ||
| Influenza | Virus | Immunosensor | 1 ng/mL (for Beijing/262/95 (H1N1)), 0.1 pg/mL (for New Caledonia/20/ 99IvR116 (H1N1)) and 50 PFU/mL (for Yokohama/110/2009 (H3N2)) | 50 to 10,000 PFU/mLm (for Yokohama/110/2009 (H3N2)) | Au-CNT surface | [37] | |
| Aptamer and antibody | Immunosensor | 10 fM | NA | Au-CNT surface | [42] | ||
| Antibody | Immunosensor | 180 TCID50/mL | NA | CNT surface | [35] | ||
| DNA | Immunosensor | 2 pM | 2 to 200 pM | Semiconducting SWCNTs or nitrogen-doped MWCNTs | [40] | ||
| DNA | FET-based | 1 pM | 1 pM to 10 nM | CNT surface | [41] | ||
| DNA | Immunosensor | 8.4 pM | 1 pM to 10 nM | Electrode | [38] | ||
| DNA | Electrochemical | 0.5 nM | NA | Electrode | [33] | ||
| Virus | Immunosensor | 1 PFU/mL | 1 to 10,000 PFU/mL | CNT surface | [36] | ||
| Virus | Optical | 3.4 PFU/mL | 3.4 to 10 PFU/mL | Au-CNT surface | [39] | ||
| DNA | Electrochemical | 7.5 fM | NA | Electrode | [34] | ||
| Antigen | Immunosensor | 55.7 pg/mL for H5N1 HA, 99.6 pg/mL for H7N9 HA, and 54.0 pg/mL for H9N2 HA | 100 pg/mL to 100 ng/m | Electrode | [43] | ||
| DNA | Electrochemical | 1.0 pg/mL | 0.01 to 500 ng/mL | Electrode | [32] | ||
| HIV | Virus | Electrochemical | 0.083 pg/ cm3 | 1.0 × 10−4 to 2 ng/cm3 | Electrode | [47] | |
| Antigen | Immunosensor | 0.0064 ng/mL | 0.01 to 60.00 ng/mL | Electrode | [46] | ||
| Protease Enzyme | Electrochemical | NA | NA | Electrode | [45] | ||
| DNA | Electrochemical | 0.13 nM | 10 nM to 1 μM | Electrode | [50] | ||
| Protease Enzyme | Electrochemical | NA | 10 pM | Electrode | [44] | ||
| Viral nucleic acids | Optical | NA | NA | Fluorescence Detector | [49] | ||
| Protein | Immunosensor | 2 pM | 10 pM to 1 nM | Electrode | [48] | ||
| Hepatitis | B | Antibody | Immunosensor | 0.89 ng/mL | 1.0 to 5.0 ng/mL | Electrode | [56] |
| Antigen | FET-based | NA | NA | CNT surface | [52] | ||
| Virus | Electrochemical | NA | NA | Electrode | [53] | ||
| Antigen | Immunosensor | 0.26 ng/mL | 1–250 ng/mL | Electrode | [57] | ||
| Antibody | Immunosensor | 0.03 ng/mL | 0.03 to 6.0 ng/mL | Electrode | [55] | ||
| Antigen | Immunosensor | 0.01 ng/mL | NA | Electrode | [54] | ||
| DNA | Electrochemical | 1.1 × 10−8 M | 7.94 × 10−8 to 1.58 × 10−6 M | Electrode | [51] | ||
| Antigen | Immunosensor | 0.86 ng/mL | 1–40 ng/mL | Electrode | [58] | ||
| C | Antigen | Immunosensor | 0.015 pg/mL | 0.05 to 1000 pg/mL | Electrode | [60] | |
| RNA | FET-based | 0.5 pM | NA | CNT surface | [59] | ||
4. Current Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Radushkevich, L.V.; Lukyanovich, V.M. The Structure of Carbon Produced by Thermal Decomposition of Carbon Monoxide on an Iron Catalyst. Russ. J. Phys. Chem. 1952, 26, 88–95. [Google Scholar]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Nurazzi Norizan, M.; Harussani Moklis, M.; Demon, S.Z.N.; Abdul Halim, N.; Samsuri, A.; Syakir Mohamad, I.; Feizal Knight, V.; Abdullah, N. Carbon Nanotubes: Functionalisation and Their Application in Chemical Sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Chemistry (IUPAC), T.I.U. of P. and A. IUPAC-Biosensor (B00663). Available online: https://goldbook.iupac.org/terms/view/B00663 (accessed on 18 October 2022).
- Clark, L.C., Jr.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N.Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Parry, R.P.; Love, C.; Robinson, G.A. Detection of Rubella Antibody Using an Optical Immunosensor. J. Virol. Methods 1990, 27, 39–48. [Google Scholar] [CrossRef]
- Davis, J.J.; Green, M.L.H.; Allen, O.; Hill, H.; Leung, Y.C.; Sadler, P.J.; Sloan, J.; Xavier, A.V.; Chi Tsang, S. The Immobilisation of Proteins in Carbon Nanotubes. Inorg. Chim. Acta 1998, 272, 261–266. [Google Scholar] [CrossRef]
- Balavoine, F.; Schultz, P.; Richard, C.; Mallouh, V.; Ebbesen, T.W.; Mioskowski, C. Helical Crystallization of Proteins on Carbon Nanotubes: A First Step towards the Development of New Biosensors. Angew. Chem. Int. Ed. 1999, 38, 1912–1915. [Google Scholar] [CrossRef]
- Tîlmaciu, C.-M.; Morris, M.C. Carbon Nanotube Biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef]
- Sharma, A.; Agrawal, A.; Kumar, S.; Awasthi, K.K.; Awasthi, K.; Awasthi, A. 23-Zinc Oxide Nanostructures–Based Biosensors. In Nanostructured Zinc Oxide; Awasthi, K., Ed.; Metal Oxides; Elsevier: Amsterdam, The Netherlands, 2021; pp. 655–695. ISBN 978-0-12-818900-9. [Google Scholar]
- Bergveld, P. Development of an Ion-Sensitive Solid-State Device for Neurophysiological Measurements. IEEE Trans. Biomed. Eng. 1970, BME-17, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cong, Y.; Huang, Y.; Du, X. Nanomaterials-Based Electrochemical Immunosensors. Micromachines 2019, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Masurkar, N.; Varma, S.; Mohana Reddy Arava, L. Supported and Suspended 2D Material-Based FET Biosensors. Electrochem 2020, 1, 260–277. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Yang, Z.; Wilkinson, J.S.; Zhou, X. Optical Biosensors Based on Refractometric Sensing Schemes: A Review. Biosens. Bioelectron. 2019, 144, 111693. [Google Scholar] [CrossRef] [PubMed]
- Dridi, F.; Marrakchi, M.; Gargouri, M.; Saulnier, J.; Jaffrezic-Renault, N.; Lagarde, F. 5-Nanomaterial-Based Electrochemical Biosensors for Food Safety and Quality Assessment. In Nanobiosensors; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 167–204. ISBN 978-0-12-804301-1. [Google Scholar]
- Pinals, R.L.; Ledesma, F.; Yang, D.; Navarro, N.; Jeong, S.; Pak, J.E.; Kuo, L.; Chuang, Y.-C.; Cheng, Y.-W.; Sun, H.-Y.; et al. Rapid SARS-CoV-2 Spike Protein Detection by Carbon Nanotube-Based Near-Infrared Nanosensors. Nano Lett. 2021, 21, 2272–2280. [Google Scholar] [CrossRef]
- Shao, W.; Shurin, M.R.; Wheeler, S.E.; He, X.; Star, A. Rapid Detection of SARS-CoV-2 Antigens Using High-Purity Semiconducting Single-Walled Carbon Nanotube-Based Field-Effect Transistors. ACS Appl. Mater. Interfaces 2021, 13, 10321–10327. [Google Scholar] [CrossRef]
- Zamzami, M.A.; Rabbani, G.; Ahmad, A.; Basalah, A.A.; Al-Sabban, W.H.; Nate Ahn, S.; Choudhry, H. Carbon Nanotube Field-Effect Transistor (CNT-FET)-Based Biosensor for Rapid Detection of SARS-CoV-2 (COVID-19) Surface Spike Protein S1. Bioelectrochemistry 2022, 143, 107982. [Google Scholar] [CrossRef]
- Thanihaichelvan, M.; Surendran, S.N.; Kumanan, T.; Sutharsini, U.; Ravirajan, P.; Valluvan, R.; Tharsika, T. Selective and Electronic Detection of COVID-19 (Coronavirus) Using Carbon Nanotube Field Effect Transistor-Based Biosensor: A Proof-of-Concept Study. Mater. Today Proc. 2022, 49, 2546–2549. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Alves, J.F.; Frasco, M.F.; Piloto, A.M.; Serrano, V.; Mateus, D.; Sebastião, A.I.; Matos, A.M.; Carmo, A.; Cruz, T.; et al. An Ultra-Sensitive Electrochemical Biosensor Using the Spike Protein for Capturing Antibodies against SARS-CoV-2 in Point-of-Care. Mater. Today Bio 2022, 16, 100354. [Google Scholar] [CrossRef] [PubMed]
- Curti, F.; Fortunati, S.; Knoll, W.; Giannetto, M.; Corradini, R.; Bertucci, A.; Careri, M. A Folding-Based Electrochemical Aptasensor for the Single-Step Detection of the SARS-CoV-2 Spike Protein. ACS Appl. Mater. Interfaces 2022, 14, 19204–19211. [Google Scholar] [CrossRef]
- Li, T.; Soelberg, S.D.; Taylor, Z.; Sakthivelpathi, V.; Furlong, C.E.; Kim, J.-H.; Ahn, S.; Han, P.D.; Starita, L.M.; Zhu, J.; et al. Highly Sensitive Immunoresistive Sensor for Point-Of-Care Screening for COVID-19. Biosensors 2022, 12, 149. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, J.-C.; Seo, G.; Woo, J.H.; Lee, M.; Nam, J.; Sim, J.Y.; Kim, H.-R.; Park, E.C.; Park, S. Computational Method-Based Optimization of Carbon Nanotube Thin-Film Immunosensor for Rapid Detection of SARS-CoV-2 Virus. Small Sci. 2022, 2, 2100111. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.C.M.S.; Gomes-Filho, S.L.R.; Silva, M.M.S.; Dutra, R.F. A Sensor Tip Based on Carbon Nanotube-Ink Printed Electrode for the Dengue Virus NS1 Protein. Biosens. Bioelectron. 2013, 44, 216–221. [Google Scholar] [CrossRef]
- Silva, M.M.S.; Dias, A.C.M.S.; Silva, B.V.M.; Gomes-Filho, S.L.R.; Kubota, L.T.; Goulart, M.O.F.; Dutra, R.F. Electrochemical Detection of Dengue Virus NS1 Protein with a Poly(Allylamine)/Carbon Nanotube Layered Immunoelectrode. J. Chem. Technol. Biotechnol. 2015, 90, 194–200. [Google Scholar] [CrossRef]
- Wasik, D.; Mulchandani, A.; Yates, M.V. A Heparin-Functionalized Carbon Nanotube-Based Affinity Biosensor for Dengue Virus. Biosens. Bioelectron. 2017, 91, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Wasik, D.; Mulchandani, A.; Yates, M.V. Point-of-Use Nanobiosensor for Detection of Dengue Virus NS1 Antigen in Adult Aedes Aegypti: A Potential Tool for Improved Dengue Surveillance. Anal. Chem. 2018, 90, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Wasik, D.; Mulchandani, A.; Yates, M.V. Salivary Detection of Dengue Virus NS1 Protein with a Label-Free Immunosensor for Early Dengue Diagnosis. Sensors 2018, 18, 2641. [Google Scholar] [CrossRef]
- Palomar, Q.; Gondran, C.; Marks, R.; Cosnier, S.; Holzinger, M. Impedimetric Quantification of Anti-Dengue Antibodies Using Functional Carbon Nanotube Deposits Validated with Blood Plasma Assays. Electrochim. Acta 2018, 274, 84–90. [Google Scholar] [CrossRef]
- Palomar, Q.; Xu, X.; Gondran, C.; Holzinger, M.; Cosnier, S.; Zhang, Z. Voltammetric Sensing of Recombinant Viral Dengue Virus 2 NS1 Based on Au Nanoparticle–Decorated Multiwalled Carbon Nanotube Composites. Microchim. Acta 2020, 187, 363. [Google Scholar] [CrossRef]
- Mendonça, P.D.; Santos, L.K.B.; Foguel, M.V.; Rodrigues, M.A.B.; Cordeiro, M.T.; Gonçalves, L.M.; Marques, E.T.A.; Dutra, R.F. NS1 Glycoprotein Detection in Serum and Urine as an Electrochemical Screening Immunosensor for Dengue and Zika Virus. Anal. Bioanal. Chem. 2021, 413, 4873–4885. [Google Scholar] [CrossRef]
- Zhu, X.; Ai, S.; Chen, Q.; Yin, H.; Xu, J. Label-Free Electrochemical Detection of Avian Influenza Virus Genotype Utilizing Multi-Walled Carbon Nanotubes–Cobalt Phthalocyanine–PAMAM Nanocomposite Modified Glassy Carbon Electrode. Electrochem. Commun. 2009, 11, 1543–1546. [Google Scholar] [CrossRef]
- Tam, P.D.; Van Hieu, N.; Chien, N.D.; Le, A.-T.; Anh Tuan, M. DNA Sensor Development Based on Multi-Wall Carbon Nanotubes for Label-Free Influenza Virus (Type A) Detection. J. Immunol. Methods 2009, 350, 118–124. [Google Scholar] [CrossRef]
- Bonanni, A.; Pividori, M.I.; Valle, M. del Impedimetric Detection of Influenza A (H1N1) DNA Sequence Using Carbon Nanotubes Platform and Gold Nanoparticles Amplification. Analyst 2010, 135, 1765–1772. [Google Scholar] [CrossRef]
- Lee, D.; Chander, Y.; Goyal, S.M.; Cui, T. Carbon Nanotube Electric Immunoassay for the Detection of Swine Influenza Virus H1N1. Biosens. Bioelectron. 2011, 26, 3482–3487. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, A.; Hong, S.; Jang, J. Electrical Immunosensor Based on Dielectrophoretically-Deposited Carbon Nanotubes for Detection of Influenza Virus H1N1. Analyst 2014, 139, 5415–5421. [Google Scholar] [CrossRef]
- Lee, J.; Ahmed, S.R.; Oh, S.; Kim, J.; Suzuki, T.; Parmar, K.; Park, S.S.; Lee, J.; Park, E.Y. A Plasmon-Assisted Fluoro-Immunoassay Using Gold Nanoparticle-Decorated Carbon Nanotubes for Monitoring the Influenza Virus. Biosens. Bioelectron. 2015, 64, 311–317. [Google Scholar] [CrossRef]
- Lee, J.; Morita, M.; Takemura, K.; Park, E.Y. A Multi-Functional Gold/Iron-Oxide Nanoparticle-CNT Hybrid Nanomaterial as Virus DNA Sensing Platform. Biosens. Bioelectron. 2018, 102, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.R.; Kim, J.; Suzuki, T.; Lee, J.; Park, E.Y. Enhanced Catalytic Activity of Gold Nanoparticle-Carbon Nanotube Hybrids for Influenza Virus Detection. Biosens. Bioelectron. 2016, 85, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Romay, V.; Liu, Y.; Ibarlucea, B.; Baraban, L.; Khavrus, V.; Oswald, S.; Bachmatiuk, A.; Ibrahim, I.; Rümmeli, M.; et al. Chemiresistive Biosensors Based on Carbon Nanotubes for Label-Free Detection of DNA Sequences Derived from Avian Influenza Virus H5N1. Sens. Actuators B Chem. 2017, 249, 691–699. [Google Scholar] [CrossRef]
- Tran, T.L.; Nguyen, T.T.; Huyen Tran, T.T.; Chu, V.T.; Thinh Tran, Q.; Tuan Mai, A. Detection of Influenza A Virus Using Carbon Nanotubes Field Effect Transistor Based DNA Sensor. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 93, 83–86. [Google Scholar] [CrossRef]
- Wang, F.; Gopinath, S.C.; Lakshmipriya, T. Aptamer-Antibody Complementation on Multiwalled Carbon Nanotube-Gold Transduced Dielectrode Surfaces to Detect Pandemic Swine Influenza Virus. IJN 2019, 14, 8469–8481. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Bhardwaj, J.; Jang, J. Paper-Based Electrochemical Immunosensor for Label-Free Detection of Multiple Avian Influenza Virus Antigens Using Flexible Screen-Printed Carbon Nanotube-Polydimethylsiloxane Electrodes. Sci. Rep. 2022, 12, 2311. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, K.A.; Luong, J.H.T. Impedance Method for Detecting HIV-1 Protease and Screening for Its Inhibitors Using Ferrocene-Peptide Conjugate/Au Nanoparticle/Single-Walled Carbon Nanotube Modified Electrode. Anal. Chem. 2008, 80, 7056–7062. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, K.A.; Luong, J.H.T. A Sensitive Electrochemical Assay for Early Detection of HIV-1 Protease Using Ferrocene-Peptide Conjugate/Au Nanoparticle/Single Walled Carbon Nanotube Modified Electrode. Anal. Lett. 2010, 43, 1680–1687. [Google Scholar] [CrossRef][Green Version]
- Kheiri, F.; Sabzi, R.E.; Jannatdoust, E.; Shojaeefar, E.; Sedghi, H. A Novel Amperometric Immunosensor Based on Acetone-Extracted Propolis for the Detection of the HIV-1 P24 Antigen. Biosens. Bioelectron. 2011, 26, 4457–4463. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shen, X.-L.; Zeng, Q.; Wang, H.-S.; Wang, L.-S. A Multi-Walled Carbon Nanotubes Based Molecularly Imprinted Polymers Electrochemical Sensor for the Sensitive Determination of HIV-P24. Talanta 2017, 164, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, M.; Costantini, M.; Mattarozzi, M.; Careri, M. Innovative Gold-Free Carbon Nanotube/Chitosan-Based Competitive Immunosensor for Determination of HIV-Related P24 Capsid Protein in Serum. RSC Adv. 2017, 7, 39970–39976. [Google Scholar] [CrossRef]
- Harvey, J.D.; Baker, H.A.; Ortiz, M.V.; Kentsis, A.; Heller, D.A. HIV Detection via a Carbon Nanotube RNA Sensor. ACS Sens. 2019, 4, 1236–1244. [Google Scholar] [CrossRef]
- Lu, Q.; Su, T.; Shang, Z.; Jin, D.; Shu, Y.; Xu, Q.; Hu, X. Flexible Paper-Based Ni-MOF Composite/AuNPs/CNTs Film Electrode for HIV DNA Detection. Biosens. Bioelectron. 2021, 184, 113229. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-M.; Zhan, Z.-M.; Ju, H.-Q.; Zhang, S.-S. Label-Free Electrochemical Detection of Short Sequences Related to the Hepatitis B Virus Using 4,4′-Diaminoazobenzene Based on Multiwalled Carbon Nanotube-Modified GCE. Oligonucleotides 2008, 18, 321–328. [Google Scholar] [CrossRef]
- Oh, J.; Yoo, S.; Chang, Y.W.; Lim, K.; Yoo, K.-H. Carbon Nanotube-Based Biosensor for Detection Hepatitis B. Curr. Appl. Phys. 2009, 9, e229–e231. [Google Scholar] [CrossRef]
- Ly, S.Y.; Cho, N.S. Diagnosis of Human Hepatitis B Virus in Non-Treated Blood by the Bovine IgG DNA-Linked Carbon Nanotube Biosensor. J. Clin. Virol. 2009, 44, 43–47. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Z.; Wan, Q. Facile Preparation of Carbon Nanotube-Conducting Polymer Network for Sensitive Electrochemical Immunoassay of Hepatitis B Surface Antigen in Serum. Bioelectrochemistry 2011, 81, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Cabral, D.G.A.; Lima, E.C.S.; Moura, P.; Dutra, R.F. A Label-Free Electrochemical Immunosensor for Hepatitis B Based on Hyaluronic Acid–Carbon Nanotube Hybrid Film. Talanta 2016, 148, 209–215. [Google Scholar] [CrossRef]
- Trindade, E.K.G.; Dutra, R.F. A Label-Free and Reagentless Immunoelectrode for Antibodies against Hepatitis B Core Antigen (Anti-HBc) Detection. Colloids Surf. B Biointerfaces 2018, 172, 272–279. [Google Scholar] [CrossRef]
- Chen, Z.; Bai, Y.; Zhao, F.; Cao, L.; Han, G.; Yin, S. Disposable Amperometric Immunosensor for Hepatitis B Antigen Detection Based on Multiwalled Carbon Nanotubes and Ferrocene Decorated Screen Printed Electrode. J. Biomed. Nanotechnol. 2019, 15, 930–938. [Google Scholar] [CrossRef]
- Upan, J.; Banet, P.; Aubert, P.-H.; Ounnunkad, K.; Jakmunee, J. Sequential Injection-Differential Pulse Voltammetric Immunosensor for Hepatitis B Surface Antigen Using the Modified Screen-Printed Carbon Electrode. Electrochim. Acta 2020, 349, 136335. [Google Scholar] [CrossRef]
- Dastagir, T.; Forzani, E.S.; Zhang, R.; Amlani, I.; Nagahara, L.A.; Tsui, R.; Tao, N. Electrical Detection of Hepatitis C Virus RNA on Single Wall Carbon Nanotube-Field Effect Transistors. Analyst 2007, 132, 738–740. [Google Scholar] [CrossRef] [PubMed]
- Pusomjit, P.; Teengam, P.; Chuaypen, N.; Tangkijvanich, P.; Thepsuparungsikul, N.; Chailapakul, O. Electrochemical Immunoassay for Detection of Hepatitis C Virus Core Antigen Using Electrode Modified with Pt-Decorated Single-Walled Carbon Nanotubes. Microchim. Acta 2022, 189, 339. [Google Scholar] [CrossRef]
- Lacerda, L.; Raffa, S.; Prato, M.; Bianco, A.; Kostarelos, K. Cell-Penetrating CNTs for Delivery of Therapeutics. Nano Today 2007, 2, 38–43. [Google Scholar] [CrossRef]
- Traylen, C.M.; Patel, H.R.; Fondaw, W.; Mahatme, S.; Williams, J.F.; Walker, L.R.; Dyson, O.F.; Arce, S.; Akula, S.M. Virus Reactivation: A Panoramic View in Human Infections. Future Virol. 2011, 6, 451–463. [Google Scholar] [CrossRef]
- Suzich, J.B.; Cliffe, A.R. Strength in Diversity: Understanding the Pathways to Herpes Simplex Virus Reactivation. Virology 2018, 522, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.R. Epstein-Barr Virus (EBV) Reactivation and Therapeutic Inhibitors. J. Clin. Pathol. 2019, 72, 651–658. [Google Scholar] [CrossRef]
- Cook, C.H.; Trgovcich, J. Cytomegalovirus Reactivation in Critically Ill Immunocompetent Hosts: A Decade of Progress and Remaining Challenges. Antivir. Res. 2011, 90, 151–159. [Google Scholar] [CrossRef]
- Cary, D.C.; Fujinaga, K.; Peterlin, B.M. Molecular Mechanisms of HIV Latency. J. Clin. Investig. 2016, 126, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Ozer, T.; Henry, C.S. Paper-Based Analytical Devices for Virus Detection: Recent Strategies for Current and Future Pandemics. TrAC Trends Anal. Chem. 2021, 144, 116424. [Google Scholar] [CrossRef] [PubMed]
- Beduk, T.; Beduk, D.; Hasan, M.R.; Guler Celik, E.; Kosel, J.; Narang, J.; Salama, K.N.; Timur, S. Smartphone-Based Multiplexed Biosensing Tools for Health Monitoring. Biosensors 2022, 12, 583. [Google Scholar] [CrossRef]
- Requardt, H.; Braun, A.; Steinberg, P.; Hampel, S.; Hansen, T. Surface Defects Reduce Carbon Nanotube Toxicity in Vitro. Toxicol. Vitr. 2019, 60, 12–18. [Google Scholar] [CrossRef]
- Qi, W.; Tian, L.; An, W.; Wu, Q.; Liu, J.; Jiang, C.; Yang, J.; Tang, B.; Zhang, Y.; Xie, K.; et al. Curing the Toxicity of Multi-Walled Carbon Nanotubes through Native Small-Molecule Drugs. Sci. Rep. 2017, 7, 2815. [Google Scholar] [CrossRef]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of Toxicity Studies of Carbon Nanotubes. J. Occup. Health 2017, 59, 394–407. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Prospective Pathways of Green Graphene-Based Lab-on-Chip Devices: The Pursuit toward Sustainability. Microchim. Acta 2022, 189, 177. [Google Scholar] [CrossRef]
- SARS-CoV-2 Variants of Concern as of 27 October 2022. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 3 November 2022).
- Xi, H.; Jiang, H.; Juhas, M.; Zhang, Y. Multiplex Biosensing for Simultaneous Detection of Mutations in SARS-CoV-2. ACS Omega 2021, 6, 25846–25859. [Google Scholar] [CrossRef]
- Banerjee, I.; Douaisi, M.P.; Mondal, D.; Kane, R.S. Light-Activated Nanotube–Porphyrin Conjugates as Effective Antiviral Agents. Nanotechnology 2012, 23, 105101. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Galvagno, S.; Ferro, S.; De Luca, L.; Monforte, A.M.; Da Ros, T.; Hadad, C.; Prato, M.; Pannecouque, C. Synthesis and Anti-HIV Activity of Carboxylated and Drug-Conjugated Multi-Walled Carbon Nanotubes. Carbon 2015, 82, 548–561. [Google Scholar] [CrossRef]



| Pros | Cons |
|---|---|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sengupta, J.; Hussain, C.M. Decadal Journey of CNT-Based Analytical Biosensing Platforms in the Detection of Human Viruses. Nanomaterials 2022, 12, 4132. https://doi.org/10.3390/nano12234132
Sengupta J, Hussain CM. Decadal Journey of CNT-Based Analytical Biosensing Platforms in the Detection of Human Viruses. Nanomaterials. 2022; 12(23):4132. https://doi.org/10.3390/nano12234132
Chicago/Turabian StyleSengupta, Joydip, and Chaudhery Mustansar Hussain. 2022. "Decadal Journey of CNT-Based Analytical Biosensing Platforms in the Detection of Human Viruses" Nanomaterials 12, no. 23: 4132. https://doi.org/10.3390/nano12234132
APA StyleSengupta, J., & Hussain, C. M. (2022). Decadal Journey of CNT-Based Analytical Biosensing Platforms in the Detection of Human Viruses. Nanomaterials, 12(23), 4132. https://doi.org/10.3390/nano12234132






